Abstract
Endemic Burkitt lymphoma (eBL) is the most common childhood cancer in sub-Saharan African countries, however, few epidemiologic studies have been undertaken and none attempted enrolling cases from multiple countries. We therefore conducted a population-based case-control study of eBL in children aged 0–15 years old in six regions in Northern Uganda, Northern Tanzania, and Western Kenya, enrolling 862 suspected cases and 2,934 population-controls (response rates 98.5–100%), and processing ~40,000 vials of samples using standardized protocols. Risk-factor questionnaires were administered, and malaria period prevalence was measured using rapid diagnostic tests (RDTs). A total of 80.9% of the recruited cases were diagnosed as eBL; 61.4% confirmed by histology. Associations with eBL risk were computed using logistic regression models adjusted for relevant confounders. Associations common in at least two countries were emphasized. eBL risk was decreased with higher maternal income and paternal education and elevated with history of inpatient malaria treatment >12 months before enrollment, and with HIV seropositivity. Reporting malaria-attributed fever up to 6 months before enrollment and malaria-RDT positivity at enrollment were associated with decreased eBL risk. Conversely, reporting exposure to mass malaria suppression programs (e.g., indoor residual insecticide) was associated with elevated risk. HIV was associated with elevated eBL risk. The study shows that it is feasible to conduct networked, multisite population-based studies of eBL in Africa. eBL was inversely associated with socioeconomic status, positively associated with inpatient malaria treatment 12 months ago and with living in areas tageted for malaria suppression, which support a role of malaria in eBL.
Keywords: Burkitt lymphoma, non-Hodgkin lymphoma, epidemiology, Epstein-Barr virus, Plasmodium falciparum malaria, HIV/AIDS
Introduction
Endemic Burkitt lymphoma (eBL) is a life-threatening B-cell lymphoma that occurs relatively commonly (~50/106)1 in children in some countries in equatorial Africa and sporadically elsewhere (~1/106)2. Deleterious chromosomal translocations that place coding regions of c-MYC under regulatory control of immunoglobulin enhancer elements3 are considered primary genetic events both in endemic and sporadic BL4. Epstein-Barr virus (EBV) and Plasmodium falciparum (Pf) malaria are considered co-factors that either increase genetic instability in B cells or increase the systemic load of abnormal B cells, thereby increasing the incidence and skewing of the geographical distribution of BL cases5. Few epidemiologic studies of eBL have been conducted, highlighting rural residence6, frequent malaria attacks, lack of access to mosquito bed nets7, 8, having 3 or more siblings, sharing a bed with siblings, living in a non-monogamous family, and having a deceased parent9 as risk factors for eBL. These risk factors share low socioeconomic status as a common characteristic in eBL children9. The mechanism of socioeconomic status for modulating eBL risk is by modulating the intensity of malaria exposure to the children10 and/or the age of EBV infection11. However, these studies have suffered some limitations, notably, their small sample sizes, not enrolling representative cases or controls, not measuring malaria infection at the time of enrollment, and largely failing to collect biospecimens suitable for use with current proteomic assays or obtaining permission for genetic studies. None recruited multiple, networked sites, thus, the feasibility of this approach to study eBL in Africa is unknown.
Therefore, we enrolled suspected eBL cases and population-based controls in six rural malaria-endemic areas in Northern Uganda, Northeastern Tanzania, and Western Kenya (Figure 1) in the Epidemiology of Burkitt Lymphoma in East African Children and Minors (EMBLEM) study during 2010–201612, 13.
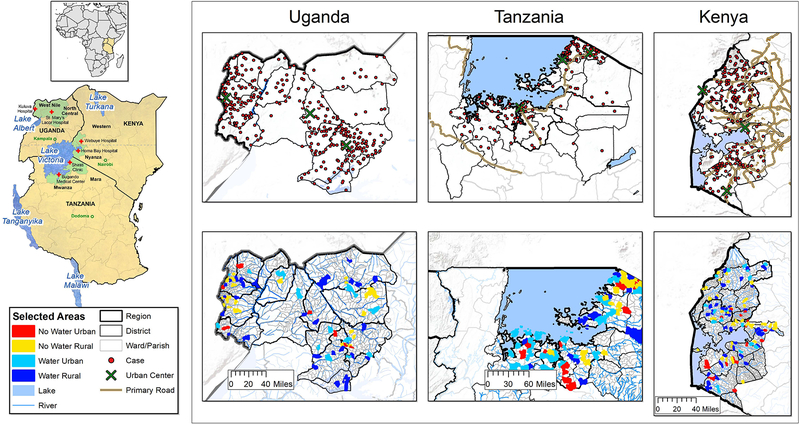
Figure 1.
Map showing the EMBLEM study area marked with green shading and the participating hospitals marked with a red cross (St. Mary’s Hospital, Lacor and Kuluva Hospital in Northern Uganda; Bugando Medical Center and Shirati District Hospital in Northern Tanzania; Homa Bay District and Webuye District Hospitals and Moi University Teaching and Referral Hospital in Western Kenya). Two regions were selected per country. A locator map shows East Africa within Africa. Multi-panel maps show the zoom out of the study areas for each country. The bottom row shows the relief features, including rivers and the location of 100 villages randomly sampled per country as a source of matched population controls. The sampled villages are indicated according to their stratification category, that is, proximity to water and population density (see “Methods” section). The upper row shows the geographical distribution of the cases that were enrolled in the EMBLEM study plotted within their district of origin (large urban centers are marked on the map). The primary all season roads serving the study areas are included to give a rough idea of the geographical dispersion of cases in relation to the villages where the matched population controls were sampled and the transport infrastructure in the study areas.
Methods
Study area, design and population
Suspected eBL cases aged 0–15 years old were recruited at six local district or regional hospitals serving a population living in a defined geographic area illustrated in Figure 1. The ecosystem in this geographic area was characterized by lakes, rivers and swamps, and supports perennial but variable holoendemic malaria transmission for >6 months in the year14, except in parts of Kenya where malaria is seasonal and lasts for shorter durations14. The selected geographic areas have historically had high BL endemicity12, 15. We considered several choices of controls, including health-facility-, school-, or neighborhood-controls. Health-facility controls were rejected because they were markedly younger than eBL cases (2.2 years versus 7.0 years) and were significantly more likely to have malaria symptoms when they were encountered at health facilities16. School-based controls were rejected because non-school going children, who represent a significant proportion of children at risk of eBL, would be excluded from this sampling space.16 Neighborhood controls were considered but rejected because of logistical challenges envisaged to maintain consistent quality of work (a control can only be enrolled after a case has been encountered) across multiple sites in different countries and concerns about low response rates16. Therefore, we enrolled population-based controls of a similar age distribution from 300 random villages (100 villages per country) sampled from national census roster according to urban/rural status and proximity to water, as previously described12, 13. The key assumption of this approach was that children residing in the same area as the cases would be exposed to similar period experiences when broadly matched for age, geography, and sex16. Before sampling, villages in the census roster were categorized as “rural” based on having a census enumeration area (EA) population count of children below 15 years being less than the EA mean population count, otherwise as “urban”12, 13. Villages were further classified as near water (swamp, river, or lake) based on the EA boundary being within 500 m of surface water, otherwise as far from water based on geographical information maps.
To ensure that cases and controls had been exposed to malaria, eligibility was restricted to children aged 0–15 years old who were usual residents (≥ four months prior to enrollment) of the study area. Because our study was conducted in rural areas, where in- and out-migration is relatively low, we did not encounter children who were recent immigrants into the study area. Children who were usual residents of another village in the study area were considered eligible as defined in Figure 1. Cases were defined histologically or cytologically, and when this was not possible, according to clinical features, imaging and laboratory results compatible with a diagnosis of eBL. To increase ascertainment and encourage referral of suspected cases to the six participating hospitals, carefully designed, culturally appropriate health education messages about eBL were developed and disseminated in the study area, stressing the availability of facilitated pathology diagnosis and treatment17.
The primary data collected for the study encompassed individual- and household-level risk factor questions, including age, sex, parental and household exposures, history of inpatient and outpatient malaria treatment, malaria-related and unrelated fevers, and use of indoor residual spraying (IRS) in the house, insecticides, and mosquito bed nets elicited by interviewers following standardized protocols12, 13. The questionnaires were designed in English at the National Cancer Institute and approved by the Technical Evaluation of Questionnaires Committee of the Division of Cancer Epidemiology and Genetics. They were then translated into Swahili and three Luo languages (Acholi, Madi, and Lugbara) that are widely spoken in the region. The season of case or control enrollment was classified as wet or dry, based on country-specific calendars. All subjects provided venous blood specimens (collected pre-treatment in the cases) for research (10 ml) and for clinical tests (4 ml) in EDTA tubes. Clinical specimens were immediately examined by light microscopy for asexual malaria parasite forms and for malaria antigens (HRP-2 and pan-LDH) using commercial malaria-rapid diagnostic tests (malaria-RDTs)12, 13. Malaria antigens remain detectable by RDTs for 35–42 days after treatment of symptomatic malaria.18 Human immunodeficiency virus (HIV) infection was assessed using three approved commercial RDTs (Determine HIV1/2, Stat Pack, Unigold commercial kits) following National Guidelines. Two concordant positive test results were required to give a participant a positive result and refer them to local counselling and treatment centers.
Research samples were transported in cold boxes to field laboratories within two hours of collection and centrifuged for 15 minutes at 1300g to separate into plasma, buffy coat, and red cell fractions for storage at −80°C. About 40,000 vials of samples were processed, and half of the vials (one half from each subject) were shipped under liquid nitrogen vapor to the Frederick National Cancer Laboratory, Frederick, MD.
Data quality
The success of the study was dependent of harmonization of procedures, taking into account prior lessons about eBL epidemiology16, 19 and pathology17. Research infrastructure to support high-quality data and biological sample collection was established and nested within the local health systems.20 Fulltime field staff were hired to implement the study in the three countries and trained by the same instructors at Makerere University College of Health Sciences in Uganda. Compliance with standard protocols was monitored through weekly teleconference calls, periodic field visits, and biennial joint investigator-staff meetings20.
Questionnaires and laboratory forms were processed at the field offices using DataFax, a technology that uses character recognition software to extract data from customized, barcoded forms to generate electronic spreadsheets, to reduce data entry errors. This eliminated the need for double manual entry and reduced the number of computers and data clerks needed to process data. Computerized data were reviewed centrally and corrected before generating analysis files.
