Abstract
Total knee arthroplasty (TKA) is among the most common elective procedures performed worldwide. Recent efforts have been made to significantly improve patient outcomes, specifically with postoperative rehabilitation. Despite the many rehabilitation modalities available, the optimal rehabilitation strategy has yet to be determined. Therefore, this systematic review focuses on evaluating existing postoperative rehabilitation protocols. Specifically, this review analyses the study designs, rehabilitation methods, and outcome measures of postoperative rehabilitation protocols for TKA recipients in the past five years. The PubMed, EMBASE, and Cochrane Library databases were queried for studies evaluating rehabilitation protocols following primary TKA. Of the 11,013 studies identified within the last five years, 70 met the inclusion and exclusion criteria. After assessing for relevance and removing duplicates, a final count of 20 studies remained for analysis. Level-of-evidence was determined by the American Academy of Orthopaedic Surgeons (AAOS) classification system. Our findings demonstrated that continuous passive motion and inpatient rehabilitation may not provide additional benefit to the patient or healthcare system. However, early rehabilitation, telerehabilitation, outpatient therapy, high intensity, and high velocity exercise may be successful forms of rehabilitation. Additionally, weight-bearing biofeedback, neuromuscular electrical stimulation, and balance control appear to be beneficial adjuncts to conventional rehabilitation. Postoperative rehabilitation following TKA facilitates patient recovery and improves quality of life. This systematic review analyzed the existing rehabilitation protocols from the past five years. Some studies did not accurately describe the conventional rehabilitation protocols, the duration of therapy sessions, and the timing of these sessions. As such, future studies should explicitly describe their methodology. This will allow high-quality assessments and the conception of standardized protocols.
Keywords: Total knee arthroplasty (TKA), postoperative rehabilitation, physical therapy, physiotherapy
Introduction
Total knee arthroplasty (TKA) is among the most common elective procedures performed worldwide (1,2). In countries such as the United States, Canada, and Australia, its incidence has grown at an annual rate of over 5% between 1998 and 2008 (3-5). Hence, recent efforts have been made to significantly improve patient outcomes. These efforts include modifications in implant design, patient optimization, and perioperative pain management. However, postoperative interventions, specifically rehabilitation, largely contribute to patient outcomes. Postoperative rehabilitation has led to shorter hospital stays, fewer complications, and reduced utilization of follow-up services (6-10).
The majority of rehabilitation protocols aim to improve quadriceps strength and range of motion (ROM). These protocols also intend to facilitate activities of daily living (ADL), and aid in the performance of more demanding exercise (11). Thus, activities that promote muscle strength, gait, and balance are specifically targeted to maximize outcomes. In recent years, numerous postoperative interventions have been evaluated. These practices include continuous passive motion, high velocity contractions, rapid rehabilitation, and telerehabilitation. Some of these interventions, such as high velocity contractions, modify the technique with which patients perform specific exercises (12). Other interventions including telerehabilitation utilize remote devices to provide standard rehabilitation (13).
Despite the many rehabilitation modalities available, the optimal rehabilitation strategy has yet to be determined. The lack of consensus on the most effective strategies is likely a result of the existing variation in the delivery, duration, and intensity of rehabilitation programs. Consequently, there is a scarcity of evidence-based practice guidelines and recommendations to guide postoperative TKA rehabilitation. Therefore, this systematic review focuses on evaluating existing postoperative rehabilitation protocols. Specifically, this review analyses the study designs, rehabilitation methods, and outcome measures of postoperative rehabilitation protocols for TKA recipients in the past five years.
Methods
Databases queried
A systematic review of the literature for rehabilitation protocols following primary TKA was conducted by querying the PubMed, EMBASE, and Cochrane Library databases. Articles published in the past five years (January 1, 2013 to December 31, 2018) were identified using various keyword combinations and Boolean operators. The following string was utilized for the search:
((“Total knee arthroplasty” OR “TKA” OR “primary total knee arthroplasty” OR “primary TKA”) AND (“rehabilitation” OR “physiotherapy” OR “physical therapy”))
Inclusion & exclusion criteria
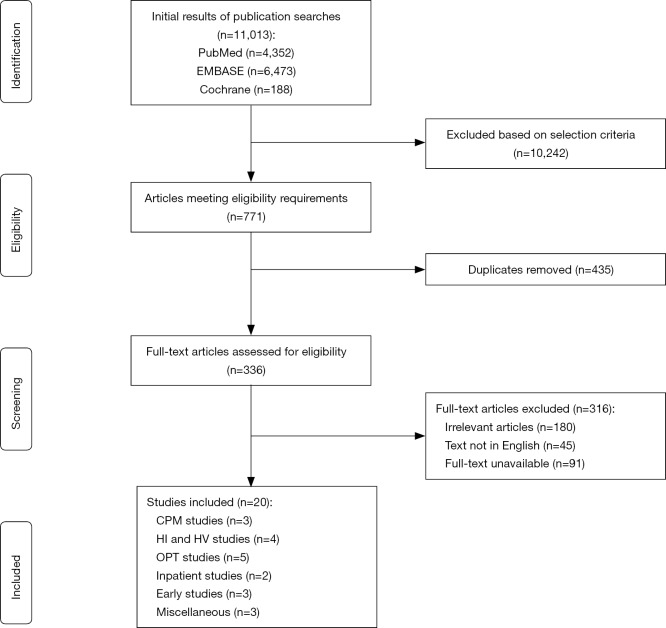
Publications were eligible for inclusion if they: (I) examined postoperative exercise-based interventions in a rehabilitation setting; (II) they included participants who underwent primary unilateral TKA; (III) the study was performed in the United States of America, Canada, United Kingdom, or Australia; and (IV) the level of evidence was III or higher based on the American Association of Orthopaedic Surgeons (AAOS) level of evidence classification (14). Studies were excluded if: (I) they were written or published in a language other than English; and (II) the full text was not available; (III) they were systematic reviews, meta-analyses, study protocols for randomized controlled trials (RCTs), feasibility or pilot studies, letters to the editor, surveys, or case reports (Figure 1). Two independent reviewers (IM Dávila Castrodad, TM Recai) screened each title and abstract to determine whether the article met the inclusion and exclusion criteria. If the two reviewers agreed about the inclusion of a study, the study was selected for final analysis. If there was any doubt about a study’s eligibility, a third reviewer (NS Mohamed) was consulted.
Figure 1.
Systematic review flowchart for study inclusion.
Eligible studies
The initial search in the PubMed database generated 4,352 results, of which 25 met our inclusion and exclusion criteria. The original query for the EMBASE database resulted in 6,473 entries. Of these, 23 met our inclusion and exclusion criteria. The initial query of the Cochrane Library yielded 188 results, of which 22 met our inclusion and exclusion criteria. After assessing for relevance and removing duplicates, a final count of 20 studies remained for analysis. A total of 17 studies were Level-of-Evidence I, one study was Level-of-Evidence I, and two studies were a Level-of-Evidence III.
The studies were stratified as best as possible based on the similarities of the intervention protocols. This resulted in the following study groupings: continuous passive motion; high velocity and high intensity exercise; outpatient therapy; inpatient therapy; early rehabilitation; and miscellaneous.
Study data/extracted data
The 20 studies included in this systematic review were assessed, and the data extracted included the type of study, diagnosis, procedure performed, rehabilitation intervention, control and intervention group characteristics, time to follow-up, and outcome measures.
Results
Continuous passive motion
Rehabilitation protocol
Continuous passive motion (CPM) is achieved by a motorized device, which passively drives the knee through a predefined arc of motion. It is believed that soft tissue healing is improved, length of stay (LOS) is reduced, and fewer complications occur with the addition of CPM (11). Studies from the previous decade have reported mixed results regarding CPM use following TKA. While some investigations report that CPM improves active knee flexion, others demonstrate no difference in functional outcomes (15-17). Some reports make mention of increased wound drainage, swelling, and analgesic use in patients who utilized CPM during their recovery (18-20). The efficacy of CPM was evaluated by several methods including the categorization of patients in non-CPM and CPM groups. However, the ROM parameters established on the device and the usage time has varied.
Results in the literature
Three Level-of-Evidence I studies evaluating the postoperative use of CPM were included in this systematic review (Table 1). Herbold et al. assessed the impact of CPM as an adjunct to conventional rehabilitation in patients who were transferred to an inpatient facility within five days of surgery (21). The CPM group (n=70) received three hours of physical therapy and two hours of CPM daily until discharge while the control group (n=71) only received physical therapy during their stay. The patients remained admitted in the facility between 6 and 11 days and were evaluated one day prior to discharge for active ROM, Timed Up and Go (TUG), and Functional Independence Measure (FIM). The authors found no significant differences in the outcome measures between the CPM and non-CPM groups: active knee flexion: (CPM: 83.5°±10.0° vs. no-CPM: 86.4°±7.9°; P=0.080), active knee extension (CPM: −2.7°±2.8° vs. no-CPM: −3.3°±3.3°; P=0.211); TUG (CPM: 19.9±7.5 s vs. no-CPM: 19.8±6.1 s; P=0.532); total FIM (CPM: 107.0±4.1 vs. no-CPM: 107.8±3.2; P=0.146). The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores were evaluated seven days post-discharge and were also similar between the groups (CPM: 30.2±14.6 vs. no-CPM: 33.3±14.4; P=0.294).
