Abstract
The detrimental health implications of chemical preservatives in fruits have necessitated exploitation of safe and natural alternatives such as edible gums. This work studied shelf-life extension in grape (Pinot noir) under cold storage by xanthan gum (XAN) coatings enriched with ascorbic acid (XANAS) and citric acid (XANCI). Standard scientific methods were used to examine some sensory (color, texture—resilience and hardness), enzyme, anthocyanine and antioxidant activities. Also, the reaction rate mechanism was examined through modeling of selected shelf-life indicators; color change, weight loss, and antioxidants. The results revealed that, Xanthan gum and its acid modified coatings significantly (p < 0.05) suppressed polyphenol oxidase, ascorbic acid oxidase, polymethyl etherase acitivies and maintained the structural integrity of the grape during the 21 days storage period. Weight loss (%) in the grape samples was 13.66 < 13.98 < 14.16 < 15.64 in the order XANAS < XANCI < XAN < CONTROL whilst ferric reducing antioxidant power (FRAP) activity was 150.23 > 143.18 > 136.49 > 104.5 mg/100 g AEAC corresponding to XAN > XANAS > XANCI > CONTROL. Significantly (p < 0.05) higher phytochemical contents were observed in the gum coatings compared to the control. Through statistical parameters such as the coefficient of determination (R2), root mean square error (RMSE) and reduced Chi square (χ2), the second-order polynomial model predicted precisely the decomposition of color, weight loss and FRAP of grape. Color deterioration was attributed to changes in b* parameter as a result of phenolics and phytochemical decompositions resulting from enzymatic activities. Conclusively, acid modified xanthan gum coatings could preserve phytochemicals, color, antioxidant and textural properties of grape in cold temperature storage.
Keywords: Antioxidant, Chromatic, Enzyme activity, PCA, Phytochemicals, Kinetics
Introduction
Grapes (Pinot noir) remain one of the popular agricultural products and more than 50 million tonnes are produced annually with China being the world’s leading producer (Beristain et al. 2001; Tsali and Goula 2018). The high phenolics and antioxidative properties of this fruit render them efficient in chronic and cardiovascular disease prevention (Kalamara et al. 2015; Khadem and Marles 2010). The fruit is also delicate and tender with high losses along its value chain. As a result of high post-harvest losses of grape, more than 75% is processed into wine after harvest (Tsali and Goula 2018). Irrespective of the challenges of preserving the fruits in the natural forms for a longer period, health conscious patrons of grape still prefer consuming grapes in its unprocessed form. The grape fraternity has since been confronted with the quest to finding novel and non-destructive grapes preservation mechanisms. Studies have identified shelf-life parameters such as browning, weight loss, decay, softening and shattering as the main indicators for deterioration of stored grapes (Del Nobile et al. 2009; Nicolosi et al. 2018). According to Sarpong et al. (2018a), grape producers have resorted to the use of chemicals, including sulphites, sodium benzoate, nitrates and aspartame in the preservation of these fruits. However, the hostile health implications of these chemicals have led to alternative safer and natural preservative such as edible gums.
The introduction as well as the use of edible gums is gaining preference among researchers and grape producers as a result of its natural, cheaper, encapsulation, film forming and emulsification properties (Al-Juhaimi 2014; Ali et al. 2010, 2013; Sarpong et al. 2018a). The preservative principles of edible gums reside in their ability to create modified atmosphere by forming a semi-permeable barrier to prevent oxygen, carbon dioxide, solute and moisture movement. This subsequently leads to the reduction of water, respiration and oxidation reaction (Ali et al. 2010; Falcão-Rodrigues et al. 2007).
In our earlier work, Sarpong et al. (2018a), we demonstrated the superior nature of xanthan gum in controlling enzyme inactivation and browning pigmentation of apple slices among acacia senegal and karaya gums. In this work we focused on (1) shelf-life extension of grapes (Pinot noir) under cold temperature storage with xanthan gum enhancement with ascorbic and citric acids, and (2) examination of the reaction rate mechanism through modelling of selected shelf-life indicators (color change, weight loss and antioxidants) for future prediction.
Materials and methods
Sample preparation
Grapes (Pinot noir) with 16.2 ± 1.2% soluble solid content were obtained from Jiangsu University fruit and vegetable market, Zhenjiang, Jiangsu Province at commercial maturity and transported to the laboratory for immediate processing. The choice of grape was based on the absence of physical or mechanical wounds and microbial decay. Also, homogeneity in firmness, color, size, and shape, were also necessary considerations in the selection of the fruits.
Experimental design and preparation of dipping solutions
Four coating solutions were formulated according to a previously described protocol of Sarpong et al. (2018a). The coating solutions included Xanthan gum (10 g) only herein referred to as XAN, Xanthan gum (10 g) enriched with ascorbic acid (1.0% w/v) designated as XANAS, Xanthan gum (10 g) enriched with citric acid (1.0% w/v) denoted as XANCI and distilled water (containing 1% glycerol and Tween-20) coating used as control (CON). To prepare the gum coating solutions, powdered gums weighing 10 g were dissolved in distilled water (100 mL) with 3% (w/v) of calcium chloride to improve wettability. Hot plate magnetic stirrer (model SP 18420-26 Barnstead thermolyne 2555 Kerper Boulevard Dubuque USA) was used in heating the solution at 40 °C for 60 min. Afterwards, glycerol (1%) was added (serving as a plasticizer) to the coating solutions. Grapes were dipped in the solutions for 3 min, air-dried for 30 min, packed in plastic bags (280 × 200 mm) and then stored in the refrigerator at 4 ± 1 °C and 85% relative humidity (RH) for 21 days. Measurement was done in four samples per formulation on day 0 (1 h after coating) and 3 days interval until 21 days.
Weight loss
For weight loss, 100 g of grapes was weighed and calculated as a percentage using Eq. (1)
| 1 |
Enzymes activities
Polyphenol oxidase (PPO) and ascorbic acid oxidase (AAO) extraction and assay was determined spectrophotometrically following a method described in our previous works (Sarpong et al. 2018a). A unit of PPO and AAO (units per g (U/g)) was defined as the amount of PPO and AAO to cause a change of 0.001 in absorbance unit per min under assay condition. Pectin methylesterase (PME) was determined following a method described by Cautela et al. (2018) with minor modification. Briefly, 5 g of grapes was blended and mixed with 20 ml of 1% pectin-salt solution (containing 10 g pectin and 15.3 g NaCl dissolved in 1.0 L distilled water) and incubated at 30 °C. The pH of the solution was adjusted to 7 using 2 N NaOH. Later, the pH was again readjusted to 7.7 with 0.05 N NaOH and the time to retain 7.7 pH throughout the test period using 0.1 ml of 0.05 N NaOH was noted. The PME enzyme unit (U/g) was calculated according to Eq. (2)
| 2 |
Determination of phytochemicals
The pH deferential method (Wang et al. 2015) was used to determine anthocyanin concentration. The absorbance of ground grape in 0.025 mol/L KCl2 solution (pH 1.0 buffer) and 0.4 mol/L sodium acetate buffer (pH 4.5) was read simultaneously at 510 and 700 nm using ultraviolet–visible spectrophotometer (TU-1810; Purkinje General Instrument Co., Ltd., Beijing, China). Anthocyanin estimation was based on the following Eq. (3) and expressed as µg/100 g C3GE (cyanidin-3-glucoside equivalent). Total phenolic contents (TPC) were determined using the Folin–Ciocalteau by a method described in our previous work (Sarpong et al. 2018b) and measured at 765 nm using ultraviolet–visible spectrophotometer (TU-1810; Purkinje General Instrument Co., Ltd., Beijing, China) using gallic acid as a standard. The results were expressed in µg/100 mL GAE (gallic acid equivalents). The total flavonoid contents (TFC) were estimated by aluminum chloride assay using a previously described technique by Sarpong et al. (2018b) and read at 420 nm. The results were expressed as µg equivalents of rutin (ER) per 100 g based on the calibration curve of rutin (µg/100 g ER).
| 3 |
where A = pH 1.0 (A530nm − A700nm) − pH 4.5 (A530nm − A700nm); MW = molecular weight of cyanidin-3-glucoside (449.2 g/mol); DF = dilution factor and ε = molar extinction coefficient of cyanidin-3-glucoside (26,900 L/mol × cm).