Ethical issues
The study was approved by Uganda Virus Research Institute Research and Ethics Committee, Uganda National Council for Science and Technology (H816), Tanzania National Institute for Medical Research (NIMR/HQ/R.8c/Vol. IX/1023), Moi University/Moi Teaching and Referral Hospital Institutional Research and Ethics Committee (000536), and in the US by National Cancer Institute Special Studies Institutional Review Board (10-C-N133). Written informed consent was given by guardians; children aged 7 years or older assented.
Statistical methods
Analyses were performed separately for each country, and then with the data from the three countries combined. Associations of eBL risk with questionnaire variables and period prevalence for malaria, based on malaria- RDT positivity, were are based on odds ratios and 95% confidence intervals (ORs, 95% CIs) adjusted for age (0–2, 3–5, 6–8, 9–11, 12–15 years), sex, and village characteristics (rural/urban or proximity to surface water; “baseline models”) and further for variables with a p<0.05 when added to the baseline models (SAS, Cary, North Carolina). Six other variables about animal exposures, not included in this paper but previously found to be associated with pfPR12, 13, were additionally included in the multivariable models.
We minimized collinearity between variables by dropping one variable of any pair with a Spearman correlation coefficient (ρ)>0.6 (2 pairs in Uganda; 1 pair in Tanzania; and 2 pairs in Kenya), based on having a lower p-value or based on other a priori considerations. Stratified analyses were performed to evaluate the impact of including clinical cases and of enrolling cases from rural versus urban villages. This study was conducted to generate a new resource for eBL research, and the current analysis was conducted to generate baseline data to explore hypotheses; thus, we did not adjust for multiple comparisons, and a two-sided p <0.05 was considered significant. To reduce dangers of overinterpretation of our results, we place greater emphasis on findings that were consistent in at least two countries.
Results
Of 862 suspicious cases recruited, 697 (80.9%) were enrolled as eBL; 428 (61.4%) with histological confirmation. We excluded 165 suspicious cases (135 from Uganda, 12 from Tanzania, and 18 from Kenya) after histological or clinical review. Of the 2970 population-based controls approached, 2,934 (98.8%) were enrolled; 36 controls (Uganda=17, Tanzania=5, and Kenya=14) refused to participate12, 13. Response patterns are shown in Supplementary Figure 1.
The male:female ratio was 1.69:1 among cases and 1.13:1 among controls (Table 1). Cases and controls had similar mean ages in Uganda (8.0 versus 7.7 years) and Tanzania (6.8 years versus 7.4 years) but they were younger in Kenya (6.6 years versus 7.4 years; Table 1). The mean age of the cases in boys and girls was similar in the three countries; 9.5% (n=66) of all the cases were younger than 3 years old [Uganda: 3.1% (n=10); Tanzania: 10.2% (n=13); and Kenya: 17.4% (n=43)]. The proportion of cases younger than 3 years old in histologically confirmed cases was: 5.8% (n=25) [Uganda: 2.0% (n=5); Tanzania: 8.3% (n=3); and Kenya: 12.5% (n=17)]. In all the countries, most cases lived in villages near water (64.8–86.3%). Most cases in Uganda lived in rural villages (62.7%, n=151), but the proportion was slightly over one-third in Tanzania (37.7%, n=46) and slightly over one-half in Kenya (59.0%, n=125).
Table 1:
Characteristics of endemic Burkitt lymphoma cases and controls the EMBLEM Study in Uganda, Tanzania, and Kenya, 2010 – 2016.
| Uganda | Tanzania | Kenya | All countries combined* | |||||
|---|---|---|---|---|---|---|---|---|
| Cases, n (%) | Controls, n (%) | Cases, n (%) | Controls, n (%) | Cases, n (%) | Controls, n (%) | Cases, n (%) | Controls, n (%) | |
| Demographics | ||||||||
| Age, years | ||||||||
| 0–2 | 10 (3.1) | 56 (4.9) | 13 (10.2) | 45 (5.5) | 43 (17.4) | 92 (9.5) | 66 (9.5) | 193 (6.6) |
| 3–5yr | 78 (24.2) | 262 (22.8) | 45 (35.2) | 198 (24.2) | 63 (25.5) | 242 (25.1) | 186 (26.7) | 702 (23.9) |
| 6–8yr | 101 (31.4) | 376 (32.7) | 25 (19.5) | 281 (34.3) | 67 (27.1) | 261 (27.1) | 193 (27.7) | 918 (31.3) |
| 9–11yr | 78 (24.2) | 286 (24.9) | 26 (20.3) | 181 (22.1) | 44 (17.8) | 203 (21.0) | 148 (21.2) | 670 (22.8) |
| 12–15 | 55 (17.1) | 170 (14.8) | 19 (14.8) | 114 (13.9) | 30 (12.2) | 167 (17.3) | 104 (14.9) | 451 (15.4) |
| Mean age, (standard deviation) | 8.0 (3.4) | 7.7 (3.3) | 6.8 (3.8) | 7.4 (3.3) | 6.6 (3.8) | 7.4 (3.7) | 7.3 (3.7) | 7.5 (3.5) |
| Sex | ||||||||
| Female | 120 (38.0) | 541 (47.0) | 56 (44.1) | 387 (47.3) | 80 (32.5) | 448 (46.4) | 256 (37.2) | 1376 (46.9) |
| Male | 196 (62.0) | 609 (53.0) | 71 (55.9) | 432 (52.8) | 166 (67.5) | 517 (53.6) | 433 (62.8) | 1558 (53.1) |
| Missing/Unknown | 6 | 1 | 1 | 8 | ||||
| Male:female ratio | 1.6 | 1.1 | 1.3 | 1.1 | 2.1 | 1.2 | 1.7 | 1.1 |
| Design variables | ||||||||
| Proximity to water† | ||||||||
| Far | 33 (13.7) | 486 (42.3) | 43 (35.3) | 411 (50.2) | 55 (26.1) | 500 (51.8) | 131 (22.8) | 1397 (47.6) |
| Near | 208 (86.3) | 664 (57.7) | 79 (64.8) | 408 (49.8) | 156 (73.9) | 465 (48.2) | 443 (77.2) | 1537 (52.4) |
| Missing/Unknown | 81 | 6 | 36 | 123 | ||||
| Population density of children 0–15 years‡ | ||||||||
| Low | 151 (62.7) | 753 (65.5) | 46 (37.7) | 418 (51.0) | 125 (59.0) | 652 (67.6) | 322 (56.0) | 1823 (62.1) |
| High | 90 (37.3) | 397 (34.5) | 76 (62.3) | 401 (49.0) | 87 (41.0) | 313 (32.4) | 253 (44.0) | 1111 (37.9) |
| Missing/Unknown | 81 | 6 | 35 | 122 | ||||
| Year of study enrollment | ||||||||
| 2010–2012 | 109 (33.9) | 68 (5.9) | 20 (15.6) | 21 (8.5) | 150 (21.5) | 68 (2.3) | ||
| 2013–2015 | 177 (55.0) | 1082 (94.1) | 96 (75.0) | 178 (21.7) | 206 (83.4) | 369 (38.2) | 479 (68.7) | 1629 (55.5) |
| 2016 | 36 (11.2) | 12 (9.4) | 641 (78.3) | 20 (8.1) | 596 (61.8) | 68 (9.8) | 1237 (42.2) | |
This column shows the observed characteristics based on data from all the three study countries combined; the percentages are for columns.
Villages were classified as ‘near water’ if any part of their boundary was within 500 meters of surface water; otherwise they were classified as ‘far from water’.
Villages were classified as ‘high population-density’, a surrogate for urban areas, if the population count of children aged 0–15 years was greater than or equal to the average population count for census enumeration areas in the regions studied for each country; otherwise as ‘low population-density’ (See Methods).
Associations of eBL with malaria-RDT and measures of mass malaria suppression
More cases were diagnosed during the wet season in Uganda (versus dry: OR=2.18, p<0.0001) and Tanzania (OR=1.49, p=0.05), but fewer in Kenya (OR= 0.45, p<0.0001; Table 2). Compared to controls, malaria-RDT positivity in blood was lower in eBL cases in Uganda (OR=0.44, p<0.0001), Tanzania (OR=0.36, p=0.0001), and Kenya (OR=0.26, p<0.0001). The markedly lower malaria-RDT positivity in eBL cases was not explained by exposure to IRS in the past 12 months (Figure 2A) or bed net ownership/use (Figure 2B), although eBL cases were more likely to report owning a bed net, particularly in Uganda (Figure 2C), suggesting a small bias.
Table 2:
Associations between endemic Burkitt lymphoma and patient characteristics in Uganda, Tanzania, and Kenya, 2010–2016.
