Abstract
Social support during childhood lays the foundation for social relationships throughout the life course and has been shown to predict a wide range of mental and physical health outcomes. Social support measured in late life is prospectively associated with better cognitive aging, but few studies have evaluated social support received earlier in the life course. We quantified the effects of childhood social support, reported retrospectively, on later-life cognitive trajectories and investigated biopsychosocial mechanisms underlying these associations. Latent growth curve models estimated 10-year cognitive trajectories in 8,538 participants (baseline ages 45–93; Mage = 63) in the REasons for Geographic and Racial Differences in Stroke (REGARDS) project. Independent of sociodemographics, childhood socioeconomic status, and household size, greater retrospective childhood social support was associated with better initial episodic memory, but not verbal fluency or cognitive change, in later adulthood. Associations with initial memory level were mediated by sociodemographic and psychosocial variables; specifically, those who reported greater childhood social support reported higher educational attainment and had better physical and emotional health in adulthood, which were each associated with better memory. These results provide support for broad and enduring effects of childhood social support on mental, physical, and cognitive health decades later.
Keywords: Lifespan Perspective, Cognitive Aging, Education, Perceived Stress, Body Mass Index
Social relations play a prominent role in human development. Individuals move through life embedded within a personal network of supportive others (i.e., their social convoy; Kahn & Antonucci, 1980; Antonucci, 2001). The social convoy emerges developmentally from a core of attachment relations in childhood and then expands to include other important relationships as the individual’s social sphere enlarges. While this model considers the importance of social relations at any point in the life course, it also posits that social relations build from previous experiences, further emphasizing the criticality of childhood (Antonucci, Ajrouch & Birditt, 2014). Supportive interactions between an individual and convoy members provide a secure base for individual functioning throughout the life course (Antonucci & Jackson, 1987).
In adulthood, the quality of social relations has been linked to important health outcomes such as depressive symptoms (Antonucci, Fuhrer & Dartigues, 1997), dementia (Amieva et al., 2010), and mortality (Berkman & Syme, 1979), above and beyond quantitative aspects of social relations (i.e., network size, see Blazer, 1982). For example, in a population-based study of 3,777 older adults in France followed over 15 years, reported satisfaction with social relationships at baseline was associated with reduced risk of incident dementia, independent of sex, socioeconomic status, initial cognitive function, functional abilities, chronic diseases, positive affect, marital status, and social network size (Amieva et al., 2010). Despite substantial prior research linking social support to socioemotional and cognitive outcomes in adulthood, the mechanisms underlying these associations are less frequently explored. Uchino (2010) proposed that social support influences morbidity and mortality through multiple processes: behavioral (e.g., positive and negative health behaviors), psychological (e.g., depressive symptoms, control beliefs), and biological (e.g., cardiovascular, neuroendocrine, and immune function).
Given the centrality of social experiences during childhood (e.g., parental attachment) to life course theories of social relations and strong evidence that social relations predict mental, physical, and cognitive health over the short-term (Antonucci et al., 1997; Amieva et al., 2010; Berkman & Syme, 1979), early childhood social support may have long-term effects on later-life development. A recent study of older adults who retrospectively reported their engagement in enriching early-life activities (e.g., taking lessons, playing team sports) found that participating in more activities was associated with higher educational attainment and better cognitive functioning in the domains of processing speed and executive functioning (Chan, Parisi, Moored & Carlson, 2018). To the extent that some of these enriching activities involve a social component, this study provides some support for the overall hypothesis that social aspects of the childhood environment link to later-life cognitive outcomes, though the current study focuses on social support rather than social activities.
Prior research has found that children with unsupportive social relations exhibit biobehavioral profiles that can shape their mental and physical health outcomes across the life course (Repetti, Taylor & Seeman, 2002). In their comprehensive review, Repetti, Taylor and Seeman (2002) differentiate between risky family environments characterized by conflict and aggression versus a cold, unsupportive, or neglectful home. They summarize evidence that the latter family environment is prospectively linked with higher rates of illness and physical complaints several years later, obesity in early adulthood, and to more serious medical conditions in midlife. The authors point to disrupted physiologic/neuroendocrine functioning (e.g., sympathetic-adrenomedullary reactivity, hypothalamic-pituitary-adrenocortical reactivity, and serotonergic functioning), as well as deficits in emotion processing, social competence, and behavioral self-regulation as potential mediating and sustaining factors underlying the enduring physical and mental health effects of unsupportive childhood environments.
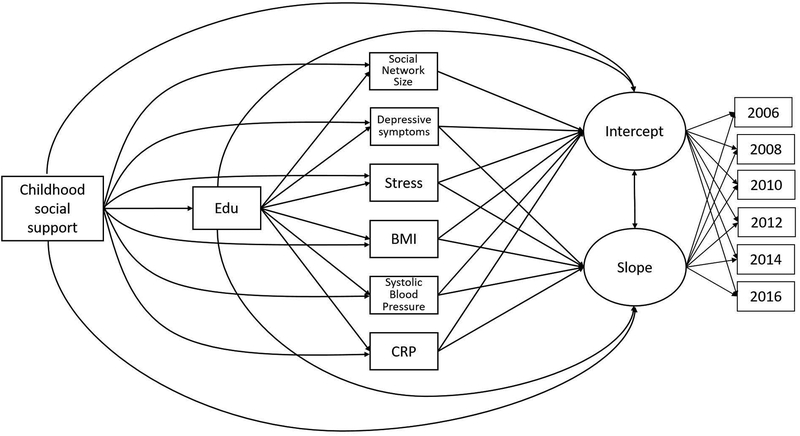
Therefore, we sought to quantify the effects of retrospective reports of childhood social support on later-life cognitive trajectories and investigate biopsychosocial mechanisms underlying these enduring effects. As shown in Figure 1, potential mediators of the relationship between childhood social support and later-life cognition examined in this study were educational attainment, adult social resources (i.e., social network size), psychological functioning (i.e., depressive symptoms and perceived stress), and cardiovascular risk factors (i.e., body mass index, systolic blood pressure, C-reactive protein).
Figure 1.
Schematic of paths estimated in the final mediation model. For simplicity, covariates (i.e., age, sex, race, parental education, and number of adults and children in childhood household) and covariances among the mediators are not shown. Note. Edu = Years of education; Stress = Perceived Stress Scale; BMI = Body mass index; CRP = C-reactive protein.
Potential Mediators of Links between Childhood Social Support and Later-Life Cognition
Educational Attainment
Educational attainment can be influenced, in part, by early-life social experiences. Higher quality relationships between parent and child can facilitate the development of cognitive skills (Estrada, Arsenio, Hess & Holloway, 1987) and/or regulatory behaviors (Hughes & Devine, 2017) that help children thrive in an academic environment. With regard to cognitive skills, positive affective relationships may enhance the flow of information from parents and provide a more stable emotional base from which children can explore the world. Even at very young ages, positive interactions with an adult caregiver can help to scaffold learning of pre-literacy and pre-mathematics skills that prepare children for the classroom environment, such as number and letter recognition (Hess, Holloway, Dickson, & Price, 1984). Compared to children in affectively negative relationships, more securely-attached children have been found to be more likely to engage adults to assist in problem solving, and more likely to initiate and persist in challenging cognitive tasks (Bretherton, 1985). With regard to regulatory behaviors, positive parenting behaviors have been linked to better attention and inhibitory control (Hughes & Devine, 2017), and these regulatory behaviors have also been associated with school success (Devine, Bignardi & Hughes, 2016).
Children’s social relationships, particularly with their primary caregivers, have been linked to a variety of educational outcomes. For example, one prospective, longitudinal study found that higher affective quality of the mother-child relationship at age 4 predicted higher school achievement at age 12, and this longitudinal association was independent of maternal IQ, socioeconomic status, and children’s mental ability at age 4 (Estrada et al., 1987). Better school performance can contribute to higher educational attainment, as it has been shown to predict motivation and potential to pursue higher education (Armbruster, Lehr, & Osborn, 2001). Indeed, analyses of the prospective Minnesota Longitudinal Study of Risk and Adaptation have shown that more positive social relations in childhood predict lower likelihood of dropping out of high school (Jimerson, Egeland, Sroufe, & Carlson, 2000) and higher educational attainment through age 32 (Raby, Roisman, Fraley & Simpson, 2015). These results are in line with the Enduring Effects model of development, which states that early relationship experiences not only organize early developmental adaptation, but continue to shape adjustment across development (Grossmann, Grossmann & Waters, 2006; Sroufe, Egeland, & Kreutzer, 1990; Sroufe, Coffino, & Carlson, 2010).
Educational attainment is one of the strongest positive predictors of late-life cognitive functioning, though evidence for an influence of educational attainment on rate of cognitive decline has been mixed (Anstey & Christensen, 2000; Piccinin et al., 2013; Zahodne et al., 2011; Zahodne, Stern & Manly, 2015). Educational experiences may lead to better cognitive functioning throughout adulthood by promoting more efficient cognitive processes (e.g., strategies) and/or more efficient or compensatory brain processes (Barulli & Stern, 2013).
Social Resources
The convoy model of social relations indicates that social exchanges between children and caregivers have an enduring effect on socioemotional outcomes (Kahn & Antonucci, 1980; Antonucci, 2001). Indeed, higher maternal relationship quality during childhood has been linked to greater availability of social resources later in adulthood, including larger social networks (Raby et al., 2015; Wiseman, Mayseless & Sharabany, 2006; Lee et al., 2015; Sharifian, Kraal, Zaheed, Sol & Zahodne, under revision). This may be because the quality of childhood relationships contributes to the development of secure internal working models of attachment (Bowlby, 1980). These working models of attachment developed during childhood remain relatively stable over time, such that attachments made during adulthood tend to parallel those attachments experienced during childhood (Waters, Merrick, Treboux, Crowell & Albersheim, 2000). Thus, individuals with greater social support during childhood may be more likely to form close social relationships and less likely to experience social isolation later in life.
Greater availability of social resources (i.e., larger social network size) has been associated with better cognitive aging (Barnes, Mendes de Leon, Wilson, Bienias & Evans, 2004; Brenowitz, Kukull, Beresford, Monsell & Williams, 2014; Sӧrman, Rönnlund, Sundströrm, Norberg, & Nilsson, 2017). In one population-based study of older adults, larger social network size (i.e., number of children, relatives and friends seen at least once a month) was associated not only with better cognition at study entry, but also slower subsequent cognitive decline over five years (Barnes, Mendes de Leon, Wilson, Bienias & Evans, 2004). In a clinico-pathological study of older adults, larger social networks buffered the negative cognitive impact of Alzheimer’s-type neuropathology (Bennett, Schneider, Tang, Arnold & Wilson, 2006). It is possible that having a larger social network provides more opportunities for mental stimulation, which could contribute to the development of more resilient cognitive networks, in line with the theory of cognitive reserve (Barulli & Stern, 2013).
Psychological Functioning
Depressive Symptoms.