Antioxidant activity assay
The antioxidant capacity of the gum coated grape samples under cold storage was determined by ferric reducing antioxidant power (FRAP) assay. The antioxidants present in the sample reduce the Fe(III)/tripyridyltriazine (TPTZ) complex to the blue ferrous form with an increase in absorbance at 593 nm. The FRAP activity was determined according to Sarpong et al. (2018b)_ENREF_25 protocol. A freshly prepared FRAP reagent containing 25 mL of acetate buffer (0.3 µM pH 3.6), 2.5 mL of 10 µM TPTZ in 40 mM HCl and 2.5 mL of 20 µM FeCl3·6H2O warmed at 37 °C. A volume of 50 µL extract was mixed with 950 µL FRAP reagent and measured at 593 nm absorbance using ultraviolet–visible spectrophotometer, (TU-1810; Purkinje General Instrument Co., Ltd., Beijing, China) for 5 min. The results of FRAP were presented as milligram per hundred grams of ascorbic acid equal antioxidant capacity (mg/100 g AEAC) using ascorbic acid as a standard curve.
Color measurements
Color assay of the grape samples was carried out using a colorimeter (DC-P3 Time Group Inc., Beijing, China) equipped with illuminant D65 according to the protocol described by Sarpong et al. (2018c). Colors were expressed in L*-value (lightness), a*-value (redness/greenness) and b*-value (yellowness/blueness). Prior to each color quantification, the colorimeter was standardized with a typical white plate (L* = 96.98, a* = 0.03 and b* = 1.84). The total color difference (ΔE) was computed using Eq. (4).
| 4 |
Kinetics models of color change, weight loss and antioxidants degradation
The second-order polynomial model (Eq. 5) was used to evaluate color change, weight loss and antioxidants degradation kinetics;
| 5 |
where is initial component measure at time zero, is the value at time (t), a, b, and c are the kinetic parameters. Origin-Pro 9.2 (Origin Lab Corporation, Northampton, MA, USA) tool was used to perform the regression analysis. The best model fitting was evaluated by the coefficient of estimation (R2), root mean square error (RMSE) and reduced Chi square () (Sarpong et al. 2019b).
Texture
The texture profile analysis (TPA) of grape (after 21 days) was determined by compressing sample up to 50% of its original height in a first cycle of texture profile analysis using a texture measuring instrument (TA-XT2i M/s. Stable Microsystems, Survey, UK). A stainless steel ball probe (0.25S) was selected and the pre-speed, post-speed and compression speed were set at 2.0, 2.0 and 1.0 mms−1, respectively as fully enumerated in our previous study. The TPA was performed for ten replicates for each gum coating sample as well as the control and average values expressed as N and J m−3 for firmness and resilience respectively.
Statistical analysis
Data was analyzed using Origin-Pro 9.2 (Origin Lab Corporation, Northampton, MA, USA) and presented as the means ± standard deviations. For further comparison, one-way Analysis of variance (ANOVA), Pearson’s coefficient of correlation and Turkey’s multiple comparison tests were performed using XLSTAT version 2014.5.03 (Microsoft Office Corporation, USA).
Results and discussion
Influence of acid enriched Xanthan gum coating on grape quality in cold temperature storage
The application of edible gums for shelf-life extension of fruit is gaining popularity in recent times (Lopes et al. 2015; Sarpong et al. 2018a; Shiekh et al. 2013). The influence of acid enriched Xanthan gum coating on grape wholesomeness under cold storage were studied. Table 1 showed the changes in the enzymes (Polyphenol oxidase–PPO and Ascorbic acid oxidase–AAO) activity, antioxidant (Ferric reducing antioxidant power–FRAP, Total phenolic content–TPC, Total flavonoid content–TFC, and Anthocynide–ANTHOCY) ability and chromatic (color–L, a, b, and ΔE) properties of grape as affected by acid enriched Xanthan gum pretreatment during 21 days of cold storage.
Table 1.
Influence of modified xanthan gum pretreatment on antioxidant, enzyme, phytochemicals and chromatic as well as textural properties of grapes under cold storage
| Parameter | Treatment | Storage period (days) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 9 | 12 | 15 | 18 | 21 | ||
| PPO (U/g) | Control | 91.871 ± 2.7a | 102.25 ± 7.9a | 142.50 ± 6.7d | 266.5 ± 3.9a | 277.37 ± 4.3a | 308.25 ± 5.3a | 407.25 ± 5.5a | 438.53 ± 4.6a |
| XAN | 91.877 ± 2.5a | 104.5 ± 8.4a | 152.25 ± 5.2c | 176.00 ± 5.2d | 179.75 ± 3.3d | 181.00 ± 4.3d | 180.75 ± 4.2d | 210.50 ± 3.2c | |
| XANAS | 91.875 ± 2.1a | 112.5 ± 5.9c | 182.75 ± 6.1a | 216.00 ± 4.2b | 213.50 ± 5.3b | 244.75 ± 3.3b | 260.50 ± 4.5b | 296.01 ± 3.3b | |
| XANCI | 91.873 ± 2.5a | 118.25 ± 5.2b | 178.01 ± 4.3b | 197.75 ± 3.1c | 184.75 ± 3.2c | 190.75 ± 4.4c | 196.50 ± 3.3c | 206.03 ± 3.1d | |