| Uganda | Tanzania | Kenya | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases, n (%) | Controls, n (%) | OR* (95% CI) | Cases, n (%) | Controls, n (%) | OR* (95% CI) | Cases, n (%) | Controls, n (%) | OR* (95% CI) | |
| Characteristics | |||||||||
| Season† | |||||||||
| Dry | 138 (42.9) | 731 (63.6) | Ref | 47 (36.7) | 395 (48.2) | Ref | 108 (43.7) | 267 (27.7) | Ref |
| Wet | 184 (57.1) | 419 (36.4) | 2.18 (1.63, 2.92) | 81 (63.3) | 424 (51.8) | 1.49 (1.00, 2.23) | 139 (56.3) | 698 (72.3) | 0.45 (0.33, 0.62) |
| p-value | <0.0001 | 0.050 | <0.0001 | ||||||
| Measures of malaria | |||||||||
| Indoor residual insecticide sprayed in house | |||||||||
| No | 191 (61.0) | 752 (65.7) | Ref | 38 (30.7) | 551 (68.2) | Ref | 190 (81.9) | 880 (92.5) | Ref |
| Yes | 122 (39.0) | 393 (34.3) | 1.02 (0.75, 1.38) | 86 (69.4) | 257 (31.8) | 5.27 (3.43, 8.09) | 42 (18.1) | 71 (7.5) | 3.13 (1.97, 4.96) |
| Missing/Unknown | 9 | 5 | 4 | 11 | 15 | 14 | |||
| p-value | 0.906 | <0.0001 | <0.0001 | ||||||
| Mosquito net ownership and use the night before | |||||||||
| No | 125 (39.8) | 767 (67.1) | Ref | 21 (16.7) | 289 (35.4) | Ref | 44 (18.9) | 322 (33.8) | |
| Yes, but not used | 35 (11.2) | 11 (1.0) | 22.5 (9.94, 51.0) | 17 (13.5) | 30 (3.7) | 8.29 (3.81, 18.1) | 38 (16.3) | 43 (4.5) | 7.09 (3.91, 12.8) |
| Yes, and used | 154 (49.0) | 366 (32.0) | 2.90 (2.12, 3.97) | 88 (69.8) | 497 (60.9) | 2.19 (1.31, 3.67) | 151 (64.8) | 589 (61.7) | 1.90 (1.26, 2.88) |
| Missing/Unknown | 8 | 6 | 2 | 3 | 14 | 11 | |||
| p-value | <0.0001 | <0.0001 | <0.0001 | ||||||
| Regularly uses mosquito insecticide sprays | |||||||||
| No | 298 (95.2) | 1126 (98.4) | Ref | 114 (89.8) | 775 (95.2) | Ref | 226 (97.0) | 924 (97.5) | Ref |
| Yes | 15 (4.8) | 18 (1.6) | 5.62 (2.62, 12.0) | 13 (10.2) | 39 (4.8) | 2.31 (1.15, 4.62) | 7 (3.0) | 24 (2.5) | 1.26 (0.49, 3.26) |
| Missing/Unknown | 9 | 6 | 1 | 5 | 14 | 17 | |||
| p-value | <0.0001 | 0.019 | 0.628 | ||||||
| Malaria rapid diagnostic test | |||||||||
| Negative | 204 (65.4) | 561 (49.0) | Ref | 95 (80.5) | 473 (58.7) | Ref | 192 (84.2) | 542 (57.0) | Ref |
| Positive | 108 (34.6) | 583 (51.0) | 0.44 (0.32, 0.60) | 23 (19.5) | 333 (41.3) | 0.36 (0.22, 0.59) | 36 (15.8) | 409 (43.0) | 0.26 (0.17, 0.39) |
| Missing/Unknown | 10 | 6 | 10 | 13 | 19 | 14 | |||
| p-value | <0.0001 | 0.0001 | <0.0001 | ||||||
| History of fevers, malaria treatment and hospital admission | |||||||||
| Has fever at enrollment | |||||||||
| No | 136 (43.5) | 1122 (97.9) | Ref | 41 (32.3) | 767 (94.0) | Ref | 70 (31.1) | 836 (87.5) | Ref |
| Yes | 177 (56.6) | 24 (2.1) | 49.7 (30.4, 81.2) | 86 (67.7) | 49 (6.0) | 32.1 (19.6, 52.7) | 155 (68.9) | 120 (12.6) | 16.5 (11.1, 24.4) |
| Missing/Unknown | 9 | 4 | 1 | 3 | 22 | 9 | |||
| p-value | <0.0001 | <0.0001 | <0.0001 | ||||||
| ≥1 fever up to 12 months before enrollment | |||||||||
| No | 31 (22.8) | 254 (22.6) | Ref | 11 (26.8) | 115 (15.0) | Ref | 54 (76.1) | 320 (38.2) | Ref |
| Yes | 105 (77.2) | 868 (77.4) | 0.82 (0.51, 1.31) | 30 (73.2) | 652 (85.0) | 0.49 (0.23, 1.03) | 17 (23.9) | 517 (61.8) | 0.22 (0.12, 0.40) |
| Missing/Unknown | 186 | 28 | 87 | 52 | 176 | 128 | |||
| p-value | 0.401 | 0.059 | <0.0001 | ||||||
| ≥1 fever due to malaria up to 6 months before enrollment | |||||||||
| No | 113 (36.2) | 313 (27.3) | Ref | 42 (33.6) | 250 (30.7) | Ref | 108 (48.7) | 288 (30.1) | Ref |
| Yes | 199 (63.8) | 832 (72.7) | 0.56 (0.41, 0.76) | 83 (66.4) | 565 (69.3) | 0.90 (0.60, 1.37) | 114 (51.4) | 668 (69.9) | 0.47 (0.34, 0.65) |
| Missing/Unknown | 10 | 5 | 3 | 4 | 25 | 9 | |||
| p-value | 0.0002 | 0.636 | <0.0001 | ||||||
| ≥1 fever not due to malaria up to 6 months before enrollment | |||||||||
| No | 181 (59.5) | 1062 (92.9) | Ref | 44 (34.9) | 599 (73.6) | Ref | 100 (44.6) | 772 (81.1) | Ref |
| Yes | 123 (40.5) | 81 (7.1) | 8.40 (5.79, 12.2) | 82 (65.1) | 215 (26.4) | 5.17 (3.42, 7.82) | 124 (55.4) | 180 (18.9) | 5.17 (3.67, 7.28) |
| Missing/Unknown | 18 | 7 | 2 | 5 | 23 | 13 | |||
| p-value | <0.0001 | <0.0001 | <0.0001 | ||||||
| ≥1 hospital admission | |||||||||
| No | 112 (35.7) | 626 (54.7) | Ref | 31 (24.4) | 516 (63.2) | Ref | 71 (30.3) | 697 (73.2) | Ref |
| Yes | 202 (64.3) | 519 (45.3) | 2.01 (1.49, 2.70) | 96 (75.6) | 300 (36.8) | 5.45 (3.50, 8.51) | 163 (69.7) | 255 (26.8) | 6.67 (4.70, 9.47) |
| Missing/Unknown | 8 | 5 | 1 | 3 | 13 | 13 | |||
| p-value | <0.0001 | <0.0001 | <0.0001 | ||||||
| Inpatient malaria treatment | |||||||||
| Yes, past 12 months | 24 (7.6) | 150 (13.1) | Ref | 10 (7.9) | 106 (13.0) | Ref | 35 (15.0) | 95 (10.0) | Ref |
| Yes, > 12 months | 82 (26.1) | 269 (23.5) | 2.55 (1.39, 4.67) | 13 (10.2) | 136 (16.7) | 1.15 (0.47, 2.82) | 12 (5.1) | 117 (12.3) | 0.24 (0.11, 0.50) |
| Never | 208 (66.2) | 725 (63.4) | 2.04 (1.17, 3.57) | 104 (81.9) | 574 (70.3) | 1.94 (0.94, 3.99) | 187 (79.9) | 743 (77.8) | 0.55 (0.35, 0.86) |
| Missing/Unknown/Unknown | 8 | 6 | 1 | 3 | 13 | 10 | |||
| p-value | 0.010 | 0.066 | 0.001 | ||||||
| Outpatient malaria treatment | |||||||||
| Yes, past 12 months | 153 (48.9) | 532 (46.5) | Ref | 62 (48.8) | 255 (31.3) | Ref | 106 (45.3) | 539 (56.4) | Ref |
| Yes, > 12 months | 73 (23.3) | 90 (7.9) | 3.69 (2.44, 5.56) | 14 (11.0) | 69 (8.5) | 0.87 (0.45, 1.68) | 23 (9.8) | 64 (6.7) | 1.86 (1.05, 3.28) |
| Never | 87 (27.8) | 523 (45.7) | 0.88 (0.63, 1.24) | 51 (40.2) | 492 (60.3) | 0.44 (0.29, 0.67) | 105 (44.9) | 352 (36.9) | 1.49 (1.06, 2.09) |
| Missing/Unknown | 9 | 5 | 1 | 3 | 13 | 10 | |||
| p-value | <0.0001 | 0.0004 | 0.021 | ||||||
| HIV status | |||||||||
| Negative | 302 (98.1) | 1135 (99.4) | Ref | 114 (96.6) | 775 (99.9) | Ref | 203 (93.1) | 936 (98.4) | Ref |
| Positive | 6 (2.0) | 7 (0.6) | 5.49 (1.52, 19.9) | 4 (3.4) | 1 (0.1) | 25.6 (2.76, 236) | 15 (6.9) | 15 (1.6) | 4.32 (1.90, 9.86) |
| Missing/Unknown | 14 | 8 | 10 | 43 | 29 | 14 | |||
| p-value | 0.009 | 0.004 | 0.001 | ||||||
| Parental characteristics | |||||||||
| Mother’s education | |||||||||
| Up to standard 4 | 200 (63.7) | 581 (50.8) | Ref | 41 (32.3) | 201 (24.6) | Ref | 36 (15.7) | 182 (19.0) | Ref |
| Standard 5–7 | 100 (31.9) | 446 (39.0) | 0.70 (0.51, 0.95) | 82 (64.6) | 570 (69.9) | 0.74 (0.48, 1.13) | 105 (45.7) | 455 (47.6) | 1.36 (0.84, 2.21) |
| ≥ Senior secondary school | 14 (4.5) | 117 (10.2) | 0.51 (0.28, 0.96) | 4 (3.2) | 45 (5.5) | 0.43 (0.14, 1.29) | 89 (38.7) | 319 (33.4) | 1.58 (0.96, 2.61) |
| Missing/Unknown | 8 | 6 | 1 | 3 | 17 | 9 | |||
| p-value | 0.019 | 0.193 | 0.201 | ||||||
| p-trend | 0.005 | 0.070 | 0.079 | ||||||
| Father’s education | |||||||||
| Up to standard 4 | 85 (27.5) | 212 (18.7) | Ref | 35 (27.8) | 111 (13.7) | Ref | 27 (11.8) | 151 (16.1) | Ref |
