Abstract
The objective of this study was to investigate the effects of rigor state on physicochemical characteristics and the oxidative stability of chicken leg and breast muscles as a function of freezing time. Breast and leg muscles were excised from 24 broiler chickens at 30 min or 1.5 h postmortem (PM), frozen overnight at − 75 °C immediately, and then stored at − 20 °C for 90 days to measure the meat quality traits. Results showed that longer freezing led to deterioration of meat quality with higher deterioration for post-rigor frozen muscles. Pre-rigor frozen muscles had higher pH, water holding capacity (around 90%), and sarcomere length with a lower thaw and cook loss than post-rigor frozen muscles. The Warner–Bartzler shear force (WBSF) values for chicken leg and breast muscles were insignificant (except pre-rigor leg muscles which had significantly higher WBSF value only at 90th day of storage). The lightness (L*) value increased significantly with increasing storage for all samples. Post-rigor muscles had significantly higher TBARS values (0.62 mg MDA/kg) than the pre-rigor muscles. The leg muscles had better physicochemical characteristics compared to breast muscles, except for the cook loss. Therefore, immediate freezing (prior to onset of rigor) could be an effective way to minimize the quality deterioration of frozen chicken muscles.
Keywords: Chicken muscle, Pre- and post-rigor muscle, Meat freezing, Physicochemical characteristics, Oxidative stability
Introduction
The consumption of chicken meat has been increasing over the decades throughout the world because of its high-protein and low-fat content which are important characteristics for a healthy diet. Compared to beef, lamb and pork meat, chicken meat is inexpensive, easily available, and acceptable to all communities (Jayasena et al. 2013). Chicken leg and breast muscles are economically important than rest of the poultry carcass, because consumers’ demand for boneless meat have increased dramatically in recent years (Yu et al. 2005). Factors such as poultry processing technique (cold or hot boning process), postmortem (PM) aging, onset of rigor mortis, freezing and thawing time and temperature, etc., are pivotal to the quality characteristics of boneless poultry carcass (Adegoke and Falade 2005; Leygonie et al. 2012; Yu et al. 2005). On the other hand, poultry meat is particularly susceptible to lipid and protein oxidation, which is often a major threat to the quality of processed poultry products (Estévez 2015). Breast and leg muscles differ in their composition in terms of the proportion of white/red fiber, myoglobin, glycogen, glycolytic and oxidative enzymes, which have a direct effect on the chicken meat quality (Ali et al. 2007). A number of studies related to the effects of multiple freezing and thawing with different time and temperature combinations on the characteristics of poultry meat quality have been conducted (Kang et al. 2006; Yu et al. 2005). However, limited information is available on the physicochemical characteristics and oxidative stability of pre- and post-rigor frozen chicken breast and leg muscles as a function of freezing time.
After slaughtering or electrical stunning of poultry, the development of rigor mortis or PM aging leads to varied physical and biochemical changes in the muscles through the activity of proteolytic system (Carvalho et al. 2017). This proteolytic system is related to the quality attributes such as tenderness, juiciness, water holding capacity (WHC), flavor, protein, and structural degradation of chicken muscles (Sams and Dzuik 1999; Kim et al. 2016). It is well known that severe thaw-rigor occurs if the muscle temperature decreases below 16 °C before the onset of rigor mortis. Thus, pre-rigor frozen muscles might be related to the development of toughness, especially in red muscles. When pre-rigor frozen muscles are thawed, muscles become shrunken and lose a large amount of drip resulting in reduced the juiciness of the meat (Yu et al. 2005). However, few studies have reported the relationship between pre-rigor frozen chicken muscles and thaw-rigor (Lesiak et al. 1996; Sams and Dzuik 1999; Yu et al. 2005; Ali et al. 2015). Therefore, it is necessary to study an appropriate rigor state for freezing chicken muscles to produce a high-quality chicken meat.
The objective of this study was to evaluate pre- and post-rigor frozen muscles in relation to chicken meat quality. In this study, changes in muscles pH, meat color, WHC, thaw loss, cook loss, shear force, sarcomere length, and oxidative stability of pre- and post-rigor frozen chicken leg and breast muscles were measured during frozen storage of 90 days.
Materials and methods
Sample preparation
Twenty-four commercially reared broiler chickens (aged 6 weeks and live weight 2.5 kg approximately) were selected at the local commercial poultry farm. They were transported to a chicken slaughterhouse and slaughtered by a commercial slaughtering method. After bleeding was complete, breast and leg (thigh and drumstick) muscles were excised within 10 min PM. All obvious fat and connective tissues were removed from the muscles. Each part (breast and leg) was cut into four equal parts, weight recorded, and packed separately in moisture impermeable polyethylene bags, which were sealed and randomly divided into two portions. One portion was stored in deep-freezer at − 75 °C after 30 min PM (pre-rigor muscles) and the other was stored after 1.5 h PM (post-rigor muscles) at the same temperature overnight. After freezing was complete, samples were moved to a freezer at − 20 °C and stored for 90 days. A set of frozen samples were thawed at 0 °C for 24 h before subsequent analysis at 7th, 30th, 60th, and 90th day of storage.