Depressive symptoms during childhood are tightly linked to the quality of social support received during childhood (Liu, 2003; Papini & Roggman, 1992). For example, childhood social support from mothers has been associated with a decrease in adolescents’ depressive symptoms over time (Eisman, Stoddard, Heinze, Caldwell & Zimmerman, 2015). Parental acceptance can increase children’s perceived self-worth, resulting in the experience of fewer depressive symptoms (Garber, Robinson & Valentiner, 1997). Greater prevalence and persistence of depressive symptoms in childhood is positively associated with the experience of depressive symptoms during adulthood (Horwath, Johnson, Klerman, & Weissman, 1992; Pine, Cohen, Cohen & Brook, 1999).
Depressive symptoms are a consistent predictor of lower late-life cognitive functioning and more rapid cognitive decline (e.g., Zahodne, Stern & Manly, 2014; Lohman et al., 2013; Wilson et al., 2014). One mechanism by which depressive symptoms could lead to late-life cognitive impairment is through behavior. Specifically, depressive symptoms have been negatively associated with health behaviors that predict better cognitive functioning in older adults, including exercise and healthy eating (Williams, Plassman, Burke, Holsinger & Benjamin, 2010).
Perceived Stress.
Stress-sensitivity in adulthood may reflect patterns of social interaction experienced during childhood. Through social learning, positive social interactions can promote the development of emotion regulation skills that can help children cope with stressors as they move through the life course (Yap, Schwartz, Byrne, Simmons & Allen, 2010; Chang, Schwartz, Dodge & McBride-Chang, 2003). For example, in a daily diary study, individuals who retrospectively reported poorer parental relationship quality experienced a higher frequency of stressors (Mallers, Charles, Neupert, & Almeida, 2010). Further, individuals with poorer retrospectively reported maternal relationship quality reported greater subsequent psychological distress (Mallers et al., 2010), suggesting that early life social environment may influence exposure and reactivity to stressful events in adulthood.
Independent of depressive symptoms, greater perceived stress predicts faster subsequent cognitive decline in older adults (Aggarwal et al., 2014; Turner, James, Capuano, Aggarwal & Barnes, 2017). Chronic stress could lead to late-life cognitive impairment via a glucocorticoid cascade (Sapolsky, 2001). Specifically, cortisol dysregulation caused by chronic stress has deleterious effects on brain morphology, particularly in the hippocampus and prefrontal cortex due to their relatively high density of glucocorticoid receptors (Jamieson & Dinan, 2001; Juster, McEwen & Lupien, 2010; Dedovic, Duchesne, Andrews, Engert & Pruessner, 2009; Lupien et al., 1998).
Cardiovascular Factors
Evidence supports broad and enduring effects of childhood social support on cardiovascular risk factors later in life, including body mass index (BMI), hypertension, and inflammation. In turn, individual differences in adult cognition have been attributed to BMI, hypertension, and inflammation. Overall, the evidence reviewed below provides rationale for the hypothesis that these cardiovascular factors may underlie associations between childhood social support and later cognitive functioning.
Body Mass Index.
Higher BMI may be an indicator of poor health behaviors related to diet and physical activity. Youth who report low maternal support show greater increases in BMI decades later, and these effects are independent of socioeconomic status, family structure, anxiety, and depression (Assari & Caldwell, 2017; Assari, Caldwell & Zimmerman, 2015). In turn, high BMI in middle age has been consistently linked to lower cognitive functioning (Chelune, Ortega, Linton & Boustany, 1986; Elias, Elias, Sullivan, Wolf & D’Agostino, 2003; Gunstad, Paul, Cohen, Tate & Gordon, 2006; Gunstad et al., 2007). High BMI and obesity (i.e., greater adiposity) may be detrimental for cognitive health by inducing structural brain changes via insulin resistance, low-grade inflammation, and/or secondary cardiovascular diseases (Biessels, Deary & Ryan, 2008; Shefer, Marcus & Stern, 2013). Indeed, obesity is associated with smaller gray matter volume and lower cortical thickness in frontal and temporal brain regions (Willette & Kapogiannis, 2015).
Hypertension.
Longitudinal work has documented that undergraduates who rated their parents higher in parental caring were less likely to suffer from hypertension 35 years later (Russek & Schwartz, 1997). In turn, hypertension has been linked to worse cognitive aging, particularly when hypertension is measured in mid-life (Iadecola et al., 2016). Hypertension leads to pathological cerebrovascular changes, including white matter damage, micro-infarcts, microbleeds, silent brain infarcts, and brain atrophy, which compromise cognitive functioning (Pantoni, 2010).
Inflammation.
Retrospective reports of greater social support during childhood have also been associated with less peripheral inflammation in midlife (Slopen, Chen, Priest, Albert, & Williams, 2016). Elevated blood levels of C-reactive protein (CRP), an index of peripheral inflammation that has been conceptualized as a biomarker for cardiovascular disease risk, has been linked to worse cognitive aging, independent of other cardiovascular risk factors (Metti et al., 2014; Yaffe et al., 2003). Associations between CRP and cognitive aging outcomes appear to be mediated by neurodegeneration (Bettcher et al., 2012; Marsland et al., 2015).
The Current Study
The overall goal of the current study was to investigate associations between retrospective childhood social support and later-life cognitive trajectories in a sample of adults aged 45 to 93. Because episodic memory and verbal fluency are highly sensitive to age-related decline (Daselaar & Cabeza, 2013; Gomez & White, 2006) and are among the strongest predictors of subsequent risk of progression to dementia (Boraxbekk et al., 2015; Henry, Crawford, & Phillips, 2004), analyses focused on these cognitive domains. Our primary hypothesis was that greater retrospectively assessed childhood social support would be associated with better cognitive functioning, as well as slower age-related cognitive decline. The above-reviewed literature provides rationale for our additional hypotheses that educational attainment, social network size, psychological factors, and cardiovascular factors would each mediate these long-term effects. Specifically, we predicted that greater retrospectively-reported childhood social support would be associated with higher educational attainment, larger social networks, fewer depressive symptoms, less perceived stress, lower BMI, lower blood pressure, and lower CRP in adulthood, which would each be independently associated with better cognitive functioning measured one to two years later, as well as slower subsequent cognitive decline over ten years.
Method
Participants
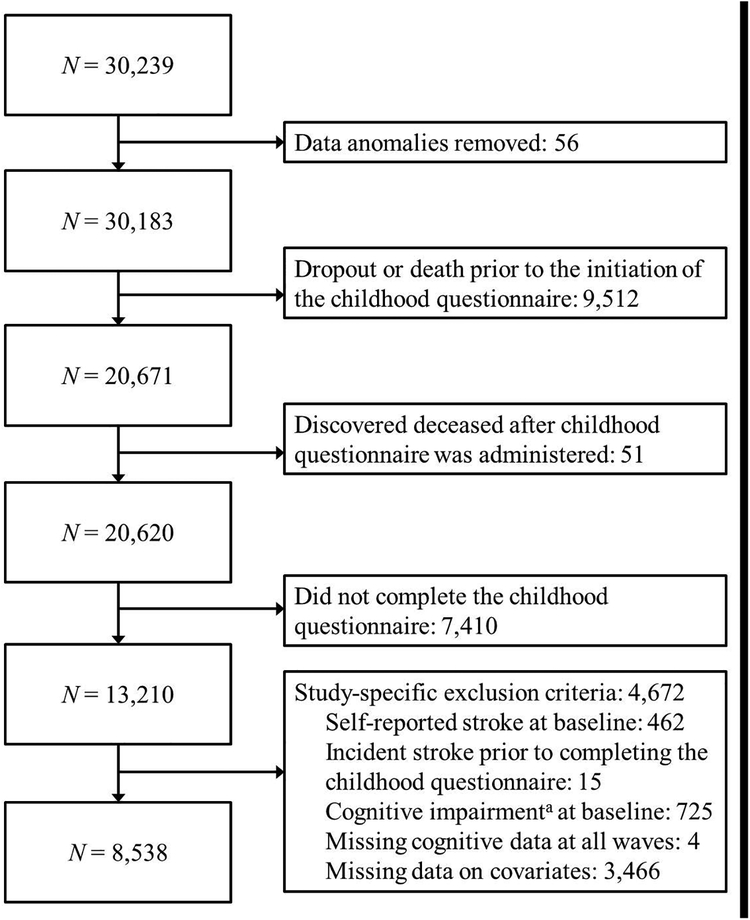
Data were drawn from the REasons for Geographic and Racial Differences in Stroke (REGARDS) project, a national, population-based, longitudinal study of non-Hispanic African American and white adults aged ≥ 45 years (Howard et al., 2005). In brief, participants were identified based on age-race-sex-geographic strata using a commercially available nationwide list. English-speaking, community-dwelling adults were recruited using mail and telephone contacts and were enrolled between 2003 and 2007. Adults living in the Southeastern United States and African Americans were oversampled by design. The final cohort of 30,239 was 45% men and 58% Whites with 56% from the southeastern US and the remainder from the other 40 contiguous states; mean age at enrollment was 65.3 years. Baseline demographic information, medical history, and health status were collected using computer-assisted telephone interviews and self-administered questionnaires. Trained health care professionals collected blood and urine samples, electrocardiograms, blood pressure, height and weight measurements, and medication data during an in-home visit. Telephone follow-up occurs every 6 months. In July 2012, an ancillary study was initiated to collect childhood and family life factors through a questionnaire mailed to all active participants. Figure 2 provides a flow chart describing exclusion criteria resulting in an analysis cohort of 8,538. Table 1 displays unweighted characteristics of the current sample. All participants provided written informed consent, and all study procedures were approved by the institutional review boards at the participating institutions. The current secondary data analysis of de-identified data was reviewed by the institutional review board at the University of Michigan and determined to be exempt from regulation.
Figure 2. Flow chart describing derivation of the final analytic sample.
a Six Item Screener ≤ 4; Callahan, Unverzagt, Hui, Perkins & Hendrie, 2002
Table 1.
Unweighted sample characteristics at baseline and their intercorrelations (N = 8,538)
| Mean (SD) or % | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (45–93) | 62.78 (8.43) | .01 | −.29* | −.27* | −.09* | .12* | −.09* | −.11* | −.07* | .20* |
| Sex (% men) | 43.76 | −.01 | −.21* | .03 | .10* | .09* | −.15* | −.17* | −.01 | .11* |
| Race/ethnicity (% Black) | 24.75 | −.01 | −.09* | −.18* | −.09* | −.09* | .06* | .04* | .19* | .13* |
| Mother education (% high school or less) | 71.33 | .15* | .12* | .24* | .26* | .01 | −.03* | −.01 | −.09* | −.09* |
| Father education (% high school or less) | 71.94 | .12* | .12* | .24* | .27* | .02 | −.04* | −.00 | −.09* | −.09 |
| Adults in childhood household | 2.27 (0.93) | −.02 | −.03* | −.04 | −.05* | .04* | −.01 | −.00 | −.01 | .04* |
| Children in childhood household | 2.60 (1.88) | −.10* | −.05* | −.12* | −.15* | .07* | .02* | .03* | .05* | .04* |
| 1. Childhood social support (1–5) | 4.16 (0.96) | − | − | − | − | − | − | − | − | − |
| 2. Memory (z-score) | 0.27 (0.85) | .06* | − | − | − | − | − | − | − | − |
| 3. Verbal fluency (z-score) | 0.30 (0.89) | .03 | .35* | − | − | − | − | − | − | − |
| 4. Education | 15.03 (2.29) | .13* | .18* | .30* | − | − | − | − | − | − |
| 5. Social network size | 13.65 (10.14) | .10* | −.02 | −.02 | −.04* | − | − | − | − | − |
| 6. Depressive symptoms (012) | 0.81 (1.68) | −.11* | −.05* | −.02 | −.11* | −.10* | ||||
| 7. Perceived stress (0–16) | 2.74 (2.63) | −.13* | −.05* | −.05 | −.09* | −.12* | .44* | − | − | − |
| 8. Body mass index (%) | −.03* | −.08* | −.13* | −.12* | −.02 | .07* | .04* | − | − | |
| Underweight (<18.5 kg/m2) | 0.89 | |||||||||
| Normal (18.5–24.9 kg/m2) | 25.95 | |||||||||
| Overweight (25–29.9 kg/m2) | 38.43 | |||||||||
| Obese (30+ kg/m2) | 34.73 | |||||||||
| 9. Systolic blood pressure (mm/Hg) | 124.52 (15.21) | .00 | −.14* | −.14* | −.11* | .04* | .00 | −.03* | .22* | − |
| 10. C-reactive protein (%) | −.02 | −.02 | −.15* | −.13* | −.03* | .07* | .05* | .33* | .13* | |
| Low (<1 mg/L) | 30.60 | |||||||||
| Moderate (1–3 mg/L) | 34.69 | |||||||||
| High (>3 mg/L) | 34.71 |
Note. CSS = Childhood social support; SD = Standard deviation.
p < .05
Measures
Childhood social support.