Table 1. Continuous passive motion.
| Study | Level of evidence | Intervention cohort | Control cohort | Duration of follow-up | Outcomes, mean (SD) | ROM (degrees), mean (SD) |
|---|---|---|---|---|---|---|
| Herbold et al. | I | CPM: 3 hrs a day of PT and OT plus 2 hrs a day of CPM (n=70) | No-CPM: 3 hours a day of PT and OT (n=71) | CPM: 8.3 (1.7) days; No-CPM: 8.7 (2.7) days | WOMAC: CPM 30.2 (14.6) vs. no-CPM 33.3 (14.4); P=0.294 | Extension: CPM −2.7 (2.8) vs. no-CPM –3.3 (3.3); P=0.211 |
| TUG: CPM 19.9 s (7.5) vs. no-CPM 19.8 s (6.1); P=0.532 | Flexion: CPM 83.5 (10.0) vs. no-CPM 86.4 (7.9); P=0.080 | |||||
| FIM: CPM 107.0 (4.1) vs. no-CPM 107.8 (3.2); P=0.146 | ||||||
| Boese et al. | I | Moving CPM: Moved through 0–110° ROM, five hours a day for a minimum of 2 days (n=55) | No-CPM: no-CPM during hospital stay (n=54) | 3 weeks | N/A | Extension§: CPM 2.3 vs. non-moving CPM 2.2 vs. no-CPM 2.1; P=0.94 |
| Non-moving CPM: Stationary at 90° POD 0 and moved through 0–110° ROM, 5 hours for subsequent days (n=51) | Flexion§: CPM: 111.9 vs. non-moving CPM 111.2 vs. no-CPM 111.7; P=0.94 | |||||
| Full ROM§: CPM 109.6 vs. non-moving CPM 109.0 vs. no-CPM 109.5; P=0.96 | ||||||
| Joshi RN et al. | I | One-on-one PT 2× a day and 3 CPM sessions per day until discharge | One-on-one physical therapy 2× a day starting on POD 0 or POD 1 (n=52) | 6 weeks | WOMAC: CPM 31.5 (14.4) vs. no-CPM 27.7 (13.9); P=0.23 | Extension: CPM −1.5 (2.9) vs. no-CPM −1.3 (2.3); P=0.94 |
| Each session lasted 2 hrs (total of 6 hrs per day), with ROM increased as tolerated (n=57) | PAQ: CPM 35.4 (13.8) vs. no-CPM 36.0 (12.9); P=0.84 | Flexion: CPM 115.0 (9.3) vs. no-CPM 115.7 (8.0); P=0.69 | ||||
| Full ROM: CPM 113.5 (10.2) vs. no-CPM 114.3 (9.5); P=0.72 | ||||||
| 3 months | WOMAC: CPM 21.4 (13.1) vs. no-CPM 18.2 (13.9); P=0.20 | Extension: CPM −1.3 (2.2) vs. no-CPM −0.4 (1.3); P=0.03 | ||||
| PAQ: CPM 24.8 (12.2) vs. no-CPM 25.7 (12.8); P=0.75 | Flexion: CPM 121.0 (7.4) vs. no-CPM 120.3 (5.7); P=0.41 | |||||
| Full ROM: CPM 119.7 (8.1) vs. no-CPM 119.9 (6.0); P=0.85 |
§, mean. POD, postoperative day; ROM, range of motion; WOMAC, Western Ontario and McMaster Universities Arthritis Index; CPM, continuous passive motion; PAQ, Physical Activity Questionnaire; PT, physical therapy; OT, occupational therapy; TUG, timed up and go; FIM, Functional Independence Measure; SD, standard deviation.
In a study conducted by Boese et al., patients were assigned by consecutive sequencing to one of three study groups (22). The CPM group consisted of 55 patients who received a moving CPM device immediately upon arrival to the orthopedic floor after surgery. The device moved through 0–110° ROM, five hours a day for a minimum of two days. The non-moving CPM group consisted of 51 patients whose operative knee was kept stationary at 90 degrees for the first night but followed the same protocol as the CPM group on all subsequent days. The group that did not receive CPM during their stay consisted of 54 patients. While the average LOS was three days, the groups were compared until postoperative day two (POD 2). At three weeks postoperatively, all groups showed similar results regarding ROM (CPM: 109.6° vs. non-moving CPM: 109.0° vs. no-CPM: 109.5°; P=0.96), flexion (CPM: 111.9° vs. non-moving CPM: 111.2° vs. no-CPM: 111.7°; P=0.94), and extension (CPM: 2.3° vs. non-moving CPM: 2.2° vs. no-CPM: 2.1°; P=0.94).
A study by Joshi et al. evaluated longer-term outcomes following CPM. Patients in this study were randomly assigned to a CPM (n=57) or non-CPM (n=52) group (23). The CPM group received six hours of CPM per day in addition to physical therapy twice a day from POD 1 to discharge. Meanwhile, the non-CPM group had physical therapy twice a day starting on POD 0 or 1. At 6 weeks and 3 months postoperatively, there were no differences in flexion between groups (CPM: 115.0° vs. no-CPM: 115.7°; P=0.69 and CPM: 121.0° vs. no-CPM: 120.3°; P=0.41, respectively). Similarly, there were no differences at 6 weeks and 3 months post-operatively between the cohorts with respect to ROM (CPM: 113.5° vs. no-CPM: 114.3°; P=0.72 and CPM: 119.7° vs. no-CPM: 119.9°; P=0.85, respectively), WOMAC scores (CPM: 31.5 vs. no-CPM: 27.7; P=0.23 and CPM: 21.4 vs. no-CPM: 18.2; P=0.20, respectively), and Physical Activity Questionnaire (PAQ) (CPM: 35.4 vs. no-CPM: 36.0; P=0.84 and CPM: 24.8 vs. no-CPM: 25.7; P=0.75, respectively). However, the authors did find a difference in extension at 3 months (CPM: −1.3° vs. no-CPM: −0.4°; P=0.03) compared to 6 weeks (CPM: −1.5° vs. no-CPM: −1.3°; P=0.94).
Summary
The utilization of CPM as an adjunct to TKA rehabilitation has had varied results in the past years. Though several reports have noted positive outcomes, some studies do not demonstrate a significant difference when compared to conventional rehabilitation (19,24-26). The studies above also conclude that CPM has no additional benefit to a rehabilitation protocol. This holds true for the immediate postoperative period and the sub-acute rehabilitation setting. These studies evaluated outcomes between 1 week and 3 months, and no significant differences were seen in LOS, pain, ROM, or function at any time point. Though the average LOS following TKA is 3.5 days, some studies note improvement with greater than seven days of use (18,27-29). As such, future studies should evaluate CPM in the outpatient setting while increasing the time or/and frequency of its use.
In addition, several aspects of the protocols were not thoroughly described. The conventional rehabilitation methods, the duration of therapy sessions, and the timing of these sessions were not explained. This is problematic for reproducibility. In order to obtain the best results, methods should be standardized to enable high-quality assessments. The lack of accurate protocol descriptions is a common theme throughout this review. In summary, given a lack of evidence demonstrating significant benefits with CPM, its use may not be justified based on its added costs.
High velocity & high intensity exercise
Rehabilitation protocol
As individuals age, several neuromotor changes occur, which leads to skeletal muscle weakness and reduced power. In TKA recipients, muscular strength and power decrease by at least 24% when compared to the contralateral side (30). Experts believe more demanding rehabilitation protocols may help overcome these deficits. Recent research focuses on rehabilitation strategies that incorporate movement velocity, a component of power. Given its preferential activation of type 2 muscle fibers, high velocity (HV) exercise is thought to improve functional mobility (12). This form of exercise is defined as performing a muscle contraction as quickly as possible, or in 1 second or less. In contrast to HV, a LV muscle contraction is performed in two seconds or more. Evidence indicates that HV exercises can improve static and dynamic balance while decreasing quadriceps impairment (31).
On the other hand, high intensity (HI) rehabilitation solely focuses on strength, defined as the contraction force. This program includes progressive resistance exercises (PRE) and rapid progression to weight-bearing (WB) exercises. Several authors have expressed that the progressive strengthening (PS) and functional exercises according to clinical milestones promote positive outcomes (32). What is not yet known is whether a PRE-program restores function to levels comparable to healthy age-matched controls. Four Level-of-Evidence I studies regarding HV and HI exercise were analyzed in this systematic review (Table 2).
Table 2. High velocity exercise and high intensity.
