Abstract
The central nervous system regulates fertility through the release of gonadotropin-releasing hormone (GnRH). This control revolves around the hypothalamo-pituitary-gonadal axis, which operates under traditional homeostatic feedback by sex steroids from the gonads in males and most of the time in females. An exception is the late follicular phase in females, when homeostatic feedback is suspended and a positive feedback response to estradiol initiates the preovulatory surges of GnRH and luteinizing hormone (LH). Here we briefly review the history of how mechanisms underlying central control of ovulation by circulating steroids have been studied, discuss the relative merit of different model systems, and integrate some of the more recent findings in this area into an overall picture of how this phenomenon occurs.
Introduction
GnRH neurons form the final common central output pathway controlling fertility in vertebrates. Their output is regulated primarily by homeostatic sex steroid feedback. During the preovulatory period of the mammalian female reproductive cycle in spontaneously ovulating species, however, the feedback action of estradiol switches from negative to positive feedback. This initiates a surge of GnRH, and subsequently LH, release and ultimately triggers ovulation. A central signal is required for ovulation in most mammals. In some species, including rabbits, ovulation is induced by copulation; this association made it possible to study the neural link to reproduction as early as the 18th century. In 1797, Jon Haighton recounted to the Royal Society his observation that, in rabbits, sex made “by sympathy the ovarian vesicles enlarge, project, and burst” (1). Haighton rejected the hypothesis that semen directly stimulated the ovary to release an egg because he had severed the Fallopian tubes. He conjectured sympathy, or crosstalk, between the vagina and ovaries through the nervous system occurred to induce ovulation. The study of the brain’s role in ovulation accelerated in the early 20th century. In 1936, Marshall and Verney induced ovulation when they passed electrical current through a rabbit’s brain (2). A year later, Harris refined their work when he induced ovulation by electrically stimulating a specific region of the brain, the hypothalamus (3).
A neural signal was also postulated to be necessary for ovulation in animals that do not require copulation to ovulate, i.e., spontaneous ovulators. Humans, non-human primates, sheep, rodents, and many other mammals ovulate spontaneously at the end of the follicular phase of the reproductive cycle (proestrus in rodents). Studying spontaneous ovulation became possible as techniques, such as the vaginal smear, were developed to follow cycle stage in live animals. In 1950, Everett and Sawyer delayed spontaneous ovulation by anesthetizing rats with phenobarbital on the afternoon of proestrus. In their control animals, ovulation occurred between 1 and 2 am on the morning of estrus (lights off at 7 pm), but anesthesia delayed ovulation by 24 hours if administered during a critical period (3 – 5 pm before lights off) the previous day (4). They hypothesized that a neural signal initiated spontaneous ovulation during this period. Eight years later, Critchlow stimulated the hypothalamus directly to trigger “spontaneous” ovulation (5). In the 1950s, hypothalamic pathologies were first associated with both hypogonadism and precocious puberty in humans (6), further supporting a central role in the regulation of fertility.
The study of the brain’s role in reproduction did not occur in isolation, as a role was also emerging for the pituitary. In 1921 and 1922, Evans and Long noted that injecting pituitary extract into a rat’s peritoneal cavity enlarged its ovaries and disrupted its estrous cycles (7-9). Similarly, surgical removal of the pituitary caused ovarian atrophy, and pituitary transplants beneath the hypothalamus (site of the sella turcica, home of the pituitary) restored estrous cycles and spontaneous ovulation (10,11). When the pituitary was transplanted to sites outside of the sella turcica, however, reproduction was not restored (12). These studies supported two early hypotheses: first, the pituitary may be important for reproduction in spontaneously ovulating species, and second, communication with the hypothalamus is necessary for pituitary control of reproduction.
Support for the hypothalamo-pituitary control of ovulation and reproduction continued to expand through the 20th century. A releasing factor in the hypothalamus had long been postulated to initiate pituitary hormone release to control reproduction. By 1971, Schally had isolated and sequenced 11.4 mg of GnRH from the hypothalami of 240,000 pigs (13). This GnRH is made and released by a small population (800 – 2500 neurons in mammals) that is scattered through the preoptic area and anterior hypothalamus (14). Many of these neurons project to and secrete GnRH into the median eminence, from where it is carried down long portal vessels into the capillary beds of the anterior pituitary. There, GnRH binds to receptors on pituitary gonadotropes to trigger the release of two hormones, follicle stimulating hormone (FSH) and LH. The release of these hormones stimulates follicular maturation and the production of sex steroids in the ovaries. Ovarian steroids provide feedback on the pituitary and hypothalamus to regulate hormone release. Collectively, hypothalamus, pituitary, and ovaries control complex hormonal interactions to precisely coordinate the reproductive cycle. The focus of this review is on systemic feedback; for recent reviews of a potentially interesting role for neural steroids in this process the reader is referred to a recent review on this by Terasawa (15).
Modes of estradiol feedback regulation of the hypothalamus and pituitary
In mammals, ovarian estradiol was soon linked with ovulation induction (16), and studies showed that estradiol differentially regulates pulsatile vs surge modes of GnRH release via negative and positive feedback, respectively. For the majority of the reproductive cycle, GnRH is released in pulsatile manner and drives the pulsatile release of gonadotropins (17-20). Estradiol is traditionally referred to as having negative feedback actions on pulsatile hormone release. A closer examination of the actions of estrogens suggests this nomenclature is somewhat misleading. The term negative feedback arises from the observation that mean LH levels are lower in estrogen-treated than in ovariectomized (open feedback loop) animals (21-23). This is attributable primarily to a reduction in pulse amplitude as frequency of GnRH and LH release are often increased, or at least not suppressed, in higher estrogen states produced by either steroid replacement in the physiologic range or natural progression towards the late follicular phase (22,24-28). For historical consistency, we will refer to this action of estradiol as negative feedback, but wish to clarify the term to mean the action of estradiol to modulate the pulsatile pattern of GnRH/LH that characterizes much of the female cycle.
In most mammals, there is a switch from pulsatile GnRH to a continuous surge of GnRH release at the end of the follicular phase that is induced by estradiol positive feedback. There is little evidence of episodic secretion during the surge suggesting it is a different mode of secretion or a continuous mode superimposed upon the episodic mode (29-32). There remains some controversy over whether or not a GnRH surge exists in humans. It is certainly clear that in old-world primates a consistent GnRH pulse frequency can generate reproductive cyclicity at least over a few months (33,34). This led to the postulate that GnRH is permissive for LH surge generation in these species, rather than deterministic. Other indirect measures of GnRH release have suggested there is actually a decrease in GnRH during the LH surge in monkeys and women (35-37). Estradiol positive feedback at the pituitary appears to be stronger in these species, evidenced by the ability of estradiol to induce an LH surge in males and the ability of transplanted ovaries to produce cyclic hormonal changes reminiscent of the menstrual cycle in males (38,39). This question is difficult to resolve without direct measurement of GnRH release itself. This is not currently possible in humans but in rhesus monkeys preovulatory, estradiol-induced and progesterone-induced increases in GnRH release during the LH surge have been observed (30,40,41), suggesting this phenomenon may also exist in humans.
