Abstract
Cervical degenerative disease is a major cause of neck disability, but it has been understudied in patients with cervical spondylotic (CS), largely due to the fact that the neurological impairment associated with this condition tends to be the primary treatment focus. This observational study examined the cerebral functional alterations occurring in advanced cervical spondylosis and myelopathy using resting state functional MRI. Associations between functional connectivity (FC) and neck disability using the Neck Disability Index (NDI) were assessed. Results of the study demonstrated an increase in FC with increasing in neck disability in regions associated with sensorimotor system (both postcentral gyri and precentral gyri, bilaterally, with the SMA; bilateral precentral gyri and the left postcentral gyrus, with the left superior frontal gyrus; bilateral SMA and the left putamen, with the superior frontal gyri). Accounting for the difference in neurological function (mJOA score), strong connectivity between the precentral gyri and the SMA associated with the neck disability. Consistent with studies in chronic pain conditions, these findings suggest neck disability is associated with altered cerebral FC in cervical spondylosis patients.
Keywords: Brain, degenerative cervical myelopathy, cervical spondylosis, functional MRI, neck disability
INTRODUCTION
Neck pain is a highly significant societal issue as it is the fourth leading cause of disability in the United States, with an annual prevalence greater than 30% [1]. Although traumatic etiologies such as whiplash are frequently encountered in clinical practice, the vast majority of neck pain is related to musculoskeletal etiologies. Cervical spondylosis is an expected consequence of aging, with an incidence of 10% at age 25 and 75% by the age of 65 [2]. Senior citizens are the fastest growing segment of the United States population, and by the middle of this century, it is predicted that they will represent nearly one-quarter of the population [3]. Thus, neck pain, already a leading cause of disability in middle aged and older adults, is likely to be encountered with increasing frequency in the coming years.
Neck pain and disability has been largely understudied in cervical spondylosis (CS) patients, as the focus has understandably been directed towards treatment of the progressive neurological dysfunction, which is the hallmark of this disorder. Pain conditions are frequently centrally mediated, and these mechanisms are poorly understood, but may contribute to chronicity and their refractory nature. Central mechanisms have been well described for other common pain syndromes including those arising from the low back and abdominal etiologies [4–7].
One of the major goals of this investigation is to better understand the supratentorial response to neck pain and disability, along with the central mechanisms involved, with the future hope of identifying regions that could be potentially amenable for treatment strategies. We have previously reported that atrophic structural changes occur in cerebral regions associated with pain pathways in CS patients, and that these changes correlate with the degree of neck pain and disability, as measured by the Neck Disability Index (NDI) [8]. In this study, we explore the differences in functional connectivity measured using resting state fMRI (rs-fMRI) in patients with advanced cervical spondylosis (CS) with and without neck disability, as well as healthy volunteers. Similar to previous studies involving aspects of chronic pain [9–17], we hypothesized patients with CS will demonstrate altered brain functional connectivity within regions often implicated in chronic pain [9–17], including the insular cortex (IC), the superior frontal gyrus (SFG), the middle frontal gyrus (MFG), the pre- and postcentral gyri, the superior parietal lobule (SPL), the supplementary motor area (SMA), the anterior and posterior cingulate (AC and PC, respectively), and decreased connectivity between subcortical and frontal lobe regions proportional to the degree of neck pain or disability.
MATERIALS AND METHODS
Patient Population
A total of 36 patients with cervical spondylosis with or without myelopathy were prospectively enrolled in a cross-sectional study involving observational MRI and evaluation of neck disability from 2016–2018. Patients were recruited from an outpatient neurosurgery clinic, and each had at least moderate cervical stenosis as defined as no visible cerebrospinal fluid signal around the spinal cord at the site of maximal compression on MRI. The patients were referred for neurosurgical consultation due to the diagnosis of cervical stenosis, and most of patients presented with either neurological symptomatology, neck pain, or both in varying degrees. All patients signed Institutional Review Board (IRB#11–001876; Medical IRB Committee #3; University of California Los Angeles) approved consent forms, all analyses were done in compliance with the Health Insurance Portability and Accountability Act (HIPAA), and the UCLA IRB approved all aspects of the current study. The cohort included 25 males and 11 females, with a mean age of 56 years (range 37 to 80). The neck disability index (NDI) was used as a measure of neck pain [18, 19], where higher value of NDI represents higher degrees of disability. The modified Japanese Orthopedic Association (mJOA) score was used as a measure of neurological function [20], where lower value of mJOA represents worse neurological deficits. Two patients reported no neurological deficits (mJOA = 18) or neck disability (NDI < 4). Nine patients had no neurological deficits (mJOA = 18) but presented with neck disability (NDI > 4). Seven patients had neurological impairment (mJOA < 18) but presented with no neck disability (NDI < 4). All other patients had some neurological deficits (mJOA < 18) and neck disability (NDI > 4). For Patients in the early stages of cervical spondylosis and patients with a high threshold of pain, symptoms may not yet be observable. Thus, even when a patient has an mJOA=18 or NDI < 4, this does not necessarily mean the patient is neurologically healthy.
