Abstract
Jatropha curcas, an economically important biofuel feedstock with oil-rich seeds, has attracted considerable attention among researchers in recent years. Nevertheless, valuable information on the yield component of this plant, particularly regarding ovule development, remains scarce. In this study, transcriptome profiles of anther and ovule development were established to investigate the ovule development mechanism of J. curcas. In total, 64,325 unigenes with annotation were obtained, and 1723 differentially expressed genes (DEGs) were identified between different stages. The DEG analysis showed the participation of five transcription factor families (bHLH, WRKY, MYB, NAC and ERF), five hormone signaling pathways (auxin, gibberellic acid (GA), cytokinin, brassinosteroids (BR) and jasmonic acid (JA)), five MADS-box genes (AGAMOUS-2, AGAMOUS-1, AGL1, AGL11, and AGL14), SUP and SLK3 in ovule development. The role of GA and JA in ovule development was evident with increases in flower buds during ovule development: GA was increased approximately twofold, and JA was increased approximately sevenfold. In addition, the expression pattern analysis using qRT-PCR revealed that CRABS CLAW and AGAMOUS-2 were also involved in ovule development. The upregulation of BR signaling genes during ovule development might have been regulated by other phytohormone signaling pathways through crosstalk. This study provides a valuable framework for investigating the regulatory networks of ovule development in J. curcas.
Subject terms: Plant regeneration, Plant physiology
Introduction
Jatropha curcas L., a species native to tropical regions in the Western Hemisphere, is now found prevalently distributed in Africa and Asia. J. curcas is described as an ideal bioenergy crop for its oil-rich seeds, high unsaturated fatty acid content in seed oil (the oil with high unsaturated fatty acid content is suitable for producing biodiesel), low nutrient requirements and high drought tolerance. In addition, J. curcas also has potential in medical applications such as anti-tumor, anti-microbial and anti-parasitic1–3. However, due to low seed yield, this plant has limited economic benefit for exploitation and further expansion of the Jatropha-based biodiesel industry. The low ratio of female to male flowers (1/10—1/30) is one of the critical factors attributed to the low seed yield of J. curcas4; therefore, in J. curcas, gene expression profile analysis was employed to study the development of seeds5, the response of seedlings to drought and salt stress6,7, and lipid metabolism in seeds and other tissues8. For high-throughput discovery of novel Jatropha genes, de novo assembly and transcriptome analysis of various tissues of J. curcas were performed, obtaining 17,457 assembled transcripts (contigs) and 54,002 singletons9. However, genomics studies on flower development in J. curcas are relatively scarce. Pan et al. analyzed the transcriptome of the inflorescence meristems of J curcas treated with cytokinin, obtained 81,736 unigenes, and identified a series of cytokinin-responsive genes, such as JcCycA3;2, JcCycD3;1, JcCycD3;2 and JcTSO1, which were thought to contribute to the increase in flower number10. Xu et al. analyzed the transcriptome of flower buds at different phases of sex differentiation in J. curcas, obtained 57962 unigenes, and found that the gibberellin-regulated protein 4-like gene and the AMP-activated protein kinase gene are associated with stamen differentiation11.
J. curcas is a monoecious plant. Sex differentiation occurs after the initiation of five petal primordia. Unlike male flowers, the female flowers undergo a hermaphrodite period: stamens first develop carpels and then gradually degenerate as the three carpels to be formed are fused11. Ovule development is an important process for the development of female flowers and is crucial for fruit yield. Any disturbances during macrosporogenesis or female gametogenesis may result in female flower abortion. Molecular studies on sporogenesis and gametogenesis of J. curcas are not available; therefore, transcriptome profiles spanning the developmental processes of pollen and ovule in J. curcas were constructed. In this study, we focused on macrosporogenesis and female gametogenesis during ovule development, and differentially expressed gene analysis was then performed on the expression profiles of flower buds at different developmental stages of the ovule in female flowers.
Results
Development of the ovule
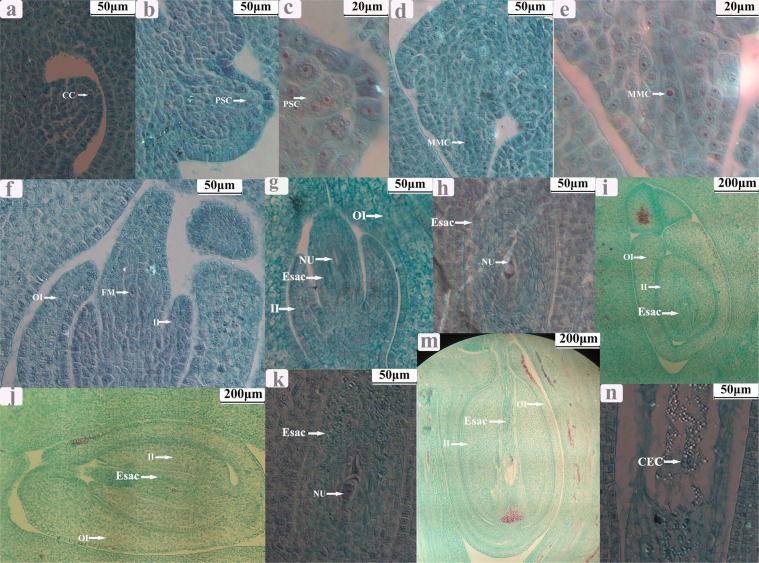
After the fusion of the three carpels, the ovule primordium was formed. When the ovary was approximately 0.3 mm long, archesporial cells occurred. When the ovary was approximately 0.6 mm long, archesporial cells gave rise to primary sporogenous cells. When the ovary was approximately 0.9 mm long, the style began to develop, and the primary sporogenous cells developed into macrospore mother cells (MMC). When the ovary was approximately 1.2 mm long, the style was deep green, and the stigmas appeared; MCC underwent two consecutive meioses with a result of 4 cells, of which three were degenerated, and the remaining one was developed into a functional macrospore. When the ovary was approximately 3.2 mm long, functional macrospores experienced three consecutive mitoses, resulting in an embryo sac with 8 nuclei. When the whole ovary became green, the stigmas were mature; of these 8 nuclei, two moved to the center of the embryo sac and formed a polar, and the remaining 6 formed an egg, two synergids, and three antipodal cells, respectively (Figs 1 and 2).
Figure 1.
Microstructure of ovule at different development stages in Jatropha curcas female flowers. (a) Chesporial cell; (b,c) the occurance of primary sporogenous cells and a parietal cell; (d,e) the occurance of macrospore mother cell; (f,g) the formation of functional macrospore (mononuclear embryo sac); (h,i) the formation of 2-nucleate embryo sac; (j,k) the formation of 8- nucleate embryo sac; (m,n) the maturation of embryo sac. CC: Chesporial cell; PSC: primary sporogenous cells; PC: parietal cell; MMC: macrospore mother cell; Esac: embryo sac; NU: nucleate; II: inner integuments; OI: outer integuments; CEC: Central cell.
Figure 2.
The morphology of ovule (or cuples) at different development stages. JCFI: from (a to b); JCFII: from (c to d); JCFIII: from (d to f); JCFIV: from (f to e).
Endogenous phytohormone level in flower buds at different development stages
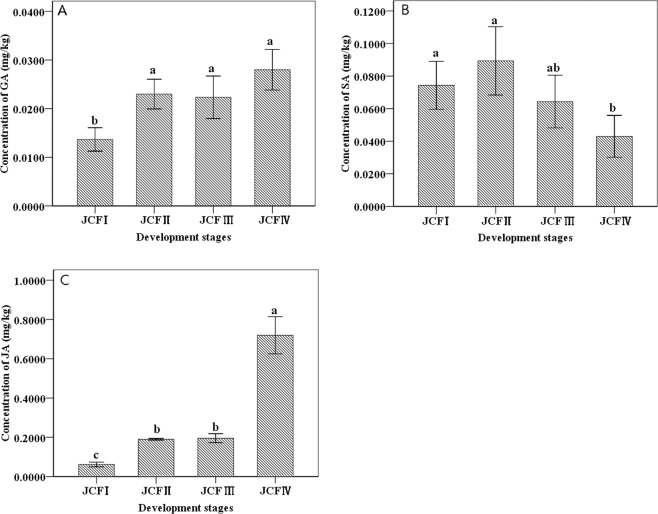
The concentrations of endogenous gibberellic acid (GA) and jasmonic acid (JA) in flower buds began to significantly increase in JCFII, but JA further experienced a dramatic increase in JCFIV. Additionally, the concentration of endogenous SA in flower buds significantly declined only at the stage of JCFIV and only slightly changed in the stages of JCFl, JCFII and JCFIII (Fig. 3).
Figure 3.
The concentration of endogenous gibberellic acid (GA), jasmonic acid (JA) and salicylic acid (SA) in flower buds from different stages of ovule development.
Identification of differently expressed genes
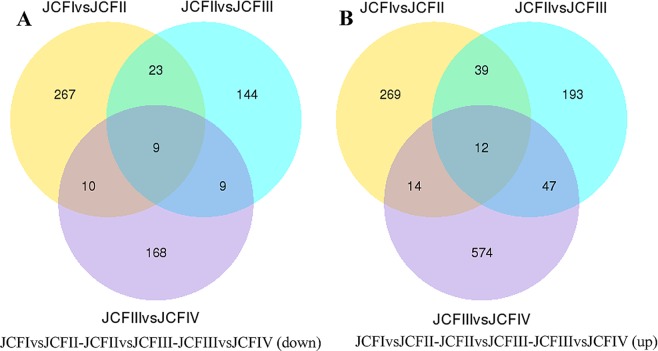
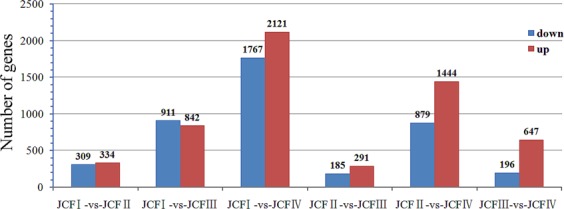
The sequencing results of the twelve sample DGE libraries are shown in Table 1. The depth, coverage and homogenization of sequencing were high, which suggested that these results could reflect the actual expression of genes, and the data were thus suitable for further analysis (Fig. S2). In these twelve libraries, approximately 83.78–87.00% of the clean reads could be mapped to the assembled transcriptome (Table S4). In total, 1723 genes that were differentially expressed during ovule development in female flower buds were screened out and are shown in Fig. 4. Of these differentially expressed genes (DEGs), 9 genes were downregulated and 12 genes were upregulated throughout the entire process of ovule development; 579 genes were downregulated and 1036 genes were upregulated in one stage; 42 genes were downregulated and 100 genes were upregulated at two stages (Fig. 5).
Table 1.
Expression level of ovule development related genes during ovule development.
| Gene_id | Annotation description | Protein ID | log2(JCFII/JCFI) | log2(JCFIII/JCFII) | log2(JCFIV/JCFIII) | log2(JCFIII/JCFI) | log2(JCFIV/JCFI) | log2(JCFIV/JCFII) |
|---|---|---|---|---|---|---|---|---|
| c26856_g5 | Argonaute 2 (Arabidopsis thaliana) | Q9SHF3 | 0.692 | 0.814 | ||||
| c25989_g2 | AGAMOUS (AGAMOUS-1) (Panax ginseng) | Q40872 | 0.902 | 1.407 | 1.178 | |||
| c27618_g1 | SLK3 (Arabidopsis thaliana) | F4JT98 | 0.618 | 0.561 | 1.179 | 1.023 | ||
| c29108_g3 | AGL14(Arabidopsis thaliana) | Q38838 | 1.073 | 1.895 | 1.742 | 0.670 | ||
| c30128_g4 | AGL11(Arabidopsis thaliana) | Q38836 | 2.751 | 1.351 | 4.105 | 4.115 | 1.367 | |
| c25989_g3 | AGL1(Arabidopsis thaliana) | P29381 | 1.126 | 1.470 | 1.544 | |||
| c24381_g1 | YABBY 5 (Arabidopsis thaliana) | Q8GW46 | 0.660 | 0.650 | 0.809 | 1.459 | 1.316 | |
| c21190_g1 | SUPERMAN (Arabidopsis thaliana) | Q38895 | 1.477 | 1.329 | ||||
| c24288_g1 | AGAMOUS (AGAMOUS-2) (Nicotiana tabacum) | Q43585 | 1.329 | 1.210 | 0.730 | 2.541 | 3.271 | 1.946 |
Figure 4.