Muscle pH
The pH values of the chicken breast and leg muscles were measured using a portable pH meter (MP 230, Mettler Toledo, Switzerland). Sample (3 g) was mixed with deionized water (27 mL) and then homogenized (IKA T25, ULTRATURAX, Germany) for 30 s. Before measuring the pH of the samples, the pH meter was calibrated with standard buffers of pH 4.01, 7.0, and 9.21, at 25 °C.
Water-holding capacity
Water-holding capacities (WHC) of pre- and post-rigor frozen chicken leg and breast muscles were determined by released water (RW) % following the method of Joo (2018) with minor modifications. The samples (3.0 g) of chicken muscles were placed in a previous desiccated and weighed filter-paper (Whatman No. 1 of 11 cm of diameter) with two thin plastic films. Then the meat samples placed in the filter-paper, and plastic film were transferred between plexiglass plates followed by a 2.5 kg load was applied for 5 min. The compressed meat sample was removed carefully from the damp filter-paper and plastic films, and weighed the damp filter-paper and plastic films immediately. The WHCs of different cut muscles were calculated using the following equation;
where W1 is the weight of damp filter-paper and plastic films; W2 is the weight of filter-paper and plastic films; W3 is the weight of the meat sample.
Thaw loss and cook loss
Thaw losses of the frozen samples were measured by calculating the difference between primary weight and the weight obtained after thawing for 24 h in the chilling room. The samples were patted with paper towels before weighing. After measurement of thaw loss, the bagged muscles were cooked at 75 °C using a water bath for 30 min. Cook loss was obtained by calculating the differences in weight between before and after cooking. The results of thaw loss and cook loss were expressed as the percentage of initial weight.
Meat color measurements
Color measurements of chicken meat were carried out using a colorimeter (Minolta CR -300, Minolta Co., Japan). Before measuring color values, a standard white plate (Y = 93.5, X = 0.3132, y = 0.3198) was used to calibrate the calorimeter. The results were expressed as L* (lightness), a* (redness), and b* (yellowness).
Warner–Bratzler shear force
Warner–Bartzler shear force (WBSF) values for different samples of chicken muscles used in this study were determined using an Instron Universal Testing Machine (Model 4400, Instron Co., USA) with a V-shaped shear blade. The samples cores (1.0 cm—diameter) were cooked for 30 min using a water bath to an internal temperature of 70 °C.
Sarcomere length
The sarcomere lengths of chicken muscles were measured by the method of Cross et al. (1981). Two solutions were used; solution A (0.1 M KCl, 0.39 M boric acid, and 5 m methylene di amine tetra acetic acid in 2.5% glutaraldehyde) and solution B (0.25 M KCl, 0.29 M boric acid, and 5 m Methylene di amine tetra acetic acid in 2.5% glutaraldehyde). The sample (1 g) was placed in a vial containing solution A for 2 h. Then the mixture was transferred to fresh vials containing solution B for 17–19 h. On the following day, the resulting muscles fiber was measured by placing the treated muscle on a microscopic slide with a drop of solution B. The microscope slide was placed horizontally in a vertically oriented laser beam path to give an array of diffraction bands on a screen. The resultant bands were perpendicular to the long axis of the muscles fibers. The following equation was used to calculate the sarcomere length.
where D is the distance (mm) from the specimen holding device to the screen (D = 98 mm) and T is the separation (mm) between the zero and the first maximum band. An average of 10 sarcomere lengths were obtained for each meat sample.
Thiobarbituric acid-reactive substance (TBARS)
Around 5 g of sample was mixed with 15 mL 0.1 M phosphate buffer (pH 7.0) and then homogenized for 2 min. An aliquot of supernatant (1 mL) was transferred into a glass tube and added 50 μL of butylated hydroxytoluene (BHT; 7.2% in ethanol) and 1.95 mL thiobarbituric acid (TBA)/trichloroacetic acid (TCA)/HCl solution (0.375% TBA, 15% TCA and 0.25 N HCl). Then the mixture was transferred for incubation at 90 °C for 15 min in a shaking water bath followed by cooling at room temperature. The solution was filtered using a filter paper, and the absorbance was measured at 532 nm using UV–Vis spectrophotometer (G1115AA, Agilant technologics, USA) against a blank containing 2 mL of TBA/TCA/HCl solution in 1 mL of distilled water without muscles sample. TBARS values of different samples were calculated as mg of malonaldehyde (MDA)/kg of the sample by comparing with a standard curve constructed with 1,1,3,3-tetramethoxyprophane.