| AAO (U/g) | Control | 69.57 ± 3.6a | 67.55 ± 2.9a | 77.51 ± 2.1a | 60.10 ± 3.1a | 42.52 ± 2.1a | 36.25 ± 2.3a | 30.00 ± 2.2a | 32.50 ± 2.2a |
| XAN | 69.51 ± 4.5a | 49.51 ± 5.1c | 69.52 ± 1.9b | 42.52 ± 2.7b | 29.51 ± 3.3b | 27.10 ± 2.3b | 25.01 ± 1.3b | 25.25 ± 2.3b | |
| XANAS | 69.53 ± 3.2a | 52.56 ± 3.9b | 65.01 ± 2.3c | 35.91 ± 1.9d | 22.50 ± 2.2c | 17.91 ± 1.2c | 17.53 ± 3.2c | 21.25 ± 1.2d | |
| XANCI | 69.55 ± 3.0a | 47.59 ± 5.0c | 63.57 ± 2.9d | 40.51 ± 2.2c | 21.90 ± 4.1c | 12.50 ± 3.2d | 18.50 ± 2.2c | 18.00 ± 1.9c | |
| FRAP (mg/100 g AEAC) | Control | 255.63 ± 1.2c | 233.25 ± 1.2b | 219.51 ± 1.1b | 198.36 ± 1.3c | 186.27 ± 1.4c | 162.53 ± 1.2c | 142.10 ± 2.4c | 104.55 ± 1.4d |
| XAN | 267.51 ± 3.2b | 241.45 ± 3.2a | 227.21 ± 2.3a | 205.34 ± 1.1a | 191.96 ± 1.3a | 177.57 ± 2.3a | 152.75 ± 2.5a | 150.23 ± 3.5a | |
| XANAS | 273.68 ± 2.2a | 222.60 ± 1.1c | 210.95 ± 2.5c | 201.38 ± 3.3b | 189.87 ± 2.2b | 171.17 ± 2.2a | 149.73 ± 1.4b | 143.18 ± 2.4b | |
| XANCI | 267.00 ± 2.8b | 233.11 ± 3.3b | 217.57 ± 1.2b | 202.75 ± 2.3b | 171.88 ± 1.4d | 160.95 ± 1.2d | 143.65 ± 3.5c | 136.49 ± 2.5c | |
| TPC (µg/100 g GAE) | Control | 172.03 ± 1.1a | 200.26 ± 2.2c | 235.88 ± 3.1c | 276.63 ± 2.2c | 312.49 ± 3.2c | 341.51 ± 1.1d | 349.32 ± 2.3d | 359.80 ± 2.3d |
| XAN | 172.05 ± 1.3a | 222.97 ± 3.3a | 370.54 ± 3.1a | 481.02 ± 1.2a | 556.63 ± 2.2d | 664.92 ± 3.1a | 742.24 ± 3.2a | 771.27 ± 3.2a | |
| XANAS | 172.10 ± 2.0a | 193.25 ± 3.1d | 208.34 ± 2.3d | 256.63 ± 1.3d | 263.95 ± 3.2b | 344.44 ± 2.2c | 415.17 ± 3.2c | 488.34 ± 1.2c | |
| XANCI | 172.09 ± 1.1a | 210.99 ± 2.1b | 259.07 ± 2.2b | 334.44 ± 2.4b | 362.73 ± 1.3b | 434.68 ± 3.0b | 476.15 ± 1.2b | 570.29 ± 3.2b | |
| TFC (µg/100 g ER) | Control | 589.76 ± 1.4a | 665.07 ± 1.2b | 785.79 ± 3.5b | 895.07 ± 2.2c | 866.14 ± 1.5b | 834.71 ± 2.3b | 758.21 ± 3.6d | 751.93 ± 3.3d |
| XAN | 589.73 ± 1.9a | 653.65 ± 2.1c | 671.50 ± 2.6d | 859.71 ± 1.5d | 839.00 ± 2.4d | 795.07 ± 1.5d | 786.14 ± 2.5c | 779.00 ± 2.5c | |
| XANAS | 589.71 ± 1.2a | 665.08 ± 1.5b | 765.07 ± 1.4c | 946.14 ± 1.4b | 849.00 ± 1.3c | 809.71 ± 2.4c | 795.79 ± 1.4b | 789.71 ± 1.4b | |
| XANCI | 589.69 ± 1.5a | 700.78 ± 1.6a | 881.14 ± 2.4a | 998.71 ± 2.3a | 928.29 ± 2.4a | 928.64 ± 2.3a | 926.14 ± 1.5a | 892.93 ± 2.5a | |
| ANTHOCY (µg/100 g C3GE) | Control | 24.93 ± 0.4a | 19.60 ± 0.9c | 18.36 ± 1.2c | 17.85 ± 0.5c | 17.04 ± 1.3c | 17.00 ± 0.4c | 17.00 ± 1.4c | 16.97 ± 0.9c |
| XAN | 24.97 ± 0.6a | 24.72 ± 0.6a | 24.03 ± 0.5a | 23.76 ± 0.4a | 22.36 ± 0.9a | 22.10 ± 1.0a | 21.99 ± 0.6a | 21.79 ± 0.9a | |
| XANAS | 24.91 ± 1.4a | 23.54 ± 1.3b | 22.53 ± 0.8b | 21.57 ± 0.6b | 21.04 ± 0.4b | 20.92 ± 0.5b | 20.80 ± 0.3b | 20.57 ± 0.6b | |
| XANCI | 24.95 ± 1.0a | 22.90 ± 1.4b | 21.02 ± 1.3b | 21.07 ± 0.4b | 20.94 ± 1.3b | 20.87 ± 0.4b | 20.46 ± 0.9b | 20.17 ± 0.7b | |
| Color (L*) | Control | 27.85 ± 2.4c | 23.86 ± 2.2c | 21.34 ± 4.7c | 20.45 ± 4.6c | 20.08 ± 6.4d | 18.28 ± 3.5c | 17.81 ± 6.7c | 16.17 ± 6.7c |
| XAN | 32.49 ± 1.4a | 33.50 ± 2.7a | 30.68 ± 5.2a | 28.20 ± 3.9a | 27.33 ± 4.7b | 26.38 ± 5.6a | 25.27 ± 6.6a | 24.63 ± 6.6a | |
| XANAS | 28.18 ± 2.4b | 29.39 ± 3.6b | 29.90 ± 2.8a | 29.25 ± 5.1a | 28.22 ± 2.9a | 26.89 ± 4.5a | 25.39 ± 3.8a | 22.96 ± 3.8b | |
| XANCI | 26.76 ± 3.4c | 27.33 ± 4.2c | 27.04 ± 3.4b | 26.88 ± 3.6b | 26.05 ± 5.1c | 25.48 ± 5.3b | 24.63 ± 3.9b | 23.65 ± 4.9a | |
| Color (a*) | Control | 2.56 ± 1.4a | 2.99 ± 2.5a | 3.40 ± 2.9a | 3.55 ± 2.7a | 3.45 ± 2.2a | 3.14 ± 2.9a | 2.64 ± 2.7b | 2.59 ± 2.7a |
| XAN | 2.05 ± 0.4a | 2.65 ± 0.6b | 3.18 ± 2.6b | 3.16 ± 2.8b | 3.12 ± 3.8b | 3.09 ± 3.2b | 3.07 ± 5.1a | 2.98 ± 5.1a | |
| XANAS | 2.02 ± 2.4a | 2.52 ± 0.1c | 2.72 ± 2.9c | 2.95 ± 5.2c | 2.82 ± 3.7c | 2.55 ± 3.1c | 2.22 ± 2.3c | 1.54 ± 4.3b | |
| XANCI | 2.17 ± 1.4a | 2.53 ± 0.4c | 2.64 ± 1.3b | 2.77 ± 3.9d | 2.55 ± 2.3d | 2.38 ± 2.7d | 1.98 ± 1.5d | 1.53 ± 4.5b | |
| Color (b*) | Control | 2.62 ± 0.2c | 2.10 ± 0.4c | 1.74 ± 0.3a | 1.34 ± 0.2b | 1.00 ± 0.3a | 0.74 ± 0.4b | 0.62 ± 0.4a | 0.59 ± 0.4a |
| XAN | 3.36 ± 0.2b | 2.91 ± 0.3a | 2.27 ± 1.6a | 1.69 ± 0.4a | 1.02 ± 0.2a | 0.63 ± 0.2c | 0.49 ± 0.5c | 0.32 ± 0.5c | |
| XANAS | 3.82 ± 0.2a | 2.78 ± 0.2b | 2.21 ± 1.1a | 1.67 ± 0.5a | 1.04 ± 0.4a | 0.86 ± 0.3a | 0.57 ± 0.5b | 0.49 ± 0.5b | |
| XANCI | 1.79 ± 0.2d | 1.60 ± 0.3d | 1.15 ± 0.3c | 0.98 ± 0.2c | 0.78 ± 0.2b | 0.55 ± 0.2d | 0.36 ± 0.3d | 0.31 ± 0.3d | |
| Color (ΔE) | Control | – | 4.60 ± 0.4a | 6.60 ± 1.3a | 7.54 ± 1.2a | 8.30 ± 1.3a | 9.72 ± 1.4a | 10.68 ± 1.4a | 11.82 ± 1.4a |
| XAN | – | 1.26 ± 0.3c | 2.40 ± 0.3b | 4.84 ± 1.4b | 5.88 ± 0.6b | 6.86 ± 1.0b | 7.86 ± 0.7b | 8.47 ± 1.5b | |
| XANAS | – | 1.93 ± 0.2b | 1.96 ± 0.1c | 1.74 ± 0.5c | 2.25 ± 0.5c | 2.80 ± 0.9c | 3.91 ± 1.4c | 5.32 ± 1.5c | |
| XANCI | – | 0.58 ± 0.3d | 1.07 ± 0.3d | 1.33 ± 0.2d | 2.25 ± 0.7c | 2.60 ± 0.3d | 3.44 ± 1.0d | 4.41 ± 0.9d | |