Genes differently expressed during ovule development.
Figure 5.
Venn diagram showing the overlaps between different develop stages of ovule.
Gene expression patterns of selected genes
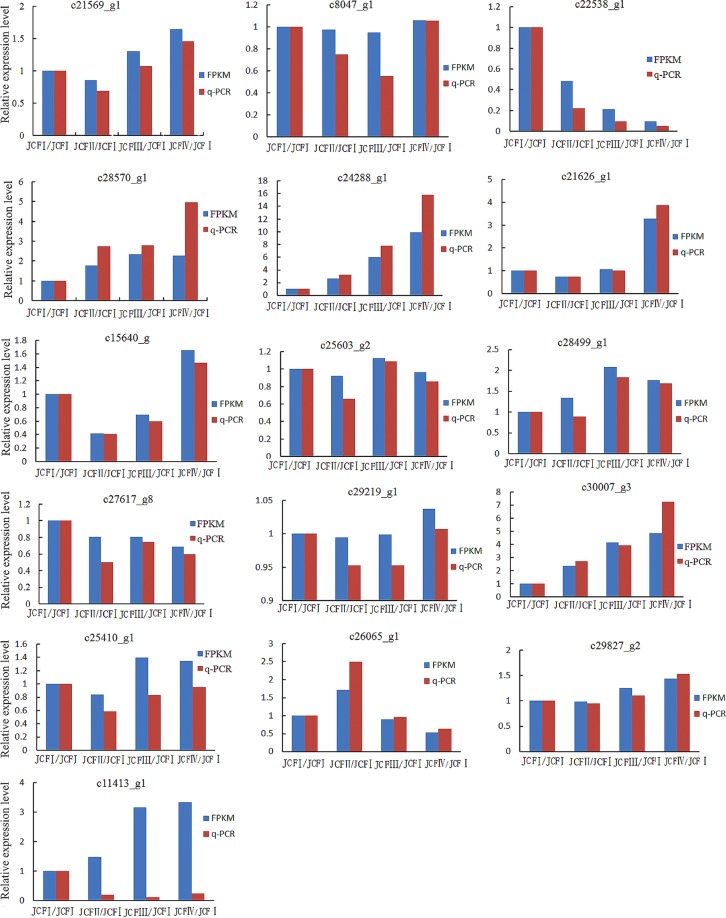
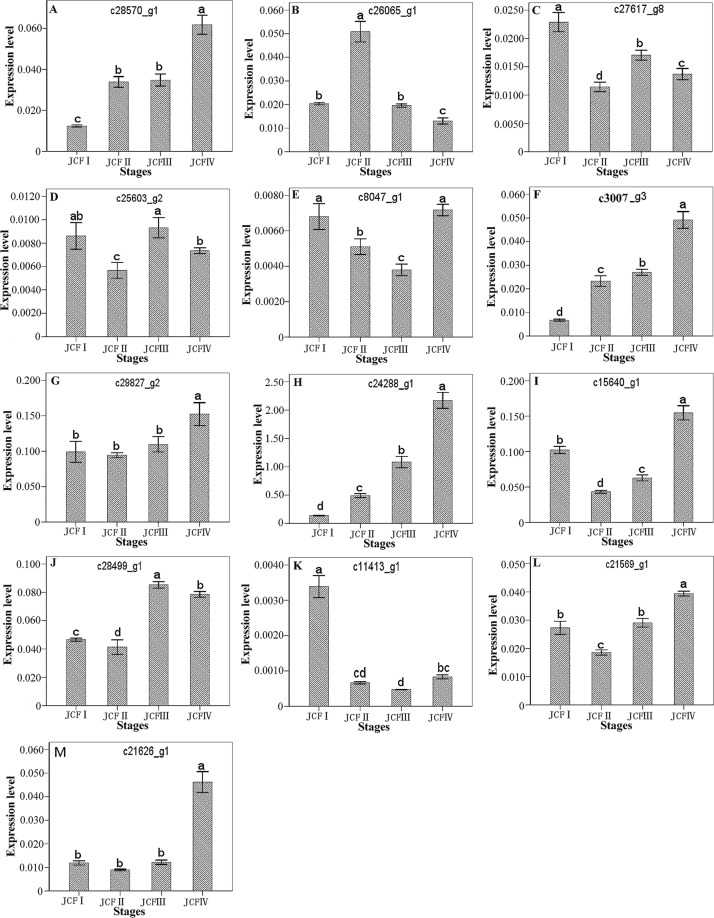
In total, 16 DEGs were selected for qRT-PCR analysis to validate the expression profiles obtained by digital gene expression analysis, and the results showed that the expression patterns of these genes were consistent with the results obtained by digital gene expression analysis, except AUX22 (c11413_g1) (Fig. 6), indicating that transcriptome data in this study were reliable.
Figure 6.
Expression pattern of randomly selected genes. The fold changes of the genes were calculated as the ratio of the JCFI/JCFI, JCFII/JCFI, JCFIII/JCFI and JCFIV/JCFI and are shown on the y-axis.
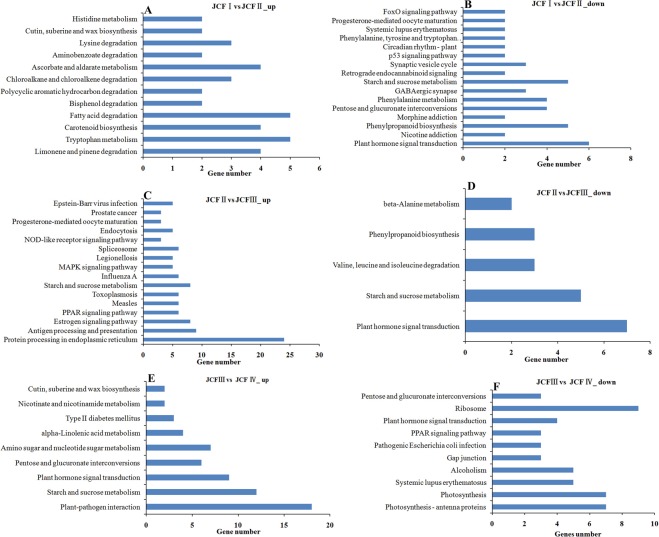
KEGG pathway enrichment analysis of differentially expressed genes
After KEGG analysis, the significantly enriched pathways were selected for further analysis. The results indicated that sugar metabolism and protein biosynthesis/processing were respectively enhanced during the maturation of ES and the formation of MES, and the metabolisms of auxin and JA were also upregulated during the formation of MMC and the maturation of EC, but the metabolism of SA declined during ovule development. Among the significantly enriched pathways (Fig. 7), plant hormone signal transduction was downregulated in pairwise JCFI vs JCFII and JCFII vs JCFIII but showed a trend to upregulate in pairwise JCFIII vs JCFIV; starch and sucrose metabolism was upregulated in pairwise JCFIII vs JCFIV; protein processing in endoplasmic reticulum was upregulated in pairwise JCFIII vs JCFII; tryptophan metabolism associated with auxin metabolism was upregulated in pairwise JCFI vs JCFII; alpha-linolenic acid metabolism associated with JA metabolism was upregulated in pairwise JCFIII vs JCFIV; phenylalanine metabolism associated with SA metabolism was downregulated in pairwise JCFI vs JCFII.
Figure 7.
KEGG classification analysis of the differently expressed genes.
Differentially expressed transcription factor genes during ovule development
A total of 156 transcription factor (TF) genes were differentially expressed during ovule development, of which the top six TF families were bHLH, WRKY, MYB, ethylene-responsive transcription factors (ERF), NAC and TCP: 26 from bHLH, 21 from WRKY, 20 from MYB, 18 from ERF, 13 from NAC, and 8 from TCP. Among the DEGs of these six TF families, the upregulated TF genes included 17 bHLH, 11 WRKY, 12 ERF, 10 NAC, 10 MYB and 2 TCP (Fig. 8). Of these 10 MYB genes, a R2R3-type MYB gene (c28570_g1) was confirmed to be upregulated with the development of the ovule by qRT-PCR analysis (Fig. 9). These results suggested that bHLHs, WRKYs, ERFs, NACs and MYBs would extensively participate in ovule development.
Figure 8.
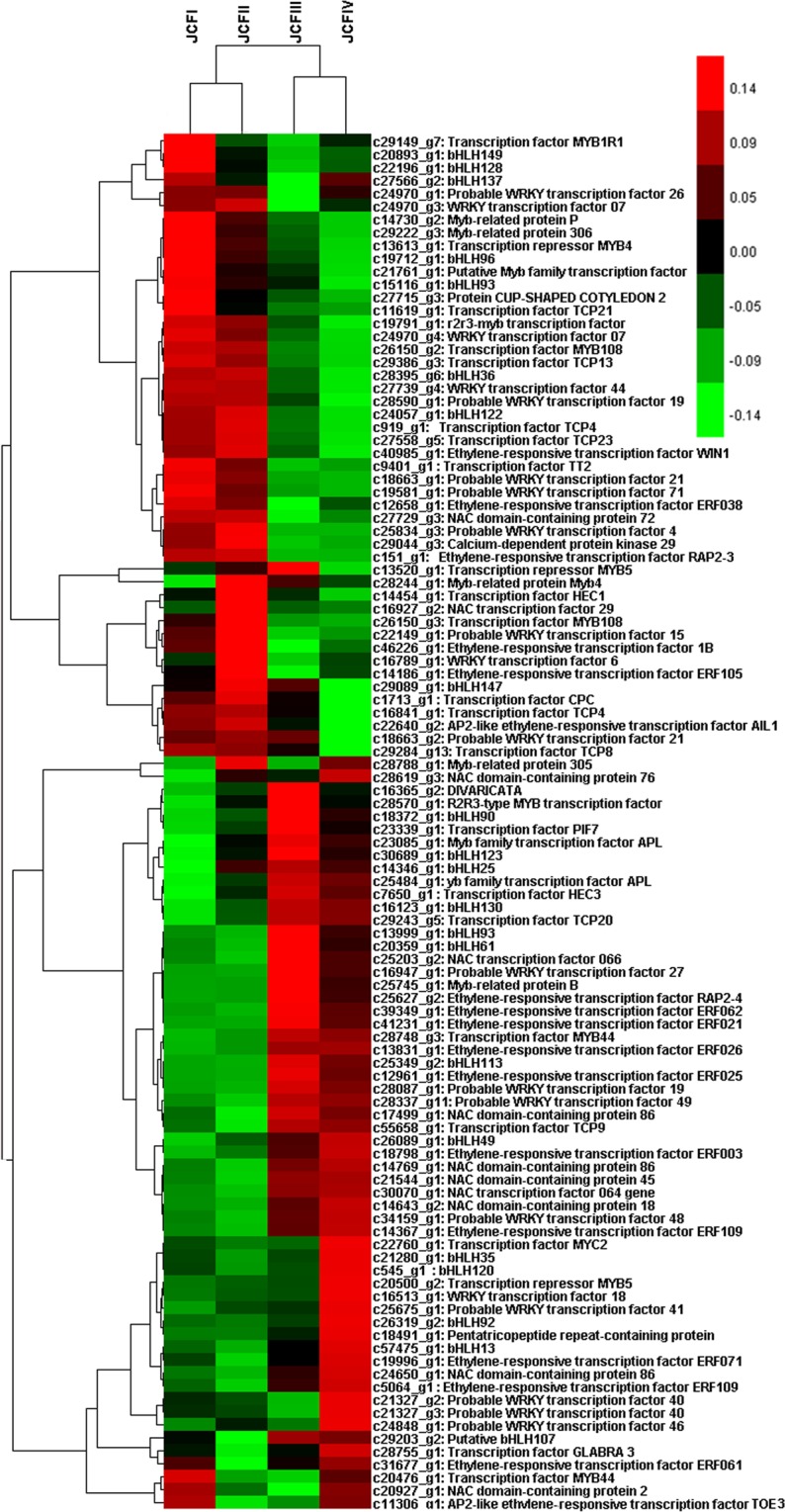
Cluster analysis showing the differentially expressed transcriptor factor genes during ovule development.