Models to study estradiol feedback
Because of the availability of a vast array of genetic and other technical tools, much of the work to understand the neurobiology underlying these different modes of GnRH release has been done in rodent species, specifically laboratory mice. Three primary hormone replacement models have been used to induce negative and positive feedback in mice and were recently compared directly (42). Early work in mice utilized paradigms consisting of ovariectomy (OVX) with low estradiol replacement for approximately a week, followed by an estradiol rise on its own (E rise model) or in combination with a subsequent progesterone rise (43). Another paradigm is to ovariectomize mice and replace with a constant high physiologic level of estradiol (OVX+E) (44). This model takes advantage of a diurnal change in the feedback action of estradiol in these species. Specifically, in rodents ovulation is tightly coupled to time-of-day, and the GnRH/LH surges begin 1-2 hours before lights out in nocturnal species (4,32) and a similar time before lights on in diurnal species (45). In mice, rats and hamsters, the OVX+E paradigm induces daily LH surges in the late afternoon, hence has been referred to as the daily surge model (44,46,47). In OVX+E mice, LH release is suppressed in the morning (AM) and increased in the afternoon (PM) relative to ovariectomized mice that do not receive estradiol (OVX). This pattern persists in brain slices with GnRH firing rates and release suppressed in the AM relative to the PM in OVX+E mice (44,48).
Of note all of these models deviate from the natural estrous cycle, and all have advantages and disadvantages. On the negative side, constant estradiol, even at physiologic levels, is not characteristic of the estrous cycle. Further, all of these OVX+E models operate on a different duration than the typical cycle, with the E rise model being longer and the daily surge being shorter. On the plus side, all of these paradigms permit the study of estradiol feedback in genetic models that are not capable of generating an estradiol rise on their own. The differences in these models also can make it possible to probe different aspects of positive feedback. In the E rise model, the switch between negative and positive feedback relies on both an increase in estradiol and on time of day. In the daily surge model, the switch between negative and positive feedback relies on time of day. An interesting biological question that remains to be answered is whether or not the underlying neurobiological mechanisms are the same in both of these models and how they compare to the natural cycle.
Daily surge vs the cycle
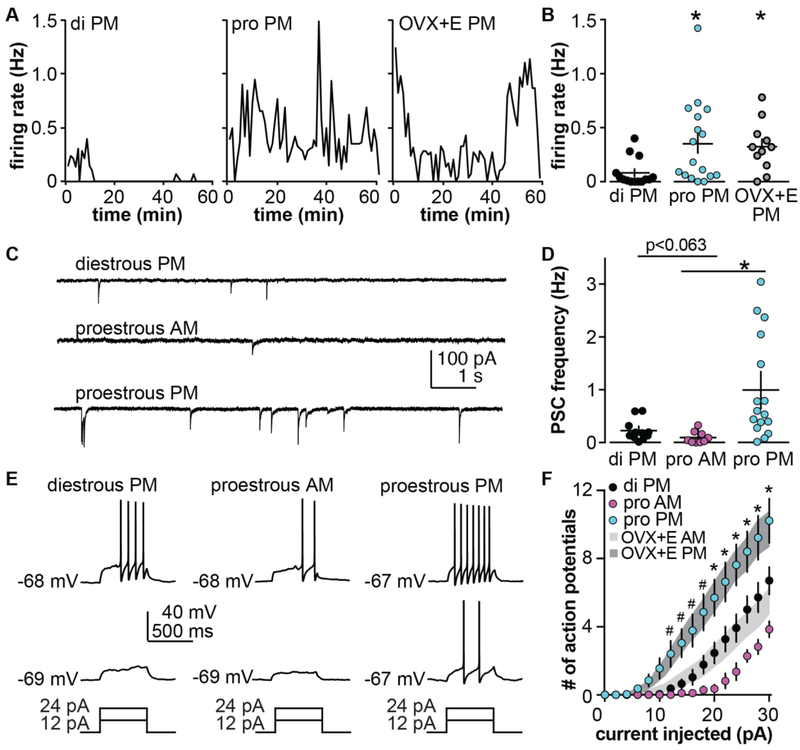
The daily surge model has been used to characterize changes in multiple intrinsic and fast-synaptic properties during the switch from negative to positive feedback (49-54). As this dataset has grown, it became increasingly important to compare at least some of the changes induced by this model to those that occur during the cycle. This was particularly important as the amplitude of the proestous surge was observed to be larger than the estradiol-induced LH surge (55,56). To do this, we examined three parts of the estrous cycle. Diestrous PM is a time of relatively low estradiol that is characterized by pulsatile LH release. Proestrous AM is a time when exposure to high estradiol needed for surge induction has occurred, but the LH surge has not yet been triggered. Proestrous PM is the time of estradiol positive feedback and the LH surge. GnRH neuron firing rate (diestrous and proestrous PM only), GABAergic fast synaptic transmission, GnRH neuron excitability, and action potential properties were examined (Figure 1). Firing rate of GnRH neurons determined by extracellular recordings of GFP-identified GnRH neurons in brain slices prepared on the afternoon of diestrous vs proestrous were strikingly similar to those observed in the daily surge model from OVX+E AM vs OVX+E PM neurons, respectively (56). Further, the larger amplitude of the proestrous LH surge was shown to be attributable at least in part to increased pituitary responsiveness to GnRH (56). These observations suggest that the final output of the reproductive neuroendocrine system (GnRH release) is likely to be similar in the daily surge model and during the natural proestrous surge. Whole-cell recordings were used to examine synaptic and intrinsic properties of GnRH neurons during the cycle. The number of action potentials fired in response to fixed current injection is one way to characterize the integrated sum of the intrinsic properties of a neuron; this is often termed excitability. GnRH neuron excitability on diestrous PM was strikingly similar to that in OVX AM, OVX PM and OVX+E AM in the daily surge model (54,57). Similarly the positive feedback states (OVX+E PM and proestrous PM) were comparable in excitability and greater than that observed during the negative feedback/open loop conditions. We were initially surprised that OVX+E AM cells were not less excitable than cells from OVX mice as other properties, including potassium and calcium currents, are altered by estradiol in the daily surge models in manners that would typically reduce excitability. Computational modeling suggested an inverse relationship between the conductance and voltage-dependence of inactivation of a transient potassium current in GnRH neurons accounted for the similarity between OVX and negative feedback states (OVX+E AM) (54).
Figure 1.
Comparison of daily surge model with estrous cycle. A, B. Representative firing patterns (A) and individual values and mean ±SEM firing rate (B) of GnRH neurons from diestrous, proestrous or OVX+E mice recorded in the PM. C, D. Representative recordings (C) and individual values and mean ±SEM frequency (D) of spontaneous GABAergic postsynaptic current (PSCs) in GnRH neurons from diestrous PM, proestrous AM and proestrous PM mice. E. Representative current-clamp recordings from diestrous PM, proestrous AM and proestrous PM mice. F. Mean±SEM number of action potentials in these groups; grey shaded areas show range of SEM for the same experiment in GnRH neurons from OVX+E AM and OVX+E PM mice. * p<0.05. A and B adapted from (56), C-F adapted from (54,57) with permission.