| Study | Level of evidence | Intervention cohort | Control cohort | Duration of follow-up | Outcomes, mean | ROM (degrees), mean (SD) |
|---|---|---|---|---|---|---|
| Doerfler et al. | I | PRE and HV exercise 2× a wk for 8 wks | PRE and LV exercise 2× a wk for 8 wks | 4-6 wks | 6MW: HV 97.1 m vs. LV 54.4 m; P=0.049 | N/A |
| Fast concentric (1 s) and slow eccentric (3 s) contractions (n=12) | Slow concentric and eccentric (3 s) contractions (n=9) | PIF¶: HV 1.0 Nm/BMI vs. LV 0.6 Nm/BMI; P=0.03 | ||||
| 10-m Gait Speed: HV 1.31 s vs. LV 1.01 s; P=0.40 | ||||||
| Functional Stair Test: HV 1.9 s vs. LV 1.2 s; P=0.33 | ||||||
| Balance test¶: HV 0.10 vs. LV 0.11; P=0.96 | ||||||
| Kelly et al. | I | HV curbs and stairs for 2 sessions a wk | LV curbs and stairs for 2 sessions a wk | 7 wks | 6MW: HV 393 m vs. LV 383.9 m; P=0.001 | N/A |
| Fast concentric (1 s) and slow eccentric (2 s) contractions | Fast concentric (2 s) and slow eccentric (2 s) contractions | SCT: HV 20.2 s vs. LV 21.6 s; P=0.001 | ||||
| The end range of the concentric contraction held for 5 s (n=19) | The end range of the concentric contraction held for 5 s (n=19) | TUG: HV 10.4 s vs. LV 10.8 s; P=0.001 | ||||
| VAS: HV 13.0 vs. LV 21.8; P=0.001 | ||||||
| Bade et al. | I | HI: Warm up, PRE targeting all lower extremity muscle groups; bilateral and unilateral WB functional exercises, balance exercises, agility exercises 2–3× a wk for 11 wks (n=84) | LI: Isometric and ROM exercise the first 4 wks, a slower transition to WB exercises, only body weight and elastic bands for resistance, restriction of activities outside of ADL’s the first 4 wks 2–3× a wk for 11 wks (n=78) | 3 mos | TUG¶: HI −1.35 s vs. LI −1.01 s; P=0.08 | Extension¶: HI −0.61 vs. LI −0.35; P=0.53 |
| 6MW¶: HI 38.83 m vs. LI 23.39 m; P=0.13 | Flexion¶: HI −1.93 vs. LI −2.10; P=0.90 | |||||
| SCT¶: HI −3.89 s vs. LI −3.28 s; P=0.21 | ||||||
| WOMAC¶: HI −19.60 vs. LI −19.48; P=0.93 | ||||||
| Quad strength¶: HI 0.02 Nm/kg vs. LI −0.05 Nm/kg; P=0.14 | ||||||
| Quad activation¶: HI 11.70% vs. LI 8.52%; P=0.14 | ||||||
| Adverse event rates¶: HI 1 fall vs. LI 3 falls; P=0.78 | ||||||
| 12 mos | TUG: HI 7.36 s (1.77) vs. LI 7.44 s (1.50) | Extension: HI –2.18 (2.43) vs. LI –1.76 (2.28) | ||||
| 6MW: HI 531.7 m (98.9) vs. LI 513.6 m (78.4) | Flexion: HI 129.28 (8.89) vs. LI 128.27 (8.61) | |||||
| SCT: HI 11.40 s (3.62) vs. LI 11.77 s (3.15) | ||||||
| WOMAC: HI 6.69 (7.75) vs. LI 7.16 (6.28) | ||||||
| Quad strength: HI 1.42 Nm/kg (0.47) vs. LI 1.43 Nm/kg (0.44) | ||||||
| Quad activation: HI 83.39% (11.73) vs. LI 83.73% (10.12) | ||||||
| Pozzi et al. | I | HI: 12 sessions of PS 2–3× a wk (n=165) | CG: No knee joint pathology, no rehabilitation (n=88) | 12 mos | KOS-ADL: HI 85.48% vs. LI 79.18% vs. CG: 98.01%; P<0.001 | Extension: HI 0.52 vs. LI 2.78 vs. CG: −1.75; P<0.001 |
| LI: 23 sessions of SPT 2–3× a wk (n=40) | TUG: HI 7.75 s vs. LI 8.67 s vs. CG: 6.63 s; P<0.001 | Flexion: HI 120.15 vs. LI 119.03 vs. CG: 139.32; P<0.001 | ||||
| SCT: HI 12.43 s vs. LI 16.49 s vs. CG: 9.68 s; P<0.001 | ||||||
| 6MW: HI 549.72 m vs. LI 494.91 m vs. CG: 655.91 m; P<0.001 | ||||||
| MVIC: HI 6.58 Nm/kg vs. LI 5.85 Nm/kg vs. CG: 9.49 Nm/kg; P<0.001 |
¶, mean difference. ROM, range of motion; WOMAC, Western Ontario and McMaster Universities Arthritis Index; TUG, timed up and go; PRE, Progressive Resistance Exercise; HV, high velocity; LV, low velocity; 6MW, 6-minute walk test; PIF, Normalized Peak Isometric Force; Nm/kg, Newton-meters/kg; SCT, Stair Climbing Test; VAS, Visual Analog Scale; HI, high intensity; LI, low intensity; CG, control group; ADL, activities of daily living; Quad, Quadriceps; PS, progressive strengthening; SPT, standard physical therapy; OPT, Outpatient Physical Therapy; KOS-ADL, knee outcome survey-activities of daily living; MVIC, maximal voluntary isometric contraction; SD, standard deviation.
Results in the literature
In a RCT conducted by Doerfler et al., the efficacy of HV and LV quadriceps exercises were compared based on functional outcomes and quadriceps power (12). Approximately 4–6 weeks following TKA, 21 participants attended rehabilitation twice a week for a total of eight weeks (16 sessions). The rehabilitation programs consisted of a standardized progressive resistance exercise (PRE) program in addition to HV quadriceps (n=12) or LV quadriceps exercises (n=9). The exercises were completed for 3 sets for a maximum of 10 repetitions and were progressed by adding additional weight. In the HV group, all quadriceps exercises were performed with fast concentric (1 second) and slow eccentric (3 seconds) contractions while the LV group performed both contractions slowly (3 seconds). The authors found that progressive resistance HV quadriceps exercises demonstrated a greater improvement in the 6-minute walk test (6MW) (HV: 97.1 vs. LV: 54.4 m; P=0.049) and normalized peak isometric force (PIF) from baseline to final testing compared to the high velocity group [HV: 1.0 vs. LV: 0.6 Newton-meters/BMI (Nm/BMI) mean improvement; P=0.03]. At 20% and 40% PIF, there were no between-group differences noted, although at 40% PIF, the HV group was trending favorably on the involved side (P=0.06) with a large effect size (Cohen’s d=0.96). No between-group differences were found for the 10-m gait speed (HV: 1.31 s vs. LV: 1.01 s; P=0.40), functional stair test (HV: 1.9 s vs. LV: 1.2 s; P=0.33), or balance tests (HV: 0.10 vs. LV: 0.11 mean improvement; P=0.96).
Kelly et al. performed a RCT and compared the effects of utilizing either HV (n=19) or LV (n=19) exercises on gait, functional performance, and pain following TKA (33). Patients engaged in 12 rehabilitation sessions within 7 weeks postoperatively, with a goal of 2 sessions per week. The HV group performed curbs and stairs as quickly as possible while the LV group performed curbs and stairs at their preferred speed. The open-chain resistive exercises were performed by the HV group with a concentric contraction in 1 second or less whereas the LV group performed the contraction in 2 seconds. Both groups performed the eccentric contractions for 2 seconds, and both held the end range of concentric contraction for 5 seconds. At the end of the 7-week period, both groups showed significantly improved outcomes for the mean 6MW (HV: 393 m vs. LV: 383.9 m; P=0.001), stair climb test (SCT) (HV: 20.2 s vs. LV: 21.6 s; P=0.001), TUG (HV: 10.4 s vs. LV: 10.8 s; P=0.001), but only the HV group reported decreased Visual Analog Scale (VAS) pain scores (HV: 13.0 vs. LV: 21.8; P=0.001).
The greatest amount of strength and functional performance loss occurs in the first month following TKA (6). As such, Bade et al. compared the safety and efficacy of a HI rehabilitation protocol to a LI rehabilitation protocol beginning four days postoperatively (34). Rehabilitation sessions occurred three times a week for the first six weeks and twice a week over the last five weeks, resulting in a total of 26 visits over a span of 11 weeks. The intervention of the HI group (n=84) consisted of a warm up, PRE targeting all lower extremity muscle groups; bilateral and unilateral WB functional exercises, balance exercises, agility exercises, and activity prescriptions. These exercises were performed for two sets of eight repetitions and based on an eight-repetition maximum. The LI intervention group (n=78) had an initial focus on isometric and ROM exercise for the first four weeks, a slower transition to WB exercises, less progression in difficulty of WB exercises, used only body weight and elastic bands for resistance, and had restriction of activities outside of ADL’s for the first four weeks gradually building to 30 minutes by the end of therapy. The mean differences between the HI and LI groups at 3 months were not significant based on the TUG (−1.35 vs. −1.01 s; P=0.08), 6MW (38.83 vs. 23.39 m; P=0.13), SCT (−3.89 vs. −3.28 s; P=0.21), WOMAC scores (−19.60 vs. −19.48; P=0.93), extension (−0.61° vs. −0.35°; P=0.53), flexion (−1.93° vs. −2.10°; P=0.90), quadriceps strength (0.02 vs. −0.05 Nm/kg; P=0.14), quadriceps activation (11.70% vs. 8.52%; P=0.14), and adverse event rates (1 fall vs. 3 falls; P=0.78). Also, there were no differences between the HI and LI groups over 12 months based on the mean TUG (7.36±1.77 vs. 7.44±1.50 s), 6MW (531.7±98.9 vs. 513.6±78.4 m), SCT (11.40±3.62 vs. 11.77±3.15 s), WOMAC scores (6.69±7.75 vs. 7.16±6.28), extension (−2.18°±2.43° vs. −1.76°±2.28°), flexion (129.28°±8.89° vs. 128.27°±8.61°), quadriceps strength (1.42±0.47 vs. 1.43±0.44 Nm/kg), and quadriceps activation (83.39%±11.73% vs. 83.73%±10.12%). By 12 months, SCT performance improved from baseline by 5.42 seconds in the HI group [95% confidence interval (CI): −7.03 to −3.81; P<0.001] and 4.36 seconds in the LI group (95% CI: −6.01 to −2.70; P<0.001).
Pozzi et al. compared progressive strengthening (PS) to standard physical therapy (SPT) (32). There PS group (n=165) participated in progressive strengthening exercise sessions 2–3 times a week for at least 12 sessions while the SPT group (n=40) had an average of 23 sessions of outpatient physical therapy (OPT). The control group (CG) (n=88), age 50–85 years without symptomatic knee joint pathology received no rehabilitation intervention. All patients were evaluated 12 months after surgery. The CG showed higher Knee Outcome Survey-Activities of Daily Living (KOS-ADL) scores (PS: 85.48% vs. SPT: 79.18% vs. CG: 98.01%; P<0.001), greater flexion (PS: 120.15°, SPT: 119.03°, CG: 139.32°; P<0.001), greater extension (PS: 0.52° vs. SPT: 2.78° vs. CG: −1.75°; P<0.001), better TUG (PS: 7.75 s vs. SPT: 8.67 s vs. CG: 6.63 s; P<0.001) and SCT (PS: 12.43 s vs. SPT: 16.49 s vs. CG: 9.68 s; P<0.001), longer 6MW (PS: 549.72 m vs. SPT: 494.91 m vs. CG: 655.91 m; P<0.001) and greater quadriceps maximal voluntary isometric contraction (MVIC) (PS: 6.5 8Nm/kg vs. SPT: 5.85 Nm/kg vs. CG: 9.49 Nm/kg; P<0.001) than the SPT or PS groups. A higher proportion of the PS group achieved the minimum cutoff for extension (PS: 30% vs. SPT: 15%; P=0.042), quadriceps strength (PS: 18% vs. SPT: 5%; P=0.032) and SCT (PS: 34% vs. SPT: 18%; P=0.029), compared to those in the SPT group.
Summary
During the past several years, strength, power, and functional mobility have been recognized as important areas of focus for the geriatric population. Due to their increased deficits, it is believed TKA recipients require more aggressive rehabilitation. The above studies evaluated higher demand rehabilitation programs. One study concluded that both HV and LV interventions were equally effective in improving strength and function while the other study found that a HV program had better short- and long-term strength and functional outcomes. The HV studies were RCTs, and therefore well-designed. However, they had very small samples sizes. This limitation may make it difficult to draw strong conclusions and formulate official guidelines with the integration of a HV program. In addition, the definition of LV slightly varied between the studies which may have led to slight differences in the performance of the LV or control group.