Figure 9.
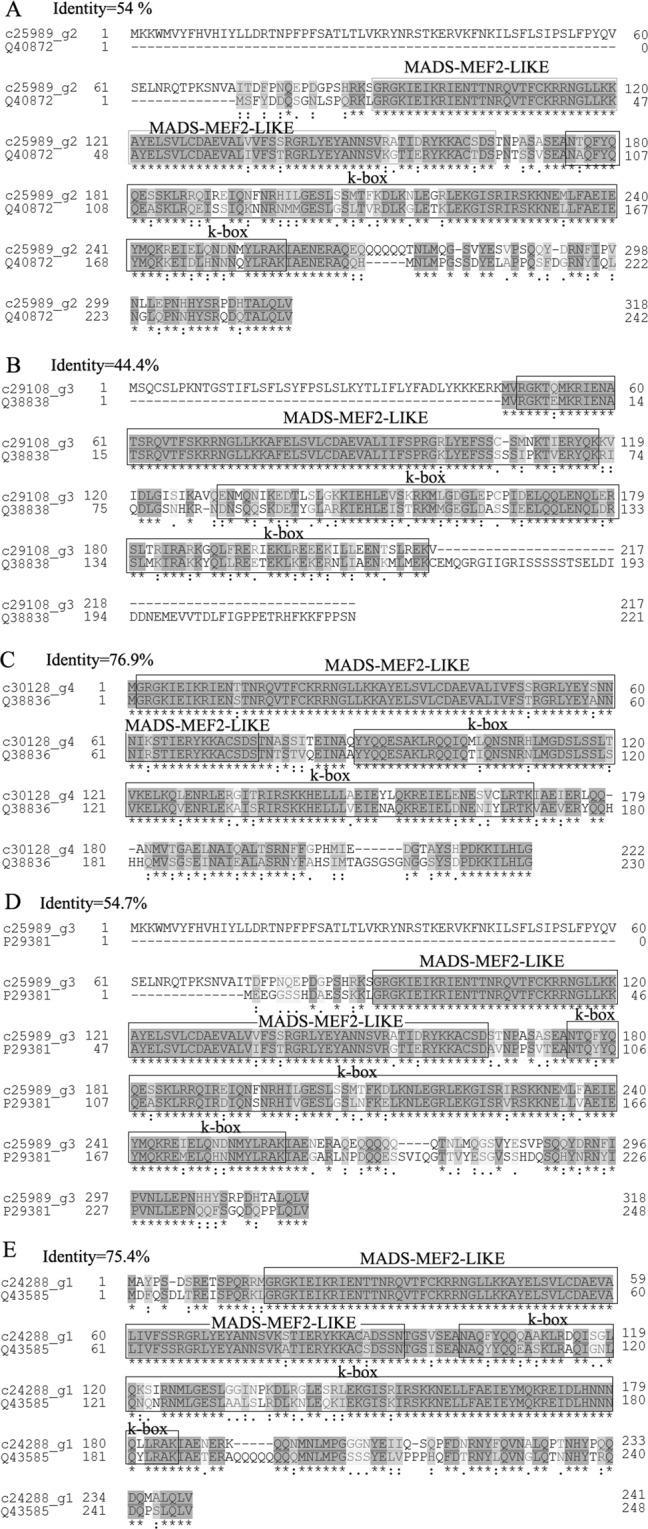
Sequence alignment between five MADS-box proteins and their homologues in protein database UniProt.
Prediction of gene regulating ovule development
In total, 9 genes annotated as ovule development-related genes were upregulated during ovule formation as suggested by transcriptome analysis, including 5 MADS-box protein genes (AGAMOUS-2 (AG-2), AG-1, AGL1/SHP1, AGL11/STK and AGL14), YABBY 5, UPERMAN (SUP), SLK3 and Argonaute 2 (Table 1). These five MADS-box proteins were then subjected to sequence alignment with their homologs in the protein database UniProt. The results showed that these five MADS-box proteins have a MADS-MEF2-LIKE and a K-box domain and belong to the MADS-MEF2-LIKE subfamily of MADS (Fig. 9). The MADS-MEF2-LIKE domain showed high homology between these five MADS-box proteins and their homologs (Fig. 9). Of these 9 genes, AG-2 was upregulated throughout ovule development, as suggested by both transcriptome analysis and qRT-PCR analysis (Fig. 10; Table 1); AGL1, AGL14, AG-1 and SUP were upregulated in pairwise JCFI vs JCFII; SLK3 was upregulated in pairwise JCFIV vs JCFIII; AGL11/STK was continuously upregulated from JCFI to JCFIII; YABBY 5 was continuously upregulated from JCFII to JCFIV; argonaute 2 was upregulated in pairwise JCFII vs JCFIV and JCFI vs JCFIV. On the other hand, CRABS CLAW (CRC), annotated as an ovule development-related gene, was upregulated from JCFI to JCFIV, as suggested by qRT-PCR analysis (Fig. 10).
Figure 10.
One-way ANOVA analysis of expression level of 15 selected genes in samples at different ovule development stages, as detected by real-time QPCR. Different letters in the same row indicate significant differences (P ≤ 0.005). c28570_g1: R2R3-type MYB; c30007_g3: CRABS CLAW; c24288_g1: AGAMOUS-2; C28499_g1: Auxin-induced protein 5NG4; c21569_g1: IAA1; c15640_g1: GASA3; c8047_g1: BZR1; C21626_g1: Auxin-induced in root cultures protein 12; c29827_g2: GAI; c26065_g1: GASL7; c27617_g8: ARF5; c25603_g2: BAK1; c11413_g1: AUX22.
Phytohormone signaling responsive genes associated with ovule development
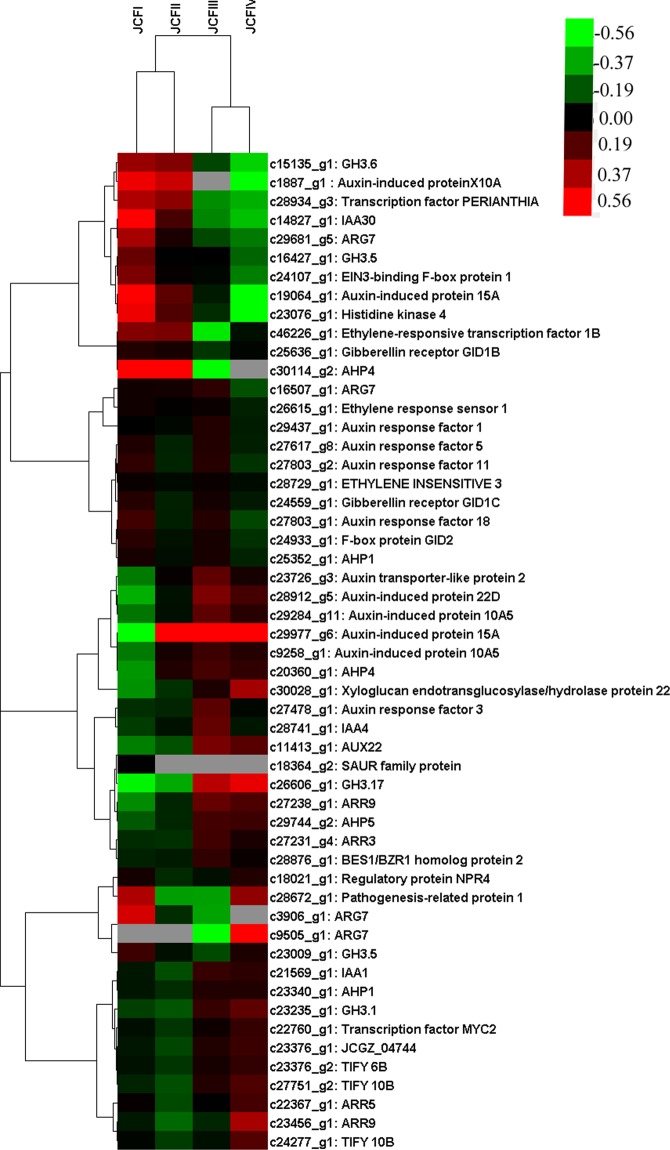
In all, 71 phytohormone signaling-responsive DEGs were identified, 61 of which were screened out by transcriptome analysis, 9 by qRT-PCR analysis, and IAA1 by both qRT-PCR and transcriptome analysis (Figs 10, 11). The expression of IAA1 was first downregulated at JCFII and then significantly upregulated during the following development stages (Fig. 10B,L).
Figure 11.
Cluster analysis showing the differentially expressed genes enriched in phytohormone signaling during ovule development.
Of these 61 DEGs screened out by transcriptome analysis, 24 were from auxin, 2 from BR, 10 from CK, 8 from ABA, 5 from ETH, 3 from GA, 6 from JA, and 3 from SA. In pairwise CFI vs JCFII, the expression of 4 SAUR genes (22D protein, 15A-1 and two 10A5 (10A5-1 and 10A5-2)) in auxin and histidine-containing phosphotransfer proteins 4 (HP4-2) in CK were upregulated, whereas ARF 11, ARF 18 and GH3.5 in auxin were downregulated. In pairwise JCFII vs JCFIII, the expression of GH3.17 in auxin was upregulated, whereas IAA30 and X10A in auxin and GH3.5 in JA were downregulated. In pairwise JCFIII vs JCFIV, the expression of GH3.1 in auxin, ARR9-2 and ARR5 in CK, gibberellin receptor GID1B in GA, xyloglucan endotransglucosylase/hydrolase protein 22 (XTH22) in BR, pathogenesis-related protein 1 in SA, and two TIFY 10B and a MYC2 in JA were upregulated, whereas ARG7-3 in auxin was downregulated. In pairwise JCFI vs JCFIII, JCFI vs JCFIV or JCFII vs JCFIV, the expression of auxin transporter-like protein 2 (ATLP2), IAA4, ARF3 and ARG7-1 in auxin, HP5, HP1-2, ARR3 and ARR9-1 in CK, BES1/BZR1 homolog protein 2 in BR, and TIFY 6B and hypothetical protein JCGZ_04744 in JA were upregulated, whereas ARG7-2, ARG7-4, 15A-2, GH3.6, ARF 5 and ARF 1 in auxin, GID1C and GID2 in GA, HP1 in CK, NPR4 and PERIANTHIA in SA were downregulated. On the other hand, HK 4 was continuously downregulated during ovule development, and HP4-1 was downregulated from JCFI to JCFIII. The genes in ETH signaling were all downregulated during the development of the ovule. Of the 8 genes involved in ABA signaling, only ABA receptor PYL9 was downregulated in pairwise JCFIII vs JCFIV, while the other genes gradually changed their expression during ovule development (Fig. 11).
