Abstract
Background
Knee osteoarthritis (OA) is a chronic debilitating condition that is estimated to affect approximately 12% of the current adult population in the United States, and is associated with severe pain and disability. Among these patients, quadriceps muscle atrophy and concomitant weakness are frequent findings that contribute significantly to the burden of this disease. One emerging method of quadriceps muscle strengthening and rehabilitation in knee OA patients is the use of neuromuscular electrical stimulation therapy (NMES). Among the currently available systems for NMES therapy are the mobile health (mHealth) platforms allowing clinicians to monitor patient compliance and utilization trends in addition to capturing certain clinical outcome points. The aim of this study was to analyze data collected by a commercially available mobile-app controlled NMES platform and to examine: (I) utilization trends, (II) range-of-motion (ROM) changes, (III) pain scores, and (IV) patient reported outcome scores in patients who used this device as part of management of their knee OA.
Methods
We retrospectively reviewed patients who received mobile-app controlled NMES therapy for knee OA who were enrolled in this multi-center study between April 2017 and July 2018 in a cloud-based provider online portal system. A total of 41 patients met all our inclusion and exclusion criteria and were included in our final analysis. For each patient, the total number of NMES sessions, the duration of NMES therapy, visual analogue pain scores, ROM, and the Knee injury Osteoarthritis Outcome Score (KOOS, JR) were collected and analyzed. Patient’s utilization trends were reported through analyzing NMES sessions and therapy durations. Descriptive statistics were utilized to analyze all relevant values.
Results
Across all patients, NMES therapy was utilized for an average of 3.5 months (range, 2 weeks to 10 months). On average, 90 sessions (range, 6 to 487) of therapy were received by patients for an average of 1,819 minutes (range, 120 to 9,740 minutes). Overall, patients achieved a mean ROM of 99˚±4.3˚ at final follow-up. Pain scores reduced from a mean of 5 points prior to device use (range, 1 to 8 points) to 2.5 points after use (range, 0 to 6 points) (P<0.001). Evaluation of KOOS questionnaires available for 17 patients showed incremental improvement from 52.46 points when therapy was started, to 63 points at 6 months following NMES therapy. No complications or adverse events were reported from any of the participants.
Conclusions
Although NMES therapy has been reported on by multiple authors, including in knee OA, there are limited have been no studies that have reported on the compliance, feasibility, and patient outcomes of using a mobile-app controlled NMES therapy devices in the setting of knee OA. Furthermore, the incorporation of cloud-based provider online platform may offer an additional advantage by allowing clinicians to monitor the progress and compliance of their patients in real-time. Therefore, patients who are making sub-optimal progress may benefit from an early intervention to modify their therapy protocol to achieve the best outcome.
Keywords: Neuromuscular electrical stimulation therapy (NMES), knee, osteoarthritis (OA), pain, mobile
Introduction
Knee osteoarthritis (OA) is a chronic debilitating condition that is estimated to affect approximately 12% of the current adult population in the United States (US) and is associated with severe pain and disability (1-3). It is estimated that 650,000 650,000 total knee arthroplasties (TKAs) are performed annually in the US at a cost exceeding $11 billion, are largely due to this condition (4). Among OA patients, quadriceps muscle atrophy and concomitant weakness are frequent findings that contribute significantly to the burden of this disease (5-7). Multiple studies have demonstrated a correlation between weakness of the quadriceps muscle and increased pain, decreased knee function, and increased overall disability (6,7). Studies have also suggested worse outcomes in these patients, even after knee arthroplasty is performed, if their quadriceps weakness has not been addressed (8). Therefore, more attention has been recently directed towards aggressive quadriceps rehabilitation in patients with knee OA, as strengthening this muscle is associated with better function, outcomes, and quality of life.
One emerging method of quadriceps muscle strengthening and rehabilitation in knee OA patients is the use of neuromuscular electrical stimulation therapy (NMES) (9-11). This therapy entails the use of low-level electrical impulses that cause involuntary contractions in the targeted muscles through stimulating surface electrodes directly applied to the desired muscle group. Several studies have demonstrated promising results with its use leading to improvement in pain, muscle strength, exercise tolerance, and quality of life in patients with knee OA as well as those undergoing TKA (9-14). Therefore, it has been proven to be successfully incorporated into multiple rehabilitation scenarios for patients requiring non-operative and operative management of their knee OA.
Among the currently available systems for NMES therapy are the mobile health (mHealth) platforms allowing clinicians to monitor patient compliance and utilization trends in addition to capturing certain clinical outcome points (9). The use of wearable mobile devices and biosensors to gather and store large amounts of health-related data has been rapidly accelerating. This data holds potential to allow us to understand patient’s behavior using home-based therapies and to answer questions related to the outcomes. With the development of sophisticated, new analytical capabilities, we are better able to analyze these data and apply the results of our analyses to understand disease progression. The aim of this study was to analyze the real-world data collected by a commercially available mobile-app controlled NMES platform and to examine: (I) utilization trends, (II) ROM changes, (III) pain scores, and (IV) patient reported outcome scores in patients who used this device as part of management of their knee OA.