Regarding HI rehabilitation, the studies above suggest that a HI program is safe for individuals following TKA. Patients who participate of progressive strengthening have similar outcomes to healthy individuals. This form of rehabilitation may be more effective in restoring functional levels. However, patients initiated therapy in the early postoperative period, thus athrogenic muscular inhibition may have limited its effectiveness. To overcome this problem, future research should analyze whether alternative strategies are more effective in patients with large quadriceps muscle activation deficits. Another common issue in these studies is that treatment exposure could not be precisely quantified. The progression of exercises is solely based on the individual patients, and this can carry variability within the experimental group.
Outpatient therapy
Telerehabilitation
Rehabilitation protocol
Telerehabilitation utilization has continued to rise over the last decade due to the growing scientific literature supporting its usage following TKA. Telerehabilitation delivers rehabilitation services remotely using information and telecommunication technologies. Several methods of telerehabilitation have been utilized. One method involves a clinician-controlled pan, tilt, zoom (PTZ) camera and software that allows real-time visual-audio interaction between the therapist and home-based patient (13). Another method uses the Virtual Exercise Rehabilitation Assistant (VERA) (35). This system allows for physical therapy protocols to be delivered to patients at home via an animated image on a display. VERA uses three-dimensional cameras to pick-up real-time movements, allowing it to provide real-time feedback for patients to fully benefit from the program. Tablet applications have also been developed to deliver remote rehabilitation. CaptureProof, which is an iPod touch application where 23 videos are created and uploaded by therapists (36). Physical therapists monitor progress through patient videos and provide feedback as necessary. Other web-based platforms send emails to patients with descriptions, pictures, and videos of required exercises based on the time from surgery (37). This review includes three Level-of-Evidence I studies and one Level-of-Evidence III study concerning telerehabilitation (Table 3).
Table 3. Outpatient therapy.
| Study | Level of evidence | Intervention cohort | Control cohort | Duration of follow-up | Outcomes, mean | ROM (degrees), mean |
|---|---|---|---|---|---|---|
| Moffet et al. | I | In-home TELE for 16 sessions (n=101) | In-home STD for 16 sessions (n=104) | 4 mos | WOMAC: TELE: 84.5 vs. STD: 82.6 | N/A |
| Chughtai et al. | III | VERA telerehabilitation system (n=18) | N/A | 3.5 visits (mean) | % Change WOMAC: 66% | N/A |
| % Change KSS pain: 368% | ||||||
| % Change KSS function: 335% | ||||||
| Bini & Majahan | I | Asynchronous video-based TELE (n=14) | OPT (n=15) | 24 wks | KOOS¶: TELE: −17.591 vs. OPT: −17.251; P=0.954 | Full ROM¤: TELE: 1–120 vs. OPT: 0–120 |
| VAS¶: TELE: −3.429 vs. OPT: −4.0; P=0.61 | ||||||
| VR-12 PCS¶: TELE: 15.115 vs. OPT: 15.493; P=0.922 | ||||||
| VR-12 MCS¶: TELE: 6.326 vs. OPT: 1.077; P=0.187 | ||||||
| Klement et al. | I | Web-based SDPT (n=195) | Web-based SDPT and OPT (n=101) | 6 mos | KOOS Jr: SDPT: 73.9 vs. SDPT and OPT: 68.0; P=0.026 | N/A |
| Ko et al. | I | 2 Sessions of one-to-one PT and 2 of home-based PT for 6 wks (n=85) | 4 Sessions of monitored home-based PT for 6 wks (n=80) | 10 wks | OKS: one-to-one: 32 vs. group-based: 36 vs. home-based: 34; P=0.20 | Extension: one-to-one: 4 vs. group-based: 4 vs. home-based: 5; P=0.91 |
| 2 Sessions of group-based PT and 2 of home-based PT for 6 wks (n=84) | 6MW: one-to-one: 397.5 m vs. group-based: 405 m vs. home-based: 425 m; P=0.37 | Flexion: one-to-one: 115 vs. group-based: 110 vs. home-based: 116; P=0.45 | ||||
| WOMAC function: one-to-one: 16 vs. group-based: 13 vs. home-based: 14; P=0.15 | Quad Lag: one-to-one: 1 vs. group-based: 2 vs. home-based: 1; P=0.57 | |||||
| WOMAC pain: one-to-one: 3.8 vs. group-based: 1.6 vs. home-based: 2.5; P=0.79 |
¶, mean difference; ¤, range. ROM, range of motion; WOMAC, Western Ontario and McMaster Universities Arthritis Index; 6MW, 6-minute walk test; VAS, Visual Analog Scale; Quad, quadriceps; OPT, outpatient physical therapy; TELE, telerehabilitation; STD, face-to-face; VERA, Virtual Exercise Rehabilitation Assistant; KSS, Knee Society Score; SDPT, self-directed physical therapy; KOOS, Knee Injury and Osteoarthritis Outcome Score; VR-12, Veterans-RAND 12 Health Survey; PCS, Physical Component Score; MCS, Mental Component Score; OKS, Oxford Knee Score; SD, standard deviation.
Results in the literature
In 2015, Moffet et al. conducted a two-month rehabilitation program, which compared in-home telerehabilitation (TELE) to in-home face-to-face (STD) visits of patients with similar demographics and clinical characteristics at baseline (13). Two hundred and five patients were randomly assigned to either an in-home telerehabilitation group (n=101) or face-to-face rehabilitation group (n=104) prior to discharge. Rehabilitation included 16 sessions of 45–60 minutes and both groups followed the same intervention. Interventions included various exercises that focused on mobility, strengthening, function, and balance. Patients were evaluated prior to TKA as well as two and four months following discharge. After the last follow up evaluation, the total WOMAC mean for both the TELE and STD group was 84.5 and 82.6, respectively. The total WOMAC mean differences between the two groups were near zero (−1.6%; 95% CI: −5.6% to 2.3%). This study concluded that TELE may be deemed an effective alternative to STD therapy following TKA.
Similar results were observed by Chughtai et al., who measured patient compliance, time spent, clinical outcome scores, and usability of a virtual rehabilitation platform of telerehabilitation (35). Eighteen TKA patients participated in this study and utilized the VERA telerehabilitation system to receive the instructions for the required exercises. Patients spent an average of 26.5 minutes per day engaging in an average of 13.5 exercises for an average of 29.5 days. Patients had a mean of 3.5 outpatient follow-up visits. The mean system usability scale (SUS) score was 93 points, above the 50th percentile point of the scale. Patient WOMAC scores improved by 66%, Knee Society Score (KSS) pain scores improved by 368%, and KSS function scores improved by 33%. All outcomes showed significant improvement, which further supports the utilization of telerehabilitation.
Bini & Majahan conducted a RCT comparing asynchronous video-based telerehabilitation to traditional in-person OPT beginning two-weeks postoperatively (36). A total of 14 patients underwent telerehabilitation while 15 patients were assigned to the OPT group. The telerehabilitation app contained videos consisting of the same exercises patients in the control group were instructed to perform. The authors found no difference between the telerehabilitation group and the outpatient group for the following patient reported outcomes: ROM (1–120° vs. 0–120°; no P value), mean Knee Injury and Osteoarthritis Outcome Score (KOOS) change (−17.591 vs. −17.251; P=0.954), mean VAS change (−3.429 vs. −4.0; P=0.61), mean Veterans-RAND 12 health survey (VR-12) PCS change (15.115 vs. 15.493; P=0.922), and mean VR-12 mental component score (MCS) change (6.326 vs. 1.077; P=0.187) after an average 24-week follow-up.
Klement et al. prospectively evaluated TKA patients enrolled in a web-based self-directed physical therapy (SDPT) program (37). Patients received daily emails with exercise instructions and weekly updates as time progressed. A total of 195 patients completed the 10-week SDPT program while 101 patients received additional outpatient therapy mostly due to inadequate ROM and patient request. Both groups significantly improved postoperatively, but at 6-month follow up the SDPT group showed greater improvement in the KOOS Jr scores compared to the combined regimen group (73.9 vs. 68.0; P=0.026).
Summary
Many technological advancements have recently been integrated and adapted for the field of medicine. The feasibility regarding telerehabilitation in the post-surgical setting has been questioned in recent years. All four studies demonstrated that telerehabilitation, in its various forms, and web-based therapy provide comparable clinical outcomes to other forms of OPT following TKA. Despite the investment, telerehabilitation is especially notable as it may afford longer-term reductions in costs to healthcare systems. Given that post-discharge care costs can account for at least 36% of the episode of care for joint arthroplasty, healthcare savings secondary to the implementation of telerehabilitation or web-based therapy can have a significant impact (37). These studies have additionally shown that compliance and exercise comprehension can be achieved through telerehabilitation. However, despite its success, the large group of patients that opted to undergo OPT in addition to SDPT demonstrates this form of therapy may not be for everyone.
One-to-one physical therapy
Rehabilitation protocol
Another form of OPT is one-to-one therapy, which is intended to provide rehabilitation to one patient at a time. Physical therapists provide manual therapy such as joint mobilization and soft tissue therapy (38). Therapeutic modalities including cryotherapy, interferential electrical stimulation, and taping are also provided to the patient. Physical therapists may also perform specific exercises such as vastus medialis oblique retraining and iliotibial band stretching. One Level-of-Evidence I study concerning one-to-one therapy was included in this review (Table 3).
Results in the literature
The efficacy of other forms of OPT therapy have also been evaluated during the past five years. In a multicenter RCT, Ko et al. evaluated whether center-based, one-to-one physical therapy provided superior functional and physical outcomes when compared to group-based therapy or a monitored home-based program (38). The patients in all three treatment arms began their respective rehabilitation therapies two weeks post-surgery. Specifically, patients in the one-to-one therapy arm (n=85) performed two center-based sessions guided by a therapist, and two home-based sessions each week for six weeks. This group received manual therapy, therapeutic modalities, lower extremity stretching, and specific exercises aimed at retraining the quadriceps. Patients in group-based therapy (n=84) received two sessions of 50-minute circuit training and two home-based sessions. Patients performed stairs and balance retraining, full-body exercises, and aerobic activities. Patients in the monitored home program arm (n=80) performed four home-based sessions each week. The exercises included warm-up and cool-down components, seven functional exercises, an outdoor walking or stationary cycling component, and muscle stretches.
