Abstract
Objectives
Diagnosis of metastatic basal cell carcinoma (BCC) remains challenging, in part due to its rarity. With the advent of molecularly targeted therapies, recognition of this entity is more important than ever.
Methods
We identified 11 cases of metastatic BCC over a 13-year period. We analyzed these tumors in conjunction with their respective primary tumors by histomorphologic, immunohistochemical, and molecular genetic analyses.
Results
We identified three morphologic patterns of metastasis in BCC. The most common (seven cases) was characterized by completely typical features of BCC. Two cases showed marked squamous differentiation within BCC. The final two cases showed exclusively features of a poorly differentiated carcinoma. One of these was definitively classified by molecular analysis, as both the primary and metastatic tumors harbored the same inactivating PTCH1 mutation.
Conclusions
This study illustrates multiple distinct morphologic patterns in metastatic BCC and highlights the utility of ancillary molecular testing for accurate diagnosis.
Keywords: Basal cell carcinoma, Metastasis, Lymph node, Lung, Bone
Basal cell carcinoma (BCC) is the most common human cancer, with an estimated annual incidence of 800 to 1,000 cases per 100,000 individuals in the United States.1 This tumor is characterized by a relatively indolent course. While local recurrence and even locally aggressive behavior are not uncommon, metastatic spread is exceedingly rare. To date, approximately 350 cases of metastatic BCC have been reported.2-8 These examples consist mainly of case reports and small cases series. Even among these cases, it is unclear how many represent bona fide examples of metastatic basal carcinoma as histologic documentation of primary and corresponding metastatic tumors has been inconsistent, and many reports have lacked detailed histopathologic descriptions and/or illustrations. Domarus and Stevens,3 in their review of the literature, could accept as bona fide examples only 170 of the 205 cases reported at the time. Ackerman and colleagues,9 in their own review of the literature, believed that many reported cases of metastatic BCC were likely misclassified.
We describe here 11 additional cases of metastatic BCC. These cases have been analyzed in conjunction with their respective primary tumors, with detailed histopathologic analysis and description, and with extensive clinical correlation and follow-up. Mutational analysis was performed in the metastatic component of one case to ascertain BCC origin. This series suggests three distinct histopathologic patterns of metastatic BCC. Awareness of these features and ancillary molecular testing in poorly differentiated cutaneous tumors with possible BCC origin may facilitate better recognition of this rare entity, which is critical given the existence of specific targeted therapies for BCC.10
Materials and Methods
Study approval was obtained from our institutional review board. Potential cases were identified by searching our institution’s pathology records between 2005 and 2018. Cases were reviewed blindly by two dermatopathologists for diagnostic confirmation. Only cases with an unequivocal diagnosis of BCC upon re-review by at least two of the authors (A.C.L. and J.H.) were included. All cases met the following criteria for inclusion in this study: (1) strong morphologic concordance between the primary and metastatic tumors or mutational status in keeping with BCC origin in poorly differentiated carcinoma (n = 1); (2) distant metastases that could not be accounted for by direct local extension; (3) the sites of metastatic disease had to be anatomically compatible with their corresponding primary sites of origin; and (4) metastatic disease could not be accounted for by any other primary tumor and where immunohistochemistry was performed for diagnostic purposes, it supported this notion.
Clinical information was obtained from the electronic medical record. Patients were deidentified, and a case number was assigned for each patient. Age, sex, primary tumor site and size, treatment of primary tumor, recurrence, metastasis, time interval between primary tumor and metastasis, and significant comorbidities (immunosuppression, genodermatosis, etc) were recorded.