Of interest in this regard, the excitability of GnRH neurons recorded on proestrous AM was reduced compared to diestrous PM. The same shifts in response to cycle stage were observed for GABAergic transmission to GnRH neurons, with transmission during the low estradiol negative feedback state of diestrous PM being lower than during positive feedback on proestrous PM, but GABA input during the high estradiol negative feedback of proestrous AM being the lowest frequency. These results were again initially surprising. The ability of a high physiologic and even pharmacologic level of estrogen to induce positive feedback is consistent (43,58,59), but in vivo the negative feedback actions of constant estradiol on GnRH release appeared to be stronger than those of the estradiol rise during the cycle (28,58). These observations had led us to postulate that a likely limitation of the daily surge model was that negative feedback was stronger than would be typical during the cycle. Together these newer data suggest that a possible limitation of the daily surge model is rather that negative feedback in this model effectively recapitulates that of lower estradiol states of diestrus, but may fall short of the stronger negative feedback that emerges on the morning of proestrus.
The existence of a daily central signal for ovulation such as observed in the daily surge model was identified in the middle of the last century in studies that demonstrated that barbiturate anesthesia during a critical period on proestrus blocked ovulation for 24 hours in rats (4). Ovulation can occur on a daily basis during the breeding season in many fish and bird species (60,61). Daily ovulation per se has not been observed in placental mammals but the LH surge and ovulation occurs at a particular time of day in some mammals. This is especially observed, as mentioned above, in rodents. Interestingly, LH surges in women occur more often during late sleep/early wake hours (62,63), and shiftwork, which can disrupt the circadian clock, is linked to menstrual cycle irregularities and increased time to pregnancy (64-66).
If a daily neural signal for ovulation can exist, why don’t mammals ovulate daily? This may be attributed in part to the time needed for a follicle to mature to the point that it can produce sufficient estradiol to trigger positive feedback. Of interest in this regard, tau mutant hamsters, in which the free-running period is ~20 hours vs. just under 24 hours in the wild type, exhibit estrous cycles lasting five circadian days, or about 100 hours. This is similar in duration to the typical four-day (96 hour) estrous cycle in wild type golden hamsters (67). Daily LH surges are induced during subjective afternoon in OVX+E tau hamsters, and the period of consecutive LH surges was shorter than in wild type hamsters (68). These observations are consistent with the postulate that follicle maturation and subsequent estradiol production are limiting and that the reproductive cycle does not result from a mere counting of circadian days. The provision of a constant high physiologic estradiol level, such as in the OVX+E daily surge model, would circumvent this limitation, allowing a central signal to occur on a daily basis as observed.
Are synaptic and/or intrinsic changes needed to produce increased GnRH neuron output during positive feedback?
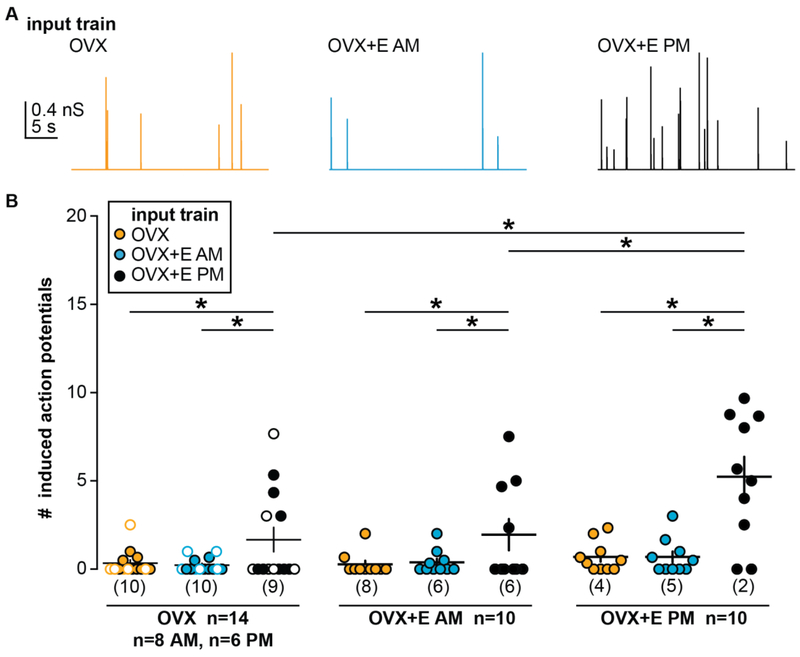
The daily surge model has produced data indicating that both synaptic and intrinsic properties of GnRH neurons are altered by estradiol feedback mode (50-54,69,70). Performing these studies typically required optimizing recording conditions to isolate a single variable. Further, most experiments were done in voltage-clamp mode, which fixes membrane potential to observe and quantify currents, but at the same time precludes the membrane potential from responding to changes in intrinsic properties. To begin to address the question of whether intrinsic changes and/or synaptic changes are needed to generate increased GnRH neuron firing during positive feedback we utilized dynamic clamp (71). GABA is the primary fast synaptic input to GnRH neurons in adults and can be excitatory even in adulthood (72,73). We mined our previous recordings of GABA transmission to GnRH neurons in the daily surge model (44), and selected traces that were representative of OVX (open loop), OVX+E AM (negative feedback) and OVX+E PM (positive feedback) conditions. Conductance trains mimicking these patterns were then applied in random order to GnRH neurons from these same animal models, effectively mixing or matching intrinsic properties of the recorded cell with the type of synaptic input (Figure 2). This approach revealed that both the synaptic inputs and intrinsic properties were important for the increased firing rate observed during positive feedback (72,73). Specifically, the GABA conductance train from positive feedback induced more firing in all animal models, suggesting increased input frequency was important, and this positive feedback train was most effective in cells recorded during positive feedback, indicating the intrinsic properties during positive feedback poise the cell to be more responsive to excitatory synaptic input.
Figure 2.
Both synaptic input and intrinsic properties contribute to increased GnRH neuron firing during positive feedback. A. Representative conductance trains from OVX (orange), OVX+E AM (blue), and OVX+E PM (black) conditions. B. Individual values and mean ± SEM spikes induced during individual postsynaptic conductances in input each train in cells from all three animal models. In the OVX group, open circles denote cells recorded in the PM and closed circles denote cells recorded in the AM. Numbers in parentheses along x-axis indicate number of cells not firing any spikes. *p<0.05 two-way repeated-measures ANOVA/Fisher’s LSD test. From (71) with permission.
It is important to point out that additional factors not examined in this study may contribute to surge generation. For example, estradiol can alter excitatory fast glutamatergic transmission to GnRH neurons, and spines where glutamate afferents may synapse onto activated GnRH neurons are increased on proestrus (53,74,75). It is also important to point out that in other animal models, no change in GABA PSC frequency has been reported during positive feedback (76). Arguing against a lack of a role for GABA in surge generation, specific knockout of estrogen receptor alpha (ERα) from GABA neurons blocks positive feedback (77), although this could be attributable to reduced release of cotransmitters such as kisspeptin that would be activated by estradiol action (78) as many kisspeptin neurons utilize GABA as a co-transmitter (79,80).
