Abstract
Free-living nematodes, an ancient animal phylum of unsegmented microscopic roundworms, have successfully adapted to nearly every ecosystem on Earth: from marine and freshwater to land, from the polar regions to the tropics, and from the mountains to the ocean depths. They are globally the most abundant animals in sediments and soils. In the present article, we identify the factors that collectively explain the successful ecological proliferation of free-living nematodes and demonstrate the impact they have on vital sediment and soil processes. The ecological success of nematodes is strongly linked to their ability to feed on various food sources that are present in both sediments and soils, and to proliferate rapidly and survive in contrasting environmental conditions. The adaptations, roles, and behaviors of free-living nematodes have important implications for the resilience of sediments and soils, and for emergent animal communities responding to human alterations to ecosystems worldwide.
Keywords: adaptability, ecological success, free-living nematodes, sediments, soils
“If all the matter in the universe except the nematodes were swept away, our world would still be dimly recognizable, … We should find its mountains, hills, vales, rivers, lakes, and oceans represented by a thin film of nematodes.” This quote from Cobb (1914) refers to the remarkable ubiquity of free-living nematodes on Earth. Nematodes are an ancient animal phylum of unsegmented microscopic roundworms that are invisible to the naked human eye. They are the only group of multicellular animals (metazoans) that are pervasive in sediments and soils, where they often outnumber other animals (Heip et al. 1985, Bongers and Ferris 1999). Nematodes are found in habitats ranging from the deep oceanic trenches to mountain peaks. In shallow marine sediments, nematode numbers can reach 0.5 million to 5 million individuals per square meter (Soetaert et al. 2009). Although their abundance and individual body size decline with water depth (Soetaert et al. 1997), the relative abundance of free-living nematodes comes to dominate among metazoans as larger animals decline more steeply with water depth (Rex et al. 2006). The abundance of freshwater nematodes is generally lower, but can exceed 1 million individuals per square meter (Traunspurger et al. 2012) and also tends to decrease with water depth. In soils, the number of nematodes typically ranges from 2 million to 20 million individuals per square meter (Burges and Raw 1967), with more found in coarse soils and less in clay soils.
The more than 27,000 described nematode species, of which about 60% are parasitic (15% plant parasites, 45% animal parasites; Hugot et al. 2001), most likely represent only a small portion of the total phylum Nematoda (Barker 1998, Zhang 2013). Historically, more research has been conducted on parasitic than free-living nematodes, so the actual diversity of free-living nematodes is presumed to be substantially underestimated (Hugot et al. 2001, Appeltans et al. 2012). In this overview, we focus on the free-living nematodes—that is, nematodes that do not parasitize animals or plants but live in the space between sediment and soil particles.
In marine sediments, nematode diversity, expressed as the number of species, tends to be highest in sandy sediments and lower in mud (Heip et al. 1985). Nematode diversity in freshwater habitats is close to that of some estuaries but lower than reported for deep-sea habitats (Eyualem-Abebe et al. 2006). Temperate deciduous forests support the highest soil nematode diversity, followed by tropical rain forests and arable land (Song et al. 2017). The density and diversity of other abundant metazoans, including polychaetes and harpacticoid copepods in marine sediments and mites and collembolans in soils, are about an order of magnitude lower.
The body plan of free-living nematodes is simple. The intestine and gonad are surrounded by a strong and flexible body wall with dorsal and ventral longitudinal muscles. The body wall and the pressurized, fluid-filled body cavity act as an antagonist for muscle action and enable movement. This mode of locomotion restricts morphological diversity in nematodes that, for example, have never evolved locomotory appendages (e.g., fins, legs, or wings) as other successful animal phyla have (Kiontke and Fitch 2013). In the present article, we identify the environmental characteristics and gradients in which sediment- and soil-dwelling nematodes live; combine general properties of sediments and soils with some specific evolutionary, physiological, biological, and morphological characteristics of nematodes to identify the factors underlying the ecological success of this ancient phylum; and demonstrate the impact this has on important sediment and soil processes.
On the basis of our findings, we propose hypotheses for future cross-realm ecological research to better understand how organism interactions (including those between free-living nematodes and microorganisms) in sediments and soils affect ecosystem processes that are of global importance.
Ecological properties of sediments and soils
Throughout the evolutionary history of Earth, physical, chemical, and biological processes have modified bedrock or other kinds of deposits into habitats in which a wide range of organisms, including nematodes, could evolve (table 1). Nematodes use the pore spaces and channels between particles, because their small size generally limits their capacity to physically mix sediment and soil particles (but see Löhr and Kennedy 2015 for marine nematodes reworking oxygen-depleted sediments). Marine and freshwater nematodes move within interstitial pores that are permanently water filled, thereby affecting solute transport near sediment–water interfaces (Aller and Aller 1992). Soil nematodes live in water films surrounding soil particles and, depending on the level of saturation, may directly use surface tension forces for movement and indirectly benefit from the more rapid gaseous exchange within thin films (Yeates 2004).
Table 1.
Generalized properties of sediments and soils (Carr et al. 2003, Bergtold and Traunspurger 2004, Shurin et al. 2006, Zhu et al. 2006, Glud 2008, Grosberg et al. 2012, Hernandez et al. 2014, Knapp et al. 2017).