Of these 9 DEGs screened by qRT-PCR analysis, 3 genes (GASA3, GAI and GASL7) were from the GA response signaling pathway, 2 (BRASSINOSTEROID INSENSITIVE 1-associated receptor kinase 1 (BAK1) and (BRASSINAZOLE-RESISTANT 1 protein (BZR1)) from BR, 4 (ARF5, 5NG4, AUX22 and auxin-induced in root cultures protein 12 (AIT12)) from auxin. Of these genes, GASA3 was upregulated from JCFII to JCFIV, and GASL7 from JCFI to JCFII; BZR1, AIT12 and GAI were upregulated from JCFIII to JCFIV; 5NG4, ARF5 and BAK1 were from JCFII to JCFIII; AUX22 was greatly downregulated at the stages after JCFI (Fig. 10).
Differentially expressed genes related to phytohormone metabolism
The DEGs related to phytohormone metabolism were screened out as follows: auxin metabolism-related DEGs were screened out from the DEGs enriched in the tryptophan metabolism pathway, CK metabolism-related DEGs from the DEGs enriched in the enriched zeatin biosynthesis pathway, gibberellin metabolism-related DEGs from the DEGs enriched in the diterpenoid biosynthesis pathway, BR metabolism-related DEGs from the DEGs enriched in metabolism pathway, JA metabolism-related DEGs from the DEGs enriched in the alpha-linolenic acid metabolism pathway, and SA biosynthesis-related DEGs from the DEGs enriched in phenylalanine biosynthesis (Table 2).
Table 2.
Phytohormone biosynthesis genes differently expressed during the ovule development.
| Genes | Swissprot Description | Protein ID | log2(JCFII/JCFI) | log2(JCFIII/JCFII) | log2(JCFIV/JCFIII) | log2(JCFIII/JCFI) | log2(JCFIV/JCFI) | log2(JCFIV/JCFI I) |
|---|---|---|---|---|---|---|---|---|
| Auxin | ||||||||
| c24045_g2 | Probable YUCCA8 (Arabidopsis thaliana) | Q9SVU0 | 1.504 | |||||
| c25692_g3 | Aldehyde dehydrogenase family 3 member H1(ALDH3 H1) (Arabidopsis thaliana) | Q70DU8 | 1.096 | |||||
| c14699_g1 | Aldehyde dehydrogenase family 2 member B4(ALDH2 B4-1), (Arabidopsis thaliana) | Q9SU63 | 0.737 | |||||
| c20408_g1 | Aldehyde dehydrogenase family 2 member B4(ALDH2 B4-2), (Arabidopsis thaliana) | Q9SU63 | −0.688 | |||||
| c29730_g3 | Cytosolic sulfotransferase 16 (Arabidopsis thaliana) | Q9C9D0 | −1.383 | −0.884 | ||||
| c24704_g1 | Aldehyde dehydrogenase family 3 member F1(ALDH3 F1) (Arabidopsis thaliana) | Q70E96 | −0.625 | |||||
| c26913_g1 | YUCCA2 (Arabidopsis thaliana) | Q9SVQ1 | −2.025 | |||||
| c28407_g1 | YUCCA4 (Arabidopsis thaliana) | Q9LFM5 | −0.937 | |||||
| Gibberellins | ||||||||
| c15206_g1 | Gibberellin 2-beta-dioxygenase 2 (Pisum sativum) | Q9XHM5 | −2.105 | −2.100 | ||||
| c25340_g1 | Ent-kaurene oxidase (Arabidopsis thaliana) | Q93ZB2 | 0.749 | 1.394 | 2.109 | 1.469 | ||
| c28071_g1 | Ent-kaurenoic acid oxidase 1 (Arabidopsis thaliana) | O23051 | 0.558 | 0.585 | ||||
| Cytokinin | ||||||||
| c39116_g1 | Cytokinin dehydrogenase 3 (Arabidopsis thaliana) | Q9LTS3 | −2.138 | |||||
| c29016_g9 | Adenylate isopentenyltransferase 5 (Arabidopsis thaliana) | Q94ID2 | 2.246 | 1.099 | 2.785 | 3.884 | 1.642 | |
| JA | ||||||||
| c27373_g1 | 3-ketoacyl-CoA thiolase 2 (Arabidopsis thaliana) | Q56WD9 | 0.746 | 1.155 | 1.679 | 1.905 | ||
| c27860_g2 | Linoleate 13S-lipoxygenase 3-1 (Solanum tuberosum) | O24371 | 1.015 | 1.275 | 1.182 | |||
| c17968_g1 | Allene oxide synthase (Linum usitatissimum) | P48417 | 1.385 | 1.404 | 1.483 | |||
| c29952_g2 | 12-oxophytodienoate reductase 3 (Solanum lycopersicum) | Q9FEW9 | 1.040 | 0.957 | ||||
| c29272_g6 | Acyl-coenzyme A oxidase 2 (Arabidopsis thaliana) | O65201 | −0.733 | |||||
| c29400_g1 | fatty acid beta-oxidation multifunctional protein AIM1 (Arabidopsis thaliana) | Q9ZPI6 | 0.522 | 0.942 | 0.723 | |||
| c24535_g1 | 4-coumarate–CoA ligase-like 5 (Arabidopsis thaliana) | Q84P21 | 0.520 | 0.476 | ||||
| c27608_g6 | Allene oxide cyclase 4 (Arabidopsis thaliana) | Q93ZC5 | 1.095 | 1.203 | ||||
| c28745_g1 | Linoleate 13S-lipoxygenase 2-1 (Solanum tuberosum) | O24370 | 1.350 | 0.903 | ||||
| c29303_g1 | Putative 12-oxophytodienoate reductase 11 (Oryza sativa subsp. Japonica) | B9FSC8 | ||||||
| SA | ||||||||
| c29339_g2 | Phenylalanine ammonia-lyase (Populus trichocarpa) | P45730 | −1.040 | −0.617 | ||||
| BR | ||||||||
| c28090_g2 | CYP90D1 (Arabidopsis thaliana) | Q9M066 | −0.672 | |||||
| c24187_g1 | CYP90B1 (Arabidopsis thaliana) | O64989 | −0.990 | |||||
In total, 26 differentially expressed genes were identified, of which 8 were from auxin, 2 from BR, 2 from CK, 3 from GA, 10 from JA, and 1 from SA. In pairwise JCFI vs JCFII, the expression levels of YUCCA8, aldehyde dehydrogenase family 3 member H1 (ALDH 3H1) and ALDH 2B4-1 in auxin were upregulated, whereas cytokinin dehydrogenase 3 in CK was downregulated. In pairwise JCFII vs JCFIII, the expression of Ent-kaurene oxidase (KO) in GA was upregulated, whereas ALDH 2 B4-2 in auxin and gibberellin 2-beta-dioxygenase 2 in GA were downregulated. In pairwise JCFIII vs JCFIV, the expression of linoleate 13 S-lipoxygenase 3-1, allene oxide synthase, and 12-oxophytodienoate reductase 3 in JA was upregulated. In pairwise JCFI vs JCFII and JCFIII vs JCFIV, the expression of adenylate isopentenyltransferase 5 (IPT5) in CK was upregulated. In pairwise JCFII vs JCFIII and JCFIII vs JCFIV, the expression of 3-ketoacyl-CoA thiolase 2 in JA was upregulated. Additionally, the expression of several genes gradually changed during ovule development. In pairwise JCFI vs JCFIII, CFI vs JCFIV or JCFII vs JCFIV, the expression of ent-kaurenoic acid oxidase 1 (KAO1) in GA and fatty acid beta-oxidation multifunctional protein AIM1 gene, 4-coumarate-CoA ligase-like 5, allene oxide cyclase 4 gene, linoleate 13 S-lipoxygenase 2-1 gene, and 12-oxophytodienoate reductase 11 gene in JA were upregulated, whereas cytosolic sulfotransferase 16, ALDH3F1, YUCCA2 and YUCCA4 in auxin, acyl-coenzyme A oxidase 2 gene in JA, phenylalanine ammonia-lyase gene in SA, and CYP90D1 and CYP90B1 in BR were downregulated (Table 2).
Discussion
The embryo sac of J. curcas belongs to the polygonum type. A macrospore mother cell (MMC) only produces one functional macrospore that then develops into an embryo sac with 8 nuclei after three consecutive mitoses. Of these 8 nuclei, two move to the center of the embryo sac and form a polar, and the remaining six form an egg, two synergids, and three antipodal cells, respectively (Figs 1 and 2). A transcriptome covering the entire development process of sporogenesis and gametogenesis in both male and female flowers was constructed using Illumina sequencing.
Overall, plant hormone signal transduction declined during ovule development but showed a trend toward enhancement during the maturation of ES. Protein biosynthesis and processing were enhanced during the formation of MES. Sugar metabolism seemed to be enhanced during the development process of the ovule. A strong relationship exists between flower development and carbohydrates12–15. In particular, the concentration of starch in the ovule at specific steps of development is closely correlated with fertility16.
Transcription factors participating in ovule development
Five TF families, such as bHLH, WRKY, MYB, NAC and ERF, would extensively contribute to ovule development, as suggested by the upregulation of 17 bHLH, 11 WRKY, 12 ERF, 10 NAC and 10 MYB during ovule development. The members of these five TF families are thought to be involved in ovule development. bHLHs play important roles in carpel, stigma and anther development and phytochrome signaling17–19. MYBs can regulate the development of anther, petal, and embryogenesis20–22. R2R3-MYBs are the largest group of plant MYB factors, and several R2R3-MYBs have been documented to function in ovule development. FOURLIPS and MYB88 are thought to regulate megasporogenesis23, and MYB98 is specifically expressed in synergid cells24. For WRKY, WRKY71 can accelerate flowering via the direct activation of FT and LEAFY25, and WRKY34 and WRKY2 are required for male gametogenesis in Arabidopsis26. Several members of the AP2/ERF family are thought to be involved in microspore, somatic and zygotic embryogenesis27. Members of the NAC family, such as CUC genes, contribute to ovule primordial development28,29.
MADS-BOX, SUP and SLK3 genes participate in ovule development
In the present study, five MADS-BOX genes (AG-1, AG-2, AGL1, AGL11 and AGL14), SUP and SLK3 are expected to function in the development of the ovule in J. curcas. AG has been reported to determine stamen, carpel and ovule identity30–32. In this study, AG-1 functioned during the stage of occurrence of MMC and AG-2 throughout the entire development of the ovule. SHP1 (AGL1) and STK (AGL11) are known as ovule identity genes that control ovule cell fate and regulate sporophyte and gametophyte development33–35. SHP1 (AGL1) is also reported to function in regulating cell divisions in the ovule and in promoting stigma, style and medial tissue development36,37. Our results show that AGL1 and AGL11 play roles during the stage of MMC, controlling ovule cell fate, and regulating sporophyte development and cell divisions in the ovule. However, AGL11 is also predicted to function during the formation of MES and regulate gametophyte development. AGL14 can control auxin transport via PIN transcriptional regulation38, and the PIN protein-dependent auxin gradient is responsible for pattern formation during early embryogenesis, organogenesis and ovule development39. Our results suggest that AGL14 functions during the occurrence of MMC and regulates the early development of the ovule. SUP is known to function in the direct regulation of ovule development by dictating the growth of the adaxial outer-ovule integument by downregulating cell division40–42. In J. curcas, SUP may function during the occurrence of MMC when outer-ovule integument is not generated, and a new role of SUP in ovule development is predicted. Members of the YABBY family are active in determining abaxial-adaxial identity in Arabidopsis lateral organs43 and involved in amorphous or arrested growth of the integument44. However, YABBY 5, a member of the YABBY family, functions during the stages after the occurrence of MMC. The SLK gene is required for the proper development of vital female reproductive tissues derived from the carpel margin by maintaining meristematic potential in both the carpel margin meristem and the shoot apical meristem45. In the present study, SLK3, a member of the SLK gene, is predicted to function during the maturation of ES (a late stage of ovule development). Although these genes are predicted to function in certain developmental stages of the ovule, the details of their roles in ovule development are not clear and warrant further exploration. CRC has been thought to function in carpel morphogenesis and in the development of ovules33,46. Similarly, CRC was upregulated with the development of ovules in J. curcas and may function in the entire process of ovule development47.
