Abstract
Constipation is a common complaint that may be primary (idiopathic or functional) or associated with a number of disorders or medications. Although most constipation is self-managed by patients, 22% seek healthcare, mostly to primary care providers (>50%) and gastroenterologists (14%) which then result in large expenditures for diagnostic testing and treatments. There is strong evidence that stimulant and osmotic laxatives, intestinal secretagogues and peripherally restricted mµ-opiate antagonists are effective and safe; the latter drugs are a major advance for managing opioid-induced constipation. Constipation which is refractory to available laxatives should be evaluated for defecatory disorders and slow transit constipation using studies of anorectal function and colonic transit. Defecatory disorders are often responsive to biofeedback therapies, whereas slow transit constipation may require surgical intervention in selected patients. Both efficacy and cost should guide the choice of treatment for functional constipation and opiate-induced constipation. No studies have compared inexpensive laxatives with newer drugs that work by other mechanisms.
Keywords: Constipation, irritable bowel syndrome, laxatives, biofeedback therapy
Definition and Classification
Constipation is defined by bowel disturbances (ie, reduced frequency of bowel habits, hard stools, excessive straining to defecate, a sense of anorectal bloackge, anal digitation, and a sense of incomplete evacuation after defecation). By contrast to some physicians, who consider reduced stool frequency as the only symptom of constipation. patients are often troubled by the other symptoms of constipation.1 Constipation may be primary alone or secondary to an underlying disorder.
There are 2 approaches for classifying chronic constipation. The American Gastroenterological Association criteria utilize colonic transit and anorectal tests to classify constipated patients into one of the three groups: normal transit constipation (NTC), slow transit constipation (STC), and pelvic floor dysfunction or defecatory disorders (DD).2 Clinicians frequently assess colonic transit and anorectal functions in constipated patients who have not responded to pharmacotherapy.
By contrast, epidemiologic studies and pharmaceutical trials use the original, or suitably modified, so-called Rome criteria (the most recent iteration is the Rome IV criteria) which incorporate symptoms and anorectal assessments of rectal evacuation3,4 (Figure 1, Table 1,5,6). DD are defined by bowel symptoms and anorectal tests indicative of impaired rectal evacuation. However, functional constipation (FC) and constipation-predominant IBS (IBS-C) are defined only by symptoms, bowel symptoms only (FC) or with abdominal pain that is temporally related with bowel disturbances (IBS-C, Table 1). Because the Rome criteria and the inclusion criteria for pharmacological studies in FC and IBS-C do not specify that anorectal tests should be normal, it is conceivable, perhaps likely that many patients with FC and IBS-C actually have an unrecognized DD.
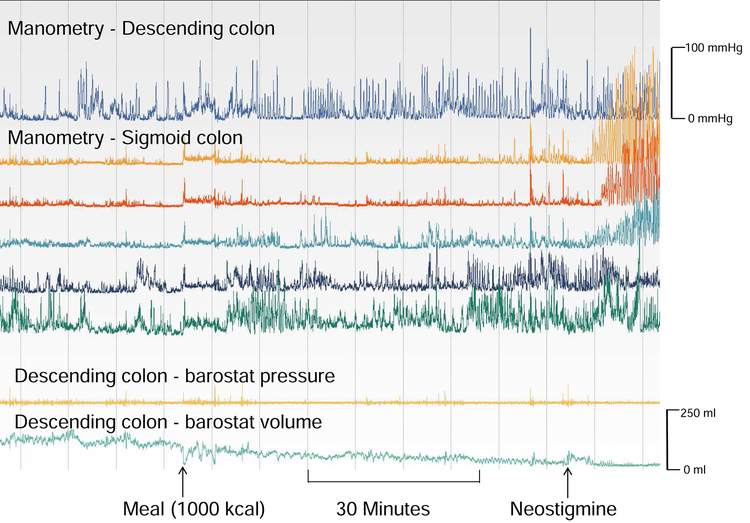
Figure 1. Normal colonic contractile responses to a meal in a patient with isolated slow transit constipation.
Motor activity was recorded with manometry and a barostat balloon under fasting conditions (30 minutes), for 1 hour after a meal, and for 15 minutes after the cholinesterase inhibitor neostigmine. Before the meal, phasic pressure activity was greater in the distal than the proximal sigmoid colon. Phasic activity increased after the meal and more so after neostigmine. The volume of a balloon, located between the uppermost and second manometry sensors and inflated to a constant pressure of 12 mmHg, declined after a meal, and more so after neostigmine, reflecting increased colonic tone.
Table 1.
Differences between Functional Constipation and Constipation-Predominant IBS
| Feature | Functional Constipation | Constipation predominant IBS |
|---|---|---|
| Symptom criteria 4 | Symptoms for ≥ 6-months and ≥ 2 following symptoms for >¼ defecations during past 3 months: • Straining • Lumpy or hard stools • Sensation of incomplete evacuation • Sensation of anorectal obstruction/blockade • Manual maneuvers to facilitate defecations; <3 defecations/week • Loose stools are not present, and there are insufficient criteria for IBS |
Recurrent abdominal pain or discomfort at least 3 days per month in the past 3 months associated with 2 or more of the following: • Improvement with defecation • Onset associated with change in frequency of stool • Onset associated with change in form (appearance) of stool • <25% of bowel movements were loose stools |
| Upper gastrointestinal symptoms (eg, heartburn, dyspepsia), anxiety and depression, urinary symptoms 5 | Less common a | More common a |
| Prevalence of defecatory disorder 5 | Approximately 50% of patients | Approximately 50% of patients |
| Prevalence of increased rectal sensation 6 | Less common a | More common a |
The prevalence of these symptoms varies by symptom; hence specific figures are not provided (a)
The prevalence of increased rectal sensation varies among studies
We suspect that most practitioners use the generic term “chronic constipation” rather than differentiate between IBS-C and FC. That is not a significant limitation since dietary fiber supplementation and/or simple laxatives are beneficial for both in primary care. However, an assessment of the phenotype, guides and predicts the response to therapy. For example, pelvic floor biofeedback therapy, not laxatives, are the cornerstone of managing DD. The dose and response to treatment with secretagogues (e.g., lubiprostone) differs between FC and IBS-C. Medically-refractory isolated slow transit constipation is an indication for colectomy.
Some patients satisfy criteria for FC and IBS-C. Indeed, in one study, nearly 90% of IBS-C patients also had symptoms of FC. Conversely, approximately 44% of patients with FC also IBS-C criteria.7 The Rome criteria specify that patients who have symptoms of IBS-C and FC should be diagnosed as IBS-C. An alternative, perhaps simpler, approach is to classify constipation based on the presence or absence of severe abdominal pain, regardless of the relationship between abdominal pain and bowel symptoms, into constipation with or without moderate or severe pain. Compared to constipated patients with no or mild pain, patients with severe pain report more somatic symptoms, worse overall health, and a greater impact of bowel symptoms on quality of life.7
Prevalence
In the community, the median prevalence is 16% in all adults. In older people, the prevalence is greater (ie, 33.5% in adults aged 60–101 years),8,9 It is greater in non-Caucasians, in institutionalized people, and in women; the median prevalence ratio in women to men is 1.5:1.10 Women more frequently use laxatives and seek health care for their constipation.
Few studies have evaluated colonic transit and anorectal functions among constipated people in the community. In one study, 516 of 11,112 constipated patients in Olmsted County, Minnesota had anorectal tests; 245 had a defecatory disorder (DD), which approximates to an overall age- and sex-adjusted incidence rate of 19.3 (95% CI: 16.8–21.8) per 100 000 person-years. That figure is higher than the incidence rate of Crohn’s disease (i.e., 5.8) in the same population.11
Risk Factors
Increasing age, female sex, Lower socioeconomic status, lower parental education rates, less self-reported physical activity, certain medications (Supplementary Table 1), stressful life events. physical and sexual abuse, and depression are associated with constipation.2 Among nursing home residents, adverse drug effects may partly explain the high prevalence of constipation.12 However, these associations do not imply causation.
Economic Impact and Impact on Quality of Life
In the United States, most constipated patients are self-treated. A minority (e.g., 22% in a U.S. household survey) seek healthcare for constipation.13 However, the prevalence is high. Hence, for outpatient clinic visits, constipation ranks among the top five most common physician diagnoses for GI disorders,14 accounting for almost 8 million ambulatory visits annually in 2001–2004 (i.e., 0.72% of all ambulatory visits)15 to adult primary care providers (33%), pediatricians (21%), and gastroenterologists (14%). Every year, more than a million patients are referred to gastroenterologists for constipation. These 8 million physician visits far exceeded the number of persons who had colon or rectal cancer (142, 570 ) in the U.S. in 2010,16 emphasizing the infrequency with which colon cancer occurs among chronically constipated patients. The direct medical costs for constipation were estimated in excess of $230 million annually.17 The medical costs were twofold greater in women with than without constipation18 In a more recent study of a commercially insured population, 33% of total annual all cause medical expenses were attributable to GI related symptoms in patients with constipation who incurred about $8700 more than non-constipated matched controls.19 Approximately 75% of both groups were female and health care costs were higher in constipated patients with abdominal symptoms.