| Marine sediment | Freshwater sediment | Soil | |
|---|---|---|---|
| Composition of top layer | 20%–60% solid, 40%–80% liquid | 20%–60% solid, 40%–80% liquid | Approximately 50% solid, 25% liquid, 25% gas |
| Temperature | Limited seasonal fluctuations and latitudinal gradients in subtidal environments (4°C–6°C, 700–1200 m throughout most oceans); intertidal areas can reach temperatures of more than 30°C in summer and below 0°C in winter | Limited seasonal fluctuations and moderate latitudinal gradients (5°C and 8°C at 16 m water depth in temperate lake in Germany, almost constant 14°C at 50 m water depth in tropical lake (Mexico) | Often large diurnal and seasonal fluctuations and latitudinal gradients (from –30°C in the arctic to 40°C in the tropics) |
| Salinity | Electric conductivity approximately 50 dS per m; typically, 30–40 g NaCl per l; site specific; fairly constant over time | Electric conductivity below 0.56 dS per m in temperate lake (Germany), 13 dS per m in tropical lake (Mexico); site specific; fairly constant over time | Electric conductivity approximately 1–15 dS per m; typically, up to 0.5 g NaCl per l; site specific; variable over time |
| Typical pH | Narrow range; pH 6.5–8.5; gradients of up to 2 pH units on a subcentimeter scale | Narrow range; pH 6.5–8.5 (temperate and tropical lakes) | Wide range; pH 3.5–9.0 |
| Redox zone | Oxic and suboxic in the approximately 10-cm top layer; vertical oxygen distribution strongly affected by the activity of fauna | Temperate lakes: small oxic and suboxic top layer; tropical lakes: often anoxic hypolimnia, top layer anoxic | Oxic and suboxic in 20–40 cm top layer (depends on soil texture); beneath 40 cm anoxic |
| Medium | Transport of materials and organisms by convective forces of waves and currents extends the spatial scale of many processes (generally less in freshwater than marine sediments) | Magnitude of the zone of capillary rise dependent on soil texture (shallower in sandy than in clay or peat soils); downward transport of materials and organisms mainly as a result of precipitation | |
Note: Given the wide variety of sediment and soil habitats, properties listed should not be taken as fixed characteristics for either of the three major habitat types. Abbreviations: °C, degrees Celsius; cm, centimeters; dS, decisiemens; g, grams; l, liters; m, meters, NaCl, sodium chloride.
Physical gradients are steeper and extremes greater in soils than in sediments because of the more complex distribution of short-lived water-filled pores and films that create many unique microhabitats. Nematodes can only migrate in soil through pores with a diameter of less than 30 micrometers (Jones 1975), and within the habitable pore space, their activity is affected by the water–air balance. Saturation and drought can result in reduced mobility and activity of nematodes (Neher 2010).
Free-living nematodes in sediments and soils
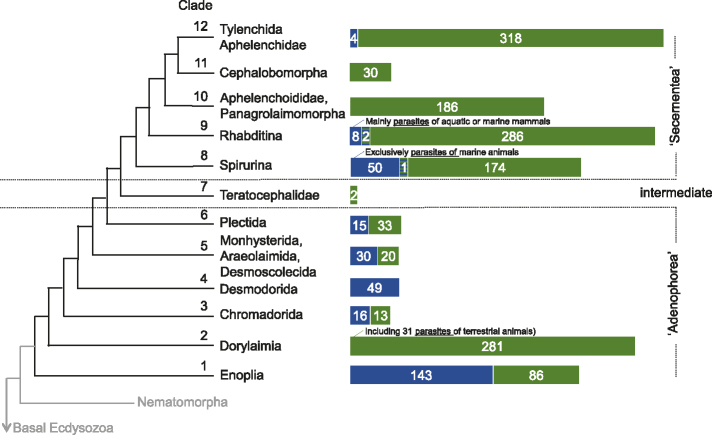
Nematodes evolved during the early Cambrian (525 million years ago; MYA) in marine habitats (Rota-Stabelli et al. 2013). Together with the arthropods (i.e., insects, spiders and crustaceans), nematodes belong to the Ecdysozoa, a superphylum that unites all molting invertebrates with over a million described species (Telford et al. 2008). Ecdysozoans have been key components of ecosystems since the Ediacaran period (approximately 587 MYA to 543 MYA). The colonization of land by different arthropod lineages occurred approximately 510 MYA to 471 MYA, and the radiation of extant nematodes and tardigrades (water bears) approximately 442 MYA (Rota-Stabelli et al. 2013). Even today, the most abundant metazoans on Earth are nematodes, whereas the most diverse are the insects (Schminke 2018). Twelve major clades have been distinguished within the phylum Nematoda (figure 1). Clades 1–6 and 8–12 correspond to classes formerly referred to as “Adenophorea” and “Secernentea,” respectively.
Figure 1.
Phylogenetic relationship between major nematode taxa based on full length small subunit ribosomal DNA sequences (e.g., Holterman 2008). The distribution of marine and terrestrial nematodes is indicated by blue and green bars, respectively. Striped blue or green bars indicate species occurring in both habitats. Numbers in bars refer to numbers of nematode taxa included in the analysis. Clade 7 consists of a single monogenic terrestrial nematode family, Teratocephalidae (here represented by two Teratocephalus species). Its members have a mixture of morphological characteristics that are considered to be typical for “Adenophorea” and “Secernentea” (e.g., Zhang and Baldwin 2001). Nematodes preferring very moist terrestrial habitats are often also found in freshwater sediments.
Diverse life styles
The life cycle of free-living nematodes is characterized by four molts, developing from a freshly hatched first or second stage juvenile, via two or three further juvenile stages, to adulthood (table 2). These basic characteristics are shared among all nematodes, irrespective of whether they live in the sediment or soil and are independent from their feeding ecology. Asexual reproduction (parthenogenesis) is prevalent in species inhabiting shallow water bodies or those subject to repeated drying and wetting (Wharton 1986). Life cycle stages often provide a means of surviving fluctuations in the environment. For example, different life stages can have distinct food preferences (Yeates 1987), and feeding strategies can be switched, depending on the availability of food sources (Buffan-Dubau and Carman 2000, Riera and Hubas 2003, Lebreton et al. 2012).
Table 2.
Generalized morphological, biological and physiological aspects of free-living nematodes in sediments and soils (Van de Velde and Coomans 1987, Bird and Bird 1991, Turpenniemi and Hyvarinen 1996, Traunspurger 2000, Eyualem-Abebe et al. 2008).