GA plays important roles at the stage from MMC in ovule development
GA is known to play various roles in plant reproductive development, and different species respond differently to GA48. The GA signaling pathway is involved in the development of stamens and pollen in Arabidopsis and tomato49–51. GA is a negative regulator in the development of stamen in maize52,53 but a positive regulator in Arabidopsis and rice54. GA is thought to play essential and complicated roles in floral organ development in J. curcas. In J. curcas, GA treatment increased both female and male flowers in the studies of Pan et al.10 and Gayakvad et al.55 promoted the development of stamens to produce bisexual flowers in research of Pi et al.56 and increased the number of female flowers in the research of Makwana et al.57. In gynoecious plants, GA treatment represses pistil development in female flowers to produce neutral flowers but does not resume the development of stamens58. In the present study, GA was predicted to promote the development of macrospores and embryo sacs in monoecious female flowers. The GA concentration in flower buds was increased at the stages from the occurrence of MMC to the maturation of ES, which was due to the upregulation of KO and KAO1. KO and KAO1 catalyzed the reaction to convert ent kaurene to GA12 during GA biosynthesis59. Furthermore, the upregulation of several GA-responsive genes (such as GASL7 GASA3 GID1b and GAI) during the stages from the formation of MMC to the maturation of SE may be the result of the increase in GA. In cotton, GASL7 is predominantly expressed in cotyledons, and GASA3 is expressed in fiber60. In Arabidopsis thaliana, GASA3 accumulates in root, meristems, and flower seeds61. In Arabidopsis thaliana, 3 GA hormone receptors, such as GID1a, GID1b, and GID1c, have partially specialized functions in both proteolytic and nonproteolytic GA signaling. GID1b plays a stronger role in nonproteolytic GA signaling62. As a member of DELLA, GAI is a negative regulator of GA signaling63.
JA plays important roles in the stage of maturation of EC
JA is thought to play critical roles in regulating reproductive development in plants, such as sex determination and stamen development in maize, and the development of inflorescences and flowers in rice and Arabidopsis64. In J. curcas, JA biosynthesis is lower in gynoecious than in monoecious florescence, indicating that the decrease of JA biosynthesis would likely contribute to the abortion of male flowers in gynoecious plants60. However, our results suggest that JA contributes to the maturation of EC in females; JA levels are increased during EC maturation, which is the result of the enhancement of JA biosynthesis, suggesting the upregulation of four JA biosynthesis genes, such as 3-ketoacyl-CoA thiolase 2, linoleate 13S-lipoxygenase 3-1, allene oxide synthase, and 12-oxophytodienoate reductase 359. On the other hand, several JA-responsive genes, such as MYC2, JAZ, TIFY 6B and TIFY 10B, were upregulated at the maturation of EC, supporting our results. TIFY 10B and TIFY 6B belong to the JAZ subfamilies of the TIFY family and could be induced by JA65,66. In Arabidopsis, JAZ1/TIFY10b can work as a transcriptional repressor in the models of JA signaling67,68. Activation of JA response genes is mediated in part by MYC269–71; in the absence of JA, MYC2’s function as a transcriptional activator is repressed by members of the JAZ family proteins, and elevated levels of JA induce the release of MYC2 from this repression by increasing the rate of degradation of JAZs70,72. Furthermore, MYC2 is a “master switch” in the crosstalk between JA and the other hormone signaling pathways73.
Auxin may regulate the occurrence of MMC in ovule development
Auxin is thought to regulate ovule development74,75, possibly by determining the cell identity of the female gametophyte cell39, and the gradient of auxin is responsible for pattern formation during ovule development39,76. Similarly, our results also suggest that auxin contributes to ovule development. The auxin biosynthesis in flower buds is expected to be enhanced during the occurrence of MMC, as suggested by the upregulation of YUCCA8, ALDH 3H1 and ALDH 2B4-1. In Arabidopsis thaliana, the YUCCA gene encodes a flavin monooxygenase-like enzyme that appears to oxidize tryptamine to N-hydroxytryptamine77,78. The ALDH family is thought to function in IAA biosynthesis by catalyzing the NAD-dependent formation of IAA from indole-3-acetaldehyde79. The expression levels of SAURs (22D, 10A5 and two 15A), two GH3s (GH3.17 and GH3.1), two AUX/IAA (IAA1, IAA4), AFR3, ARG7-1, AIT12, ATLP2 and 5NG4 were upregulated during the development of the ovule, which would be associated with the elevated level of JA. Also, 10A5 and 15A are members of SAURs and could be rapidly induced by auxin treatment within 2.5 min80,81. The AUX/IAA family is short-lived transcriptional factors and repressors of early auxin response genes at low auxin concentrations, and increased auxin could reduce the level of AUX/IAA proteins by accelerating their degradation. Different GH3s respond differentially to auxin treatment. In Arabidopsis, under IAA treatment, GH3.1 showed strong transcriptional induction, while GH3.5 and GH3.6 showed weaker responses, and GH3.17 showed little or no induction82,83. AFR3 is a member of group II AFRs and is thought to be involved in floral meristem, gynoecium, stamen and perianth patterning in Arabidopsis84. Exogenous auxin treatment upregulates ARF385.
Cytokinin may regulate the occurrence of MMC and the maturation of ES in ovule development
In Arabidopsis thaliana, cytokinin is reported to regulate ovule formation and ovule number86–91 and to specify the functional megaspore in the female gametophyte92. In J. curcas, the application of CK increased the number of female flower10,55. Similarly, our results suggest that CK contributes to female development. CK biosynthesis in female flower buds would be enhanced at stages of MMC occurrence and ES maturation, as suggested by the upregulation of IPT and the downregulation of cytokinin dehydrogenase at these two stages. IPT catalyzes the initial step in the de novo biosynthesis of cytokinin in higher plants, whereas cytokinin dehydrogenase catalyzes the degradation of cytokinin59. In response to the increase in CK, several CK response genes, such as HP4-2, HP5, HP1-2, and five A-type ARRs (ARR9-2, ARR5, ARR9-1 and two ARR3) were upregulated. The cytokine signaling cascade typically consists of three functional modules: HK, HP, and response regulator (ARR)93. HP proteins serve as phosphorelay carriers between cytokinin receptors and downstream nuclear responses, activating B-type ARR proteins, which in turn, activate A-type ARRs94. Additionally, A-type ARRs are reported to participate in ovule development. Megagametophyte defects could be observed in the Arabidopsis mutants arr7 and arr1595. In ovules lacking a functional embryo sac, ARR7 and other A-type ARRs (ARR4, ARR5 and ARR6) are overexpressed96. Most of the A-type ARRs could be rapidly and specifically induced by exogenous cytokinin97,98. RRA3 and RRA5 were robustly induced by cytokinin in Arabidopsis and J. curcas2,10,99,100.
Brassinosteroids may function in the stages from meiosis of MMC to ES maturation
Brassinosteroids (BRs) suppress the development of the female flower in cucumber101, whereas they positively regulate the ovule number in Arabidopsis by regulating related gene expression by BZR1102. BRs influence ovule development by regulating the transcription of genes, such as HUELLENLOS (HLL) and AINTEGUMENTA (ANT), which are redundant in the control of the ovule primordial growth103. ANT is a direct target of BRZ1, while HLL is regulated in an indirect way102. However, in this research, BR biosynthesis in flower buds is expected to decline during ovule development, as suggested by the downregulation of CYP90D1 and CYP90B1. In Arabidopsis, CYP90D1 plays an important role in the early C-22 oxidation of BR biosynthesis, and CYP90B1 catalyzes multiple 22 a-hydroxylation steps in BR biosynthesis104,105. In contrast to the decline of BR biosynthesis, BR signaling-responsive DEGs were all upregulated during ovule development, such as XTH22, BZR1, BAK1 and BES1/BZR1, indicating that cross-talk might exist between BRs and other phytohormone signaling pathways. Several reports proposed that BRs and GAs could interact at the signaling leve106–108. The DELLA protein GAI is thought to inactivate BZR1 by inhibiting the ability of BZR1 to bind to target promoters and negatively regulate BR signaling106–108. Additionally, auxin can also crosstalk with BRs through G-protein signaling109. XTHs, which can be upregulated by BRs, are thought to modify the length of xyloglucans, enabling the cell wall to expand110,111. BAK1 is a coreceptor of BRI1, and the binding of BRs to BRI1 relieves the repression of BKI and causes the association of BRI1 with BAK1, as well as a series of phosphorylation events112,113. BZR1 and BES1 are two major transcription factors regulated by BIN2 and mediate BR-regulated gene expression77,114,115.
Conclusion
To the best of our knowledge, this report is the first to perform a transcriptome analysis of ovule development in J. curcas. A set of ovule development-related genes has been identified in female flower buds through expression profiling analysis, which provided comprehensive information on the ovule development of J. curcas, revealing that five TF families (bHLH, WRKY, MYB, NAC and ERF), five hormones signaling (auxin, GA, CK, BR and JA), five MADS-box genes (AG-1, AG-2, AGL1, AGL11 and AGL14), SUP and SLK3 participate in ovule development. GA and JA are involved in ovule development, as confirmed by their accumulation in flower buds during ovule development. Additionally, CRC and SUP are demonstrated to be involved in ovule development by qRT-PCR analysis. Transcriptome analysis is only an initial step in the exploration of the molecular mechanism of ovule development in J. curcas, but the ovule development-related genes identified in this study will establish a foundation for investigations into the molecular mechanisms of ovule development in J. curcas, and further analyses of these genes are needed to elucidate their roles in ovule development in J. curcas.
Materials and Methods
Flower bud samples collected
The flower bud samples of different development stages were collected from thirty J. curcas trees in May 2015 in Zhenfeng, southwestern China (36°14′50.2″N, 87°51′47.8″E). Each flower bud sample was separated into two portions. The portion used for the anatomic structure analysis of the ovary was fixed immediately in formaldehyde-acetic acid-50% alcohol mixtures (4: 6: 90, v/v), and the other portion used for transcriptome analysis was dipped immediately in RNAlocker (Tiandz, Inc., Beijing China) on ice. The flower bud samples used for the phytohormone quantification were stored in liquid nitrogen.
Analysis of paraffin sections of ovaries
The flower bud samples used for the anatomic structure analysis of ovaries were fixed in formaldehyde-acetic acid-50% alcohol mixtures (4: 6: 90, v/v) for 24–48 h and then stored in 70% ethanol. The ovaries cut from fixed flower samples were dehydrated by gradient alcohol and then embedded in paraffin. Serial sections (8~10 μm thick) were cut using a Leica RM 2016 rotary microtome and stained in Safranin-Fast Green. The stained paraffin sections were analyzed under an Olympus – CX41 light microscope. The anatomic structure analysis of anther development will be discussed in detail in further research.
Grouping on the flower bud samples and total RNA extraction
The flower bud samples stored in RNAlocker were then divided into eight development phases according to the morphology of flower buds at different development phases using an anatomical lens as follows: JCMI, the formation of ten stamen primordia; JCMII, from the occurrence of sporogenous cells to the occurrence of microspore mother cell; JCMIII, from the meiosis of microspore mother cell to the formation of meiotic tetrads; JCMIV, from free microspore stage to the maturation of pollen grain; JCFI, the growth of three carpels; JCFII, from the occurrence of sporogenous cells (SC) to the occurrence of macrospore mother cell (MMC); JCFIII, from the meiosis of MMC to the formation of mononuclear embryo sac (MES); JCFIV, from the formation of MES to the maturation of embryo sac (ES)(Figs 1 and 2).