Where does estradiol act for negative and positive feedback?
A persistent question about estradiol feedback has been where it occurs. This is because this feedback requires classical signaling via ERα (81), which GnRH neurons typically do not express in detectable levels (82,83). Estradiol feedback is thus likely transmitted to GnRH neurons by ERα-expressing afferents (84). Kisspeptin is a neuromodulator that stimulates GnRH neurons (85,86). These neurons project to GnRH neurons and are directly but differentially responsive to estradiol (87-89). Specifically, the mRNA for kisspeptin is increased by estradiol in the kisspeptin neurons of the anteroventral periventricular (AVPV), postulated to underlie positive feedback, but decreased in kisspeptin neurons of the arcuate nucleus, postulated to underlie negative feedback. To begin to determine the role of ERα in these cells, whole-body knockout of ERα from kisspeptin cells was done using Cre-lox technology. These KERKO mice have disrupted cycles and do not exhibit estradiol-induced LH surges (90-92). This suggests ERα in kisspeptin cells may be critical for both estradiol negative and positive feedback. Relatively little was known about the properties of these kisspeptin neurons and how they respond to estradiol. We thus began to characterize these properties in control and KERKO mice.
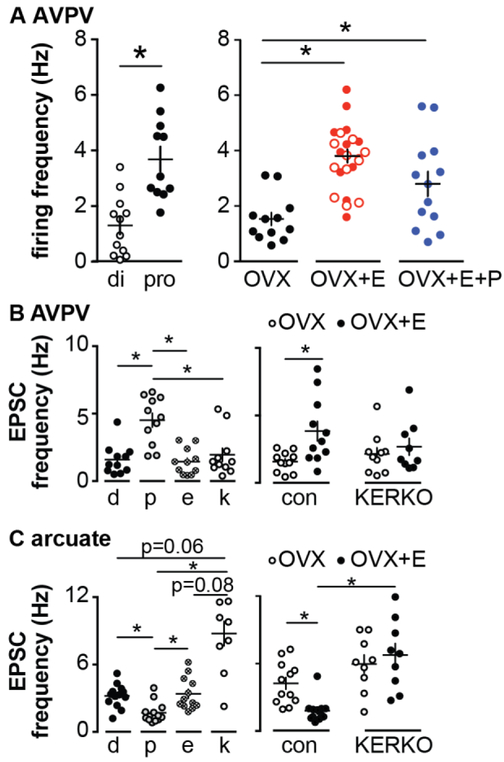
AVPV kisspeptin neurons were found to be more excitable during estradiol positive feedback on proestrus PM than during negative feedback on diestrus PM (93) (Figure 3). This increased firing was attributable to estradiol; adding progesterone did not produce a further elevation in firing rate. Burst firing by these neurons followed the same pattern, being increased during positive feedback whether occurring during the cycle or induced by estradiol. Both electrophysiological recordings measuring ionic currents and mRNA expression of these ion channel genes in pooled cells suggest several ionic conductances that can underlie burst firing are expressed by AVPV kisspeptin neurons, including hyperpolarization-activated cation channels, T-type calcium channels, and persistent sodium channels, and are regulated by estradiol (93-96). Further support of a role for estradiol comes from studies in KERKO mice. AVPV kisspeptin neurons were less excitable, fired fewer bursts and no longer changed firing rate in response to estradiol (97).
Figure 3.
Estradiol regulation of firing rate and EPSC frequency in kisspeptin neurons of the hypothalamus. A. AVPV kisspeptin neuron firing rate is elevated during proestrus (left) and by estradiol (right). Open symbols in OVX+E were injected with vehicle at the time of progestin injection, closed symbols were uninjected controls. B, C. Spontaneous glutamatergic EPSC frequency is regulated by cycle stage and estradiol in both AVPV (B) and arcuate (C) kisspeptin neurons. Estradiol regulation is lost in KERKO mice. From (93,98) with permission.
Estradiol feedback also modulates synaptic transmission to AVPV kisspeptin neurons, increasing glutamate transmission and suppressing hyperpolarizing GABAergic transmission to these cells, indicating that estradiol tilts the balance toward excitatory inputs during positive feedback (98,99). Coupled with estradiol upregulation of intrinsic conductances underlying bursting firing, AVPV kisspeptin neurons are poised to increase output during positive feedback to drive the GnRH/LH surge.
KERKO mice are a useful tool but lack both temporal and spatial regulation of ERα. Because Cre-lox will delete ERα as soon as Kiss1 is expressed there can be developmental changes in these cells or their networks (100,101). Further, the deletion of ERα from all kisspeptin cells makes it impossible to assess independently the role of AVPV and arcuate kisspeptin neurons. We thus used CRISPR/Cas9 to target Esr1 in the AVPV of adult mice (97). This approach successfully reduced ERα expression in AVPV kisspeptin neurons from ~75% in controls to about 25% in knockdown mice. These mice exhibited typical cycles but had markedly blunted proestrous and estradiol-induced LH surges. Further, their electrophysiologic properties resembled those in KERKO mice. These studies suggest ERα in AVPV kisspeptin neurons is required for estradiol action on their intrinsic membrane excitability and that these effects are activational, rather than organizational.
Kisspeptin neurons in the hypothalamic arcuate nucleus (also called KNDy neurons for their coexpression of kisspeptin, neurokinin B and dynorphin) are postulated to mediate estradiol negative feedback regulation of pulsatile GnRH/LH pulse as well as to generate LH pulses (87,102). Short-term extracellular recordings of these cells in OVX vs. OVX+E mice during negative feedback did to reveal any differences in firing pattern (98), although an effect of steroids on a longer-term firing pattern of these cells, similar to that observed in males, cannot be excluded (103). In, KERKO mice, however, firing rate of arcuate kisspeptin neurons in brain slices was markedly increased, as was LH pulse frequency in vivo (98). Estradiol also altered synaptic transmission to these cells, suppressing spontaneous glutamatergic transmission. Of note, the direction of regulation of glutamate transmission to these two kisspeptin populations is opposite.
Targeting the same CRISPR approach to the arcuate kisspeptin neurons produced a similar reduction in percent of neurons expressing ERα. In striking contrast to the mice in which the AVPV was targeted, mice with reduced ERα expression in the arcuate kisspeptin neurons had disrupted estrous cycles, with an increasing tendency to remain in estrus. This is similar to mice in which ERα was deleted from Tac2-expressing neurons via Cre-lox technology (92); the overlap of ERα and Tac2 expression in the brain is largely represented by the arcuate kisspeptin neurons. In the targeted CRISPR knock down, arcuate kisspeptin neurons also exhibited increased firing rate and increased levels of glutamatergic transmission. Together with the above, these findings suggest arcuate kisspeptin neurons mediate at least some aspects of negative feedback via ERα. These observations are further consistent with a key role for these cells in generating pulsatile secretion, as normal LH pulse frequency modulation is critical for producing cyclic changes in steroids.