| WL (%) | Control | – | 4.33 ± 0.6a | 7.40 ± 1.4a | 9.48 ± 0.2a | 11.78 ± 1.3a | 13.84 ± 0.8a | 15.22 ± 0.4a | 15.64 ± 1.4a |
| XAN | – | 4.23 ± 0.3a | 6.79 ± 1.3b | 8.86 ± 1.4b | 11.51 ± 0.9b | 13.19 ± 1.5b | 13.87 ± 1.5b | 14.16 ± 0.9b | |
| XANAS | – | 4.12 ± 0.2a | 6.29 ± 1.2c | 8.92 ± 0.5b | 11.22 ± 1.1c | 12.64 ± 0.3c | 12.92 ± 0.7d | 13.66 ± 1.5c | |
| XANCI | – | 3.99 ± 1.2a | 6.66 ± 1.3b | 8.67 ± 0.2c | 11.39 ± 1.0d | 12.88 ± 1.2c | 13.20 ± 0.6c | 13.98 ± 0.9c | |
Different superscript in a column denotes significant difference at p < 0 .05. (Turkey, n = 4). PPO polyphenol oxidase, AAO ascorbic acid oxidase, PME pectin methylesterase, TPC total phenolic content, FRAP ferric reducing antioxidant power, TFC Total flavonoid content, FIRM firmness, ANTH Anthocyanin, WL weight loss, XAN Xanthan, XANAS Xanthan + Ascorbic acid, XANCI Xanthan + Citric acid, C3GE cyanidin-3-glucoside equivalent, ER equivalents of rutin, GAE gallic acid equivalents, AEAC ascorbic acid equivalent antioxidant capacity
Enzyme activity of Xanthn gum coated grapes samples in cold temperature storage
From Table 1, it was obvious that storage period and pretreatment matrixes played significant roles in grape enzyme activity during the storage period. The initial PPO activity increased whilst AAO activity decreased significantly (p < 0.05) with increasing storage time in a fairly linear tendency. For instance, PPO activity was significantly minimal in XANCI (206 ± 3.1) at the end of the storage period, compared with the control (438 ± 4.6 U/g). Similarly, XAN and XANAS pretreatment also recorded significantly minimal enzyme activities 296 ± 3.3 and 210 ± 3.2 U/g correspondingly, relative to the control. Sarpong et al. (2018a) reported a similar result of the suppressed activity of PPO by Xanthan gum in apple slices. Also, literature reported the suppression of PPO by ascorbic and citric acids in banana slices (Sarpong et al. 2018c), apricots in cold storage (Wu et al. 2015) and apples (Sapers et al. 1989) as observed in this study. The Xanthan gum and its enriched forms (Xanthan + ascorbic acid − XANAS and Xanthan + citric acid − XANCI) suppressed the activity of the PPO. Furthermore, the activity of AAO was suppressed effectively by Xanthan gum and its modifications in comparison to the control. AAO suppression followed the order; XANCI > XANAS > XAN > Control (Table 1). Since PPO thrives on copper ions, citric acid may have further exerted a chelating effect and also reduced the pH of the grape, consequently, suppressing PPO and AAO activities (Igual et al. 2010; Ioannou 2013). A similar reason could be attributed to the action of ascorbic acid enriched Xanthan gum (XANAS). Beside storage time, the role of acid enriched xanthan gum coating on grape became evident by the variation in the Pectin methylesterase (PME) (Fig. 1) composition as observed. There was an initial appreciation in PME activities reaching a peak on the 9th day of storage, then demonstrated a gradual decline for all treatments. This trend could be ascribed to the depletion of pectin during the storage period. A similar outcome was reported by Wu et al. (2015) of reduction in PME activity after 4 days of storage in apricots. The acid enriched Xanthan gums significantly (p < 0.05) suppress PME activity when compared to control. This phenomenon is critical and very vital for grape quality during storage as the action of PME would result in softening grape tissues during storage. Evidently, the textural properties recorded in this study proved that acid modified xanthan gum pretreatments significantly maintained the structural and textural properties of the grape samples over the storage period in comparison to the control (Fig. 2). This is an indication of effective restriction of PME activity in the fruit. The reason could be accredited to the lower oxygenated atmosphere created as a result of semi-permeable barrier by the gum coatings and a reduction in pH as suggested by Katsaros et al. (2017). The physical integrity of the grape samples was evaluated employing the texture profile analysis (TPA) approach. The order of the TPA for grape under cold storage in this study was XANAS > XANCI > XAN > Control and XANCI > XANAS > XAN > Control for resilience and firmness respectively. Our result is comparable with firmness results in apricots (Wu et al. 2015). Reduced PME activity due to the gum coatings may describe the superior textural properties when compared with the control.
Fig. 1.
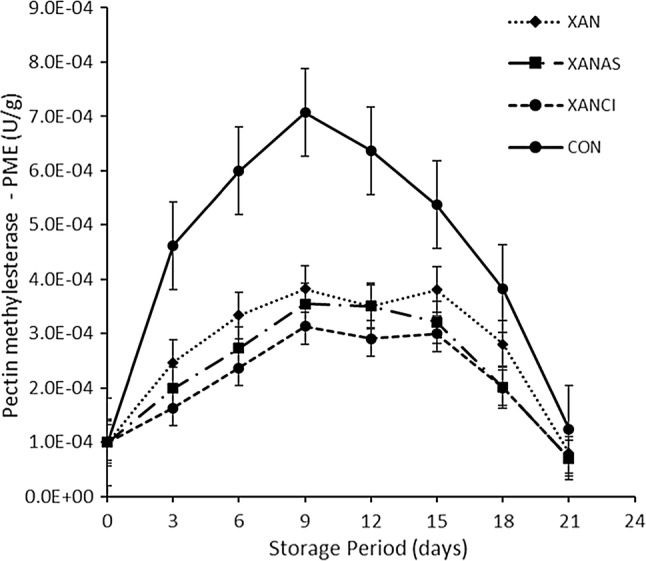
Pectin methylesterase (PME) activity of grape under cold storage with modified xanthan gum pretreatment. Different lower cases on bars denote significant difference (p < 0.05). XAN xanthan, XANAS Xanthan + Ascorbic acid, XANCI Xanthan + Citric acid; n = 4
Fig. 2.