For total RNA extraction, the flower buds from those eight development stages (each phase has three replicates) were ground in liquid nitrogen, and total RNA was isolated using the E.A.N. A Plant RNA Kit (Omega, USA) according to the manufacturer’s manual. The obtained RNA samples were then used for library construction.
Library construction and Illumina sequencing
The RNA of flower buds from those eight flower development stages was applied for library construction. A total amount of 3 μg RNA per sample was used for the RNA sample preparations. RNA purity was checked using a NanoPhotometer® spectrophotometer (IMPLEN, CA, USA). RNA concentration was measured using a Qubit® RNA Assay Kit in Qubit® 2.0 Flourometer (Life Technologies, CA, USA). RNA integrity was assessed using the RNA Nano 6000 Assay Kit of the Agilent Bioanalyzer 2100 system (Agilent Technologies, CA, USA). Sequencing libraries were generated using the NEBNext® Ultra™ RNA Library Prep Kit for Illumina® (NEB, USA) following the manufacturer’s recommendations, and index codes were added to attribute sequences to each sample. The quality of the library was assessed on the Agilent Bioanalyzer 2100 system and ABI Step OnePlus Real-Time PCR System in Novogene (Beijing, China). The clustering of the index-coded samples was performed on a cBot Cluster Generation System using the TruSeq PE Cluster Kit v3-cBot-HS (Illumina) according to the manufacturer’s instructions. After cluster generation, the library preparations were sequenced on an Illumina Hiseq platform, and paired-end reads were generated (Novogene Bioinformatics Technology, Beijing, China).
De Novo Transcriptome Assembly and Abundance Estimation
The raw reads produced from sequencing machines were cleaned by removing reads with adaptors or unknown nucleotides larger than 10% and low quality with the percentage of low-quality bases (base quality ≤10) more than 50%. The left files (read1 files) from all libraries/samples were pooled into one large left. fq file, and right files (read2 files) into one big right. fq file. Transcriptome assembly was accomplished based on the left. fq and right. fq using Trinity116 with min-kmer-cov set to 2 by default and all other parameters set default. Transcriptome de novo assembly was carried out with the short read assembly program Trinity. The resulting sequences of trinity were called transcripts. When a gene has several transcripts, the longest one was selected as a unigene of the gene. Multiple samples from the same species are sequenced, and unigenes from each sample’s assembly can be taken into a further process of sequence splicing and redundancy removal with sequence clustering software to acquire nonredundant unigenes as long as possible.
Unigene annotation and Protein - Coding Region Prediction
Gene function was annotated based on the following databases: NT (E-value ≤ 1.0E−5), NR, Swiss-Prot (E-value ≤ 1.0E−5), Pfam (Protein family) (E-value ≤ 0.01), KOG/COG (Clusters of Orthologous Groups of proteins) (E-value ≤ 1.0E−3), KO (KEGG Ortholog database) (E-value ≤ 1.0E−10) and GO (Gene Ontology) (E-value ≤ 1.0E−6).
For protein coding region sequence (CDS) prediction, unigenes were first aligned by blastx (E-value < 1.0E−5) to protein databases in the priority order of NR, Swiss-Prot. Proteins with the highest ranks in blast results were taken to decide the CDS of unigenes, and then both the nucleotide sequences (5′-3′) and amino sequences of the unigene CDS were acquired by translating the CDS into amino sequences with the standard codon table. Unigenes that cannot be aligned to any database were scanned by ESTScan (3.0.3) to obtain nucleotide sequences (5′-3′) and amino sequences of the CDS.
Different expression genes (DGEs) determination
Clean read data of each sample were mapped back onto the assembled transcriptome. The resulting read count for each gene was obtained and then applied for gene expression level estimation in each sample using RSEM117. Differential expression analysis between two groups was performed using the DESeq R package (1.10.1). The resulting P values were adjusted using Benjamini and Hochberg’s approach for controlling the false discovery rate. Genes with an adjusted P-value < 0.05 were classified as differentially expressed genes (DEGs). The obtained DEGs were then subjected to KEGG enrichment analysis, and KOBAS software was used to test the statistical enrichment of differential expression genes in KEGG pathways118.
Expression profile of genes involved in ovule development
Total RNA samples from flower buds at four different development phases (JCFI, JCFII, JCFIII and JCFIV) (Fig. 2) were used for expression pattern analysis of 16 selected DEGs (Table S5). The total RNA (1 µg) of each sample was used for first-strand cDNA synthesis using AMV RNA PCR Kit 3.0 (Takara). qPCR was performed on an ABI StepOnePlus Real-Time PCR System (Applied Biosystems, Inc. USA) using 2 × SYBR green PCR mix (QIAGEN, Shanghai, China). The β-tubulin gene was chosen as the endogenous reference gene for the qPCR analysis. The primers used for qPCR analysis are listed in Supplementary Table S1. Each sample has three biological replicates. The average threshold cycle (Ct) was calculated, and the relative expression level of each gene was then calculated according to the 2−ΔΔCt method. A one-way ANOVA was performed on the gene expression level of the samples at different development stages using the software Statistical Package for the Social Science (SPSS) version 11.5 (SPSS Inc., Chicago, IL, USA). The individual treatment means were compared using the LSD (least significance difference) test.
Quantification of phytohormone
The female flower bud samples at four development phases, JCFI, JCFII, JCFIII and JCFIV, were used for quantification of phytohormones. A 0.1-g sample frozen in liquid nitrogen was ground in liquid nitrogen and then dipped in 1 mL mixture composed of cold acetone: deionized water: hydrochloric acid (37%) = 2:1:0.002 (V:V:V). After incubation at 4 °C for 30 min, 1 mL dichloromethane was added. After incubation at 4 °C for 30 min, the sample was then centrifuged for 15 min at 13,000 g and 4 °C, and the resulting subnatant was collected for drying by liquid nitrogen. Phytohormones in the dried residue were extracted with 0.1 mL methanol. Quantification of phytohormones was performed as described previously using a liquid chromatography–mass chromatography system (Shiseido SP HPLC- Thermo TSQ Quantum Ultra MS/Ms) with an ODS column (SHISEIDO C18, 5 μm, 2.0 × 150 mm)119,120,121.
Availability of Supporting Data All RNA-Seq data for this project has been deposited in NCBI under the SRA accession SRP115141. Summary description of library Illumina sequencing, De novo transcriptome assembly Table S1 and annotation are listed in Supplementary Data Tables S2–S5, Figs S1–S7.
Supplementary information
The revised version of supplementary information.pd
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31460184) and the First Class Disipline Construction Project of ecology in Guizhou Province (GNYL[2017]007).
Author contributions
Gang Xu designed and managed this study and wrote this manuscript. Shi-kang Lei performed the real-time q-PCR analysis. Jian Huang participated in the design and management of this study. Xue-guang Sun participated in the design and management of this study. Xue Li collected the flower bud samples and performed the transcriptome data analysis.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-52421-0.
References
- 1.Lin J, Yan F, Tang L, Chen F. Antitumor effects of curcin from seeds of Jatropha curcas. Acta Pharmacol Sin. 2003;24:241–246. [PubMed] [Google Scholar]
- 2.Igbinosa OO, Igbinosa EO, Aiyegoro OA. Antimicrobial activity and phytochemical screening of stem bark extracts from Jatropha curcas (Linn) Afr J Pharm Pharacol. 2009;3:58–62. [Google Scholar]
- 3.Fagbenro-Beyioku AF, Oyibo WA, Anuforom BC. Disinfectant/antiparasitic activities of Jatropha curcas. East Africa Med J. 1998;75:508–511. [PubMed] [Google Scholar]
- 4.Dehgan, B. & Webster, G. Morphology and infrageneric relationships of the genus J. curcas. University of California Press, Berkeley, CA, USA (1992).
- 5.Natarajan P, et al. Gene discovery from Jatropha curcas by sequencing of ESTs from normalized and full-length enriched cDNA library from developing seeds. BMC Genomics. 2010;11:606. doi: 10.1186/1471-2164-11-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang LH, You J, Chan ZL. Identification and characterization of TIFY family genes in Brachypodium distachyon. J Plant Res. 2015;128:995–1005. doi: 10.1007/s10265-015-0755-2. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, et al. Global Analysis of Gene Expression Profiles in Physic Nut (Jatropha curcas L.) Seedlings Exposed to Salt Stress. Plos one. 2014;9(5):e97878. doi: 10.1371/journal.pone.0097878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa GG, et al. Transcriptome analysis of the oil-rich seed of the bioenergy crop Jatropha curcas L. BMC Genomics. 2010;11:462–471. doi: 10.1186/1471-2164-11-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Natarajan P, Parani M. De novo assembly and transcriptome analysis of five major tissues of Jatropha curcas L. using GS FLX titanium platform of 454 pyrosequencing. BMC Genomics. 2011;12:191–202. doi: 10.1186/1471-2164-12-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan BZ, Chen MS, Ni J, Xu ZF. Transcriptome of the inflorescence meristems of the biofuel plant Jatropha curcas treated with cytokinin. BMC Genomics. 2014;15:974–993. doi: 10.1186/1471-2164-15-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu G, Huang J, Yang Y, Yao Y. Transcriptome analysis of flower sex differentiation in Jatropha curcas L. using RNA sequencing. Plos One. 2016;11(2):e0145613. doi: 10.1371/journal.pone.0145613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigo J, Hormaza JI, Herrero M. Ovary starch reserves and flower development in apricot (Prunus armeniaca) Physiol Plant. 2000;108:35–41. doi: 10.1034/j.1399-3054.2000.108001035.x. [DOI] [Google Scholar]
- 13.Imamura A, et al. Compilation and characterization of Arabiopsis thaliana response regulators implicated in His-Asp phosphorelay signal transduction. Plant Cell Physiol. 1999;40(7):733–742. doi: 10.1093/oxfordjournals.pcp.a029600. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz R, Garcia-Luis A, Honerri C, Guardiola JL. Carbohydrate availability in relation to fruitlet abscission in Citrus. Ann Bot. 2001;87:805–812. doi: 10.1006/anbo.2001.1415. [DOI] [Google Scholar]