Perceived social support during childhood was assessed with a self-administered questionnaire on childhood and family life factors, which was mailed to participants between their 3rd and 5th cognitive assessment, on average. Social support items were modified from the Enhancing Recovery in Coronary Heart Disease (ENRICHD) Social Support Instrument (ESSI; Vaglio et al., 2004) to refer to the retrospective childhood period. Items were prefaced by the statement, “Please read the following questions and mark the answer that best describes what you recall about people in your life who helped you feel important or special during your childhood.” The five items included: “How often was there someone whom you could talk to, trust and confide,” “How often was there someone who showed you love and affection,” “How often was there someone who could help you with your homework,” “How often was there someone who encouraged and pushed you to succeed in school,” and “How often did you have as much contact as you would like with someone you felt close to, someone in whom you could trust and confide.” Items were rated for frequency on a 5-point scale: (1) None of the time; (2) A little of the time; (3) Some of the time; (4) Most of the time; (5) All of the time and averaged and used as a continuous variable, with higher scores indicating greater support. In the current sample, these items showed high internal consistency (α = .91).
Cognition.
Starting one to two years after the initial REGARDS visit (M = 1.56 years; SD = 0.06), the following cognitive measures were administered over the phone by trained interviewers every two years. Episodic memory was assessed with the Consortium to Establish a Registry for Alzheimer’s Disease Word List (CERAD; Fillenbaum et al., 2008), in which participants are asked to recall a list of 10 unrelated words after each of three learning trials. After a 5-minute delay, participants are again asked to recall the list, without hearing the list repeated. Total words recalled across the three learning trials were summed to create an immediate recall score. Immediate and delayed recall scores were converted to z-scores using means and standard deviations from the larger REGARDS cohort at the initial cognitive assessment. At each occasion, immediate and delayed recall z-scores at each visit were averaged to create an episodic memory composite score.
Verbal fluency was assessed with tests of semantic and letter fluency, in which participants were asked to generate the names of animals or words that begin with the letter “F,” respectively, during one minute. Semantic and letter fluency scores were converted to z-scores using means and standard deviations from the larger REGARDS cohort at the initial cognitive assessment. At each occasion, semantic and letter fluency z-scores were averaged to create a verbal fluency composite score.
Mediators.
The current study examined the following socioeconomic, socioemotional, and cardiovascular mediators measured during the REGARDS baseline visit, which were measured one to two years prior to the initial cognitive assessment (M= 1.60 years; SD = 0.06): educational attainment, social network size, depressive symptoms, perceived stress, BMI, systolic blood pressure, and CRP.
Education was operationalized as years of education ranging from 1–14, 16, 18. Social network size was operationalized as the sum of (1) the number of close friends (i.e., “people that you feel at ease with, can talk to about private matters, and can call on for help”) and (2) the number of relatives that “you feel close to,” top-coded at 50. Depressive symptoms were assessed with the four-item version of the Center for Epidemiologic Studies Depression questionnaire (CES-D; Melchior, Huba, Brown & Reback, 1993). CES-D scores in the current study could range from 0 to 12; higher scores indicated a higher level of depressive symptoms (α = .79). Perceived stress was assessed with a short version (4 items) of the Cohen Perceived Stress Scale (PSS; Cohen, Kamarck & Mermelstein, 1983). PSS scores in the current study could range from 0 to 16; higher scores on the PSS indicated greater perceived stress (α = .62).
Height and weight were objectively measured by the health care professionals during the in-home visit. Height was measured using an electronic stadiometer, and weight was measured using a digital scale. BMI was computed as kg/m2 and categorized according to Centers for Disease Control and Prevention guidelines: underweight (>18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), or obese (30+ kg/m2). Individuals who were underweight (<1% of sample) were excluded from mediation analyses. Systolic blood pressure was measured twice using a standard aneroid sphygmomanometer, and the two values were averaged. Systemic inflammation was operationalized as C-reactive protein (CRP) level, obtained from venous blood by particle-enhanced immunonephelometry using the BNII nephelometer (N High Sensitivity CRP, Dade Behring, Deefield, IL). CRP values were categorized according to American Heart Association and Centers for Disease Control and Prevention guidelines for cardiovascular risk: low (<1 mg/L), moderate (1–3 mg/L), and high (>3 mg/L) (Pearson et al., 2003).
A sensitivity analysis also included chronic disease burden as a separate mediator, as it could be a potential confounder of associations between the objective health variables and cognition. Chronic disease burden was operationalized as a count of the following diseases: heart disease, dyslipidemia, diabetes, non-life threatening cancer, and kidney failure. The presence of heart disease was defined via self-report or objective evidence on electrocardiogram. The presence of dyslipidemia was defined via self-report or objective evidence from a lipid panel. The presence of diabetes was determined via self-report or objective evidence from fasting blood glucose levels. The presence of cancer that had not required treatment within two years prior to baseline and kidney failure were determined via self-report.
Covariates.
All models controlled for age, sex, race, parental education, number of adults in childhood household, and number of children in childhood household. Baseline age in years was modeled as a continuous variable and centered at 65, sex was categorized as female or male (reference category was female). Race was self-reported as African American or white (reference category was white). Parental education was self-reported high school or less versus other categories (0 = high school or less; 1 = more than high school) for both the participant’s mother (or other primary female caregiver) and father (or other primary male caregiver). The number of adults and children living in the participant’s childhood household at age 10 were separate continuous variables. Age, sex, and race were obtained from the initial REGARDS visit. Parental education and number of adults in childhood household were both obtained from the mailed questionnaire on childhood and family life factors.
Analytic Strategy
Of the 30,239 REGARDS participants, 20,620 were still in active follow-up at the time of the initial mailing of the childhood questionnaire in 2012, and 13,210 questionnaires were returned (64%). Table 2 summarizes differences between participants who completed the childhood questionnaire and the larger REGARDS analytic sample. Given the potential for selection bias, inverse probability weighting (IPW) was used to re-weight the sample back to the original REGARDS sample (Little & Rubin, 1987; Hernán, Hernández-Diaz, & Robins, 2004). Specifically, weights were created based on logistic regression models of (1) being alive and active at the time the questionnaire was fielded (i.e., censorship weight) and (2) completion of the questionnaire with the following predictors: sex, race, education, self-rated health, stroke, cognitive status based on the Six Item Screener, residence location (i.e., urban, rural, or mixed), smoking, income, health insurance status, cardiovascular health (i.e., Life’s Simple Seven score), kidney failure, previous heart attack, and interaction terms. Thus, weights account for both questionnaire eligibility and questionnaire non-response. Missing values were mean imputed for the weighting models, and the models included a missing indicator for each covariate.
Table 2.
Comparisons between REGARDS participants who did and did not complete the childhood questionnaire
| Did not complete questionnaire (N=16,973) | Completed questionnaire (N=13,210) | Group differencea | Completer sample weighted by IPW based on questionnaire elibility and non-responseb | |
|---|---|---|---|---|
| Age | 65.6 | 63.9 | 15.36 | 64.5 |
| Male | 45.9% | 43.7% | 14.60 | 44.8% |
| White | 49.9% | 69.6% | 1191.9 | 59.0% |
| Education | 1319.36 | |||
| College graduate | 27.7% | 43.8% | 34.8% | |
| Some college | 27.2% | 26.3% | 27.01% | |
| High school graduate | 27.7% | 23.5% | 26.2% | |
| Less than high school | 17.3% | 6.5% | 12.0% | |
| Missing | 0.14% | 0.01% | 0.02% | |
| General Self-Reported | 1201.68 | |||
| Health | ||||
| Excellent | 12.3% | 20.6% | 16.1% | |
| Very Good | 26.5% | 35.4% | 30.8% | |
| Good | 37.1% | 32.4% | 35.2% | |
| Fair | 18.9% | 9.9% | 14.7% | |
| Poor | 5.0% | 1.7% | 3.1% | |
| Missing | 0.28% | 0.11% | 0.13% | |
| Stroke Prevalence | 8.7% | 3.5% | 331.96 | 6.0% |
| Missing | 0.38% | 0.29% | 0.3% | |
| Impaired Cognitive Status | 8.0% | 4.5% | 305.91 | 6.1% |
| Missing | 21.2% | 16.2% | 18.7% | |
| Census Track Size | 123.01 | |||
| Rural | 8.5% | 11.2% | 9.7% | |
| Mixed | 8.9% | 11.0% | 9.9% | |
| Urban | 73.3% | 67.9% | 70.8% | |
| Missing | 9.4% | 9.9% | 9.6% | |
| Income | 1195.34 | |||
| Less than $20k | 22.9% | 12.0% | 17.6% | |
| $20k-$34k | 25.9% | 22.0% | 24.8% | |
| $35k-$74k | 26.2% | 33.9% | 30.0% | |
| $75k and above | 11.4% | 21.3% | 15.8% | |
| Refused | 13.6% | 10.8% | 12.3% | |
| Life Simple Seven | 14.2 | 14.8 | −21.55 | 14.5 |
| Missing | 51.0% | 27.5% | 40.2% | |
| Has Health Insurance | 92.1% | 94.8% | 88.22 | 93.2% |
| Missing | 0.15% | 0.03% | 0.07% | |
| Kidney Failure | 2.6% | 0.9% | 122.24 | 1.4% |
| Missing | 0.6% | 0.6% | 0.6% | |
| Previous MI | 15.3% | 9.3% | 254.33 | 12.4% |
| Missing | 2.1% | 1.7% | 1.9% |
Note. IPW = Inverse probability weight
Chi-squared tests and t-tests used to compare categorical and continuous covariates, respectively. All group differences were statistically significant.