Standard 4-μm-thick H&E-stained sections were prepared for histopathologic analysis. Immunohistochemical staining for p63 (clone BC4A4; Biocare Medical) was performed at a dilution of 1:250. Antigen retrieval was achieved using a pressure cooker in the presence of citrate buffer (pH 6.0), staining was performed via an automated platform (Dako Autostainer Plus; Dako), and detection was via the Envision Plus system (Dako). DNA extracted from formalin-fixed, paraffin-embedded tissue was examined by targeted next-generation sequencing interrogating the exonic sequences of 447 cancer-associated genes and 191 introns across 60 genes as previously described.11
Results
Clinical Findings
We identified 11 patients over a 13-year period with metastatic BCC. Table 1 summarizes the clinical features. There was a male predilection (seven males, four females). Ten patients were white, and one patient was African American (case 6). Age at the time of primary BCC ranged from 34 to 85 years (mean and median of 57 years, n = 9; initial presentation in two of the cases was of metastatic disease). The mean age at presentation with metastatic disease was 59.4 years (median, 58 years), with a range of 36 to 88 years. Seven patients had primary tumors in the head and neck area, three in the upper extremity, and one in the trunk. One patient had a right axillary metastasis, and the exact primary site could not be ascertained because of multiple potential primary sources; he had many primary BCCs potentially related to previous Grenz radiotherapy and UV light treatment for underlying Darier disease. Of interest, this patient subsequently developed a primary BCC of the left mastoid region 2 years after the axillary metastasis and later a metastasis from this tumor to the leptomeninges (dura). Three patients experienced local recurrence of the primary tumor prior to documentation of metastasis. As expected, most primary tumors in the head and neck region metastasized to neck and submandibular lymph nodes; tumors from the upper extremity and trunk spread to axillary lymph nodes. Other sites of metastatic involvement included lungs, spine, bone marrow, and pelvic bones.
Table 1 .
Clinical Features of Patients With Metastatic BCCa
| Case No. | Age, y/Sex | Primary Site | Primary Size | Treatment of Primary | Recurrence | Metastasis | Time Interval to Metastasis | Treatment of Metastasis | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 68/M | Nose | 1.5 cm ulcerated | Excision and XRT | No | Right axillary LN | 8 years | Reexcision and XRT | NED at 1 year |
| 2 | 36/M | Right occipital scalp | >8 cm | Excision and vismodegib | Yes; 1.5-cm mass in scar and hyoid bone (1 year) | Neck LN and soft tissues, lung; spinal accessory nerve PNI | 1.5 years | Reexcision, vismodegib | Gorlin syndrome; >1,000 BCCs; NED at 4 years |
| 3 | 66/M | Left ear | 2 cm | Excision, chemotherapy, and radiation | Yes; left pre- and postauricular region (1 year) | Neck LNs and soft tissues, lung, facial nerve involvement PNI | 2 years | Reexcision, vismodegib, taladegib | AWD at 6 years |
| 4 | 49/F | Right arm | Unknown | Excision, chemotherapy, and XRT | No | Right axillary LN | Metastatic at presentation; longstanding history of BCC | Reexcision, chemotherapy, and radiation | NED at 13 years |
| 5 | 88/M | Right axilla | 1.9 cm | Mohs surgery | Yes; right axilla (3 years) | Left lung | 3 years | None (symptomatic) | AWD at 5 years |
| 6 | 54/F | Left ear | Unknown | Excision | Unknown | Spine (L3), lungs, ribs, sacrum, and pubic ramus | 1 year | Chemotherapy and XRT | DOD at 2 years |
| 7 | 58/M | Right scalp | 4 cm | Mohs surgery, wide local excision, and XRT | No | Left neck LN, sternum | 2 years | XRT, pembroluzimab | CLL dx 10 years prior to metastatic BCC; AWD at 2 years |
| 8 | 50/F | Chin | 3.5 cm | Excision, chemotherapy, and XRT | No | Submandibular soft tissues, bone marrow | 4 years and 9 years, respectively | Chemotherapy and XRT | AWD at 9 years |
| 9 | 67/M | Unknown and left mastoid region | Unknown | Excision, XRT, vismodegib | No | Right axilla and dura | Unknown (axilla) and 3 years dura (mastoid) | Reexcision, XRT | Darier disease; AWD at 5 years |
| 10 | 58/F | Left shoulder | Unknown | Mohs | No | Left axillary LNs | 1 year | Reexcision and lymph node dissection | B-ALL 1 year after diagnosis; NED at 5 years |
| 11 | 59/M | Right shoulder | Unknown | Excision, XRT | No | Right axillary LNs | Metastatic at presentation; 4 years | Reexcision and lymph node dissection | NED at 1 year |
AWD, alive with disease; B-ALL, B-cell acute lymphoblastic leukemia; BCC, basal cell carcinoma; CLL, chronic lymphocytic leukemia/lymphoma; DOD, dead of disease; Dx, diagnosis; LN, lymph node(s); NED, no evidence of disease; PNI, perinerual involvement; XRT, radiation therapy.
aAll patients were white except for case 6, who was African American.