- 15.Iglesias DJ, Tadeo FR, Primo-Millo E, Talon M. Fruit set dependence on carbohydrate availability in citrus trees. Tree Physiol. 2003;23:199–204. doi: 10.1093/treephys/23.3.199. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigo J, Herrero M. Influence of intraovular reserves on ovule fate in apricot (Prunus armeniaca L.) Sexual Plant Reprod. 1998;11:86–93. doi: 10.1007/s004970050124. [DOI] [Google Scholar]
- 17.Feller A, Machemer K, Braun EL, Grotewold E. Evolutionary and comparative analysis of MYB and bHLH plant transcription factors. Plant J. 2011;66:94–116. doi: 10.1111/j.1365-313X.2010.04459.x. [DOI] [PubMed] [Google Scholar]
- 18.Gremski K, Ditta G, Yanofsky MF. The HECATE genes regulate female reproductive tract development in Arabidopsis thaliana. Development. 2007;134:3593–3601. doi: 10.1242/dev.011510. [DOI] [PubMed] [Google Scholar]
- 19.Reyes-Olalde, J. I. et al. The bHLH transcription factor SPATULA enables cytokinin signaling, and both activate auxin biosynthesis and transport genes at the medial domain of the gynoecium. PLOS Genetics 1–31 (2017). [DOI] [PMC free article] [PubMed]
- 20.Mandaokar A, et al. Transcriptional regulators of stamen development in Arabidopsis identified by transcriptional profiling. Plant J. 2006;46(6):984–1008. doi: 10.1111/j.1365-313X.2006.02756.x. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, et al. Over expression of PGA37/MYB118 and MYB115 promotes vegetative-to-embryonic transition in Arabidopsis. Cell Res. 2008;19:224–235. doi: 10.1038/cr.2008.276. [DOI] [PubMed] [Google Scholar]
- 22.Baumann K, et al. Control of cell and petal morphogenesis by R2R3 MYB transcription factors. Development. 2007;134:1691–1701. doi: 10.1242/dev.02836. [DOI] [PubMed] [Google Scholar]
- 23.Makkena S, Lee E, Sack FD, Lamb RS. The R2R3 MYB transcription factors FOUR LIPS and MYB88 regulate female reproductive development. J Exp Bot. 2012;63(15):5545–5558. doi: 10.1093/jxb/ers209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Punwani JA, Rabiger DS, Drews GN. MYB98 positively regulates a battery of synergid-expressed genes encoding filiform apparatus localized proteins. Plant Cell. 2007;19:2557–2568. doi: 10.1105/tpc.107.052076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Y, et al. WRKY71 accelerates flowering via the direct activation of Flowering Locus T and LEAFY in Arabidopsis thaliana. Plant J. 2016;85:96–106. doi: 10.1111/tpj.13092. [DOI] [PubMed] [Google Scholar]
- 26.Guan Y, et al. Phosphorylation of a WRKY transcription factor by MAPKs is required for pollen development and function in Arabidopsis. PLos Genet. 2014;10(5):e1004384. doi: 10.1371/journal.pgen.1004384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuwamoto R, Fukuoka H, Takahata Y. Identification and characterization of genes expressed in early embryogenesis from microspores of Brassica napus. Planta. 2007;225:641–652. doi: 10.1007/s00425-006-0388-8. [DOI] [PubMed] [Google Scholar]
- 28.Gomi K, et al. GID2, an F-box subunit of the SCF E3 complex, specifically interacts with phosphorylated SLR1 protein and regulates the gibberellinsindependent degradation of SLR1 in rice. Plant J. 2004;37:626–634. doi: 10.1111/j.1365-313X.2003.01990.x. [DOI] [PubMed] [Google Scholar]
- 29.Galbiati F, et al. An integrative model of the control of ovule primordia formation. Plant J. 2013;76:446–455. doi: 10.1111/tpj.12309. [DOI] [PubMed] [Google Scholar]
- 30.Yanofsky MF, et al. The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature. 1990;346:35–39. doi: 10.1038/346035a0. [DOI] [PubMed] [Google Scholar]
- 31.Bowman JL, Drews GN, Meyerowitz EM. Expression of the Arabidopsis floral homeotic gene AGAMOUS is restricted to specific cell types late in flower development. Plant Cell. 1991;3:749–758. doi: 10.1105/tpc.3.8.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinyopich A, et al. Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature. 2003;424:85–88. doi: 10.1038/nature01741. [DOI] [PubMed] [Google Scholar]
- 33.Bowman JL, Smyth DR. CRABS CLAW, a gene that regulates carpel and nectary development in Arabidopsis, encodes a novel protein with zinc finger and helix-loop-helix domains. Development. 1999;126:2387–2396. doi: 10.1242/dev.126.11.2387. [DOI] [PubMed] [Google Scholar]
- 34.Battaglia, R., Brambilla, V. & Colombo, L. Morphological analysis of female gametophyte development in the bel1 stk shp1 shp2 mutant. Plant Biosyst142, 643–649 (2008).
- 35.Matias-Hernandez L, et al. VERDANDI is a direct target of the MADS domain ovule identity complex and affects embryo sac differentiation in Arabidopsis. Plant Cell. 2010;22(6):1702–1715. doi: 10.1105/tpc.109.068627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehlers K, et al. The MADS box genes ABS, SHP1, and SHP2 are essential for the coordination of cell divisions in ovule and seed coat development and for endosperm formation in Arabidopsis thaliana. PLoS One. 2016;11(10):e0165075. doi: 10.1371/journal.pone.0165075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colombo M, et al. A new role for the SHATTERPROOF genes during Arabidopsis gynoecium development. Dev Biol. 2010;337(2):294–302. doi: 10.1016/j.ydbio.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 38.Garay-Arroyo, A. et al. The MADS transcription factor XAL2/AGL14 modulates auxin transport during Arabidopsis root development by regulating PIN expression. The EMBO Journal 1–12 (2013). [DOI] [PMC free article] [PubMed]
- 39.Pagnussat. GC, Alandete-Saez M, Bowman JL, Sundaresan V. Auxin-dependent patterning and gamete specification in the Arabidopsis female gametophyte. Science. 2009;324:1684–1689. doi: 10.1126/science.1167324. [DOI] [PubMed] [Google Scholar]
- 40.Bereterbide A, Hernould M, Castera S, Mouras A. Inhibition of cell proliferation, cell expansion and differentiation by the Arabidopsis SUPERMAN gene in transgenic tobacco plants. Planta. 2001;1:22–29. doi: 10.1007/s004250100584. [DOI] [PubMed] [Google Scholar]
- 41.Meyerowitz, E. M. et al. A genetic and molecular model for flower development in Arabidopsis thaliana. Development 157–167 (1991). [PubMed]
- 42.Gaiser JC, Robinson-Beers K, Gasser CS. The Arabidopsis SUPERMAN gene mediates asymmetric growth of the outer lntegument of ovules. Plant Cell. 1995;7:333–345. doi: 10.2307/3869855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meister RJ, Oldenhof H, Bowman JL, Gasser CS. Multiple protein regions contribute to differential activities of YABBY proteins in reproductive development. Plant Physiol. 2005;2:651–662. doi: 10.1104/pp.104.055368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kelley DR, Skinner DJ, Gasser CS. Roles of polarity determinants in ovule development. Plant J. 2009;6:1054–1064. doi: 10.1111/j.1365-313X.2008.03752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bao. F, Azhakanandam S, Franks RG. SEUSS and SEUSS-LIKE transcriptional adaptors regulate floral and embryonic development in Arabidopsis. Plant Physiol. 2010;152:821–836. doi: 10.1104/pp.109.146183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alvarez J, Smyth DR. CRABS CLAW and SPATULA, two Arabidopsis genes that control carpel development in parallel with AGAMOUS. Development. 1999;126:2377–2386. doi: 10.1242/dev.126.11.2377. [DOI] [PubMed] [Google Scholar]
- 47.Durbak AR, Taxt FE. CLAVATA signaling pathway receptors of Arabidopsis regulate cell proliferation in fruit organ formation as well as in meristems. Genetics. 2011;189:177–194. doi: 10.1534/genetics.111.130930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pharis RP, King RW. Gibberellins and reproductive development in seed plants. Annu. Rev. Plant Physiol. 1985;36:517–568. doi: 10.1146/annurev.pp.36.060185.002505. [DOI] [Google Scholar]
- 49.Koornneef M, van der Veen JH. Induction and analysis of gibberellin sensitive mutants in Arabidopsis thaliana (L) Heynh. Theor Appl Genet. 1980;58:257–263. doi: 10.1007/BF00265176. [DOI] [PubMed] [Google Scholar]
- 50.Barendse GWM, et al. Growth hormones in pollen, styles and ovaries of Petunia hybrida and Lilium species. Acta Bot Neerl. 1970;19:175–186. doi: 10.1111/j.1438-8677.1970.tb00639.x. [DOI] [Google Scholar]
- 51.Nester JE, Zeevaart JAD. Flower development in normal tomato and a gibberellin-deficient (ga-2) mutant. Am J Bot. 1988;75:45–55. doi: 10.1002/j.1537-2197.1988.tb12160.x. [DOI] [Google Scholar]
- 52.Tanurdzic M, Banks JA. Sex-determining mechanisms in land plants. Plant Cell. 2004;16(Suppl. 1):S61–S71. doi: 10.1105/tpc.016667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y, Tan B. New insight in the Gibberellin biosynthesis and signal transduction. Plant Signal Behav. 2015;10:e1000140. doi: 10.1080/15592324.2014.1000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng H, et al. Gibberellin regulates Arabidopsis floral development via suppression of DELLA protein function. Development. 2004;131:1055–1064. doi: 10.1242/dev.00992. [DOI] [PubMed] [Google Scholar]
- 55.Gayakvad P, Jadeja DB, Bhalawe S. Effect of foliar application of GA3, ethrel and copper sulphate on flowering behaviour and sex ratio of Jatropha curcas L. Journal of Applied and Natural Science. 2014;6(1):286–289. doi: 10.31018/jans.v6i1.416. [DOI] [Google Scholar]
- 56.Pi X, Pan B, Xu Z. Induction of bisexual flowers by gibberellins in monoecious biofuel plant Jatropha curcas (Euphorbiaceae) Plant Divers Resour. 2013;35:26–32. [Google Scholar]
- 57.Makwana V, Shukla P, Robin P. GA application induces alteration in sex ratio and cell death in Jatropha curcas. Plant Growth Regul. 2010;61(2):121–125. doi: 10.1007/s10725-010-9457-x. [DOI] [Google Scholar]
- 58.Chen M, et al. Comparative Transcriptome Analysis between Gynoecious and Monoecious Plants Identifies Regulatory Networks Controlling Sex Determination in Jatropha curcas. Front Plant Sci. 2017;7:1–14. doi: 10.3389/fpls.2016.01953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Howe, G. A. “The roles of hormones in defense against insects and disease” in Plant Hormones Biosynthesis, Signal Transduction, Action. Revised 3rd ed. P. J. Davies, (Cornell University, Dept. Plant Biology), 646-670 (2010).