In comparison, a cohort of 17 neurologically intact, healthy control volunteers (HC) underwent the same MRI protocol, which included 11 men and 6 women with average age of 40 years, ranging from 25 to 62. A significant difference in age was present between CS and HC (P<0.001, t-test), so connectivity results were corrected for age. The patient and healthy volunteer demographic data are summarized in Table 1. Patients included in the current study are a subset of previous patients used in a previous publication [21].
Table 1:
Demographics for cervical spondylosis (CS) patients and healthy control (HC) volunteers used for rs-fMRI analyses.
| N | Age (mean years +/− SD) [min, max] | Sex | NDI (mean +/− SD) [min, max] | mJOA (mean +/− SD) [min, max] | |
|---|---|---|---|---|---|
| CS Patients | 36 | 60.1 ± 12.2 [37, 80] | 25M / 11F | 11.1 ± 9.2 [0, 37] | 15.9 ± 2.5 [10, 18] |
| HC Volunteers | 17 | 40.7 ± 10.9 [25, 62] | 11M / 6F | 0 | 18 |
Resting-State fMRI Acquisition and Post-Processing
All functional MR images were collected on a Siemens Prisma 3T MR scanner (Siemens Healthcare, Erlangen, Germany) with a repetition time (TR)=1500–2000ms; echo time (TE)=28–30ms; slice thickness of 4mm with no interslice gap; field-of-view (FOV) of 220–245mm with an acquisition matrix of 64×64 for an in-plane resolution of 3.4–3.8mm, interleaved acquisition; flip angle of 90°; and parallel imaging via CAIPIRINHA with a factor of 2. Additionally, a 1mm 3D isotropic MPRAGE sequence was acquired for alignment with functional MRI data using standard acquisition parameters (TE=minimum, TR=1500–200ms, inversion time (TI)=1100–1500ms, flip angle 8–15 degrees, slice thickness = 1mm, FOV=25cm and matrix size of 256×256).
The CONN Toolbox (https://www.nitrc.org/projects/conn) [22] was used for functional connectivity analysis of the brain, which implements functions from the Statistic Parametric Mapping (SPM, http://www.fil.ion.ucl.ac.uk/spm/) toolbox. Because rs-fMRI is interested in low-frequency oscillations (≤ 0.1Hz, but generally lower than 0.08Hz), and because scans in this study used a relatively fast TR (1.5–2s), slice-timing correction was not applied as previous studies have suggested negligible effects on rs-fMRI results [23]. Functional realignment (motion correction, 12 degrees of freedom) and unwarping, registration of functional data to the structural volume, and registration of the structural volume to the standardized space defined by the Montreal Neurological Institute (MNI) averaged T1 brain [24] was then performed using the CONN pipeline. Segmentation of structural volumes, which included skull stripping and processing of tissue types (GM, WM, and CSF), were then performed. Artifacts Detection Tool (ART), an SPM package implemented in the CONN pipeline, was used to remove signal intensity spikes and fMRI volumes with excessive motion from the scan, with thresholds for signal intensity outliers set at 9 standard-deviations above or below the mean. A motion limit of 2mm translation and 2° rotation in any direction was also enforced. Spatial smoothing of the functional data was performed using an 8mm full width at half maximum (FWHM) Gaussian kernel. For denoising, signal from the WM, CSF, and motion parameters were regressed from the functional data. Additional signal filtering was performed using a band-pass filter of 0.008 – 0.09 Hz, to reduce noise due to physiological effects, such as respiration and pulsation, and noise due to scanner drift.