| Standard 5–7 | 148 (47.9) | 547 (48.3) | 0.73 (0.51, 1.05) | 76 (60.3) | 619 (76.1) | 0.37 (0.23, 0.60) | 86 (37.6) | 371 (39.6) | 1.80 (1.02, 3.15) |
| ≥ Senior secondary school | 76 (24.6) | 373 (33.0) | 0.59 (0.39, 0.89) | 15 (11.9) | 83 (10.2) | 0.55 (0.28, 1.11) | 116 (50.7) | 416 (44.4) | 1.93 (1.11, 3.36) |
| Missing/Unknown | 13 | 18 | 2 | 6 | 18 | 27 | |||
| p-value | 0.040 | <0.001 | 0.063 | ||||||
| p-trend | 0.013 | 0.011 | 0.040 | ||||||
| Mother’s occupation | |||||||||
| Trader/Sales | 11 (3.5) | 94 (8.2) | Ref | 13 (10.2) | 62 (7.6) | Ref | 45 (19.4) | 254 (26.5) | Ref |
| Peasant farmer | 286 (90.8) | 1009 (88.1) | 1.30 (0.65, 2.61) | 103 (81.1) | 724 (88.7) | 0.64 (0.34, 1.23) | 145 (62.5) | 570 (59.6) | 1.39 (0.94, 2.07) |
| Manual laborer | 18 (5.7) | 43 (3.8) | 3.28 (1.28, 8.42) | 11 (8.7) | 30 (3.7) | 1.65 (0.64, 4.21) | 42 (18.1) | 133 (13.9) | 1.48 (0.87, 2.52) |
| Missing/Unknown | 7 | 4 | 1 | 3 | 15 | 8 | |||
| p-value | 0.021 | 0.026 | 0.207 | ||||||
| Father’s occupation | |||||||||
| Trader/Sales | 40 (12.7) | 173 (15.1) | Ref | 22 (17.3) | 100 (12.3) | Ref | 54 (23.4) | 243 (25.4) | Ref |
| Peasant farmer | 243 (77.4) | 831 (72.7) | 0.83 (0.54, 1.27) | 97 (76.4) | 669 (82.1) | 0.60 (0.36, 1.02) | 111 (48.1) | 422 (44.1) | 1.11 (0.75, 1.65) |
| Manual laborer | 31 (9.9) | 139 (12.2) | 1.00 (0.56, 1.79) | 8 (6.3) | 46 (5.6) | 0.75 (0.30, 1.84) | 66 (28.6) | 291 (30.4) | 0.92 (0.60, 1.42) |
| Missing/Unknown | 8 | 7 | 1 | 4 | 16 | 9 | |||
| p-value | 0.535 | 0.161 | 0.602 | ||||||
| Mother’s income, US dollars‡ | |||||||||
| ≤ 7.5 | 213 (66.2) | 565 (49.1) | Ref | 81 (63.3) | 279 (34.1) | Ref | 88 (35.6) | 244 (25.3) | Ref |
| 7.6-≤15.0 | 76 (23.6) | 247 (21.5) | 1.05 (0.74, 1.48) | 30 (23.4) | 260 (31.8) | 0.43 (0.27, 0.69) | 55 (22.3) | 222 (23.0) | 0.76 (0.50, 1.17) |
| >15.0 | 33 (10.3) | 338 (29.4) | 0.33 (0.20, 0.52) | 17 (13.3) | 280 (34.2) | 0.23 (0.13, 0.41) | 104 (42.1) | 499 (51.7) | 0.65 (0.46, 0.93) |
| p-value | <0.0001 | <0.0001 | 0.004 | ||||||
| p-trend | <0.0001 | <0.0001 | 0.021 | ||||||
| Home characteristics | |||||||||
| Distance of home from main road | |||||||||
| Far from the main road | 239 (75.9) | 812 (70.9) | Ref | 73 (57.5) | 642 (78.8) | Ref | 84 (36.2) | 518 (54.1) | Ref |
| Near the main road | 53 (16.8) | 281 (24.5) | 0.86 (0.58, 1.28) | 40 (31.5) | 114 (14.0) | 3.37 (2.13, 5.33) | 96 (41.4) | 332 (34.7) | 2.15 (1.50, 3.08) |
| In town or city | 23 (7.3) | 53 (4.6) | 1.83 (1.02, 3.28) | 14 (11.0) | 59 (7.2) | 2.30 (1.17, 4.50) | 52 (22.4) | 108 (11.3) | 3.30 (2.08, 5.25) |
| Missing/Unknown | 7 | 4 | 1 | 4 | 15 | 7 | |||
| p-value | 0.078 | <0.0001 | <0.0001 | ||||||
| Number of rooms in house | |||||||||
| 1–2 room | 273 (86.9) | 987 (86.1) | Ref | 47 (37.0) | 481 (59.0) | Ref | 116 (50.2) | 539 (56.3) | Ref |
| ≥ 3 rooms | 41 (13.1) | 159 (13.9) | 1.43 (0.94, 2.17) | 80 (63.0) | 335 (41.1) | 2.43 (1.62, 3.63) | 115 (49.8) | 419 (43.7) | 1.46 (1.06, 2.01) |
| Missing/Unknown | 8 | 4 | 1 | 3 | 16 | 7 | |||
| p-value | 0.094 | <0.0001 | 0.021 | ||||||
| Number of children and adult resident | |||||||||
| 2–4 people | 173 (55.1) | 703 (61.3) | Ref | 28 (22.1) | 302 (37.0) | Ref | 48 (20.8) | 257 (26.8) | Ref |
| 5–7 people | 115 (36.6) | 385 (33.6) | 1.45 (1.06, 1.96) | 56 (44.1) | 334 (40.9) | 1.96 (1.18, 3.24) | 125 (54.1) | 500 (52.2) | 1.49 (0.99, 2.24) |
| ≥ 8 people | 26 (8.3) | 58 (5.1) | 2.19 (1.21, 3.97) | 43 (33.9) | 180 (22.1) | 2.81 (1.62, 4.86) | 58 (25.1) | 201 (21.0) | 1.57 (0.97, 2.54) |
| Missing/Unknown | 8 | 4 | 1 | 3 | 16 | 7 | |||
| p-value | 0.006 | 0.001 | 0.120 | ||||||
| p-trend | 0.002 | 0.0002 | 0.060 | ||||||
| Number of people sleeping in the same room as child | |||||||||
| 0–2 people | 49 (15.6) | 202 (17.6) | Ref | 28 (22.1) | 269 (33.0) | Ref | 92 (39.8) | 389 (40.6) | Ref |
| 3 people | 99 (31.5) | 275 (24.0) | 1.55 (0.99, 2.42) | 46 (36.2) | 288 (35.3) | 1.41 (0.84, 2.37) | 83 (35.9) | 259 (27.0) | 1.28 (0.88, 1.85) |
| ≥ 4 people | 166 (52.9) | 669 (58.4) | 1.07 (0.71, 1.63) | 53 (41.7) | 259 (31.7) | 1.88 (1.13, 3.13) | 56 (24.2) | 310 (32.4) | 0.71 (0.47, 1.06) |
| Missing/Unknown | 8 | 4 | 1 | 3 | 16 | 7 | |||
| p-value | 0.058 | 0.049 | 0.022 | ||||||
| p-trend | 0.691 | 0.014 | 0.142 | ||||||
| Connected to electricity grid | |||||||||
| No | 306 (97.1) | 1092 (95.4) | Ref | 122 (96.1) | 745 (91.3) | Ref | 202 (86.7) | 835 (87.6) | Ref |
| Yes | 9 (2.9) | 53 (4.6) | 0.75 (0.26, 2.17) | 5 (3.9) | 71 (8.7) | 0.44 (0.17, 1.13) | 31 (13.3) | 118 (12.4) | 1.22 (0.77, 1.93) |
| Missing/Unknown | 7 | 5 | 1 | 3 | 14 | 12 | |||
| p-value | 0.598 | 0.087 | 0.397 | ||||||
| Source of drinking water | |||||||||
| Unprotected spring/well | 244 (77.5) | 882 (77.0) | Ref | 59 (46.5) | 453 (55.5) | Ref | 130 (55.6) | 572 (59.9) | Ref |
| Protected spring/well | 61 (19.4) | 202 (17.6) | 1.58 (1.07, 2.34) | 42 (33.1) | 218 (26.7) | 1.45 (0.93, 2.27) | 46 (19.7) | 171 (17.9) | 1.43 (0.95, 2.16) |
| Public tap/piped household | 10 (3.2) | 61 (5.3) | 1.05 (0.47, 2.31) | 26 (20.5) | 145 (17.8) | 1.25 (0.74, 2.11) | 58 (24.8) | 212 (22.2) | 1.63 (1.10, 2.40) |
| Missing/Unknown | 7 | 5 | 1 | 3 | 13 | 10 | |||
| p-value | 0.073 | 0.254 | 0.029 | ||||||
| Distance to water source, meters | |||||||||
| < 500 | 38 (12.1) | 383 (33.5) | Ref | 52 (40.9) | 136 (16.7) | Ref | 149 (64.5) | 356 (37.2) | Ref |
| 500–999 | 63 (20.0) | 126 (11.0) | 4.86 (2.93, 8.08) | 22 (17.3) | 159 (19.5) | 0.37 (0.21, 0.65) | 33 (14.3) | 230 (24.0) | 0.32 (0.20, 0.49) |
| 1000–4999 | 122 (38.7) | 511 (44.6) | 2.85 (1.85, 4.37) | 32 (25.2) | 440 (53.9) | 0.16 (0.09, 0.26) | 38 (16.5) | 268 (28.0) | 0.38 (0.25, 0.59) |
| ≥ 5000 | 92 (29.2) | 125 (10.9) | 11.4 (6.83, 19.1) | 21 (16.5) | 81 (9.9) | 0.69 (0.38, 1.27) | 11 (4.8) | 104 (10.9) | 0.18 (0.08, 0.41) |
| Missing/Unknown | 7 | 5 | 1 | 3 | 16 | 7 | |||
| p-value | <0.0001 | <0.0001 | <0.0001 | ||||||
| p-trend | <0.0001 | <0.0001 | <0.0001 | ||||||
Abbreviation: CI = confidence interval
Note: p-values are for heterogeneity
Associations are minimally adjusted for age, sex, proximity to water and population density of children 0–15 years.
The months of April to June and September to December were classified as wet season months, while the months of January to March and July to August were classified as dry season months.
Income was categorized based on the international poverty line of $1.90 per a day to calculate the average 30-day monthly income. Total household income or father’s income were not analyzed because the results for these were considered unreliable.
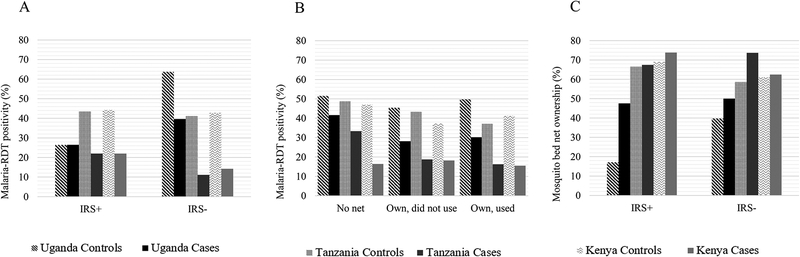
Figure 2.