- 60.Liu Z, et al. Cotton GASL genes encoding putative gibberellins-regulated proteins are involved in response to GA signaling in fiber development. Mol Biol Rep. 2013;40:4561–4570. doi: 10.1007/s11033-013-2543-1. [DOI] [PubMed] [Google Scholar]
- 61.Roxrud I, Lid SE, Fletcher JC, Schmidt EDL, Opsahl-Sorteberg HG. GASA4, one of the 14-member Arabidopsis GASA family of small polypeptides, regulates flowering and seed development. Plant Cell Physiol. 2007;48(3):471–483. doi: 10.1093/pcp/pcm016. [DOI] [PubMed] [Google Scholar]
- 62.Hauvermale AL, Ariizumi T, Steber CM. The roles of the GA receptors GID1a, GID1b, and GID1c in sly1-independent GA signaling. Plant Signal Behav. 2014;9:e28030. doi: 10.4161/psb.28030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peng J, et al. The Arabidopsis GAI gene defines a signalling pathway that negatively regulates gibberellin responses. Genes Dev. 1997;11:3194–3205. doi: 10.1101/gad.11.23.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuan Z, Zhang D. Roles of jasmonate signaling in plant in florescence and flower development. Curr Opin Plant Biol. 2015;27:44–51. doi: 10.1016/j.pbi.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 65.Zhang L, You J, Chan Z. Identification and characterization of TIFY family genes in Brachypodium distachyon. J Plant Res. 2015;128:995–1005. doi: 10.1007/s10265-015-0755-2. [DOI] [PubMed] [Google Scholar]
- 66.Ye H, Du H, Tang N, Li X, Xiong L. Identification and expression profiling analysis of TIFY family genes involved in stress and phytohormone responses in rice. Plant Mol Biol. 2009;71:291–305. doi: 10.1007/s11103-009-9524-8. [DOI] [PubMed] [Google Scholar]
- 67.Thines B, et al. JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature. 2007;448:661–665. doi: 10.1038/nature05960. [DOI] [PubMed] [Google Scholar]
- 68.Takahashi F, et al. The mitogen-activated protein kinase cascade MKK3-MPK6 is an important part of the jasmonate signal transduction pathway in Arabidopsis. Plant Cell. 2007;19:805–818. doi: 10.1105/tpc.106.046581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lorenzo O, Chico JM, Sánchez-Serrano JJ, Solano R. JASMONATEINSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell. 2004;16:1938–1950. doi: 10.1105/tpc.022319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chini A, et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature. 2007;448:666–671. doi: 10.1038/nature06006. [DOI] [PubMed] [Google Scholar]
- 71.Dombrecht B, et al. MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell. 2007;19:2225–2245. doi: 10.1105/tpc.106.048017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Melotto M, et al. A critical role of two positively charged amino acids in the Jas motif of Arabidopsis JAZ proteins in mediating coronatine- and jasmonoyl isoleucine-dependent interactions with the COI1 F-box protein. Plant J. 2008;55:979–988. doi: 10.1111/j.1365-313X.2008.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kazan K, Manners JM. MYC2: the master in action. Mol Plant. 2013;6:686–703. doi: 10.1093/mp/sss128. [DOI] [PubMed] [Google Scholar]
- 74.Nemhauser JL, Feldman LJ, Zambryski PC. Auxin and ETTIN in Arabidopsis gynoecium morphogenesis. Development. 2000;127:3877–3888. doi: 10.1242/dev.127.18.3877. [DOI] [PubMed] [Google Scholar]
- 75.Nole-Wilson S, Azhakanandam S, Franks RG. Polar auxin transport together with AINTEGUMENTA and REVOLUTA coordinate early Arabidopsis gynoecium development. Dev Biol. 2010;346:181–195. doi: 10.1016/j.ydbio.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 76.Sundaresan V, Alandete-Saez M. Pattern formation in miniature: the female gametophyte of flowering plants. Development. 2010;137:179–189. doi: 10.1242/dev.030346. [DOI] [PubMed] [Google Scholar]
- 77.Mano Y, Nemoto K. The pathway of auxin biosynthesis in plants. Journal of Experimental Botany. 2012;63(8):2853–2872. doi: 10.1093/jxb/ers091. [DOI] [PubMed] [Google Scholar]
- 78.Zhao J, et al. Two putative BIN2 substrates are nuclear components of brassinosteroid signaling. Plant Physiol. 2002;130:1221–1229. doi: 10.1104/pp.102.010918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McClure BA, et al. Transcription, Organization, and Sequence of an Auxin-Regulated Gene Cluster in Soybean. Plant Cell. 1989;1:229–239. doi: 10.1105/tpc.1.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McClure BA, Guilfoyle TJ. Characterization of a class of small auxin-inducible polyadenylated RNAs. Plant Mol Biol. 1987;9:611–623. doi: 10.1007/BF00020537. [DOI] [PubMed] [Google Scholar]
- 81.Staswick PE, Tiryaki I, Rowe M. Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell. 2002;14:1405–1415. doi: 10.1105/tpc.000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Staswick PE, et al. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell. 2005;17:616–627. doi: 10.1105/tpc.104.026690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mun JH, et al. Auxin response factor gene family in Brassica rapa: genomic organization, divergence, expression, and evolution. Mol Genet Genomics. 2012;287:765–784. doi: 10.1007/s00438-012-0718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Larsson E, Franks RG, Sundberg E. Auxin and the Arabidopsis thaliana gynoecium. J Exp Bot. 2013;64(9):2619–2627. doi: 10.1093/jxb/ert099. [DOI] [PubMed] [Google Scholar]
- 85.Bartrina I, Otto E, Strnad M, Werner T, Schmülling T. Cytokinin regulates the activity of reproductive meristems, flower organsize, ovule for mation, and thus seed yield in Arabidopsis thaliana. Plant Cell. 2011;23:69–80. doi: 10.1105/tpc.110.079079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheng CY, Kieber JJ. The role of cytokinin in ovule development in Arabidopsis. Plant Signal Behav. 2013;8(3):e23393. doi: 10.4161/psb.23393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miyawaki K, et al. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. PNAS. 2006;103:16598–16603. doi: 10.1073/pnas.0603522103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Riefler M, Novak O, Strnad M, Schmulling T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell. 2006;18:40–54. doi: 10.1105/tpc.105.037796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Werner T, et al. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell. 2003;15:2532–2550. doi: 10.1105/tpc.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hutchison CE, et al. The Arabidopsis histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling. Plant Cell. 2006;18:3073–3087. doi: 10.1105/tpc.106.045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheng CY, Mathews D, Eric Schaller G, Kieber JJ. Cytokinin-dependent specification of the functional megaspore in the Arabidopsis female gametophyte. Plant J. 2013;73:929–940. doi: 10.1111/tpj.12084. [DOI] [PubMed] [Google Scholar]
- 92.Mizuno T. His–Asp phosphotransfer signal transduction. J Biochem. 1998;123:555–563. doi: 10.1093/oxfordjournals.jbchem.a021972. [DOI] [PubMed] [Google Scholar]
- 93.Haberer G, Kieber JJ. Cytokinins. New insights into a classical phytohormone. Plant Physiol. 2002;128:354–362. doi: 10.1104/pp.010773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leibfried A, et al. WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature. 2005;438:1172–1175. doi: 10.1038/nature04270. [DOI] [PubMed] [Google Scholar]
- 95.Johnston AJ, et al. Genetic subtraction profiling identifies genes essential for Arabidopsis reproduction and reveals interaction between the female gametophyte and the maternal sporophyte. Genome Biol. 2007;8(10):R204. doi: 10.1186/gb-2007-8-10-r204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taniguchi M, et al. Expression of Arabidopsis response regulator homologs is induced by cytokinins and nitrate. FEBS Lett. 1998;429:259–262. doi: 10.1016/S0014-5793(98)00611-5. [DOI] [PubMed] [Google Scholar]
- 97.D’Agostino I, Deruere J, Kieber J. Characterization of the response of the Arabidopsis ARR gene family to cytokinin. Plant Physiol. 2000;124:1706–1717. doi: 10.1104/pp.124.4.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bhargava A, et al. Identification of cytokinin-responsive genes using microarray meta-analysis and RNA-seq in Arabidopsis. Plant Physiol. 2013;162(1):272–294. doi: 10.1104/pp.113.217026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kiba T, et al. Differential expression of genes for response regulators in response to cytokinins and nitrate in Arabidopsis thaliana. Plant Cell Physiol. 1999;40(7):767–771. doi: 10.1093/oxfordjournals.pcp.a029604. [DOI] [PubMed] [Google Scholar]
- 100.Papadopoulou E, Grumet R. Brassinosteriod- induced femaleness in cucumber and relationship to ethylene production. HortScience. 2005;40(6):1763–1767. doi: 10.21273/HORTSCI.40.6.1763. [DOI] [Google Scholar]
- 101.Huang HY, et al. BR signal influences Arabidopsis ovule and seed number through regulating related genes expression by BZR1. Mol Plant. 2012;6:456–469. doi: 10.1093/mp/sss070. [DOI] [PubMed] [Google Scholar]
- 102.Schneitz K, Baker SC, Gasser CS, Redweik A. Pattern formation and growth during floral organogenesis: HUELLENLOS and AINTEGUMENTA are required for the formation of the proximal region of the ovule primordium in Arabidopsis thaliana. Development. 1998;125:2555–2563. doi: 10.1242/dev.125.14.2555. [DOI] [PubMed] [Google Scholar]
- 103.Kim GT, et al. CYP90C1 and CYP90D1 are involved in different steps in the brassinosteroid biosynthesis pathway in Arabidopsis thaliana. Plant J. 2005;41:710–721. doi: 10.1111/j.1365-313X.2004.02330.x. [DOI] [PubMed] [Google Scholar]
- 104.Choe S, et al. The DWF4 gene of Arabidopsis encodes a cytochrome p450 that mediates multiple 22 a-hydroxylation steps in brassinosteroid biosynthesis. Plant Cell. 1998;10:231–243. doi: 10.1105/tpc.10.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bai MY, et al. Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nat Cell Biol. 2012;14:810–817. doi: 10.1038/ncb2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gallego-Bartolomé J, et al. Molecular mechanism for the interaction between gibberellin and brassinosteroid signaling pathways in Arabidopsis. PNAS. 2012;109:13446–13451. doi: 10.1073/pnas.1119992109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li QF, et al. An interaction between BZR1 and DELLAs mediates direct signaling crosstalk between brassinosteroids and gibberellins in Arabidopsis. Sci Signal. 2012;5:ra72. doi: 10.1126/scisignal.2002908. [DOI] [PubMed] [Google Scholar]
- 108.Wang, L. & Chong, K. Auxin, Brassinosteroids, and G-Protein Signaling in: Integrated G Proteins Signaling in Plants . eds S. Yalovsky et al. (Springer-Verlag Berlin Heidelberg), 135–54 (2010).
- 109.Vissenberg K, Martinez-Vilchez IM, Verbelen JP, Miller JG, Fry SC. In vivo colocalization of xyloglucan endotransglycosylase activity and its donor substrate in the elongation zone of Arabidopsis roots. Plant Cell. 2000;12:1229–1237. doi: 10.1105/tpc.12.7.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vissenberg K, Fry SC, Verbelen JP. Root Hair Initiation Is Coupled to a Highly LocalizedIncrease of Xyloglucan Endotransglycosylase Action in Arabidopsis Roots. Plant Physiol. 2001;127:1125–1135. doi: 10.1104/pp.010295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li J, et al. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell. 2002;110:213–222. doi: 10.1016/S0092-8674(02)00812-7. [DOI] [PubMed] [Google Scholar]
- 112.Nam KH, Li J. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell. 2002;110:203–212. doi: 10.1016/S0092-8674(02)00814-0. [DOI] [PubMed] [Google Scholar]
- 113.Wang ZY, et al. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev Cell. 2002;2:505–513. doi: 10.1016/S1534-5807(02)00153-3. [DOI] [PubMed] [Google Scholar]
- 114.He JX, et al. BZR1 is a transcriptional repressor with dual roles in brassinosteroid homeostasis and growth responses. Science. 2005;307:1634–1638. doi: 10.1126/science.1107580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grabherr MG, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature Biotech. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li B, Dewey C. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12(1):323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mao X, et al. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics. 2005;21:3787–3793. doi: 10.1093/bioinformatics/bti430. [DOI] [PubMed] [Google Scholar]
- 118.Naito T, et al. A link between cytokinin and ASL9 (ASYMMETRIC LEAVES 2 LIKE 9) that belongs to the AS2/LOB (LATERAL ORGAN BOUNDARIES) family genes in Arabidopsis thaliana. Biosci Biotech Biochem. 2007;71:1269–1278. doi: 10.1271/bbb.60681. [DOI] [PubMed] [Google Scholar]
- 119.Nakagawa H, et al. Overexpression of a petunia zinc-finger gene alters cytokinin metabolism and plant forms. Plant J. 2005;41:512–523. doi: 10.1111/j.1365-313X.2004.02316.x. [DOI] [PubMed] [Google Scholar]
- 120.Hirano K, et al. The GID1 mediated GA perception mechanism is conserved in the lycophyte selaginella moellendorffii but not in the bryophyte physcomitrella patens. Plant Cell. 2007;19:3058–3079. doi: 10.1105/tpc.107.051524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McClerklin SA, et al. Indole-3-acetaldehyde dehydrogenase-dependent auxin synthesis contributes to virulence of Pseudomonas syringae strain DC3000. PLOS Pathogens. 2018;14(1):e1006811. doi: 10.1371/journal.ppat.1006811. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The revised version of supplementary information.pd