Abstract
Intracranial aneurysms (IA) are local dilatations in cerebral arteries that predominantly affect the circle of Willis. Occurring in approximately 2-5% of adults, these weakened areas are susceptible to rupture, leading to subarachnoid hemorrhage (SAH), a type of hemorrhagic stroke. Due to its early age of onset and poor prognosis, SAH accounts for >25% of years lost for all stroke victims under the age of 65. In this review, we describe the cerebrovascular pathology associated with intracranial aneurysms. To understand IA genetics, we summarize syndromes with elevated incidence, genome-wide association studies (GWAS), whole exome studies on IA-affected families, and recent research that established definitive roles for Thsd1 (Thrombospondin Type 1 Domain Containing Protein 1) and Sox17 (SRY-box 17) in IA using genetically engineered mouse models. Lastly, we discuss the underlying molecular mechanisms of IA, including defects in vascular endothelial and smooth muscle cells caused by dysfunction in mechanotransduction, Thsd1/FAK (Focal Adhesion Kinase) signaling, and the Transforming Growth Factor β (TGF-β) pathway. As illustrated by THSD1 research, cell adhesion may play a significant role in IA.
Keywords: Intracranial aneurysm, subarachnoid hemorrhage, etiology, genetics, animal models, THSD1
Introduction
An intracranial aneurysm (IA) is a dilatation in the wall of a cerebral artery that occurs predominantly in the circle of Willis. A ruptured IA is the most common cause of subarachnoid hemorrhage (SAH), a devastating condition that can lead to death or permanent disability.(2017) Due to early age of onset and high mortality, SAH accounts for >25% of years lost for all stroke victims under the age of 65 years.(Johnston et al. 1998) Despite treatment advances, the SAH mortality rate is ~40% and only half of survivors return to independent life. The IA prevalence in the adult population is approximately 2%-5% with an annual rupture rate of 8-10 per 100,000.(Hop et al. 1997; Tromp et al. 2014; Zacharia et al. 2010; Linn et al. 1996; Korja et al. 2013; Andreasen et al. 2013; Wiebers et al. 2004; Wiebers et al. 2003) Prior to rupture, IAs are usually asymptomatic and population-wide IA screening is unrealistic due to brain imaging costs. Currently, unruptured IAs are primarily discovered as an incidental finding or in affected families. When treated before rupture, survival rates improve dramatically. (1998)
IAs usually develop in adulthood. Structurally, there are two types of intracranial aneurysms: saccular and fusiform. Saccular (aka berry) aneurysms are sac-like pocket that arises from a cerebral wall, while the less common fusiform aneurysms are dilations that affect a short length of vessel where the entire vessel diameter is increased. Female sex, smoking, hypertension, and alcohol consumption are risk factors, but a positive family history confers the greatest risk.(Andreasen et al. 2013; Korja et al. 2013) It is known that 7%-20% of patients have a family history and first-degree relatives are at increased risk, regardless of ethnic background.(Norrgard et al. 1987; Ronkainen et al. 1993; Schievink et al. 1994; Schievink et al. 1995; Bromberg et al. 1995; Teasdale et al. 2005; Ronkainen et al. 1997; D. H. Kim et al. 2003) The Nordic Twin Study reports a SAH heritability estimate of 41% [95% CI, 23.7% to 55.5%], while the odds ratio for SAH is 51.0 [95% CI, 8.56-1117] when ≥2 affected first-degree relatives have SAH.(Korja et al. 2010; Bor et al. 2008) In a recent study of 21 twin pairs (Mackey et al. 2015), 11 of 12 monozygotic twins developed IA, irrespective of smoking or hypertension, while only 5 of 9 dizygotic twins were both affected. Increased IA incidence is a prominent phenotype in Autosomal Dominant Polycystic Kidney Disease (ADPKD); however, the vast majority of IA occurs in non-syndromic families or sporadic cases. In this review, we present our current understanding of intracranial aneurysms, highlighting pathological features, several syndromes with elevated IA incidence, and genetic studies that elucidate molecular mechanisms that contribute to disease. We will also describe the discovery and functional significance of THSD1 (Thrombospondin type I Domain containing 1), a gene whose deleterious rare variants cause a subset of human IA/SAH cases.
Methods
The medical literature on intracranial aneurysms was reviewed up to March 25, 2019 using PubMed searches using combinations of the following terms: intracranial, cerebral, brain, aneurysm, syndrome, genetics, exome sequencing, pathology, physiology, biology, histology, vascular, endothelial, smooth muscle, inflammatory, cell signaling, molecular mechanism, animal model, mouse, and zebrafish. For whole exome sequencing results of IA families, both the data in the paper and supplementary information is summarized in Table 3. In several subsections, we refer to review articles that provide additional information on a particular topic. Nonetheless, we added newly discovered facts and discussed their significances as well as referred the previous reviews, so as to maintain the overall integrity and continuity.
Table 3.
Candidate IA-genes implicated by whole exome sequencing studies.
| Study | Family | Total (Affected/Unaffected) | Sequenced (Affected/Unaffected) | Candidate Gene(s) |
|---|---|---|---|---|
| Santiago-Sim, et al.(Santiago-Sim et al. 2016) | IA001 | (9/20) | (9/13) | THSD1 (Nonsense variant in family, Missense mutations were found in 8/507 probands in our IA/SAH cohort and each variant affected protein function) |
| A | (6/2) | (5/1) | C1orf35, CALHM1, CCDC39, CTNND2, KLF11, PPIL4, ROBO3, SLK, TRMT1, WDR96, ZNF653 | |
| Farlow, et al.(Farlow et al. 2015) | B | (4/2) | (4/2) | ABCC3, DUSP16, MCM10, NME4, SNX21, TANC2, TSC2, TXNDC11, UNC93A, ZNF362 |
| Familial Intracranial Aneurysm (FIA) Study | C | (7/2) | (5/2) | C11orf65, SEC16B |
| D | (4/0) | (4/0) | ALMS1, ARHGEF17, DGKA, FOXM1, HAL, IFNA21, MAP7D1, NLRP1, TAS1R1, TMEM132B | |
| E | (4/0) | (4/0) | HTRA2, NDST1, SMEK2 | |
| F | (5/2) | (5/2) | AGMAT, CYP1A1, FOXRED1, LMBR1L, RFC4, SOX30, UNC13B | |
| G | (4/2) | (4/2) | ACSM3, ASPM, C1orf38, C10orf58, CCDC37, CHD9, COL17A1, DIAPH1, FAM71A, GSTCD, ITGB6, MLLT4, MTRF1L, OVGP1, PCDHGA11, PIK3C2B, PKP3, PPYR1, PTAFR, RSPH3, TBC1D7, TET2, TRPA1, ZNF264, ZNF835 | |
| Bourcier, et al.(Bourcier et al. 2018) | A | (5/26) | (2/0) | ANGPTL6, GSTA5, UNC5B, BATF2, DPF2, SART1, KEAP1, C19orf57 |
| B | (2/2) | (2/2)# | ANGPTL6 (p.Glu131Val) | |
| C | (2/8) | (2/8)# | ANGPTL6 (p.Glu131Val) | |
| D | (2/1) | (1/1)# | ANGPTL6 (p.Leu348Phe) | |
| E | (3/1) | (2/1)# | ANGPTL6 (p.Ala153ValfsTer66) | |
| F | (2/7) | (2/7)# | ANGPTL6 (p.Ala153ValfsTer66) | |
| Wu, et al.(Y. Wu et al. 2017) | A | (5/4) | (3/1) | MASP2, DHRS3*, OR2G3*, OR2T11, VWA3B, PHF3, LOXL2*, TBC1D31, CAAP1, TTC16, SH3GLB2, LSM14A, MIA, PPP1R37, RIPK4, FGL1*, RRP12, KLC3* |
| 10 | (6/10) | (5/0) | GPATCH8, RNF213, OR11H1, ZNF335 | |
| 60 | (5/9) | (5/0) | ABCA10, HELZ2, RNF213, PLEC, ZNF335 | |
| Zhou, et al.%(zhou et al. 2016) | 89 | (9/11) | (6/0) | AIM1, CDAN1, OR11H1, PLEC, RTTN |
| 94 | (5/8) | (4/0) | ABCA10, AIM1, RNF213, RTTN, SF3A2 | |
| 28 | (8/9) | (4/0) | CDAN1, GPATCH8, HELZ2, RNF213, SF3A2 | |
| 9 | (4/7) | (2/0) | Unknown | |
| P1 | (5/8) | (4/0) | CFTR, KCNH3, MLL2, PDE11A, TMEM146, ZNF222, ZNF233 | |
| P2 | (5/6) | (4/0) | C5orf42, CYC1, IL10RA, KNTC1, PNKD | |
| P3 | (5/1) | (3/0) | BPIFB3, BPIFB4, C10orf122, KCNV2, RDH16, RSU1, SGK223, TG | |
| Yan, et al.(Yan et al. 2015) | P4 | (5/3) | (5/0) | ABCA12, ADAMTS15, DEFB132, DNAH9, ZNF224 |
| P5 | (4/2) | (3/0) | AMPD1, JMJD1C | |
| P6 | (3/5) | (3/0) | CBLL1, DLG1, GLB1L2, LIMCH1, LRP5, MTA3, PLAU, SLC22A11, XAB2 | |
| P7 | (4/1) | (4/0) | C4orf45, CYP1B1, FILIP1L, FOXN1, KIAA1244, MYBBP1A, SGSM3 | |
| P8 | (3/1) | (3/0) | C2orf62, FCRL1, IQGAP3, KIF20A, SPAG17, STON1, THBD, USP4, VPS13B | |
| P9 | (3/1) | (3/0) | C17orf53, CD320, DSG1, MLL2, MYO7B, RNF10 | |
| P10 | (3/9) | (3/0) | IL11RA, ITGB6, NEB, ZNF292 | |
| P11 | (6/5) | (4/0) | GPR63, VIPR1, ZXDC | |
| P12 | (3/1) | (3/0) | ASTN2, CRAT, CRELD1, CYP4F11, FAM59A, GPR63, LRP4, MYCBPAP, PAFAH2, PEG3, PVRL1, TBX4, TTN | |
| P1 | (3/2) | (3/0) | NCF1, SRGAP2D, CLEC18B, CCDC88C, UNC79, ARHGEF17, PLEK, KIAA0922, MKI67, PPARGC1B | |
| P2 | (2/4) | (1/0) | NCF1, IL17F, ARHGEF17 | |
| P3 | (1/5) | (1/0) | SRGAP2D, IL17F, ZNF175, PLEK, KIAA0922, MLH1, ATXN7 | |
| P4 | (2/18) | (2/0) | ARHGEF17, GIPR, FAM47E | |
| P5 | (2/11) | (2/0) | AOX1, RANBP3, PLEK, KIAA0922, ADAM15, XRCC1, SIGLEC1, CSMD2, UBE3B, ISOC2, PLTP | |
| Yang, et al.(Yang et al. 2018) | P6 | (2/18) | (2/0) | AOX1, ARHGEF17, AKAP13, MLH1, ATXN7, FAM47E, SCAF11, PPARGC1B |
| P7 | (2/3) | (1/0) | NCF1, TMED3, PLEK, AKAP13, ACSM5, GIPR, PLTP | |
| P8 | (2/3) | (2/0) | NCF1, CLEC18B, ZNF175, MKI67, ACSM5, ISOC2 | |
| P9 | (2/20) | (1/0) | IL17F, TMED3, KIAA0922, ADAM15, TNRC6A | |
| P10 | (2/20) | (2/0) | SRGAP2D, CLEC18B, UNC79, TMED3, RANBP3, PLEK, XRCC1, SIGLEC1, TNRC6A, CSMD2, UBE3B | |
| P11 | (2/2) | (2/0) | SRGAP2D, CLEC18B, CCDC88C, TMED3, AKAP13, XRCC1, ACSM5, SCAF11 | |
| P12 | (2/4) | (1/0) | NCF1, SRGAP2D, AOX1 |
By targeted sequencing only
In the four families with RNF213 variants, the RNF213 did not segregate with disease in 7 of 18 IA cases.