| Marine nematodes | Freshwater nematodes | Soil nematodes | |
|---|---|---|---|
| Morphology | Caudal adhesive glands present in most species with primary secretory function, to aid attachments of nematodes and eggs to substratum and agglutination of sediment particles in burrows | Unicellular caudal glands (up to 3) producing secretions that facilitate attachment to substratum | Epidermal and caudal adhesive glands generally absent |
| Reproduction | Mostly amphimictic (i.e., reproduction in which sperm and eggs come from separate individuals), fertilization by copulation | Mostly amphimictic, fertilization by copulation; occasionally parthenogenetic (i.e., asexual reproduction without fertilization); hermaphroditism is rare | Mostly amphimictic, fertilization by copulation, meiotic and mitotic parthenogenesis as well as self-fertilizing hermaphroditism are fairly common |
| Feeding | Feed on diverse food sources, including bacteria, microalgae, protozoans, small metazoans (including other nematodes); soil nematodes may also feed on fungi and higher and lower plants (including algae) | ||
| Chemosensory organs | Amphid (complex sense organ in the head region that is exposed to the external environment by a pore in the nematode cuticle) functions as primary chemoreceptor; amphids vary in shape and size | “Adenophorea”: Amphid similar to marine and freshwater nematodes but often smaller; phasmid absent “Secernentea”: Amphid; phasmid (complex sense organ in the tail region), similar to amphid but smaller | |
| Secretory–excretory (S–E) system | Relatively simple S–E system consisting of a single ventral gland cell, a renette, usually with a noncuticularized terminal duct; S–E system mainly involved in secretion of glycoproteins that coat the cuticle surface and act as a lubricant to assist movement | “Adenophorea”: Relatively simple S–E system similar to marine and freshwater nematodes “Secernentea”: More complex, tubular H-shaped S–E system with a cuticle-lined duct; S–E system mainly involved in osmotic regulation as well as secretion of glycoproteins | |
| Osmoregulation | Usually isosmotic to seawater; high cuticular permeability for water; species-specific differences in the efficiency and rate of osmoregulation | Slightly hyperosmotic to surroundings; high cuticular permeability for water | Hyperosmotic to surroundings; low cuticular permeability for water, slowing down the rate of water flux allows time for osmoregulatory mechanisms to operate |
Note: Given that phenomena such as convergent evolution, and secondary loss and gain are widespread among nematodes, attributes should not be taken as fixed characteristics for either of the three major habitat types.
Nematodes show a variety of adaptations to frequently changing or extreme environmental conditions. These include their protective cuticle, a flexible and chemically inert exoskeleton, and the ability to go into developmental dormancy and diapause (Dauer stage) as a strategy to survive adverse conditions including prolonged scarcity of food (McSorley 2003). A range of reproductive strategies allows nematodes to proliferate rapidly in changing and extreme environmental conditions.
The nematodes’ sensory organs (table 2) allow them to use primarily chemical but also electrical, light, mechanical, and temperature cues to orientate, move, locate a sexual partner, and forage successfully (Moens et al. 1999, Rasmann et al. 2012). Because roots produce and exude a great variety of compounds into the rhizosphere, chemoreception has particular relevance to the ecological proliferation of soil nematodes.
One might wonder whether high nematode diversity and ubiquitous distribution are the result of a few major habitat transitions followed by extensive diversification or the outcome of a high number of transitions followed by a lower degree of diversification. Using small subunit ribosomal DNA data on basal Chromadoria (clades 3 to 6; figure 1), Holterman and colleagues (2006) pinpointed a number of transitions from terrestrial to marine habitats and vice versa. Transitions (especially those from sea to land) occur at various taxonomic levels and are particularly prevalent in nematodes that possess a relatively simple secretory–excretory (S–E) system (“Adenophorea”; figure 1, table 2). The evolution of a more complex system with osmoregulatory function increases the tolerance of successful colonists to varying ionic compositions of their interstitial habitat, as is generally encountered in soils (“Secernentea”; table 2). However, Lee (1961) and Nkem and colleagues (2006) reported high osmotic stress survival rates of some terrestrial species with simple S–E systems of limited osmoregulatory capacity (“Adenophorea”; figure 1, table 2).
Position of nematodes in sediment and soil food webs
Soil and sediment food webs are predominately heterotrophic (i.e., rely on the external or allochtonous input of organic compounds, such as those obtained from plant or animal matter). Although shallow intertidal food webs are sites of high primary production (Herman et al. 2000, Christianen et al. 2017), organic matter mineralization often exceeds local production, because organic matter from the water column provides an additional organic input for the benthic food web. Some highly localized deep-sea ecosystems, such as cold seeps and hydrothermal vents, are partially supported by chemoautotrophy on the basis of oxidation of reduced compounds from the sea floor (Bell et al. 2017). However, the vast majority of the deep sea is heterotrophic (Smith et al. 2008).
In soils, the phototrophic (aboveground) and the heterotrophic (belowground) compartments are physically connected by plants (Wall 2007). Influx of carbon and energy is primarily provided directly by plants that, via their root systems, release substantial quantities of primary and secondary metabolites to the rhizosphere (Derrien et al. 2004). This boosts a selected part of the soil microbial community, mainly bacteria and fungi. Selectivity is often brought about by plant-family-specific categories of secondary metabolites. After senescence, the remaining plant biomass gradually feeds into the soil food web. The main primary decomposers in soil are bacteria and fungi. Generally, bacteria feed on relatively easily degradable parts of the (mainly plant derived) detritus pool, whereas fungi depolymerize recalcitrant polymers of plant origin such as cellulose, hemicellulose, pectin, and lignin.
Apart from coastal areas with macroalgae, mangroves, or seagrasses, there is no direct physical link between the phototrophic compartment—the euphotic zone—and the subseafloor. Sediment food webs are fueled by allochthonous organic matter (from land via rivers or from the sea via currents) and by internal or autochthonous pelagic and benthic primary production. The relative importance of organic carbon derived from pelagic and benthic primary production and from imported organic matter to sediment-dwelling heterotrophs is habitat-specific. In shallow coastal systems in which most of the seafloor lies within the photic zone, benthic photoautotrophy via submerged vascular plants (seagrasses), macroalgae, and benthic microalgae is high and typically exceeds that of phytoplankton (Underwood and Kromkamp 1999, Christianen et al. 2017). With the exception of chemosynthetic sites, such as hydrothermal vents and cold seeps, heterotrophic fauna in marine sediments is supported by sinking aggregates of microbe-colonized organic matter (Thornton 2002) that originates from surface production and sinks through the water column or is laterally advected (Ramirez-Llodra et al. 2010). As aggregates gradually sink through the water column and undergo decomposition by bacteria, the deposition of organic matter onto the seafloor is inversely related with water depth (Buesseler et al. 2007).