The interval from diagnosis of primary to metastatic disease ranged from zero (ie, metastatic at presentation) to 8 years with a mean of 2.4 years (median, 2 years). This interval could not be ascertained in one patient. There was no difference in interval for the development of regional vs distant metastases. One patient (case 6) with a primary tumor in the external auditory canal experienced widespread metastases with pulmonary and bone involvement and pathologic fractures (rib, sacrum, and pubic ramus). One patient had a history of immunosuppression due to longstanding chronic lymphocytic leukemia prior to diagnosis of primary or metastatic disease (case 7). One patient was diagnosed with B-cell acute lymphoblastic leukemia 1 year after metastatic BCC (case 9), and two patients had a history of a genodermatosis (cases 2 and 9). One patient died of metastatic BCC 2 years after diagnosis (case 6). Five patients were alive with metastatic disease 5 years after diagnosis (mean follow-up of 5.4 years, median of 5 years; cases 3, 5, 7, 8, and 9). Five patients were alive and without evidence of disease at last follow-up (mean follow-up of 4.8 years, median of 4 years; cases 1, 2, 4, 10, and 11).
Pathologic Findings
Primary tumor size ranged from 1.5 to more than 8 cm (mean, 3.5 cm; median, 2.8 cm) for the six cases with available information. Perineural invasion was documented in one primary tumor and in three metastatic tumors. The histologic subtype of the primary tumors was nodular (three cases), infiltrative (five cases), or nodular and infiltrative (three cases). Two cases showed squamous differentiation within an otherwise typical BCC Table 2.
Table 2 .
Pathologic Features of Patients With Metastatic Basal Cell Carcinoma
| Case No. | Pattern of Growth (Primary Tumor) | Squamous Differentiation (Primary Tumor) | Perineural Invasion (Primary) | Histologic Pattern (Metastatic Tumor) | Perineural Invasion (Metastasis) |
|---|---|---|---|---|---|
| 1 | Nodular | No | No | Typical (basaloid) | No |
| 2 | (Micro)nodular and infiltrative | No | No | Typical (basaloid) | Yes |
| 3 | Infiltrative | No | No | Typical (basaloid) | Yes |
| 4 | Infiltrative | No | No | Typical (basaloid) | No |
| 5 | Nodular and focally infiltrative | No | No | Typical (basaloid) | No |
| 6 | Infiltrative | No | No | Typical (basaloid) | No |
| 7 | Nodular and infiltrative | No | No | Typical (basaloid) | Yes |
| 8 | Infiltrative | Yes, extensive | No | Squamoid | No |
| 9 | Infiltrative | Yes, extensive | No | Squamoid | No |
| 10 | Nodular | No | No | Poorly differentiated | No |
| 11 | (Micro)nodular | No | Yes | Poorly differentiated | No |
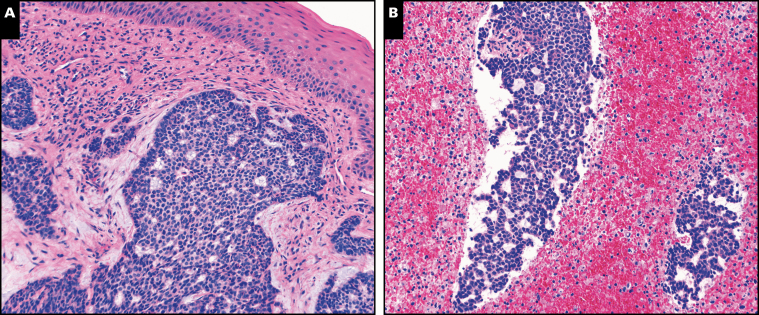
Three morphologic patterns were seen among the 11 cases of metastatic BCC. In seven cases, the metastases showed typical features of BCC. These metastases to lymph node, lung, and bone were indistinguishable from their primary tumors Image 1, Image 2, and Image 3. Central necrosis was a prominent feature in three of these seven cases (Image 1).
Image 1 .
A, Case 2. Typical features of basal cell carcinoma (BCC) in the primary skin tumor (H&E, x200). B, Case 2. Typical features of BCC in the lymph node metastasis. Prominent central necrosis is noted in the metastasis, a feature present in other cases of metastatic BCC in this series (H&E, x200).
Image 2 .
A, Case 5. Typical features of basal cell carcinoma (BCC) in the primary skin tumor (H&E, x400). B, Case 5. Typical features of BCC are also present in the cell block preparation from a fine-needle aspiration of the lung metastasis (H&E, x400).