Figure shows malaria-RDT positivity among cases and controls by indoor residual spraying (IRS) use (IRS+) or not (IRS−) in the past year (Panel A) and by mosquito bed net ownership (Panel B) and mosquito bed net ownership by IRS use in the past year (Panel C). The results are ordered as controls first followed by cases for Uganda, Tanzania and Kenya.
Contrary to our expectations, exposure to malaria suppression interventions was associated with elevated eBL risk. Although reporting application of IRS in the house one year before enrollment was not different between eBL cases and healthy controls in Uganda (versus no IRS: OR=1.02, p=0.91), it was significantly associated with eBL risk in Tanzania (OR= 5.27, p<0.0001) and Kenya (OR=3.13, p<0.0001). Consistent with this pattern, reporting use of a mosquito bed net the night before interview was associated with eBL risk in children Uganda (versus not owning a mosquito bed net: OR=2.90, 95% CI 2.12–3.97), Tanzania (OR=2.19, 95% CI 1.31–3.67), and Kenya (OR=1.90, 95% CI 1.26–2.88). Moreover, the association with eBL risk was stronger among children who reported owning but not using their mosquito bed net the previous night (versus not owning: 22-fold in Uganda, 8-fold in Tanzania, and 7-fold in Kenya; Table 2). Despite regular use of insecticide being relatively rare, reported by only 1.6–4.8% of the controls, eBL risk was associated with reporting regular use of insecticide sprays in Uganda (versus non-use: OR=5.62, p<0.0001) and Tanzania (OR=2.31, p=0.02), but not in Kenya (OR=1.26, p=0.63).
Associations of eBL with fevers, inpatient or outpatient malaria treatment, and HIV
A history of fevers may provide clues about exposure and immunity to malaria as well as to other pathogens and the state of health in the children. When considering any fever experienced up to 12 months before enrollment, no difference was observed between eBL cases and controls in Uganda (versus no fever: OR=0.82, p=0.40), but eBL cases were less likely than controls to report these fevers in this period in Tanzania (OR=0.49, p=0.06) and Kenya (OR=0.22, p<0.0001). Considering fevers attributed to malaria in the period up to 6 months before enrollment eBL cases were less likely than controls to report these fevers in Uganda (versus none: OR=0.56, p≤0.001) and Kenya (OR=0.47, p<0.0001) but not in Tanzania (OR=0.90, p=0.64, Table 2). Inpatient malaria treatment is necessary in lacking immunity to malaria and becomes less frequent in older children who have acquired immunity21. Inpatient malaria treatment >12 months before enrollment or lack of such treatment was reported more frequently by cases than controls in Uganda (versus ≤12 months before enrollment: OR=2.55 and OR=2.04, respectively, p=0.01) while lack of treatment was more frequently reported by cases in Tanzania (OR=1.94, 95% CI 0.94–3.99; p=0.07). However, the findings were heterogenous in Kenya with cases being less likely to report inpatient malaria treatment >12 months before enrollment or lack of receiving such treatment ever (OR=0.24 and OR=0.55, respectively, p=0.001).
In contrast to inpatient malaria treatment, outpatient malaria treatment is given to people with immunity to malaria when they experience break through infections and it is a surrogate of intensity of exposure. Compared to controls, cases frequently reported malaria outpatient treatment >12 months before enrollment in Uganda (versus ≤12 months: OR=3.69) and Kenya (OR=1.86). A history of no outpatient malaria treatment was associated with decreased eBL risk in Tanzania (OR=0.44 p=0.004; Table 2).
In all the three countries, fevers not attributed to malaria experienced up to 6 months before enrollment were associated with eBL risk in Uganda (OR=8.40, p<0.0001), Tanzania (OR=5.17, p<0.0001) and Kenya (OR=5.17, p<0.0001). This association was stronger for fever reported at enrollment (OR=49.7, p<0.0001, OR=32.1, p<0.0001, and OR=16.5, p<0.0001, for Uganda, Tanzania and Kenya, respectively, Table 2).
These non-malaria fevers were not explained by HIV infection, which was rare (25 cases, 3.6%; 23 controls, 0.78%; Table 2); it was less common in Uganda (2.0%) than in Tanzania (3.4%) and Kenya (6.9%). HIV infection was associated with eBL risk in Uganda (OR=5.49, p=0.01), Tanzania (OR=25.6, p=0.004), and in Kenya (OR=4.32, p=0.001).
Associations of eBL with parental and household characteristics
In all three countries, higher maternal income (Uganda: p-trend<0.0001; Tanzania: p-trend<0.0001; and Kenya: p-trend=0.021) and higher maternal- and paternal-education in Uganda and Tanzania were associated with decreased eBL risk (Table 2). Consistently, reporting lower status maternal occupations, like farming or manual labor, was associated with elevated eBL risk in Uganda (versus sales: OR=3.28) and Tanzania (OR=1.65) but not in Kenya (OR=1.48, p=0.21). Although living in house connected to electricity is an indicator of higher socioeconomic status, this was not associated with eBL risk.
Location of ones’ house, house size, and crowding within the house may be an indicator of socioeconomic status. Living in a house near a road (versus far: OR=3.37 and 2.15 in Tanzania and Kenya, respectively) or living in house in a town (OR=1.83, 2.30, and 3.30 in Uganda, Tanzania, and Kenya, respectively) were associated with elevated eBL risk. Living in a house with 3+ rooms (versus 1–2 rooms: OR=1.43, 2.43, and 1.46 for Uganda, Tanzania, and Kenya, respectively) or with 5–7 people (range OR=1.45 – 1.96) or ≥8 people (range OR=1.57 – 2.81, Table 2). Compared to children who obtained drinking water from an unprotected spring or well, eBL was associated with those whose drinking water was obtained from a protected spring or in Uganda (OR=1.58, p=0.07) or from piped-in/public tap in Kenya (OR=1.63, p=0.03). No difference in the sources of drinking water was observed between cases and controls in Tanzania (p=0.25). Distance of the home to the source of drinking water may be associated with greater exposure to environmental risk factors. Consistently, higher eBL risk in Uganda but lower eBL risk in Tanzania and Kenya were associated with increasing distance from home to the source of drinking water (all p<0.0001, Table 2).
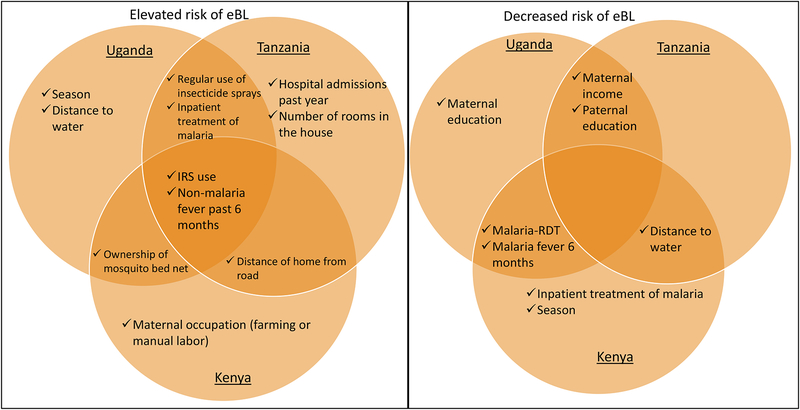
Table 3 shows the results from the multivariable models and Figure 3 shows the associations that were significant in at least one country; we emphasize associations observed in at least two countries. Higher maternal income was associated with decreased eBL risk in Uganda (p=0.0004) and Tanzania (p=0.001), whereas living in a house near the road or in a town or city were associated with elevated eBL risk in Tanzania (p<0.0001) and Kenya (p=0.03). Despite consistent evidence in our study that exposure to IRS was associated with reduced low-grade malaria prevalence whereas not such association was observed with use of mosquito bed nets or regular use of insecticide sprays12, 13, all were associated with elevated eBL risk. Specifically, reporting IRS in the house in the past year was associated with eBL risk in all the three countries (Uganda: OR=1.71, p=0.026; Tanzania: OR=3.93, p<0.0001; and Kenya: OR= 6.78, p<0.0001), as was regular use of insecticide sprays was associated with eBL risk in Uganda (OR=5.62, p=0.002) and Tanzania (OR=3.47, p=0.026). Interestingly, the association with eBL was stronger in children who owned but did not use their mosquito bed net the night before interview in Uganda (OR=39.7, p=0.0001) and Kenya (OR=5.74, p=0.002).
Table 3:
Multivariate associations with endemic Burkitt lymphoma the EMBLEM Study, 2010–2016.