LOXL2 contained the only genetic variant that segregated with disease among the five candidates tested as denoted by an asterisk.
Genes highlighted in red were the authors preferred candidate gene.
Results
1. Cerebrovascular Pathology of Intracranial Aneurysms
Pathological analyses on human IA samples provide critical insights into the molecular mechanism of IA formation and rupture. A common feature of IA is disintegration of the internal elastic lamina (IEL), a subendothelial connective tissue that separate intima from media. Other characteristics may include irregular luminal surface, myointimal hyperplasia, disorganization of the muscular media, hypocellularization, and infiltration of inflammatory cells (reviewed in (Santiago-Sim T 2011)).
1A. Normal intracranial artery in human
An intracranial artery consists of three layers: the intima, media, and adventitia. The intima is the innermost layer that faces the luminal side and has direct contact with blood flow. It is composed of a monolayer of endothelial cells and a subendothelial extracellular matrix. Glycoproteins, proteoglycans, and elastin can be deposited into the extracellular matrix that forms the internal elastic lamina (IEL) that separates intima from media. The media is mainly composed of smooth muscle cells with an extracellular matrix that contains predominantly type III collagen.(Canham et al. 1991) The adventitia is the outmost layer and consists of a complex network of type I collagen fibers, elastin, nerves, and fibroblasts.(Finlay et al. 1995) It is notable that the external elastic lamina (EEL) that separates media from adventitia in aortic arteries does not exist in intracranial arteries, potentially rendering intracranial arteries more vulnerable to hemodynamic stress.
IA occurs in high frequency at a bifurcation of the circle of Willis, a ring-like arterial structure located at the base of the brain which supplies blood to the brain and surrounding structures.(Williams and Brown 2013) It is well documented that these bifurcations are characterized by high wall shear stress due to the impingement of blood flow that may contribute to IA formation.(Sforza et al. 2009) In 1930, Forbus proposed that the smooth muscle layer is lacking at bifurcation apexes in the circle of Willis, resulting in inherent IA susceptibility.(Forbus 1930) Subsequently, the “media gap” was shown to be the physiological junction between two smooth muscle layers that primarily contains tendon-like collagen that provides strength and stability.(Finlay et al. 1998) Meng and colleagues later designed elegant rabbit experiments that showed that IA does not originate from the apex but from the proximal region, where both high wall shear stress and positive shear stress gradient applies.(Meng et al. 2007; Meng et al. 2014)
1B. Abnormal Intracranial Artery in IA Patients
Disintegration of internal elastic lamina (IEL)
Internal elastic lamina contains elastic fibers that provide flexibility to arteries. In a normal intracranial artery, IEL is well-preserved and homogeneous. In IA, IEL becomes torn, fragmented, or disappears, especially in the fundus of the aneurysm. The disruption of IEL is considered as a hallmark for IA pathology.(Krings et al. 2011)
Irregular luminal surface of intima
In IAs from human patients or induced in animal models, the luminal surface of intima shows large evaginations and deep, narrow invaginations as detected by transmission electron microscopy.(Draghia et al. 2008) In addition, small holes and enlarged gaps were detected at the junction of endothelial cells at the luminal surface which accordingly, attracts a thick layer of platelets and/or leukocytes. Overall, the luminal surface of intima becomes rugged in comparison to the well-preserved and smooth surface in the normal intracranial artery.(Scanarini et al. 1978)
Myointimal hyperplasia
In normal intracranial arteries, IEL separates intima from media. When IEL becomes disintegrated, smooth muscle cells in media can migrate into the intima layer and proliferate, causing myointimal hyperplasia. This phenomenon results in intimal thickening that is frequently observed in IA samples.(Fennell et al. 2016) It is unclear whether myotintimal hyperplasia contributes to IA formation or merely is a result of IEL degeneration.
Disorganization of the muscular media
The smooth muscle cells in the media are organized onto the reticular fiber that mainly consists of type III collagen. In IA, the medial layer is disorganized and smooth muscle cells often undergo a “phenotypic switch” from contractile to a synthetic type that is pro-inflammatory and pro-remodeling.(Chalouhi et al. 2012) Morphologically, their appearance changes from their original spindle-like shape into a spider-like one.(Song et al. 2018), resulting in media that is no longer arranged in tightly compacted bands. In comparison with unruptured IA, the media of ruptured IA is thin and often devoid of smooth muscle cells, probably due to increased apoptosis. A similar phenotype has also been observed in mouse models.(Aoki and Nishimura 2011; Morimoto et al. 2002)
Hypocellularization
Hypocellularization in IA walls occur commonly. Both apoptotic and necrotic cells have been observed in human IAs, especially in their necks and domes, while few have been described in control arteries. (Pentimalli et al. 2004) Data from experimentally induced IA animal model support that apoptosis of smooth muscle cells is associated with IA formation.(Kondo et al. 1998) Necrosis is in general considered an acute response to injury. Recently, necroptosis was identified as a new type of necrosis that could occur in a programmed manner similar to apoptosis. Given its link to aortic aneurysms via smooth muscle cell death(Q. Wang et al. 2015), necroptosis may also play a role in IA pathogenesis.
Infiltration of Inflammatory Cells
Inflammatory cells are frequently found in aneurysmal tissues in both animal models and humans where they are thought to contribute to disease. These cell populations include macrophages, neutrophils, T cells, and B cells. Of note, macrophages can secret matrix-degrading enzymes such as MMP2 and MMP9, and cytokines that further recruit other inflammatory cells (reviewed in (Chalouhi et al. 2012)). Macrophages are also thought to be important for aneurysmal rupture. Further descriptions of the roles of immune cells in IA is presented in section 4C.
2. Genetic Syndromes with Elevated Intracranial Aneurysm Incidence
In addition to environmental risk factors, genetics plays a significant role in disease, providing valuable clues on the molecular mechanisms of IA. The available data demonstrates that IAs can be caused by rare variants of major effect as illustrated by multiple genetic syndromes (Table 1) or common variants of minor effect as shown by GWAS. According to the American Heart Association (AHA) and American Stroke Association (ASA) guidelines(Thompson et al. 2015), IA screening is recommended for those affected by Autosomal Dominant Polycystic Kidney Disease (ADPKD), Type IV Ehlers-Danlos (vascular subtype), and Microcephalic Osteodysplastic Primordial Dwarfism (MOPD). The mechanisms by which the underlying syndromic genes as well as others contribute to IA will be discussed in Section 4. Computed tomography (CT) or magnetic resonance angiography (MRA) based imaging is strongly encouraged for IA detection.
Table 1.
Genetic Syndromes, underlying defective gene, and IA Incidence
| Syndrome | Major Gene(s) | IA incidence | References |
|---|---|---|---|
| Autosomal Dominant Polycystic Kidney Disease (ADPKD) | PKD1, PKD2 | 11% | (Cagnazzo et al. 2017) |
| Type IV Ehlers-Danlos Syndrome (Vascular Subtype) | Col3a1 | 17.5% | (S. T. Kim et al. 2016) |
| Microcephalic/Majewski’s Osteodysplastic Primordial Dwarfism, Type II (MOPD2) | PCNT | up to 50% | (Teo et al. 2016; Bober et al. 2010; Brancati et al. 2005; Li et al. 2015) |
| Loeys-Dietz Syndrome (LDS) | TGFBR1, TGFBR2, SMAD3, TGFB2 | 10-28% | (Loeys et al. 2006; Rodrigues et al. 2009; Vanakker et al. 2011; S. T. Kim et al. 2016) |
| Marfan Syndrome | FBN1 | 0-14% | (van den Berg et al. 1996; Conway et al. 1999; S. T. Kim et al. 2016) |
| Neurofibromatosis Type I | NF1 | 9% | (Conway et al. 2001; Schievink et al. 2005) |
2A. Autosomal Dominant Polycystic Kidney Disease (ADPKD)
Autosomal dominant polycystic kidney disease (ADPKD) has an IA prevalence of roughly 11% as determined by a systematic literature review.(Cagnazzo et al. 2017) Caused by loss-of-function in polycystin 1 (PKD1) or polycystin 2 (PKD2), ADPKD is primarily characterized by polycystic kidney, leading to renal dysfunction and eventual failure (reviewed in (Pirson 2010; Bergmann et al. 2018)). In addition, other organs, most notably the liver, are adversely affected. ADPKD patients frequently have high blood pressure that is a risk factor for IA as well as other diseases. This disorder has reduced average life expectancy to 53 and 69 years for European PKD1 and PKD2 patients, respectively.(Hateboer et al. 1999)
2B. Type IV Ehlers-Danlos Syndrome (Vascular Subtype)
Affecting 1 in 50,000-200,000, vascular EDS is an autosomal dominant connective tissue disorder characterized by extreme vascular fragility that often leads to hemorrhage and death (reviewed in (De Paepe and Malfait 2012; Malfait 2018)). This disease is caused by variants/mutations in Col3a1 that encodes an abundant, extracellular matrix protein that is required to support and strengthen connective tissue.(Tsipouras et al. 1986; Nicholls et al. 1988; Superti-Furga et al. 1988) In a retrospective study, 7 out of 40 (17.5%) individuals with vascular EDS had IA.(S. T. Kim et al. 2016) Annual, non-invasive imaging (e.g. CT or MRA) of the vascular tree is strongly recommended. Unfortunately, the discovery of IA in these patients has little clinical utility as surgical intervention is highly risky in these patients.
2C. Microcephalic/Majewski’s Osteodysplastic Primordial Dwarfism, Type II (MOPD2)
MOPD2 is a rare, autosomal recessive disorder characterized by short stature with other skeletal abnormalities including a disproportionately small head size (reviewed in (Rauch 2011)). Due to the extreme rarity of this disease, the precise incidence of IA in MOPD2 remains unknown but the current data suggest that it may occur in up to half.(Teo et al. 2016; Bober et al. 2010; Brancati et al. 2005; Li et al. 2015) This disease is caused by the inheritance of two defective copies of the Pericentrin 1 (PCNT) gene that encodes a centrosome-associated protein that is important for proper chromosomal segregation.(Rauch et al. 2008; Willems et al. 2010) In addition, PCNT loss-of-function in epithelial cells disrupts cilia formation and PDK2 localization to basal bodies.(Jurczyk et al. 2004) The significance of the PCNT-PDK2 interaction and potentially PCNT haploinsufficiency in IA is further suggested as PCNT rare missense variants were found in multiple IA families where several affected also had kidney cysts.(Lorenzo-Betancor et al. 2018) In these particular families, it should be noted that other rare variants beyond PCNT exist where another gene variant may be responsible for disease.