Image 3 .
A, Case 6. Typical features of basal cell carcinoma (BCC) are evident in the primary skin tumor (H&E, x200). B, Case 6. Lymph node metastasis with typical features of BCC (H&E, x400). C, Case 6. Typical features of BCC in bone metastasis (H&E, x400).
Two metastases showed pronounced squamous differentiation with prominent keratinization Image 4A. In some areas, these metastases could be confused for squamous cell carcinoma (SCC). However, other areas within the same tumor showed typical features of BCC Image 4B.
Image 4 .
A, Case 8. Soft tissue metastasis showing prominent squamous differentiation with keratinization (H&E, x200). B, Case 8. Other areas within the same metastatic lesion show typical features of basal cell carcinoma (H&E, x200).
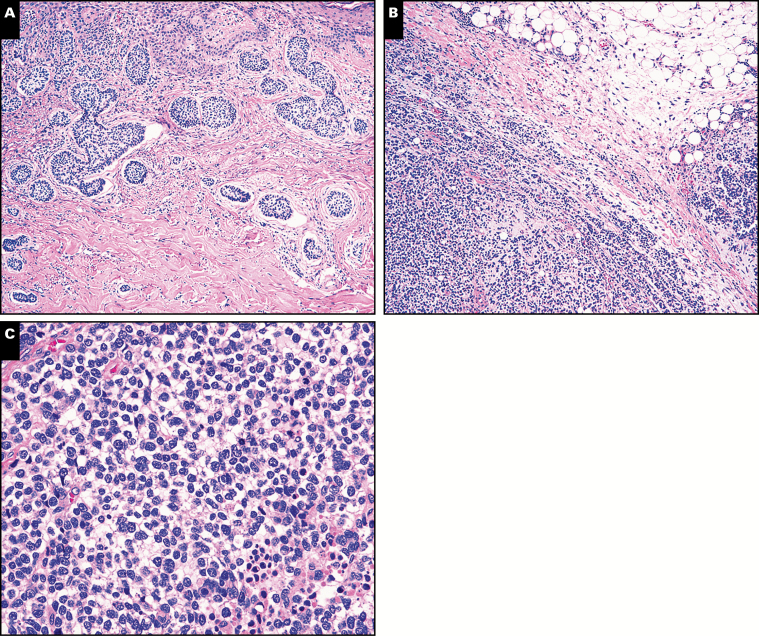
In one patient (case 10), the primary tumor displayed a spectrum of histomorphologic features: a superficial aspect with basaloid features typical of BCC, some areas with a lesser degree of differentiation, and still others that were poorly differentiated with sheet-like growth and single-cell infiltration at the perimeter Image 5A and Image 5B. This poorly differentiated component merged imperceptibly with the remainder of the tumor and retained expression of keratins and p63 (data not shown), arguing against a second independent tumor arising within a BCC. Indeed, this component might have been difficult to recognize as BCC had the more typical areas of BCC not been present. The corresponding lymph node metastasis showed exclusively poorly differentiated morphology without any areas resembling typical BCC Image 5C and Image 5D. This metastasis might have been difficult to classify without correlation with the primary tumor. Keratin and p63 immunostains were diffusely positive in the lymph node metastasis (Image 5E and data not shown).
Image 5 .
A, Case 10. Primary basal cell carcinoma (BCC) with an area of lesser differentiation (single asterisk) and another area of a poorly differentiated tumor (double asterisk) (H&E, x200). B, Case 10. Higher magnification of a poorly differentiated area (double asterisk above), not recognizable as BCC (H&E, x600). C, Case 10. Lymph node metastasis showing diffuse nodal involvement (H&E, x100). D, Case 10. Higher magnification of lymph node metastasis showing a poorly differentiated neoplasm (H&E, x600). E, Case 10. The lymph node metastasis diffusely expresses p63 by immunohistochemistry (x200).
In the final case (case 11), the primary tumor showed a micronodular pattern Image 6A. Perineural invasion was present. The metastasis, by contrast, was characterized by irregular nodules and sheets of poorly differentiated carcinoma with large, hyperchromatic cells; zonal necrosis; and areas with clear cell change Image 6B and Image 6C. Of note, typical features of BCC were not present, and thus this case was originally interpreted as a poorly differentiated carcinoma, not otherwise specified.