Among constipated people, general health, mental health and social functioning are worse than in healthy controls, and more so in hospitalized patients than in the community.20 The mental and physical subcomponent scores in hospitalized constipated patients were comparable to patients with Crohn’s disease. Among constipated people in the community, scores were comparable to patients with gastroesophageal reflux, hypertension, diabetes, and depression.21
Pathophysiology
Among patients who seek medical care, the most frequently implicated disturbances are colonic motor dysfunction (i.e., slow transit constipation) and impaired defecation (i.e., defecatory disorders), which may occur in isolation or coexist.22–24 A substantial proportion of constipated patients have normal colon transit and anorectal functions. Abnormal colonic sensation and disturbances of the colonic microbiome may also contribute. Whereas some defecatory disorders are also associated with slow colonic transit,24–26 it is useful to consider mechanisms of slow transit constipation and defecatory disorders separately.
Normal (NTC) and Slow Transit Constipation (STC)
Isolated STC is defined as slow colonic transit in the absence of a defecatory disorder or megacolon. Isolated STC, is regarded as a manifestation of colonic motor dysfunction, and may result from inadequate caloric intake.27 However, only some patients with STC have colonic motor dysfunction as evaluated with manometry.24, 28, 29 Perhaps this discrepancy between colonic transit and motor assessments with barostat-manometry reflect the intra-individual variability in colonic transit and manometry and the limited fidelity of non-high-resolution manometry catheters for detecting propagation of motor events. Also, factors other than colonic motor functions (e.g., the colonic microbiome) may affect colonic transit. NTC is not synonymous with IBS-C, since 23% of patients with IBS-C had delayed colonic transit in one study.30.
Manometric abnormalities in STC include fewer high amplitude propagated contractions (HAPCs), retrogradely propagated or nonpropagated sigmoid or rectal phasic pressure activity. These disturbances may impede colonic flow.31 Contractile responses to a meal and/or to pharmacological stimuli (e.g., bisacodyl or neostigmine) may also be impaired (Figure 1).24, 32 Colonic inertia is defined by markedly reduced or absent responses to a meal and to a pharmacological stimulus (e.g., bisacodyl or neostigmine) rather than solely by slow transit constipation.24, 33A marked reduction in colonic intrinsic nerves and interstitial cells of Cajal may cause colonic motor dysfunction.34 In medically-refractory patients with STC who do not have a defecatory disorder, this should prompt consideration of colectomy, as discussed later. The rationale for colonic manometry prior to colectomy is stronger in children than in adults.35 Overexpression of progesterone receptors, which is associated with impaired smooth muscle contractile responses to acetylcholine and serotonin, is another explanation for slow transit constipation in women.36
Defecatory Disorders (DD)
DD are defined by symptoms of constipation and objective evidence of impaired rectal evacuation. Impaired evacuation may result from increased resistance to evacuation and/or inadequate rectal propulsive forces. High anal resting pressure, incomplete relaxation or paradoxical contraction of the puborectalis and external anal sphincters (“dyssynergia”) cause increased resistance to evacuation (Figure 2).26, 37 However, these disturbances and other pseudonyms (e.g., obstructed defecation, outlet obstruction) refer to the same disorder. Other disturbances in DD include delayed colonic transit,24, 38 rectal hyposensitivity,39 and structural disturbances (e.g., rectoceles and excessive perineal descent).40, 41
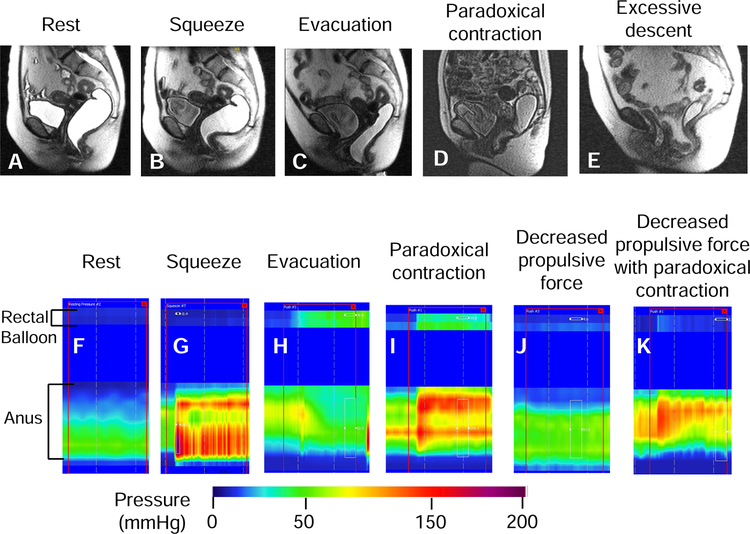
Figure 2. Representative examples of normal and abnormal anorectal evacuation recorded with MRI (upper panel) and high resolution manometry (lower panel).
With MRI, observe increased puborectalis indentation during squeeze (arrow, panel B) and normal relaxation of the puborectalis, perineal descent, opening of the anal canal and evacuation of ultrasound gel during evacuation (panel C). During evacuation in constipated patients, observe paradoxical contraction of the puborectalis (panel D) and exaggerated perineal descent with an enterocele (panel E). High resolution manometry shows increased anal pressure during squeeze (G) compared to rest (F). The white rectangle demarcates the duration of squeeze (G) and evacuation (H-K). Observe increased rectal pressure with anal relaxation during evacuation (H) in a healthy person. By contrast, during evacuation in constipated patients, observe increased rectal pressure with paradoxical anal contraction (I), no change in rectal pressure versus rest (J), and no change in rectal pressure with paradoxical anal contraction (K).
To what extent these anorectal sensorimotor dysfunctions cause defecatory symptoms is unclear. Some asymptomatic people and patients with symptoms (e.g., rectal pain) other than DD have dysynergia, perhaps because it is challenging to simulate defecation during a test.42–44 Some abnormalities (e.g., delayed colonic transit and rectal hyposensitivity) may be a consequence rather than a cause of DD.38 The findings of different tests (e.g., anorectal manometry and defecography) may diverge. There is no gold standard for the diagnosis. Stool form influences the expression of symptoms in constipated patients; it is more challenging to expel hard than soft stools.45
The etiology of DD is unclear. Perhaps they result from neglecting the call to defecate and/or represent an inappropriate pattern, of sphincter contraction that is initiated by avoidance of pain or trauma.46 Symptoms often begin in childhood. Indeed, one in three children with childhood constipation had persistent symptoms beyond puberty.47
Among patients with DD, slow colon transit may be secondary (e.g., related to physical obstruction to passage of contents by stool or rectocolonic inhibitory reflexes initiated by rectal distention from retained stool)48 or the primary manifestation. For example, some patients with DD lack the colonic propagated sequences that normally precede defecation.29 Perhaps the colonic motor dysfunction occurs first and predisposes to excessive straining, which leads to DD.
Other Disturbances
Some patients may have abnormal colonic and/or rectal sensation. Increased rectal sensation is associated with abdominal pain and bloating, suggestive of irritable bowel syndrome.49, 50 Conversely, reduced rectal sensation may explain why some patients do not experience the desire to defecate.23 Constipation is associated with alterations of the colonic mucosal microbiome independent of colonic transit; genera from Bacteroidetes were more abundant in constipated patients.51 Disturbed synthesis of bile acids, which stimulated colonic secretion when they are not absorbed in the terminal ileum, has been observed.52
Clinical Evaluation
The clinical assessment should elicit the specific symptoms of constipation, clarify which are most distressing, and assess for medications that cause constipation (Supplementary Table 1). Alarm symptoms include blood admixed with stools, a sudden change in bowel habits, especially after the age of 50 years, anemia, weight loss, and a family history of colon cancer. The timing of symptom onset (e.g., onset during childhood), dietary calorie and fiber intake, a history of abuse, and obstetric events should be recorded. Patients should be asked about maneuvers (e.g., straining to begin and/or to end defecation) they use to defecate. Some symptoms (i.e., sense of anal blockage during defecation, need for anal digitation, or a sense of incomplete evacuation after defecation) are more suggestive of DD.24 The utility of bowel diaries and pictures of stool form (e.g., by the Bristol Stool Form Scale) for efficiently and reliably characterizing bowel habits cannot be overemphasized. By contrast, self-reported stool frequency is unreliable and does not predict colonic transit.53–55 Not infrequently, patients misperceive they have constipation because they do not have a bowel movement every day. In the United States, the normal range is 3–21 bowel movements per week.56 The ease of defecation is also influenced by stool form.45 Among constipated women, straining to begin defecation is more frequent for hard stools than normal stools.45 Patients with severe DD find it difficult to pass even soft stools and enema fluid. After a complete purge, it takes several days for residue to accumulate to form a normal fecal mass. This may explain why some patients skip a bowel movement for a few days after a bout of diarrhea. In constipated patients, laxatives can predispose to alternating constipation and diarrhea, which may lead to a misdiagnosis of irritable bowel syndrome.57
Many constipated patients also have symptoms such as abdominal bloating, distention or discomfort, which may be partly attributable to constipation per se.58 For many patients, abdominal bloating, which may be associated with abdominal distention, is the most bothersome symptom.59 Other symptoms include fatigue, malaise, fibromyalgia and psychosocial distress.
The clinical evaluation should identify diseases that cause constipation (Supplementary Table 2). A thorough perineal and rectal examination is necessary to identify DD. The resistance to insertion of the finger per anus reflects anal resting tone. Pelvic contraction is normally accompanied by elevation of the puborectalis and increased anal tone. When patients try to “expel the examining finger,” both muscles should relax with perineal descent by 2–4 cm.60, 61 Features of DD include high anal resting tone, which manifests as increased resistance to insertion of the examining finger into the anal canal; during simulated evacuation there may be impaired relaxation or paradoxical contraction of the sphincter, and/or reduced perineal descent. Other findings include impacted stool in the rectum, fecal soiling, a rectocele, or puborectalis tenderness. A digital rectal examination is useful but not sufficient to identify DD. Among constipated patents, a rectal examination performed by a skilled examiner had a sensitivity of 80% and a specificity of 56% for predicting an abnormal rectal balloon expulsion test, which reflects a DD.61 With less skilled examiners, the utility of a digital rectal examination is probably lower..