2D. Loeys-Dietz Syndrome (LDS)
Similar to Type IV Ehlers-Danlos, LDS is an autosomal dominant, connective tissue disease. Characterized by mutations in the Tgf-β pathway genes (predominantly TGFBR1, TGFBR2, SMAD3, and TGFB2), these patients have severe vascular phenotypes where arterial aneurysm dissection and bleeding are common that often manifest early in life. Roughly one-third of LDS deaths are caused by cerebrovascular bleeding with an overall IA incidence of 10-28%.(Loeys et al. 2006; Rodrigues et al. 2009; Vanakker et al. 2011; S. T. Kim et al. 2016) Given the widespread defects in the vasculature, biennial screening of the entire vascular tree is recommended, including brain CTs or MRAs.(MacCarrick et al. 2014)
2E. Other Disease with Putative IA Association: Marfan Syndrome and Neurofibromatosis Type I
Marfan syndrome (MFS) is an autosomal dominant connective disorder that leads to skeletal, ocular, and cardiovascular malformations due to mutations in the fibrillin-1 (FBN1) gene that leads to increased Tgf-β signaling.(Dietz et al. 1992; Hayward et al. 1992) The initial association of MFS with IA was largely based on numerous case reports.(Finney et al. 1976; Matsuda et al. 1979; Ohtsuki et al. 1984; Higashida et al. 1988; Stehbens et al. 1989; Hainsworth and Mendelow 1991; Schievink et al. 1997) Subsequent analysis of 129 Marfan patients with >3,400 observation years found no evidence for symptomatic IA in any patient,(van den Berg et al. 1996) while an autopsy study of 25 Marfan patients revealed only one case with a 2mm unruptured aneurysm.(Conway et al. 1999) In contrast, a recent study found that 14% (8/59) Marfan individuals were characterized by IA.(S. T. Kim et al. 2016) Further research will be required to reconcile these results.
Like Marfan, Neurofibromatosis Type 1 (NF1) is an autosomal dominant disease. Caused by pathogenic variants in the NF1 gene, this disease is characterized by cutaneous neurofibromas (typically benign), café-au-lait spots, iris Lisch nodules, and vasculopathy. The association of NF1 with IA remains controversial. Intracranial aneurysms have been reported in 9% (2/22) of NF1 cases as detected by MRI.(Schievink et al. 2005) An autopsy study of 25 NF1 individuals found none with an intracranial aneurysm nor were there any NF1-affected individuals among 925 intracranial aneurysm patients at Johns Hopkins Medical Institutions from 1990-2000.(Conway et al. 2001)
3. Genetic Studies on Non-Syndromic Intracranial Aneurysms
Genetic studies on non-syndromic IA can be broadly classified into four categories: linkage analyses, genome-wide association studies (GWAS), candidate gene approaches, and whole exome sequencing research.
3A. Linkage analysis
In IA, linkage analyses on aneurysm families or sib-pairs have mapped 15 IA susceptibility loci (reviewed in (Hitchcock and Gibson 2017; Zhou et al. 2018)).(Nahed et al. 2005; Ruigrok et al. 2008; Roos et al. 2004; Foroud et al. 2008; Foroud et al. 2009; Verlaan et al. 2006; Onda et al. 2001; Farnham et al. 2004; C. J. Kim et al. 2011; Ozturk et al. 2006; Yamada et al. 2004; Olson et al. 2002; van der Voet et al. 2004; Mineharu et al. 2007; Santiago-Sim et al. 2009a) These studies suggest that a subset of IAs may be caused by rare mutations/variants with major effect. The identified IA linkage peaks are often quite broad encompassing tens or hundreds of genes and the definitive causative gene(s) remain largely unidentified. The one exception is a linkage peak at 13q14-21 that we identified in a large family with 9 IA-affected and 13 unaffected members who provided DNA.(Santiago-Sim et al. 2009a) Subsequently, we discovered that this chromosomal region harbored a nonsense THSD1 variant that co-segregates exclusively with disease as determined by whole exome and targeted sequencing. Furthermore, we demonstrated Thsd1 heterozygous and null mice develop IA and SAH, validating a direct disease role (unpublished observations and (Santiago-Sim et al. 2016)).
3B. Genome-Wide Association Studies (GWAS)
To date, many groups have performed GWAS on those affected by IA and controls, identifying more than 20 IA-candidate loci (reviewed in (Hitchcock and Gibson 2017; Zhou et al. 2018)). An overarching conclusion is that there is no common genetic variant that has a large effect on IA. Of note, only two loci have been replicated by more than two studies: CDKN2A/CDKN2B/CDKN2B-AS on chromosome 9p21,3(Bilguvar et al. 2008; Yasuno et al. 2010; Deka et al. 2010; Foroud et al. 2012; Foroud et al. 2014; Low et al. 2012; Abrantes et al. 2015; Akiyama et al. 2010; Kurki et al. 2014) and SOX17 on 8q11.23(Bilguvar et al. 2008; Yasuno et al. 2010; Deka et al. 2010; Foroud et al. 2012). A recent meta-analysis of 116,000 IA cases reported an odds ratio of 1.29 (95% CI 1.21-1.38) for rs10757278 in CDKN2B-AS and 1.21 (95% CI 1.15-1.27) for rs9298506 near SOX17.(Alg et al. 2013) These loci have been implicated in diverse populations that include North American, Finnish, Dutch, Japanese, New Zealanders, and Australians. SOX17 was recently implicated as a bona fide IA-causing gene as endothelial-specific deletion of Sox17 in mice induces IA formation under hypertension stress.(Lee et al. 2015) Functional validation of CDKN2A/B in IA pathogenesis remains elusive and is complicated by the structural complexity of this locus. Specifically, CDKN2A undergoes alternative promoter utilization that yields transcripts encoding either p14ARF or p16/INK4A. The promoter for P14ARF in the divergent orientation directs expression of CDKN2B-AS that is also known as long non-coding RNA Anril. The CDKN2B gene lies within this IncRNA gene in the reverse orientation.
3C. Case-Control Studies for Candidate Gene Association
Based on linkage analysis, expression studies, or biological function, multiple case-control studies have interrogated whether specific polymorphisms are IA-associated (Table 2). The implicated genes include: ACE(Cun et al. 2017), ADAMTS2(Arning et al. 2016), ARHGEF(Zholdybayeva et al. 2018), COL1A2(Joo et al. 2009; Glasker et al. 2014; Gan et al. 2017), COL3A1(Zholdybayeva et al. 2018), CSPG2(Ruigrok et al. 2009; Zholdybayeva et al. 2018), ELN(Akagawa et al. 2006; Paterakis et al. 2017), ENG(Joo et al. 2008; X. Hu et al. 2015; Zholdybayeva et al. 2018), HSPG2(Ruigrok et al. 2009), IL6(Sun et al. 2008; G. Zhang et al. 2011; Zheng et al. 2013), JDP2(Krischek et al. 2010; Zholdybayeva et al. 2018), KLK5-10(Weinsheimer et al. 2007; Suo et al. 2014), let-7a-1/let-7f-1/let-7d(Sima et al. 2015), LIMK1(Akagawa et al. 2006; Low et al. 2011; Zholdybayeva et al. 2018), LOX(Hong et al. 2017), MLL2(Zholdybayeva et al. 2018), MMP2(Low et al. 2011; Alg et al. 2018), NOS3(Paschoal et al. 2018), PRDM9(Zholdybayeva et al. 2018), RNF213(Zhou et al. 2016), SERPINA3(Zholdybayeva et al. 2018), SOX17(Zholdybayeva et al. 2018), StAR(Zholdybayeva et al. 2018), TNF-α(Low et al. 2011; J. Hu et al. 2017), TSLC2A9(L. T. Zhang et al. 2015), UBR3(Zholdybayeva et al. 2018), VCAN(Ruigrok et al. 2006; Sathyan et al. 2014), and WWOX(Fan et al. 2016). Further research using mouse models will be required to determine which of the aforementioned genes contribute directly to IA.
Table 2.
Intracranial Aneurysm Candidate Gene Studies
3D. Whole Exome Sequencing IA Studies
With advances in high throughput sequencing that have significantly reduced costs, whole exome sequencing has recently been applied to identify candidate disease-causing genes in affected families. Using relative stringent filtering criteria, first cousins who share 12.5% of their genomic DNA have in common an average of 27 rare coding variants with a minor allele frequency (MAF) < 0.2%. This reality highlights the inherent limitations of many familial IA studies. Table 3 highlights published studies that have used whole exome sequencing (and often subsequent targeted sequencing) to identify candidate IA genes. As illustrated in Table 3, many candidate genes have been identified on a per family basis in these studies. Of note, most families selected for study appear to have an autosomal dominant pattern of inheritance and a single gene defect is most likely responsible. In addition, the number of candidate genes are less than what is predicted in several cases due to how variants were filtered and reported. To date, only a single family has yielded a definitive IA-gene. Specifically, our group identified multiple rare variants in thrombospondin type I domain containing 1 (THSD1) from IA patients by combining linkage analysis, whole exome sequencing, and targeted sequencing.(Santiago-Sim et al. 2009a; Santiago-Sim et al. 2016) The THSD1 nonsense variant R450X was identified in a large IA family where it co-segregated in 9 affected members and was absent in the 13 unaffected family members who provided DNA. Our loss-of-function studies in zebrafish and mice validated a direct role for Thsd1 in intracranial hemorrhage.
4. Biology of Intracranial Aneurysms
Intracranial arteries are mainly composed of two types of cells, endothelial cells forming intima and smooth muscle cells forming media. When IA occurs, infiltration of inflammatory cells such as macrophages are inevitable as well as neutrophils, T cells, and B cells. Each type of cells has different biological characteristics and contributes to IA via different molecular and cellular mechanisms.
4A. Contribution of Vascular Endothelial Cells
Vascular endothelial cells organize into a polarized monolayer that surrounds the vessel lumen. The apical side of endothelial cells is in direct contact with and senses blood flow through mechanosensory pathways. At the lateral side, three different cell-cell adhesions exist: adherens junctions, tight junctions, and gap junctions. The basal side of endothelial cells contains focal adhesion that mediates the interaction between the cell and extracellular matrix. Figure 1 depicts some of the genes and pathways in endothelial cells whose dysfunction contributes to IA.
Figure 1. Schematic of IA genes in vascular endothelial cells.
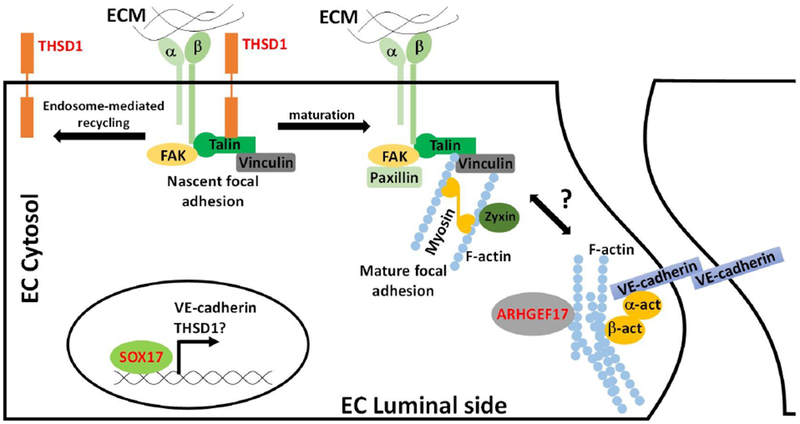
Three IA genes including THSD1, SOX17, and ARHGEF17 are highlighted in red. THSD1 physically interacts with integrin complex through talin at nascent focal adhesion. When nascent focal adhesion matures, THSD1 leaves for next nascent focal adhesion site via endosome-mediated recycling process. Loss of THSD1 leads to defects in focal adhesion, a key determinant of the actin cytoskeleton, and modulator of several downstream pathways. Sox17 functions as a transcriptional factor and modulates VE-cadherin expression. Loss of VE-cadherin decreases cell-cell adhesion and increases permeability. ARHGEF17 is guanidine exchange factor that potentially regulates the remodeling of actin cytoskeleton.