Conclusions and future directions
Application of newer methodologies to the old question of how the action of estradiol switches from negative to positive feedback has brought increased understanding and generated new questions. At the GnRH neuron, both fast-synaptic and intrinsic changes appear to contribute to initiating a robust GnRH surge, but the nature of these signals can be further refined. The postulated roles of AVPV kisspeptin neurons in positive feedback and arcuate neurons in negative feedback have been supported, but how these signals are generated in these cells and then conveyed to GnRH neurons remains largely a mystery. Mechanistic studies of population synchrony and the neurobiology of the interactions between kisspeptin neurons and GnRH neurons need to be pursued. Further investigation of the nature of the estradiol-sensitive inputs to kisspeptin neurons may reveal additional interactions among these cells and/or new populations to study in the question of estradiol feedback.
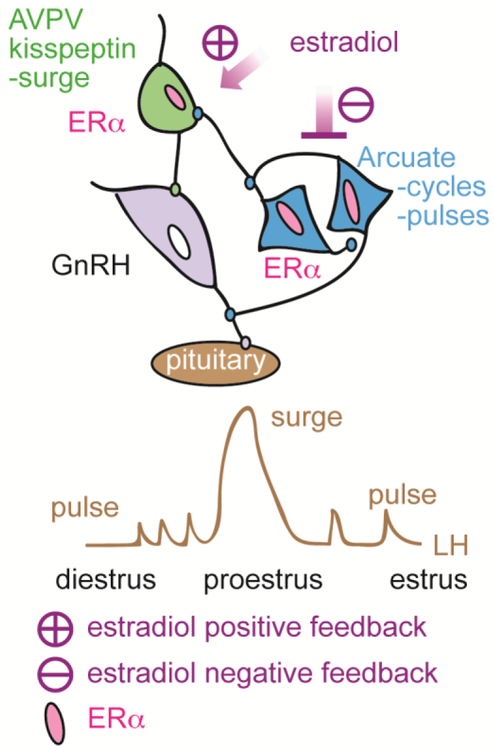
Figure 4.
Schematic diagram of proposed feedback actions of estradiol via AVPV and arcuate kisspeptin neurons. From (97).
Acknowledgements:
We thank Elizabeth Wagenmaker and Laura Burger for expert technical assistance.
Funding sources: Supported by National Institute of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development R01 HD41469 (SMM). CEA was supported by T32 GM007863, T32 HD079342 and F30 HD085721. MS was supported in part by the Brazilian Federal Agency for Support and Evaluation of Graduate Education and the Ministry of Education (CAPES).
Abbreviations:
- ERα
estrogen receptor alpha
- GFP
green-fluorescent protein
- GnRH
gonadotropin-releasing hormone
- LH
luteinizing hormone
- OVX
ovariectomized
- OVX+E
ovariectomized with estradiol implant
- OVX+E+E
ovariectomized with estradiol implant plus estradiol injection
- GABA
gamma-aminobutyric acid
- PSC
postsynaptic currents
- EPSC
excitatory postsynaptic currents
- KERKO
kisspeptin-specific ERα knock out
- AVPV
anteroventral periventricular nucleus
- CRISPR
clustered regularly interspaced short palindromic repeats
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Data sharing statement Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1.Haighton j, Garthshore M. An experimental inquiry concering animal impregnation. Philosoph Trans Royal Soc London 1979; 87 [Google Scholar]
- 2.Marshall FHA, Verney EB. The occurence of ovulation and pseudo-pregnancy in the rabbit as a result of central nervous stimulation. J Physiol 1936; 86:327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris GW. The induction of ovulation in the rabbit by electrical stimulation of the hypothalamo-hypophysial mechanism. . Proceedings of the Royal Society of London Series B, Biological sciences 1937; 122:374–394 [Google Scholar]
- 4.Everett JW, Sawyer CH. A 24-hour periodicity in the "LH-release apparatus" of female rats, disclosed by barbiturate sedation. Endocrinology 1950; 47:198–218 [DOI] [PubMed] [Google Scholar]
- 5.Critchlow V Ovulation induced by hypothalamic stimulation in the anesthetized rat. . Am J Physiol 1958; 195:171–174 [DOI] [PubMed] [Google Scholar]
- 6.Bauer HG. Endocrine and other clinical manifestations of hypothalamic disease; a survey of 60 cases, with autopsies. J Clin Endocrinol Metab 1954; 14:13–31 [DOI] [PubMed] [Google Scholar]
- 7.Evans HM, Long JA. Characteristic Effects upon Growth, Oestrus and Ovulation Induced by the Intraperitoneal Administration of Fresh Anterior Hypophyseal Substance. Proc Natl Acad Sci U S A 1922; 8:38–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans HM, Long JA. The effect of feeding the anterior lobe of the hypophysis on the oestrous cycle of the rat. Anat Rec 1921; 21:62 [Google Scholar]
- 9.Evans HM, Long JA. The effect of the anterior lobe of the hypophysis administered intraperitoneally upon growth and maturity and oestrous cycles of the rat. . Anat Rec 1921; 21:62–63 [Google Scholar]
- 10.Smith PE. Ablation and transplantation of the hypophyses in the rat. Anat Rec 1926; 32:221 [Google Scholar]
- 11.Greep RO. Functional pituitary grafts in rats. . Proc Soc Exp Biol Med 1936; 34:754–755 [Google Scholar]
- 12.Harris GW, Jacobsohn D. Functional grafts of the anterior pituitary gland. Proceedings of the Royal Society of London Series B, Biological sciences 1952; 139:263–276 [DOI] [PubMed] [Google Scholar]
- 13.Baba Y, Arimura A, Schally AV. On the tryptophan residue in porcine LH and FSH-releasing hormone. Biochem Biophys Res Comm 1971; 45:483–487 [DOI] [PubMed] [Google Scholar]
- 14.Silverman AJ, ed. The gonadotropin-releasing hormone (GnRH) neuronal systems: immunocytochemistry and in situ hybridization 2 ed: Raven Press, Ltd, New York, NY; 1994. E Knobil JN, ed. The Physiology of Reproduction; No. 1 [Google Scholar]
- 15.Terasawa E Neuroestradiol in regulation of GnRH release. Hormones and Behavior 2018; 104:138–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Docke F, Dorner G. The mechanism of the induction of ovulation by oestrogens. J Endocrinol 1965; 33:491–499. [DOI] [PubMed] [Google Scholar]
- 17.Moenter SM, Brand RM, Midgley AR, Karsch FJ. Dynamics of gonadotropin-releasing hormone release during a pulse. Endocrinology 1992; 130:503–510 [DOI] [PubMed] [Google Scholar]
- 18.Midgley AR Jr., McFadden K, Padmanabhan V, Karsch FJ, Mauger DT. Neuroendocrine Control of Follicle-Stimulating Hormone (FSH) Secretion. I. Direct Evidence for Separate Episodic and Basal Components of FSH Secretion1. Endocrinology 1997; 138:424–432 [DOI] [PubMed] [Google Scholar]
- 19.Levine JE, Norman RL, Gliessman PM, Oyama TT, Bangsberg DR, Spies HG. In vivo gonadotropin-releasing hormone release and serum luteinizing hormone measurements in ovariectomized, estrogen-treated rhesus macaques. Endocrinology 1985; 117:711–721 [DOI] [PubMed] [Google Scholar]
- 20.Terasawa E Steroid modulation of pulsatile LHRH release in the rhesus monkey. Hormones & Behavior 1994; 28:406–416 [DOI] [PubMed] [Google Scholar]
- 21.Goodman RL, Karsch FJ. Pulsatile secretion of luteinizing hormone: differential suppression by ovarian steroids. Endocrinology 1980; 107:1286–1290 [DOI] [PubMed] [Google Scholar]
- 22.Leipheimer RE, Bona-Gallo A, Gallo RV. Influence of estradiol and progesterone on pulsatile LH secretion in 8-day ovariectomized rats. Neuroendocrinology 1986; 43:300–307 [DOI] [PubMed] [Google Scholar]