Diagnostic Tests
A complete blood count may be useful. The diagnostic utility and cost-effectiveness of fasting serum glucose, sensitive thyroid-stimulating hormone, and calcium is probably very low.62 Among constipated patients, colonoscopy, to identify colon cancer is required only in patients with alarm clinical features, constipation refractory to medical management, and for patients who have not had an age-appropriate colon cancer screening procedure after the onset of constipation; this age specification is lower in some patients with a family history of colon cancer.63
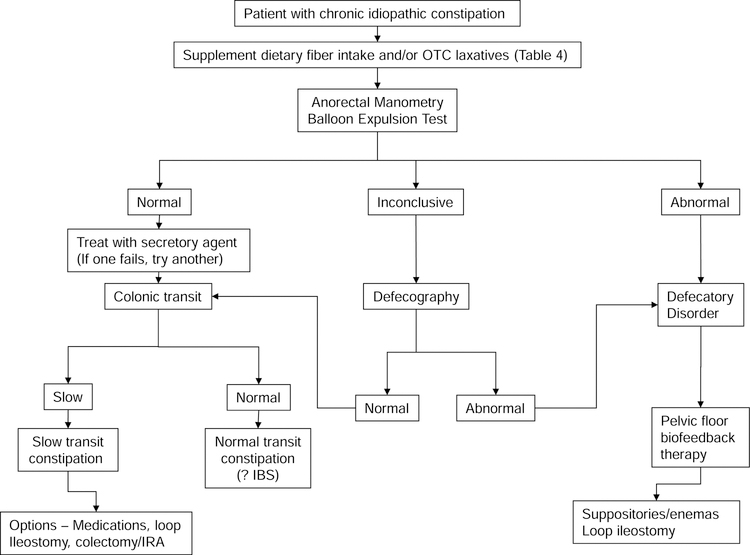
A rectal balloon expulsion test and an anorectal manometry should be performed in constipated patients who do not respond to a high fiber diet and non-prescription laxatives. (Figure 3). When access to anorectal tests is not readily available, a trial of new secretory agents, which are expensive, may be considered before anorectal testing.
Figure 3.
Suggested algorithm for treating patients with chronic constipation
Rectal Balloon Expulsion Test
This test measures the time required to evacuate a water-filled balloon in the seated position; the normal value depends on the technique, and is generally less than 1 minute.64, 65 While the test is highly sensitive and specific for identifying DD, the results may be falsely normal in patients with pelvic laxity, for example, as in over 90% of patients with a large rectocele, enterocele, peritoneocele, and/or sigmoidocele had a normal balloon expulsion test in 1 study.66 Also, some patients with a DD may strain excessively to overcome increased resistance and expel the balloon. In these patients, the normal balloon expulsion test may not reflect normal anorectal functions.
Anorectal Manometry
A normal rectoanal inhibitory reflex excludes Hirschsprung’s disease, which is very rare in adults. In addition to high anal resting pressure, manometry may reveal a reduced rectoanal gradient during evacuation. The latter may result from reduced rectal propulsive force and/or impaired anal relaxation (Figure 2). Even among healthy controls, the rectaoanal gradient (i.e., rectal – anal pressure) during evacuation is negative, for example up to −55 mmHg in asymptomatic women. This is counterintuitive because it would seem that a positive gradient is necessary for normal evacuation. This limits the utility of the rectoanal gradient during evacuation for diagnosing DD.67, 68 We recommend that 2 or more of these 5 manometric abnormalities (i.e., anal resting pressure or anal pressure during evacuation greater than 90th percentile, rectal pressure, anal relaxation or rectoanal gradient less than the 10th percentile value in sex-matched controls) suggest a DD.
Defecography
In the United States, defecography is generally used when the results of anorectal manometry do not concur with the clinical impression and/or when anatomic abnormalities (e.g., a clinically significant rectocele) are suspected.3 The most relevant findings in DD include inadequate or excessive perineal descent or widening of the anorectal angle during defecation.41, 66, 69 Other features include internal rectal intussusception, solitary rectal ulcers, rectoceles and rectal prolapse. If the vagina and small intestine are opacified, enteroceles, bladder and uterovaginal prolapse are also visible. Methodological limitations to barium defecography can be minimized by using standardized techniques.70, 71 Besides avoiding radiation exposure, MR defecography is preferable for visualizing the bony landmarks, which are necessary for measuring pelvic floor motion (Figure 2). However, with conventional, closed-configuration MR systems imaging is only possible in the supine position.72
Colonic Transit
Before the test, medications that slow or accelerate colonic transit should be discontinued. The most common and cost effective approach is to use radiopaque markers (Sitz-Mark, Konsyl Pharmaceuticals, Fort Worth, TX). The “Hinton technique”, entails ingestion of a capsule containing 24 radiopaque markers. Normally, an abdominal x-ray taken 5 days later reveals less than 5 remaining markers in the colon.73 Alternatively (i.e., “Metcalf technique”), a capsule containing 24 radiopaque markers is ingested on days 1, 2 and 3. More than 68 markers combined on days 4 and 7 reflects slow colon transit.74 The test is more reproducible in patients with simple constipation53 than in defecatory disorders and colonic inertia.75 Other equivalent options are scintigraphy30 or a wireless pH-pressure capsule.76 While a radiopaque marker study takes 5–7 days, scintigraphy requires 24 or 48 hours.74 In constipated patients, measurements of colonic transit with radiopaque markers and scintigraphy and separately with the wireless motility-pH capsule are reasonably correlated.53, 76 The capsule can also measure, small bowel transit, in a limited fashion, gastric emptying and colonic motor activity.77 However, this study takes 5 days and requires patients to wear a data collection device.
Colonic Manometry and Barostat Testing
As detailed above, this test is used selectively in patients with medically-refractory slow transit constipation who are being considered for colectomy at specialized centers.28 In adults, personal experience suggests the test is helpful in selected cases (e.g., among patients who have severe symptoms but only a borderline delay in colonic transit (Figure 1).
Putting it Together
After the clinical assessment, constipated patients may be tentatively classified into one (or possibly more) of the following categories:
NTC with normal colonic transit and defecation. Some patients with NTC also have symptoms of IBS-C (e.g., abdominal pain, bloating and incomplete defecation).
STC with slow colonic transit, normal defecation and absence of megacolon.
DD (anismus/dyssynergia; ineffective propulsive pressures; failure of relaxation; descending perineal syndrome )
STC and DD. Some patients also have features of IBS.
Opioid-induced constipation, which is defined by new, or worsening, symptoms of constipation when initiating, changing, or increasing opioid therapy4
Organic constipation (mechanical obstruction, or drug side effect; (Supplementary Table 1) or metabolic disorders; (Supplementary Table 2).
During the primary consultation, the clinical assessment is probably sufficient to exclude organic and secondary constipation in most patients, providing the basis for symptomatic treatment. Diagnostic studies for constipation will only be required in some cases.
Medical Management
Table 2 78–82 summarizes common laxatives and newer pharmacological agents for chronic constipation. Drugs (e.g., bile-acid transporter inhibitors) that were effective in phase II trials but need further study will not be discussed.83
Table 2. OTC and Prescription Treatments for Chronic Constipation and Constipation-Predominant IBS.
Courtesy of Dr Michael Hirsch, Department of Pharmacy, University of Wisconsin Hospitals, Madison, Wi
| Treatment, frequency | Dose | NNT (95% CI) for CC and IBS-C b | Cost per month 2018$ | Comments |
|---|---|---|---|---|
| Bulking agents: psyllium, daily | CC: Variable dose IBS-C: Variable dose |
CC: 2 (1–3) 79 IBS-C: 10 (6–33) 79 |
8.34 | Start with low dose and increase gradually. |
| Polyethylene glycol, daily | CC: 17gm IBS-C | CC: 3 (2–4) 2 IBS-C: NA |
8.73 | More evidence in CC than IBS-C. Improved bowel symptoms but not abdominal pain in IBS-C80 |
| Lactulose, daily | 20g | NA | 13.28 | Can produce bloating and distension |
| Bisacodyl, daily | CC: 10mg IBS-C | CC: 4 (NA)81 IBS-C: NA |
5.17 | Available as suppository, preferably administered 30 minutes after breakfast |
| Senna, daily | 17.2 – 34.4mg | 6.96 | Widely used anthraquinone laxative | |
| Prucalopride, daily | CC: 2mg IBS-C: NA |
CC: 6 (5–9) 2 IBS-C: NA |
Not approved in the United States. Available in Mexico, Canada, and Europe | |
| Linaclotide, daily | CC: 72µg IBS-C: 290 µg |
CC: 12 (6–29) (72 µg); 10 (6–19) (145 µg) 78 IBS-C: 6 (4–16) (290 µg) |
466.47 | Improves abdominal pain, bloating, and global IBS symptoms in IBS-C |
| Lubiprostone, twice daily | CC: 24µg IBS-C: 8 µg |
CC: 4 (3–6) (24µg) 79 IBS-C: 12 (8–25) (8 µg) |
445.32 | Also improves abdominal bloating, discomfort, constipation severity in opioid-induced constipation82 |
| Plecanatide, daily | CC: 3 mg or 6 mg IBS-C: or 6mg | CC: 11 (8–19) (3 mg); 12 (8–23) (6 mg) 78 IBS-C:9 (6–16) (3mg);9 (6–17) (6mg) |
466.16 | Same as linaclotide |
Abbreviations: CC = chronic constipation; IBS-C = irritable bowel syndrome of constipation.