Methods
Study sample
A de-identified data set was obtained for the retrospective review of patients who received mobile-app controlled NMES devices for knee OA between April 2017 and July 2018. This inclusion criteria were as follows: (I) patients were age 18 years or older, (II) patients had Kellgren and Lawrence grade II OA or higher, and (III) patients who had completed at least one session of daily NMES therapy (20 minutes). Patients who were active drug abusers, had inflammatory arthropathies, or prematurely stopped using their device were excluded. Additionally, patients who underwent NMES therapy without specific parameters were also excluded. A total of 41 patients met all our inclusion and exclusion criteria and were included in our final analysis.
NMES device
The CyMedica e-vive™ (CyMedica Orthopedics, Inc. Scottsdale, Arizona), a closed-loop NMES device that can be integrated to a knee garment, was used in this study. Three large electrodes are garment-incorporated and overlay the quadriceps muscle group (vastus medialis and rectus femoris). A controller transmitter pairs the garment through wireless technology to the patient’s mobile device. A user-friendly mobile device application (The CyMedica e-vive app; available to be downloaded from Apple/iOS: App Store and Android: Google Play Store) operates over the patient’s mobile device and monitors activity progress, records patient generated data, and collects range-of-motion (ROM) values through two accelerometers also incorporated into the knee garment (above and below the knee joint). This mobile application in-turn sends performance data through a secure connection to a cloud-based provider online portal system (CyMedica Provider Portal, CyMedica Orthopedics, Inc. Scottsdale, Arizona) allowing providers access through a standard web browser to monitor patient performance and progress. Patients were recommended to perform the NMES therapy one to two times a day for 5 days a week. Each NMES session is 20 minutes in length. Patients are recommended to apply the therapy for as long as needed to sustain the improved outcomes (reduced pain and improved knee function).
Data collection, analysis, and study endpoints
For each patient, the number of NMES sessions, the duration of NMES therapy, visual analogue pain scores, ROM, and the Knee injury Osteoarthritis Outcome Score (KOOS, JR) were collected and analyzed. Patient’s utilization trends were reported through analyzing NMES sessions and therapy duration. Any device related complication and adverse event were recorded and reported as frequencies. Descriptive statistics were utilized to analyze all relevant outcome measures. A Student’s t-test was conducted to compare pain scores before and after NMES intervention. All statistical analyses were conducted using SPSS version 24 (Armonk, NY, USA).
Results
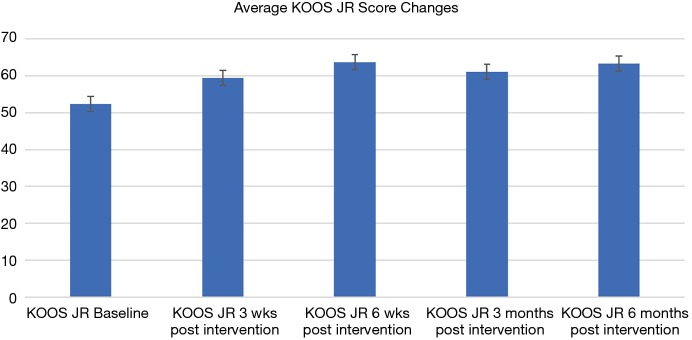
Across all patients, NMES therapy was utilized for an average of 3.5 months (range, 2 weeks to 10 months). On average, 90 sessions (range, 6 to 487) of therapy was received by patients for an average of 1,819 minutes (range, 120 to 9,740 minutes). Overall, patients achieved a mean ROM of 99˚±4.3˚ at final follow-up. Pain scores reduced from a mean of 5 points prior to device use (range, 1 to 8 points) to 2.5 points after use (range, 0 to 6 points) (P<0.001) (Table 1). Evaluation for KOOS questionnaires available for 17 patients showed incremental improvement from 52.46 points when therapy was started to 63 points at 6 months following NMES therapy (see Figure 1). No complications or adverse events were reported for any of the participants.