Oxford Knee Score (OKS) at 10 weeks was similar for all three groups (one-to-one: 32 vs. group-based: 36 vs. home-based: 34; P=0.20). There also were no differences in 6MW (one-to-one: 397.5 m vs. group-based: 405 m vs. home-based: 425 m; P=0.37), WOMAC function (one-to-one: 16 vs. group-based: 13 vs. home-based: 14; P=0.15), WOMAC pain (one-to-one: 3.8 vs. group-based: 1.6 vs. home-based: 2.5; P=0.79), flexion (one-to-one: 115° vs. group-based: 110° vs. home-based: 116°; P=0.45), extension (one-to-one: 4° vs. group-based: 4° vs. home-based: 5°; P=0.91), and quadriceps lag (one-to-one: 1° vs. group-based: 2° vs. home-based: 1°; P=0.57).
Summary
Clinic-based rehabilitation is a common practice for the majority of patients undergoing TKA for a duration of 6 to 8 weeks post-discharge (39). Yet, this study assessed one-to-one therapy and found that other forms, such as home-based therapy and group therapy, are just as efficient in improving postoperative TKA outcomes. According to this study, the monitored home program following TKA yields similar results to one-to-one therapy and group-based therapy. This study demonstrates comparable results to older smaller sampled studies which assessed various forms of HEPs with some degree of monitoring (40,41). However, these older studies acknowledge that, for ethical reasons, those patients in the HEP programs making unsatisfactory progress were recommended to attend additional therapy and therefore may not represent exclusive home-based intervention. Nonetheless, there are added benefits to a monitored home program, particularly for patients. According to the study above, patients appreciated the convenience of a home program and found that the lack of traveling and parking fees were additional advantages. Typical 6-week driving restrictions require TKA patients to rely on others for travel, but these home-based programs seem to mitigate this matter. Despite potential cost reductions with the implementation of both telerehabilitation and home-based therapies, the caveat with home programs is the lack of start-up costs associated with telecommunication technologies. A comparison of various forms of early HEPs can determine whether there is an optimal home-based method to maximize patient outcomes in the early postoperative period.
Inpatient rehabilitation
Rehabilitation protocol
The utilization of inpatient rehabilitation and therapy services received during a hospital stay following TKA varies internationally. Inpatient rehabilitation is uncommon in nations such as Canada and the United Kingdom. In contrast, Australia, Switzerland, and the United States commonly utilize this post-operative rehabilitation services (42). Hence, studies from several nations have made an effort to determine whether inpatient rehabilitation yields superior outcomes to other alternatives. One Level-of-Evidence I study and one Level-of-Evidence II study investigated the effectiveness of inpatient rehabilitation following TKA and were included in this review (Table 4).
Table 4. Inpatient therapy.
| Study | Level of evidence | Intervention cohort | Control cohort | Duration of follow-up | Outcomes, mean | ROM (degrees), mean (SD) |
|---|---|---|---|---|---|---|
| Buhagiar et al. | I | 10-day Inpatient PT (twice-daily supervised sessions and class-based exercises) and home-based (n=81) | Monitored home-based (n=84) | 26 wks | 6MW: inpatient: 316.8 m vs. home: 318.8 m; P>0.05 | N/A |
| OKS: inpatient: 17.4 vs. home: 16.7; P>0.05 | ||||||
| EQ-5D VAS: inpatient: 66.3 vs. home: 64.0; P>0.05 | ||||||
| Naylor et al. | II | Inpatient PT (n=129) | No inpatient PT (n=129) | 90 days | OKS¥: 0; P=0.54 | N/A |
| EQ-VAS¥: −2.5; P=0.09 | ||||||
| 365 days | OKS¥: 0; P=0.40 | N/A | ||||
| EQ-VAS¥: 0; P=0.32 |
¥, median difference. ROM, range of motion; PT, physical therapy; 6MW, 6-minute walk test; OKS, Oxford Knee Score; EQ-5D VAS, EuroQol Group 5-Dimension Self Report Questionnaire; EQ-VAS, EuroQol Visual Analogue Scale; SD, standard deviation.
Results in the literature
Buhagiar et al. conducted a multicenter RCT to determine whether a 10-day inpatient program was superior to a monitored home program by evaluating a total 165 patients. One group received inpatient hospital rehabilitation and home-based rehabilitation (n=81), while the other group only received home-based rehabilitation (n=84) (42). Inpatient rehabilitation consisted of twice-daily supervised sessions of 60–90 minutes of physical therapy and 60–90 minutes of class-based exercises. The home program involved the routine care provided at the hospital, with exercises focused on restoring knee mobility, lower limb strength, normal neuromuscular coordination, and gait patterns. All exercises were rehearsed and individualized due to comorbidities. Twenty-six weeks after surgery, the authors found no significant difference in patient satisfaction, complication rates, 6MW (inpatient: 316.8 m vs. home: 318.8 m), OKS (inpatient: 17.4 vs. home: 16.7), or EuroQol Group 5-Dimension Self Report Questionnaire (EQ-5D) VAS (inpatient: 66.3 vs. home: 64.0) between the inpatient rehabilitation and the home-based program. It was concluded that inpatient rehabilitation did not improve mobility at 26 weeks postoperatively compared with a monitored home-based rehabilitation program.
In 2017, Naylor et al. prospectively compared the effectiveness of an inpatient rehabilitation pathway for privately insured TKA patients (5). Patients were propensity-score matched based on whether they received inpatient rehabilitation or not. A total of 258 patients were divided into pairs according to their susceptibility scores for undergoing inpatient rehabilitation. Covariates such as age, sex, body mass index (BMI), and markers of health impairment were also used to pair patients. The authors found no difference at 90 and 365 days in OKS scores (0 and 0 median difference; P=0.54 and P=0.40, respectively) and EuroQol Visual Analogue Scale for “today” health (EQ-VAS) (−2.5 and 0 median difference; P=0.09 and P=0.32, respectively). Additionally, they found that the EuroQol health scores were significantly worse on day 35 for patients who participated in inpatient rehabilitation (-5 median difference; P=0.01).
Summary
Inpatient rehabilitation is one of many current forms of rehabilitation employed today. The aforementioned studies concluded that inpatient rehabilitation does not yield superior results when compared to community- or home-based therapy following TKA. Thorough cost analyses have shown that inpatient therapy substantially increases costs. Hence, given the high cost differential and no difference in outcomes, inpatient therapy looks to be an expensive intervention that does not provide additional benefit. These studies had appropriate sample sizes to demonstrate a statistical difference and may therefore be suitable to draw conclusions and incline providers to refer patients to community- or home-based therapy rather than inpatient rehabilitation. Of all 20 studies included in this review, patient satisfaction was only measured in one study of this subgroup. Healthcare systems are currently placing more weight on patient satisfaction particularly because reimbursement is more commonly linked to the patient experience. This metric is therefore a valuable endpoint, pertinent in these analyses and should be included in upcoming postoperative rehabilitation studies.
Early rehabilitation
Rehabilitation protocol
Early postoperative rehabilitation has been defined in various ways. While some studies define this intervention within 6 weeks of surgery, others have introduced the concept of rapid rehabilitation (43). This form of treatment is referred to rehabilitation therapies that begin in the post anesthesia care unit (PACU) on POD 0. Patients treated with RR perform bed mobility exercises with appropriate assistance, which include rolling to either side, scooting up and down, and moving from the supine position to the sitting position. The principle goal with RR is to achieve ambulation of 10 feet with appropriate assistance and a rolling walker. In contrast, conventional in-hospital rehabilitation typically begins on POD 1 with similar goals, but ambulation of 40-80 feet with moderate assistance with a rolling walker is encouraged. Previous studies have demonstrated a reduction in LOS in TKA patients undergoing RR (Pagnotta’s review). In this systematic review, two Level-of-Evidence I and one Level-of-Evidence III studies on this topic have been included (Table 5).
Table 5. Early therapy.
| Study | Level of evidence | Intervention cohort | Control cohort | Duration of follow-up | Outcomes, mean | ROM (degrees), mean |
|---|---|---|---|---|---|---|
| Pagnotta et al. | I | RR: PT on POD 0 (n=30) | Non-RR: PT on POD 1 (n=45) | 12 wks | LOS: RR: 3.1 days vs. non-RR: 3.6 days; P<0.05 | N/A |
| KOOS: RR: 57.33; 95% CI: 29.32 to 85.4 vs. non-RR: 77.14; 95% CI: 68.54 to 85.74 | ||||||
| McGinn et al. | III | OPT within 6 wks (n=411) | OPT after 6 wks (n=74) | 8.2 mos (mean) | N/A | Extension: before6 wks: 0.7 vs. after 6 wks: 1.5; P=0.019 |
| Flexion: before 6 wks: 114 vs. after 6 wks: 111; P=0.008 | ||||||
| Han et al. | I | HEP (n=194) | Usual care (OPT) (n=196) | 6 wks | WOMAC pain: HEP: 7.2 vs. OPT: 7.4; P>0.05 | Extension: HEP: −6.3 vs. OPT: −6.5; P>0.05 |
| WOMAC physical function: HEP: 22.4 vs. OPT: 22.5; P>0.05 | Flexion: HEP: 96.8 vs. OPT: 95.7; P>0.05 | |||||
| 50-foot walk time: HEP: 12.9s vs. OPT: 12.9 s; P>0.05 | ||||||
| Readmissions: HEP: 9% vs. OPT: 7%; P>0.05 |
POD, postoperative day; ROM, range of motion; WOMAC, Western Ontario and McMaster Universities Arthritis Index; PT, physical therapy; OPT, outpatient physical therapy; KOOS, Knee Injury and Osteoarthritis Outcome Score; RR, rapid rehabilitation; LOS, length of stay; HEP, Home Exercise Program; SD, standard deviation.