Although habitat dependent, influx of organic carbon into sediment or soil food webs can be categorized by orders of magnitude. The influx of organic carbon to deep-sea benthos, for example, is in the range of 1–10 grams of organic carbon (g Corg) per square meter (m2) per year (Glover and Smith 2003), whereas the input in shallow sediments can be up to 400 g Corg per m2 per year (Andersson et al. 2004). For terrestrial soils, carbon inputs can be up to two orders of magnitude greater than deep-sea benthos (e.g., on average 96 g Corg per m2 per year for restored native grasslands, Matamala et al. 2009; 58–132 g Corg per m2 per year for low productive coniferous forests, Leppälammi-Kujansuu et al. 2014).
Energy flow through the food web
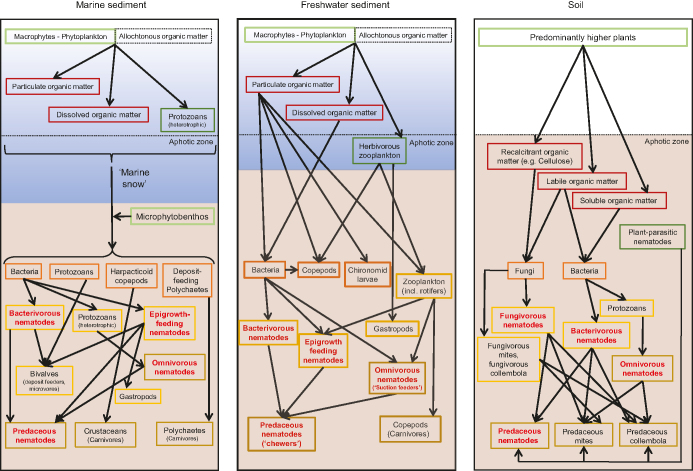
Organic matter decomposition in sediments and soils is a complex process, involving chemical alteration of organic matter, physical fragmentation, and finally release of mineral nutrients. Indirect interactions, such as bioturbation (the biological reworking of sediments and soils by burrowing animals and, in soils, rooting plants) and diseases caused by pathogens, or direct interactions, such as grazing and predation, result in flows of organic matter through subsurface food webs that are remarkably similar in sediments and soils (Krumins et al. 2013). Macroinvertebrates in sediments (e.g., polychaetes, crustaceans, gastropods, bivalves) and soils (e.g., termites, earthworms, millipedes) assist in the decomposition of organic matter by fragmenting it, thereby increasing the surface area and allowing microorganisms to colonize it, and by incorporating organic matter into the substrate, where conditions for microbial processes are favorable for decomposition (figure 2). This fragmentation is much less needed in marine systems as the primary producers (mostly algae) are already of microscopic size.
Figure 2.
Schematic diagram generalizing the complex trophic interactions between free-living nematodes and microorganisms, other meiofauna and macroinvertebrates in marine (left), freshwater (middle) and terrestrial (right) subsurface food webs. Dashed horizontal lines separate the (eu)photic and the aphotic zones. Diagrams focus on primary producers (light green box), primary consumers (dark green box), primary decomposers (brown box) and next trophic levels (yellow and orange box) in the food webs as far as micro- and meiofauna are concerned. Marine and freshwater herbivore nematodes are omitted, as are fungal decomposers in freshwater sediments, and megabenthic and vertebrate consumers across realms. Diagrams do not assign ecological importance to the illustrated trophic links; neither do all arrows imply a direct trophic interaction. For example, the trophic link between nematodes and organic material is mostly indirect—that is, nematodes feed primarily on the biofilm surrounding particles rather than digesting organic matter directly (see text). Given the wide variety of subsurface food webs, the diagrams should not be taken as fixed characteristics for either of the three major habitat types.
Smaller organisms such as rotifers, tardigrades and nematodes living in water (films) around sediment or soil particles or microarthropods such as springtails and mites living in air-filled pores in soils occupy diverse trophic positions in the food web (figure 2). In general, free-living nematodes feed on types of food sources that are available both in sediments and soils such as bacteria, protists, and other nematodes. At the top of the food web are predators, represented by species of macro- and microfauna, which feed on smaller invertebrates and protists. In both sediment and soil food webs, nematodes are present at various trophic levels. They occupy an intermediary position as consumer of a range of carbon sources and as a food source for secondary consumers (figure 2).
Catalytic effect of nematodes on microbe-mediated degradation and remineralization processes
The high density and species diversity of nematodes, their diversity in lifestyles (table 2), and their presence at various trophic levels (figure 2) collectively suggest that they play an important role in sediments and soils (Neher et al. 2012, Heidemann et al. 2014, Schratzberger and Ingels 2018). Through their biological activities (e.g., movement, ingestion and defecation of food particles, excretion of metabolic wastes), nematodes affect ecological processes both directly and indirectly. Central questions in sediment and soil ecology therefore relate to the types of interaction nematodes have with other organisms and how these relationships affect the regulation of ecosystem processes.
Experimental studies with nematodes estimate their direct importance to organic matter mineralization to be limited and their direct grazing rates on settled organic matter to be low in comparison to other biotic compartments (Verhoef and Brussard 1990, Schratzberger and Ingels 2018). We suggest in the present article that, quantitatively, the indirect roles of nematodes on nutrient mineralization in the food web are more relevant. Nematodes selectively graze microorganisms (resulting in an accelerated turnover of microbial cells) and inoculate substrate with microorganisms (figure 3). Increased mineralization observed in field situations and experimental treatments containing high nematode abundance is primarily a result, therefore, of nematodes feeding on and dispersing microorganisms that mediate organic matter mineralization and nutrient cycling (Nascimento et al. 2012). This has four important consequences:
Figure 3.