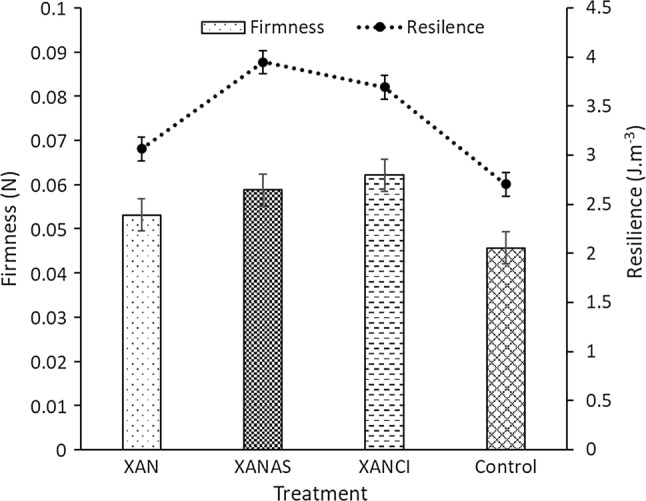
Textural properties of grape under cold storage pretreated with modified xanthan gum. XAN Xanthan, XANAS Xanthan + Ascorbic acid, XANCI Xanthan + Citric acid; n = 10
Antioxidant and phytochemical activities of Xanthn gum coated grape samples
The antioxidant properties of the acid enriched xanthan gum coated grape under cold storage were determined and results presented as ferric reducing antioxidant property (FRAP), total phenolic content (TPC) and total flavonoid content (TFC) (Table 1). There were significant (p < 0.05) differences in the antioxidant properties of the pretreated samples compared to the control. The FRAP reduced generally over the storage period with highest value observed in XAN treated grape samples followed by XANAS and XANCI with 150.23, 143.18 and 136.49 mg/100 g AEAC respectively, compared to 104.5 mg/100 g AEAC for the control at the end of storage. The TPC and TFC exhibited a similar rising trend over the storage period as the FRAP (Table 1). All the enriched gum pretreated samples exhibited significantly (p < 0.05) higher values compared to the control. XANCI recorded higher value of 893 µg/100 g GAE for TFC while XAN recorded 771 µg/100 ml GAE for TPC. XANCI > XANAS > XAN > Control and XAN > XANCI > XANAS > Control depicted the typical trend for TFC and TPC respectively in this study. This could be attributed to Xanthan formulations forming a protective coating around the grape restricting oxygen supply, hence preserving the phenolics (Quoc et al. 2015). This could be a contributing factor for the reduced action of enzymes of the gum pretreated samples compare to the control as observed earlier. Table 1 also illustrated the anthocyanin content of the four samples over the storage period. There was significant (p < 0.05) difference in the anthocyanin content of the acid enriched gum pretreated samples compared to the control. Generally, XAN pretreated sample had the highest anthocyanin content followed by XANAS and XANCI both of which were not significantly (p > 0.05) different throughout the storage period. At the end of the 21 days storage period, the anthocyanin contents were 21.79, 20.57, 20.17 and 16.97 (µg/100 g GAE) (Table 1) following the trend XAN, XANAS, XANCI and Control respectively. Anthocyanin content largely declined over the storage period, a phenomenon reported earlier in apple cultivas (Bal 2017). The higher anthocyanin contents recorded for the gum coated samples were noteworthy because anthocyanins contribute largely to total phenolic content and the color of fruit. Therefore, the higher TPC in the gum coated samples were comprehensible and an evidence of an appreciable retention of grape color in the grape samples.
Changes in color and weight loss of Xanthn gum coated grape samples in cold storage
Changes in the content of color were shown in Table 1. Observably, different behavior was detected among the color parameters resulting from the acid enriched gum coatings during the storage period. However, a similar trend of decrease in L* and b* and a parabolic (increase and fall) inclination in a* of color were observed with a significant difference (p < 0.05). Also, the total change in color (ΔE) increased slightly in the gum coated samples, but momentously in the control with a significant difference of p < 0.05. XANAS recorded minimal changes in L* (22.96) and a* (1.54) whereas XANCI recorded the least change in b* (0.31) in cold storage from initial values of 28.18, 2.02 and 1.79 for L*, a* and b* correspondingly. Meanwhile the control recorded 16.17, 2.59 and 0.59 for L*, a* and b* respectively. This was also reflected in the total color change (∆E) where the gum coating exhibits were lower with the trend XANCI < XANAS < XAN < Control corresponding to 4.41, 5.32, 8.47 and 11.82 under cold storage respectively. Color changes are often attributed to oxidative reactions leading to disintegration of pigments (Kumar et al. 2011) and from the results, the gum coatings and lower pH ameliorated and abridged this reaction significantly. Likewise, the gum coatings by a possible reduction in respiration of the grapes in cold storage significantly reduced weight loss (WL). From Table 1, the WL after the 21 days storage period was 13.66, 13.98, 14.16 and 15.64% for XANAS, XANCI, XAN and control respectively. There was a significant difference (p < 0.05) in the WL of the gum coated samples compared to the control. Quoc et al. (2015) reported that xanthan gum could reduce respiration rate and subsequent weight loss in fruit under cold storage.
Degradation kinetics of color, weight loss and FRAP
For accurate prediction of color, weight loss and FRAP, the degradation phenomena were modelled using second-order polynomial (Eq. 5) and the fit of the model was measured using three statistical parameters (R2, and RMSE). The results shown in Table 2 demonstrated a good fitting of the model with high R2 (0.9384–0.9967) and low χ2 (0.0016–13.6872) and RMSE (0.0079–10.5208). This meant that the degradation pattern of color, weight loss and FRAP exhibited exponential and linear tendencies at various stages of storage. From Table 2, varied gum coating formulations affected the variables and coefficients of the model resulting in different degradation of these parameters. For a typical description of second-order polynomial, two constants [scale factor (a) and shape factor (b)] are of critical importance since constant (c) only demonstrates the interception with y-axis and most often shows the commencement/initiation point of the growth curve. Hence, a positive/negative constant (c) provides evidence of the starting point of the curve and the magnitude of the distance from the central point (zero). For the scale factor (a), when a > 1, it determines the rate of degradation and an increasing nature of the reaction whilst a < 1 demonstrates the cessation of the reaction over the storage period (Buzrul and Alpas 2007). Therefore, the rate of reaction/degradation is directly proportional to the scale factors (a) constant of the model. On the hand, the shape factor (b) suggests a shoulder or tail-forming nature of the degradation curve when “b” is positive and negative respectively. This suggested that the degradation reaction of color and some conditions under FRAP will continue to increase beyond the 21 days storage period according to Table 2; because a positive shape factor (b) suggestes an upward turn of the growth curve. According to Sarpong et al. (2018a), the magnitude of the b-constant exposses the tailing nature of the degradation curves. Furthermore, the b-constant is also opined to indicate the kinetic pattern of reaction at the cell level of fruit which could cause the degradation of nutrients.
Table 2.