Shear Stress-Related Mechanosensory Defects in Endothelial Cells
The luminal endothelium experiences blood flow with associated shear stress. Shear stress plays a critical role in intracranial aneurysm formation and growth. High shear stress may induce IA formation while low shear stress can promote IA growth and rupture.(Diagbouga et al. 2018) Shear stress mechanosensors have been identified in disease (reviewed in (Deng et al. 2014; Givens and Tzima 2016)). These include different transmembrane proteins such as G-protein coupled receptors. Moreover, plasma membrane microdomains such as lipid raft and cilia are likely critical. Several IA-predisposing genetic syndromes, most notably ADPKD and MOPD2 that were discussed previously, implicate mechanotransduction in IA. For ADPKD, the two responsible genes (PKD1 and PKD2) serve as mechanosensors that transduce blood flow-induced signaling via cilia.(Nauli et al. 2008; AbouAlaiwi et al. 2009) In MOPD2 where between 25%-50% of cases have IA(Lorenzo-Betancor et al. 2018), autosomal recessive variants in PCNT are disease-causing and it is now known that PCNT loss inhibits cilia formation and leads to mislocalization of PKD2.(Jurczyk et al. 2004) When taken together, these results suggest mechanosensors including PKD1 and PKD2 can contribute to IA, likely by perturbing downstream signaling.
Cell-cell adhesion defects in endothelial cells
In some IA patients, red blood cells are detected in the intima or media and this dissection could be caused by defects in endothelial cell-cell adhesion and integrity involving adherens, tight, and gap junctions. In mouse models, loss of Sox17 in endothelial cells lead to IA formation and disruption of VE-cadherin, a major player in adherens junctions, supporting that cell-cell adhesion can contribute to IA formation. It is possible that Sox17 as a transcription factor regulates a plethora of downstream targets including VE-cadherin and its modulators.(Nakajima-Takagi et al. 2013) Interestingly, another IA-causing gene THSD1 regulates adherens junction assembly since VE-cadherin was reduced or mislocalized in endothelial cells that lack THSD1(Haasdijk et al. 2016). In the future, it will be interesting to see if THSD1 is also a downstream target of SOX17 and adds an extra layer of regulation on VE-cadherin activity in endothelial cells.
Role of TGF-β pathway in endothelial cells
TGF-β pathway plays a pleotropic role in different cell types within the cerebrovasculature and its disease significance is confirmed by Loeys-Dietz syndrome. Interestingly, loss of TGF-β pathway genes in mouse endothelial cells via either inactivation of Smad2/3(Itoh et al. 2012) or Smad4(Crist et al. 2018) cause vascular defects, highlighting a pivotal role in endothelial cells. Importantly, rare variants of endoglin and beta-glycan, both of which are type III receptors for TGF-β pathway, were found in IA patients, suggesting the potential role of TGF-β signaling transduction in IA pathogenesis.(Santiago-Sim et al. 2009b)
Focal adhesion is essential for cerebrovascular integrity in endothelial cells
In comparison with adherens junctions, focal adhesions are composed of superamolecular complexes that mediate the interaction between cell and extracellular matrix (reviewed in (Wozniak et al. 2004)). Integrin is a major protein involved in focal adhesion and transduces signaling in bidirectional manner. When extracellular matrix proteins such as fibronectin binds to its receptor integrin, an outside-in signaling can be illicit through integrin and eventually activate cytoskeleton remodeling (Figure 2). In addition, an inside-out signaling pathway exists where the intracellular adaptor protein talin binds to integrin, leading to a conformational change and its activation.(Harburger and Calderwood 2009) Focal adhesions are known to maintain cerebrovascular integrity.(Iida et al. 2018; Liu et al. 2012) For instance, inactivation of integrin αv and β1 leads to intracranial hemorrhage in zebrafish. Furthermore, depletion of talin, an integrin binding protein that is essential for focal adhesion, also causes intracranial hemorrhage in zebrafish.(Q. Wu et al. 2015) Focal adhesion kinase (FAK) is a master kinase to regulate the dynamics of integrin-mediated focal adhesion, and inactivation of FAK in mouse endothelium causes brain hemorrhage.(Shen et al. 2005)
Figure 2. THSD1/FAK signaling with its downstream targets.
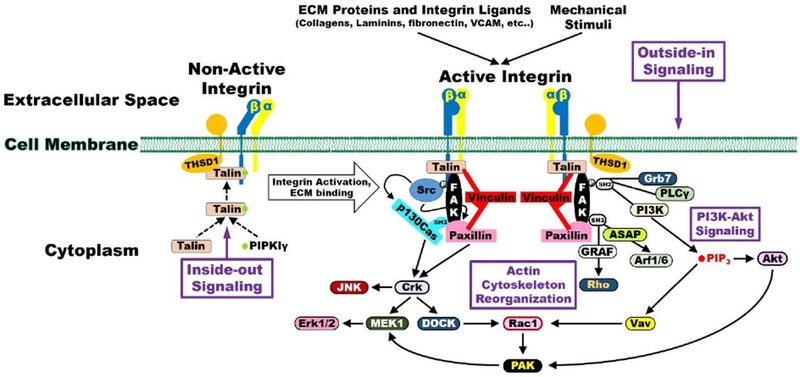
THSD1 is required for normal levels of focal adhesions and THSD1 loss reduces the overall amount of focal adhesions, FAK, and active phosphorylated FAK in endothelial cells. As a result, several pathways may be implicated including SRC, PI3K/AKT, Rho, and Rac1 signaling that affects actin cytoskeleton organization and cell adhesion mediated in part through integrins and mechanosensors.
THSD1 and Focal Adhesions
THSD1 is a single-span transmembrane protein that is functionally linked to FAK signaling and cell adhesion (Figure 2). In terms of protein partners, Thsd1 was initially identified as a putative talin-interactor based on a mass spectrometry of talin immunoprecipitation.(de Hoog et al. 2004) Subsequently, we validated this interaction using reciprocal IPs in endothelial cells.(Santiago-Sim et al. 2016) Protein-protein interaction databases do not provide further candidates for Thsd1 interactions in part due to biases in cell types used as THSD1 since it is predominantly present in endothelial cells. Since Talin is a key protein for integrin-mediated focal adhesion, we hypothesized that THSD1 might affect endothelial focal adhesion and FAK signaling. Functional studies in human umbilical vein endothelial cells suggest that THSD1 regulates focal adhesion stability. Knockdown of THSD1 by small interfering RNA reduces the focal adhesion number, concomitant with reduced ability in cell attachment onto collagen but not fibronectin. Interestingly, a rescue experiment demonstrated that most of THSD1 variants showed impaired ability in focal adhesion and cell attachment.
To characterize THSD1 further, Rui and co-workers utilized a novel precision tagging technique to epitope-tag THSD1 and tracked its subcellular localization.(Rui et al. 2017; Xu et al. 2017; Xu et al. 2018) Interestingly, THSD1 localizes at nascent focal adhesion that displays a dot-like appearance rather than mature focal adhesion that displays as streak-like patterns, suggesting that THSD1 may function at the early phase of focal adhesion assembly.(Rui et al. 2017) Multiple THSD1 missense variants identified in IA-patients encode proteins with reduced binding affinity for talin, which may contribute to their compromised function in focal adhesion stability. Surprisingly, THSD1 was also located at the endosome, in addition to nascent focal adhesions, suggesting that THSD1 may function at the interface between these two organelles and orchestrate the efficiency of focal adhesion assembly. On one hand, THSD1 facilitates nascent focal adhesion assembly by directly promoting integrin complex formation. On the other hand, THSD1 leaves mature focal adhesion for next nascent one via endosome-mediated protein trafficking. Recently, a novel mechanism was revealed that integrin can maintain an active conformation in endosomes via FAK/Talin/PIPK1gamma complex.(Nader et al. 2016) It will be interesting to know if THSD1 is also a part of such protein complex to modulate integrin activity in endosomes.
4B. Contribution of vascular smooth muscle cells
In IA, changes in the vascular endothelium often occur in concert with phenotypic changes in smooth muscle cells that provides structural support to vessel walls (reviewed in (Starke et al. 2014)). Pathology demonstrated that smooth muscle cells can switch from contractile to synthetic type, which is pro-inflammatory, pro-remodeling, and de-differentiated.(Fennell et al. 2016) This transition may lead to a morphological switch from spindle-like smooth muscle cells into spider-like cells that can migrate to and proliferate in the intima, causing myointimal hyperplasia. Moreover, smooth muscle cells with synthetic type can secret cytokines and metalloproteases that recruit inflammatory cells and degrades theextracellular matrix, respectively.(Chalouhi et al. 2012) This will further compromise the integrity of media layer of vessel wall. In comparison with contractile type, synthetic smooth muscle cells have reduced collagen biosynthesis and often undergo increased cell death that further weakens the aneurysm wall and predispose it to IA rupture.(Kondo et al. 1998; Pentimalli et al. 2004) It is worth noting that the critical role of smooth muscle cells in preserving vascular integrity was also highlighted by a clinical case where a child carrying a mutation in myosin heavy chain 11 (MYH11) developed rapidly progressing IAs at early age with one rupture.(Ravindra et al. 2016)
4C. Contribution of Inflammatory Cells
Macrophages
Macrophages have been noted in numerous human IA samples. Their function in IA is two-fold. Macrophages secrete metalloproteases such as MMP2 and MMP9 that can degrade extracellular matrix in vessel wall and render IA prone to rupture. Also, macrophages release a variety of cytokines that further recruit other inflammatory cells such as neutrophils, T cells and mast cells. The causal role of macrophages in IA formation and rupture has been well established.(Chalouhi et al. 2012; Signorelli et al. 2018) Kanematsu et al found that clodronate liposome-induced macrophage depletion in mice have a much lower risk of developing IAs.(Kanematsu et al. 2011) Monocyte Chemoattractant Protein-1 (MCP1) is one of the key chemokines that regulate infiltration and polarization of macrophages. MCP1-deficient mice exhibit a significant decrease in IA formation.(Aoki et al. 2009) Furthermore, the incidence of IA in mice lacking NF-κB, a master transcription factor regulating the inflammatory function of macrophage, was significantly reduced.(Aoki et al. 2007) Of note, this study utilized advanced imaging to detect macrophages in animal models and human patients, such as the application of an FDA-approved nanoparticle ferumoxytol that can be efficiently cleared off by macrophages while remaining intact in other non-phagocytic cells.(Aoki et al. 2017) Despite the established link between macrophages and IA, more mechanistic studies are needed especially on understanding how imbalance of the two subtypes of macrophage with opposing functions (M1 vs. M2 macrophage) contributes to IA disease.(Shao et al. 2017) It is worth mentioning that M2 macrophages can be subdivided into M2a, M2b, M2c, and M2d in response to different ligands stimulations, such as interleukins or TGF-beta.(Roszer 2015) Identification of other new phenotypes of macrophages such hemorrhage-associated macrophages called Mhem continue to expand the list. (Batra et al. 2018) It will be intriguing to see whether intracranial aneurysm formation or rupture can be preferentially associated with a specific subtype of macrophages, which may provide a novel therapeutic target.
Other Inflammatory Cells
Other inflammatory cells such as neutrophils, T cells, B cells, and mast cells are also detected in the aneurysm wall in human samples and animal models.(Chalouhi et al. 2012; Signorelli et al. 2018) Neutrophils and mast cells are regarded as functional players in IA pathogenesis.(Ishibashi et al. 2010; Chu et al. 2015; Meng et al. 2014) Nonetheless, the role of T cells in IA remains controversial. Sawyer and colleagues found that in Rag1 knockout mice, which lacks T cells, the incidence of IA formation and rupture was reduced.(Sawyer et al. 2016) Later, another research group induced IA, utilizing rats lacking T cells caused by a mutation in the Whn gene. (Miyata et al. 2017) Surprisingly, they found T cells are not required for IA formation and progression. It is unclear why discordant results were found between rodent species on the requirement for T cells in IA. Since T cells have different subpopulations that exert different, even opposite functions, further dissection of their specific contribution might help resolve this discrepancy.