Image 6 .
A, Case 11. Primary basal cell carcinoma (BCC) showing a micronodular pattern (H&E, x200). B, Case 11. In contrast, the metastasis (right axillary lymph nodes) shows a poorly differentiated carcinoma (H&E, x200). C, Case 11. The metastasis shows sheets of large, discohesive, and hyperchromatic cells with a “clear cell” appearance, which is not recognizable as metastatic BCC (H&E, x600).
Given the clinical history of BCC in the right shoulder and poorly differentiated metastatic carcinoma in the ipsilateral axilla 4 years later, the possibility of metastatic BCC was eventually considered, and targeted next-generation sequencing was performed on both the primary and metastatic tumors. The primary tumor showed a high mutational burden of 57.8 mutations per Megabase (Mb), and the metastasis had 60.1 mutations/Mb. An increased number of C > T transitions, enriched at dipyrimidine (CC, CT, TC) sites, characteristic of UV-induced DNA damage was identified in both tumors. Sequencing identified an identical PTCH1 nonsense mutation (c.1726C > T; p. Q576*) Figure 1 in both the primary (allele fraction 0.15) and the metastasis (allele fraction 0.85), leading to biallelic genomic PTCH1 inactivation in the metastasis. The same TP53 splice site mutation (c.993+IG > A) was identified in both the primary (allele fraction 0.28) and the metastasis (allele fraction 0.9), causing TP53 loss of function; the apparent increase in mutant allele fractions is likely attributed to a higher percentage of neoplastic cells in the metastasis compared with the primary (80% vs 30%, respectively). With regard to copy number variants, high-level amplification of MYCN at 2p24.3 (estimated 20 copies) was detected only in the metastasis and probably occurred later during progression. Collectively, these data confirm the origin of the axillary metastasis from the skin primary, establishing the diagnosis of metastatic BCC.
Figure 1 .
Case 11. An identical PTCH1 nonsense mutation (c.1726C > T;p.Q576*) is present in both the primary (A, B) and the metastasis (C, D), leading to biallelic genomic PTCH1 inactivation in the metastasis (A, C). An increased number of C > T transitions, enriched at dipyrimidine (CC, CT, TC) sites, characteristic of UV-induced DNA damage was identified in both tumors (B, D).
Discussion
The indolent behavior of BCC is well recognized, and most patients are cured by simple excision or other local therapy. Local recurrences tend to be nondestructive and often follow incomplete therapy. On occasion, locally destructive behavior can occur, particularly if neglected. Metastasis, by contrast, is exceedingly rare. To date, only about 350 cases of metastatic BCC have been reported,3-5,12,13 and even among these cases, doubts have been expressed regarding the accuracy of some of the diagnoses.3,9 The sheer rarity of metastatic BCC, combined with its indolent reputation, likely contributes to the challenges in making this diagnosis. In some cases, the possibility of a cutaneous BCC as the primary source may not even be considered, while in others, BCC may be considered but quickly dismissed. And yet, with the development of molecularly targeted therapies such as vismodegib, sonidegib, and immune modulators (checkpoint inhibitors), the recognition of metastatic BCC is more important than ever.14
Our study largely confirms several previously reported aspects of the epidemiology of metastatic BCC, including male predominance, occurrence mainly in white patients, and the head and neck as the most common primary site.3,4,15-17 However, some differences are worth noting. Primary tumor size in our study was smaller than in previous reports (mean and median sizes were 3.3 cm and 2 cm vs 7 and 8 cm in one large study3 and >20 cm for both in another4). Median interval from primary onset to metastatic disease was also shorter in our cohort (2.5 years in our cohort vs 9 years in the literature).3 Finally, prognosis in metastatic BCC has traditionally been viewed as grim, with estimates of 1- and 5-year survival being 20% and less than 10%, respectively.3 One of our patients died of metastatic disease after 2 years. However, the other 10 were alive at last follow-up (median of 5 years): five had persistent metastatic disease, and five showed no evidence of disease after treatment. Most of the cases in this series were received in consultation after a diagnosis of metastasis, so some of these differences may be attributable to bias in case selection.