Results in the literature
In their RCT, Pagnotta et al. assessed the effect of rapid rehabilitation on LOS and functional outcomes at 4 and 12 weeks following TKA (43). Patients were divided into two groups according to the time the procedure took place. The experimental group (n=30) contained patients whose surgery occurred earlier in the day, which allowed for a session of RR. Patients whose surgeries were scheduled later in the day were assigned to the control group (n=45). In addition to bed mobility exercises, the patients of the RR group worked on transferring (from sitting to standing and vice versa), both with the appropriate amount of assistance. Non-RR patients began therapy on POD 1 based on the institution’s clinical pathway. The authors found a significant difference in average LOS between the RR and non-RR groups (RR: 3.1 days vs. non-RR: 3.6 days; P<0.05). At 4 and 12 weeks postoperatively, KOOS scores did not differ significantly between the groups (RR: 57.33; 95% CI: 29.32 to 85.4 vs. non-RR: 77.14; 95% CI: 68.54 to 85.74).
While Pagnotta et al. focused on LOS and functional outcomes, McGinn et al. retrospectively analyzed ROM following TKA (44). The authors divided the patients according to the initiation of the outpatient physical therapy (OPT), those who started OPT within 6 weeks of TKA (n=411), and those who began after 6 weeks (n=74). The authors reported that those who started OPT within 6 weeks had a significantly higher mean flexion compared to those who started OPT after 6 weeks (114° vs. 111°; P=0.008). Those who started OPT within 6 weeks were found to have significantly lower extension compared to those who started PT after 6 weeks (0.7° vs. 1.5°; P=0.019).
Han et al. also assessed the efficacy of early rehabilitation (45). The patients randomized to a home exercise program (HEP) (n=194) were compared to a usual care group (n=196) which had access to clinic-based outpatient physiotherapy (not otherwise explained). Patients in the early rehabilitation group received a HEP where weeks 1−2 focused on achieving full active and passive ROM while weeks 3−6 focused on functional and WB exercises for strength. At the 6-week postoperative visit, the authors found no difference between the HEP and usual care groups for WOMAC pain (7.2 vs. 7.4) and physical function scores (22.4 vs. 22.5), ROM (96.8° vs. 95.7° flexion; −6.3° vs. −6.5° extension), 50-foot walk time (12.9 vs. 12.9 s), or adverse events (9% vs. 7% readmissions; P>0.05 for all).
Summary
Despite the adoption of early rehabilitation, its definition encompasses several approaches. Early rehabilitation can range from POD 0 up to the six-week benchmark. While the above studies evaluated different variables regarding early rehabilitation benefits, they demonstrate that this strategy decreases LOS and allows better ROM and decreased stiffness. For these reasons, earlier intervention seems appealing and beneficial for TKA patients. Some experts have voiced their concerns regarding patient recovery and potential adverse events if rehabilitation is commenced too early (43). Earlier rehabilitation does not increase readmission rates, but rapid rehabilitation should be further assessed for safety (43). One major difference to point out between these studies is their study design. In the one retrospective review, demographic characteristics such as body mass index (BMI) and age were not analyzed as potential confounders, which is limitation the authors described.
Miscellaneous
Rehabilitation protocol
Other studies evaluating the impact of weight-bearing biofeedback (WBB), neuromuscular electrical stimulation (NMES), and balance control are described in this section. Postoperative TKA patients are susceptible to poor gait mechanics, which is a result of asymmetrical lower extremity movement patterns (46-49). The discrepancy in limb movement can lead to long-term functional limitations, and WBB has emerged as a symmetry retraining technique to improve long-term performance (50).
Another noninvasive modality is NMES, which uses dosed electrical currents to evoke visible tetanic muscle contractions (51). The electrical current is delivered through cutaneous electrode pads to the neuromuscular junction and surrounding muscle fibers. This method was implemented in postoperative rehabilitation because quadriceps strength can decrease by up to 62% after TKA (52). Though this weakness can persist for years, NMES has been shown to increase quadriceps activation and strength post-surgery (53).
Finally, balance control plays another integral role in postoperative TKA rehabilitation. Specifically, standing balance can be compromised in elderly patients who suffer from knee osteoarthritis. Reports have demonstrated that 25% of TKA recipients fall within two years of surgery (54). As such, balance exercises are currently incorporated in rehabilitation programs to challenge stability. These exercises involve movements such as twisting, turning, and walking on uneven surfaces. Three Level-of-Evidence I studies are analyzed below (Table 6).
Table 6. Miscellaneous.
| Study | Level of evidence | Intervention cohort | Control cohort | Duration of follow-up | Outcomes, mean (SD) | ROM (degrees), mean (SD) |
|---|---|---|---|---|---|---|
| Christensen et al. | I | WBB: supervision while playing the Nintendo Wii Fit Plus game and HEP for 6 wks (n=13) | No-WBB: HEP for 6 wks (n=13) | 6 wks | FTSST: WBB: 11.5 s (1.6) vs. no-WBB: 12.7 s (3.3); P=0.021 | N/A |
| 26 wks | FTSST: WBB: 9.5s (2.4) vs. no-WBB: 9.6s (1.6); P=0.021 | N/A | ||||
| Walking speed: WBB: 1.29 m/s (0.25) vs. no-WBB: 1.24 m/s (0.13); P=0.068 | ||||||
| Knee Movement: WBB: 0.61 Nm/kg (0.25) vs. no-WBB: 0.42 Nm/kg (0.44); P=0.008 | ||||||
| Levine et al. | I | NMES without supervision for 14 days preop and 60 days postop and ROM (n=35) |
No-NMES: ROM and PRE with supervision (n=35) | 6 mos | GUG: NMES: 10.64 s (2.88) vs. no-NMES: 10.25 s (2.11); 95% CI: −1.068 to 1.848 | Extension: NMES: 2.58 (2.86) vs.
no-NMES: 3.54 (6.05); 95% CI: −3.73 to 1.797 |
| WOMAC: NMES: 86.61 (14.68) vs. no-NMES: 80.8 (16.95); 95% CI: −3.19 to 14.81 | Flexion: NMES: 114.5 (13.01) vs. no-NMES: 112.2 (10.56); 95% CI: −4.44 to 9.1 | |||||
| KSS pain: NMES: 79.08 (10.97) vs. no-NMES: 75.5 (14.77); 95% CI: −3.78 to 10.93 | ||||||
| KSS function: NMES: 80.0 (18.6) vs. no-NMES: 72.08 (18.23); 95% CI: −2.57 to 18.4 | ||||||
| Jogi et al. | I | Balance and ROM and strengthening for 5 wks (n=30) | No-balance: ROM and strengthening for 5 wks (n=33) | 5 wks | BBS: balance: 53 [6] vs. no-balance: 48 [6]; P>0.05 | N/A |
| WOMAC function: balance: 14 [13] vs. no-balance: 16 [10]; P>0.05 | ||||||
| ABC: balance: 74 [23] vs. no-balance: 69 [24]; P>0.05 | ||||||
| TUG: balance: 13 [4] vs. no-balance: 15 [5]; P<0.05 |
ROM, range of motion; WOMAC, Western Ontario and McMaster Universities Arthritis Index; TUG, timed up and go; PRE, Progressive Resistance Exercise; Nm/kg, Newton-meters/kg; KSS, Knee Society Score; OKS, Oxford Knee Score; HEP, Home Exercise Program; WBB, weight-bearing biofeedback; NMES, Neuromuscular Electrical Stimulation; FTSST, five times sit-to-stand test; GUG, get-up-and-go; BBS, Berg Balance Scale; ABC, activities-specific balance confidence; SD, standard deviation.
Results in the literature
In a RCT, Christensen et al. evaluated the effects of WBB exercises on functional movement patterns in TKA patients (55). Patients who received daily biofeedback therapy and a standard HEP for six weeks (n=13) were compared to patients who received a daily standard HEP also for six weeks (n=13). Patients in the WBB group received a Nintendo Wii system with the Nintendo Wii Fit Plus game and were supervised by a physical therapist who provided feedback while they performed tasks in the game. The WBB group showed greater improvement in the Five Times Sit-to-Stand Test (FTSST) time at 6 weeks (11.5±1.6 vs. 12.7±3.3 s; P=0.021) and at 26 weeks (9.5±2.4 vs. 9.6±1.6 s; P=0.021) when compared to the control group. Both the WBB and the control groups showed improvement in walking speed (1.29±0.25 vs. 1.24±0.13 m/s; P=0.068), but the WBB group obtained greater knee movement (0.61±0.25 vs. 0.42±0.44 Nm/kg; P=0.008) at 26 weeks.
In their RCT, Levine et al. compared NMES to conventional physical therapy (53). Patients in the NMES group (n=35) performed ROM exercises and utilized a NMES machine without supervision for 14 days preoperatively and 60 days postoperatively. Patients in the control group (n=35) performed ROM and PREs while hospitalized and after discharge under the direct supervision of a physical therapist. The authors found no difference at 6 months between the NMES and control groups for flexion (114.5°±13.01° vs. 112.2°±10.56°; 95% CI: −4.44 to 9.1), extension (2.58°±2.86° vs. 3.54°±6.05°; 95% CI: −3.73 to 1.797), Get-Up-and-Go (GUG) (10.64±2.88 vs. 10.25±2.11 s; 95% CI: −1.068 to 1.848), WOMAC (86.61±14.68 vs. 80.8±16.95; 95% CI: −3.19 to 14.81), KSS pain (79.08±10.97 vs. 75.5±14.77; 95% CI: −3.78 to 10.93), and KSS function (80.0±18.6 vs. 72.08±18.23; 95% CI: −2.57 to 18.4).
The final study of this review was conducted by Jogi et al. to evaluate the effectiveness of balance exercises postoperatively (54). Patients in the balance group (n=30) completed daily ROM, muscle strengthening, and balance exercises for a five-week period while the control group (n=33) only completed daily ROM and muscle strengthening exercises. Both groups initiated rehabilitation 7−10 days after surgery. After five weeks of rehabilitation, the authors found no difference between the balance and control group in the Berg Balance Scale (BBS) (53±6 vs. 48±6), WOMAC function (14±13 vs. 16±10), and Activities-specific Balance Confidence (ABC) scores (74±23 vs. 69±24) (P>0.05 for all). However, the TUG between the balance group and the control was significantly different (13±4 vs. 15±5; P<0.05).