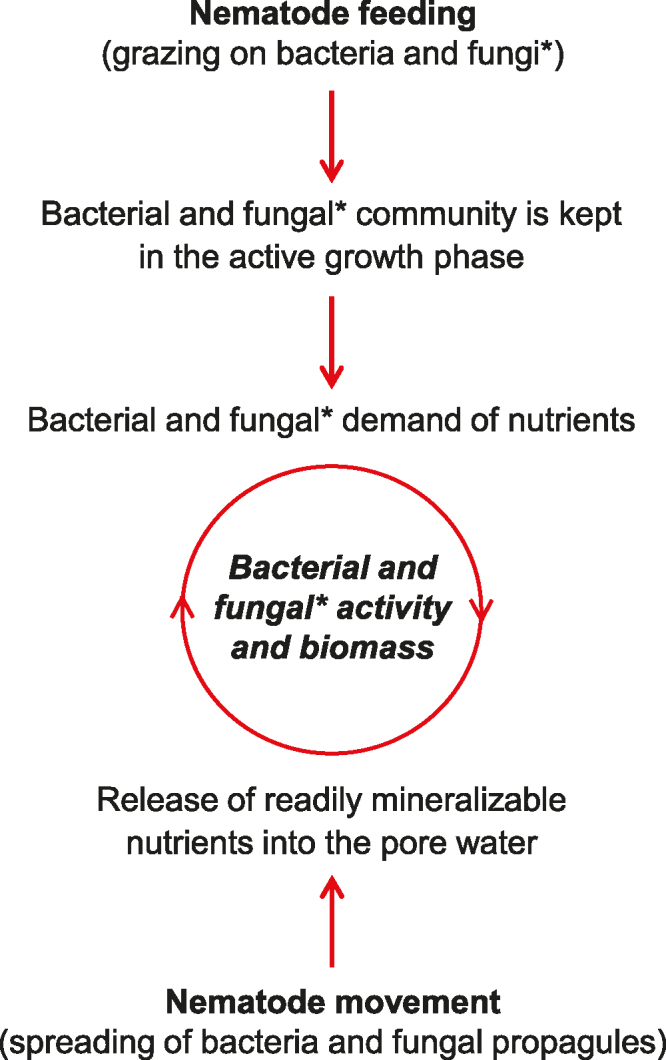
Schematic diagram illustrating the effects of free-living nematodes on microorganisms in sediments and soils. *Applies to soil and freshwater nematodes only.
First, the microbial community is kept in the active growth phase (Gerlach 1978), implying a higher demand of nutrients by microorganisms and therefore resulting in a higher decomposition rate of the organic matter. Ingham and colleagues (1985) and Gebremikael and colleagues (2016) demonstrated strong stimulatory effects of nematodes on bacteria- and fungi-mediated mineralization in soil microcosms. Similarly, Bonaglia and colleagues (2014) revealed a catalytic effect of marine nematodes on nitrifying and denitrifying bacteria in sediment microcosms.
Second, bacterivorous nematodes tend to have a higher carbon-to-nitrogen ratio than their microbial prey. In addition, the growth efficiency of nematodes is lower than that of bacteria. Consequently, nematodes ingest more nutrients than required, and the excess (following lysis of microbial cells) is excreted in a mineral or readily mineralizable form such as ammonium, phosphate, and sulphate into the pore water that otherwise would have been locked up in the microbial biomass. Bacteria, in contrast, usually respire most of the assimilated carbon and immobilize most of the assimilated nitrogen resulting in a significant contribution of nematodes to nitrogen mineralization compared to bacteria (Bonaglia et al. 2014, Gebremikael et al. 2016).
Third, microorganisms are redistributed to places rich in nutrients, thereby stimulating the growth and activity of those microorganisms that mediate organic matter mineralization and nutrient cycling (Gebremikael et al. 2016).
Finally, in marine sediments only, nematodes excrete large quantities of nitrogen in their mucus that is available for the microbial community or can bind to detritus (Riemann and Schrage 1978, Riemann and Helmke 2002, Moens et al. 2005). These excretions provide matrix and inorganic nutrients that are easily metabolized by microorganisms, thereby stimulating their activity and growth.
In decomposer food webs in sediments and soils, organic matter mineralization is affected by bacterivorous, fungivorous, and omnivorous nematodes feeding on microorganisms and excreting nutrients in excess of their metabolic need. The abundance and activity of these microbivorous nematodes may, in turn, be regulated by predatory nematodes, therefore preventing overgrazing by those groups and further controlling nutrient availability. At the same time, nutrients excreted by nematodes regulate microbial biomass and activity (figure 3). Without the continuous liberation of nutrients by nematodes via movement and feeding, microorganisms would be less able to compensate grazed biomass (de Ruiter et al. 1998).
Ecological flexibility pays off for nematodes
A suite of evolutionary and environmental processes has facilitated the diversification of nematodes (and other metazoans). These include vertical burrowing by animals, which enhanced oxygenation and primary productivity; the formation of new ecological links, which connected pelagic and benthic systems; and the advent of metazoan predation, which expanded food webs (Erwin et al. 2011, Sperling and Stockey 2018).
Nematodes arose as marine bacterivores in the oceans over 500 MYA (Erwin et al. 2011, Rota-Stabelli et al. 2013). Assuming that the physical and physiological adaptations required to live as a bacterivorous nematode in marine sediments are comparable to the adaptations needed to feed on bacteria in freshwater and terrestrial habitats, the collection and digestion of food would not constitute a major hurdle in the new habitat. If nematodes would have lived as primary decomposers on the seafloor, a major habitat transition would have required fundamental physiological adaptations to their new food source. The composition of organic material reaching the seafloor differs considerably from the composition of the organic matter in soils. The ability of free-living nematodes to feed on types of food that are available in both sediments and soils such as bacteria, protists, and other nematodes will have contributed to their proliferation.
We conclude that the nematode's selectivity of food sources available in both aquatic and terrestrial habitats, combined with their capacity to easily adapt to and survive in realm-specific environmental conditions (table 2), has contributed to their success in terms of abundance and diversity in sediments and soils worldwide.
Nematodes possess a range of physiological and life-history characteristics (table 2) that renders them less vulnerable to environmental change than larger fauna at higher trophic levels (figure 2). In both sediments and soils, predators of nematodes tend to be larger and have longer generation times or lower fecundity than their prey. The generally high fecundity of nematodes means that intermediate levels of disturbance often have only transitory effects on their populations. Compared with larger animals, the recolonization of severely disturbed sediments and soils by nematodes tends to proceed more rapidly.
Nematode adaptability (indicated by frequent habitat transitions, coupled with their resilience to environmental change) has important implications for the functioning of sediments and soils worldwide. Although empirical evidence on the specific roles of nematodes in most ecosystem processes is currently scarce, our overview suggests that nematode adaptations, roles, and behaviors have important implications for the resistance and resilience of sediments and soils to natural and anthropogenic change.