- 23.Yamaji T, Dierschke DJ, Bhattacharya AN, Knobil E. The negative feedback control by estradiol and progesterone of LH secretion in the ovariectomized rhesus monkey. Endocrinology 1972; 90:771–777 [DOI] [PubMed] [Google Scholar]
- 24.Kaynard AH, Follett BK, Karsch FJ. Feedback regulation of pulsatile LH secretion in the ewe: stimulation of frequency by estradiol. Neuroendocrinology 1988; 48:81–86 [DOI] [PubMed] [Google Scholar]
- 25.Rossmanith WG, Liu CH, Laughlin GA, Mortola JF, Suh BY, Yen SSC. Relative changes in LH pulsatility during the menstrual cycle: using data from hypogonadal women as a reference point. Clinical Endocrinology 1990; 32:647–660 [DOI] [PubMed] [Google Scholar]
- 26.Veldhuis JD, Beitins IZ, Johnson ML, Serabian MA, Dufau ML. Biologically Active Luteinizing Hormone Is Secreted in Episodic Pulsations that Vary in Relation to Stage of the Menstrual Cycle*. The Journal of Clinical Endocrinology & Metabolism 1984; 58:1050–1058 [DOI] [PubMed] [Google Scholar]
- 27.Sollenberger MJ, Carlsen EC, Johnson ML, Veldhuis JD, Evans WS. Specific Physiological Regulation of Luteinizing Hormone Secretory Events Throughout the Human Menstrual Cycle: New Insights into the Pulsatile Mode of Gonadotropin Release. Journal of Neuroendocrinology 1990; 2:845–852 [DOI] [PubMed] [Google Scholar]
- 28.Moenter SM, Caraty A, Locatelli A, Karsch FJ. Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology 1991; 129:1175–1182 [DOI] [PubMed] [Google Scholar]
- 29.Moenter SM, Brand RC, Karsch FJ. Dynamics of gonadotropin-releasing hormone (GnRH) secretion during the GnRH surge: insights into the mechanism of GnRH surge induction. Endocrinology 1992; 130:2978–2984 [DOI] [PubMed] [Google Scholar]
- 30.Pau KY, Berria M, Hess DL, Spies HG. Preovulatory gonadotropin-releasing hormone surge in ovarian-intact rhesus macaques. Endocrinology 1993; 133:1650–1656 [DOI] [PubMed] [Google Scholar]
- 31.Kaynard AH, Pau KY, Hess DL, Spies HG. Gonadotropin-releasing hormone and norepinephrine release from the rabbit mediobasal and anterior hypothalamus during the mating-induced luteinizing hormone surge. Endocrinology 1990; 127:1176–1185 [DOI] [PubMed] [Google Scholar]
- 32.Sarkar DK, Chiappa SA, Fink G, Sherwood NM. Gonadotropin-releasing hormone surge in pro-oestrous rats. Nature 1976; 264:461–463 [DOI] [PubMed] [Google Scholar]
- 33.Adams JM, Taylor AE, Schoenfeld DA, Crowley WF Jr., Hall JE. The midcycle gonadotropin surge in normal women occurs in the face of an unchanging gonadotropin-releasing hormone pulse frequency. J Clin Endocrinol Metab 1994; 79:858–864 [DOI] [PubMed] [Google Scholar]
- 34.Knobil E, Plant TM, Wildt L, Belchetz PE, Marshall G. Control of the rhesus monkey menstrual cycle: permissive role of hypothalamic gonadotropin-releasing hormone. Science 1980; 207:1371–1373 [DOI] [PubMed] [Google Scholar]
- 35.Hall JE, Taylor AE, Martin KA, Rivier J, Schoenfeld DA, Crowley WF Jr. Decreased release of gonadotropin-releasing hormone during the preovulatory midcycle luteinizing hormone surge in normal women. Proceedings of the National Academy of Sciences of the United States of America 1994; 91:6894–6898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kesner JS, Wilson RC, Kaufman JM, Hotchkiss J, Chen Y, Yamamoto H, Pardo RR, Knobil E. Unexpected responses of the hypothalamic gonadotropin-releasing hormone "pulse generator" to physiological estradiol inputs in the absence of the ovary. Proceedings of the National Academy of Sciences of the United States of America 1987; 84:8745–8749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin KA, Welt CK, Taylor AE, Smith JA, Crowley WF Jr., Hall JE. Is GnRH reduced at the midcycle surge in the human? Evidence from a GnRH-deficient model. Neuroendocrinology 1998; 67:363–369 [DOI] [PubMed] [Google Scholar]
- 38.Karsch FJ, Dierschke DJ, Knobil E. Sexual differentiation of pituitary function: apparent difference bewteen primates and rodents. Science 1973; 179:484–486 [DOI] [PubMed] [Google Scholar]
- 39.Norman RL, Spies HG. Cyclic ovarian function in a male macaque: additional evidence for a lack of sexual differentiation in the physiological mechanisms that regulate the cyclic release of gonadotropins in primates. Endocrinology 1986; 118:2608–2610 [DOI] [PubMed] [Google Scholar]
- 40.Xia L, Van Vugt D, Alston EJ, luckhaus J, Ferin M A surge of gonadotropin-releasing hormone accompanies the estradiol-induced gonadotropin surge in the rhesus monkey. Endocrinology 1992; 131:2812–2820 [DOI] [PubMed] [Google Scholar]
- 41.Woller MJ, Terasawa E. Changes in pulsatile release of neuropeptide-Y and luteinizing hormone (LH)-releasing hormone during the progesterone-induced LH surge in rhesus monkeys. Endocrinology 1994; 135:1679–1686 [DOI] [PubMed] [Google Scholar]
- 42.Franks J, Dror T, Kauffman AS. Analysis of Multiple Positive Feedback Paradigms Demonstrates a Complete Absence of LH Surges and GnRH Activation in Mice Lacking Kisspeptin Signaling1. Biology of Reproduction 2013; 88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bronson FH. The regulation of luteinizing hormone secretion by estrogen: relationships among negative feedback, surge potential, and male stimulation in juvenile, peripubertal, and adult female mice. Endocrinology 1981; 108:506–516 [DOI] [PubMed] [Google Scholar]
- 44.Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA 2005; 102:15682–15687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahoney MM, Sisk C, Ross HE, Smale L. Circadian Regulation of Gonadotropin-Releasing Hormone Neurons and the Preovulatory Surge in Luteinizing Hormone in the Diurnal Rodent, Arvicanthis niloticus, and in a Nocturnal Rodent, Rattus norvegicus. Biology of Reproduction 2004; 70:1049–1054 [DOI] [PubMed] [Google Scholar]
- 46.Legan SJ, Coon GA, Karsch FJ. Role of estrogen as initiator of daily LH surges in the ovariectomized rat. Endocrinology 1975; 96:50–56 [DOI] [PubMed] [Google Scholar]
- 47.Norman RL, Blake CA, Sawyer CH. Estrogen-dependent 24-hour periodicity in pituitary LH release in the female hamster. Endocrinology 1973; 93:965–970 [DOI] [PubMed] [Google Scholar]