The constants for second-order polynomials of color, weight loss, FRAP and TPC of grapes treated with modified xanthan gum under cold storage
| Parameters | Determinant | Treatment | R2 | RMSE | χ2 | Model constants | ||
|---|---|---|---|---|---|---|---|---|
| a | b | c | ||||||
| Color | L* | CONTROL | 0.9426 | 0.8902 | 0.7925 | − 0.8754 | 0.0187 | 26.9742 |
| XAN | 0.9501 | 0.9479 | 0.8984 | − 0.5721 | 0.0065 | 33.5483 | ||
| XANAS | 0.9866 | 0.2728 | 0.0744 | 0.3605 | − 0.0298 | 28.4235 | ||
| XANCI | 0.9791 | 0.1886 | 0.0356 | 0.8754 | − 0.0115 | 26.9454 | ||
| a* | CONTROL | 0.9945 | 0.0402 | 0.0016 | 0.2135 | − 0.0115 | 2.5235 | |
| XAN | 0.9901 | 0.0543 | 0.0030 | 0.2527 | − 0.011 | 2.0384 | ||
| XANAS | 0.9877 | 0.0518 | 0.0027 | 0.1862 | − 0.0099 | 2.0254 | ||
| XANCI | 0.9866 | 0.0473 | 0.0022 | 0.1236 | − 0.0074 | 2.1929 | ||
| b* | CONTROL | 0.9967 | 0.0429 | 0.0018 | − 0.1831 | 0.0041 | 2.6355 | |
| XAN | 0.9861 | 0.1366 | 0.0187 | − 0.2506 | 0.0005 | 3.4979 | ||
| XANAS | 0.9944 | 0.0079 | 0.0889 | − 0.3044 | 0.0071 | 3.7551 | ||
| XANCI | 0.9874 | 0.0617 | 0.0038 | − 0.1121 | 0.0018 | 1.8283 | ||
| ΔE | CONTROL | 0.9852 | 0.3027 | 0.0916 | 0.5263 | − 0.0057 | 2.9694 | |
| XAN | 0.9857 | 0.3257 | 0.0106 | 0.7286 | − 0.0132 | − 1.0034 | ||
| XANAS | 0.9632 | 0.1205 | 0.0145 | − 0.6222 | 0.0318 | 4.0163 | ||
| XANCI | 0.9967 | 0.0792 | 0.0063 | 0.5263 | 0.0068 | 0.4403 | ||
| Weight Loss | WL | CONTROL | 0.9959 | 0.2705 | 0.0732 | 1.1882 | − 0.0228 | 0.9403 |
| XAN | 0.9909 | 0.3674 | 0.1340 | 1.2168 | − 0.0268 | 0.5863 | ||
| XANAS | 0.9901 | 0.0364 | 0.1328 | 1.1991 | − 0.074 | 0.5005 | ||
| XANCI | 0.9927 | 0.3370 | 0.1136 | 1.3375 | − 0.0313 | − 0.4005 | ||
| Antioxidant | FRAP | CONTROL | 0.9889 | 5.2497 | 9.5595 | − 4.3324 | − 0.1142 | 251.2584 |
| XAN | 0.9889 | 4.4133 | 5.4771 | − 7.2572 | 0.0764 | 265.8867 | ||
| XANAS | 0.9384 | 10.5208 | 13.6872 | − 8.1983 | 0.1242 | 261.8426 | ||
| XANCI | 0.9875 | 4.8979 | 7.9891 | − 7.6190 | 0.0810 | 257.6718 | ||
TPC total phenolic content, FRAP ferric reducing antioxidant power, XAN Xanthan, XANAS Xanthan + Ascorbic acid, XANCI Xanthan + Citric acid
Correlation analysis of the effect of Xanthan gum coatings on some quality properties of grape in cold temperature storage
In acid enriched xanthan gum coated grapes under cold temperature storage as depicted in Table 3, both a* and b* contribution to color change (∆E) were not significant (p > 0.05) with correlation values of 0.468 and 0.605 respectively, when compared with the L* correlation of − 0.722, which is in sharp contrast with our earlier report (Sarpong et al. 2019b) on dried banana. Meanwhile, FRAP (r = − 0.688), TFC (r = − 792), resilience (r = − 0.944) and firmness (r = 0.997) correlated negatively whilst PPO (r = 0.744), AAO (r = 0.992), PME (r = 0.940) and weight loss (r = 0.909) correlated positively with color change (∆E). It can be deduced that FRAP and TFC decompositions or degradation resulting from the activities of the studied enzymes significantly caused color change in cold temperature stored grapes when compared with the contribution of TPC (r = − 0.207) and anthocyanin (r = − 0.636) which were not significant.
Table 3.
Pearson’s coefficient of correlation of antioxidant, chromatic, enzymes and textural properties of grape pretreated with modified xanthan gum under cold storage
| Variables | PPO | AAO | PME | FRAP | TPC | TFC | ANTH | L* | a* | b* | ΔE | WL | FIRM | RESILE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PPO | 1 | |||||||||||||
| AAO | 0.811* | 1 | ||||||||||||
| PME | 0.878* | 0.948* | 1 | |||||||||||
| FRAP | − 0.885* | − 0.714* | − 0.895* | 1 | ||||||||||
| TPC | − 0.765* | − 0.283 | − 0.514 | 0.814* | 1 | |||||||||
| TFC | − 0.661 | − 0.836* | − 0.644 | 0.326 | 0.053 | 1 | ||||||||
| ANTH | − 0.900* | − 0.673* | − 0.861* | 0.995* | 0.867* | 0.312 | 1 | |||||||
| L* | − 0.963* | − 0.813* | − 0.936* | 0.975* | 0.781* | 0.519 | 0.973* | 1 | ||||||
| a* | − 0.209 | 0.392 | 0.153 | 0.280 | 0.768* | − 0.458 | 0.363 | 0.199 | 1 | |||||
| b* | 0.966* | 0.697* | 0.734* | − 0.758* | − 0.764* | − 0.678* | − 0.795* | − 0.867* | − 0.304 | 1 | ||||
| ΔE | 0.744* | 0.992* | 0.940* | − 0.688* | − 0.207 | − 0.792* | − 0.636 | − 0.772* | 0.468 | 0.605 | 1 | |||
| WL | 0.817* | 0.901* | 0.987* | − 0.910* | − 0.506 | − 0.525 | − 0.868* | − 0.916* | 0.144 | 0.643 | 0.909* | 1 | ||
| FIRM | − 0.773* | − 0.998* | − 0.935* | 0.682* | 0.226 | 0.833* | 0.635 | 0.780* | − 0.447 | − 0.651 | − 0.997* | − 0.893* | 1 | |
| RESILIENCE | − 0.531 | − 0.895* | − 0.862* | 0.602 | 0.032 | 0.595 | 0.525 | 0.632 | − 0.595 | − 0.333 | − 0.944* | − 0.878* | 0.916* | 1 |
*Pearson’s correlation significant accepted at p < 0.05. PPO polyphenol oxidase, AAO ascorbic acid oxidase, PME pectin methylesterase, TPC total phenolic content, FRAP ferric reducing antioxidant power, TFC total flavonoid content, FIRM firmness, ANTH anthocyanin, WL weight loss
The study of enzyme activities was of significance as these brought to the forefront the thoughtful interaction between the phytochemicals, color and pretreatment (coating) material in this study. The elevated levels of enzyme activities (PPO, AAO and PME) in the control samples as depicted in Table 1 confirms the positive and significant influence (p < 0.05) of the enriched gum coating on the stored grapes taking the cold storage period into account. Considering all the four pretreatments, the gum coatings demonstrated minimal enzymatic activity compared to the control. This can be attributed to lower oxygenated atmosphere created by the gum coatings (XAN, XANAS and XANCI) thereby reducing the oxidative reaction and enzyme activities (Katsaros et al. 2017). PME enzyme activities play a significant role in the firmness (r = − 935), resilience (− 0.862), weight loss (r = 0.987)) and b* (r = 0.734) of grape and this was confirmed by the significant correlation among them as a result of increase in senescence (Katsaros et al. 2017). Additionally, from Table 3, one could infer that FRAP (r = − 0.895) and anthocyanin (r = − 0.861) were the main reactive compounds used by PME for de-esterification of pectin of cell walls to reduce firmness whereas TFC (− 0.644) was less significantly used.