Functional Connectivity Analysis
In order to evaluate associations between functional connectivity and neck disability, ROI-to-ROI (also termed seed-to-seed) functional connectivity was performed within the supratentorial brain. ROI-to-ROI analyses implemented general linear models (GLMs) of the functional connectivity with respect to NDI, with age included as a covariate, as well as mJOA under certain conditions. Seed and target ROIs were selected from Harvard-Oxford atlas based on previous studies that have observed cortical morphological changes as a result of neck disability and/or pain [8, 25] as well as common ROIs in functional networks often implicated in the chronic pain [9–17], including the insular cortex (IC), the superior frontal gyrus (SFG), the middle frontal gyrus (MFG), the pre- and postcentral gyri, the superior parietal lobule (SPL), the supplementary motor area (SMA), the anterior and posterior cingulate (AC and PC, respectively), the precuneus, the cuneus, the thalamus, and the putamen. It is important to note that although brainstem[26] and cerebellar regions[27] have been implicated in chronic pain, including the periaqueductal gray (PAG)[28], these regions were not included in the current study as we only focused on supratentorial brain regions. Associations were evaluated between the functional connectivity and NDI for all 36 CS patients and 17 healthy volunteers. Additionally, using the series of GLMs outlined above, we determined the accuracy of prediction of NDI using age and connectivity measurements most commonly identified, with and without addition of mJOA. Significance was set at P<0.05 (two-sided) for the individual connections with a false discovery rate (FDR)<0.05 based on the number of target regions.
RESULTS
The mean NDI score of the patient cohort was 11.1 with a range between 0 and 37. Based on the scoring system developed by Vernon and Mior [18, 19], there were 9 patients with no reported disability (0–4 points), 20 patients with mild disability (5–14 points), 3 patients with moderate disability (15–24 points), 2 patients with severe disability (25–34 points), and 2 patients with complete reported disability (35–50 points). Average mJOA score was 15.9 with a range of 10–18.
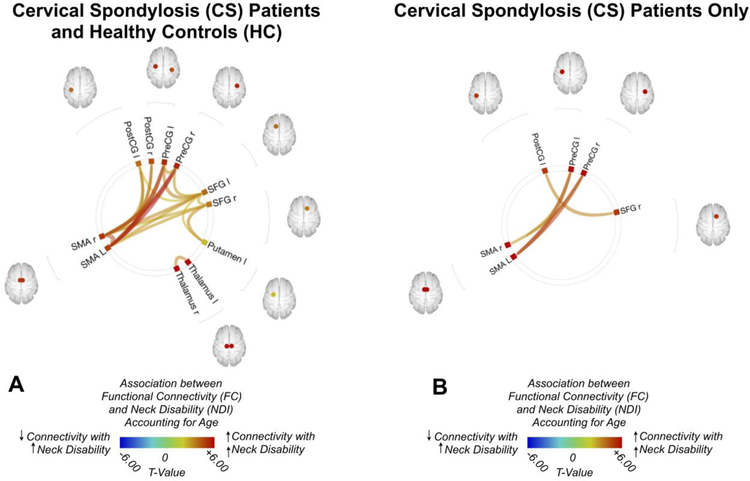
When computing the association between NDI and FC for ROI-to-ROI analyses by controlling the effect of age differences, NDI generally demonstrated a strong positive association with FC (Fig. 1A), or an increase in FC with increase in neck disability. Both the postcentral gyri and precentral gyri, bilaterally, exhibited a strong positive connectivity with the SMA with increasing neck disability, while the bilateral precentral gyri and the left postcentral gyrus exhibited a strong positive association with the left superior frontal gyri with increasing neck disability, and the bilateral SMA and the left putamen displayed a strong connectivity with the superior frontal gyrus with increasing neck disability. When measuring the ROI-to-ROI functional connectivity within the CS cohort exclusively (Fig. 1B), similar trends emerged, where the precentral gyri demonstrated an increasing connectivity with the SMA with increasing neck disability, and the right superior frontal gyri showed increasing connectivity with the left precentral gyri with increasing disability score.
Fig. 1:
(A) ROI-to-ROI functional connectivity (FC) association with Neck Disability Index (NDI) for both patients with cervical spondylosis (CS) and healthy control (HC) volunteers after controlling for age (B) ROI-to-ROI FC association with NDI for patients with CS only, after by controlling for age. Colors denote value of the T-statistic, yellow-red represents positive association (increasing FC with increased neck disability), light blue-blue denotes negative association (increasing FC with decreasing neck disability). Position of ROIs displayed on mid-axial slices. PreCG = Precentral Gyrus; PostCG = Postcentral Gyrus; SFG = Superior Frontal Gyrus; SMA = Supplementary Motor Area; r = Right Hemisphere; l = Left Hemisphere.