Table 1. Pain scores before and after NMES intervention.
| Patient ID | Before intervention | After intervention |
|---|---|---|
| 1 | 7 | 2 |
| 2 | 4 | 3 |
| 3 | 6 | 3 |
| 4 | 5 | 3 |
| 5 | 6 | 4 |
| 6 | 5 | 2 |
| 7 | 3 | 1 |
| 8 | 7 | 5 |
| 9 | 3 | 1 |
| 10 | 6 | 3 |
| 11 | 5 | 2 |
| 12 | 8 | 5 |
| 13 | 4 | 2 |
| 14 | 5 | 2 |
| 15 | 7 | 5 |
| 16 | 4 | 3 |
| 17 | 6 | 2 |
| 18 | 5 | 2 |
| 19 | 4 | 2 |
| 20 | 4 | 3 |
| 21 | 2 | 0 |
| 22 | 5 | 3 |
| 23 | 4 | 3 |
| 24 | 4 | 2 |
| 25 | 4 | 2 |
| 26 | 1 | 0 |
| 27 | 8 | 6 |
| 28 | 4 | 3 |
| 29 | 5 | 2 |
| 30 | 4 | 2 |
| 31 | 3 | 0 |
| Mean (range) | 5 (1 to 8) | 2.5 (0 to 6) |
Figure 1.
Improvement in the Knee injury Osteoarthritis Outcome Score (KOOS, JR) in the cohort.
Discussion
Mobile-app controlled NMES platforms have recently gained increasing attention and demonstrated clinical success to restore and rehabilitate quadriceps muscle strength in a variety of conditions, particularly following total knee arthroplasty. In this study, we expanded on current knowledge by studying the impact of NMES on patients who received non-operative care for knee osteoarthritis. Patients who underwent the therapy showed adequate compliance and demonstrated good ROM, significant reduction in pain scores, and improved patient reported outcome scores at final follow-up. Although NMES therapy has been reported on by multiple authors, including in patients with knee OA, there are limited to that have reported on the compliance, feasibility, and patient outcomes of using a mobile-app controlled NMES therapy device in the setting of knee OA. Furthermore, the incorporation of a cloud-based provider online platform may offer an additional advantage by allowing clinicians to monitor the progress and compliance of their patients in real-time. Therefore, patients who are making sub-optimal progress may benefit from an early intervention to modify their therapy protocol to achieve the best outcome.
There are several limitations to this study. In addition to the sample size and the retrospective analysis, other factors that may have contributed to the improvement in outcomes were not accounted for such as the use of pain medications and use of physical therapy. In addition, our analysis lacked a comparative control group and therefore, multiple confounders may have impacted our results making it less generalizable. Furthermore, completed questionnaires and pain scores were available for only small percentages of our study population. However, this is a novel study that investigated the use of NMES therapy in OA patients and none of the participants encountered a complication. Therefore, it will provide an impetus for future studies with better study design to truly show the full extent of the NMES therapy benefit in this setting.
Multiple studies have reported on the use of NMES therapy in various settings. Labanca et al. (15) conducted a randomized study where 63 patients were randomly allocated to either a NMES therapy and exercise group, an exercise-only group, or a no additional treatment group, for patients who had anterior cruciate ligament reconstructions. The authors assessed the quadriceps strength in all patients using the maximal isometric strength achieved at 15, 30, 60, and 180 days after surgery. Patients who received the NMES therapy achieved higher muscle strength and lower pain scores when compared with other groups. In another study by Yoshida et al. (16), the authors conducted a prospective randomized single-blind clinical trial and compared the use of sensory-level NMES vs. motor-level NMES for quadriceps rehabilitation following TKA in comparison to a control group that received only a standard rehabilitation program. Both NMES groups achieved higher isometric contraction compared to the control group (P=0.001 and P=0.028). They also achieved better 2-minute walk test results. The authors concluded that both NMES modalities can be a useful addition to standard rehabilitation protocols following TKA allowing better functional outcomes.
In a Cochrane systematic review, Martimbianco et al. (17) analyzed 8 randomized clinical trials reporting on 345 patients who had patellofemoral pain syndrome and underwent NMES therapy. The included studies suffered from heterogeneous NMES therapy protocols and characteristics. Patients in these studies also had a wide variation in terms of the duration and severity of their symptoms and associated additional therapies. The authors concluded that there is currently a lack of high-quality evidence on the exact role of NMES therapy in these patients and that more studies accounting for all patients characteristics and adjuvant therapies are needed to provide more solid evidence on the exact role of NMES therapy. However, in a comprehensive meta-analysis by Bistolfi et al. (18) the authors analyzed the effectiveness of using NMES therapy following TKA. Out of the 496 patients that were included in the final analysis, those who received NMES achieved the best scores (timed up and go test, stair climbing test, and walk test) compared to those who did not undergo the therapy. This was particularly effective in the early postoperative period (first 3 months) and the benefit was more evident in patients who had a lack of muscular activation.
In conclusion, NMES therapy has demonstrated promising clinical results in multiple settings and this study will help to expand our knowledge particularly on newer platforms incorporating mobile-app control and cloud-based provider portal to remotely monitor patient progress. Despite the limitations, findings from the present study provide a preliminary assessment of this tool and will provide an impetus for future studies.