- 48.Glanowska KM, Venton BJ, Moenter SM. Fast scan cyclic voltammetry as a novel method for detection of real-time gonadotropin-releasing hormone release in mouse brain slices. J Neurosci 2012; 32:14664–14669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chu Z, Moenter SM. Physiologic regulation of a tetrodotoxin-sensitive sodium influx that mediates a slow afterdepolarization potential in gonadotropin-releasing hormone neurons: possible implications for the central regulation of fertility. J Neurosci 2006; 26:11961–11973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pielecka-Fortuna J, DeFazio RA, Moenter SM. Voltage-Gated Potassium Currents Are Targets of Diurnal Changes in Estradiol Feedback Regulation and Kisspeptin Action on Gonadotropin-Releasing Hormone Neurons in Mice. Biology of Reproduction 2011; 85:987–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun J, Chu Z, Moenter SM. Diurnal In Vivo and Rapid In Vitro Effects of Estradiol on Voltage-Gated Calcium Channels in Gonadotropin-Releasing Hormone Neurons. J Neurosci 2010; 30:3912–3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Christian CA, Moenter SM. Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci 2007; 27:1913–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Christian CA, Pielecka-Fortuna J, Moenter SM. Estradiol suppresses glutamatergic transmission to gonadotropin-releasing hormone neurons in a model of negative feedback in mice. Biol Reprod 2009:1128–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adams C, Stroberg W, DeFazio RA, Schnell S, Moenter SM. Gonadotropin-releasing hormone (GnRH) neuron excitability is regulated by estradiol feedback and kisspeptin. J Neurosci 2018; 38:1249–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagenmaker ER, Moenter SM. Exposure to acute psychosocial stress disrupts the luteinizing hormone surge independent of estrous cycle alterations in female mice. Endocrinology 2017; 158:2593–2602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Silveira MA, Burger LL, DeFazio RA, Wagenmaker ER, Moenter SM. GnRH neuron activity and pituitary response in estradiol-induced vs proestrous luteinizing hormone surges in female mice. Endocrinology 2017; 158:356–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adams C, Chen X, Moenter SM. Changes in GABAergic Transmission to and Intrinsic Excitability of Gonadotropin-Releasing Hormone (GnRH) Neurons during the Estrous Cycle in Mice. eNeuro 2018; 5:ENEURO.0171–0118.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moenter SM, Caraty A, Karsch FJ. The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology 1990; 127:1375–1384 [DOI] [PubMed] [Google Scholar]
- 59.Caraty A, Locatelli A, Martin GB. Biphasic response in the secretion of gonadotrophin-releasing hormone in ovariectomized ewes injected with oestradiol. J ENdocrinol 1989; 123:375–382 [DOI] [PubMed] [Google Scholar]
- 60.Takahashi A, Abe H, Kanda S, Karigo T, Oka Y, Okubo K. Time-of-Day-Dependent Changes in GnRH1 Neuronal Activities and Gonadotropin mRNA Expression in a Daily Spawning Fish, Medaka. Endocrinology 2012; 153:3394–3404 [DOI] [PubMed] [Google Scholar]
- 61.Krishnan KA, Proudman JA, Bolt DJ, Bahr JM. Development of an homologous radioimmunoassay for chicken follicle-stimulating hormone and measurement of plasma FSH during the ovulatory cycle. Comparative biochemistry and physiology Comparative physiology 1993; 105:729–734 [DOI] [PubMed] [Google Scholar]
- 62.Cahill DJ, Wardle PG, Harlow CR, Hull MG. Onset of the preovulatory luteinizing hormone surge: diurnal timing and critical follicular prerequisites. Fertil Steril 1998; 70:56–59 [DOI] [PubMed] [Google Scholar]
- 63.Kerdelhue B, Brown S, Lenoir V, Queenan JT, Jones GS, Scholler R, Jones HW. Timing of initiation of the preovulatory luteinizing hormone surge and its relationship with the circadian cortisol rhythm in the human. Neuroendocrinology 2002; 75:158–163 [DOI] [PubMed] [Google Scholar]
- 64.Bisanti L, Olsen J, Basso O, Thonneau P, Karmaus W. Shift work and subfecundity: a European multicenter study. European Study Group on Infertility and Subfecundity. Journal of occupational and environmental medicine 1996; 38:352–358 [DOI] [PubMed] [Google Scholar]
- 65.Boden MJ, Kennaway DJ. Circadian rhythms and reproduction. Reproduction 2006; 132:379–392 [DOI] [PubMed] [Google Scholar]
- 66.Labyak S, Lava S, Turek F, Zee P. Effects of shiftwork on sleep and menstrual function in nurses. Health care for women international 2002; 23:703–714 [DOI] [PubMed] [Google Scholar]
- 67.Refinetti R, Menaker M. Evidence for separate control of estrous and circadian periodicity in the golden hamster. Behavioral and neural biology 1992; 58:27–36 [DOI] [PubMed] [Google Scholar]
- 68.Lucas RJ, Stirland JA, Darrow JM, Menaker M, Loudon AS. Free running circadian rhythms of melatonin, luteinizing hormone, and cortisol in Syrian hamsters bearing the circadian tau mutation. Endocrinology 1999; 140:758–764 [DOI] [PubMed] [Google Scholar]
- 69.Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev 2010; 31:544–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Christian CA, Moenter SM. Vasoactive Intestinal Polypeptide Can Excite Gonadotropin-Releasing Hormone Neurons in a Manner Dependent on Estradiol and Gated by Time of Day. Endocrinology 2008; 149:3130–3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adams C, DeFazio RA, Christian CA, Milescu LS, Schnell S, Moenter SM. Changes in Both Neuron Intrinsic Properties and Neurotransmission Are Needed to Drive the Increase in GnRH Neuron Firing Rate during Estradiol-Positive Feedback. The Journal of Neuroscience 2019; 39:2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herbison AE, Moenter SM. Depolarising and hyperpolarising actions of GABA(A) receptor activation on gonadotrophin-releasing hormone neurones: towards an emerging consensus. J Neuroendocrinol 2011; 23:557–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DeFazio RA, Heger S, Ojeda SR, Moenter SM. Activation of A-Type {gamma}-Aminobutyric Acid Receptors Excites Gonadotropin-Releasing Hormone Neurons. Mol Endocrinol 2002; 16:2872–2891 [DOI] [PubMed] [Google Scholar]
- 74.Tada H, Kuroki Y, Funabashi T, Kamiya Y, Goto T, Suyama K, Sano A, Mitsushima D, Etgen AM, Takahashi T. Phasic synaptic incorporation of GluR2-lacking AMPA receptors at gonadotropin-releasing hormone neurons is involved in the generation of the luteinizing hormone surge in female rats. Neuroscience 2013; 248:664–669 [DOI] [PubMed] [Google Scholar]
- 75.Chan H, Prescott M, Ong Z, Herde MK, Herbison AE, Campbell RE. Dendritic Spine Plasticity in Gonadatropin-Releasing Hormone (GnRH) Neurons Activated at the Time of the Preovulatory Surge. Endocrinology 2011; 152:4906–4914 [DOI] [PubMed] [Google Scholar]