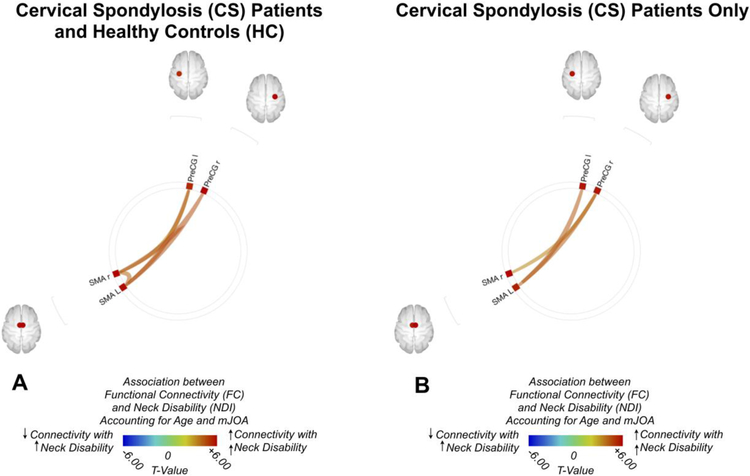
To further evaluate the association between FC and the neck disability independent of neurological impairment as measured by mJOA, ROI-to-ROI analyses were also performed by accounting for both age and mJOA (Fig. 2). When both CS and HC subjects were combined (Fig. 2A), results showed a positive association between functional connectivity and NDI between the right precentral gyrus and the SMA, as well as between the left precentral gyrus and the left SMA along with the right precentral gyrus and the right SMA. Examination of CS patients only showed a similar positive correlation between connectivity and NDI between the right precentral gyrus and the SMA, as well as between the left precentral gyrus and the left SMA, but no association between connectivity between the right precentral gyrus and the right SMA and NDI (Fig. 2B).
Fig. 2:
(A) ROI-to-ROI functional connectivity (FC) association with Neck Disability Index (NDI) for both patients with cervical spondylosis (CS) and healthy control (HC) volunteers after controlling for both age modified Japanese Orthopedic Association (mJOA) score. (B) ROI-to-ROI FC association with NDI for CS patients only, after controlling for both age and mJOA. Colors denote value of the T-statistic, yellow-red represents positive association (increasing FC with increased neck disability), light blue-blue denotes negative association (increasing FC with decreasing neck disability). Position of ROIs displayed on mid-axial slices. PreCG = Precentral Gyrus; PostCG = Postcentral Gyrus; SFG = Superior Frontal Gyrus; SMA = Supplementary Motor Area; r = Right Hemisphere; l = Left Hemisphere.
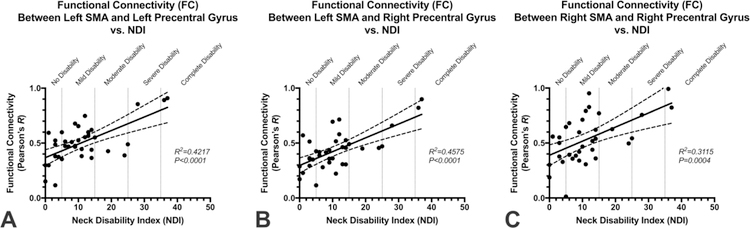
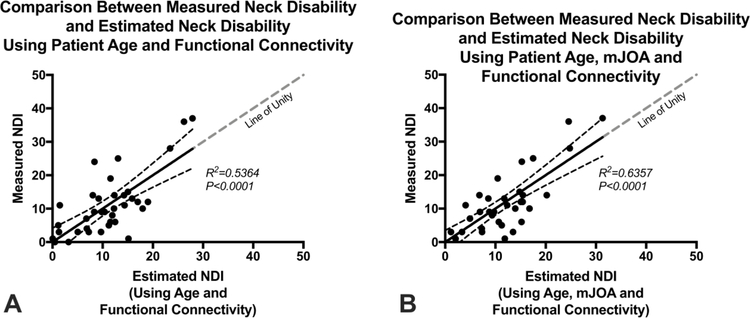
Fig. 3 shows the association between connectivity measures and NDI for patients with CS in three distinct connections that were present in the majority of connectivity analyses performed: left SMA-to-left precentral gyrus (Fig. 3A; SMA L-to-PreCG L); left SMA-to-right precentral gyrus (Fig. 3B; SMA L-to-PreCG R); and right SMA-to-right precentral gyrus (Fig. 3C; SMA R-to-PreCG R). Using the connectivity measures in these three regions combined with patient age, we constructed two GLMs to predict NDI, one with and one without the use of mJOA. In the first model (Fig. 4A), age and functional connectivity measures from the left SMA-to-left precentral gyrus, left SMA-to-right precentral gyrus, and right SMA-to-right precentral gyrus were used to predict NDI and showed a high correlation with measured values (R2=0.5364, P<0.0001; Slope = 1.00 ± 0.16 (95% C.I.); Intercept = 2.92×10–10± 2.07). Results of this model showed a sensitivity of 57%, specificity of 83%, positive predictive value of 44%, negative predictive value of 89%, and accuracy of 78% for identifying patients with moderate disability (NDI > 14). The second mode (Fig. 4B), which included mJOA, age, and functional connectivity measures from the left SMA-to-left precentral gyrus, left SMA-to-right precentral gyrus, and right SMA-to-right precentral gyrus showed a higher correlation with measured values (R2=0.6357, P<0.0001; Slope = 1.00 ± 0.13 (95% C.I.); Intercept = −8.96×10−10± 1.72). This resulted in a slightly better performance compared with the model excluding mJOA, showing a sensitivity of 71%, specificity of 79%, positive predictive value of 45%, negative predictive value of 92%, and accuracy of 78% for identifying patients with moderate disability (NDI > 14).
Fig. 3:
Correlation observed between measured Neck Disability Index (NDI) and functional connectivity (FC) between (A) the left supplementary motor area (SMA) and left precentral gyrus (PreCG) (R2=0.4217, P<0.0001) ; (B) left SMA and right PreCG (R2=0.4575, P<0.0001); and (C) right SMA and right PreCG (R2=0.3115, P=0.0004).
Fig. 4:
Association between measured Neck Disability Index (NDI) and estimated NDI using (A) a general linear model (GLM) consisting of patient age and functional connectivity between the left supplementary motor area (SMA) and left precentral gyrus (PreCG), left SMA-to-right PreCG, and right SMA-to-right PreCG (R2=0.5364, P<0.0001); and (B) a second GLM consisting of patient age, neurological status measured with mJOA, and functional connectivity between left SMA-to-left PreCG, left SMA-to-right PreCG, and right SMA-to-right PreCG (R2=0.6357, P<0.0001).
DISCUSSION
Advanced cervical spondylosis can cause both neurological decline and severe pain, leading to significant disability and impairment of activities of daily living. Due to the potentially devastating neurological deterioration that can occur in CS, the vast majority of CS research has been directed towards treatment of the progressive neurological dysfunction associated with this condition. In an effort to better understand the role of the entire central nervous system in the pathogenesis of CS, there has been increasing interest in the cerebral changes that occur during disease progression. Using task-based fMRI, we have found that cerebral motor functional changes occur in CS patients, likely as a compensatory mechanism in response to chronic spinal cord injury, designed to preserve neurological function [29, 30]. One of the most common findings has been a wide recruitment of adjacent cortical regions prior to surgery followed by reorganization of the cortical representation map after surgery towards that of normal patterns, which paralleled neurological recovery. This central plasticity has also been described for pain syndromes such as phantom pain from an amputated limb, where the deafferentation results in shifting of the cortical representation areas into adjacent regions [31, 32] and reverses with clinical improvement [33].
To our best knowledge, this is the first report that demonstrates measurable cerebral functional changes in CS patients in proportion to the severity of neck disability as measured by NDI. In the present study, worsening neck disability (increasing NDI) was associated with increased FC, particularly to the pre- and post-central gyri, the superior frontal gyrus, and the supplementary motor area. While there is variation in the characteristics of functional connectivity findings in chronic pain conditions, the motor cortex is an area that repeatedly appears as altered in chronic pain [34]. This is particularly relevant in the study of CS patients, as motor dysfunction, specifically hand coordination and gait, are the cardinal features of this disorder. In a study of patients with chronic pain from rheumatoid arthritis, for example, there was largely increased functional connectivity across sensorimotor-related regions, such as the primary sensorimotor cortex, the supplementary motor area, and cingulate cortex [35]. This appears similar to structural [25, 36, 37] and functional [38] alterations observed in the sensorimotor network in ankylosing spondylitis, a painful form of arthritis that often affects the spine. Similar functional connectivity alterations in the sensorimotor network have also been observed chronic visceral pain [39, 40] and other chronic pain conditions [41].