See supplementary material for information on IPW procedures
Descriptive statistics and correlations were computed in SPSS. Episodic memory and verbal fluency trajectories were modeled in Mplus (Muthén & Muthén, 2007) using latent growth curves estimated with maximum likelihood with robust standard errors. Time was parameterized as years from baseline. In the current sample, participants had up to six (M = 3.08; SD = 1.34) time points of memory data and up to five (M = 2.76; SD = 1.00) time points of verbal fluency data, collected biennially between 2006 and 2016. Overall, 5,664 participants had 3 or more occasions of memory data, while 5,331 participants had 3 or more occasions of verbal fluency data. Missing data were managed with full information maximum likelihood. Rather than imputing missing values, missing data were handled within the analysis model using all available data at each occasion to estimate population parameters that would most likely produce the estimates from the sample data.
Separate models estimated episodic memory and verbal fluency trajectories. Initial models adjusting only for baseline age were run to characterize initial cognitive level (intercept) and subsequent rate of cognitive change over the follow-up (linear slope).
To quantify associations between childhood social support and later-life cognition (Aim 1), growth parameters within each model were regressed onto childhood social support, as well as covariates (i.e., age, sex1, race1, mother’s education, father’s education, and number of adults and children in childhood household).
To identify potential mediators of associations between childhood social support and cognitive trajectories (Aim 2), the following variables were simultaneously added to the models: educational attainment, social network size, depressive symptoms, perceived stress, BMI, systolic blood pressure, and CRP. Figure 1 illustrates the Aim 2 mediation model. Within each model, the cognitive score intercept and slope were regressed onto all mediators, and each mediator was regressed onto childhood social support. Because most individuals in this age cohort completed their formal education before age 30, educational attainment is likely to be more proximal to childhood than the other mediators (i.e., social network size, depressive symptoms, perceived stress, systolic blood pressure, BMI, and CRP). Therefore, these psychological and physiological mediators were also regressed onto education. Thus, the mediation models allow for simultaneous, independent indirect effects of reported childhood social support on cognitive trajectories through each of the seven mediators, as well as sequential (i.e., multiple-mediator) indirect effects through educational attainment and each of the other six mediators. Indirect effects correspond to the product of all regression coefficients along the path from childhood social support to cognitive intercept or slope. The intercept and slope were also regressed onto childhood social support to quantify direct effects of childhood social support on cognitive trajectories independent of all mediators and covariates. A subsequent model added chronic disease burden as a separate mediator.
Fit of each model was evaluated with the following commonly used indices: comparative fit index (CFI), Tucker-Lewis index (TLI), root-mean-square error of approximation (RMSEA), and standardized root-mean square residual (SRMR). CFI > 0.95, TLI > 0.95, RMSEA < 0.06, and SRMR < 0.05 were used as criteria for adequate model fit (Hu & Bentler, 1999). Given the non-independence of the parameters estimated within each structural equation model, the False Discovery Rate (FDR) was used to control for Type 1 error.
Results
Bivariate Associations
Table 1 presents sample characteristics and their bivariate associations. Greater reported childhood social support was associated with more education, larger social networks, fewer depressive symptoms, lower perceived stress, and lower BMI. Childhood social support was not significantly associated with systolic blood pressure or CRP. With regard to covariates, greater childhood social support was associated with higher maternal (ρ = .12; p < .001) and paternal (ρ = .10; p < .001) education and fewer children in the household (r = −.10; p < .001). Childhood social support was not significantly associated with age (r = .02; p = .17), race (ρ = −.01; p = .27), or reported number of adult household members (r = −.01; p = .50).
Cognitive Trajectories
Initial models examining change in episodic memory and verbal fluency adjusted only for baseline age. As shown in Table 3, there was significant inter-individual variability in change for both episodic memory and verbal fluency. Importantly, alternative parameterizations of time did not substantially affect the estimates of the associations of interest between childhood social support and cognition reported below.
Table 3.
Standardized effects from weighted latent growth curve models adjusted only for baseline age centered at age 63
| Episodic memory | Verbal fluency | |||
|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | |
| Fixed effects | ||||
| Intercept | 0.006 | −0.014 – 0.026 | 0.011 | 0.088 – 0.133 |
| Slope | 0.106 | 0.067 – 0.144 | −0.794 | −0.928 – −0.660 |
| Random effects | ||||
| Intercept | 0.829 | 0.813 – 0.846 | 0.931 | 0.919 – 0.942 |
| Slope | 0.850 | 0.816 – 0.884 | 0.850 | 0.795 – 0.906 |
| Model fit | ||||
| Chi square (df) | 80.432 (20) | 72.152 (13) | ||
| CFI | 0.995 | 0.996 | ||
| TLI | 0.995 | 0.995 | ||
| RMSEA | 0.014 | 0.017 | ||
| SRMR | 0.022 | 0.018 | ||
Note. CI = confidence interval; df = degrees of freedom; CFI = comparative fit index; TLI = Tucker-Lewis index, RMSEA = root mean square error of approximation; SRMS = standardized root mean square residual.
Childhood Social Support and Cognitive Trajectories
Episodic Memory.
The initial episodic memory model that included childhood social support and covariates fit very well: CFI = 1.00; TLI = .99; RMSEA = .01 (90% CI: .01 to .01), SRMR = .01. As shown in Table 4, greater reported childhood social support was associated with better initial memory. Reported childhood social support was positively but not significantly associated with memory change. Figure 3 depicts model-estimated episodic memory trajectories as a function of childhood social support. Younger age, female sex, white race, higher reported maternal and paternal college education, and fewer reported adult and child household members were also associated with better initial memory performance. The only substantial predictor of memory change was younger age.
Table 4.
Multivariable-adjusted standardized estimates from weighted latent growth curve models
| Initial cognitive level | Annual rate of cognitive change | ||||
|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | ||
| Model 1: Episodic memory | |||||
| Age | −0.361 | −0.386 – −0.336 | −0.371 | −0.424 – −0.319 | |
| Male (ref: female) | −0.290 | −0.316 – −0.264 | 0.022 | −0.022 – 0.067 | |
| Black (ref: white) | −0.178 | −0.207 – −0.148 | 0.036 | −0.014 – 0.086 | |
| Father’s education (ref: high school or less) | 0.056 | 0.029 – 0.083 | −0.036 | −0.082 – 0.010 | |
| Mother’s education (ref: high school or less) | 0.089 | 0.061 – 0.117 | −0.033 | −0.080 – 0.015 | |
| Adults in household | −0.039 | −0.068 – 0.010 | 0.020 | −0.024 – 0.063 | |
| Children in household | −0.057 | −0.085 – −0.028 | −0.031 | −0.080 – 0.018 | |
| Childhood social support | 0.056 | 0.029 – 0.082 | 0.033 | −0.014 – 0.081 | |
| Model 2: Verbal fluency | |||||
| Age | −0.255 | −0.286 – −0.224 | −0.353 | −0.454 – −0.252 | |
| Male (ref: female) | −0.011 | −0.042 – 0.021 | 0.013 | −0.049 – 0.076 | |
| Black (ref: white) | −0.212 | −0.247 – −0.177 | −0.082 | −0.153 – −0.011 | |
| Father’s education (ref: high school or less) | 0.138 | 0.105 – 0.170 | −0.044 | −0.109 – 0.020 | |
| Mother’s education (ref: high school or less) | 0.156 | 0.123 – 0.189 | −0.082 | −0.150 – −0.015 | |
| Adults in household | 0.014 | −0.021 – 0.049 | −0.053 | −0.131 – 0.025 | |
| Children in household | −0.028 | −0.063 – 0.006 | −0.048 | −0.115 – 0.019 | |
| Childhood social support | 0.022 | −0.010 – 0.054 | 0.029 | −0.035 – 0.092 | |
Note. CI = Confidence interval
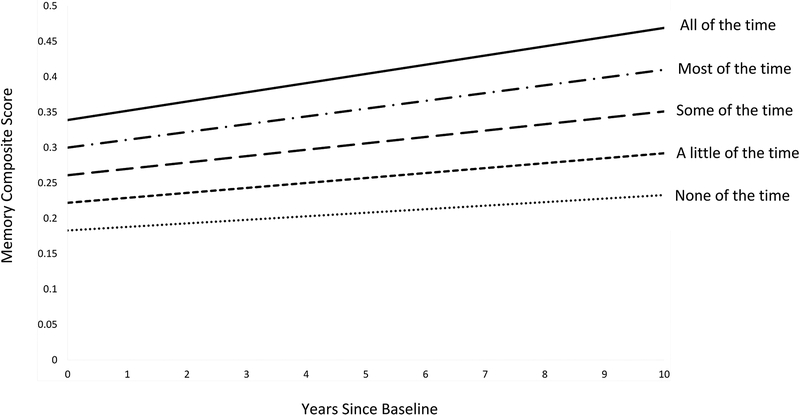
Figure 3.
Model-estimated episodic memory trajectories as a function of the frequency with which social support was available during childhood, averaged across five social support items. Greater childhood social support was significantly associated with higher initial memory (β=0.056; 95% CI: 0.029 – 0.082), but not subsequent memory change (β=0.033; 95% CI: −0.014 – 0.081). Depicted trajectories control for covariates, which were set at the following values: age = 63; sex = female; race = white; father’s education = high school or less; mother’s education = high school or less; number of adults in the childhood household = 2; number of children in the childhood household = 3.
Verbal Fluency.
The initial verbal fluency model that included childhood social support and covariates fit very well: CFI = .99; TLI = .99; RMSEA = .02 (90% CI: .01 to .02), SRMR = .01. As shown in Table 4, younger age, white race, and higher reported maternal and paternal college education were associated with better initial verbal fluency. Reported childhood social support was positively but not significantly associated with initial level of verbal fluency or rate of decline in verbal fluency. The only substantial predictor of less verbal fluency decline was younger age.
Mediators of the Effect of Childhood Social Support on Episodic Memory
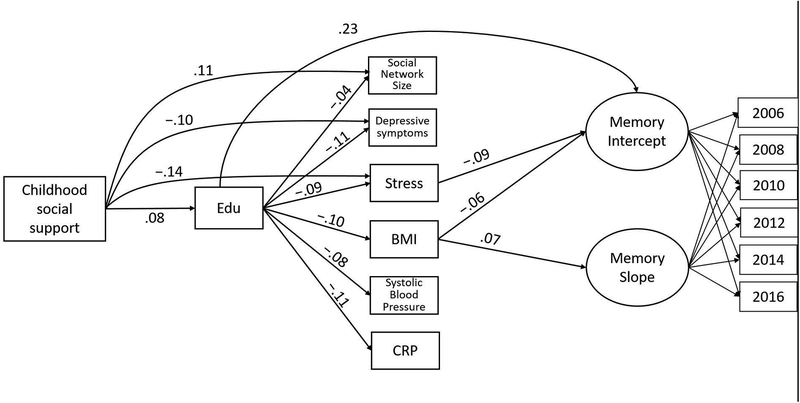
The episodic memory mediation model fit very well: CFI = 0.99; TLI = .98; RMSEA = .01 (90% CI: .01 to .02), SRMR = .01. Results are shown in Table 5 and Figure 4. There were multiple indirect pathways from greater reported childhood social support to better initial memory. Greater reported childhood social support was associated with more education (β=.08; 95% CI: .06 – .11), which was associated with better initial memory (β=.23; 95% CI: .20 – .26). In addition, educational attainment was associated with lower BMI (β=−.10; 95% CI: −.12 – −.08) and lower perceived stress (β=−.09; 95% CI: −.11 – −.07). In turn, lower BMI (β=−.06; 95% CI: −.09 – −.04) and lower perceived stress (β=−.09; 95% CI: −.12 – −.06) were each independently associated with better initial memory. Thus, education mediated the association between reported childhood social support and initial memory through these psychological and health mediators, as well as independent of all mediators. Independent of education, participants reporting greater childhood social support reported less perceived stress at baseline (β=−.14; 95% CI: −.16 – −.11), which was associated with better initial memory (estimate provided above). Independent of the indirect pathways, the positive direct effect of childhood social support on initial memory was no longer significant (see Table 5).