A key consideration in the discussion of metastatic BCC is the issue of squamous differentiation, a feature emphasized in many early studies.18-21 Farmer and Helwig,21 for example, reported significant squamous differentiation in nearly all their cases (15 of 17). In the 10 cases for which a primary tumor could be evaluated, eight showed squamous differentiation. Moreover, for those cases with recurrences prior to metastasis, there was felt to be a progressive increase in squamous differentiation. The apparent implication was that the acquisition of more SCC-like histologic features might portend a more aggressive course, including metastasis. A number of later studies, however, did not reach the same conclusion. Domarus and Stevens,3 in their review of the literature, reported that less than 15% of metastatic BCCs showed squamous differentiation. Two subsequent case series from the Mohs Surgery Clinic at the University of Wisconsin supported that conclusion.4,5 Our results are in line with those of Domarus and Stevens.3 We observed squamous differentiation in only two of 11 cases (Image 4). In contrast, seven cases showed typical features of BCC (Images 1-3). These results suggest that squamous differentiation is not a prerequisite for the development of metastatic BCC and is unlikely to represent the most common histologic pattern of metastasis. This point is worth emphasizing since the impression that squamous differentiation is a central factor in metastatic BCC remains prevalent, including in major textbooks of dermatopathology.22-24
SCC is nevertheless an important entity in the differential diagnosis of BCC. Care is needed when a metastasis shows squamous differentiation to ensure that it is not a true SCC, particularly given that SCC is common and is associated with a significantly higher rate of metastasis than BCC. Indeed, Ackerman and colleagues9 believed that many cases reported as metastatic BCCs were more likely to have represented poorly differentiated SCCs. Close study of the metastatic tumor may be helpful in this regard: in our two cases with squamous differentiation, areas typical of BCC could be identified (Image 4), raising confidence in the diagnosis. Review of the primary tumor and close clinical correlation may also be helpful, and in some cases, molecular analysis might play a decisive role (see below).
In addition to SCC, the differential diagnosis of metastatic BCC is broad and includes malignant adnexal neoplasms, breast adenocarcinoma, Merkel cell carcinoma, and others Table 3. Eccrine and apocrine neoplasms typically show ductal differentiation, distinguishing them from BCC; if needed, confirmatory immunostains with epithelial membrane antigen and carcinoembryonic antigen can be performed. Likewise, sebaceous differentiation would point toward sebaceous carcinoma. A basaloid mammary adenocarcinoma metastatic to axillary nodes could be confused with BCC. Typically, clinical history of breast cancer would raise suspicion for that diagnosis, and appropriate immunohistochemical analysis could be pursued for confirmation. Finally, Merkel cell carcinoma should be considered in the differential diagnosis of BCC, particularly in small biopsy specimens.25 Here, immunohistochemistry should allow for ready distinction, as Merkel cell carcinoma is typically positive for cytokeratin 20, whereas BCC is not.
Table 3 .
Selected Differential Diagnoses of Metastatic BCC
| Entity | Distinguishing Features (From Metastatic BCC) |
|---|---|
| Squamous cell carcinoma | Distinction can be very difficult; prototypical features of BCC, even if focal, may distinguish BCC with squamous differentiation from SCC |
| Adnexal carcinomas | Ductal differentiation tends to favor an adnexal carcinoma; ducts may be highlighted by EMA or polyclonal CEA Sebaceous differentiation may point toward sebaceous carcinoma |
| Breast carcinoma | History of breast cancer typically precedes metastasis; GCDFP and mammoglobin expression by immunohistochemistry may be helpful |
| MCC | CK20 typically positive in MCC, negative in BCC |
| Other metastatic poorly differentiated carcinomas | Close clinical correlation and lineage-specific immunohistochemistry may identify potential site of origin |
BCC, basal cell carcinoma; CEA, carcinoembryonic antigen; EMA, epithelial membrane antigen; GCDFP, gross cystic disease fluid protein; MCC, Merkel cell carcinoma; SCC, squamous cell carcinoma.
Our study highlights a third pattern of metastatic BCC, one that shows neither typical morphology nor prominent squamatization but rather the features of a poorly differentiated carcinoma (cases 10 and 11). Taken in isolation, the possibility of metastatic BCC would likely have not been considered in these cases. For case 10, a clue was the identification of a corresponding poorly differentiated component (with essentially identical morphologic features) within the primary tumor, which otherwise showed typical features of BCC. This again highlights the importance of careful histologic analysis and extensive sampling of both the primary and metastatic tumors in the diagnosis of metastatic BCC. However, there may be cases in which a poorly differentiated component is not well represented in the primary tumor either due to sampling, because it developed later in the disease process, or in cases where the tumor was treated with a nonsurgical approach (eg, radiation). In such cases, recognition of metastases as having derived from BCC may not be possible using standard histomorphologic analysis, even with the aid of immunohistochemistry.