Electromyographic recordings of the lower back in chronic back pain patients during transcranial magnetic stimulation (TMS) of the brain have demonstrated reorganization of the motor cortex associated with severity of lower back pain [42]. Patients with chronic pelvic pain have demonstrated altered resting state FC of motor regions related to pelvic floor muscle control [5, 6]. Thus, a growing area of research is the targeting of motor regions for pain relief. While invasive motor cortex stimulation has been used for cases of intractable pain, research has also been conducted with repetitive transcranial magnetic stimulation (TMS) of the motor cortex over a wide variety of chronic pain conditions [43], and has yielded effects in conditions ranging from fibromyalgia [44], to neuropathic pain [45–47], and chronic back pain [7]. This may be an important factor in patients with chronic neck pain, especially in patients with superimposed myelopathy, as this implicates motor cortex involvement in both neurological symptoms and chronic pain.
Although results from the current study appear consistent with other chronic pain syndromes, these preliminary findings need to be validated in a larger series of patients. Future studies that also include longitudinal assessment of CS patients over time to predict changes in neck disability or prediction of response to intervention are warranted. Lastly, the fact that the motor cortex has been demonstrated to be involved in the neurobiology of pain in this study and others is likely not coincidental. This is of significant interest due to the alterations in the sensorimotor network throughout the entire central nervous system encountered in CS, including spinal cord neuronal and axonal loss [48, 49], and both micro and macro structural injury to the subcortical white matter tracts and cerebral cortex [8, 50]. Since correlation analysis is limited in terms of causal inferences, as well as determining whether changes are due directly by the disease or brain compensation for the disease, further investigation is necessary to understand these complex interactions in patients with CS.
HIGHLIGHTS.
An increase in functional connectivity was associated with increasing in neck disability in regions associated with sensorimotor system including both postcentral gyri and precentral gyri, bilaterally, with the SMA; bilateral precentral gyri and the left postcentral gyrus, with the left superior frontal gyrus; bilateral SMA and the left putamen, with the superior frontal gyri.
Strong connectivity was associated with worsening neck disability within precentral gyri and the SMA when accounting for neurological function (i.e. mJOA).
Results are consistent with studies in chronic pain conditions, suggesting worsening neck disability is associated with altered functional connectivity in cervical spondylosis patients.
Acknowledgments
Sources of Support: This work was supported by the National Institutes of Health (1R21NS065419–01A1; 2R01NS078494–06) and the Department of Radiological Sciences at UCLA.
Footnotes
Declarations of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Murray CJ, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, et al. The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA 2013;310:591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Irvine DH, Foster JB, Newell DJ, Klukvin BN. Prevalence of Cervical Spondylosis in a General Practice. Lancet 1965;1:1089–92. [DOI] [PubMed] [Google Scholar]
- [3].Stevens JA, Olson S. Reducing falls and resulting hip fractures among older women. MMWR Recomm Rep 2000;49:3–12. [PubMed] [Google Scholar]
- [4].Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 2012;15:1117–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kilpatrick LA, Kutch JJ, Tillisch K, Naliboff BD, Labus JS, Jiang Z, et al. Alterations in Resting State Oscillations and Connectivity in Sensory and Motor Networks in Women with Interstitial Cystitis/Painful Bladder Syndrome. J Urology 2014;192:947–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kutch JJ, Yani MS, Asavasopon S, Kirages DJ, Rana M, Cosand L, et al. Altered resting state neuromotor connectivity in men with chronic prostatitis/chronic pelvic pain syndrome: A MAPP Research Network Neuroimaging Study. Neuroimage-Clin 2015;8:493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Johnson S, Summers J, Pridmore S. Changes to somatosensory detection and pain thresholds following high frequency repetitive TMS of the motor cortex in individuals suffering from chronic pain. Pain 2006;123:187–92. [DOI] [PubMed] [Google Scholar]