Statistical analysis
All measurements were carried out in triplicates. Data were presented as mean values of three replicates ± standard error, and were analyzed using SAS statistical software (v9.4, SAS Institute Inc., Cary, NC, USA). Two-way analysis of variance (ANOVA) was performed using the general linear model (GLM) procedure. Duncan’s multiple range tests were used to determine the difference of means at 5% level of significance.
Results and discussion
The pH values of the frozen chicken leg and breast muscles as affected by rigor state and storage time are presented in Table 1. The pH was in the range of 6.26–5.95 at 7th day of storage, which continued to drop towards 90 days storage with values ranging from 6.08 to 5.69. The pre-rigor muscles had significantly higher pH as compared to the post-rigor muscles at all storage periods; with highest value for pre-rigor leg muscle and the lowest value for post-rigor breast muscle. Initially (7th and 30th day of storage) the pH values of chicken leg and breast muscles were insignificant but the differences were significant at the end of storage. The lower pH for post-rigor muscles is attributed to the increasing proteolytic activity with postmortem aging, which can lead to protein denaturation resulting in release of hydrogen ions, thereby resulting in reduced pH (Kim et al. 2016; Ali et al. 2015). It was reported that the pH of frozen and thawed meat tends to be lower than that prior to freezing (Leygonie et al. 2011). Carvalho et al. (2017) reported that PM aging leads to rapid reduction of pH due to protein oxidation, alteration of cellular compartmentalization, and the release of pro-oxidant enzyme. The results of this study are in agreement with Lesiak et al. (1996) who reported that the pH of chicken muscles declined with increasing time of post mortem aging.
Table 1.
pH of pre- and post-rigor chicken leg and breast muscles as a function of freezing time
| Muscles cut | Storage time (days) | |||
|---|---|---|---|---|
| 7 | 30 | 60 | 90 | |
| Pre-rigor leg | 6.26 ± 0.05Aa | 6.25 ± 0.03Aa | 6.12 ± 0.13ABab | 6.08 ± 0.03Ab |
| Post-rigor leg | 6.01 ± 0.04Ba | 5.98 ± 0.01Ba | 5.87 ± 0.06BCb | 5.81 ± 0.01Cb |
| Pre-rigor breast | 6.25 ± 0.02Aa | 6.20 ± 0.01Aa | 6.15 ± 0.11Aa | 5.98 ± 0.03Bb |
| Post-rigor breast | 5.95 ± 0.03Ba | 5.94 ± 0.03Ba | 5.83 ± 0.02Ca | 5.69 ± 0.02Db |
All values are mean ± standard error (n = 3). Different letters superscript (a–d in a row for same samples at different time, A–D in a column for different samples at the same time) indicate that means are significantly different (p < 0.05)
Thaw and cook losses of frozen chicken muscles as affected by rigor time are presented in Fig. 1, and were measured at 7th, 30th, 60th, and 90th day of storage. Thaw loss increased with increasing storage for all samples (Fig. 1A). During storage (7th to 90th day), thaw losses (%) were in the range of 1.91 to 4.5 and 2.12 to 5.56 for pre- and post-rigor leg muscles, respectively. While the values ranged from 2.6 to 5.02 and 2.67 to 5.99 for pre- and post-rigor breast muscles, respectively. The results indicate that the chicken leg muscles had significantly lower thaw loss than the breast muscles. The post-rigor muscles revealed higher thaw loss compared to the pre-rigor muscles, which is due to the effect of PM aging. Similarly, Lesiak et al. (1996) reported that drip loss of chicken muscles increased with increasing PM aging due to enhancing proteolytic activity. The cross-linkage between actin and myosin due to shrinkage of myofibrils during PM aging leads to a higher amount of free water that can be lost from the muscles (Yu et al. 2005). Moreover, rigor begins with the depletion of muscle ATP which may be responsible for quantitative differences of drip loss during rigor and after rigor (Huff-Lonergan and Lonergan 2005).
Fig. 1.