In case 11, the primary BCC was initially treated by simple excision and subsequently with radiotherapy because of positive margins, perineural invasion, and patient preference. Four years later, the patient developed axillary lymph node metastases showing a poorly differentiated carcinoma, not otherwise classifiable on morphologic or immunohistochemical grounds. Molecular genetic analysis proved decisive in this case. BCCs typically harbor mutations in the hedgehog pathway, with Patched-1 being most common, although other mutations, such as in Smoothened, may also occur. The same Patched-1 mutation was identified in both the primary and metastatic tumors of this patient. BCC also tends to show a very high mutational burden, with the majority showing a UV mutational signature.26,27 This patient’s primary and metastatic tumors showed 57 and 60 mutations/Mb, respectively, with a prominent UV signature.26 Of note, a high mutational burden has been suggested to be predictive of response to immune checkpoint blockade (PD-1 and PD-L1 inhibitors) in some advanced and metastatic BCCs.28MYCN missense mutations resulting in accumulation of N-MYC protein have been documented in 30% of BCCs, while aberrant copy number gain, as observed in our case, is thought to occur rarely.29-31 The value of molecular analysis in the diagnosis of metastatic BCC is further emphasized by a recent report documenting a case of sarcomatoid metastatic BCC.32
Despite considerable study, we still have little ability to predict which BCCs are likely to metastasize. Many risk factors have been proposed over the years, including large size, deep invasion, repeated local recurrence, infiltrative subtype, history of radiation, and, as discussed above, squamatization.3,5,10 However, each of these features is much more likely to be encountered in nonmetastasizing BCC, and so their clinical utility in predicting and managing risk seems limited. Given these limitations, the timely and accurate diagnosis of metastatic BCC remains crucial to ensure that patients receive appropriate therapy.
Source of funding: Supported by the National Institutes of Health (T32) (5T32HL007627-34) (I.M.S.).
References
- 1. Wu S, Han J, Li WQ, et al. Basal-cell carcinoma incidence and associated risk factors in U.S. women and men. Am J Epidemiol. 2013;178:890-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cotran RS. Metastasizing basal cell carcinomas. Cancer. 1961;14:1036-1040. [DOI] [PubMed] [Google Scholar]
- 3. Domarus V, Stevens PJ. Metastatic basal cell carcinoma. J Am Acad Dermatol 1984;10:1043-1060. [DOI] [PubMed] [Google Scholar]
- 4. Lo JS, Snow SN, Reizner GT, et al. Metastatic basal cell carcinoma: report of twelve cases with a review of the literature. J Am Acad Dermatol. 1991;24:715-719. [DOI] [PubMed] [Google Scholar]
- 5. Snow SN, Sahl W, Lo JS, et al. Metastatic basal cell carcinoma: report of five cases. Cancer. 1994;73:328-335. [DOI] [PubMed] [Google Scholar]
- 6. Gropper AB, Girouard SD, Hojman LP, et al. Metastatic basal cell carcinoma of the posterior neck: case report and review of the literature. J Cutan Pathol. 2012;39:526-534. [DOI] [PubMed] [Google Scholar]
- 7. Kurian RR, Di Palma S, Barrett AW. Basal cell carcinoma metastatic to parotid gland. Head Neck Pathol. 2014;8:349-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wysong A, Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013;149:615-616. [DOI] [PubMed] [Google Scholar]
- 9. Ackerman AB, Reddy VB, Soyer HP.. Neoplasms With Follicular Differentiation. New York, NY: Ardor Scribendi; 2001. [Google Scholar]
- 10. Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garcia EP, Minkovsky A, Jia Y, et al. Validation of oncopanel: a targeted next-generation sequencing assay for the detection of somatic variants in cancer. Arch Pathol Lab Med. 2017;141:751-758. [DOI] [PubMed] [Google Scholar]