| Uganda | Tanzania | Kenya | All countries combined† | |
|---|---|---|---|---|
| Characteristics | aOR* (95% CI) | aOR* (95% CI) | aOR* (95% CI) | aOR* (95% CI) |
| Season‡ | ||||
| Dry | Ref | Ref | Ref | Ref |
| Wet | 2.54 (1.67, 3.86) | 1.51 (0.79, 2.86) | 0.28 (0.18, 0.45) | 1.28 (0.99, 1.66) |
| p-value | <0.0001 | 0.211 | <0.0001 | 0.058 |
| Measure of malaria | ||||
| Indoor residual insecticide sprayed in house | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 1.71 (1.07, 2.73) | 3.93 (2.03, 7.62) | 6.78 (3.51, 13.10) | 2.32 (1.75, 3.09) |
| p-value | 0.026 | <0.0001 | <0.0001 | <0.0001 |
| Mosquito net ownership and use the night before | ||||
| No | Ref | Ref | Ref | Ref |
| Yes, but not used | 39.7 (13.3, 118) | 4.09 (1.25, 13.35) | 5.74 (2.48, 13.3) | 9.23 (5.59, 15.24) |
| Yes, and used | 4.12 (2.64, 6.42) | 1.32 (0.60, 2.90) | 1.47 (0.86, 2.53) | 2.36 (1.76, 3.18) |
| p-value | <0.0001 | 0.058 | 0.0002 | <0.0001 |
| Regularly uses mosquito insecticide sprays | ||||
| No | Ref | Ref | Ref | |
| Yes | 5.62 (1.85, 17.01) | 3.47 (1.16, 10.37) | 2.97 (1.65, 5.32) | |
| p-value | 0.002 | 0.026 | 0.0003 | |
| Malaria rapid diagnostic test | ||||
| Negative | Ref | Ref | Ref | Ref |
| Positive | 0.43 (0.28, 0.68) | 0.56 (0.27, 1.16) | 0.33 (0.20, 0.55) | 0.46 (0.35, 0.60) |
| p-value | 0.0003 | 0.120 | <0.0001 | <0.0001 |
| History of fevers, and malaria treatment | ||||
| ≥1 fever due to malaria up to 6 months before enrollment | ||||
| No | Ref | Ref | Ref | |
| Yes | 0.48 (0.29, 0.77) | 0.59 (0.35, 0.98) | 0.63 (0.47, 0.84) | |
| p-value | 0.003 | 0.042 | 0.001 | |
| ≥1 fever not due to malaria up to 6 months before enrollment | ||||
| No | Ref | Ref | Ref | Ref |
| Yes | 8.82 (5.37, 14.49) | 3.88 (2.05, 7.36) | 6.98 (4.39, 11.09) | 5.66 (4.31, 7.42) |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| ≥1 hospital admission | ||||
| No | Ref | Ref | ||
| Yes | 21.29 (9.61, 47.17) | 9.35 (6.85, 12.77) | ||
| p-value | <0.0001 | <0.0001 | ||
| Inpatient malaria treatment | ||||
| Yes, past 12 months | Ref | Ref | Ref | Ref |
| Yes, > 12 months | 3.97 (1.66, 9.51) | 2.89 (0.80, 10.41) | 0.18 (0.07, 0.48) | 0.99 (0.62, 1.57) |
| Never | 3.41 (1.52, 7.67) | 26.43 (7.73, 90.36) | 0.48 (0.25, 0.91) | 5.59 (3.61, 8.66) |
| p-value | 0.007 | <0.0001 | 0.003 | <0.0001 |
| Outpatient malaria treatment | ||||
| Yes, past 12 months | Ref | Ref | Ref | Ref |
| Yes, > 12 months | 2.17 (1.17, 4.03) | 0.45 (0.14, 1.48) | 1.61 (0.70, 3.72) | 2.23 (1.50, 3.31) |
| Never | 0.59 (0.35, 0.99) | 0.54 (0.27, 1.07) | 1.48 (0.87, 2.53) | 0.96 (0.71, 1.29) |
| p-value | 0.0001 | 0.154 | 0.277 | <0.0001 |
| Parental characteristics | ||||
| Mother’s education | ||||
| Up to standard 4 | Ref | Ref | ||
| Standard 5–7 | 0.81 (0.52, 1.26) | 0.81 (0.60, 1.08) | ||
| ≥ Senior secondary school | 0.46 (0.19, 1.12) | 0.77 (0.50, 1.20) | ||
| p-value | 0.197 | 0.319 | ||
| p-trend | 0.082 | 0.227 | ||
| Father’s education | ||||
| Up to standard 4 | Ref | Ref | Ref | Ref |
| Standard 5–7 | 0.75 (0.44, 1.26) | 0.25 (0.11, 0.55) | 1.13 (0.54, 2.37) | 0.71 (0.51, 1.00) |
| ≥ Senior secondary school | 0.53 (0.28, 0.99) | 0.19 (0.05, 0.68) | 1.03 (0.49, 2.17) | 0.70 (0.47, 1.04) |
| p-value | 0.136 | 0.002 | 0.908 | 0.116 |
| p-trend | 0.033 | 0.002 | 0.943 | 0.068 |
| Mother’s occupation | ||||
| Trader/Sales | Ref | Ref | Ref | Ref |
| Peasant farmer | 0.64 (0.23, 1.75) | 0.47 (0.17, 1.34) | 2.80 (1.53, 5.11) | 1.31 (0.86, 1.97) |
| Manual laborer | 0.79 (0.19, 3.21) | 0.75 (0.18, 3.02) | 2.36 (1.07, 5.22) | 1.54 (0.89, 2.69) |
| p-value | 0.668 | 0.334 | 0.004 | 0.272 |
| Mother’s income, US dollars§ | ||||
| 7.5 | Ref | Ref | Ref | Ref |
| 7.6-≤15.0 | 0.97 (0.60, 1.58) | 0.68 (0.31, 1.46) | 0.78 (0.42, 1.43) | 0.88 (0.65, 1.20) |
| >15.0 | 0.27 (0.14, 0.52) | 0.20 (0.08, 0.47) | 0.70 (0.40, 1.24) | 0.46 (0.33, 0.64) |
| p-value | 0.0004 | 0.001 | 0.468 | <0.0001 |
| p-trend | 0.0003 | 0.001 | 0.249 | <0.0001 |
| Home characteristics | ||||
| Distance of home from main road | ||||
| Far from the main road | Ref | Ref | Ref | |
| Near the main road | 4.33 (2.00, 9.37) | 1.88 (1.15, 3.07) | 1.66 (1.23, 2.24) | |
| In town or city | 9.17 (2.84, 29.63) | 1.94 (0.97, 3.90) | 2.92 (1.83, 4.67) | |
| p-value | <0.0001 | 0.026 | <0.0001 | |
| Number of rooms in house | ||||
| 1–2 room | Ref | Ref | Ref | |
| ≥ 3 rooms | 1.33 (0.67, 2.65) | 1.46 (0.91, 2.33) | 1.19 (0.88, 1.62) | |
| p-value | 0.419 | 0.117 | 0.256 | |
| Number of children and adult resident | ||||
| 2–4 people | Ref | Ref | Ref | |
| 5–7 people | 1.51 (0.99, 2.31) | 1.12 (0.49, 2.58) | 1.23 (0.92, 1.66) | |
| ≥ 8 people | 1.47 (0.62, 3.49) | 1.35 (0.56, 3.29) | 1.21 (0.81, 1.80) | |
| p-value | 0.144 | 0.788 | 0.365 | |
| p-trend | 0.08 | 0.497 | 0.428 | |
| Number of people sleeping in the same room as child | ||||
| 0–2 people | Ref | Ref | ||
| 3 people | 1.27 (0.75, 2.16) | 1.42 (1.02, 1.97) | ||
| ≥ 4 people | 1.10 (0.63, 1.93) | 1.31 (0.94, 1.84) | ||
| p-value | 0.673 | 0.109 | ||
| p-trend | 0.649 | 0.177 | ||
| Source of drinking water | ||||
| Unprotected spring/well | Ref | Ref | ||
| Protected spring/well | 1.44 (0.82, 2.53) | 1.53 (1.11, 2.10) | ||
| Public tap/piped household | 0.97 (0.53, 1.76) | 1.08 (0.72, 1.63) | ||
| p-value | 0.395 | 0.032 | ||
| Distance to water source, meters | ||||
| < 500 | Ref | Ref | Ref | Ref |
| 500–999 | 5.58 (2.81, 11.08) | 0.32 (0.13, 0.78) | 0.57 (0.32, 1.02) | 1.30 (0.91, 1.86) |
| 1000–4999 | 3.48 (1.99, 6.10) | 0.10 (0.05, 0.24) | 0.38 (0.21, 0.70) | 0.84 (0.61, 1.14) |
| ≥ 5000 | 14.75 (7.34, 29.66) | 0.46 (0.17, 1.28) | 0.19 (0.07, 0.50) | 2.39 (1.60, 3.57) |
| p-value | <0.0001 | <0.0001 | 0.0004 | <0.0001 |
| p-trend | <0.0001 | <0.0001 | <0.0001 | 0.034 |
Abbreviation: aOR= adjusted odds ratio; CI = confidence interval
Note: p-values are for heterogeneity
In addition to the adjustment for age, sex, proximity to water and population density of children 0–15 years, the associations are mutually adjusted for all variables included in the multivariate model. In addition, the multivariate models were adjusted for keeping animals inside the house or nearby (chicken, pigs, goat, cows, birds, dogs).
This column shows the observed associations based on data from all the three study countries combined, adjusted for each country
The months of April to June and September to December were classified as wet season months, while the months of January to March and July to August were classified as dry season months.
Income was categorized based on the international poverty line of $1.90 per a day to calculate the average 30-day monthly income. Total household income or father’s income were not analyzed because the results for these were considered unreliable.
Figure 3.
Venn diagram showing the characteristics associated with elevated or decreased risk of eBL in Uganda, Tanzania, and Kenya, highlighting findings common in the three or two countries.
The associations of elevated eBL risk with reporting a history of inpatient malaria treatment >12 months before enrollment remained significant in Uganda (p=0.007) and Tanzania (p=0.001), consistent with being exposed to malaria at an early age. The inverse associations between eBL and malaria-RDT positivity remained significant in Uganda (p=0.0003) and Kenya (p<0.0001) as did the associations with fever attributed to malaria up to 6 months before enrollment with eBL remained significant in Uganda (p=0.003) and Kenya (p=0.042). However, a history of fever not attributed to malaria in the period up to 6 months before enrollment was associated with eBL in all the three countries (Uganda: OR=8.82, p<0.0001; Tanzania OR=3.88, p<0.0001; Kenya OR= 6.98, p=<0.0001).
These results remained unchanged when we changed the analytic approach and used a combined data set (Supplementary Tables 1 and 2) or when we stratified analyses by diagnosis or rural/urban status of the case/control villages.
Discussion
Our EMBLEM study demonstrated the feasibility of conducting a networked, multisite population-based case-control study of eBL in in Uganda, Tanzania, and Kenya. The novel aspects of EMBLEM include implementing harmonized protocols to collect data and biospecimens, collecting local- and regional-area factors to adjust for macro- and micro-geographic factors, and conducting the study in rural areas where children are exposed from birth to intense malaria12, 13. Our study revealed significant associations both with elevated and decreased eBL risk, but it also exposed tremendous heterogeneity in many of the associations based on observation of significance in one, two, or all the three countries (Figure 3). The heterogeneity of associations may indicate false positive associations; thus, we emphasize associations found in at least two countries. Or it might indicate weakness in questionnaire-based methodology to accurately measure malaria and/or EBV exposures for eBL, hence there is a need to use other techniques to accurately measure these biologic exposures.
Focusing on the findings that were consistent in at least two countries, our obsevation that eBL risk is elevated with low maternal income and paternal education is not surprising. There is evidence that lower socioeconomic status is associated with higher malaria intensity12, 13, providing a mechanistic link with the elevated eBL risk15. Maternal income could be the reason for the long-term declines in trends in eBL incidence observed in Northern Tanzania during 2000–200922. These declines in eBL incidence coincided, paradoxically, with increasing drug and insecticide resistance which led to worse malaria morbidity trends and it predated the introduction of mass malaria suppression programs in 200523. However, because they the BL trends are concomitant with long-term increases in maternal income globally and in Tanzania24,25, we speculate that they may be causally related.