5. Animal Models of Intracranial Aneurysms
To interrogate candidate disease genes, animal models provide an invaluable genetic tool. Although mice are the most attractive model organism for mammalian IA, their cost and the limited availability of appropriate genetically engineered mouse strains is often prohibitive when encountering a list of candidate IA genes that warrant further evaluation. To overcome this limitation, we recently used zebrafish to determine if candidate IA genes play critical roles in early cerebrovasculature development as defined by cerebral hemorrhages. Afterwards, we validated our results in a genetically engineered mouse strain.
5A. Zebrafish Models
Intracranial aneurysm and hemorrhage are tightly associated with compromised cerebrovascular integrity. Recently, zebrafish have gained additional popularity as a vertebrate model organism for studying the cerebrovasculature. Since zebrafish embryos are transparent, intracranial hemorrhage can be directly observed using a standard microscope. Furthermore, zebrafish fecundity and rapid development permits rapid phenotypic evaluation as intracranial hemorrhage in zebrafish fry are detectable as early as 2-3 days post fertilization. Importantly, gain-of-function and loss-of-function approaches are well established in zebrafish that include morpholino and mRNA injections and more recently, through applications of CRISPR/Cas9 technology. A drawback of zebrafish studies is that there are no established protocols for identifying IA due in part to their small size.
Loss of Thsd1 induces intracranial hemorrhage in zebrafish
Two research groups have independently observed intracranial hemorrhage in zebrafish deficient in Thsd1 caused by embryonic morpholino injection using two distinct, non-overlapping antisense oligonucleotides in a concentration-dependent manner.(Haasdijk et al. 2016; Santiago-Sim et al. 2016) Future zebrafish studies of genes involved in integrin and FAK signaling are clearly warranted given the significance of THSD1 in IA. Other pathways also contribute to cerebrovascular integrity in zebrafish. For example, intracranial hemorrhage was observed in zebrafish upon inactivation of VE-cadherin-mediated cell adhesion, Birc2-mediated cell death, fibrinogen-mediated thrombosis, ift81-related hedgehog signaling pathway (Montero-Balaguer et al. 2009; Santoro et al. 2007; Vo et al. 2013; Kallakuri et al. 2015) It remains unknown if Thsd1 is involved in these pathways or vice versa.
Loss of Arhgef17 induces intracranial hemorrhage in zebrafish
Yang et al identified rare variants in ARHGEF17 from intracranial aneurysm families. To validate its causal role, they tested two different splicing morpholinos against arhgef17 into zebrafish embryo and found a significant intracranial hemorrhage. Importantly, expression of human ARHGEF17 but not its rare variant forms can partially rescue the hemorrhage phenotype(Yang et al. 2018). Since ARHGEF17 encodes a protein functions as guanine nucleotide exchange factor that regulates cytoskeleton remodeling, it is possible that loss of function in ARHGEF17 may lead to cell adhesion defects during IA pathogenesis.
5B. Mouse IA Models
To dissect the molecular mechanisms of IA, several induced mouse models have been employed and more recently, genetically engineered mice have demonstrated that two genes, Sox17 and Thsd1, play a direct role in disease. For induced IA-models, systemic hypertension, local cerebrovascular hemodynamic stress, and/or extracellular matrix integrity are manipulated (reviewed in (Y. Wang et al. 2015)). Seminal work in the induced IA field has been performed by Nobuo Hashimoto and Tomoki Hashimoto to develop and test these models.(Morimoto et al. 2002; Nuki et al. 2009; Kanematsu et al. 2011; Tada et al. 2011) Systemic hypertension can be induced by surgery and/or medicine such as renal artery ligation, angiotensin II infusion, or a diet containing high salt and Nitic Oxide synthase inhibitor L-NAME. To alter the local cerebral blood flow, carotid artery ligation is often applied. To weaken cerebrovascular walls, mice are either injected with elastase, a matrix-degrading enzyme to disrupt internal elastic lamina, or fed a diet containing BPAN, an irreversible inhibitor of lysyl oxidase that can prevent cross-linking between elastin and collagen fibers. These experimental manipulations have been applied to mice singly and in different combinations to generate IA models with distinct kinetic profiles. Since multiple stresses are often utilized in these mouse models, there are concerns about how accurately they reflect natural disease progression and may bypass key signaling pathways important for IA. This shortcoming is especially relevant to understanding IA development and progression.
Loss of Sox17 in endothelial cells induces intracranial aneurysm and subarachnoid hemorrhage in an angiotensin II-induced mouse model
SOX17 was identified as a genetic risk factor by genome-wide association studies in multiple cohorts and populations. Lee and colleagues found that endothelial-specific deletion of SOX17 in mice causes IA formation and its incidence was dramatically increased by hypertension stress such as infusion of angiotensin II together with injection of elastase.(Lee et al. 2015) Subarachnoid hemorrhage was infrequently observed in mutant mice while hypertension stress significantly increases it.
Loss of Thsd1 causes intracranial aneurysm and subarachnoid hemorrhage
Previously, we identified a large IA family where a nonsense THSD1 variant co-segregated in all 9 affected individuals and was absent in 13 unaffected family members as determined by whole exome and targeted sequencing.(Santiago-Sim et al. 2016) Thsd1 missense variants were identified in 8 among 507 probands in our IA/SAH patient cohort. Each missense protein led to adhesion defects to collagen I endothelial cells in vitro, due in part to compromised ability to promote the FAK-talin interaction.(Santiago-Sim et al. 2016; Rui et al. 2017) In the mouse cerebrovasculature, Thsd1 is strictly expressed in endothelial cells and not smooth muscle cells. We further demonstrated that Thsd1-deficient mice (+/− and −/−) had cerebral hemorrhage localized to the subarachnoid space. More recently, we found that Thsd1-deficient mice by 12 months of age are characterized by high incidence of IA as determined by cerebrovascular casting using Microfil (unpublished observations). Further experiments are needed to examine whether IA development is accelerated in response to modifiable risk factors such as hypertension; however, Thsd1-deficient mice are a unique resource as the only genetically engineered mouse IA model that develop IA at high rates without induction by additional factors.
5C. Other mammalian models
Besides IA induction in mouse models, other animals such as rats, rabbits, dogs and pigs have also been used to assess pathological changes under IA-related stresses or evaluating the technical proficiency of endovascular devices, due to their larger vessels.(Bouzeghrane et al. 2010) To dissect molecular mechanisms of IA formation and rupture, mouse models have noteworthy benefits due to their genetic tools and costs.
Conclusion and Future Perspectives
Although mechanotransduction, Thsd1/FAK signaling, and extracellular matrix integrity are implicated in IA, several noteworthy opportunities and challenges remain to understand more fully IA genetics and pathobiology. The available data suggests that many high-risk genes remain to be discovered and that these genes may have prominent roles in distinct pathways. Advances in high-throughput sequencing will facilitate future studies on IA-affected families and large patient cohorts to identify putative disease genes. As discussed previously, whole exome sequencing studies often report numerous candidate rare variants per IA family with several affected individuals. After prioritization based on gene expression and the predicted impact of the variant, the ability to narrow the candidate list is difficult. A critical bottleneck exists as experimental evidence of causality using animal models is severely lacking. To illustrate this point, only Thsd1 and Sox17 are implicated directly in IA using mouse models. Specifically, IA and SAH occur in Thsd1 heterozygous and null mice with high incidence in comparison to wild-type controls (unpublished observations and (Santiago-Sim et al. 2016)). Endothelial specific deficiency of Sox17 increases IA incidence in an Angiotensin II infusion model.(Lee et al. 2015) To evaluate the functional significance of a candidate IA gene in vertebrates, we and others have employed zebrafish, an increasingly popular model organism for studying cerebrovascular integrity in an efficient and economical way. Genetically engineered mouse models also will continue to have a prominent role. With recent advances in the CRISPR/Cas9 system, the generation of site-specific mouse knockins of candidate IA variants is becoming increasingly affordable to test directly if specific candidate variants are responsible for disease.
Currently, mouse studies on IA formation and rupture is an end-point assay that requires sacrifice of the experimental animal. Therefore, new techniques that permit the non-invasive determination of IA growth over time would be advantageous. Micro-computerized tomography (micro-CT) might provide such an opportunity, since remodeling processes in mouse carotid arteries can be well documented by using it together with AuroVist, a non-toxic x-ray contrast agent.(Schurmann et al. 2015) Recently, a commercial company Nanoprobes utilizes the same approach to successfully track the aortic aneurysm growth over time in living mouse. The innovations in imaging techniques will certainly bring paradigm shifts for understanding molecular mechanisms of intracranial aneurysm. In conclusion, past and future research holds promise for identification of high-risk patients and subsequent prevention of IA formation or therapeutic intervention prior to rupture.
Acknowledgments
This work was supported by grant 1R01NS104280-01A1 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health. We thank Dr. Joanna O’Leary for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Compliance with Ethical Standards
There are no conflicts of interest to report.