- 12. McCusker M, Basset-Seguin N, Dummer R, et al. Metastatic basal cell carcinoma: prognosis dependent on anatomic site and spread of disease. Eur J Cancer. 2014;50:774-783. [DOI] [PubMed] [Google Scholar]
- 13. Wadhera A, Fazio M, Bricca G, et al. Metastatic basal cell carcinoma: a case report and literature review. How accurate is our incidence data? Dermatol Online J. 2006;12:7. [PubMed] [Google Scholar]
- 14. Nikanjam M, Cohen PR, Kato S, et al.. Advanced basal cell cancer: concise review of molecular characteristics and novel targeted and immune therapeutics. Ann Oncol. 2018;29:2192-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paver K, Poyzer K, Burry N, et al. Letter: the incidence of basal cell carcinoma and their metastases in Australia and New Zealand. Australas J Dermatol. 1973;14:53. [DOI] [PubMed] [Google Scholar]
- 16. Weedon D, Wall D. Metastatic basal cell carcinoma. Med J Aust. 1975;2:177-179. [PubMed] [Google Scholar]
- 17. Stevens VW, Stenehjem DD, Patterson OV, et al. Characterization and survival of patients with metastatic basal cell carcinoma in the Department of Veterans Affairs: a retrospective electronic health record review. Arch Dermatol Res. 2018;310:505-513. [DOI] [PubMed] [Google Scholar]
- 18. Borel DM. Cutaneous basosquamous carcinoma: review of the literature and report of 35 cases. Arch Pathol. 1973;95:293-297. [PubMed] [Google Scholar]
- 19. Montgomery H. Basal cell squamous epithelioma. Arch Dermatol Syph. 1928;18:50-73. [Google Scholar]
- 20. Graham JH, Urbach F, Alek DS. Metastatic basal cell carcinoma. Arch Dermatol. 1969;99:777-778. [PubMed] [Google Scholar]
- 21. Farmer ER, Helwig EB. Metastatic basal cell carcinoma: a clinicopathologic study of seventeen cases. Cancer. 1980;46:748-757. [DOI] [PubMed] [Google Scholar]
- 22. Calonje E, Brenn T, Lazar A, et al.. McKee’s Pathology of the Skin. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012. [Google Scholar]
- 23. Martin RC II, Edwards MJ, Cawte TG, et al.. Basosquamous carcinoma: analysis of prognostic factors influencing recurrence. Cancer. 2000;88:1365-1369. [DOI] [PubMed] [Google Scholar]
- 24. Fantini F, Gualdi G, Cimitan A, et al. Metastatic basal cell carcinoma with squamous differentiation: report of a case with response of cutaneous metastases to electrochemotherapy. Arch Dermatol. 2008;144:1186-1188. [DOI] [PubMed] [Google Scholar]
- 25. Succaria F, Radfar A, Bhawan J. Merkel cell carcinoma (primary neuroendocrine carcinoma of skin) mimicking basal cell carcinoma with review of different histopathologic features. Am J Dermatopathol. 2014;36:160-166. [DOI] [PubMed] [Google Scholar]
- 26. Jayaraman SS, Rayhan DJ, Hazany S, et al. Mutational landscape of basal cell carcinomas by whole-exome sequencing. J Invest Dermatol. 2014;134:213-220. [DOI] [PubMed] [Google Scholar]
- 27. Brash DE. UV signature mutations. Photochem Photobiol. 2015;91:15-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goodman AM, Kato S, Cohen PR, et al. Genomic landscape of advanced basal cell carcinoma: implications for precision treatment with targeted and immune therapies. Oncoimmunology. 2018;7:e1404217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bonilla X, Parmentier L, King B, et al. Genomic analysis identifies new drivers and progression pathways in skin basal cell carcinoma. Nat Genet. 2016;48:398-406. [DOI] [PubMed] [Google Scholar]
- 30. Hatton BA, Knoepfler PS, Kenney AM, et al. N-myc is an essential downstream effector of Shh signaling during both normal and neoplastic cerebellar growth. Cancer Res. 2006;66:8655-8661. [DOI] [PubMed] [Google Scholar]
- 31. Freier K, Flechtenmacher C, Devens F, et al. Recurrent NMYC copy number gain and high protein expression in basal cell carcinoma. Oncol Rep. 2006;15:1141-1145. [PubMed] [Google Scholar]
- 32. Kiuru M, McDermott G, Coit DC, et al.. Basal cell carcinosarcoma with PTCH1 mutations in both epithelial and sarcomatoid primary tumor components and in the sarcomatoid metastasis. Am J Surg Pathol. 2014;38:1. [DOI] [PubMed] [Google Scholar]