Enzymatic browning reactions in fruit are often related to phenol metabolic enzymes such as PPO and AAO. These enzyme activities are governed by pH, temperature, ascorbic acid, oxygen and phenolic composition of fruit (Ramos et al. 2013; Sarpong et al. 2018a; Sharma and Rao 2015; Zhu et al. 2018). This was colloborated by the negative correlation of PPO and AAO with anthocyanin (r = − 0.900 and r = − 0.673) respectively, PPO with TPC (r = − 0.765) and AAO with TFC (r = − 0.836). Also, PPO and AAO contributed significantly to the ∆E, L*, b*, weight loss, firmness and resilience of cold preservation of grape with gum coatings except the non-significant effect of resilience by PPO (r = − 0.531). Thus, one could attribute the enzymatic browning reaction in cold stored grape to the changes in b* color parameter resulting from the deterioration of TPC, TFC and anthocyanin as shown in Table 3.
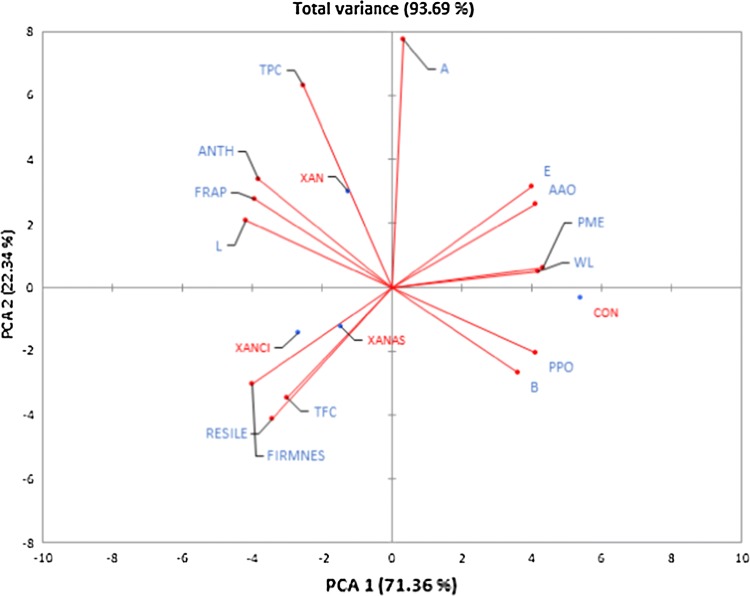
Multivariate analysis of the effect of modified xanthan gum coatings on quality properties of cold stored grape
The complexity of the effect of xanthan gum and its acid enriched forms coating on color, enzymes, phytochemicals and textural parameters was simplified by principal component analysis (PCA) and is shown in Fig. 3. The first two components were chosen based on total variance (93.69) and eigenvalue (10.1 and 3.1 respectively) which are of significant importance (Kaiser 1960; Sarpong et al. 2019a) in selecting and describing PCA. The first two components were further clustered into four groups and the first group is located on the positive side of PC2 containing the xanthan only treatment and negative side of PC1 containing the xanthan gum + citric acid (XANCI) and XANAS (xanthan gum + ascorbic acid) the acid modified form of the xanthan gum. The results showed that the xanthan gum coating led to the conservation or improvement of TPC, FRAP, anthocyanin and L*. Meanwhile, the acid enriched forms of the xanthan gum were associated with conservation or improvement in texture (resilience and firmness) as well as TFC of grape in cold storage. However, the control located on positive and negative sides of PC1 and PC2 respectively was associated with b* and higher PPO activity. Additionally, the control treatment was more related to elevated rapid depletion of color (ΔE), weight loss as well as higher enzymatic (PPO and AAO) activity. This means that color deviations in cold temperature storage of grapes were a result of enzymatic reaction and changes in b* color parameter.
Fig. 3.
Principal component analysis of antioxidant, chromatic, enzymes and textural properties of modified xanthan gum pretreated grape under cold storage. PPO Polyphenol oxidase, AAO ascorbic acid oxidase, PME Pectin methylesterase, TPC total phenolic content, FRAP ferric reducing antioxidant power, TFC total flavonoid content, FIRMNES firmness, RESILIE resilience, ANTH anthocyanin, WL weight loss, L, A, B & E Color parameters (L*, a*, b* and ΔE respectively), PCA principal component analysis
Conclusion
This study looked into the effect of ascorbic and citric acid enriched xanthan gum coatings on grape in cold storage with emphasis on color, enzymes, phytochemicals and textural properties. The results showed that the gum coatings impacted significantly on the quality parameters of the grape such that the effectiveness of these gum coatings in extending the shelf life of gape under cold storage followed the order XANCI > XANAS > XAN > Control. The kinetic decomposition of color, weight loss and FRAP were modeled and with three statistical parameters (R2, χ2 and RMSE), the second polynomial equation proved a better fitting model. The model predicted a continuing deterioration of weight loss and FRAP as a result of an upward turn of the growth curve suggested by a positive shape factor (b) and the opposite was predicted for weight loss. The b* color parameter significantly caused color changes in grape which was also attributed to TPC, TFC and anthocyanin decompositions resulting from enzymatic activities. The PPO and AAO correlated significantly to ∆E, L*, b*, weight loss, firmness and resilience which also revealed the enzymatic browning pigmentation reaction species in grapes. In effect acid enriched xanthan gum coatings could preserve phytochemicals, color, antioxidant and texture properties of grape in cold storage.
Acknowledgements
This work was supported by the National Key Research and Development Plan of China (2018YFD0700101).
Abbreviations
- AAO
Ascorbic acid oxidase
- PPO
Polyphenol oxidase
- Eq.
Equation
- ∆E
Total color difference
- r
Person’s coefficient of correlation
- R2
Coefficient of correlation
- RMSE
Root mean square error
Reduced Chi square
- PME
Pectin methylesterase
- FRAP
Ferric reducing antioxidant power
- TA
Total anthocyanin
- TPC
Total Phenolic content
- TFC
Total flavonoid content
- WL
Weight loss
- XAN
Xanthan gum
- XANAS
Xanthan gum + ascorbic acid
- XANCI
Xanthan gum + citric acid
Compliance with ethical standards
Conflict of interest
There exists no conflicting interest on the part of any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ali A, Maqbool M, Ramachandran S, Alderson PG. Gum arabic as a novel edible coating for enhancing shelf-life and improving postharvest quality of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol Technol. 2010;58:42–47. doi: 10.1016/j.postharvbio.2010.05.005. [DOI] [Google Scholar]
- Ali A, Maqbool M, Alderson PG, Zahid N. Effect of gum arabic as an edible coating on antioxidant capacity of tomato (Solanum lycopersicum L.) fruit during storage. Postharvest Biol Technol. 2013;76:119–124. doi: 10.1016/j.postharvbio.2012.09.011. [DOI] [Google Scholar]
- Al-Juhaimi FY. Physicochemical and sensory characteristics of arabic gum-coated tomato (Solanum lycopersicum L.) fruits during storage. J Food Process Preserv. 2014;38:971–979. doi: 10.1111/jfpp.12053. [DOI] [Google Scholar]
- Bal E (2017) Changes in phenolic compounds, anthocyanin and antioxidant capacity of some apple cultivars during cold storage. In: 2nd international balkan agriculture congress. Tekirdag, Turkey, AgriBalkan, Association of Thrace Universities, pp 532–539
- Beristain CI, Garcia HS, Vernon-Carter EJ. Spray-dried encapsulation of cardamom (Elettaria cardamomum) essential oil with mesquite (Prosopis juliflora) gum. LWT Food Sci Technol. 2001;34:398–401. doi: 10.1006/fstl.2001.0779. [DOI] [Google Scholar]