NNT indicates number needed to treat
Adjunctive approaches
Except for patients with dehydration, increased fluid intake does not treat constipation.62 There is an inverse relationship between physical activity and the severity of constipation.62, 84 Moderate-to-vigorous intensive physical activity (20–60 minutes on 3 to 5 days per week) improved symptoms and quality of life in IBS.85 The effects of probiotics on constipation are poorly understood.86
Dietary Fiber Supplementation and Osmotic Laxatives
Soluble dietary fiber (e.g., psyllium or ispaghula) supplements reduce bowel symptoms in chronic constipation87 and IBS;88 insoluble dietary fiber (e.g., wheat bran) do not. However, only one of 4 trials in constipated patients lasted more than 4 weeks; none were at low risk of bias. A meta-analysis of 17 trials concluded that soluble fiber improved global symptoms and constipation in IBS. However, the effects on abdominal pain were variable.88 Hence, fiber supplementation, either through the diet or as a standardized fiber supplement (Table 2), should be considered as the first step in constipated patients, particularly in primary care. Beginning with a single daily dose taken with fluids and/or meals, the dose should be gradually adjusted after a 7–10 day period, recognizing that the response may manifest over several weeks’. Patients should be reminded that fiber supplements may increase gaseousness. This often improves over time and can be reduced by switching to another fiber supplement.
Another initial option is an osmotic agent, administered daily, and supplemented, when necessary, with stimulant laxatives. No studies have compared osmotic and stimulant laxatives. A meta-analysis of 7 controlled studies with 1141 patients who had chronic idiopathic constipation observed that the number needed to treat (NNT) for osmotic and stimulant laxatives was 3 (95% CI 2–4).89 Osmotic agents (i.e., polyethylene glycol-based solutions (PEG), magnesium citrate-based products, sodium phosphate-based products, and nonabsorbable carbohydrates (ie lactulose)) draw fluid into the intestinal lumen to maintain gut isosmolality, thereby increasing stool water and colon propulsion. The dose should be titrated to produce soft but not liquid stools. For PEG, there is extensive evidence, including 1 controlled trial lasting 6 months, 89–92 and retrospective studies which confirm that treatment with PEG is safe and effective for up to 24 months.91, 93 Patients prefer PEG preparations without electrolyte supplements.94 For colonic cleansing, larger volumes of PEG with electrolytes are used.95 Magnesium hydroxide and other salts improve stool frequency and consistency. 96 Among 244 constipated women, a natural mineral water rich in magnesium and sulfate was safe and improved symptoms of chronic constipation over 2 weeks compared to mineral water which was low in magnesium. While absorption of magnesium is limited, patients with renal disease may develop severe hypermagnesemia.97 Side effects of sodium phosphate-based bowel cleansing preparations include hyperphosphatemia, hypocalcemia, and hypokalemia; less than one in 1000 individuals develop acute phosphate nephropathy.97, 98 Hence, they should be avoided.
PEG was better than lactulose for improving stool frequency, stool consistency, and abdominal pain in a Cochrane Database review of 10 randomized trials.99 In a randomized crossover study of 30 men, lactulose and sorbitol were equally effective but lactulose was associated with more nausea.100 Bacterial metabolism of these unabsorbed carbohydrates leads to gas production.
Stimulant laxatives such as senna, bisacodyl, and sodium picosulfate induce propagated colonic contractions. Even long-term use is very safe; bisacodyl and sodium picosulfate have anti-absorptive and secretory effects.92, 101–105 These agents may be used as rescue agents, (e.g., if patients do not have a bowel movement for 2–3 days)105 or more regularly if required. Stimulant suppositories (i.e., bisacodyl and glycerin) should be given about 30 minutes after breakfast in order to synchronize their effects with the gastrocolonic response. In a large study, sodium picosulfate improved stool consistency and frequency as well as ease of evacuation and quality of life compared to placebo.92 Stimulant laxatives do not appear to damage the enteric nervous system.106, 107 Unfortunately, it remains not uncommon for providers and pharmacists to warn of the “potential dangers” of using stimulant laxatives which may lead to underutilization of these effective and inexpensive agents.
In carefully selected patients with STC, the personal experience of one of the authors (AW) suggests that the prostaglandin E1 analog misoprostol, in varying doses, may be effectively used to avoid subtotal colectomy.
Intestinal Secretagogues
Secretagogues such as lubiprostone, linaclotide, and plecanatide are approved by the FDA for treating chronic constipation and IBS-C.78, 108 These agents increase intestinal chloride secretion by activating channels on the apical (luminal) enterocyte surface. 78, 108 To maintain electroneutrality, sodium is also secreted into the intestinal lumen by other ion channels and transporters. To preserve isosmolality, water secretion follows. By increasing intestinal secretion, secretagogues accelerate transit and facilitate ease of defecation. Lubiprostone, a bicyclic fatty acid derivative of prostaglandin E1, primarily activates the apical CIC-2 chloride channels;108 it accelerates small intestinal and colonic transit in healthy subjects,109 In women of childbearing age, a negative pregnancy test should be documented before starting treatment and contraceptive measures are necessary.
Similar to the heat-stable enterotoxins that cause diarrhea, linaclotide is a 14-amino acid peptide.78, 110 These ST, which are also homologs of the endogenous paracrine hormones uroguanylin in the small intestine and guanylin in the colon act on guanylyl cyclase C, which is expressed in brush border membranes of intestinal mucosal cells from the duodenum to the rectum. Linaclotide activates the intracellular catalytic domain of guanylyl cyclase C, which in turn converts guanosine triphosphate to cyclic guanosine monophosphate, inducing downstream effectors that open the cystic fibrosis transmembrane conductance regulator chloride channel and produce a net efflux of ions and water into the intestinal lumen.
Plecanatide is a newly approved guanylyl cyclase C agonist for the treatment of both chronic constipation and IBS-C. Plecanatide demonstrated efficacy and safety in a randomized placebo controlled trial of over 1300 patients with chronic constipation.111 Both 3 mg and 6 mg doses demonstrated approximately 7% more efficacy than did placebo (20% for both doses vs 12.8% placebo; p<0.004) over a 12 weeks trial. A recent systematic review and meta-analysis concluded that linaclotide and plecanatide were equally effective and safe, as might have been anticipated.78
Serotonin 5-HT4 receptor agonists
By stimulating serotonin 5-HT4 receptors, which are widely distributed on enteric neurons, 5-HT4 agonists release the excitatory neurotransmitter acetylcholine and induce mucosal secretion. The European Agency for Evaluation of Medicinal Products approved prucalopride, a 5-HT4 agonist, for treating chronic constipation in women in whom laxatives fail to provide adequate relief.112–115 It is currently not approved by the FDA but can be legally imported by patients, for example from Canada or Mexico. Prucalopride is safe and does not have cardiovascular side effects.
Comparison of pharmacological agents for chronic constipation
Based on meta-analyses,89, 116 systematic reviews,87 and the only head-to-head comparative study,117 therapeutic trial(s) of fiber supplementation, osmotic laxatives, and/or stimulant laxatives, which are effective, safe, and generally less expensive, should be implemented before newer agents (secretagogues, serotonin 5-HT4 receptor agonists in Europe) are considered (Table 278–82). Several points in Table 2 deserve emphasis. First, these numbers may not be strictly comparable since different studies used different endpoints. Second, except for soluble fiber, there is more evidence for efficacy in chronic constipation than in IBS-C. While lubiprostone, linaclotide and plecanatide have been studied in IBS-C, there are no large high-quality trials of PEG, stimulant laxatives or prucalopride in IBS-C. Third, the evidence for efficacy in chronic constipation is strong for osmotic and stimulant laxatives which also have the most favorable cost-benefit ratios. Fourth, several well designed trials demonstrate that lubiprostone, linaclotide, and plecanatide are effective for treating chronic constipation and IBS-C. Lastly, since lack of response to traditional agents (e.g., laxatives) was not an entry criterion for the studies of the 3 secretogogues, the incremental utility of these newer agents over traditional approaches is unknown.