REFERENCES
- AbouAlaiwi WA, Takahashi M, Mell BR, Jones TJ, Ratnam S, Kolb RJ, et al. (2009). Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ Res, 104(7), 860–869, doi: 10.1161/CIRCRESAHA.108.192765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrantes P, Santos MM, Sousa I, Xavier JM, Francisco V, Krug T, et al. (2015). Genetic Variants Underlying Risk of Intracranial Aneurysms: Insights from a GWAS in Portugal. [Research Support, Non-U.S. Gov’t]. PLoS One, 10(7), e0133422, doi: 10.1371/journal.pone.0133422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagawa H, Tajima A, Sakamoto Y, Krischek B, Yoneyama T, Kasuya H, et al. (2006). A haplotype spanning two genes, ELN and LIMK1, decreases their transcripts and confers susceptibility to intracranial aneurysms. [Research Support, Non-U.S. Gov’t]. Hum Mol Genet, 15(10), 1722–1734, doi: 10.1093/hmg/dd1096. [DOI] [PubMed] [Google Scholar]
- Akiyama K, Narita A, Nakaoka H, Cui T, Takahashi T, Yasuno K, et al. (2010). Genome-wide association study to identify genetic variants present in Japanese patients harboring intracranial aneurysms. [Research Support, Non-U.S. Gov’t]. J Hum Genet, 55(10), 656–661, doi: 10.1038/jhg.2010.82. [DOI] [PubMed] [Google Scholar]
- Alg VS, Ke X, Grieve J, Bonner S, Walsh DC, Bulters D, et al. (2018). Association of functional MMP-2 gene variant with intracranial aneurysms: case-control genetic association study and meta-analysis. [Multicenter Study Observational Study]. Br J Neurosurg, 32(3), 255–259, doi: 10.1080/02688697.2018.1427213. [DOI] [PubMed] [Google Scholar]
- Alg VS, Sofat R, Houlden H, & Werring DJ (2013). Genetic risk factors for intracranial aneurysms: a meta-analysis in more than 116,000 individuals. [Meta-Analysis Research Support, Non-U.S. Gov’t Review]. Neurology, 80(23), 2154–2165, doi: 10.1212/WNL.0b013e318295d751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen TH, Bartek J Jr., Andresen M, Springborg JB, & Romner B (2013). Modifiable risk factors for aneurysmal subarachnoid hemorrhage. [Review]. Stroke, 44(12), 3607–3612, doi: 10.1161/STROKEAHA.113.001575. [DOI] [PubMed] [Google Scholar]
- Aoki T, Kataoka H, Ishibashi R, Nozaki K, Egashira K, & Hashimoto N (2009). Impact of monocyte chemoattractant protein-1 deficiency on cerebral aneurysm formation. Stroke, 40(3), 942–951, doi: 10.1161/STROKEAHA.108.532556. [DOI] [PubMed] [Google Scholar]
- Aoki T, Kataoka H, Shimamura M, Nakagami H, Wakayama K, Moriwaki T, et al. (2007). NF-kappaB is a key mediator of cerebral aneurysm formation. Circulation, 116(24), 2830–2840, doi: 10.1161/CIRCULATIONAHA.107.728303. [DOI] [PubMed] [Google Scholar]
- Aoki T, & Nishimura M (2011). The development and the use of experimental animal models to study the underlying mechanisms of CA formation. [Review]. J Biomed Biotechnol, 2011, 535921, doi: 10.1155/2011/535921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T, Saito M, Koseki H, Tsuji K, Tsuji A, Murata K, et al. (2017). Macrophage Imaging of Cerebral Aneurysms with Ferumoxytol: an Exploratory Study in an Animal Model and in Patients. J Stroke Cerebrovasc Dis, 26(10), 2055–2064, doi: 10.1016/j.jstrokecerebrovasdis.2016.10.026. [DOI] [PubMed] [Google Scholar]
- Arning A, Jeibmann A, Kohnemann S, Brokinkel B, Ewelt C, Berger K, et al. (2016). ADAMTS genes and the risk of cerebral aneurysm. [Research Support, Non-U.S. Gov’t]. J Neurosurg, 125(2), 269–274, doi: 10.3171/2015.7.JNS154. [DOI] [PubMed] [Google Scholar]
- Batra R, Suh MK, Carson JS, Dale MA, Meisinger TM, Fitzgerald M, et al. (2018). IL-1beta (Interleukin-1beta) and TNF-alpha (Tumor Necrosis Factor-alpha) Impact Abdominal Aortic Aneurysm Formation by Differential Effects on Macrophage Polarization. [Comparative Study Research Support, N.I.H., Extramural]. Arterioscler Thromb Vasc Biol, 38(2), 457–463, doi: 10.1161/ATVBAHA.117.310333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann C, Guay-Woodford LM, Harris PC, Horie S, Peters DJM, & Torres VE (2018). Polycystic kidney disease. [Review]. Nat Rev Dis Primers, 4(1), 50, doi: 10.1038/s41572-018-0047-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilguvar K, Yasuno K, Niemela M, Ruigrok YM, von Und Zu Fraunberg M, van Duijn CM, et al. (2008). Susceptibility loci for intracranial aneurysm in European and Japanese populations. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Nat Genet, 40(12), 1472–1477, doi: 10.1038/ng.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bober MB, Khan N, Kaplan J, Lewis K, Feinstein JA, Scott CI Jr., et al. (2010). Majewski osteodysplastic primordial dwarfism type II (MOPD II): expanding the vascular phenotype. [Case Reports]. Am J Med Genet A, 152A(4), 960–965, doi: 10.1002/ajmg.a.33252. [DOI] [PubMed] [Google Scholar]
- Bor AS, Rinkel GJ, Adami J, Koffijberg H, Ekbom A, Buskens E, et al. (2008). Risk of subarachnoid haemorrhage according to number of affected relatives: a population based case-control study. [Research Support, Non-U.S. Gov’t]. Brain, 131(Pt 10), 2662–2665, doi: 10.1093/brain/awn187. [DOI] [PubMed] [Google Scholar]
- Bourcier R, Le Scouarnec S, Bonnaud S, Karakachoff M, Bourcereau E, Heurtebise-Chretien S, et al. (2018). Rare Coding Variants in ANGPTL6 Are Associated with Familial Forms of Intracranial Aneurysm. [Research Support, Non-U.S. Gov’t]. Am J Hum Genet, 102(1), 133–141, doi: 10.1016/j.ajhg.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzeghrane F, Naggara O, Kallmes DF, Berenstein A, Raymond J, & International Consortium of Neuroendovascular, C. (2010). In vivo experimental intracranial aneurysm models: a systematic review. AJNR Am J Neuroradiol, 31(3), 418–423, doi: 10.3174/ajnr.A1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain Aneurysm Foundation (2017). Brain aneurysm statistics and facts. https://www.bafound.org/about-brain-aneurysms/brain-aneurysm-basics/brain-aneurysm-statistics-and-facts/ Accessed 8/5/2017 2017.
- Brancati F, Castori M, Mingarelli R, & Dallapiccola B (2005). Majewski osteodysplastic primordial dwarfism type II (MOPD II) complicated by stroke: clinical report and review of cerebral vascular anomalies. [Case Reports Research Support, Non-U.S. Gov’t Review]. Am J Med Genet A, 139(3), 212–215, doi: 10.1002/ajmg.a.31009. [DOI] [PubMed] [Google Scholar]
- Bromberg JE, Rinkel GJ, Algra A, Greebe P, van Duyn CM, Hasan D, et al. (1995). Subarachnoid haemorrhage in first and second degree relatives of patients with subarachnoid haemorrhage. [Research Support, Non-U.S. Gov’t]. BMJ, 311(7000), 288–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnazzo F, Gambacciani C, Morganti R, & Perrini P (2017). Intracranial aneurysms in patients with autosomal dominant polycystic kidney disease: prevalence, risk of rupture, and management. A systematic review. [Review Systematic Review]. Acta Neurochir (Wien), 159(5), 811–821, doi: 10.1007/s00701-017-3142-z. [DOI] [PubMed] [Google Scholar]
- Canham PB, Talman EA, Finlay HM, & Dixon JG (1991). Medial collagen organization in human arteries of the heart and brain by polarized light microscopy. Connect Tissue Res, 26(1–2), 121–134. [DOI] [PubMed] [Google Scholar]
- Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, et al. (2012). Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab, 32(9), 1659–1676, doi: 10.1038/jcbfm.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Wilson K, Gu H, Wegman-Points L, Dooley SA, Pierce GL, et al. (2015). Myeloperoxidase is increased in human cerebral aneurysms and increases formation and rupture of cerebral aneurysms in mice. Stroke, 46(6), 1651–1656, doi: 10.1161/STROKEAHA.114.008589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway JE, Hutchins GM, & Tamargo RJ (1999). Marfan syndrome is not associated with intracranial aneurysms. [Comparative Study]. Stroke, 30(8), 1632–1636. [DOI] [PubMed] [Google Scholar]
- Conway JE, Hutchins GM, & Tamargo RJ (2001). Lack of evidence for an association between neurofibromatosis type I and intracranial aneurysms: autopsy study and review of the literature. [Comparative Study Review]. Stroke, 32(11), 2481–2485. [DOI] [PubMed] [Google Scholar]
- Crist AM, Lee AR, Patel NR, Westhoff DE, & Meadows SM (2018). Vascular deficiency of Smad4 causes arteriovenous malformations: a mouse model of Hereditary Hemorrhagic Telangiectasia. Angiogenesis, 21(2), 363–380, doi: 10.1007/s10456-018-9602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cun YP, Xiong CJ, Diao B, Yang Y, Pan L, & Ma LT (2017). Association between angiotensin-converting enzyme insertion/deletion polymorphisms and intracranial aneurysm susceptibility: A meta-analysis. BiomedRep, 6(6), 663–670, doi: 10.3892/br.2017.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hoog CL, Foster LJ, & Mann M (2004). RNA and RNA binding proteins participate in early stages of cell spreading through spreading initiation centers. [Research Support, Non-U.S. Gov’t]. Cell, 117(5), 649–662. [DOI] [PubMed] [Google Scholar]
- De Paepe A, & Malfait F (2012). The Ehlers-Danlos syndrome, a disorder with many faces. [Research Support, Non-U.S. Gov’t Review]. Clin Genet, 82(1), 1–11, doi: 10.1111/j.1399-0004.2012.01858.x. [DOI] [PubMed] [Google Scholar]
- Deka R, Koller DL, Lai D, Indugula SR, Sun G, Woo D, et al. (2010). The relationship between smoking and replicated sequence variants on chromosomes 8 and 9 with familial intracranial aneurysm. [Multicenter Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Stroke, 41(6), 1132–1137, doi: 10.1161/STROKEAHA.109.574640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Huo Y, & Luo J (2014). Endothelial mechanosensors: the gatekeepers of vascular homeostasis and adaptation under mechanical stress. Sci China Life Sci, 57(8), 755–762, doi: 10.1007/s11427-014-4705-3. [DOI] [PubMed] [Google Scholar]
- Diagbouga MR, Morel S, Bijlenga P, & Kwak BR (2018). Role of hemodynamics in initiation/growth of intracranial aneurysms. Eur J Clin Invest, 48(9), e12992, doi: 10.1111/eci.12992. [DOI] [PubMed] [Google Scholar]
- Dietz HC, Saraiva JM, Pyeritz RE, Cutting GR, & Francomano CA (1992). Clustering of fibrillin (FBN1) missense mutations in Marfan syndrome patients at cysteine residues in EGF-like domains. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. Hum Mutat, 1(5), 366–374, doi: 10.1002/humu.1380010504. [DOI] [PubMed] [Google Scholar]
- Draghia F, Draghia AC, & Onicescu D (2008). Electron microscopic study of the arterial wall in the cerebral aneurysms. Rom J Morphol Embryol, 49(1), 101–103. [PubMed] [Google Scholar]
- Fan J, Sun W, Lin M, Yu K, Wang J, Duan D, et al. (2016). Genetic association study identifies a functional CNV in the WWOX gene contributes to the risk of intracranial aneurysms. Oncotarget, 7(13), 16104–16111, doi: 10.18632/oncotarget.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farlow JL, Lin H, Sauerbeck L, Lai D, Koller DL, Pugh E, et al. (2015). Lessons learned from whole exome sequencing in multiplex families affected by a complex genetic disorder, intracranial aneurysm. [Multicenter Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. PLoS One, 10(3), e0121104, doi: 10.1371/journal.pone.0121104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnham JM, Camp NJ, Neuhausen SL, Tsuruda J, Parker D, MacDonald J, et al. (2004). Confirmation of chromosome 7q11 locus for predisposition to intracranial aneurysm. [Research Support, U.S. Gov’t, P.H.S.]. Hum Genet, 114(3), 250–255, doi: 10.1007/s00439-003-1044-z. [DOI] [PubMed] [Google Scholar]