- 76.Liu X, Porteous R, Herbison AE. Dynamics of GnRH Neuron Ionotropic GABA and Glutamate Synaptic Receptors Are Unchanged during Estrogen Positive and Negative Feedback in Female Mice. eNeuro 2017; 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheong RY, Czieselsky K, Porteous R, Herbison AE. Expression of ESR1 in Glutamatergic and GABAergic Neurons Is Essential for Normal Puberty Onset, Estrogen Feedback, and Fertility in Female Mice. J Neurosci 2015; 35:14533–14543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Piet R, Kalil B, McLennan T, Porteous R, Czieselsky K, Herbison AE. Dominant Neuropeptide Cotransmission in Kisspeptin-GABA Regulation of GnRH Neuron Firing Driving Ovulation. J Neurosci 2018; 38:6310–6322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J Jr., Atkin S, Bookout AL, Rovinsky S, Frazao R, Lee CE, Gautron L, Zigman JM, Elias CF. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience 2011; 173:37–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frazao R, Cravo RM, Donato J Jr., Ratra DV, Clegg DJ, Elmquist JK, Zigman JM, Williams KW, Elias CF. Shift in Kiss1 cell activity requires estrogen receptor alpha. J Neurosci 2013; 33:2807–2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Christian CA, Glidewell-Kenney C, Jameson JL, Moenter SM. Classical estrogen receptor alpha signaling mediates negative and positive feedback on gonadotropin-releasing hormone neuron firing. Endocrinology 2008; 149:5328–5334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszan T, Carpenter CD, Liposits Z, Petersen SL. Detection of estrogen receptor-beta messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 2000; 141:3506–3509 [DOI] [PubMed] [Google Scholar]
- 83.Lehman MN, Ebling FJ, Moenter SM, Karsch FJ. Distribution of estrogen receptor-immunoreactive cells in the sheep brain. Endocrinology 1993; 133:876–886 [DOI] [PubMed] [Google Scholar]
- 84.Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone H-J, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 2006; 52:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin Acts Directly and Indirectly to Increase Gonadotropin-Releasing Hormone Neuron Activity and Its Effects Are Modulated by Estradiol. Endocrinology 2008; 149:1979–1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dumalska I, Wu M, Morozova E, Liu R, van den Pol A, Alreja M. Excitatory Effects of the Puberty-Initiating Peptide Kisspeptin and Group I Metabotropic Glutamate Receptor Agonists Differentiate Two Distinct Subpopulations of Gonadotropin-Releasing Hormone Neurons. J Neurosci 2008; 28:8003–8013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev 2009; 30:713–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lehman MN, Hileman SM, Goodman RL. Neuroanatomy of the Kisspeptin Signaling System in Mammals: Comparative and Developmental Aspects In: Kauffman AS, Smith JT, eds. Kisspeptin Signaling in Reproductive Biology. New York, NY: Springer New York; 2013:27–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 2005; 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- 90.Dubois SL, Acosta-Martinez M, DeJoseph MR, Wolfe A, Radovick S, Boehm U, Urban JH, Levine JE. Positive, but not negative feedback actions of estradiol in adult female mice require estrogen receptor alpha in kisspeptin neurons. Endocrinology 2015; 156:1111–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, Levine JE. Timing and completion of puberty in female mice depend on estrogen receptor CE±-signaling in kisspeptin neurons. Proceedings of the National Academy of Sciences 2010; 107:22693–22698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Greenwald-Yarnell ML, Marsh C, Allison MB, Patterson CM, Kasper C, MacKenzie A, Cravo R, Elias CF, Moenter SM, Myers MG Jr. ERalpha in Tac2 Neurons Regulates Puberty Onset in Female Mice. Endocrinology 2016; 157:1555–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang L, DeFazio RA, Moenter SM. Excitability and Burst Generation of AVPV Kisspeptin Neurons Are Regulated by the Estrous Cycle Via Multiple Conductances Modulated by Estradiol Action. eNeuro 2016; 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang C, Bosch MA, Qiu J, Ronnekleiv OK, Kelly MJ. 17beta-Estradiol increases persistent Na(+) current and excitability of AVPV/PeN Kiss1 neurons in female mice. Mol Endocrinol 2015; 29:518–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang C, Tonsfeldt KJ, Qiu J, Bosch MA, Kobayashi K, Steiner RA, Kelly MJ, Ronnekleiv OK. Molecular mechanisms that drive estradiol-dependent burst firing of Kiss1 neurons in the rostral periventricular preoptic area. Am J Physiol Endocrinol Metab 2013; 305:E1384–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Piet R, Boehm U, Herbison AE. Estrous cycle plasticity in the hyperpolarization-activated current ih is mediated by circulating 17beta-estradiol in preoptic area kisspeptin neurons. J Neurosci 2013; 33:10828–10839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang L, Vanacker C, Burger LL, Barnes T, Shah YM, Myers MG, Moenter SM. Genetic dissection of the different roles of hypothalamic kisspeptin neurons in regulating female reproduction. eLife 2019;8:e43999 DOI: 107554/eLife43999 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang L, Burger LL, Greenwald-Yarnell ML, Myers MG Jr., Moenter SM. Glutamatergic transmission to hypothalamic kisspeptin neurons is differentially regulated by estradiol through estrogen receptor α in adult female mice. J Neurosci 2018; 38:1061–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.DeFazio RA, Elias CF, Moenter SM. GABAergic transmission to kisspeptin neurons is differentially regulated by time of day and estradiol in female mice. J Neurosci 2014; 34:16296–16308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kumar D, Freese M, Drexler D, Hermans-Borgmeyer I, Marquardt A, Boehm U. Murine arcuate nucleus kisspeptin neurons communicate with GnRH neurons in utero. J Neurosci 2014; 34:3756–3766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS. BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology 2010; 151:5807–5817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, Porteous RW, Kim JS, Colledge WH, Iremonger KJ, Herbison AE. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci U S A 2017; 114:E10216–E10223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vanacker C, Moya MR, DeFazio RA, Johnson ML, Moenter SM. Long-Term Recordings of Arcuate Nucleus Kisspeptin Neurons Reveal Patterned Activity That Is Modulated by Gonadal Steroids in Male Mice. Endocrinology 2017; 158:3553–3564 [DOI] [PMC free article] [PubMed] [Google Scholar]