Table 5.
Standardized direct and indirect effects of childhood social support on initial memory level (weighted)
| Estimate | 95% CI | |
|---|---|---|
| Total effect | 0.054 | 0.027 – 0.080 |
| Direct effect | 0.015 | −0.012 – 0.041 |
| Specific indirect effects | ||
| Education | 0.019 | 0.014 – 0.025 |
| Education, social network size | 0.000 | 0.000 – 0.000 |
| Education, depressive symptoms | 0.000 | 0.000 – 0.001 |
| Education, perceived stress | 0.001 | 0.000 – 0.001 |
| Education, body mass index | 0.001 | 0.000 – 0.001 |
| Education, systolic blood pressure | 0.000 | 0.000 – 0.000 |
| Education, C-reactive protein | 0.000 | 0.000 – 0.000 |
| Social network size | 0.002 | −0.001 – 0.005 |
| Depressive symptoms | 0.004 | 0.001 – 0.007 |
| Perceived stress | 0.012 | 0.007 – 0.016 |
| Body mass index | 0.001 | −0.001 – 0.002 |
| Systolic blood pressure | 0.000 | −0.001 – 0.000 |
| C-reactive protein | 0.000 | 0.000 – 0.000 |
Note. CI = Confidence interval
Figure 4.
Schematic of significant paths in the final mediation model. Standardized parameter estimates are shown. For simplicity, covariates (i.e., age, sex, race, parental education, and number of adults and children in childhood household) and covariances among the mediators are not shown. Note. Edu = Years of education; Stress = Perceived Stress Scale; BMI = Body mass index; CRP = C-reactive protein.
While BMI (estimate provided above) was negatively associated with initial memory, there was no single-mediator paths from reported childhood social support to initial memory through BMI because reported childhood social support was not associated with BMI (β=−.01; 95% CI: −.03 – .01) independent of education. While childhood social support was associated with larger social network size (β=.11; 95% CI: .09 – .13) and fewer depressive symptoms (β=−.10; 95% CI: −.12 – −.08), there were no single-mediator paths through either of these variables because neither social network size (β=.02; 95% CI: −.01 – .05) nor depressive symptoms (β=−.04; 95% CI: −.08 – −.01) was independently associated with initial memory. (Note that the association between depressive symptoms and initial memory was not robust to FDR correction.)
There were no single-mediator paths through SBP or CRP because childhood social support was not associated with SBP (β=.01; 95% CI: −.01 – .03) or CRP (β=−.00; 95% CI: −.02 – .02), and neither SBP (β=−.02; 95% CI: −.05 – .01) nor CRP (β=−.00; 95% CI: −.03 – .03) was associated with initial episodic memory.
Due to the absence of a total effect of reported childhood social support on rate of memory change, indirect effects were not examined. The only mediator associated with memory change was BMI. Specifically, higher BMI at baseline was positively associated with memory change (β=.07; 95% CI: .02 – .11).
Post Hoc Analyses
In order to clarify the positive association between higher baseline BMI and memory change, age-stratified models were conducted. It may be the case that this positive association would only be identified among older adults. Positive associations between BMI and cognition among older adults have previously been interpreted in terms of reverse causation, given that weight loss can characterize the preclinical phase of dementia (Kivimäki et al., 2018; Singh-Manoux et al., 2018). Both stratified models fit well; age < 65: CFI = 0.99; TLI = .98; RMSEA = .01 (90% CI: .01 to .02), SRMR = .02; age ≥ 65: CFI = 1.00; TLI = .99; RMSEA = .01 (90% CI: .00 to .01), SRMR = .01. Importantly, the positive association between BMI and memory change was stronger among participants aged 65 and older (β=.09; 95% CI: .02 – .17), compared with participants aged 64 and younger (β=.06; 95% CI: −.01 – .13). This pattern of results is consistent with the evidence that low BMI can be a characteristic of preclinical dementia even before dementia onset (Suemoto, Gilsanz, Mayeda & Glymour, 2015).
A subsequent model added chronic disease burden as a separate mediator in order to determine whether the indirect effects of childhood social support on initial memory level described above were independent of chronic disease burden. This model fit well: CFI = 0.98; TLI = .96; RMSEA = .02 (90% CI: .02 to .02), SRMR = .02. All indirect effects reported above were unchanged, and there were no independent indirect effects through chronic disease burden (β=−.00; 95% CI: −.00 – .00) or through education and chronic disease burden (β=.00; 95% CI: .00 – .00).
Discussion
This study provides evidence that retrospectively assessed childhood social support is associated with episodic memory functioning, independent of reported parental education and household size during childhood. These results are consistent with the notion that development is cumulative, with later functioning building on what came before (Sroufe et al., 2010). Mediation analyses revealed multiple, independent pathways from childhood social support to initial memory functioning through education, perceived stress, and BMI. Together, these variables mediated associations between reported childhood social support and episodic memory. These results provide preliminary evidence for enduring effects of early social environment on mental, physical, and cognitive health decades later and highlight the need for life course longitudinal studies with direct measurements in childhood, mid-life, and late-life to more fully understand individual differences in cognitive aging.
This study extends a substantial body of research linking social support to cognitive development throughout the life course. Most studies have focused on opposite ends of the life course: childhood or late life. For example, greater parental engagement with toddlers predicted stronger perceptual skills, language skills, and executive functioning in kindergarten, independent of maternal education and socioeconomic status (Ayoub, Vallotton & Mastergeorge, 2011; Hirsh-Pasek et al., 2015; Hubbs-Tait, Culp, Culp & Miller, 2002; Narvaez et al., 2013; NICHD Early Child Care Research Network, 2001; O’Connor & McCartney, 2007; Tamis-LeMonda, Shannon, Cabrera & Lamb, 2004). Further, intervention research aimed at increasing the expression of positive parental behaviors (i.e., warmth, sensitivity, responsiveness) has been shown to increase children’s cognitive skills over two years (Sheridan, Knoche, Kupzyk, Edwards & Marvin, 2011). In older adulthood, greater emotional support was prospectively associated with cognitive performance on tests of language, abstraction, spatial ability, and episodic memory 7.5 years later, independent of socioeconomic status, physical health, physical activity, depressive symptoms, and self-efficacy (Seeman, Lusignolo, Albert & Berkman, 2001). The current study bridges these two bodies of literature by examining life course pathways connecting early-life social support to later-life cognitive functioning.
This study also points to life course mechanisms by which childhood social support could have enduring effects on later-life memory functioning. Specifically, people who reported greater social support during childhood were also more highly educated, which was associated with less perceived stress and lower BMI. Each of these factors was independently associated with higher initial memory. People who reported greater social support during childhood also reported larger social networks, fewer depressive symptoms, and less perceived stress later in life. However, of these, only less perceived stress was independently associated with better initial memory. These results suggests that perceived stress may play a more prominent role in linking childhood social support to later life memory than other related socioemotional factors, such as depressive symptoms and social network size. The lack of association between social network size and memory is consistent with several studies that have included more comprehensive measures of socioemotional functioning. These studies have suggested that satisfaction with social networks, perceived social support, and/or contact frequency are more strongly associated with cognitive aging than the number of social ties (Amieva et al., 2010; Zahodne, Ajrouch, Sharifian & Antonucci, in press).
The indirect effect of reported childhood social support on later-life memory through educational attainment is consistent with theoretical and empirical work demonstrating how children’s relationships with their primary caregivers can influence academic competence through the fostering of regulatory behaviors (e.g., inhibitory control, attentional capacity), foundational skills (e.g., literacy and numeracy), and flexibility/creativity associated with enhanced well-being (Estrada et al., 1987; Gregory & Rimm-Kaufman, 2008; Jimerson et al., 2000). Compared with the other mediators examined in this study, educational attainment is also likely to be the most proximal to childhood, as most individuals in this age cohort completed their formal education before age 30. Education may promote aspects of cognitive and neural development during the early developmental period that result in higher cognitive performance throughout adulthood. Education also lays the foundation for a lifetime of advantageous experiences, both cognitive (e.g., cognitively demanding occupations) and non-cognitive (e.g., higher income, better quality neighborhoods, increased access to high-quality health care) that can help individuals maintain cognitive health as they age. Indeed, part of the positive association between education and initial memory level occurred through its inverse association with psychological (i.e., perceived stress) and physical (i.e., BMI) health.
Of the cardiovascular risk factors examined, only BMI was found to be a significant factor on the path from reported childhood social support to initial memory. Specifically, greater reported social support during childhood was associated with higher educational attainment, which in turn was associated with lower BMI, which was associated with better initial memory. These findings are consistent with previous literature demonstrating long-term effects of childhood social support on adult BMI (Assari & Caldwell, 2017; Assari et al., 2015). They further suggest that educational attainment may be a mechanism underlying this effect. Lower education has been associated with poorer health behaviors involving diet and physical activity (Moss, Xiao & Matthews, 2018), which can lead to higher BMI. Thus, the finding that BMI was a significant mediator of link between childhood social support, education, and later-life memory may highlight the importance of behavioral pathways. These findings are also consistent with literature linking mid-life obesity to poorer brain and cognitive aging (Willette & Kapogiannis, 2015; Biessels et al., 2008; Shefer et al., 2013).
Independent of education, there were also indirect effects of reported childhood social support on later-life memory through perceived stress. Cross-sectional and longitudinal associations between received social support and psychological outcomes have been demonstrated in samples spanning the life course, from very early childhood to late life (Liu, 2003; Papini & Roggman, 1992; Yap et al., 2010; Chang, Schwartz et al., 2003; Lee, Kahana & Kahana, 2016). Warm and supportive interactions may foster children’s ability to regulate their emotions and stress responses (Dix, 1991; Power, 2004, Yap et al., 2010), and these skills can be employed to maintain psychological and cognitive health throughout adulthood (Horwath et al., 1992; Pine et al., 1999). The indirect effect of reported childhood social support on episodic memory through perceived stress is consistent with previous work showing that perceived stress is prospectively associated with worse subsequent episodic memory (Aggarwal et al., 2014; Turner et al., 2017). These effects have been attributed to cognitive (e.g., allocation of attentional resources; Jones, Siegle, Muelly, Haggerty & Ghinassi, 2010), behavioral (e.g., health behaviors; Williams et al., 2010), and physiological (e.g., glucocorticoid cascade; Sapolsky, 2001) mechanisms.