Treatments for Opioid-induced constipation (OIC)
Over the past two decades, the use of opiates and opioids for chronic pain has assumed epidemic proportions.118 Between 40–90% of patients on opioids have constipation.119 Opioids delay gastrointestinal transit, stimulate non-propulsive motor activity, increase intestinal segmentation and decrease electrolyte and water secretion into the gut. These effects work predominantly through opioid mµ receptors located in the gut as well as the central nervous system and may be difficult to overcome with most available laxatives. Lubiprostone is slightly better than placebo and is of similar efficacy to prucalopride, which is available in Europe.120
A more biologically plausible approach to OIC is to use an effective peripheral µ-opioid receptor antagonist. These drugs do not significantly counteract the benefits of pain reduction (Supplementary Table 3). For example, naloxegol is a pegylated derivative of naloxone that does not cross the blood-brain barrier. Two randomized, placebo-controlled trials involving 1,352 subjects found that naloxegol in doses of 12.5 mg or 25 mg daily were superior to placebo over a 12-week trial.121 Response rates to the 25 mg dose were significantly higher with drug vs. placebo (44.4% vs. 29.4%; 39.7% vs. 29.3%) with an NNT of 6.7 and 9.7 respectively. Similar results were seen among patients who previously had an inadequate response to laxatives. Similarly, in a meta-analysis, methylnaltrexone in doses of 0.15 mg/kg and 0.20 mg/kg body weight every other day when given subcutaneously, and 12 mg daily when given orally, were significantly superior to placebo.122 These agents together with naloxone, naldemedine, and lubiprostone are approved for treating OIC in the United States. The peripheral µ-opioid receptor antagonist alvimopan shortens postoperative ileus but is not approved for treating OIC.123
Management of Defecatory Disorders
Non-structural DD are best managed by biofeedback-aided pelvic floor therapy, which is more effective than polyethylene glycol, sham feedback, or diazepam.124 In one study, colonic transit normalized after biofeedback therapy in 65% of patients with disordered defecation, which suggests that pelvic floor dysfunction may delay colonic transit.38 These trials employed 5–6 training sessions lasting 30–60 minutes at 2 week intervals. The therapist’s skill and experience and the patient’s motivation influence the response to biofeedback therapy. Aided by visual or auditory feedback of anorectal and pelvic floor muscle activity, which are recorded with surface electromyographic sensors or manometry, patients are taught to increase intra-abdominal pressure and relax the pelvic floor muscles during defecation. Thereafter, patients learn how to expel an air-filled balloon. When rectal sensation is reduced, sensory retrainingmay also be provided.
Regrettably, biofeedback therapy is not widely used to manage DD, perhaps because the therapy is not widely available and/or its benefits are not widely recognized. Many therapists inappropriately teach patients with DD to strengthen the external anal sphincter rather than improve coordination between abdominal and pelvic floor motion during evacuation. Third-party coverage for biofeedback therapy in DD has improved and may be more accepted when using the entirely appropriate term “muscle rehabilitation therapy”. For example, in several states, the Centers for Medicare and Medicaid Servicesnow regard biofeedback therapy as medically necessary for treating adults with constipation due to DD unresponsive to laxatives. When insurance carriers deny approval for biofeedback therapy in patients with DD, the decision should be appealed because they may be unaware of the considerable evidence demonstrating the efficacy of pelvic floor retraining for DD.
Role of Surgery
Abdominal colectomy and ileorectal anastomosis is the next option in patients with medically-refractory slow transit constipation who do not have diffuse upper GI dysmotility or a DD.125 Some studies suggest that quality of life improves and is sustained over time.126 However, results are variable.127 In general, studies in which colorectal physiologic assessments were incomplete observed poorer outcomes. Potential complications include ileus, small bowel obstruction, anastomotic leakage, and wound infections. Most episodes of small bowel obstruction are managed conservatively and do not require reoperation. Other surgical or minimally-invasive approaches for slow transit constipation include antegrade colonic enemas that are administered by infusing water into the colon, either through an appendiceal conduit (Malone procedure) or indwelling cecostomy catheter (percutaneous endoscopic cecostomy, PEC).127 Since a PEC can be performed under local anaesthesia and conscious sedation, it may be preferred to colectomy in patients who have a higher surgical risk due to co-morbidities. Also, a PEC is reversible. By comparison, 30% of patients have complications after the Malone procedure.10 In patients with slow transit constipation, severe bloating and/or abdominal pain, a venting ileostomy may be useful to determine if symptoms are attributable to the small intestine or colon. An iliorectal anastomosis may be inadvisable if symptoms do not improve with a venting ileostomy.128 In these situations, a colostmy is ill-advised because colonic transit is slow and persistent constipation may occur.
Other approaches
Sacral nerve stimulation, dividing the puborectalis muscle or performing a postanal repair 129,130 do not improve symptoms of constipation and are not FDA approved for use in the United States. Injection of botulinum toxin into the puborectalis muscle131, 132 cannot be recommended for managing DD. The efficacy of the stapled transanal resection procedure, wherein staples are applied to the redundant rectal mucosa associated with rectocele and intussusception is uncertain and the link between symptoms and actual anatomic abnormalities is tenuous.125 It is likely that anatomic abnormalities, such as intussusception and rectal prolapse are secondary to a DD and excessive straining, which is not remedied by the procedure. The adrenergic α1 receptor antagonist reduced anal pressure at rest and during simulated evacuation but did not improve symptoms in patients with defecatory disorders.133
Conclusions
Constipation is a common symptom that can substantially affect quality of life. An algorithmic approach facilitates the management. A structural evaluation of the colon is only required in a minority of patients. Laxatives, biofeedback and surgery are all effective in treating selected patients.
Supplementary Material
Acknowledgments:
This study was supported in part by USPHS NIH Grant R01 DK78924 from the National Institutes of Health.
Abbreviations
- NTC
normal transit constipation
- STC
slow transit constipation
- DD
defecatory disorders
- FC
functional constipation
- IBS-C
constipation-predominant IBS
- U.S.
United States
- NNT
number needed to treat
- PEG
polyethylene glycol
- 5-HT4
5-Hydroxytryptamine receptor 4
- FDA
United States Food and Drug Administration
- OIC
Opioid-induced constipation
- PEC
percutaneous endoscopic cecostomy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Dr. Bharucha reports personal fees from Allergan, personal fees from Forum Pharmaceuticals, personal fees from Macmillan Medical Communications, personal fees from Salix Pharma, outside the submitted work; In addition, Dr. Bharucha has a patent Portable anorectal manometry device with royalties paid to Medspira, and a patent Anorectal manometry probe fixation device licensed to Medtronic. Dr. Wald reports personal fees from Ironwood Pharma, personal fees from Takeda/Sucampo, personal fees from Theravance, personal fees from Shire, personal fees from EnteraHealth, outside the submitted work.
REFERENCES
- 1.Sandler RS, Drossman DA. Bowel habits in young adults not seeking health care. Dig. Dis. Sci 1987;32(8):841–845. [DOI] [PubMed] [Google Scholar]
- 2.Bharucha AE, Locke GR, Pemberton JH. AGA Practice Guideline on Constipation: Technical Review. Gastroenterology 2013;144:218–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rao S, Bharucha AE, Chiarioni G, et al. Functional anorectal disorders. Gastroenterology 2016;150(6):1430–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mearin F, Lacy BE, Chang L, et al. Bowel Disorders. Gastroenterology 2016;18:18. [DOI] [PubMed] [Google Scholar]
- 5.Bouchoucha M, Devroede G, Mary F, Bon C, Bejou B, Benamouzig R. Painful or mild-pain constipation? A clinically useful alternative to classification as irritable bowel syndrome with constipation versus functional constipation. Dig. Dis. Sci 2018. [DOI] [PubMed] [Google Scholar]
- 6.Whitehead WE, Palsson OS, Simren M. Biomarkers to distinguish functional constipation from irritable bowel syndrome with constipation. Neurogastroenterol. Motil 2016;28(6):783–792. [DOI] [PubMed] [Google Scholar]
- 7.Bharucha AE, Sharma M. Painful and Painless Constipation: All Roads Lead to (A Change in) Rome. Dig. Dis. Sci 2018;21:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mugie SM, Benninga MA, Di Lorenzo C. Epidemiology of constipation in children and adults: A systematic review. Best Pract. Res. Clin. Gastroenterol 2011;25(1):3–18. [DOI] [PubMed] [Google Scholar]