- [8].Woodworth DC, Holly LT, Mayer EA, Salamon N, Ellingson BM. Alterations in Cortical Thickness and Subcortical Volume are Associated With Neurological Symptoms and Neck Pain in Patients With Cervical Spondylosis. Neurosurgery 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 2005;9:463–84. [DOI] [PubMed] [Google Scholar]
- [10].Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci 2008;28:1398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cauda F, Sacco K, Duca S, Cocito D, D’Agata F, Geminiani GC, et al. Altered resting state in diabetic neuropathic pain. PLoS One 2009;4:e4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum 2010;62:2545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Burgmer M, Gaubitz M, Konrad C, Wrenger M, Hilgart S, Heuft G, et al. Decreased gray matter volumes in the cingulo-frontal cortex and the amygdala in patients with fibromyalgia. Psychosom Med 2009;71:566–73. [DOI] [PubMed] [Google Scholar]
- [14].Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum 2002;46:1333–43. [DOI] [PubMed] [Google Scholar]
- [15].Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci 2007;27:10000–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Staud R, Craggs JG, Perlstein WM, Robinson ME, Price DD. Brain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and healthy controls. Eur J Pain 2008;12:1078–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cifre I, Sitges C, Fraiman D, Munoz MA, Balenzuela P, Gonzalez-Roldan A, et al. Disrupted functional connectivity of the pain network in fibromyalgia. Psychosom Med 2012;74:55–62. [DOI] [PubMed] [Google Scholar]
- [18].Vernon H The Neck Disability Index: state-of-the-art, 1991–2008. J Manipulative Physiol Ther 2008;31:491–502. [DOI] [PubMed] [Google Scholar]
- [19].Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther 1991;14:409–15. [PubMed] [Google Scholar]
- [20].Yonenobu K, Abumi K, Nagata K, Taketomi E, Ueyama K. Interobserver and intraobserver reliability of the japanese orthopaedic association scoring system for evaluation of cervical compression myelopathy. Spine (Phila Pa 1976) 2001;26:1890–4; discussion 5. [DOI] [PubMed] [Google Scholar]
- [21].Woodworth DC, Holly LT, Salamon N, Ellingson BM. Resting-State Functional Magnetic Resonance Imaging Connectivity of the Brain Is Associated with Altered Sensorimotor Function in Patients with Cervical Spondylosis. World Neurosurg 2018;119:e740–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2012;2:125–41. [DOI] [PubMed] [Google Scholar]
- [23].Wu CW, Chen CL, Liu PY, Chao YP, Biswal BB, Lin CP. Empirical evaluations of slice-timing, smoothing, and normalization effects in seed-based, resting-state functional magnetic resonance imaging analyses. Brain Connect 2011;1:401–10. [DOI] [PubMed] [Google Scholar]
- [24].Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL. Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Comput Assist Interv 2006;9:58–66. [DOI] [PubMed] [Google Scholar]
- [25].Wu Q, Inman RD, Davis KD. Neuropathic pain in ankylosing spondylitis: a psychophysics and brain imaging study. Arthritis Rheum 2013;65:1494–503. [DOI] [PubMed] [Google Scholar]
- [26].Mills EP, Di Pietro F, Alshelh Z, Peck CC, Murray GM, Vickers ER, et al. Brainstem Pain-Control Circuitry Connectivity in Chronic Neuropathic Pain. J Neurosci 2018;38:465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Moulton EA, Schmahmann JD, Becerra L, Borsook D. The cerebellum and pain: passive integrator or active participator? Brain Res Rev 2010;65:14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hemington KS, Coulombe MA. The periaqueductal gray and descending pain modulation: why should we study them and what role do they play in chronic pain? J Neurophysiol 2015;114:2080–3. [DOI] [PubMed] [Google Scholar]
- [29].Holly LT, Dong Y, Albistegui-DuBois R, Marehbian J, Dobkin B. Cortical reorganization in patients with cervical spondylotic myelopathy. J Neurosurg Spine 2007;6:544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dong Y, Holly LT, Albistegui-Dubois R, Yan X, Marehbian J, Newton JM, et al. Compensatory cerebral adaptations before and evolving changes after surgical decompression in cervical spondylotic myelopathy. J Neurosurg Spine 2008;9:538–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Flor H, Nikolajsen L, Jensen T Staehelin. Phantom limb pain: a case of maladaptive CNS plasticity? Nat Rev Neurosci 2006;7:873–81. [DOI] [PubMed] [Google Scholar]