The availability of cognitive data in two domains (i.e., episodic memory and verbal fluency) allowed for a preliminary investigation of domain-specific effects. While childhood social support was associated with better episodic memory, it was not associated with better verbal fluency. Given that much of the association between childhood social support and initial memory was mediated by perceived stress, this pattern of results may suggest that episodic memory is more sensitive to stress-related behavioral and physiological dysregulation than verbal fluency due to the particular vulnerability of the hippocampus (Jamieson & Dinan, 2001; Juster et al., 2010; Dedovic et al., 2009; Lupien et al., 1998). Compared with episodic memory tasks, performance on semantic memory tasks may also be more strongly driven by crystallized abilities that are less susceptible to the influence of psychosocial factors. Additional research is needed to determine the extent to which childhood social environment influences other cognitive domains later in life.
In the current study, childhood social support was only associated with initial level of episodic memory functioning, but not with rate of memory change. Although the causal relationships among the variables studied are unclear, it is important to note that intervening on a factor that improves a cognitive intercept but does not slow the rate of cognitive aging per sé could still have long-lasting implications for cognitive morbidity among older adults. Indeed, initial memory level is a major determinant of dementia risk, as individuals who enter late life at a lower level of functioning will reach an impairment threshold sooner than individuals who enter late life at a higher level of functioning, even if both groups exhibit identical rates of decline (Rusmaully et al., 2017). Higher-functioning individuals may thus develop impairment later and experience impairment for a shorter period of time prior to death. At the population level, microsimulation models indicate that delaying the onset of dementia by only five years would result in a 41% reduction of prevalent dementia cases (Zissimopoulos, Crimmins & St. Clair, 2014). Thus, identifying modifiable protective factors that can increase cognitive level has implications for both dementia incidence and prevalence.
Limitations and Future Directions
A primary limitation of this life course study is the use of retrospective reports of social support received during childhood, which can impede causal inferences. While perceptions of childhood relationships remain stable from adolescence to adulthood (Rossi & Rossi, 1990; Bell & Bell, 2018) and through adulthood (Yancura & Aldwin, 2009), retrospective reports can be prone to recall bias. Contemporary factors have the potential to influence reconstructive memory processes (Hardt & Rutter, 2004), but this bias is not present for all outcomes studied, and some evidence suggests that current mood states are only weakly related to these retrospective reports (Yancura & Aldwin, 2009). It is not yet known whether cognitive ability in older adulthood among cognitively intact individuals might systematically bias recall of childhood events and environments. Moreover, most studies comparing prospective and retrospective reports have focused on adverse childhood experiences (ACEs). Less is known about how contemporary factors might affect retrospective recall of other dimensions of the childhood environment, such as childhood social support. The comparability of prospective and retrospective data may depend on the specific aspects of the family environment that are queried. In a 25-year longitudinal study directly comparing prospectively and retrospectively collected data on the family environment, socioemotional aspects of cohesion and conflict predicted adult outcomes (e.g., adult well-being) just as well from retrospective as from prospective measures (Bell & Bell, 2018), but this study did not include adult cognitive outcomes. With growing interest in the influence of childhood on later-life cognitive health, it will be critical to better understand whether and how cognitive ability affects recall accuracy of a range of childhood domains.
Another limitation is that the childhood social support data were collected several years after the initial cognitive assessment. While it is likely that declining cognition could reduce the accuracy of retrospective reports of childhood experiences, it is not clear that it would systematically bias these reports in a negative direction so as to explain the current pattern of results. Rather, this inaccuracy is likely to have introduced non-differential error into the measurement of social support, which could have made it more difficult to detect an association between reported childhood social support and initial cognitive level. Nevertheless, these results should be confirmed through prospective studies that collect social support data directly from children or adolescents and follow them into late life. An additional limitation of this study is the lack of data on social support received during adulthood. While social network size was included as an indicator of adult social resources, the quantity or quality of social support received from network members – regardless of social network size – may have emerged as an additional mediator of the association between childhood social support and memory.
Another limitation of this study is the lack of face-to-face measures of cognitive functioning, as resource limitations in large-scale, national studies often necessitate the collection of cognitive data over the telephone. However, previous studies comparing face-to-face and telephone administration of cognitive instruments indicates that these administration methods are comparable, and telephone administration may even reduce biases related to selection and test anxiety (Unverzagt et al., 2007; Rapp et al., 2012; Castanho et al., 2014). Nevertheless, future studies are needed to replicate the current results using gold-standard neuropsychological assessment.
Strengths of this study include the large, national sample of African American and non-Hispanic white adults, the availability of up to 10 years of longitudinal data on cognitive functioning in two domains, the availability of data on childhood social environment (albeit retrospective), and the use of structural equation modeling to quantify direct and indirect effects of childhood social support on later-life cognitive trajectories. We also included multiple mediators reflecting social, psychological and biological processes measured prior to the collection of the cognitive data in a single model to demonstrate a wide range of associations of reported childhood social support on later-life mental, physical, and cognitive outcomes.
The primary implication of the current findings is that there may be multiple points of intervention to maximize healthy aging. Promoting adequate social support during childhood has the potential to yield broad and lasting beneficial effects across multiple life domains, including cognitive aging. However, even in the absence of adequate childhood social support, fostering educational attainment may have the highest potential for interrupting risk pathways, as the numerically largest specific indirect effect in the current study was the single-mediator path through education, and there were several additional multiple-mediator paths involving education (see Table 5).
In conclusion, although the current study is unable to establish causal effects, our results suggest that greater childhood social support may have enduring effects on later-life memory functioning. These results underscore the importance of the childhood social environment for laying the foundation for better emotional and physical health across the life course. Future studies should evaluate the extent to which policies and interventions designed to enhance the supportiveness of children’s social environments effect healthier trajectories into late life. This study highlights the value of taking a biopsychosocial life course approach to understanding human cognitive development.
Supplementary Material
Acknowledgments
This work was supported by cooperative agreement U01 NS041588 co-funded by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA), National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIA. Representatives of the NINDS were involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. This work occurred as part of the 2016 Advanced Psychometric Methods in Cognitive Aging Research conference funded by the National Institute on Aging (NIA) (R13 AG030995, PI: Mungas). Additional funding was provided by the National Institute on Aging of the National Institutes of Health under Award Numbers R01 AG039588, R01AG054520, and R00AG047963. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at https://www.uab.edu/soph/regardsstudy/.
Footnotes
Exploratory multiple group models tested whether effects of childhood social support differed by race or sex. Specifically, each model was simultaneously estimated in the two groups (either African American/white or male/female). Initially, all regression paths were forced to be equivalent across the two groups. Then, the regression path between childhood social support and intercept, or between childhood social support and slope, was allowed to vary across the two groups. A significant change in the model chi square provided evidence that the specific regression path significantly differed across groups. Multiple group models indicated that associations between childhood social support and cognitive intercept or slope were not significantly different according to race or sex and thus, all subsequent analyses were conducted across all participants with race and sex as covariates.
References
- Aggarwal NT, Wilson RS, Beck TL, Rajan KMB, Mendes de Leon CF, Evans DA, & Everson-Rose SA (2014). Perceived stress and change in cognitive function among adults aged 65 years and older. Psychosomatic medicine, 76, 80–85. 10.1097/PSY.0000000000000016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amieva H, Stoykova R, Matharan F, Helmer C, Antonucci TC, Dartigues JF (2010). What aspects of social network are protective for dementia? Not the quantity but the quality of social interactions is protective up to 15 years later. Psychosomatic Medicine, 72, 905–911. 10.1097/PSY.0b013e3181f5e121 [DOI] [PubMed] [Google Scholar]
- Anstey K & Christensen H (2000). Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitive change in old age: a review. Gerontology, 46, 163–177. 10.1159/000022153 [DOI] [PubMed] [Google Scholar]
- Antonucci TC (2001). Social relations: an examination of social networks social support and sense of control In Birren JE Schaie KW (Eds.), Handbook of the psychology of aging (5th ed., pp. 427–453). New York: Academic Press. [Google Scholar]
- Antonucci TC, Ajrouch KJ, & Birditt KS (2014). The convoy model: explaining social relations from a multidisciplinary perspective. The Gerontologist, 54, 82–92. 10.1093/geront/gnt118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonucci TC, Fuhrer R, & Dartigues J (1997). Social relations and depressing symptomatology in a sample of community-dwelling French older adults. Psychology & Aging, 122, 189–195. 10.1037/0882-7974.12.1.189 [DOI] [PubMed] [Google Scholar]
- Antonucci TC & Jackson JS (1987). Social support, interpersonal efficacy, and health: A life course perspective In Carstensen LL & Edelstein BA (Eds.), Handbook of clinical gerontology (pp. 291–311). New York: Pergamon Press. [Google Scholar]
- Assari S & Caldwell CH (2017). Low family support and risk of obesity among black youth: role of gender and ethnicity. Children, 4, 36 10.3390/children4050036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assari S, Caldwell CH, & Zimmerman MA (2015). Low parental support in late adolescence predicts obesity in young adulthood; gender differences in a 12-year cohort of African Americans. Journal of Diabetes and Metabolic Disorders, 14, 47 10.1186/s40200-015-0176-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub C, Vallotton CD, & Mastergeorge AM (2011). Developmental pathways to integrated social skills: The roles of parenting and early intervention. Child Development, 82, 583–600. doi: 10.1111/j.1467-8624.2010.01549.x [DOI] [PubMed] [Google Scholar]
- Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, & Evans DA (2004). Social resources and cognitive decline in a population of older African Americans and whites. Neurology, 63, 2322–2326. 10.1212/01.WNL.0000147473.04043.B3 [DOI] [PubMed] [Google Scholar]
- Barulli D & Stern Y (2013). Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends in Cognitive Science, 17, 502–509. 10.1016/j.tics.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell DC, & Bell LG (2018). Accuracy of retrospective reports of family environment. Journal of Child and Family Studies, 27, 1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Tang Y, Arnold SE, & Wilson RS (2006). The effect of social networks on the relation between alzheimer’s disease pathology and level of cognitive function in old people: A longitudinal cohort study. The Lancet Neurology, 5, 406–412. 10.1016/S1474-4422. [DOI] [PubMed] [Google Scholar]
- Berkman LF, & Syme SL (1979). Social networks, host resistance, and mortality. A nine-year follow-up study of Alameda county residents. American Journal of Epidemiology, 109, 186–204. 10.1093/aje/kwx103 [DOI] [PubMed] [Google Scholar]
- Bettcher BM, Wilheim R, Rigby T, Green R, Miller JW, Racine CA, Yaffe K, Miller BL, & Kramer JH (2012). C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain, Behavior & Immunity, 26, 103–108. 10.1016/J.BBI.2011.07.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessels GJ, Deary IJ, Ryan CM, (2008). Cognition and diabetes: a lifespan perspective. Lancet Neurol. 7, 184–190. [DOI] [PubMed] [Google Scholar]
- Blazer DG (1982). Social support and mortality in an elderly community population. American Journal of Epidemiology, 115, 684–694. [DOI] [PubMed] [Google Scholar]
- Bowlby J (1980). Attachment and Loss. New York: NY: Basic Books. [Google Scholar]
- Boraxbekk C, Lundquist A, Nordin A, Nyberg L, Nilsson L, & Adolfsson R (2015). Free recall episodic memory performance predicts dementia ten years prior to clinical diagnosis: Findings from the betula longitudinal study. Dementia and Geriatric Cognitive Disorders Extra, 5, 191–202. 10.1159/0002381535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz WD, Kukull WA, Beresford SA, Monsell SE, & Williams EC (2014). Social relationships and risk of incident mild cognitive impairment in U.S. Alzheimer’s disease centers. Alzheimer Disease and Associated Disorders, 28, 253–260. https://doi.org/1097/WAD.0000000000000020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretherton I (1985). Attachment theory: retrospect and prospect. Monographs of the Society for Research in Child Development, 50, 1–2. [PubMed] [Google Scholar]
- Callahan CM, Unverzagt FW, Hui SL, Perkins AJ & Hendrie HC (2002). Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care, 40, 771–781. 10.1097/01.MLR.0000024610.33213.C8 [DOI] [PubMed] [Google Scholar]
- Castanho TC, Amorim L, Zihl J, Palha JA, Sousa N, & Santos NC (2014). Telephone-based screening tools for mild cognitive impairment and dementia in aging studies: a review of validated instruments. Frontiers in Aging Neuroscience, 6, 16 10.3389/fnagi.2014.00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T, Parisi JM, Moored KD, & Carlson MC (2018). Variety of enriching early-life activities linked to late-life cognitive functioning in urban community-dwelling African Americans. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. [Epub ahead of print.] 10.1093/geronb/gby056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Schwartz D, Dodge KA, & McBride-Chang C (2003). Harsh parenting in relation to child emotion regulation and aggression. Journal of Family Psychology, 17, 598–606. 10.1037/0893-3200.17.4.598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelune G, Ortega D, Linton J, & Boustany M (1986). Personality and cognitive findings among patients electing gastroplasty for morbid obesity. International Journal of Eating Disorders, 5, 701–712. 10.1002/1098-108X(198605)5 [DOI] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24, 385–396. 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- Daselaar S & Cabeza R (2013). Age-related decline in working memory and episodic memory In Ochsner Kevin N. & Kosslyn Stephen (Eds) The Oxford Handbook of Cognitive Neuroscience. 10.1093/oxfordhb/9780199988693.013.0022 [DOI] [Google Scholar]
- Dedovic K, Duchesne A, Andrews J, Engert V & Pruessner JC (2009).The brain and the stress axis: the neural correlates of cortisol regulation in response to stress. Neuroimage, 47, 864–871. 10.1016/j.neuroimage.2009.05.074 [DOI] [PubMed] [Google Scholar]
- Devine RT, Bignardi G, & Hughes C (2016). Executive function mediates the relations between parental behaviors and children’s early academic ability. Frontiers in Psychology, 7, 1–15. 10.3389/fpsyg.2016.01902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix T (1991). The affective organization of parenting: Adaptive and maladaptive processes. Psychological Bulletin, 110, 3–25. 10.1037/0033-2909.110.1.3 [DOI] [PubMed] [Google Scholar]
- Eisman AB, Stoddard SA, Heinze J, Caldwell CH & Zimmerman MA (2015). Depressive symptoms, social support and violence exposure among urban youth: A longitudinal study of resilience. Developmental Psychology, 51, 1307–1316. 10.1037/a0039501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias M, Elias P, Sullivan L, Wolf P, & D’Agostino R (2003). Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. International Journal of Obesity, 27, 260–268. 10.1038/sj.ijo.802225 [DOI] [PubMed] [Google Scholar]
- Estrada P Arsenio WF, Hess RD, Holloway SD (1987). Affective quality of the mother-child relationship: Longitudinal consequences for children’s school-relevant cognitive functioning. Developmental Psychology, 23, 210–215. 10.1037/0012-1649.23.2.210 [DOI] [Google Scholar]
- Fillenbaum GG, van Belle G, Morris JC, Mohs RC, Mirra SS, Davis PC, Tariot PN, Silverman JM, Clark CM, Welsh-Bohmer KA, & Heyman A (2008). CERAD (Consortium to establish a registry for alzheimer’s disease) the first 20 years. Alzheimer’s & Dementia, 4, 96–109. 10.1016/j.jalz.2007.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber J, Robinson NS, Valentiner D (1997). The relation between parenting and adolescent depression: self-worth as a mediator. Journal of Adolescent Research, 12, 12–33. 10.1177/0743554897121003 [DOI] [Google Scholar]
- Gomez RG, & White DA (2006). Using verbal fluency to detect very mild dementia of the alzheimer type. Archives of Clinical Neuropsychology, 21, 771–775. https://doi.og/10.1016/j.acn.2006.06.012 [DOI] [PubMed] [Google Scholar]
- Gregory A, & Rimm-Kaufman S (2008). Positive mother-child interactions in kindergarten: Predictors of school success in high school. School Psychology Review, 37, 499–515. [Google Scholar]
- Grossmann KE, Grossmann K, & Waters E (2006). Attachment from infancy to adulthood: The major longitudinal studies. New York, NY: Guilford Press. [Google Scholar]
- Gunstad J, Paul R, Cohen R, Tate D, & Gordon E (2006). Obesity is associated with memory deficits in young and middle-aged adults. Journal of Eating and Weight Disorders/Studies on Anorexia, Bulimia, and Obesity, 11, e15–e19. 10.1007/BF03327747 [DOI] [PubMed] [Google Scholar]
- Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, & Gordon E (2007). Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Comprehensive Psychiatry, 48, 57–61. [DOI] [PubMed] [Google Scholar]
- Hardt J & Rutter M (2004). Validity of adult retrospective reports of adverse childhood experiences: Review of the evidence. Journal of Child Psychology and Psychiatry, 45, 260–273. 10.1111/j.1469-7610.2004.00218.x [DOI] [PubMed] [Google Scholar]
- Henry JD, Crawford JR, & Phillips LH (2004). Verbal fluency performance in dementia of the alzheimer’s type: A meta-analysis. Neuropsychologia, 42, 1212–1222. 10.1016/j.neuropsychologia.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Hernán MA, Hernández-Diaz S, & Robins J (2004). A structural approach to selection bias. Epidemiology, 15, 615–625. [DOI] [PubMed] [Google Scholar]
- Hess RD, Holloway SD, Dickson WP, & Price GG, (1984) Maternal variables as predictors of children’s school readiness and later achievement in vocabulary and mathematics in sixth grade. Child Development, 55, 1902–1912. 10.2307/1129937 [DOI] [Google Scholar]
- Hirsh-Pasek K, Adamson L, Bakeman R, Owen M, Golinkoff R, Pace A, Yust PK & Suma K (2015). The contribution of early communication quality to low income children’s language success. Psychological Science, 26, 1071–1083. 10.1177/0956797615581493 [DOI] [PubMed] [Google Scholar]
- Horwath E, Johnson J, Klerman GL, & Weissman MM (1992). Depressive symptoms as a relative and attributable risk factors for first-onset major depression. Archives of General Psychiatry, 49, 817–823. 10.1001/archpsyc.1992.01820100061011 [DOI] [PubMed] [Google Scholar]
- Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, & Howard G (2005). The Reasons for Geographic and Racial Differences in Stroke Study: objective and design. Neuroepidemiology, 25, 135–143. 10.1159/000086678 [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM (1999). Cutoff criteria for it indexes in covariance structure and analysis: conventional criteria versus new alternatives. SEM, 6, 1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- Hubbs-Tait L, Culp AM, Culp RE, & Miller CE (2002). Relation of maternal cognitive stimulation, emotional support, and intrusive behavior during head start to children’s kindergarten cognitive abilities. Child Development, 73, 110–131. 10.1111/1467-8624.00395 [DOI] [PubMed] [Google Scholar]
- Hughes C, & Devine RT (2017). For better or for worse? Positive and negative parental influences on young children’s executive function. Child Development. [Epu ahead of print] [DOI] [PubMed] [Google Scholar]
- Iadecola C, Yaffe K, Biller J, Bratzke LS, Faraci FM, Gorelick PB, Gulati M, Kamel H, Knopman DS, Launer LJ, Saczynski JS, Seshadri S, & Al Hazzouri AZ (2016). Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. Hypertension, 68, e67–e94. 10.1161/HYP.000000000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson K & Dinan TG (2001). Glucocorticoids and cognitive function: from physiology to pathophysiology. Hum Psychopharmacol, 16, 293–302. 10.1002/hup.304 [DOI] [PubMed] [Google Scholar]
- Jimerson S, Egeland B, Sroufe LA, & Carlson B (2000). A prospective longitudinal study of high school dropouts examining multiple predictors across development. Journal of School Psychology, 6, 525–549. 10.1016/S0022-4405(00)00051-0 [DOI] [Google Scholar]
- Jones NP, Siegle GH, Muelly ER, Haggerty A, & Ghinassi F (2010). Poor performance on cognitive tasks in depression: doing too much or note enough? Cognitive, Affective, & Behavioral Neuroscience, 10, 129–140. 10.3758/CABN.10.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and Biobehavioral Reviews, 35, 2–16. 10.1016/j.neubiorev.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Kahn RL & Antonucci TC (1980). Convoys over the life course: attachment, roles, and social support In Baltes PB Brim O (Eds.), Life-span development and behavior (Vol. 3, pp. 254–283). New York: Academic Press. [Google Scholar]
- Kivimäki M, Luukkonen R, Batty GD, Ferrie JE, Pentti J, … Jokela M (2018). Body mass index and risk of dementia: analysis of individual level data from 1.3 million individuals. Alzheimer’s and Dementia, 14, 601–609. 10.1016/j.jalz.2017.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L, Aldwin CM, Kubzansky LD, Chen E, Mroczek DK, Wang JM, & Spiro A (2015). Do cherished children age successfully? Longitudinal findings from the veterans affair normative aging study. Psychology and Aging, 30, 894–910. 10.1037/pag0000050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Kahana B, & Kahana E (2016). Social support and cognitive functioning as resources for elderly persons with chronic arthritis pain. Aging & Mental Health, 20, 370–379. 10.1080/13607863.2015.1013920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little RJA, & Rubin DB (1987). Statistical analysis with missing data. New York: Wiley. [Google Scholar]
- Liu Y (2003). Parent-child interaction and children’s depression: The relationships between parent-child interaction and children’s depressive symptoms in Taiwan. Journal of Adolescence, 26, 447–457. 10.1016/S0140-1971(03)00029-0 [DOI] [PubMed] [Google Scholar]