- Buzrul S, Alpas H. Modeling inactivation kinetics of food borne pathogens at a constant temperature. LWT Food Sci Technol. 2007;40:632–637. doi: 10.1016/j.lwt.2006.02.019. [DOI] [Google Scholar]
- Cautela D, Castaldo D, Laratta B. Hydroxy- and polyhydroxybenzoic acids: occurrence and recent bioactivity studies. J Food Meas Charact. 2018;12:2795–2800. doi: 10.1007/s11694-018-9894-1. [DOI] [Google Scholar]
- Del Nobile MA, et al. A study on quality loss of minimally processed grapes as affected by film packaging. Postharvest Biol Technol. 2009;51:21–26. doi: 10.1016/j.postharvbio.2008.06.004. [DOI] [Google Scholar]
- Falcão-Rodrigues MM, Moldão-Martins M, Beirão-da-Costa ML. DSC as a tool to assess physiological evolution of apples preserved by edibles coatings. Food Chem. 2007;102:475–480. doi: 10.1016/j.foodchem.2006.05.016. [DOI] [Google Scholar]
- Igual M, García-Martínez E, Camacho MM, Martínez-Navarrete N. Effect of thermal treatment and storage on the stability of organic acids and the functional value of grapefruit juice. Food Chem. 2010;118:291–299. doi: 10.1016/j.foodchem.2009.04.118. [DOI] [Google Scholar]
- Ioannou I. Prevention of enzymatic browning in fruit and vegetables. Eur Sci J. 2013;9:310–341. doi: 10.19044/esj.2013.v9n30p%p. [DOI] [Google Scholar]
- Kaiser HF. The application of electronic computers to factor analysis. Educ Psychol Measur. 1960;20:141–151. doi: 10.1177/001316446002000116. [DOI] [Google Scholar]
- Kalamara E, Goula AM, Adamopoulos KG. An integrated process for utilization of pomegranate wastes—Seeds. Innov Food Sci Emerg Technol. 2015;27:144–153. doi: 10.1016/j.ifset.2014.12.001. [DOI] [Google Scholar]
- Katsaros GJ, Alexandrakis ZS, Taoukis PS. Kinetic assessment of high pressure inactivation of different plant origin pectinmethylesterase enzymes. Food Eng Rev. 2017;9:170–189. doi: 10.1007/s12393-016-9153-3. [DOI] [Google Scholar]
- Khadem S, Marles RJ. Monocyclic phenolic acids hydroxy- and polyhydroxybenzoic acids: occurrence and recent bioactivity studies. Molecules. 2010;15:7985–8005. doi: 10.3390/molecules15117985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Sharma A, Patil RT. Color kinetics of aonla shreds with amalgamated blanching during drying AU—Gupta, R.K. Int J Food Prop. 2011;14:1232–1240. doi: 10.1080/10942911003637343. [DOI] [Google Scholar]
- Lopes BdM, Lessa VL, Silva BM, La Cerda LG. Xanthan gum: properties, production conditions, quality and economic perspective. J Food Nutr Res. 2015;54:185–194. [Google Scholar]
- Nicolosi E, Ferlito F, Amenta M, Russo T, Rapisarda P. Changes in the quality and antioxidant components of minimally processed table grapes during storage. Sci Hortic. 2018;232:175–183. doi: 10.1016/j.scienta.2017.12.050. [DOI] [Google Scholar]
- Quoc L, Hoa D, Ngoc H, Phi T. Effect of xanthan gum solution on the preservation of acerola (Malpighia glabra L.) Cercetari Agronomice in Moldova. 2015;48:89–97. doi: 10.1515/cerce-2015-0045. [DOI] [Google Scholar]
- Ramos B, Miller FA, Brandão TRS, Teixeira P, Silva CLM. Fresh fruits and vegetables—an overview on applied methodologies to improve its quality and safety. Innov Food Sci Emerg Technol. 2013;20:1–15. doi: 10.1016/j.ifset.2013.07.002. [DOI] [Google Scholar]
- Sapers GM, et al. Control of enzymatic browning in apple with ascorbic acid derivatives, polyphenol oxidase inhibitors, and complexing agents. J Food Sci. 1989;54:997–1002. doi: 10.1111/j.1365-2621.1989.tb07931.x. [DOI] [Google Scholar]
- Sarpong F, Oteng-Darko P, Golly MK, Amenorfe LP, Rashid MT, Zhou C. Comparative study of enzymes inactivation and browning pigmentation of apple (Malus domestica) slices by selected gums during low temperature storage. J Food Biochem. 2018;42:e12681. doi: 10.1111/jfbc.12681. [DOI] [Google Scholar]
- Sarpong F, Yu X, Zhou C, Amenorfe LP, Bai J, Wu B, Ma H. The kinetics and thermodynamics study of bioactive compounds and antioxidant degradation of dried banana (Musa ssp.) slices using controlled humidity convective air drying. J Food Meas Charact. 2018;12:1935–1946. doi: 10.1007/s11694-018-9809-1. [DOI] [Google Scholar]
- Sarpong F, et al. Influence of anti-browning agent pretreatment on drying kinetics, enzymes inactivation and other qualities of dried banana (Musa ssp.) under relative humidity-convective air dryer. J Food Meas Charact. 2018;12:1229–1241. doi: 10.1007/s11694-018-9737-0. [DOI] [Google Scholar]
- Sarpong F, Jiang H, Oteng-Darko P, Zhou C, Amenorfe LP, Mustapha AT, Rashid MT. Mitigating effect of relative humidity (RH) on 2-furoylmethyl-amino acid formation. LWT. 2019;101:551–558. doi: 10.1016/j.lwt.2018.11.077. [DOI] [Google Scholar]
- Sarpong F, Zhou C, Bai J, Amenorfe LP, Golly MK, Ma H. Modeling of drying and ameliorative effects of relative humidity (RH) against β-carotene degradation and color of carrot (Daucus carota var.) slices. Food Sci Biotechnol. 2019;28:75–85. doi: 10.1007/s10068-018-0457-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Rao TVR. Xanthan gum based edible coating enriched with cinnamic acid prevents browning and extends the shelf-life of fresh-cut pears. LWT Food Sci Technol. 2015;62:791–800. doi: 10.1016/j.lwt.2014.11.050. [DOI] [Google Scholar]
- Shiekh RA, Malik MA, Al-Thabaiti SA, Shiekh MA. Chitosan as a novel edible coating for fresh fruits. Food Sci Technol Res. 2013;19:139–155. doi: 10.3136/fstr.19.139. [DOI] [Google Scholar]
- Tsali A, Goula AM. Valorization of grape pomace: encapsulation and storage stability of its phenolic extract. Powder Technol. 2018;340:194–207. doi: 10.1016/j.powtec.2018.09.011. [DOI] [Google Scholar]
- Wang L, Sun X, Li F, Yu D, Liu X, Huang W, Zhan J. Dynamic changes in phenolic compounds, colour and antioxidant activity of mulberry wine during alcoholic fermentation. J Funct Foods. 2015;18:254–265. doi: 10.1016/j.jff.2015.07.013. [DOI] [Google Scholar]
- Wu B, Guo Q, Wang G-X, Peng X-Y, Wang J-D, Che F-B. Effects of different postharvest treatments on the physiology and quality of ‘Xiaobai’ apricots at room temperature. J Food Sci Technol. 2015;52:2247–2255. doi: 10.1007/s13197-014-1288-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Wang L, Xiao Z, Niu Y. Characterization of the key aroma compounds in mulberry fruits by application of gas chromatography–olfactometry (GC-O), odor activity value (OAV), gas chromatography-mass spectrometry (GC–MS) and flame photometric detection (FPD) Food Chem. 2018;245:775–785. doi: 10.1016/j.foodchem.2017.11.112. [DOI] [PubMed] [Google Scholar]