- [32].Pons TP, Garraghty PE, Ommaya AK, Kaas JH, Taub E, Mishkin M. Massive cortical reorganization after sensory deafferentation in adult macaques. Science 1991;252:1857–60. [DOI] [PubMed] [Google Scholar]
- [33].Flor H, Denke C, Schaefer M, Grusser S. Effect of sensory discrimination training on cortical reorganisation and phantom limb pain. Lancet 2001;357:1763–4. [DOI] [PubMed] [Google Scholar]
- [34].Martucci KT, Ng P, Mackey S. Neuroimaging chronic pain: what have we learned and where are we going? Future Neurol 2014;9:615–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Flodin P, Martinsen S, Altawil R, Waldheim E, Lampa J, Kosek E, et al. Intrinsic Brain Connectivity in Chronic Pain: A Resting-State fMRI Study in Patients with Rheumatoid Arthritis. Front Hum Neurosci 2016;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wu Q, Inman RD, Davis KD. Fatigue in ankylosing spondylitis is associated with the brain networks of sensory salience and attention. Arthritis Rheumatol 2014;66:295–303. [DOI] [PubMed] [Google Scholar]
- [37].Wu Q, Inman RD, Davis KD. Tumor necrosis factor inhibitor therapy in ankylosing spondylitis: differential effects on pain and fatigue and brain correlates. Pain 2015;156:297–304. [DOI] [PubMed] [Google Scholar]
- [38].Hemington KS, Wu Q, Kucyi A, Inman RD, Davis KD. Abnormal cross-network functional connectivity in chronic pain and its association with clinical symptoms. Brain Struct Funct 2016;221:4203–19. [DOI] [PubMed] [Google Scholar]
- [39].Hong JY, Kilpatrick LA, Labus J, Gupta A, Jiang Z, Ashe-McNalley C, et al. Patients with chronic visceral pain show sex-related alterations in intrinsic oscillations of the resting brain. J Neurosci 2013;33:11994–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hong JY, Labus JS, Jiang Z, Ashe-Mcnalley C, Dinov I, Gupta A, et al. Regional neuroplastic brain changes in patients with chronic inflammatory and non-inflammatory visceral pain. PLoS One 2014;9:e84564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain 2011;152:S49–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Schabrun SM, Elgueta-Cancino EL, Hodges PW. Smudging of the Motor Cortex Is Related to the Severity of Low Back Pain. Spine 2017;42:1172–8. [DOI] [PubMed] [Google Scholar]
- [43].Antal A, Terney D, Kuhnl S, Paulus W. Anodal Transcranial Direct Current Stimulation of the Motor Cortex Ameliorates Chronic Pain and Reduces Short Intracortical Inhibition. J Pain Symptom Manag 2010;39:890–903. [DOI] [PubMed] [Google Scholar]
- [44].Passard A, Attal N, Benadhira R, Brasseur L, Saba G, Sichere P, et al. Effects of unilateral repetitive transcranial magnetic stimulation of the motor cortex on chronic widespread pain in fibromyalgia. Brain 2007;130:2661–70. [DOI] [PubMed] [Google Scholar]
- [45].Leo RJ, Latif T. Repetitive transcranial magnetic stimulation (rTMS) in experimentally induced and chronic neuropathic pain: A review. J Pain 2007;8:453–9. [DOI] [PubMed] [Google Scholar]
- [46].Lefaucheur JP, Drouot X, Menard-Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS restores defective intracortical inhibition in chronic neuropathic pain. Neurology 2006;67:1568–74. [DOI] [PubMed] [Google Scholar]
- [47].Lefaucheur JP, Drouot X, Keravel Y, Nguyen JP. Pain relief induced by repetitive transcranial magnetic stimulation of precentral cortex. Neuroreport 2001;12:2963–5. [DOI] [PubMed] [Google Scholar]
- [48].Holly LT, Freitas B, McArthur DL, Salamon N. Proton magnetic resonance spectroscopy to evaluate spinal cord axonal injury in cervical spondylotic myelopathy. J Neurosurg Spine 2009;10:194–200. [DOI] [PubMed] [Google Scholar]
- [49].Ellingson BM, Salamon N, Grinstead JW, Holly LT. Diffusion tensor imaging predicts functional impairment in mild-to-moderate cervical spondylotic myelopathy. Spine J 2014;14:2589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Aleksanderek I, Stevens TK, Goncalves S, Bartha R, Duggal N. Metabolite and functional profile of patients with cervical spondylotic myelopathy. J Neurosurg Spine 2017;26:547–53. [DOI] [PubMed] [Google Scholar]