- Lohman MW, Rebok GW, Spira AP, Parisi JM, Gross AL, & Kueider AM, (2013). Depressive symptoms and memory among older adults: Results from the ACTIVE memory training intervention. Journal of Aging and Health, 25, 209S–229S. 10.1177/0898264312460573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ (1998). Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neuroscience, 1, 69–73. 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Mallers MH, Charles ST, Neupert SD, & Almeida DM (2010). Perceptions of childhood relationships with mother and father: Daily emotional and stressor experiences in adulthood. Developmental Psychology, 46, 1651–1661. 10.1037/a0021020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Kuan DC, Sheu LK, Krajina K, & Manuck SB (2015). Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav Immun, 48, 195–204. 10.1016/J.BBI.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior LA, Huba GJ, Brown VB, & Reback CJ (1993). A short depression index for women. Educational and Psychological measurement, 53, 1117–1125. [Google Scholar]
- Metti AL, Yaffe K, Boudreau RM, Simonsick EM, Carnahan RM, Satterfield S, Harris TB, Ayonayon HN, Rosano C, Cauley JA, & Health ABC Study. (2014). Trajectories of inflammatory markers and cognitive decline over 10 years. Neurology of Aging, 35, 2785–2790. 10.1016/j.neurobiolaging.2014.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss JL, Xiao Q, & Matthews CE (2018). Patterns of cancer-related health behaviors among middle-aged and older adults: individual- and area-level socioeconomic disparities. Preventative Medicine, 115, 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK., & Muthén BO (2007). Mplus User’s Guide (Sixth Edition). Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Narvaez D, Wang L, Gleason T, Cheng Y, Lefever J & Deng L (2013). The evolved developmental niche and child sociomoral outcomes in chinese 3-year-olds. European Journal of Developmental Psychology, 10, 106–127. 10.1080/17405629.2012.761606 [DOI] [Google Scholar]
- NICHD Early Child Care Research Network. (2001). Nonmaternal care and family factors in early development: An overview of the NICHD study of early child care. Applied Developmental Psychology, 22, 457–492. 10.1016/S0193-3973(01)00092-2 [DOI] [Google Scholar]
- O’Connor E, & McCartney K (2007). Attachment and cognitive skills: An investigation of mediating mechanisms. Journal of Applied Developmental Psychology, 28, 458–476. 10.1016/j.appdev.2007.06.007 [DOI] [Google Scholar]
- Pantoni L (2010). Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurology, 9, 689–701. 10.1016/S1474-4422(10)70104-6 [DOI] [PubMed] [Google Scholar]
- Papini DR & Roggman LA (1992). Adolescent perceived attachment to parents in relation to competence, depression, and anxiety: a longitudinal study. Journal of Early Adolescence, 12, 420–440. 10.1177/0272431692012004005 [DOI] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, … American Heart Association. (2003). Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation, 107(3), 499–511. [DOI] [PubMed] [Google Scholar]
- Piccinin AM, Muniz G, Clouston S, Reynolds CA, Thorvaldsson V, Deary IJ, Deeg DJ, Johansson B, Mackinnon A Spiro A, Starr JM, Skoog I & Hofer SM, (2013). Coordinated analysis of age, sex, and education effects on change in MMSE scores. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 68, 374–390. 10.1093/geronb/gbs077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Cohen E, Cohen P, & Brook J (1999). Adolescent depressive symptoms as predictors of adult depression: Moodiness or mood disorder? American Journal of Psychiatry, 156, 133–135. 10.1176/ajp.156.1.133 [DOI] [PubMed] [Google Scholar]
- Power TG (2004). Stress and coping in childhood: The parents’ role. Parenting: Science and Practice, 4, 271–317. 10.1207/s15327922par0404_1 [DOI] [Google Scholar]
- Raby KL, Roisman GI, Fraley RC, & Simpson JA (2015). The enduring predictive significance of early maternal sensitivity: Social and academic competence through age 32 years. Child Development, 86, 695–708. 10.1111/cdev.12325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp SR, Legault C, Espeland MA, Resnick SM, Hogan PE, … Shumaker SA (2012). Validation of a cognitive assessment battery administered over the telephone. Journal of the American Geriatrics Society, 60, 1616–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, & Seeman TE (2002). Risky families: family social environments and the mental and physical health of offspring. Psychological Bulletin, 128, 330–366. [PubMed] [Google Scholar]
- Rossi AS, & Rossi PH (1990). Of human bonding: Parent-child relations across the life course. New York, NY: de Gruyter. [Google Scholar]
- Rusmaully J, Dugravot A, Moatti J, Marmot MG, Elbaz A, Kivikmaki M, Sabia S, & Singh-Manoux A (2017). Contribution of cognitive performance and cognitive decline to associations between socioeconomic factors and dementia: a cohort study. PLoS Medicine, 14, e1002334 10.1371/journal.pmed.1002334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russek LG & Schwartz GE (1997). Perceptions of parental caring predict health status in midlife: a 35-year follow-up of the Harvard Mastery of Stress Study. Psychosomatic Medicine, 59, 144–149. 10.1097/00006842-199703000-00005 [DOI] [PubMed] [Google Scholar]
- Sapolsky RM (2001). Depression, antidepressants, and the shrinking hippocampus. Proc Natl Acad Sci USA, 98, 12320–12322. 10.1073/pnas.231475998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, Lusignolo TM, Albert M, & Berkman L (2001). Social relationships, social support, and patterns of cognitive aging in healthy, high-functioning older adults: MacArthur studies of successful aging. Health Psychology, 20, 243–255. 10.1037/0278-6133.20.4.243 [DOI] [PubMed] [Google Scholar]
- Sharifian N, Kraal AZ, Zaheed AB, Sol K & Zahodne LB (under revision). Longitudinal socioemotional pathways between retrospective early-life maternal relationship quality and episodic memory in older adulthood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer G, Marcus Y, Stern N (2013). Is obesity a brain disease? Neurosci. Biobehav. Rev 37, 2489–2503. 10.1016/j.neubiorev.2013.07.015 [DOI] [PubMed] [Google Scholar]
- Sheridan S, Knoche L, Kupzyk K, Edwards C, Marvin C (2011). A randomized trial examining the effects of parent engagement on early language and literacy: The getting ready intervention. Journal of School Psychology, 49, 361–383. 10.1016/j.jsp.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A, Dugravot A, Shipley M, Brunner EJ, Elbaz A, Sabis S, & Kivimaki M (2018). Obesity trajectories and risk of dementia: 28 years of follow-up in the Whitehall II Study. Alzheimer’s & Dementia, 14, 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Chen Y, Priest N, Albert MA, & Williams DR (2016). Emotional and instrumental support during childhood and biological dysregulation in midlife. Preventive Medicine, 84, 90–96. 10.1016/j.ypmed.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sörman DE, Rönnlund M, Sundström A, Norberg M, Nilsson L (2017). Social network size and cognitive functioning in middle-aged adults: Cross-sectional and longitudinal associations. Journal of Adult Development, 24, 77–88. 10.1007/s10804-016-9248-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroufe LA, Coffino B, & Carlson EA (2010). Conceptualizing the role of early experience: lessons from the Minnesota longitudinal study. Developmental Review, 30, 36–51. 10.1016/j.dr.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sroufe LA, Egeland B & Kreutzer T (1990). The fate of early experience following developmental change: Longitudinal approaches to individual adaptation in childhood. Child Development, 61, 1363–1373. 10.2307/11w30748. [DOI] [PubMed] [Google Scholar]
- Suemoto CK, Gilsanz P, Mayeda ER, & Glymour MM (2015). Body mass index and cognitive function: the potential for reverse causation. International Journal of Obesity, 39, 1383–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamis-LeMonda C, Shannon J, Cabrera N, & Lamb M (2004). Fathers and mothers at play with their 2- and 3-year-olds: Contributions to language and cognitive development. Child Development, 75, 1806–1820. 10.1111/j.1467-8624.2004.00818.x [DOI] [PubMed] [Google Scholar]
- Turner AD, James BD, Capuano AW, Aggarwal NT & Barnes LL (2017). Perceived stress and cognitive decline in different cognitive domains in a cohort of older African Americans. American Journal of Geriatric Psychiatry, 25, 25–34. 10.1016/j.jagp.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino BN (2010). Social support and healtlh: a review of physiological processes potentially underlying links to disease outcomes. Journal of Behavioral Medicine, 29, 377–387. 10.1007/s10865-006-9056-5 [DOI] [PubMed] [Google Scholar]
- Unverzagt FW, Monahan PO, Moser LR, Zhao Q, Carpenter JS, … Champion VL (2007). The Indiana University telephone-based assessment of neuropsychological status: a new method for large scale neuropsychological assessment. Journal of the International Neurospychological Society, 13, 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaglio J, Conard M, Poston WS, O’Keefe J, Haddock CK, House J, & Spertus JA (2004). Testing the performance of the ENRICHD Social Support Instrument in cardiac patients. Health and Quality of Life Outcomes, 2, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters E, Merrick S, Treboux D, Crowell J, & Albersheim L (2000). Attachment security in infancy and early adulthood: A twenty-year longitudinal study. Child Development, 3, 684–689. 10.1111/1467-8624.00176 [DOI] [PubMed] [Google Scholar]
- Willette AA, Kapogiannis D, (2015). Does the brain shrink as the waist expands? Ageing Res. Rev 20, 1–12. 10.1016/j.arr.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JW, Plassman BL, Burke J, Holsinger T, & Benjamin S (2010). Preventing Alzheimer’s Disease and Cognitive Decline. Evidence Report/Technology Assessment No. 193. (Prepared by the Duke Evidence-based Practice Center under Contract No. HHSA 290-2007-10066-I; AHRQ Publication No. 10-E005). Rockville, MD: Agency for Healthcare Research and Quality. [Google Scholar]
- Wiseman H, Mayseless O & Sharabany R (2006). Why are they lonely? Perceived quality of early relationships with parents, attachment, personality predispositions and loneliness in first-year university students. Personality and Individual Differences, 40, 237–248. 10.1016/j.paid.2005.05.015 [DOI] [Google Scholar]
- Wilson RS, Capuano AW, Boyle PA, Hoganson GM, Hizel LP, … Bennett DA (2014). Clinical-pathologic study of depressive symptoms and cognitive decline in old age. Neurology, 83, 702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Penninx BW, Simonsick EM, Pahor M, Kritchevsky S, Launer L, Kuller L, Rubin S, & Harris T (2003). Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology, 61, 76–80. 10.1212/01.WNL.0000073620.42047.D7 [DOI] [PubMed] [Google Scholar]
- Yancura LA, & Aldwin CM (2009). Stability and change in retrospective reports of childhood experiences over a 5-year period: findings from the Davis Longitudinal Study. Psychology and Aging, 24, 715–721. [DOI] [PubMed] [Google Scholar]
- Yap MBH, Schwartz OS, Byrne ML, Simmons JG, & Allen NB (2010). Maternal positive and negative interaction behaviors and early adolescents’ depressive symptoms: Adolescent regulation as a mediator. Journal of Research on Adolescence, 20, 1014–1043. 10.1111/j.1532-7795.2010.00665.x [DOI] [Google Scholar]
- Zahodne LB, Ajrouch K, Sharifian N & Antonucci T (in press). Social relations and age-related change in memory. Psychology and Aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Glymour MM, Sparks C, Bontempo D, Dixon RA, MacDonald SWS, & Manly JJ (2011). Education does not slow cognitive decline with aging: 12-year evidence from the Victoria Longitudinal Study. Journal of the International Neuropsychological Society, 17, 1039–1046. 10.1017/S1355617711001044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Stern Y, & Manly JJ (2014). Depressive symptoms precede memory decline, but not vice versa, in non-demented older adults. Journal of the American Geriatrics Society, 62, 130–134. 10.1111/jgs.12600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Stern Y, Manly JJ (2015). Differing effects of education on cognitive decline in diverse elders with low versus high educational attainment. Neuropsychology, 29 (4), 649–657. 10.1037/neu0000141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zissimopoulos J, Crimmins E, & St. Clair P (2014). The value of delaying Alzheimer’s disease onset. Forum for Health Economics and Policy, 18, 25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.