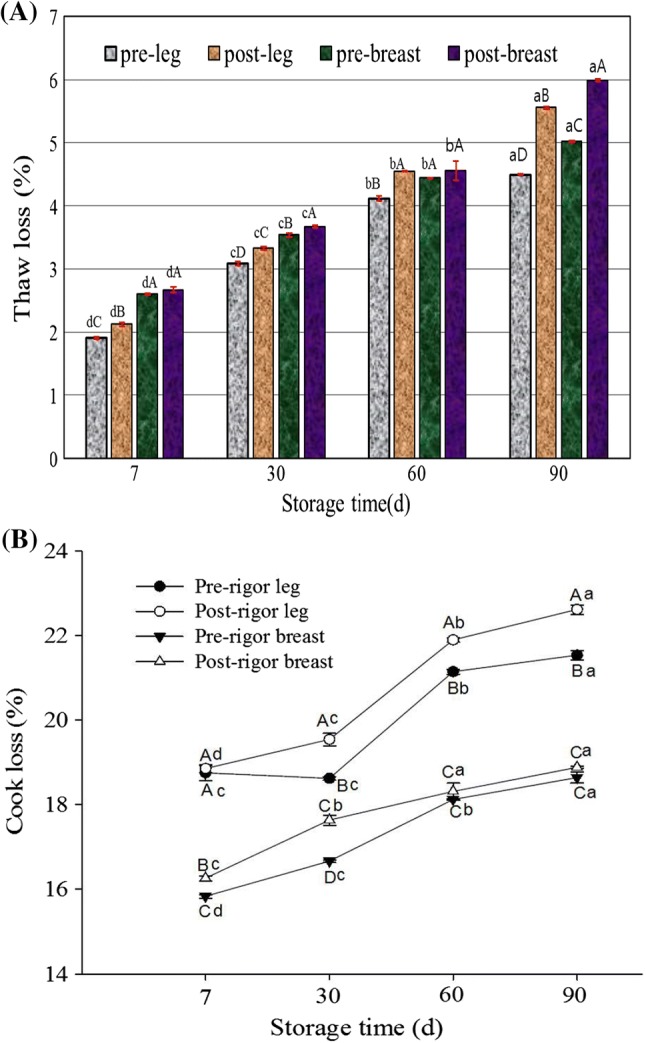
Thaw loss (A) and cook loss (B) of pre- and post-rigor chicken leg and breast muscles as a function of freezing time. Different letters in bars/lines (a–d for the same samples at different time, A–D for different samples at the same time) indicate significant differences
Cook loss is an important factor from the commercial point of view. For both the samples (pre- and post-rigor), initially cook loss was lower, which increased with increasing storage time (Fig. 1B). The post-rigor leg muscles showed significantly higher cook loss (22.61%) than those of the breast muscles (18.88%) during storage. The results indicate that leg muscles had a lower drip loss and higher held water than breast muscles, conversely, cooked leg muscles had lower held water and higher drip loss than breast muscles. Similar observations have been shown by Northcutt et al. (1994), who reported that higher held water for chicken leg muscles may have contributed to higher cook loss due to release of more water by protein unfolding. The results of this study are in agreement with Yu et al. (2005) that chicken breast muscles had lower cook loss than leg muscles as affected by thawing temperature, pre-rigor and frozen storage.
The effects of rigor time on the WHC of chicken leg and breast muscles are shown in Fig. 2. It was observed that the water release increased with an increase in storage time, leading to a decreased WHC capacity for all the samples. The chicken breast muscles showed significantly higher RW% indicating a lower WHC than the leg muscles. The initial range of RW% was 8.53 to 12.33, and the RW% values increased to 12.33 for pre-rigor leg muscle, 13.33 for post-rigor leg muscle, 13.36 for pre-rigor breast muscle, and 16.23 for post-rigor breast muscle at the end of storage (90th day). These results indicate that pre-rigor breast and leg muscles had significantly higher WHC than those of the post-rigor breast and leg muscles respectively. The post mortem aging of meat leads to different biochemical changes, oxidative deterioration, and degradation of proteins, resulting in decreased WHC of muscles (Huff-Lonergan and Lonergan 2005; Kim et al. 2016). Rahman et al. (2019) reported that the protein denaturation leads to reduced functional properties such as water/oil binding capacity, solubility etc. The actual reasons for higher WHC for leg muscles are unclear, but the composition such as proportion of white/red fiber, myofibrilar proteins (actin and myosin), glycogen, glycolytic and oxidative enzymes, etc., of leg muscles differ in breast muscles (Yu et al. 2005; Ali et al. 2007), which might lead to variations in WHC. Similar results have also been reported by Northcutt et al. (1994) that chicken leg meat had greater WHC than breast meat.
Fig. 2.