- 9.Bharucha AE, Locke GR, Zinsmeister AR, et al. Differences between painless and painful constipation among community women. Am. J. Gastroenterol 2006;101(3):604–612. [DOI] [PubMed] [Google Scholar]
- 10.Kinnunen O Study of constipation in a geriatric hospital, day hospital, old people’s home and at home. Aging-Clinical & Experimental Research 1991;3(2):161–170. [DOI] [PubMed] [Google Scholar]
- 11.Noelting J, Eaton J, Choung RS, Zinsmeister AR, Locke GR 3rd, Bharucha AE. The Incidence Rate and Characteristics of Clinically Diagnosed Defecatory Disorders in the Community. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 2016;28(11):1690–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Dijk KN, de Vries CS, van den Berg PB, Dijkema AM, Brouwers JR, de Jong-van den Berg LT. Constipation as an adverse effect of drug use in nursing home patients: an overestimated risk. Br. J. Clin. Pharmacol 1998;46(3):255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drossman DA, Li Z, Andruzzi E, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig. Dis. Sci 1993;38(9):1569–1580. [DOI] [PubMed] [Google Scholar]
- 14.Shaheen NJ, Hansen RA, Morgan DR, et al. The burden of gastrointestinal and liver diseases, 2006. Am. J. Gastroenterol 2006;101(9):2128–2138. [DOI] [PubMed] [Google Scholar]
- 15.Shah ND, Chitkara DK, Locke GR, Meek PD, Talley NJ. Ambulatory care for constipation in the United States, 1993–2004. Am. J. Gastroenterol 2008;103(7):1746–1753. [DOI] [PubMed] [Google Scholar]
- 16.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010.[Erratum appears in CA Cancer J Clin. 2011 Mar-Apr;61(2):133–4]. CA Cancer J. Clin 2010;60(5):277–300. [DOI] [PubMed] [Google Scholar]
- 17.Martin BC, Barghout V, Cerulli A. Direct medical costs of constipation in the United States. Manag. Care Interface 2006;19(12):43–49. [PubMed] [Google Scholar]
- 18.Choung RS, Branda ME, Chitkara D, et al. Longitudinal direct medical costs associated with constipation in women. Aliment. Pharmacol. Ther 2010;33(2):251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai Q, Buono JL, Spalding WM, et al. Healthcare costs among patients with chronic constipation: a retrospective claims analysis in a commercially insured population. J. Med. Econ 2014;17(2):148–158. [DOI] [PubMed] [Google Scholar]
- 20.Belsey J, Greenfield S, Candy D, Geraint M. Systematic review: impact of constipation on quality of life in adults and children. Aliment. Pharmacol. Ther 2010;31(9):938–949. [DOI] [PubMed] [Google Scholar]
- 21.Yost KJ, Haan MN, Levine RA, Gold EB. Comparing SF-36 scores across three groups of women with different health profiles. Qual. Life Res 2005;14(5):1251–1261. [DOI] [PubMed] [Google Scholar]
- 22.Nyam DC, Pemberton JH, Ilstrup DM, Rath DM. Long-term results of surgery for chronic constipation.[Erratum appears in Dis Colon Rectum 1997 May;40(5):529]. Dis. Colon Rectum 1997;40(3):273–279. [DOI] [PubMed] [Google Scholar]
- 23.Mertz H, Naliboff B, Mayer E. Physiology of refractory chronic constipation. Am. J. Gastroenterol 1999;94(3):609–615. [DOI] [PubMed] [Google Scholar]
- 24.Ravi K, Bharucha AE, Camilleri M, Rhoten D, Bakken T, Zinsmeister AR. Phenotypic variation of colonic motor functions in chronic constipation. Gastroenterology 2010;138(1):89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preston DM, Lennard-Jones JE. Severe chronic constipation of young women: ‘idiopathic slow transit constipation’. Gut 1986;27(1):41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao SS, Welcher KD, Leistikow JS. Obstructive defecation: a failure of rectoanal coordination. Am. J. Gastroenterol 1998;93(7):1042–1050. [DOI] [PubMed] [Google Scholar]
- 27.Hadley SJ, Walsh BT. Gastrointestinal disturbances in anorexia nervosa and bulimia nervosa. Current Drug Targets - Cns & Neurological Disorders 2003;2(1):1–9. [DOI] [PubMed] [Google Scholar]
- 28.Camilleri M, Bharucha AE, di Lorenzo C, et al. American Neurogastroenterology and Motility Society consensus statement on intraluminal measurement of gastrointestinal and colonic motility in clinical practice. Neurogastroenterol. Motil 2008;20(12):1269–1282. [DOI] [PubMed] [Google Scholar]
- 29.Dinning PG, Smith TK, Scott SM. Pathophysiology of colonic causes of chronic constipation. Neurogastroenterol. Motil 2009;21 Suppl 2:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manabe N, Wong BS, Camilleri M, Burton D, McKinzie S, Zinsmeister AR. Lower functional gastrointestinal disorders: evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol. Motil 2010;22(3):293–e282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bharucha AE. High amplitude propagated contractions. Neurogastroenterol. Motil 2012;24(11):977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dinning PG, Wiklendt L, Maslen L, et al. Colonic motor abnormalities in slow transit constipation defined by high resolution, fibre-optic manometry. Neurogastroenterol. Motil 2015;27(3):379–388. [DOI] [PubMed] [Google Scholar]
- 33.Bassotti G If it is inert, why does it move? Neurogastroenterol. Motil 2004;16(4):395–396. [DOI] [PubMed] [Google Scholar]
- 34.Farrugia G Interstitial cells of Cajal in health and disease. Neurogastroenterol. Motil 2008;20 Suppl 1:54–63. [DOI] [PubMed] [Google Scholar]
- 35.Corsetti M, and Costa GB Marcello, Bharucha Adil E. 4, Borrelli Osvaldo 5, Dinning Phil 2,6, Lorenzo Carlo Di 7, Huizinga Jan D. 8, Jimenez Marcel 9, Rao Satish 10, Spiller Robin 1, Spencer Nick 11, Lentle Roger 12, Pannemans Jasper 14, Thys Alexander 14, Benninga Marc 13 and Tack Jan 14 First “translational” consensus on terminology and definition of colonic motility as studied in humans and animals by means of manometric and non-manometric techniques. Neurogastroenterol. Motil 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guarino M, Cheng L, Cicala M, Ripetti V, Biancani P, Behar J. Progesterone receptors and serotonin levels in colon epithelial cells from females with slow transit constipation. Neurogastroenterol. Motil 2011;23(6):575–e210. [DOI] [PubMed] [Google Scholar]
- 37.Ratuapli SK, Bharucha AE, Noelting J, Harvey DM, Zinsmeister AR. Phenotypic Identification and Classification of Functional Defecatory Disorders Using High Resolution Anorectal Manometry. Gastroenterology 2012;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiarioni G, Ssalandini L, Whitehead WE. Biofeedback benefits only patients with outlet dysfunction, not patients with isolated slow transit constipation. Gastroenterology 2005;129(1):86–97. [DOI] [PubMed] [Google Scholar]
- 39.Gladman MA, Lunniss PJ, Scott SM, Swash M. Rectal hyposensitivity. Am. J. Gastroenterol 2006;101(5):1140–1151. [DOI] [PubMed] [Google Scholar]
- 40.Henry MM, Parks AG, Swash M. The pelvic floor musculature in the descending perineum syndrome. Br. J. Surg 1982;69(8):470–472. [DOI] [PubMed] [Google Scholar]
- 41.Bharucha AE, Fletcher JG, Seide B, Riederer SJ, Zinsmeister AR. Phenotypic variation in functional disorders of defecation. Gastroenterology 2005;128(5):1199–1210. [DOI] [PubMed] [Google Scholar]
- 42.Jones PN, Lubowski DZ, Swash M, Henry MM. Is paradoxical contraction of puborectalis muscle of functional importance? Dis Colon Rectum 1987;30(9):667–670. [DOI] [PubMed] [Google Scholar]
- 43.Chiarioni G, Nardo A, Vantini I, Romito A, Whitehead W. Biofeedback is superior to electrogalvanic stimulation and massage for treatment of levator ani syndrome. Gastroenterology 2010;138(4):1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bharucha AE, Lee TH. Anorectal and Pelvic Pain. Mayo Clin. Proc 2016;91(10):1471–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bharucha AE, Seide BM, Zinsmeister AR, Melton LJ 3rd. Insights into normal and disordered bowel habits from bowel diaries. Am. J. Gastroenterol 2008;103(3):692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitehead WE, di Lorenzo C, Leroi AM, Porrett T, Rao SS. Conservative and behavioural management of constipation. Neurogastroenterol. Motil 2009;21 Suppl 2:55–61. [DOI] [PubMed] [Google Scholar]
- 47.van Ginkel R, Reitsma JB, Buller HA, van Wijk MP, Taminiau JAJM, Benninga MA. Childhood constipation: longitudinal follow-up beyond puberty. Gastroenterology 2003;125(2):357–363. [DOI] [PubMed] [Google Scholar]
- 48.Law NM, Bharucha AE, Zinsmeister AR. Rectal and colonic distension elicit viscerovisceral reflexes in humans. Am. J. Physiol. Gastrointest. Liver Physiol 2002;283(2):G384–389. [DOI] [PubMed] [Google Scholar]
- 49.Lanfranchi GA, Bazzocchi G, Brignola C, Campieri M, Labo G. Different patterns of intestinal transit time and anorectal motility in painful and painless chronic constipation. Gut 1984;25(12):1352–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Posserud I, Syrous A, Lindstrom L, Tack J, Abrahamsson H, Simren M. Altered rectal perception in irritable bowel syndrome is associated with symptom severity. Gastroenterology 2007;133(4):1113–1123. [DOI] [PubMed] [Google Scholar]