Summary
Current rehabilitation protocols are diverse and WBB, NMES, and balance control are additional interventions briefly discussed in this review. The WBB intervention has shown to provide several benefits for the TKA population. Increased knee movement and FTSST times demonstrate that there may be potential for feedback mechanisms during rehabilitation. The use of a gaming system to observe and provide feedback in real-time is an appealing way to achieve better results. In time, these types of interactive tools may become more popular within this setting, therefore, these types of studies may begin to become more impactful in the scientific literature. Although these machines are costly, their utility in postoperative rehabilitation may be effective.
During the past several years, positive data on the use of NMES has come to light. Some studies have shown improvements in measures such as muscle strength, ROM and walking tests (56,57). Similarly, the study above demonstrates NMES use with unsupervised at-home ROM is equally effective to conventional rehabilitation with a licensed therapist, 6 months postoperatively. This method allowed patients to remain home during recovery, which has been shown to increase patient satisfaction in select patients. Additionally, quadriceps strength in the NMES group was not compromised, further suggesting this treatment has potential advantages. Thus, NMES may be another form of therapy that contributes to reducing costs associated with postoperative TKA care.
Balance exercises in rehabilitation programs can help decrease falls and the associated financial burden in TKA patients (58-60). Many facilities assess patient safety and mobility before discharge and in some circumstances, stability exercises can help reduce LOS. Therefore, incorporating balance exercises in the immediate postoperative period may be of benefit to patients as well as healthcare facilities. The aforementioned study assessed balance training and the results mirror the literature, as other studies note that walking capacity, balance-specific, and functional outcomes are improved with balance therapy (61). The balance therapy was initiated about a week after surgery. This timing slightly differs to other studies where the earliest intervention was started two weeks after TKA (62,63). Also, the recommendation for balance therapy is at least eight weeks, but this study showed improvement in outcomes by the fifth week postoperatively (63).
Conclusions
Postoperative rehabilitation following TKA facilitates patient recovery and improves quality of life. Despite the numerous postoperative rehabilitation modalities currently employed, there remains a lack of consensus regarding their duration, intensity, and delivery. Consequently, evidence-based practice guidelines remain absent. This systematic review analyzed the existing rehabilitation protocols from the past five years. Our findings demonstrated that continuous passive motion and inpatient rehabilitation may not provide additional benefit to the patient or healthcare system. However, early rehabilitation, telerehabilitation, outpatient therapy, high intensity, and high velocity exercise may be successful forms of rehabilitation. Additionally, weight-bearing biofeedback, neuromuscular electrical stimulation, and balance control appear to be beneficial adjuncts to conventional rehabilitation. Some studies did not accurately describe the conventional rehabilitation protocols, the duration of therapy sessions, and the timing of these sessions. As such, future studies should explicitly describe their methodology. This will allow high-quality assessments and the conception of standardized protocols.
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: Dr. Delanois reports other from Baltimore City Medical Society, other from Orthofix, Inc, other from Stryker, other from United orthopedics, other from Flexion Therapeutics, other from Tissue Gene, outside the submitted work. All other authors have no conflicts of interest to declare.
References
- 1.Culliford D, Maskell J, Judge A, et al. Future projections of total hip and knee arthroplasty in the UK: results from the UK Clinical Practice Research Datalink. Osteoarthr Cartil 2015;23:594-600. 10.1016/j.joca.2014.12.022 [DOI] [PubMed] [Google Scholar]
- 2.Culliford DJ, Maskell J, Beard DJ, et al. Temporal trends in hip and knee replacement in the United Kingdom: 1991 to 2006. J Bone Joint Surg Br 2010;92:130-5. 10.1302/0301-620X.92B1.22654 [DOI] [PubMed] [Google Scholar]
- 3.Kurtz SM, Ong KL, Lau E, et al. International survey of primary and revision total knee replacement. Int Orthop 2011;35:1783-9. 10.1007/s00264-011-1235-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurtz SM, Ong KL, Lau E, et al. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am 2014;96:624-30. 10.2106/JBJS.M.00285 [DOI] [PubMed] [Google Scholar]
- 5.Naylor JM, Hart A, Mittal R, et al. The value of inpatient rehabilitation after uncomplicated knee arthroplasty: a propensity score analysis. Med J Aust 2017;207:250-5. 10.5694/mja16.01362 [DOI] [PubMed] [Google Scholar]
- 6.Bade MJ, Kohrt WM, Stevens-Lapsley JE. Outcomes before and after total knee arthroplasty compared to healthy adults. J Orthop Sports Phys Ther 2010;40:559-67. 10.2519/jospt.2010.3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winters JD, Christiansen CL, Stevens-Lapsley JE. Preliminary investigation of rate of torque development deficits following total knee arthroplasty. Knee 2014;21:382-6. 10.1016/j.knee.2013.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens-Lapsley JE, Balter JE, Kohrt WM, et al. Quadriceps and hamstrings muscle dysfunction after total knee arthroplasty. Clin Orthop Relat Res 2010;468:2460-8. 10.1007/s11999-009-1219-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcus RL, Yoshida Y, Meier W, et al. An Eccentrically Biased Rehabilitation Program Early after TKA Surgery. Arthritis 2011;2011:353149. [DOI] [PMC free article] [PubMed]
- 10.Pozzi F, Snyder-Mackler L, Zeni J. Physical exercise after knee arthroplasty: a systematic review of controlled trials. Eur J Phys Rehabil Med 2013;49:877-92. [PMC free article] [PubMed] [Google Scholar]
- 11.Mistry JB, Elmallah R, Bhave A, et al. Rehabilitative Guidelines after Total Knee Arthroplasty: A Review. J Knee Surg 2016;29:201-17. 10.1055/s-0036-1579670 [DOI] [PubMed] [Google Scholar]
- 12.Doerfler D, Gurney B, Mermier C, et al. High-Velocity Quadriceps Exercises Compared to Slow-Velocity Quadriceps Exercises Following Total Knee Arthroplasty. J Geriatr Phys Ther 2016;39:147-58. 10.1519/JPT.0000000000000071 [DOI] [PubMed] [Google Scholar]
- 13.Moffet H, Tousignant M, Nadeau S, et al. In-Home Telerehabilitation Compared with Face-to-Face Rehabilitation After Total Knee Arthroplasty: A Noninferiority Randomized Controlled Trial. J Bone Joint Surg Am 2015;97:1129-41. 10.2106/JBJS.N.01066 [DOI] [PubMed] [Google Scholar]
- 14.Okike K, Kocher MS. Evidence-Based Orthopaedics: Levels of Evidence and Guidelines in Orthopaedic Surgery. 2011. Available online: https://pdfs.semanticscholar.org/1213/9f1c826b41df891e5b8024f0ae048d4a8719.pdf
- 15.Ritter MA, Gandolf VS, Holston KS. Continuous passive motion versus physical therapy in total knee arthroplasty. Clin Orthop Relat Res 1989;(244):239-43. [PubMed] [Google Scholar]
- 16.Harvey LA, Brosseau L, Herbert RD. Continuous passive motion following total knee arthroplasty in people with arthritis. Cochrane Database Syst Rev 2014;(2):CD004260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mau-Moeller A, Behrens M, Finze S, et al. The effect of continuous passive motion and sling exercise training on clinical and functional outcomes following total knee arthroplasty: a randomized active-controlled clinical study. Health Qual Life Outcomes 2014;12:68. 10.1186/1477-7525-12-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romness DW, Rand JA. The role of continuous passive motion following total knee arthroplasty. Clin Orthop Relat Res 1988;(226):34-7. [PubMed] [Google Scholar]
- 19.Pope RO, Corcoran S, McCaul K, et al. Continuous passive motion after primary total knee arthroplasty. Does it offer any benefits? J Bone Joint Surg Br 1997;79:914-7. 10.1302/0301-620X.79B6.0790914 [DOI] [PubMed] [Google Scholar]
- 20.Maniar RN, Baviskar J V., Singhi T, et al. To Use or Not to Use Continuous Passive Motion Post–Total Knee Arthroplasty. J Arthroplasty 2012;27:193-200.e1. 10.1016/j.arth.2011.04.009 [DOI] [PubMed] [Google Scholar]
- 21.Herbold JA, Bonistall K, Blackburn M, et al. Randomized controlled trial of the effectiveness of continuous passive motion after total knee replacement. Arch Phys Med Rehabil 2014;95:1240-5. 10.1016/j.apmr.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 22.Boese CK, Weis M, Phillips T, et al. The efficacy of continuous passive motion after total knee arthroplasty: a comparison of three protocols. J Arthroplasty 2014;29:1158-62. 10.1016/j.arth.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 23.Joshi RN, White PB, Murray-Weir M, et al. Prospective Randomized Trial of the Efficacy of Continuous Passive Motion Post Total Knee Arthroplasty: Experience of the Hospital for Special Surgery. J Arthroplasty 2015;30:2364-9. 10.1016/j.arth.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 24.Denis M, Moffet H, Caron F, et al. Effectiveness of continuous passive motion and conventional physical therapy after total knee arthroplasty: a randomized clinical trial. Phys Ther 2006;86:174-85. [PubMed] [Google Scholar]
- 25.Walker RH, Morris BA, Angulo DL, et al. Postoperative use of continuous passive motion, transcutaneous electrical nerve stimulation, and continuous cooling pad following total knee arthroplasty. J Arthroplasty 1991;6:151-6. 10.1016/S0883-5403(11)80010-0 [DOI] [PubMed] [Google Scholar]