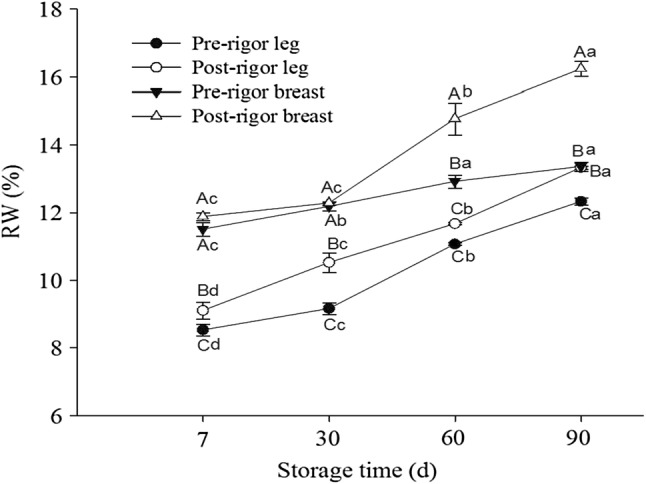
Water holding capacity (WHC) of frozen chicken leg and breast muscles as affected by rigor state prior to freezing. Different letters in lines (a–d for the same samples at different time, A–D for different samples at the same time) indicate significant differences
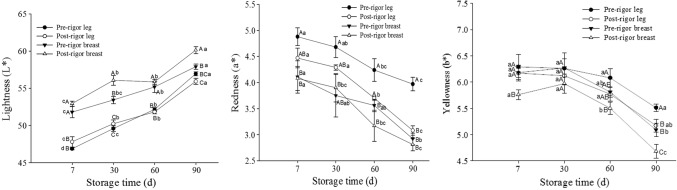
Meat color is the most important appearance quality attribute and is used as an indicator of the freshness of meat by the consumer (Joo et al. 2013). The color values of different cuts of chicken muscles are presented in Fig. 3. During aging, lightness (L*) increased significantly from 46.85 to 56.95 for pre-rigor leg muscles, 47.79 to 55.92 for post-rigor leg muscles, 51.78 to 57.90 for pre-rigor breast muscles, and from 52.93 to 60.13 for post-rigor breast muscles. By contrast, the redness (a*) and yellowness (b*) decreased with aging for all samples. Similarly, Ali et al. (2015) reported that L* value increased, and a* and b* values decreased, with a freeze–thaw cycle. It was also reported that whiteness of meat is due to an increased reflection of light, arising from the scattering of the light from denatured proteins during storage (Young and West 2001). Hughes et al. (2014) reported that decreasing of L* value due to the reduction of water retention which leads to a lower surface light reflectivity.
Fig. 3.
Color values (L* = lightness, a* = redness, b* = yellowness) of chicken muscles as affected by rigor state prior to freezing as a function of storage time. Different letters in lines (a–d for the same samples at different time, A–D for different samples at the same time) indicate significant differences
Shear force values are related to the tenderness of meat which is an important organoleptic characteristic that mostly affects consumer acceptance (Yu et al. 2005). In this study, WBSF values of frozen chicken muscles were measured as affected by the rigor state, and the results obtained are presented in Fig. 4A. The results indicated that the WBSF values decreased with the aging period. During storage (7th to 90th day), the WBSF (kg) values were in the range of 4.69 to 3.3 for pre-rigor leg muscles, 4.55 to 2.88 for post-rigor leg muscles, 4.25 to 2.77 for pre-rigor breast muscles, and 4.19 to 2.68 for post-rigor breast muscles. The results indicated that chicken breast muscles showed significantly higher tenderness values than those of the leg muscles. Amongst pre- and post-rigor muscles, no significant differences were found till 60th day of storage. At 90th day of storage, only pre-rigor leg muscles showed significantly higher WBSF value than other samples. It was reported that aging led to the degradation of proteins and increased oxidative deterioration resulting in the reduced shear force value (Davis et al. 2004; Hwang et al. 2018). The results obtained in our study reflect a trend similar to that reported by Yu et al. (2005).
Fig. 4.