- Fennell VS, Kalani MY, Atwal G, Martirosyan NL, & Spetzler RF (2016). Biology of Saccular Cerebral Aneurysms: A Review of Current Understanding and Future Directions. Front Surg, 3, 43, doi: 10.3389/fsurg.2016.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay HM, McCullough L, & Canham PB (1995). Three-dimensional collagen organization of human brain arteries at different transmural pressures. J Vasc Res, 32(5), 301–312, doi: 10.1159/000159104. [DOI] [PubMed] [Google Scholar]
- Finlay HM, Whittaker P, & Canham PB (1998). Collagen organization in the branching region of human brain arteries. Stroke, 29(8), 1595–1601. [DOI] [PubMed] [Google Scholar]
- Finney LH, Roberts TS, & Anderson RE (1976). Giant intracranial aneurysm associated with Marfan’s syndrome. Case report. [Case Reports]. J Neurosurg, 45(3), 342–347, doi: 10.3171/jns.1976.45.3.0342. [DOI] [PubMed] [Google Scholar]
- Forbus WD (1930). On the origin of miliary aneurysms of the superficial cerebral arteries. Johns Hopk. Hosp. Bull, 47, 239–284. [Google Scholar]
- Foroud T, Koller DL, Lai D, Sauerbeck L, Anderson C, Ko N, et al. (2012). Genome-wide association study of intracranial aneurysms confirms role of Anril and SOX17 in disease risk. [Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t]. Stroke, 43(11), 2846–2852, doi: 10.1161/STROKEAHA.112.656397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroud T, Lai D, Koller D, Van’t Hof F, Kurki MI, Anderson CS, et al. (2014). Genome-wide association study of intracranial aneurysm identifies a new association on chromosome 7. [Meta-Analysis Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Stroke, 45(11), 3194–3199, doi: 10.1161/STROKEAHA.114.006096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroud T, Sauerbeck L, Brown R, Anderson C, Woo D, Kleindorfer D, et al. (2009). Genome screen in familial intracranial aneurysm. [Multicenter Study Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural]. BMC Med Genet, 10, 3, doi: 10.1186/1471-2350-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroud T, Sauerbeck L, Brown R, Anderson C, Woo D, Kleindorfer D, et al. (2008). Genome screen to detect linkage to intracranial aneurysm susceptibility genes: the Familial Intracranial Aneurysm (FIA) study. [Multicenter Study Research Support, N.I.H., Extramural]. Stroke, 39(5), 1434–1440, doi: 10.1161/STROKEAHA.107.502930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Q, Liu Q, Hu X, & You C (2017). Collagen Type I Alpha 2 (COL1A2) Polymorphism Contributes to Intracranial Aneurysm Susceptibility: A Meta-Analysis. [Meta-Analysis]. MedSci Monit, 23, 3240–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givens C, & Tzima E (2016). Endothelial Mechanosignaling: Does One Sensor Fit All? Antioxid Redox Signal, 25(7), 373–388, doi: 10.1089/ars.2015.6493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasker S, Schatlo B, Klingler JH, Braun V, Spangenberg P, Kim IS, et al. (2014). Associations of collagen type I alpha2 polymorphisms with the presence of intracranial aneurysms in patients from Germany. [Multicenter Study]. J Stroke Cerebrovasc Dis, 23(2), 356–360, doi: 10.1016/j.jstrokecerebrovasdis.2013.04.038. [DOI] [PubMed] [Google Scholar]
- Haasdijk RA, Den Dekker WK, Cheng C, Tempel D, Szulcek R, Bos FL, et al. (2016). THSD1 preserves vascular integrity and protects against intraplaque haemorrhaging in ApoE−/− mice. [Research Support, Non-U.S. Gov’t]. Cardiovasc Res, 110(1), 129–139, doi: 10.1093/cvr/cvw015. [DOI] [PubMed] [Google Scholar]
- Hainsworth PJ, & Mendelow AD (1991). Giant intracranial aneurysm associated with Marfan’s syndrome: a case report. [Letter]. J Neurol Neurosurg Psychiatry, 54(5), 471–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburger DS, & Calderwood DA (2009). Integrin signalling at a glance. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, Non-P.H.S. Review]. J Cell Sci, 122(Pt 2), 159–163, doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hateboer N, v Dijk MA, Bogdanova N, Coto E, Saggar-Malik AK, San Millan JL, et al. (1999). Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. [Research Support, Non-U.S. Gov’t]. Lancet, 353(9147), 103–107. [DOI] [PubMed] [Google Scholar]
- Hayward C, Keston M, Brock DJ, & Dietz HC (1992). Fibrillin (FBN1) mutations in Marfan syndrome. [Letter]. Hum Mutat, 1(1), 79, doi: 10.1002/humu.1380010115. [DOI] [PubMed] [Google Scholar]
- Higashida RT, Halbach VV, Hieshima GB, & Cahan L (1988). Cavernous carotid artery aneurysm associated with Marfan’s syndrome: treatment by balloon embolization therapy. [Case Reports]. Neurosurgery, 22(2), 297–300. [DOI] [PubMed] [Google Scholar]
- Hitchcock E, & Gibson WT (2017). A Review of the Genetics of Intracranial Berry Aneurysms and Implications for Genetic Counseling. [Review]. J Genet Couns, 26(1), 21–31, doi: 10.1007/s10897-016-0029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong EP, Jeon JP, Kim SE, Yang JS, Choi HJ, Kang SH, et al. (2017). A Novel Association between Lysyl Oxidase Gene Polymorphism and Intracranial Aneurysm in Koreans. Yonsei Med J, 58(5), 1006–1011, doi: 10.3349/ymj.2017.58.5.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hop JW, Rinkel GJ, Algra A, & van Gijn J (1997). Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. [Review]. Stroke, 28(3), 660–664. [DOI] [PubMed] [Google Scholar]
- Hu J, Luo J, Wang H, Wang C, Sun X, Li A, et al. (2017). Association of TNF-alpha-3959T/C Gene Polymorphisms in the Chinese Population with Intracranial Aneurysms. J Mol Neurosci, 63(3-4), 349–354, doi: 10.1007/s12031-017-0985-y. [DOI] [PubMed] [Google Scholar]
- Hu X, Fang Y, Li YK, Liu WK, Li H, Ma L, et al. (2015). Role of Endoglin Insertion and rs1800956 Polymorphisms in Intracranial Aneurysm Susceptibility: A Meta-Analysis. [Meta-Analysis Research Support, Non-U.S. Gov’t Review]. Medicine (Baltimore), 94(45), e1847, doi: 10.1097/MD.0000000000001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida A, Wang Z, Hirata H, & Sehara-Fujisawa A (2018). Integrin beta1 activity is required for cardiovascular formation in zebrafish. Genes Cells, 23(11), 938–951, doi: 10.1111/gtc.12641. [DOI] [PubMed] [Google Scholar]
- International Study of Unruptured Intracranial Aneurysms, I. (1998). Unruptured intracranial aneurysms--risk of rupture and risks of surgical intervention. [Multicenter Study Research Support, U.S. Gov’t, P.H.S.]. N Engl J Med, 339(24), 1725–1733, doi: 10.1056/NEJM199812103392401. [DOI] [PubMed] [Google Scholar]
- Ishibashi R, Aoki T, Nishimura M, Hashimoto N, & Miyamoto S (2010). Contribution of mast cells to cerebral aneurysm formation. Curr Neurovasc Res, 7(2), 113–124. [DOI] [PubMed] [Google Scholar]
- Itoh F, Itoh S, Adachi T, Ichikawa K, Matsumura Y, Takagi T, et al. (2012). Smad2/Smad3 in endothelium is indispensable for vascular stability via S1PR1 and N-cadherin expressions. [Research Support, Non-U.S. Gov’t]. Blood, 119(22), 5320–5328, doi: 10.1182/blood-2011-12-395772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston SC, Selvin S, & Gress DR (1998). The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology, 50(5), 1413–1418. [DOI] [PubMed] [Google Scholar]
- Joo SP, Kim TS, Lee IK, Lee JK, Seo BR, Kim JH, et al. (2009). The role of collagen type I alpha2 polymorphisms: intracranial aneurysms in Koreans. Surg Neurol, 72(1), 48–53; discussion 53, doi: 10.1016/j.surneu.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Joo SP, Lee JK, Kim TS, Kim MK, Lee IK, Seo BR, et al. (2008). A polymorphic variant of the endoglin gene is associated with increased risk for intracranial aneurysms in a Korean population. [Research Support, Non-U.S. Gov’t]. Surg Neurol, 70(1), 39–44, doi: 10.1016/j.surneu.2008.01.060. [DOI] [PubMed] [Google Scholar]
- Jurczyk A, Gromley A, Redick S, San Agustin J, Witman G, Pazour GJ, et al. (2004). Pericentrin forms a complex with intraflagellar transport proteins and polycystin-2 and is required for primary cilia assembly. [Research Support, U.S. Gov’t, Non-P.H.S. Research Support, U.S. Gov’t, P.H.S.]. J Cell Biol, 166(5), 637–643, doi: 10.1083/jcb.200405023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallakuri S, Yu JA, Li J, Li Y, Weinstein BM, Nicoli S, et al. (2015). Endothelial cilia are essential for developmental vascular integrity in zebrafish. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. J Am Soc Nephrol, 26(4), 864–875, doi: 10.1681/ASN.2013121314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanematsu Y, Kanematsu M, Kurihara C, Tada Y, Tsou TL, van Rooijen N, et al. (2011). Critical roles of macrophages in the formation of intracranial aneurysm. Stroke, 42(1), 173–178, doi: 10.1161/STROKEAHA.110.590976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CJ, Park SS, Lee HS, Chung HJ, Choi W, Chung JH, et al. (2011). Identification of an autosomal dominant locus for intracranial aneurysm through a model-based family collection in a geographically limited area. [Research Support, Non-U.S. Gov’t]. J Hum Genet, 56(6), 464–466, doi: 10.1038/jhg.2011.27. [DOI] [PubMed] [Google Scholar]
- Kim DH, Van Ginhoven G, & Milewicz DM (2003). Incidence of familial intracranial aneurysms in 200 patients: comparison among Caucasian, African-American, and Hispanic populations. [Comparative Study Research Support, U.S. Gov’t, P.H.S.]. Neurosurgery, 53(2), 302–308. [DOI] [PubMed] [Google Scholar]
- Kim ST, Brinjikji W, & Kallmes DF (2016). Prevalence of Intracranial Aneurysms in Patients with Connective Tissue Diseases: A Retrospective Study. AJNR Am J Neuroradiol, 37(8), 1422–1426, doi: 10.3174/ajnr.A4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Hashimoto N, Kikuchi H, Hazama F, Nagata I, & Kataoka H (1998). Apoptosis of medial smooth muscle cells in the development of saccular cerebral aneurysms in rats. Stroke, 29(1), 181–188; discussion 189. [DOI] [PubMed] [Google Scholar]
- Korja M, Silventoinen K, Laatikainen T, Jousilahti P, Salomaa V, Hernesniemi J, et al. (2013). Risk factors and their combined effects on the incidence rate of subarachnoid hemorrhage--a population-based cohort study. [Research Support, Non-U.S. Gov’t]. PLoS One, 8(9), e73760, doi: 10.1371/journal.pone.0073760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korja M, Silventoinen K, McCarron P, Zdravkovic S, Skytthe A, Haapanen A, et al. (2010). Genetic epidemiology of spontaneous subarachnoid hemorrhage: Nordic Twin Study. [Research Support, Non-U.S. Gov’t Twin Study]. Stroke, 41(11), 2458–2462, doi: 10.1161/STROKEAHA.110.586420. [DOI] [PubMed] [Google Scholar]
- Krings T, Mandell DM, Kiehl TR, Geibprasert S, Tymianski M, Alvarez H, et al. (2011). Intracranial aneurysms: from vessel wall pathology to therapeutic approach. Nat Rev Neurol, 7(10), 547–559, doi: 10.1038/nrneurol.2011.136. [DOI] [PubMed] [Google Scholar]