We found a heterogenous pattern in the relationship between eBL and some variables used as surrogates for malaria exposure and immunity. For example, inpatient malaria treatment > 12 months before enrollment or absence of that history were associated with a 2-fold higher risk of eBL among children in Uganda and Tanzania. This is consistent with the high and persistent malaria transmission experienced for more than 7 months in the year in those countries14 and the notion that inpatient malaria treatment is required in younger children before they acquire immunity26, 27, and such treatment is rarely required in older children who are immune despite being subject to heavy exposure to malaria12, 13. However, the association with this variable was opposite in Kenya, where 0.18–0.48-lower risk of eBL was observed. This may reflect seasonal and less persistent malaria in Kenya13, associated with slower acquisition of immunity, such that exposures are likely to result in symptomatic infections that require treatment. In this setting, the same variable may be compatible with lighter malaria exposure. Definitive research using serological assays may help clarify these heterogenous patterns. Our findings with respect to HIV confirm the association between eBL and HIV and that it is not a major population factor for eBL in this region8, 28.
Mass malaria suppression programs have been widely implemented in the study region. Unexpectedly, we found that exposure to mass malaria suppression variables, notably, use of mosquito bed nets, insecticide sprays, and IRS was associated with elevated eBL risk. This was surprising because IRS was associated with decreased pfPR12, 13, whereas use of mosquito bed nets or regular use of insecticide sprays were not12, 13. The consistent association of these variables with eBL, on one hand, and lack of consistent association with pfPR, on the other, suggests that the associations with eBL risk are not mediated by alteration of malaria intensity12, 13.
We suggest that these apparently paradoxical patterns reflect the preferential targeting for malaria suppression areas with both high malaria intensity and eBL incidence15. If so, then, we speculate that the continuing elevated eBL risk in these areas reflects heightened risk established prior to implementation of IRS. Because IRS successfully suppressed malaria in areas where it was applied12, 13, our results indicate that the heightened risk may continue for up to 20 months, equivalent to IRS effects lasting 4–6 months per cycle and assuming 3–4 consecutive IRS cycles were implemented29. Plausibly, heavy chronic malaria before IRS led to a high burden of genetically unstable B cells30 triggered by the historical malaria3, followed by development of irreversible secondary mutations31, oligoclonal expansion by recurrent malaria5, and through Darwinian selection.32 progression to eBL, despite absence of intense malaria pressure. The period of heightened risk of 1–2 years approximates the estimated latency period for eBL33, 34.
Because we previously observed heterogenous malaria patterns in IRS areas in Uganda12, it is possible that heightened eBL risk in IRS-areas is driven by incidence in areas where malaria intensity persists at levels compatible with eBL development. This hypothesis may be refuted or confirmed by careful geographic analysis of case activity in IRS areas.
The markedly reduced frequencies of malaria-RDT positivity and of reported malaria fevers up to 6 months before enrollment in eBL cases are paradoxical, considering the malaria model for eBL etiology35. Because cases are more likely to have contact with health facilities and be treated or receive mosquito bed nets, which would lower their malaria risk, stratified analysis by IRS and mosquito bed nets and observed similar patterns. An alternative explanation is that cases received malaria treatment before hospital admission. This was favored by authors of two reports that found lower malaria parasitemia in their eBL cases than controls in a case-control study in Kenya36 and a prospective study in Uganda37. However, this does not apply to our study because the primary results are based on malaria antigens, which remain detectable in blood for 4–6 weeks after malaria treatment18. Furthermore, we collected data about malaria morbidity up to 6 months before enrollment and found it to be lower in eBL cases than controls. These results indicate that immunity acquired by eBL cases following an early exposure to malaria, before eBL onset26, 27 critical to protecting these young children from severe malaria38, continues to protect eBL children from the risk of malaria after disease onset.
Our results highlight non-malaria-related fevers as a common problem associated with eBL. These fevers are often referred to as “B” symptoms, fever of undetermined origin in patients with neoplasia39. Determining the causal factors could inform clinical management of eBL cases or lead to discovery of infections that may play role in the late stages of progression to eBL.
Our results have some limitations. We relied on questionnaire data, which are subject to multiple errors, including recall bias, inaccurate responses, and variability in their distributions in different geographic, social, and political contexts. Despite its population-based design, the study is susceptible to differential case ascertainment within and between region, and the incompleteness in obtaining pathology diagnosis was a concern17. The impact of geographic and diagnostic distortions is minimal, based qualitatively similar results from analyses stratified by rural/urban status and histological diagnosis. We performed multiple comparisons, thus some of the associations should be considered for hypothesis-generation. The strengths of our study are collecting data under a uniform protocol in three countries, and performing analyses following a common approach are strengths of our study.
To conclude, we show the feasibility of conducting population-based studies of eBL in multiple countries using uniform protocols. We observed elevated risk of eBL with non-malaria-related fevers up to before 6 months of enrollment, with exposure to mass malaria suppression variables, and HIV status; and decreased risk with indicators of higher socioeconomic status, current malaria antigenemia, and malaria history within 6 months of admission. The other associations identified likely reflect biological and ecological relationships, including effects mediated by malaria, HIV, or unknown infections.
Supplementary Material
Novelty and Impact:
Endemic Burkitt lymphoma (eBL) is the commonest childhood cancer in sub-Saharan Africa, but few epidemiologic studies have been conducted, and none have attempted recruiting from multiple countries using harmonized methods. We recruited population-based cases and controls from six regions in Uganda, Tanzania, and Kenya using harmonized protocols to investigate infectious, environment and genetic risk factors for eBL. Our results confirm the feasibility of multi-site population-based enrolment of eBL and provide new baseline data about eBL epidemiology.
Acknowledgements
We thank the study population and communities for their participation. We thank Ms. Janet Lawler-Heavner at Westat Inc, (Rockville, MD, USA) and Mr. Erisa Sunday at the African Field Epidemiology Network (Kampala, Uganda) for managing the study. We are grateful to the leadership of the collaborating countries and institutions for hosting local field offices and laboratories and supporting the fieldwork. We thank Ms. Laurie Buck, Dr. Carol Giffen, Mr. Greg Rydzak and Mr. Jeremy Lyman at Information Management Services Inc. (Calverton, MD, USA) for coordinating data, and preparing data analysis files, and Jeremy for drawing study maps.
Funding This study was funded by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute (NCI) (Contracts HHSN261201100063C and HHSN261201100007I) and, in part, by the Intramural Research Program, National Institute of Allergy and Infectious Diseases (SJR), National Institutes of Health, Department of Health and Human Services. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Abbreviations:
- CI
confidence interval
- eBL
Endemic Burkitt lymphoma
- EA
enumeration area
- EMBLEM
Epidemiology of Burkitt Lymphoma in East African Children and Minors
- EBV
Epstein-Barr virus
- HIV
human immunodeficiency virus
- IRS
indoor residual spraying
- OR
odds ratio
- Pf
Plasmodium falciparum
- pfPR
Plasmodium falciparum prevalence
- RDTs
rapid diagnostic tests
REFERENCES
- 1.Parkin DM, Sitas F, Chirenje M, Stein L, Abratt R, Wabinga H. Part I: Cancer in Indigenous Africans--Burden, Distribution, and Trends. Lancet Oncol 2008;9: 683–92. [DOI] [PubMed] [Google Scholar]
- 2.Mbulaiteye SM, Biggar RJ, Bhatia K, Linet MS, Devesa SS. Sporadic Childhood Burkitt Lymphoma Incidence in the United States During 1992–2005. Pediatr Blood Cancer 2009;53: 366–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robbiani DF, Deroubaix S, Feldhahn N, Oliveira TY, Callen E, Wang Q, Jankovic M, Silva IT, Rommel PC, Bosque D, Eisenreich T, Nussenzweig A, Nussenzweig MC. Plasmodium Infection Promotes Genomic Instability and Aid-Dependent B Cell Lymphoma. Cell 2015;162: 727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manolov G, Manolova Y. Marker Band in One Chromosome 14 from Burkitt Lymphomas. Nature 1972;237: 33–4. [DOI] [PubMed] [Google Scholar]
- 5.Rochford R, Cannon MJ, Moormann AM. Endemic Burkitt’s Lymphoma: A Polymicrobial Disease? Nat Rev Microbiol 2005;3: 182–7. [DOI] [PubMed] [Google Scholar]
- 6.Biggar RJ, Nkrumah FK. Burkitt’s Lymphoma in Ghana: Urban-Rural Distribution, Time-Space Clustering and Seasonality. Int J Cancer 1979;23: 330–6. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter LM, Newton R, Casabonne D, Ziegler J, Mbulaiteye S, Mbidde E, Wabinga H, Jaffe H, Beral V. Antibodies against Malaria and Epstein-Barr Virus in Childhood Burkitt Lymphoma: A Case-Control Study in Uganda. Int J Cancer 2008;122: 1319–23. [DOI] [PubMed] [Google Scholar]