Conclusions
Managing sediments and soils is mostly a matter of maintaining suitable habitat for the many creatures that comprise their food webs, including free-living nematodes. Knowledge of sediments and soils has been hampered by difficulties in studying organisms in their natural environment (i.e., while keeping the complex structure of sediments and soils intact). Consequently, contemporary studies of sediment and soil ecology lag somewhat behind those of other subdisciplines of ecology.
In the present article, we highlighted strong parallels between organism interactions in sediments and soils, notably the accelerating effects interactions between free-living nematodes and microorganisms have on globally important ecosystem processes. Future hypothesis-driven research will need to determine how consistent and widespread these interactions are and test the importance of interactions among microorganisms relative to interactions among fauna and interactions between microorganisms and fauna; how interaction strength is linked to ecosystem processes, both qualitatively and quantitatively; whether the nature and strength of interactions is habitat specific; whether—and, if so, how—interactions vary along abiotic gradients; how interactions evolve under environmental stress; and which interactions are critical to the provision of benefits that soils and sediments provide to humans. Research under these headings would reduce the current disparity between what is known about free-living nematodes in sediments and soils relative to their importance in sustaining life on Earth.
Acknowledgements
This article is based on discussions during the Third International Symposium on Nematodes as Environmental Bioindicators. We are grateful to Thomae Kakouli-Duarte for bringing together scientists working across aquatic and terrestrial realms and to all symposium participants for sharing their knowledge freely. We thank Jeroen Ingles, Richard Warwick, and Ewan Hunter for their valuable comments on an earlier version of the manuscript and three anonymous reviewers for their constructive comments. DvO was supported by the Netherlands Organisation for Scientific Research (VIDI grant no. 864.13.007).
Notes
Author Biographical
Michaela Schratzberger is a principal marine ecologist at the Centre for Environment, Fisheries, and Aquaculture Science, in Lowestoft, United Kingdom, and is affiliated with the Collaborative Centre for Sustainable Use of the Seas, at the University of East Anglia's Norwich Research Park, in Norwich, United Kingdom. Martijn Holterman is a postdoctoral researcher at the Laboratory of Nematology of Wageningen University, in Wageningen, the Netherlands. Dick van Oevelen is a senior scientist at the Royal Netherlands Institute for Sea Research, in Yerseke. Johannes Helder is associate professor at the Laboratory of Nematology of Wageningen University, in Wageningen, the Netherlands.
References cited
- Abebe E, Decraemer W, De Ley P. 2008. Global diversity of nematodes (Nematoda) in freshwater. Hydrobiologia 595: 67–78. [Google Scholar]
- Abebe E, Traunspurger W, Michiels I. 2006. Dynamics of freshwater nematodes: Abundance, biomass, and diversity. Pages 77–93 in Abebe E, Andrássy I, Traunspurger W, eds. Freshwater Nematodes: Ecology and Taxonomy. CABI Publishing. [Google Scholar]
- Aller RC, Aller JY.. 1992. Meiofauna and solute transport in marine muds. Limnology and Oceanography 37: 1018–1033. [Google Scholar]
- Andersson JH, Wijsman JWM, Herman PMJ, Middelburg JJ, Soetaert K, Heip C. 2004. Respiration patterns in the deep ocean. Geophysical Research Letters 31: 1–4. [Google Scholar]
- Appeltans W et al.. 2012. The magnitude of global marine species diversity. Current Biology 22: 2189–2202. [DOI] [PubMed] [Google Scholar]
- Barker K. 1998. Introduction and synopsis of advancements in nematology. Agronomy 36: 1–20. [Google Scholar]
- Bell JB, Woulds C, Van Oevelen D. 2017. Hydrothermal activity, functional diversity and chemoautotrophy are major drivers of seafloor carbon cycling. Scientific Reports 7: 12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergtold M, Traunspurger W.. 2004. The benthic community in the profundal of Lake Brunnsee: Seasonal and spatial patterns. Archiv für Hydrobiologie 160: 527–554. [Google Scholar]
- Bird AF, Bird J.. 1991. Secretory–excretory system. Pages 167–182Bird AF, Bird J eds. Structure of Nematodes. Academic Press. [Google Scholar]
- Bonaglia S, Nascimento FJA, Bartoli M, Klawonn I, Brüchert V. 2014. Meiofauna increases bacterial denitrification in marine sediments. Nature Communications 5: 5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongers T, Ferris H.. 1999. Nematode community structure as a bioindicator in environmental monitoring. Trends in Ecology and Evolution 14: 224–228. [DOI] [PubMed] [Google Scholar]
- Buesseler KO et al.. 2007. An assessment of the use of sediment traps for estimating upper ocean particle fluxes. Journal of Marine Research 65: 345–416. [Google Scholar]
- Buffan-Dubau E, Carman KR.. 2000. Diel feeding behaviour of meiofauna and their relationships with microalgal resources. Limnology and Oceanography 45: 381–395. [Google Scholar]
- Burges A, Raw F.. 1967. Soil Biology. Academic Press. [Google Scholar]
- Carr MH, Neigel JE, Estes JA, Andelman S, Warner RR, Largier JL. 2003. Comparing marine and terrestrial ecosystems: Implications for the design of coastal marine reserves. Ecological Applications 13: 90–107. [Google Scholar]
- Christianen MJA et al.. 2017. Benthic primary producers are key to sustain the Wadden Sea food web: Stable carbon isotope analysis at landscape scale. Ecology 98: 1498–1512. [DOI] [PubMed] [Google Scholar]
- Cobb NA. 1914. Nematodes and Their Relationships. US Government Printing Office. [Google Scholar]