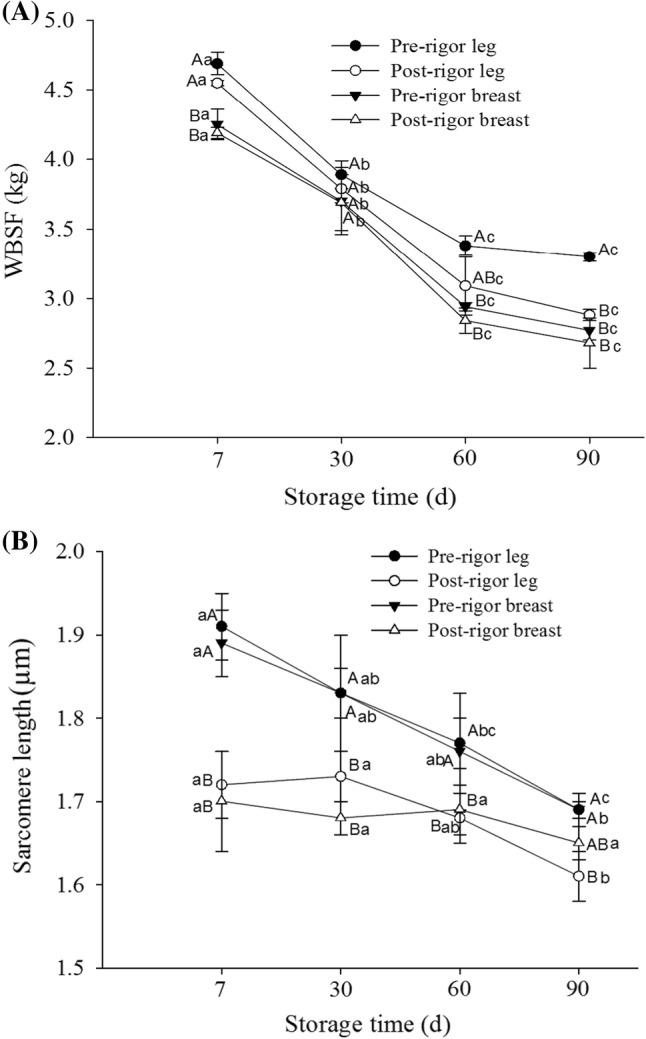
Warner–Bartzler shear force (WBSF) (A) and sarcomere length (µm) (B) of different samples of chicken muscles as affected by rigor time prior to freezing. Different letters in lines (a–d for same samples at different time, A–D for different samples at the same time) indicate significant differences
In order to reflect the contractile state of the frozen chicken muscles, sarcomere length was determined and the results are shown in Fig. 4B. At 7th day of storage, the sarcomere lengths of all muscles were found highest in the range of 1.91–1.70 µm, which then decreased gradually till 90 days (1.73–1.63 µm). All pre-rigor muscles had significantly higher sarcomere length (1.91 and 1.89 µm for leg and breast muscles respectively) than those of the post-rigor muscles (1.72 for leg muscles and 1.70 µm for breast muscles) which is attributed to early freezing after slaughtering of chickens. The sarcomere lengths of chicken leg and breast muscles were found insignificant (P > 0.05). Similarly, Koohmaraie et al. (1996) reported that immediate freezing of muscles after slaughtering and storage at − 5 °C may prevent sarcomere shortening. In case of frozen storage, it was reported that during freezing of muscles, glycolysis may proceed but muscles do not shorten as a result of the restricting effect of ice (Yu et al. 2005). They have also reported similar sarcomere length of chicken leg (2.00 µm) and breast muscles (1.80 µm) as affected by rigor, temperature, thawing, and chilling.
Oxidative stability of pre- and post-rigor chicken muscles was evaluated by measuring the thiobarbituric acid reactive substances (TBARS) during frozen storage, and the results are presented in Fig. 5. The TBARS values (mg MDA/kg) for all frozen chicken muscles increased significantly (P < 0.05) with storage. Initially (at 7th day of storage), the TBARS values amongst samples were not significantly different. The oxidative rancidity as measured by the concentration of malondialdehyde (mg MDA/kg) enhanced from 0.13 to 0.45 in pre-rigor leg muscles, 0.11 to 0.62 in post-rigor leg muscles, 0.10 to 0.43 in pre-rigor breast muscles, and 0.13 to 0.5 in post-rigor breast muscles after 90th day of storage. The results indicated that the pre-rigor muscles had significantly lower TBARS values than the post-rigor muscles. The post-rigor chicken leg muscles showed higher TBARS value (P ≤ 0.05) than that of the post-rigor breast muscles at the end of storage (90th day). Before freezing, postmortem aging of skeletal muscles rapidly increases the biochemical changes through a proteolytic system (Sams and Dzuik 1999; Kim et al. 2016), leading to higher oxidative degradation in post-rigor muscles. The results of this study are in agreement with those reported by Soyer et al. (2010). Utrera and Estévez (2013) reported that lipid oxidation can be promoted more intensively during freezing, and the differences in endogenous factors like heme iron concentration are responsible for the differences in lipid oxidation. Moreover, pro-oxidative environment produced in PM muscles leads to the occurrence of oxidative reactions during aging, processing, handling, and storage (Estévez 2015).
Fig. 5.