- 8.Mutalima N, Molyneux E, Jaffe H, Kamiza S, Borgstein E, Mkandawire N, Liomba G, Batumba M, Lagos D, Gratrix F, Boshoff C, Casabonne D, Carpenter LM, Newton R. Associations between Burkitt Lymphoma among Children in Malawi and Infection with Hiv, Ebv and Malaria: Results from a Case-Control Study. PLoS One 2008;3: e2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rainey JJ, Rochford R, Sumba PO, Kowuor D, Wilson ML, Moormann AM. Family Environment Is Associated with Endemic Burkitt Lymphoma: A Population-Based Case-Control Study. Am J Trop Med Hyg 2008;78: 338–43. [PubMed] [Google Scholar]
- 10.Maziarz M, Nabalende H, Otim I, Legason ID, Kinyera T, Ogwang MD, Talisuna AO, Reynolds SJ, Kerchan P, Bhatia K, Biggar RJ, Goedert JJ, Pfeiffer RM, Mbulaiteye SM. A Cross-Sectional Study of Asymptomatic Plasmodium Falciparum Infection Burden and Risk Factors in General Population Children in 12 Villages in Northern Uganda. Malar J 2018;17: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piriou E, Asito AS, Sumba PO, Fiore N, Middeldorp JM, Moormann AM, Ploutz-Snyder R, Rochford R. Early Age at Time of Primary Epstein-Barr Virus Infection Results in Poorly Controlled Viral Infection in Infants from Western Kenya: Clues to the Etiology of Endemic Burkitt Lymphoma. J Infect Dis 2012;205: 906–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maziarz M, Kinyera T, Otim I, Kagwa P, Nabalende H, Legason ID, Ogwang MD, Kirimunda S, Emmanuel B, Reynolds SJ, Kerchan P, Joloba MM, Bergen AW, Bhatia K, Talisuna AO, Biggar RJ, Goedert JJ, Pfeiffer RM, Mbulaiteye SM. Age and Geographic Patterns of Plasmodium Falciparum Malaria Infection in a Representative Sample of Children Living in Burkitt Lymphoma-Endemic Areas of Northern Uganda. Malar J 2017;16: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peprah S, Tenge C, Genga IO, Mumia M, Were PA, Kuremu RT, Wekesa WN, Sumba PO, Kinyera T, Otim I, Legason ID, Biddle J, Reynolds SJ, Talisuna AO, Biggar RJ, Bhatia K, Goedert JJ, Pfeiffer RM, Mbulaiteye SM. A Cross-Sectional Population Study of Geographic, Age-Specific, and Household Risk Factors for Asymptomatic Plasmodium Falciparum Malaria Infection in Western Kenya. Am J Trop Med Hyg 2019;100: 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craig MH, Snow RW, Le Sueur D. A Climate-Based Distribution Model of Malaria Transmission in Sub-Saharan Africa. Parasitol Today 1999;15: 105–11. [DOI] [PubMed] [Google Scholar]
- 15.Kafuko GW, Burkitt DP. Burkitt’s Lymphoma and Malaria. Int J Cancer 1970;6: 1–9. [DOI] [PubMed] [Google Scholar]
- 16.Baik S, Mbaziira M, Williams M, Ogwang MD, Kinyera T, Emmanuel B, Ziegler JL, Reynolds SJ, Mbulaiteye SM. A Case-Control Study of Burkitt Lymphoma in East Africa: Are Local Health Facilities an Appropriate Source of Representative Controls? Infect Agent Cancer 2012;7: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogwang MD, Zhao W, Ayers LW, Mbulaiteye SM. Accuracy of Burkitt Lymphoma Diagnosis in Constrained Pathology Settings: Importance to Epidemiology. Arch Pathol Lab Med 2011;135: 445–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grandesso F, Nabasumba C, Nyehangane D, Page AL, Bastard M, De Smet M, Boum Y, Etard JF. Performance and Time to Become Negative after Treatment of Three Malaria Rapid Diagnostic Tests in Low and High Malaria Transmission Settings. Malar J 2016;15: 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogwang MD, Bhatia K, Biggar RJ, Mbulaiteye SM. Incidence and Geographic Distribution of Endemic Burkitt Lymphoma in Northern Uganda Revisited. Int J Cancer 2008;123: 2658–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adewole I, Martin DN, Williams MJ, Adebamowo C, Bhatia K, Berling C, Casper C, Elshamy K, Elzawawy A, Lawlor RT, Legood R, Mbulaiteye SM, Odedina FT, Olopade OI, Olopade CO, Parkin DM, Rebbeck TR, Ross H, Santini LA, Torode J, Trimble EL, Wild CP, Young AM, Kerr DJ. Building Capacity for Sustainable Research Programmes for Cancer in Africa. Nat Rev Clin Oncol 2014;11: 251–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Barraquer I, Arinaitwe E, Jagannathan P, Boyle MJ, Tappero J, Muhindo M, Kamya MR, Dorsey G, Drakeley C, Ssewanyana I, Smith DL, Greenhouse B. Quantifying Heterogeneous Malaria Exposure and Clinical Protection in a Cohort of Ugandan Children. J Infect Dis 2016;214: 1072–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aka P, Kawira E, Masalu N, Emmanuel B, Brubaker G, Magatti J, Mbulaiteye SM. Incidence and Trends in Burkitt Lymphoma in Northern Tanzania from 2000 to 2009. Pediatr Blood Cancer 2012;59: 1234–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onori E The Problem of Plasmodium Falciparum Drug Resistance in Africa South of the Sahara. Bull World Health Organ 1984;62 Suppl: 55–62. [PMC free article] [PubMed] [Google Scholar]
- 24.Gakidou E, Cowling K, Lozano R, Murray CJ. Increased Educational Attainment and Its Effect on Child Mortality in 175 Countries between 1970 and 2009: A Systematic Analysis. Lancet 2010;376: 959–74. [DOI] [PubMed] [Google Scholar]
- 25.Boyle MH, Racine Y, Georgiades K, Snelling D, Hong S, Omariba W, Hurley P, Rao-Melacini P. The Influence of Economic Development Level, Household Wealth and Maternal Education on Child Health in the Developing World. Soc Sci Med 2006;63: 2242–54. [DOI] [PubMed] [Google Scholar]
- 26.Goncalves BP, Huang CY, Morrison R, Holte S, Kabyemela E, Prevots DR, Fried M, Duffy PE. Parasite Burden and Severity of Malaria in Tanzanian Children. N Engl J Med 2014;370: 1799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filipe JA, Riley EM, Drakeley CJ, Sutherland CJ, Ghani AC. Determination of the Processes Driving the Acquisition of Immunity to Malaria Using a Mathematical Transmission Model. PLoS Comput Biol 2007;3: e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newton R, Ziegler J, Beral V, Mbidde E, Carpenter L, Wabinga H, Mbulaiteye S, Appleby P, Reeves G, Jaffe H. A Case-Control Study of Human Immunodeficiency Virus Infection and Cancer in Adults and Children Residing in Kampala, Uganda. Int J Cancer 2001;92: 622–7. [DOI] [PubMed] [Google Scholar]
- 29.Sherrard-Smith E, Griffin JT, Winskill P, Corbel V, Pennetier C, Djenontin A, Moore S, Richardson JH, Muller P, Edi C, Protopopoff N, Oxborough R, Agossa F, N’guessan R, Rowland M, Churcher TS. Systematic Review of Indoor Residual Spray Efficacy and Effectiveness against Plasmodium Falciparum in Africa. Nat Commun 2018;9: 4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller JR, Janz S, Goedert JJ, Potter M, Rabkin CS. Persistence of Immunoglobulin Heavy Chain/C-Myc Recombination-Positive Lymphocyte Clones in the Blood of Human Immunodeficiency Virus-Infected Homosexual Men. Proc Natl Acad Sci U S A 1995;92: 6577–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmitz R, Young RM, Ceribelli M, Jhavar S, Xiao W, Zhang M, Wright G, Shaffer AL, Hodson DJ, Buras E, Liu X, Powell J, Yang Y, Xu W, Zhao H, Kohlhammer H, Rosenwald A, Kluin P, Muller-Hermelink HK, Ott G, Gascoyne RD, Connors JM, Rimsza LM, Campo E, Jaffe ES, Delabie J, Smeland EB, Ogwang MD, Reynolds SJ, Fisher RI, Braziel RM, Tubbs RR, Cook JR, Weisenburger DD, Chan WC, Pittaluga S, Wilson W, Waldmann TA, Rowe M, Mbulaiteye SM, Rickinson AB, Staudt LM. Burkitt Lymphoma Pathogenesis and Therapeutic Targets from Structural and Functional Genomics. Nature 2012;490: 116–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burnet M A Darwinian Approach to Immunity. Nature 1964;203: 451–4. [DOI] [PubMed] [Google Scholar]
- 33.Geser A, Brubaker G, Draper CC. Effect of a Malaria Suppression Program on the Incidence of African Burkitt’s Lymphoma. Am J Epidemiol 1989;129: 740–52. [DOI] [PubMed] [Google Scholar]
- 34.Day NE, Smith PG, Lachet B. The Latent Period of Burkitt’s Lymphoma: The Evidence from Epidemiological Clustering. IARC Sci Publ 1985: 187–95. [PubMed] [Google Scholar]
- 35.Legason ID, Pfeiffer RM, Udquim KI, Bergen AW, Gouveia MH, Kirimunda S, Otim I, Karlins E, Kerchan P, Nabalende H, Bayanjargal A, Emmanuel B, Kagwa P, Talisuna AO, Bhatia K, Yeager M, Biggar RJ, Ayers LW, Reynolds SJ, Goedert JJ, Ogwang MD, Fraumeni JF Jr., Prokunina-Olsson L, Mbulaiteye SM. Evaluating the Causal Link between Malaria Infection and Endemic Burkitt Lymphoma in Northern Uganda: A Mendelian Randomization Study. EBioMedicine 2017;25: 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Asito AS, Piriou E, Odada PS, Fiore N, Middeldorp JM, Long C, Dutta S, Lanar DE, Jura WG, Ouma C, Otieno JA, Moormann AM, Rochford R. Elevated Anti-Zta Igg Levels and Ebv Viral Load Are Associated with Site of Tumor Presentation in Endemic Burkitt’s Lymphoma Patients: A Case Control Study. Infect Agent Cancer 2010;5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De-The G, Geser A, Day NE, Tukei PM, Williams EH, Beri DP, Smith PG, Dean AG, Bronkamm GW, Feorino P, Henle W. Epidemiological Evidence for Causal Relationship between Epstein-Barr Virus and Burkitt’s Lymphoma from Ugandan Prospective Study. Nature 1978;274: 756–61. [DOI] [PubMed] [Google Scholar]
- 38.Derkach A, Otim I, Pfeiffer RM, Onabajo OO, Legason ID, Nabalende H, Ogwang MD, Kerchan P, Talisuna AO, Ayers LW, Reynolds SJ, Nkrumah F, Neequaye J, Bhatia K, Theander TG, Prokunina-Olsson L, Turner L, Goedert JJ, Lavstsen T, Mbulaiteye SM. Associations between Igg Reactivity to Plasmodium Falciparum Erythrocyte Membrane Protein 1 (Pfemp1) Antigens and Burkitt Lymphoma in Ghana and Uganda Case-Control Studies. EBioMedicine 2019;39: 358–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horowitz HW. Fever of Unknown Origin or Fever of Too Many Origins? N Engl J Med 2013;368: 197–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.