- Derrien D, Marol C, Balesdent J. 2004. The dynamics of neutral sugars in the rhizosphere of wheat. An approach by 13C pulse-labelling and GC/C/IRMS. Plant and Soil 267: 243–253. [Google Scholar]
- Erwin DH, Laflamme M, Tweedt SM, Sperling EA, Pisani D, Peterson KJ. 2011. The Cambrian conundrum: Early divergence and later ecological success in the early history of animals. Science 334: 1091–1097. [DOI] [PubMed] [Google Scholar]
- Gebremikael MT, Steel H, Buchan D, Bert W, De Neve S. 2016. Nematodes enhance plant growth and nutrient uptake under C and N-rich conditions. Scientific Reports 6: 32862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach S. 1978. Food-chain relationships in subtidal silty sand marine sediments and the role of meiofauna in stimulating bacterial productivity. Oecologia 33: 55–69. [DOI] [PubMed] [Google Scholar]
- Glover AG, Smith CR.. 2003. The deep-sea floor ecosystem: Current status and prospects of anthropogenic change by the year 2025. Environmental Conservation 30: 219–241. [Google Scholar]
- Glud RN. 2008. Oxygen dynamics of marine sediments. Marine Biology Research 4: 243–289. [Google Scholar]
- Grosberg RK, Vermeij GJ, Wainwright PC. 2012. Biodiversity in water and on land. Current Biology 22: R900–R903. [DOI] [PubMed] [Google Scholar]
- Heidemann K, Hennies A, Schakowske J, Blumenberg L, Ruess L, Scheu S, Maraun M. 2014. Free-living nematodes as prey for higher trophic levels of forest soil food webs. Oikos 123: 1199–1211. [Google Scholar]
- Heip C, Vincx M, Vranken G. 1985. The ecology of marine nematodes. Oceanography and Marine Biology: An Annual Review 23: 399–489. [Google Scholar]
- Herman PMJ, Middelburg JJ, Widdows J, Lucas CH, Heip CHR. 2000. Stable isotopes as trophic tracers: Combining field sampling and manipulative labelling of food resources for macrobenthos. Marine Ecology Progress Series 204: 79–92. [Google Scholar]
- Hernandez M, del C, Alcocer J, Oseguera LA, Escobar E. 2014. Profundal benthic invertebrates in an oligotrophic tropical lake: Different strategies for coping with anoxia. Journal of Limnology 73: 387–399. [Google Scholar]
- Holterman M et al.. 2006. Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown clades. Molecular Biology and Evolution 23: 1792–1800. [DOI] [PubMed] [Google Scholar]
- Holterman M. 2008. Phylogenetic Relationships within the Phylum Nematoda as Revealed by Ribosomal DNA, and Their Biological Implications. PhD dissertation, Wageningen University, the Netherlands. [Google Scholar]
- Hugot J-P, Baujard P, Morand S. 2001. Biodiversity in helminths and nematodes as a field of study: An overview. Nematology 3: 199–208. [Google Scholar]
- Ingham RE, Trofymow JA, Ingham ER, Coleman DC. 1985. Interactions of bacteria, fungi, and their nematode grazers: Effects on nutrient cycling and plant growth. Ecological Monographs 55: 119–140. [Google Scholar]
- Jones FGW. 1975. The soil as an environment for plant parasitic nematodes. Annals of Applied Biology 79: 113–139. [Google Scholar]
- Kiontke K, Fitch DHA.. 2013. Nematodes. Current Biology 23: R862–R864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp S et al.. 2017. Do drivers of biodiversity change differ in importance across marine and terrestrial systems: Or is it just different research communities’ perspectives? Science of the Total Environment 574: 191–203. [DOI] [PubMed] [Google Scholar]
- Krumins JA et al.. 2013. Soil and freshwater and marine sediment food webs: Their structure and function. BioScience 63: 35–42. [Google Scholar]
- Lebreton B et al.. 2012. Food sources used by sediment meiofauna in an intertidal Zostera noltii seagrass bed: A seasonal stable isotope study. Marine Biology 159: 1537–1550. [Google Scholar]
- Leppälammi-Kujansuu J, Aro L, Salemaa M, Hansson K, Kleja DB, Helmisaari HS. 2014. Fine root longevity and carbon input into soil from below- and aboveground litter in climatically contrasting forests. Forest Ecology and Management 326: 79–90. [Google Scholar]
- Lee DL. 1961. Two new species of cryptobiotic (anabiotic) freshwater nematodes, Actinolaimus hintoni sp. nov. and Dorylaimus keilini sp. nov. (Dorylaimidae). Parasitology 51: 237–240. [DOI] [PubMed] [Google Scholar]
- Löhr SC, Kennedy MJ.. 2015. Micro-trace fossils reveal pervasive reworking of Pliocene sapropels by low-oxygen-adapted benthic meiofauna. Nature Communications 6: 6589. [DOI] [PubMed] [Google Scholar]
- Matamala R, Jastrow JD, Miller RM, Garten CT. 2009. Temporal changes in C and N stocks of restored prairie: Implications for C sequestration strategies. Ecological Applications 2009 18: 1470–1488. [DOI] [PubMed] [Google Scholar]
- McSorley R. 2003. Adaptations of nematodes to environmental extremes. Florida Entomologist 86: 138–142. [Google Scholar]
- Moens T, Verbeeck L, de Maeyer A, Swings J, Vincx M. 1999. Selective attraction of marine bacterivorous nematodes to their bacterial food. Marine Ecology Progress Series 176: 165–178. [Google Scholar]
- Moens T, Dos Santos GAP, Thompson F, Swings J, Fonsêca-Genevois V, Vincx M, De Mesel I. 2005. Do nematode mucus secretions affect bacterial growth? Aquatic Microbial Ecology 40: 77–83. [Google Scholar]
- Nascimento FJ, Näslund J, Elmgren R. 2012. Meiofauna enhances organic matter mineralization in soft sediment ecosystems. Limnology and Oceanography 57: 338–346. [Google Scholar]
- Neher DA. 2010. Ecology of plant and free-living nematodes in natural and agricultural soil. Annual Review of Phytopathology 48: 371–394. [DOI] [PubMed] [Google Scholar]
- Neher DA, Weicht TR, Barbercheck ME. 2012. Linking invertebrate communities to decomposition rate and nitrogen availability in pine forest soils. Applied Soil Ecology 54: 14–23. [Google Scholar]
- Nkem J, Virginia R, Barrett J, Wall D, Li G. 2006. Salt tolerance and survival thresholds for two species of Antarctic soil nematodes. Polar Biology 29: 643–651. [Google Scholar]