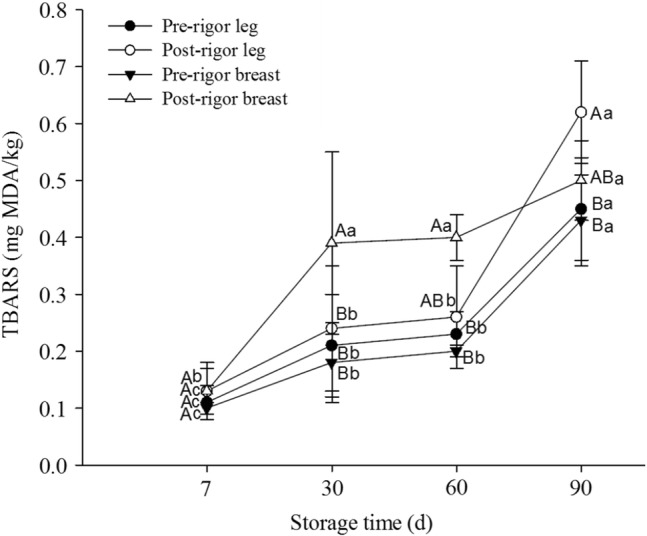
Oxidative stability of pre- and post-rigor chicken muscles evaluated by measuring the thiobarbituric acid reactive substances (TBARS) during frozen storage. Different letters in lines (a–d for same samples at different time, A–D for different samples at the same time) indicate significant differences
Conclusion
The longer postmortem aging prior to freezing could have adverse effects on the quality characteristics of frozen chicken muscles. Our study showed that the positive impacts of freezing before the onset of rigor (within 30 min after slaughtering) on the quality of chicken muscles. Pre-rigor frozen muscles had higher pH, lower drip, thaw and cook loss, than post-rigor frozen muscles. Post-rigor muscles showed lower shear force and sarcomere length compared to pre-rigor muscles. The lightness (L*) of all muscles significantly increased, and redness (L*) and yellowness (L*) decreased (P ≤ 0.05), during frozen storage. Leg muscles showed significantly higher cook loss, shear force, and sarcomere length, and lower thaw loss during storage. The oxidative deterioration increased with increasing storage time with higher TBARS values for the post-rigor frozen muscles. Therefore, immediate freezing after slaughtering may be an effective way to reduce the loss of physicochemical quality and oxidative deterioration associated with frozen chicken muscles.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adegoke GO, Falade KO. Quality of meat. J Food Agric Environ. 2005;3:87–89. [Google Scholar]
- Ali MS, Kang G, Yang H, Jeong J, Hwang Y, Park G, Joo S. A comparison of meat characteristics between duck and chicken breast. Asian Australas J Anim Sci. 2007;20:1002–1006. doi: 10.5713/ajas.2007.1002. [DOI] [Google Scholar]
- Ali S, Zhang W, Rajput N, Khan MA, Li CB, Zhou GH. Effect of multiple freeze–thaw cycles on the quality of chicken breast meat. Food Chem. 2015;173:808–814. doi: 10.1016/j.foodchem.2014.09.095. [DOI] [PubMed] [Google Scholar]
- Carvalho RH, Ida EI, Madruga MS, Martínez SL, Shimokomaki M, Estévez M. Underlying connections between the redox system imbalance, protein oxidation and impaired quality traits in pale, soft and exudative (PSE) poultry meat. Food Chem. 2017;215:129–137. doi: 10.1016/j.foodchem.2016.07.182. [DOI] [PubMed] [Google Scholar]
- Cross HR, West RL, Dutson TR. Comparison of methods for measuring sarcomere length in beef semitendinosus muscle. Meat Sci. 1981;5:261–266. doi: 10.1016/0309-1740(81)90016-4. [DOI] [PubMed] [Google Scholar]
- Davis KJ, Sebranek JG, Huff-Lonergan E, Lonergan SM. The effects of aging on moisture-enhanced pork loins. Meat Sci. 2004;66:519–524. doi: 10.1016/S0309-1740(03)00154-2. [DOI] [PubMed] [Google Scholar]
- Estévez M. Oxidative damage to poultry: from farm to fork. Poultry Sci. 2015;94:1368–1378. doi: 10.3382/ps/pev094. [DOI] [PubMed] [Google Scholar]
- Huff-Lonergan E, Lonergan SM. Mechanisms of water-holding capacity of meat: the role of postmortem biochemical and structural changes. Meat Sci. 2005;71:194–204. doi: 10.1016/j.meatsci.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Hughes JM, Oiseth SK, Purslow PP, Warner RD. A structural approach to understanding the interactions between colour, water-holding capacity and tenderness. Meat Sci. 2014;98:520–532. doi: 10.1016/j.meatsci.2014.05.022. [DOI] [PubMed] [Google Scholar]