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: A Bhave was a Consultant for DJ Orthopedics, Stryker Orthopedics and Cymedica Orthopedics. The other authors have no conflicts of interest to declare.
References
- 1.Gupta S, Hawker GA, Laporte A, et al. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatology (Oxford) 2005;44:1531-7. 10.1093/rheumatology/kei049 [DOI] [PubMed] [Google Scholar]
- 2.Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis 2001;60:91-7. 10.1136/ard.60.2.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014;73:1323-30. 10.1136/annrheumdis-2013-204763 [DOI] [PubMed] [Google Scholar]
- 4.Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007;89:780-5. [DOI] [PubMed] [Google Scholar]
- 5.Mizner RL, Petterson SC, Snyder-Mackler L. Quadriceps strength and the time course of functional recovery after total knee arthroplasty. J Orthop Sports Phys Ther 2005;35:424-36. 10.2519/jospt.2005.35.7.424 [DOI] [PubMed] [Google Scholar]
- 6.Saleh KJ, Lee LW, Gandhi R, et al. Quadriceps strength in relation to total knee arthroplasty outcomes. Instr Course Lect 2010;59:119-30. [PubMed] [Google Scholar]
- 7.Lewek MD, Rudolph KS, Snyder-Mackler L. Quadriceps femoris muscle weakness and activation failure in patients with symptomatic knee osteoarthritis. J Orthop Res 2004;22:110-5. 10.1016/S0736-0266(03)00154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petterson SC, Mizner RL, Stevens JE, et al. Improved function from progressive strengthening interventions after total knee arthroplasty: a randomized clinical trial with an imbedded prospective cohort. Arthritis Rheum 2009;61:174-83. 10.1002/art.24167 [DOI] [PubMed] [Google Scholar]
- 9.Chughtai M, Elmallah RD, Mistry JB, et al. Nonpharmacologic Pain Management and Muscle Strengthening following Total Knee Arthroplasty. J Knee Surg 2016;29:194-200. 10.1055/s-0035-1569147 [DOI] [PubMed] [Google Scholar]
- 10.Chughtai M, Piuzzi N, Yakubek G, et al. Use of an App-Controlled Neuromuscular Electrical Stimulation System for Improved Self-Management of Knee Conditions and Reduced Costs. Surg Technol Int 2017;31:221-6. [PubMed] [Google Scholar]
- 11.Coquart JB, Grosbois JM, Olivier C, et al. Home-based neuromuscular electrical stimulation improves exercise tolerance and health-related quality of life in patients with COPD. Int J Chron Obstruct Pulmon Dis 2016;11:1189-97. 10.2147/COPD.S105049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gwam CU, McGinnis T, Etcheson JI, et al. Use of Neuromuscular Electrical Stimulation During Physical Therapy May Reduce the Incidence of Arthrofibrosis After Total Knee Arthroplasty. Surg Technol Int 2018;32:356-60. [PubMed] [Google Scholar]
- 13.Pal S, Chughtai M, Sultan AA, et al. Impact of Neuromuscular Electrical Stimulation (NMES) on 90-Day Episode Costs and Post-Acute Care Utilization in Total Knee Replacement Patients with Disuse Atrophy. Surg Technol Int 2017;31:384-8. [PubMed] [Google Scholar]
- 14.Mistry JB, Elmallah RD, Bhave A, et al. Rehabilitative Guidelines after Total Knee Arthroplasty: A Review. J Knee Surg 2016;29:201-17. 10.1055/s-0036-1579670 [DOI] [PubMed] [Google Scholar]
- 15.Labanca L, Rocchi JE, Laudani L, et al. Neuromuscular Electrical Stimulation Superimposed on Movement Early after ACL Surgery. Med Sci Sports Exerc 2018;50:407-16. 10.1249/MSS.0000000000001462 [DOI] [PubMed] [Google Scholar]
- 16.Yoshida Y, Ikuno K, Shomoto K. Comparison of the Effect of Sensory-Level and Conventional Motor-Level Neuromuscular Electrical Stimulations on Quadriceps Strength After Total Knee Arthroplasty: A Prospective Randomized Single-Blind Trial. Arch Phys Med Rehabil 2017;98:2364-70. 10.1016/j.apmr.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 17.Martimbianco AL, Torloni MR, Andriolo BN, et al. Neuromuscular electrical stimulation (NMES) for patellofemoral pain syndrome. Cochrane Database Syst Rev 2017;12:CD011289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bistolfi A, Zanovello J, Ferracini R, et al. Evaluation of the Effectiveness of Neuromuscular Electrical Stimulation After Total Knee Arthroplasty: A Meta-Analysis. Am J Phys Med Rehabil 2018;97:123-30. 10.1097/PHM.0000000000000847 [DOI] [PubMed] [Google Scholar]