- 26.Bennett LA, Brearley SC, Hart JAL, et al. A comparison of 2 continuous passive motion protocols after total knee arthroplasty: a controlled and randomized study. J Arthroplasty 2005;20:225-33. 10.1016/j.arth.2004.08.009 [DOI] [PubMed] [Google Scholar]
- 27.Johnson DP. The effect of continuous passive motion on wound-healing and joint mobility after knee arthroplasty. J Bone Joint Surg Am 1990;72:421-6. 10.2106/00004623-199072030-00016 [DOI] [PubMed] [Google Scholar]
- 28.Ververeli PA, Sutton DC, Hearn SL, et al. Continuous passive motion after total knee arthroplasty. Analysis of cost and benefits. Clin Orthop Relat Res 1995;(321):208-15. [PubMed] [Google Scholar]
- 29.Cram P, Lu X, Kates SL, et al. Total Knee Arthroplasty Volume, Utilization, and Outcomes Among Medicare Beneficiaries, 1991-2010. JAMA 2012;308:1227-36. 10.1001/2012.jama.11153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walsh M, Woodhouse LJ, Thomas SG, et al. Physical impairments and functional limitations: a comparison of individuals 1 year after total knee arthroplasty with control subjects. Phys Ther 1998;78:248-58. 10.1093/ptj/78.3.248 [DOI] [PubMed] [Google Scholar]
- 31.Orr R, de Vos NJ, Singh NA, et al. Power training improves balance in healthy older adults. J Gerontol A Biol Sci Med Sci 2006;61:78-85. 10.1093/gerona/61.1.78 [DOI] [PubMed] [Google Scholar]
- 32.Pozzi F, White DK, Snyder-Mackler L, et al. Restoring physical function after knee replacement: a cross sectional comparison of progressive strengthening vs standard physical therapy. Physiother Theory Pract 2018:1-12. [Epub ahead of print]. 10.1080/09593985.2018.1479475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly MA, Finley M, Lichtman SW, et al. Comparative Analysis of High-Velocity Versus Low-Velocity Exercise on Outcomes After Total Knee Arthroplasty: A Randomized Clinical Trial. J Geriatr Phys Ther 2016;39:178-89. 10.1519/JPT.0000000000000070 [DOI] [PubMed] [Google Scholar]
- 34.Bade MJ, Struessel T, Dayton M, et al. Early High-Intensity Versus Low-Intensity Rehabilitation After Total Knee Arthroplasty: A Randomized Controlled Trial. Arthritis Care Res (Hoboken) 2017;69:1360-8. 10.1002/acr.23139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chughtai M, Kelly JJ, Newman JM, et al. The Role of Virtual Rehabilitation in Total and Unicompartmental Knee Arthroplasty. J Knee Surg 2019;32:105-10. 10.1055/s-0038-1637018 [DOI] [PubMed] [Google Scholar]
- 36.Bini SA, Mahajan J. Clinical outcomes of remote asynchronous telerehabilitation are equivalent to traditional therapy following total knee arthroplasty: A randomized control study. J Telemed Telecare 2017;23:239-47. 10.1177/1357633X16634518 [DOI] [PubMed] [Google Scholar]
- 37.Klement MR, Rondon AJ, McEntee RM, et al. Web-Based, Self-Directed Physical Therapy After Total Knee Arthroplasty Is Safe and Effective for Most, but Not All, Patients. J Arthroplasty 2019;34:S178-82. 10.1016/j.arth.2018.11.040 [DOI] [PubMed] [Google Scholar]
- 38.Ko V, Naylor J, Harris I, et al. One-to-One Therapy Is Not Superior to Group or Home-Based Therapy After Total Knee Arthroplasty. J Bone Joint Surg Am 2013;95:1942-9. 10.2106/JBJS.L.00964 [DOI] [PubMed] [Google Scholar]
- 39.Mahomed NN, Davis AM, Hawker G, et al. Inpatient compared with home-based rehabilitation following primary unilateral total hip or knee replacement: a randomized controlled trial. J Bone Joint Surg Am 2008;90:1673-80. 10.2106/JBJS.G.01108 [DOI] [PubMed] [Google Scholar]
- 40.Kramer JF, Speechley M, Bourne R, et al. Comparison of clinic- and home-based rehabilitation programs after total knee arthroplasty. Clin Orthop Relat Res 2003;410:225-34. 10.1097/01.blo.0000063600.67412.11 [DOI] [PubMed] [Google Scholar]
- 41.Rajan RA, Pack Y, Jackson H, et al. No need for outpatient physiotherapy following total knee arthroplasty: a randomized trial of 120 patients. Acta Orthop Scand 2004;75:71-3. 10.1080/00016470410001708140 [DOI] [PubMed] [Google Scholar]
- 42.Buhagiar MA, Naylor JM, Harris IA, et al. Effect of Inpatient Rehabilitation vs a Monitored Home-Based Program on Mobility in Patients With Total Knee Arthroplasty: The HIHO Randomized Clinical Trial. JAMA 2017;317:1037-46. 10.1001/jama.2017.1224 [DOI] [PubMed] [Google Scholar]
- 43.Pagnotta G, Rich E, Eckardt P, et al. The Effect of a Rapid Rehabilitation Program on Patients Undergoing Unilateral Total Knee Arthroplasty. Orthop Nurs 2017;36:112-21. 10.1097/NOR.0000000000000325 [DOI] [PubMed] [Google Scholar]
- 44.McGinn T, Chughtai M, Khlopas A, et al. Early Outpatient Physical Therapy May Improve Range-of-Motion in Primary Total Knee Arthroplasty. J Knee Surg 2017;30:618-21. 10.1055/s-0037-1603793 [DOI] [PubMed] [Google Scholar]
- 45.Han ASY, Nairn L, Harmer AR, et al. Early rehabilitation after total knee replacement surgery: a multicenter, noninferiority, randomized clinical trial comparing a home exercise program with usual outpatient care. Arthritis Care Res (Hoboken) 2015;67:196-202. 10.1002/acr.22457 [DOI] [PubMed] [Google Scholar]
- 46.Boonstra MC, Schwering PJA, De Waal Malefijt MC, et al. Sit-to-Stand Movement as a Performance-Based Measure for Patients With Total Knee Arthroplasty. Phys Ther 2010;90:149-56. 10.2522/ptj.20090119 [DOI] [PubMed] [Google Scholar]
- 47.Hatfield GL, Hubley-Kozey CL, Astephen Wilson JL, et al. The Effect of Total Knee Arthroplasty on Knee Joint Kinematics and Kinetics During Gait. J Arthroplasty 2011;26:309-18. 10.1016/j.arth.2010.03.021 [DOI] [PubMed] [Google Scholar]
- 48.Mizner RL, Snyder-Mackler L. Altered loading during walking and sit-to-stand is affected by quadriceps weakness after total knee arthroplasty. J Orthop Res 2005;23:1083-90. 10.1016/j.orthres.2005.01.021 [DOI] [PubMed] [Google Scholar]
- 49.Yoshida Y, Zeni J, Snyder-Mackler L. Do patients achieve normal gait patterns 3 years after total knee arthroplasty? J Orthop Sports Phys Ther 2012;42:1039-49. 10.2519/jospt.2012.3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McClelland J, Zeni J, Haley RM, et al. Functional and biomechanical outcomes after using biofeedback for retraining symmetrical movement patterns after total knee arthroplasty: a case report. J Orthop Sports Phys Ther 2012;42:135-44. 10.2519/jospt.2012.3773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yue C, Zhang X, Zhu Y, et al. Systematic Review of Three Electrical Stimulation Techniques for Rehabilitation After Total Knee Arthroplasty. J Arthroplasty 2018;33:2330-7. 10.1016/j.arth.2018.01.070 [DOI] [PubMed] [Google Scholar]
- 52.Mizner RL, Petterson SC, Stevens JE, et al. Early quadriceps strength loss after total knee arthroplasty. The contributions of muscle atrophy and failure of voluntary muscle activation. J Bone Joint Surg Am 2005;87:1047-53. 10.2106/00004623-200505000-00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Levine M, McElroy K, Stakich V, et al. Comparing conventional physical therapy rehabilitation with neuromuscular electrical stimulation after TKA. Orthopedics 2013;36:e319-24. 10.3928/01477447-20130222-20 [DOI] [PubMed] [Google Scholar]
- 54.Jogi P, Overend TJ, Spaulding SJ, et al. Effectiveness of balance exercises in the acute post-operative phase following total hip and knee arthroplasty: A randomized clinical trial. SAGE open Med 2015;3:2050312115570769. 10.1177/2050312115570769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Christiansen CL, Bade MJ, Davidson BS, et al. Effects of Weight-Bearing Biofeedback Training on Functional Movement Patterns Following Total Knee Arthroplasty: A Randomized Controlled Trial. J Orthop Sports Phys Ther 2015;45:647-55. 10.2519/jospt.2015.5593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stevens-Lapsley JE, Balter JE, Wolfe P, et al. Early neuromuscular electrical stimulation to improve quadriceps muscle strength after total knee arthroplasty: a randomized controlled trial. Phys Ther 2012;92:210-26. 10.2522/ptj.20110124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Avramidis K, Karachalios T, Popotonasios K, et al. Does Electric Stimulation of the Vastus Medialis Muscle Influence Rehabilitation After Total Knee Replacement? Orthopedics 2011;34:175. 10.3928/01477447-20110124-06 [DOI] [PubMed] [Google Scholar]
- 58.Duncan PW, Chandler J, Studenski S, et al. How do physiological components of balance affect mobility in elderly men? Arch Phys Med Rehabil 1993;74:1343-9. 10.1016/0003-9993(93)90090-W [DOI] [PubMed] [Google Scholar]
- 59.Carroll NV, Slattum PW, Cox FM. The Cost of Falls Among the Community-Dwelling Elderly. J Manag Care Pharm 2005;11:307-16. 10.18553/jmcp.2005.11.4.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takura T, Miki K. The future of medical reimbursement for orthopedic surgery in Japan from the viewpoint of the health economy. J Orthop Sci 2016;21:273-81. 10.1016/j.jos.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 61.Doma K, Grant A, Morris J. The Effects of Balance Training on Balance Performance and Functional Outcome Measures Following Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. Sports Med 2018;48:2367-85. 10.1007/s40279-018-0964-7 [DOI] [PubMed] [Google Scholar]
- 62.Monticone M, Ferrante S, Rocca B, et al. Home-based functional exercises aimed at managing kinesiophobia contribute to improving disability and quality of life of patients undergoing total knee arthroplasty: a randomized controlled trial. Arch Phys Med Rehabil 2013;94:231-9. 10.1016/j.apmr.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 63.Roig-Casasús S, Blasco JM, López-Bueno L, et al. Balance Training With a Dynamometric Platform Following Total Knee Replacement: A Randomized Controlled Trial. J Geriatr Phys Ther 2018;41:204-9. 10.1519/JPT.0000000000000121 [DOI] [PubMed] [Google Scholar]