- Hwang YH, Sabikun N, Ismail I, Joo ST. Comparison of meat quality characteristics of wet- and dry-aging pork belly and shoulder blade. Korean J Food Sci Anim Resour. 2018;38:950–958. doi: 10.5851/kosfa.2018.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasena DD, Jung S, Kim HJ, Bae YS, Yong HI, Lee JH, Kim JG, Jo C. Comparison of quality traits of meat from Korean native chickens and broilers used in two different traditional Korean cuisines. Asian Australas J Anim Sci. 2013;26:1038–1046. doi: 10.5713/ajas.2012.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo ST. Determination of water-holding capacity of porcine musculature based on released water method using optimal load. Korean J Food Sci Anim Resour. 2018;38:823–828. doi: 10.5851/kosfa.2018.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo ST, Kim GD, Hwang YH, Ryu YC. Control of fresh meat quality through manipulation of muscle fiber characteristics. Meat Sci. 2013;95:828–836. doi: 10.1016/j.meatsci.2013.04.044. [DOI] [PubMed] [Google Scholar]
- Kang GH, Jeong JY, Ali S, Kim SH, Jang EG, Kang HS, Lee DS, Lee SJ, Park GB, Joo ST. Effect of boning time and storage temperature on meat quality of duck breast. Korean J Food Sci Anim Resour. 2006;26:43–48. [Google Scholar]
- Kim HW, Yan FF, Hu JY, Cheng HW, Kim YHB. Effects of probiotics feeding on meat quality of chicken breast during postmortem storage. Poultry Sci. 2016;95:1457–1464. doi: 10.3382/ps/pew055. [DOI] [PubMed] [Google Scholar]
- Koohmaraie M, Doumit ME, Wheeler TL. Meat toughening does not occur when rigor shortening is prevented. J Anim Sci. 1996;74:2935–2942. doi: 10.2527/1996.74122935x. [DOI] [PubMed] [Google Scholar]
- Lesiak MT, Olson DG, Lesiak CA, Ahn DU. Effects of postmortem temperature and time on the water-holding capacity of hot-boned turkey breast and thigh muscle. Meat Sci. 1996;43:51–60. doi: 10.1016/0309-1740(95)00062-3. [DOI] [PubMed] [Google Scholar]
- Leygonie C, Britz TJ, Hoffman LC. Oxidative stability of previously frozen ostrich Muscularis iliofibularis packaged under different modified atmospheric conditions. Int J Food Sci Technol. 2011;46:1171–1178. doi: 10.1111/j.1365-2621.2011.02603.x. [DOI] [Google Scholar]
- Leygonie C, Britz TJ, Hoffman LC. Impact of freezing and thawing on the quality of meat: review. Meat Sci. 2012;91:93–98. doi: 10.1016/j.meatsci.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Northcutt JK, Foegeding EA, Edens FW. Waterholding properties of thermally preconditioned chicken breast and leg meat. Poultry Sci. 1994;73:308–316. doi: 10.3382/ps.0730308. [DOI] [PubMed] [Google Scholar]
- Rahman MS, Gul K, Yang HS, Chun J, Kerr WL, Choi SG. Thermal and functional characteristics of defatted bovine heart using supercritical CO2 and organic solvent. J Sci Food Agric. 2019;99:816–823. doi: 10.1002/jsfa.9250. [DOI] [PubMed] [Google Scholar]
- Sams AR, Dzuik CS. Meat quality and rigor mortis development in broiler chickens with gas-induced anoxia and postmortem electrical stimulation. Poultry Sci. 1999;78:1472–1476. doi: 10.1093/ps/78.10.1472. [DOI] [PubMed] [Google Scholar]
- Soyer A, Özalp B, Dalmış Ü, Bilgin V. Effects of freezing temperature and duration of frozen storage on lipid and protein oxidation in chicken meat. Food Chem. 2010;120:1025–1030. doi: 10.1016/j.foodchem.2009.11.042. [DOI] [Google Scholar]
- Utrera M, Estévez M. Oxidative damage to poultry, pork, and beef during frozen storage through the analysis of novel protein oxidation markers. J Agric Food Chem. 2013;61:7987–7993. doi: 10.1021/jf402220q. [DOI] [PubMed] [Google Scholar]
- Young OA, West J. Meat color. In: Hui YH, Nip W-K, Rogers RW, Young OA, editors. Meat science and applications. New York: Marcel Dekker Inc; 2001. pp. 39–69. [Google Scholar]
- Yu LH, Lee ES, Jeong JY, Paik HD, Choi JH, Kim CJ. Effects of thawing temperature on the physicochemical properties of pre-rigor frozen chicken breast and leg muscles. Meat Sci. 2005;71:375–382. doi: 10.1016/j.meatsci.2005.04.020. [DOI] [PubMed] [Google Scholar]