- Ramirez-Llodra E et al.. 2010. Deep, diverse and definitely different: Unique attributes of the world's largest ecosystem. Biogeosciences 7: 2851–2899. [Google Scholar]
- Rasmann S, Ali JG, Helder J, van der Putten WH. 2012. Ecology and evolution of soil nematode chemotaxis. Journal of Chemical Ecology 38: 615–628. [DOI] [PubMed] [Google Scholar]
- Rex MA et al.. 2006. Global bathymetric patterns of standing stock and body size in the deep-sea benthos. Marine Ecology Progress Series 317: 1–8. [Google Scholar]
- Riemann F, Schrage M.. 1978. The mucus-trap hypothesis on feeding of aquatic nematodes and implications for biodegradation and sediment texture. Oecologia 34: 75–88. [DOI] [PubMed] [Google Scholar]
- Riemann F, Helmke E.. 2002. Symbiotic relations of sediment-agglutinating nematodes and bacteria in detrital habitats: The enzyme-sharing concept. Marine Ecology 23: 93–113. [Google Scholar]
- Riera P, Hubas C.. 2003. Trophic ecology of nematodes from various microhabitats of the Roscoff Aber Bay (France): Importance of stranded macroalgae evidenced through δ13C and δ15N. Marine Ecology Progress Series 260: 151–159. [Google Scholar]
- Rota-Stabelli O, Daley AC, Pisani D. 2013. Molecular timetrees reveal a Cambrian colonization of land and a new scenario for ecdysozoan evolution. Current Biology 23: 392–398. [DOI] [PubMed] [Google Scholar]
- Ruiter de PC, Neutel A-M, Moore JC. 1998. Biodiversity in soil ecosystems: The role of energy flow and community stability. Applied Soil Ecology 10: 217–228. [Google Scholar]
- Schminke HK. 2018. Entomology for the copepodologist. Journal of Plankton Research 29: 149–162. [Google Scholar]
- Schratzberger M, Ingels J. 2018. Meiofauna matters: The roles of meiofauna in benthic ecosystems. Journal of Experimental Marine Biology and Ecology 502: 12–25. [Google Scholar]
- Shurin JB, Gruner DS, Hillebrand H. 2006. All wet or dried up? Real differences between aquatic and terrestrial food webs. Proceedings of the Royal Society B 273: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CR, De Leo FC, Bernardino AF, Sweetman AK, Arbizu PM. 2008. Abyssal food limitation, ecosystem structure and climate change. Trends in Ecology and Evolution 23: 518–528. [DOI] [PubMed] [Google Scholar]
- Soetaert K, Vanaverbeke J, Heip C, Herman PMJ, Middelburg JJ, Sandee A, Duineveld G. 1997. Nematode distribution in ocean margin sediments of the Goban Spur (northeast Atlantic) in relation to sediment geochemistry. Deep Sea Research Part I: Oceanographic Research Papers 44: 1671–1683. [Google Scholar]
- Soetaert K et al.. 2009. Factors affecting nematode biomass, length and width from the shelf to the deep sea. Marine Ecology Progress Series 392: 123–132. [Google Scholar]
- Song D et al.. 2017. Large-scale patterns of distribution and diversity of terrestrial nematodes. Applied Soil Ecology 114: 161–169. [Google Scholar]
- Sperling EA, Stockey RG.. 2018. The temporal and environmental context of early animal evolution: Considering all the ingredients of an “explosion.” Integrative and Comparative Biology 58: 605–622. [DOI] [PubMed] [Google Scholar]
- Telford MJ, Bourlat SJ, Economou A, Papillon D, Rota-Stabelli O. 2008. The evolution of the Ecdysozoa. Philosophical Transactions of the Royal Society B 363: 1529–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton DCO. 2002. Diatom aggregation in the sea: Mechanisms and ecological implications. European Journal of Phycology 37: 149–161. [Google Scholar]
- Traunspurger W. 2000. The biology and ecology of lotic nematodes. Freshwater Biology 44: 29–45. [Google Scholar]
- Traunspurger W, Höss S, Witthöft-Mühlmann A, Wessels M, Güde H. 2012. Meiobenthic community patterns of oligotrophic and deep Lake Constance in relation to water depth and nutrients. Fundamental and Applied Limnology/Archiv für Hydrobiologie 180: 233–248. [Google Scholar]
- Turpenniemi TA, Hyvarinen H.. 1996. Structure and role of the renette cell and caudal glands in the nematode Sphaerolaimus gracilis (Monhysterida). Journal of Nematology 28: 318–327. [PMC free article] [PubMed] [Google Scholar]
- Underwood GJC, Kromkamp J.. 1999. Primary production by phytoplankton and microphytobenthos in estuaries. Advances in Ecology Research 29: 93–154. [Google Scholar]
- Van de Velde AC, Coomans A. 1987. Ultrastructure of the excretory system of the marine nematode Monhystera disjuncta. Tissue and Cell 19: 713–725. [DOI] [PubMed] [Google Scholar]
- Verhoef HA, Brussaard L.. 1990. Decomposition and nitrogen mineralization in natural and agroecosystems: The contribution of soil animals. Biogeochemistry 11: 175–211. [Google Scholar]
- Wall DH. 2007. Global change tipping points: Above- and below-ground biotic interactions in a low diversity ecosystem. Philosophical Transactions of the Royal Society B 362: 2291–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton D. 1986. A Functional Biology of Nematodes. Croom Helm. [Google Scholar]
- Yeates GW. 1987. Nematode feeding and activity: The importance of development stages. Biology and Fertility of Soils 3: 143–146. [Google Scholar]
- Yeates GW. 2004. Ecological and behavioural adaptations. Pages 1–24 in Gaugler R, Bilgrami AL, eds. Nematode Behaviour. CABI Publishing. [Google Scholar]
- Zhang YC, Baldwin JG.. 2001. Ultrastructure of the postcorpus of the esophagus of Teratocephalus lirellus (Teratocephalida) and its use for interpreting character evolution in Secernentea. Canadian Journal of Zoology 79: 16–25. [Google Scholar]
- Zhang Z. 2013. Animal biodiversity: An update of classification and diversity in 2013. Zootaxa 3703: 5–11. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Aller RC, Yanzhen F. 2006. Two-dimensional pH distributions and dynamics in bioturbated marine sediments. Geochimica et Cosmochimica Acta 70: 4933–4949. [Google Scholar]