- 51.Parthasarathy G, Chen J, Chen X, et al. Relationship Between Microbiota of the Colonic Mucosa vs Feces and Symptoms, Colonic Transit, and Methane Production in Female Patients With Chronic Constipation. Gastroenterology 2016;150(2):367–379 e361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abrahamsson H, Ostlund-Lindqvist AM, Nilsson R, Simren M, Gillberg PG. Altered bile acid metabolism in patients with constipation-predominant irritable bowel syndrome and functional constipation. Scand. J. Gastroenterol 2008;43(12):1483–1488. [DOI] [PubMed] [Google Scholar]
- 53.Degen LP, Phillips SF. How well does stool form reflect colonic transit? Gut 1996;39(1):109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ashraf W, Park F, Lof J, Quigley EM. An examination of the reliability of reported stool frequency in the diagnosis of idiopathic constipation. Am. J. Gastroenterol 1996;91(1):26–32. [PubMed] [Google Scholar]
- 55.Saad RJ, Rao SSC, Koch KL, et al. Do stool form and frequency correlate with whole-gut and colonic transit? Results from a multicenter study in constipated individuals and healthy controls. Am. J. Gastroenterol 2010;105(2):403–411. [DOI] [PubMed] [Google Scholar]
- 56.Mitsuhashi S, Ballou S, Jiang ZG, et al. Characterizing Normal Bowel Frequency and Consistency in a Representative Sample of Adults in the United States (NHANES). Am. J. Gastroenterol 2018;113(1):115–123. [DOI] [PubMed] [Google Scholar]
- 57.Guilera M, Balboa A, Mearin F. Bowel habit subtypes and temporal patterns in irritable bowel syndrome: systematic review. Am. J. Gastroenterol 2005;100(5):1174–1184. [DOI] [PubMed] [Google Scholar]
- 58.Bharucha AE, Chakraborty S, Sletten CD. Common Functional Gastroenterological Disorders Associated With Abdominal Pain. Mayo Clin. Proc 2016;91(8):1118–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Houghton LA. Bloating in constipation: relevance of intraluminal gas handling. Best Practice & Research in Clinical Gastroenterology 2011;25(1):141–150. [DOI] [PubMed] [Google Scholar]
- 60.Orkin BA, Sinykin SB, Lloyd PC. The digital rectal examination scoring system (DRESS). Dis. Colon Rectum 2010;53(12):1656–1660. [DOI] [PubMed] [Google Scholar]
- 61.Tantiphlachiva K, Rao P, Attaluri A, Rao SSC. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clin. Gastroenterol. Hepatol 2010;8(11):955–960. [DOI] [PubMed] [Google Scholar]
- 62.Muller-Lissner SA, Kamm MA, Scarpignato C, Wald A. Myths and misconceptions about chronic constipation. Am. J. Gastroenterol 2005;100(1):232–242. [DOI] [PubMed] [Google Scholar]
- 63.Wald A Constipation: Advances in Diagnosis and Treatment. JAMA 2016;315(2):185–191. [DOI] [PubMed] [Google Scholar]
- 64.Rao SS, Hatfield R, Soffer E, Rao S, Beaty J, Conklin JL. Manometric tests of anorectal function in healthy adults. Am. J. Gastroenterol 1999;94(3):773–783. [DOI] [PubMed] [Google Scholar]
- 65.Noelting J, Ratuapli SK, Bharucha AE, Harvey DM, Ravi K, Zinsmeister AR. Normal values for high-resolution anorectal manometry in healthy women: effects of age and significance of rectoanal gradient. Am. J. Gastroenterol 2012. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Prichard DO, Lee T, Parthasarathy G, Fletcher JG, Zinsmeister AR, Bharucha AE. High-resolution Anorectal Manometry for Identifying Defecatory Disorders and Rectal Structural Abnormalities in Women. Clin. Gastroenterol. Hepatol 2017;15(3):412–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee TH, Bharucha AE. How to Perform and Interpret a High-resolution Anorectal Manometry Test. J. Neurogastroenterol. Motil 2016;22(1):46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Basilisco G, Bharucha AE. High-resolution anorectal manometry: An expensive hobby or worth every penny? Neurogastroenterol. Motil 2017;29(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Palit S, Thin N, Knowles CH, Lunniss PJ, Bharucha AE, Scott SM. Diagnostic disagreement between tests of evacuatory function: a prospective study of 100 constipated patients. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society 2016;In press. [DOI] [PubMed] [Google Scholar]
- 70.Bharucha AE. Update of tests of colon and rectal structure and function. J. Clin. Gastroenterol 2006;40(2):96–103. [DOI] [PubMed] [Google Scholar]
- 71.Noelting J, Bharucha AE, Lake DS, et al. Semi-automated vectorial analysis of anorectal motion by magnetic resonance defecography in healthy subjects and fecal incontinence. Neurogastroenterol. Motil 2012;24:e467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tirumanisetty P, Prichard D, Fletcher JG, Chakraborty S, Zinsmeister AR, Bharucha AE. Normal values for assessment of anal sphincter morphology, anorectal motion, and pelvic organ prolapse with MRI in healthy women. Neurogastroenterol. Motil 2018;02:02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hinton JM, Lennard-Jones JE, Young AC. A new method for studying gut transit times using radioopaque markers. Gut 1969;10(10):842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Metcalf AM, Phillips SF, Zinsmeister AR, MacCarty RL, Beart RW, Wolff BG. Simplified assessment of segmental colonic transit. Gastroenterology 1987;92(1):40–47. [DOI] [PubMed] [Google Scholar]
- 75.Nam YS, Pikarsky AJ, Wexner SD, et al. Reproducibility of colonic transit study in patients with chronic constipation. Dis. Colon Rectum 2001;44(1):86–92. [DOI] [PubMed] [Google Scholar]
- 76.Rao SS, Kuo B, McCallum RW, et al. Investigation of colonic and whole gut transit with wireless motility capsule and radioopaque markers in constipation. Clin. Gastroenterol. Hepatol 2009. [DOI] [PubMed] [Google Scholar]
- 77.Hasler WL, Saad RJ, Rao SS, et al. Heightened colon motor activity measured by a wireless capsule in patients with constipation: relation to colon transit and IBS. Am J Physiol - Gastrointest Liver Physiol 2009;297(6):G1107–1114. [DOI] [PubMed] [Google Scholar]
- 78.Shah ED, Kim HM, Schoenfeld P. Efficacy and Tolerability of Guanylate Cyclase-C Agonists for Irritable Bowel Syndrome with Constipation and Chronic Idiopathic Constipation: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol 2018;113(3):329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ford AC, Moayyedi P, Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am. J. Gastroenterol 2014;109 Suppl 1:S2–26; quiz S27. [DOI] [PubMed] [Google Scholar]
- 80.Chapman RW, Stanghellini V, Geraint M, Halphen M. Randomized clinical trial: macrogol/PEG 3350 plus electrolytes for treatment of patients with constipation associated with irritable bowel syndrome. Am. J. Gastroenterol 2013;108(9):1508–1515. [DOI] [PubMed] [Google Scholar]
- 81.Kamm MA, Mueller-Lissner S, Wald A, Richter E, Swallow R, Gessner U. Oral bisacodyl is effective and well-tolerated in patients with chronic constipation. Clin. Gastroenterol. Hepatol 2011;9(7):577–583. [DOI] [PubMed] [Google Scholar]
- 82.Spierings EL, Rauck R, Brewer R, Marcuard S, Vallejo R. Long-Term Safety and Efficacy of Lubiprostone in Opioid-induced Constipation in Patients with Chronic Noncancer Pain. Pain practice : the official journal of World Institute of Pain 2015. [DOI] [PubMed] [Google Scholar]
- 83.Bharucha AE, Wouters MM, Tack J. Existing and emerging therapies for managing constipation and diarrhea. Curr. Opin. Pharmacol 2017;37:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Schryver AM, Samsom M, Smout AI. Effects of a meal and bisacodyl on colonic motility in healthy volunteers and patients with slow-transit constipation. Dig. Dis. Sci 2003;48(7):1206–1212. [DOI] [PubMed] [Google Scholar]
- 85.Johannesson E, Simren M, Strid H, Bajor A, Sadik R. Physical activity improves symptoms in irritable bowel syndrome: a randomized controlled trial. Am. J. Gastroenterol 2011;106(5):915–922. [DOI] [PubMed] [Google Scholar]
- 86.Quigley EMM. The enteric microbiota in the pathogenesis and management of constipation. Best Practice & Research in Clinical Gastroenterology 2011;25(1):119–126. [DOI] [PubMed] [Google Scholar]
- 87.Suares NC, Ford AC. Systematic review: the effects of fibre in the management of chronic idiopathic constipation. Aliment. Pharmacol. Ther 2011;33(8):895–901. [DOI] [PubMed] [Google Scholar]
- 88.Bijkerk CJ, Muris JWM, Knottnerus JA, Hoes AW, de Wit NJ. Systematic review: the role of different types of fibre in the treatment of irritable bowel syndrome. Aliment. Pharmacol. Ther 2004;19(3):245–251. [DOI] [PubMed] [Google Scholar]
- 89.Ford A, Suares N. Effect of laxatives and pharmacological therapies in chronic idiopathic constipation: systematic review and meta-analysis. Gut 2011;60:209–218. [DOI] [PubMed] [Google Scholar]
- 90.Corazziari E, Badiali D, Bazzocchi G, et al. Long term efficacy, safety, and tolerabilitity of low daily doses of isosmotic polyethylene glycol electrolyte balanced solution (PMF-100) in the treatment of functional chronic constipation. Gut 2000;46(4):522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.DiPalma JA, Cleveland MV, McGowan J, Herrera JL. A randomized, multicenter, placebo-controlled trial of polyethylene glycol laxative for chronic treatment of chronic constipation. Am. J. Gastroenterol 2007;102(7):1436–1441. [DOI] [PubMed] [Google Scholar]
- 92.Mueller-Lissner S, Kamm MA, Wald A, et al. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of sodium picosulfate in patients with chronic constipation. Am. J. Gastroenterol 2010;105(4):897–903. [DOI] [PubMed] [Google Scholar]
- 93.Migeon-Duballet I, Chabin M, Gautier A, et al. Long-term efficacy and cost-effectiveness of polyethylene glycol 3350 plus electrolytes in chronic constipation: a retrospective study in a disabled population. Curr. Med. Res. Opin 2006;22(6):1227–1235. [DOI] [PubMed] [Google Scholar]
- 94.Szojda MM, Mulder CJJ, Felt-Bersma RJF. Differences in taste between two polyethylene glycol preparations. J Gastrointest Liver Dis 2007;16(4):379–381. [PubMed] [Google Scholar]
- 95.Di Palma JA, Smith JR, Cleveland Mv. Overnight efficacy of polyethylene glycol laxative. Am. J. Gastroenterol 2002;97(7):1776–1779. [DOI] [PubMed] [Google Scholar]
- 96.Dupont C, Campagne A, Constant F. Efficacy and safety of a magnesium sulfate-rich natural mineral water for patients with functional constipation. Clin. Gastroenterol. Hepatol 2014;12(8):1280–1287. [DOI] [PubMed] [Google Scholar]
- 97.Nyberg C, Hendel J, Nielsen OH. The safety of osmotically acting cathartics in colonic cleansing. Nat. Rev. Gastroenterol. Hepatol 2010;7(10):557–564. [DOI] [PubMed] [Google Scholar]
- 98.Ainley EJ, Winwood PJ, Begley JP. Measurement of serum electrolytes and phosphate after sodium phosphate colonoscopy bowel preparation: an evaluation. Dig. Dis. Sci 2005;50(7):1319–1323. [DOI] [PubMed] [Google Scholar]
- 99.Lee-Robichaud H, Thomas K, Morgan J, Nelson RL. Lactulose versus polyethylene glycol for chronic constipation. Cochrane Database Syst. Rev (7):CD007570. [DOI] [PubMed] [Google Scholar]
- 100.Lederle FA, Busch DL, Mattox KM, West MJ, Aske DM. Cost-effective treatment of constipation in the elderly: a randomized double-blind comparison of sorbitol and lactulose. Am. J. Med 1990;89(5):597–601. [DOI] [PubMed] [Google Scholar]
- 101.Louvel D, Delvaux M, Staumont G, et al. Intracolonic injection of glycerol: a model for abdominal pain in irritable bowel syndrome? Gastroenterology 1996;110(2):351–361. [DOI] [PubMed] [Google Scholar]
- 102.Ewe K, Holker B. [The effect of a diphenolic laxative (Bisacodyl) on water- and electrolyte transport in the human colon (author’s transl)]. Klin. Wochenschr 1974;52(17):827–833. [DOI] [PubMed] [Google Scholar]
- 103.Manabe N, Cremonini F, Camilleri M, Sandborn WJ, Burton DD. Effects of bisacodyl on ascending colon emptying and overall colonic transit in healthy volunteers. Aliment. Pharmacol. Ther 2009;30(9):930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kienzle-Horn S, Vix JM, Schuijt C, Peil H, Jordan CC, Kamm MA. Efficacy and safety of bisacodyl in the acute treatment of constipation: a double-blind, randomized, placebo-controlled study. Aliment. Pharmacol. Ther 2006;23(10):1479–1488. [DOI] [PubMed] [Google Scholar]
- 105.Kamm M, Mueller-Lissner S, Wald A, et al. Stimulant laxatives are effective in chronic constipation: multi-center, 4-week, double-blind, randomized, placebo-controlled trial of bisacodyl. Gastroenterology 2010;138(Suppl 1):S228. [DOI] [PubMed] [Google Scholar]
- 106.Dufour P, Gendre P. Ultrastructure of mouse intestinal mucosa and changes observed after long term anthraquinone administration. Gut 1984;25(12):1358–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kiernan JA, Heinicke EA. Sennosides do not kill myenteric neurons in the colon of the rat or mouse. Neuroscience 1989;30(3):837–842. [DOI] [PubMed] [Google Scholar]
- 108.Schey R, Rao SSC. Lubiprostone for the treatment of adults with constipation and irritable bowel syndrome. Dig. Dis. Sci 2011;56(6):1619–1625. [DOI] [PubMed] [Google Scholar]
- 109.Camilleri M, Bharucha AE, Ueno R, et al. Effect of a selective chloride channel activator, lubiprostone, on gastrointestinal transit, gastric sensory, and motor functions in healthy volunteers. American Journal of Physiology - Gastrointestinal & Liver Physiology 2006;290(5):G942–947. [DOI] [PubMed] [Google Scholar]
- 110.Bharucha AE, Waldman SA. Taking a lesson from microbial diarrheagenesis in the management of chronic constipation. Gastroenterology 2010;138(3):813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.DeMicco M, Barrow L, Hickey B, Shailubhai K, Griffin P. Randomized clinical trial: efficacy and safety of plecanatide in the treatment of chronic idiopathic constipation. Therap. Adv. Gastroenterol 2017;10(11):837–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Camilleri M, Kerstens R, Rykx A, Vandeplassche L. A placebo-controlled trial of prucalopride for severe chronic constipation. N. Engl. J. Med 2008;358(22):2344–2354. [DOI] [PubMed] [Google Scholar]
- 113.Quigley EMM, Vandeplassche L, Kerstens R, Ausma J. Clinical trial: the efficacy, impact on quality of life, and safety and tolerability of prucalopride in severe chronic constipation--a 12-week, randomized, double-blind, placebo-controlled study. Aliment. Pharmacol. Ther 2009;29(3):315–328. [DOI] [PubMed] [Google Scholar]
- 114.Tack J, van Outryve M, Beyens G, Kerstens R, Vandeplassche L. Prucalopride (Resolor) in the treatment of severe chronic constipation in patients dissatisfied with laxatives. Gut 2009;58(3):357–365. [DOI] [PubMed] [Google Scholar]
- 115.Muller-Lissner S, Rykx A, Kerstens R, Vandeplassche L. A double-blind, placebo-controlled study of prucalopride in elderly patients with chronic constipation. Neurogastroenterol. Motil 2010;22(9):991–998. [DOI] [PubMed] [Google Scholar]
- 116.Nelson AD, Camilleri M, Chirapongsathorn S, et al. Comparison of efficacy of pharmacological treatments for chronic idiopathic constipation: a systematic review and network meta-analysis. Gut 2016. [DOI] [PubMed] [Google Scholar]
- 117.Di Palma JA, Cleveland MV, McGowan J, Herrera JL. A randomized, multicenter comparison of polyethylene glycol laxative and tegaserod in treatment of patients with chronic constipation. Am. J. Gastroenterol 2007;102(9):1964–1971. [DOI] [PubMed] [Google Scholar]
- 118.Rummans TA, Burton MC, Dawson NL. How Good Intentions Contributed to Bad Outcomes: The Opioid Crisis. Mayo Clin. Proc 2018;93(3):344–350. [DOI] [PubMed] [Google Scholar]
- 119.Prichard D, Norton C, Bharucha AE. Management of opioid-induced constipation. Br. J. Nurs 2016;25(10):S4–5, S8–11. [DOI] [PubMed] [Google Scholar]
- 120.Nee J, Zakari M, Sugarman MA, et al. Efficacy of Treatments for Opioid-induced Constipation: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol 2018;25:25. [DOI] [PubMed] [Google Scholar]
- 121.Chey WD, Webster L, Sostek M, Lappalainen J, Barker PN, Tack J. Naloxegol for opioid-induced constipation in patients with noncancer pain. N. Engl. J. Med 2014;370(25):2387–2396. [DOI] [PubMed] [Google Scholar]
- 122.Mehta N, O’Connell K, Giambrone GP, Baqai A, Diwan S. Efficacy of methylnaltrexone for the treatment of opiod-induced constipation: a meta-analysis and systematic review. Postgrad. Med 2016;128(3):282–289. [DOI] [PubMed] [Google Scholar]
- 123.Prichard D, Bharucha A. Management of opioid-induced constipation for people in palliative care. Int. J. Palliat. Nurs 2015;21(6):272, 274–280. [DOI] [PubMed] [Google Scholar]
- 124.Rao SS, Benninga MA, Bharucha AE, Chiarioni G, Di Lorenzo C, Whitehead WE. ANMS-ESNM position paper and consensus guidelines on biofeedback therapy for anorectal disorders. Neurogastroenterol. Motil 2015;27(5):594–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bharucha AE, Rao SSC, Shin AS. Surgical Interventions and the Use of Device-Aided Therapy for the Treatment of Fecal Incontinence and Defecatory Disorders. Clin. Gastroenterol. Hepatol 2017;15(12):1844–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hassan I, Pemberton JH, Young-Fadok TM, et al. Ileorectal anastomosis for slow transit constipation: long-term functional and quality of life results. J. Gastrointest. Surg 2006;10(10):1330–1336; discussion 1336–1337. [DOI] [PubMed] [Google Scholar]
- 127.Wilkinson-Smith V, Bharucha AE, Emmanuel A, Knowles C, Yiannakou Y, Corsetti M. When all seems lost: management of refractory constipation – surgery, rectal irrigation, percutaneous endoscopic colostomy, and more. Neurogastroenterol. Motil 2018;In press. [DOI] [PubMed] [Google Scholar]
- 128.Wong S, Lubowski D. Slow-transit constipation: evaluation and treatment. ANZ J. Surg 2007;77(5):320–328. [DOI] [PubMed] [Google Scholar]
- 129.Keighley MR, Shouler P. Outlet syndrome: is there a surgical option? J. R. Soc. Med 1984;77(7):559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kamm MA, Hawley PR, Lennard-Jones JE. Lateral division of the puborectalis muscle in the management of severe constipation. Br. J. Surg 1988;75(7):661–663. [DOI] [PubMed] [Google Scholar]
- 131.Maria G, Cadeddu F, Brandara F, Marniga G, Brisinda G. Experience with type A botulinum toxin for treatment of outlet-type constipation. Am. J. Gastroenterol 2006;101(11):2570–2575. [DOI] [PubMed] [Google Scholar]
- 132.Faried M, El Nakeeb A, Youssef M, Omar W, El Monem H. Comparative study between surgical and non-surgical treatment of anismus in patients with symptoms of obstructed defecation: a prospective randomized study. J. Gastrointest. Surg 2010;14(8):1235–1243. [DOI] [PubMed] [Google Scholar]
- 133.Chakraborty S, Feuerhak K, Muthyala A, Harmsen WS, Bailey KR, Bharucha AE. Effects of Alfuzosin, an alpha1-Adrenergic Antagonist, on Anal Pressures and Bowel Habits in Women With and Without Defecatory Disorders. Clinical Gastroenterology and Hepatology In press [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.