- Krischek B, Tajima A, Akagawa H, Narita A, Ruigrok Y, Rinkel G, et al. (2010). Association of the Jun dimerization protein 2 gene with intracranial aneurysms in Japanese and Korean cohorts as compared to a Dutch cohort. [Comparative Study Research Support, Non-U.S. Gov’t]. Neuroscience, 169(1), 339–343, doi: 10.1016/j.neuroscience.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Kurki MI, Gaal EI, Kettunen J, Lappalainen T, Menelaou A, Anttila V, et al. (2014). High risk population isolate reveals low frequency variants predisposing to intracranial aneurysms. [Research Support, Non-U.S. Gov’t]. PLoS Genet, 10(1), e1004134, doi: 10.1371/journal.pgen.1004134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim IK, Ahn JS, Woo DC, Kim ST, Song S, et al. (2015). Deficiency of endothelium-specific transcription factor Sox17 induces intracranial aneurysm. [Research Support, Non-U.S. Gov’t]. Circulation, 131(11), 995–1005, doi: 10.1161/CIRCULATIONAHA.114.012568. [DOI] [PubMed] [Google Scholar]
- Li FF, Wang XD, Zhu MW, Lou ZH, Zhang Q, Zhu CY, et al. (2015). Identification of two novel critical mutations in PCNT gene resulting in microcephalic osteodysplastic primordial dwarfism type II associated with multiple intracranial aneurysms. [Case Reports Research Support, Non-U.S. Gov’t]. Metab Brain Dis, 30(6), 1387–1394, doi: 10.1007/s11011-015-9712-y. [DOI] [PubMed] [Google Scholar]
- Linn FH, Rinkel GJ, Algra A, & van Gijn J (1996). Incidence of subarachnoid hemorrhage: role of region, year, and rate of computed tomography: a meta-analysis. [Comparative Study Meta-Analysis]. Stroke, 27(4), 625–629. [DOI] [PubMed] [Google Scholar]
- Liu J, Zeng L, Kennedy RM, Gruenig NM, & Childs SJ (2012). betaPix plays a dual role in cerebral vascular stability and angiogenesis, and interacts with integrin alphavbeta8. Dev Biol, 363(1), 95–105, doi: 10.1016/j.ydbio.2011.12.022. [DOI] [PubMed] [Google Scholar]
- Loeys BL, Schwarze U, Holm T, Callewaert BL, Thomas GH, Pannu H, et al. (2006). Aneurysm syndromes caused by mutations in the TGF-beta receptor. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. N Engl J Med, 355(8), 788–798, doi: 10.1056/NEJMoa055695. [DOI] [PubMed] [Google Scholar]
- Lorenzo-Betancor O, Blackburn PR, Edwards E, Vazquez-do-Campo R, Klee EW, Labbe C, et al. (2018). PCNT point mutations and familial intracranial aneurysms. Neurology, 91(23), e2170–e2181, doi: 10.1212/WNL.0000000000006614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low SK, Takahashi A, Cha PC, Zembutsu H, Kamatani N, Kubo M, et al. (2012). Genome-wide association study for intracranial aneurysm in the Japanese population identifies three candidate susceptible loci and a functional genetic variant at EDNRA. [Research Support, Non-U.S. Gov’t]. Hum Mol Genet, 21(9), 2102–2110, doi: 10.1093/hmg/dds020. [DOI] [PubMed] [Google Scholar]
- Low SK, Zembutsu H, Takahashi A, Kamatani N, Cha PC, Hosono N, et al. (2011). Impact of LIMK1, MMP2 and TNF-alpha variations for intracranial aneurysm in Japanese population. [Research Support, Non-U.S. Gov’t]. J Hum Genet, 56(3), 211–216, doi: 10.1038/jhg.2010.169. [DOI] [PubMed] [Google Scholar]
- MacCarrick G, Black JH 3rd, Bowdin S, El-Hamamsy I, Frischmeyer-Guerrerio PA, Guerrerio AL, et al. (2014). Loeys-Dietz syndrome: a primer for diagnosis and management. [Research Support, Non-U.S. Gov’t Review]. Genet Med, 16(8), 576–587, doi: 10.1038/gim.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey J, Brown RD, Sauerbeck L, Hornung R, Moomaw CJ, Koller DL, et al. (2015). Affected twins in the familial intracranial aneurysm study. [Research Support, N.I.H., Extramural Twin Study]. Cerebrovasc Dis, 39(2), 82–86, doi: 10.1159/000369961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malfait F (2018). Vascular aspects of the Ehlers-Danlos Syndromes. [Review]. Matrix Biol, 71-72, 380–395, doi: 10.1016/j.matbio.2018.04.013. [DOI] [PubMed] [Google Scholar]
- Matsuda M, Matsuda I, Handa H, & Okamoto K (1979). Intracavernous giant aneurysm associated with Marfan’s syndrome. [Case Reports]. Surg Neurol, 12(2), 119–121. [PubMed] [Google Scholar]
- Meng H, Tutino VM, Xiang J, & Siddiqui A (2014). High WSS or low WSS? Complex interactions of hemodynamics with intracranial aneurysm initiation, growth, and rupture: toward a unifying hypothesis. AJNR Am J Neuroradiol, 35(7), 1254–1262, doi: 10.3174/ajnr.A3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Wang Z, Hoi Y, Gao L, Metaxa E, Swartz DD, et al. (2007). Complex hemodynamics at the apex of an arterial bifurcation induces vascular remodeling resembling cerebral aneurysm initiation. Stroke, 38(6), 1924–1931, doi: 10.1161/STROKEAHA.106.481234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineharu Y, Inoue K, Inoue S, Yamada S, Nozaki K, Hashimoto N, et al. (2007). Model-based linkage analyses confirm chromosome 19q13.3 as a susceptibility locus for intracranial aneurysm. [Research Support, Non-U.S. Gov’t]. Stroke, 38(4), 1174–1178, doi: 10.1161/01.STR.0000259657.73682.03. [DOI] [PubMed] [Google Scholar]
- Miyata H, Koseki H, Takizawa K, Kasuya H, Nozaki K, Narumiya S, et al. (2017). T cell function is dispensable for intracranial aneurysm formation and progression. PLoS One, 12(4), e0175421, doi: 10.1371/journal.pone.0175421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Balaguer M, Swirsding K, Orsenigo F, Cotelli F, Mione M, & Dejana E (2009). Stable vascular connections and remodeling require full expression of VE-cadherin in zebrafish embryos. [Research Support, Non-U.S. Gov’t]. PLoS One, 4(6), e5772, doi: 10.1371/journal.pone.0005772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto M, Miyamoto S, Mizoguchi A, Kume N, Kita T, & Hashimoto N (2002). Mouse model of cerebral aneurysm: experimental induction by renal hypertension and local hemodynamic changes. [Research Support, Non-U.S. Gov’t]. Stroke, 33(7), 1911–1915. [DOI] [PubMed] [Google Scholar]
- Nader GP, Ezratty EJ, & Gundersen GG (2016). FAK, talin and PIPKIgamma regulate endocytosed integrin activation to polarize focal adhesion assembly. Nat Cell Biol, 18(5), 491–503, doi: 10.1038/ncb3333. [DOI] [PubMed] [Google Scholar]
- Nahed BV, Seker A, Guclu B, Ozturk AK, Finberg K, Hawkins AA, et al. (2005). Mapping a Mendelian form of intracranial aneurysm to 1p34.3-p36.13. [Research Support, Non-U.S. Gov’t]. Am J Hum Genet, 76(1), 172–179, doi: 10.1086/426953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima-Takagi Y, Osawa M, Oshima M, Takagi H, Miyagi S, Endoh M, et al. (2013). Role of SOX17 in hematopoietic development from human embryonic stem cells. [Research Support, Non-U.S. Gov’t]. Blood, 121(3), 447–458, doi: 10.1182/blood-2012-05-431403. [DOI] [PubMed] [Google Scholar]
- Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, & Zhou J (2008). Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation, 117(9), 1161–1171, doi: 10.1161/CIRCULATIONAHA.107.710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls AC, De Paepe A, Narcisi P, Dalgleish R, De Keyser F, Matton M, et al. (1988). Linkage of a polymorphic marker for the type III collagen gene (COL3A1) to atypical autosomal dominant Ehlers-Danlos syndrome type IV in a large Belgian pedigree. [Case Reports Research Support, Non-U.S. Gov’t]. Hum Genet, 78(3), 276–281. [DOI] [PubMed] [Google Scholar]
- Norrgard O, Angquist KA, Fodstad H, Forsell A, & Lindberg M (1987). Intracranial aneurysms and heredity. Neurosurgery, 20(2), 236–239. [DOI] [PubMed] [Google Scholar]
- Nuki Y, Tsou TL, Kurihara C, Kanematsu M, Kanematsu Y, & Hashimoto T (2009). Elastase-induced intracranial aneurysms in hypertensive mice. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Hypertension, 54(6), 1337–1344, doi: 10.1161/HYPERTENSIONAHA.109.138297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuki H, Sugiura M, Iwaki K, Nishikawa M, & Yasuno M (1984). [A case of Marfan’s syndrome with a ruptured distal middle cerebral aneurysm]. [Case Reports]. No Shinkei Geka, 12(8), 983–985. [PubMed] [Google Scholar]
- Olson JM, Vongpunsawad S, Kuivaniemi H, Ronkainen A, Hernesniemi J, Ryynanen M, et al. (2002). Search for intracranial aneurysm susceptibility gene(s) using Finnish families. BMC Med Genet, 3, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda H, Kasuya H, Yoneyama T, Takakura K, Hori T, Takeda J, et al. (2001). Genomewide-linkage and haplotype-association studies map intracranial aneurysm to chromosome 7q11. [Research Support, Non-U.S. Gov’t]. Am J Hum Genet, 69(4), 804–819, doi: 10.1086/323614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk AK, Nahed BV, Bydon M, Bilguvar K, Goksu E, Bademci G, et al. (2006). Molecular genetic analysis of two large kindreds with intracranial aneurysms demonstrates linkage to 11q24-25 and 14q23-31. [Research Support, Non-U.S. Gov’t]. Stroke, 37(4), 1021–1027, doi: 10.1161/01.STR.0000206153.92675.b9. [DOI] [PubMed] [Google Scholar]
- Paschoal EHA, Yamaki VN, Teixeira RKC, Paschoal Junior FM, Jong ALGS, Teixeira MJ, et al. (2018). Relationship between endothelial nitric oxide synthase (eNOS) and natural history of intracranial aneurysms: meta-analysis. [Meta-Analysis Review Systematic Review]. Neurosurg Rev, 41(1), 87–94, doi: 10.1007/s10143-016-0761-4. [DOI] [PubMed] [Google Scholar]
- Paterakis K, Koutsias S, Doxani C, Xanthopoulou P, Kokkali C, Mpoulimari I, et al. (2017). Variants of the elastin (ELN) gene and susceptibility to intracranial aneurysm: a synthesis of genetic association studies using a genetic model-free approach. [Meta-Analysis]. Int J Neurosci, 127(7), 567–572, doi: 10.1080/00207454.2016.1212027. [DOI] [PubMed] [Google Scholar]
- Pentimalli L, Modesti A, Vignati A, Marchese E, Albanese A, Di Rocco F, et al. (2004). Role of apoptosis in intracranial aneurysm rupture. J Neurosurg, 101(6), 1018–1025, doi: 10.3171/jns.2004.101.6.1018. [DOI] [PubMed] [Google Scholar]
- Pirson Y (2010). Extrarenal manifestations of autosomal dominant polycystic kidney disease. [Review]. Adv Chronic Kidney Dis, 17(2), 173–180, doi: 10.1053/j.ackd.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Rauch A (2011). The shortest of the short: pericentrin mutations and beyond. Best Pract Res Clin Endocrinol Metab, 25(1), 125–130, doi: 10.1016/j.beem.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Rauch A, Thiel CT, Schindler D, Wick U, Crow YJ, Ekici AB, et al. (2008). Mutations in the pericentrin (PCNT) gene cause primordial dwarfism. [Research Support, Non-U.S. Gov’t]. Science, 319(5864), 816–819, doi: 10.1126/science.1151174. [DOI] [PubMed] [Google Scholar]
- Ravindra VM, Karsy M, Schmidt RH, Taussky P, Park MS, & Bollo RJ (2016). Rapid de novo aneurysm formation after clipping of a ruptured middle cerebral artery aneurysm in an infant with an MYH11 mutation. J Neurosurg Pediatr, 18(4), 463–470, doi: 10.3171/2016.5.PEDS16115. [DOI] [PubMed] [Google Scholar]
- Rodrigues VJ, Elsayed S, Loeys BL, Dietz HC, & Yousem DM (2009). Neuroradiologic manifestations of Loeys-Dietz syndrome type 1. AJNR Am J Neuroradiol, 30(8), 1614–1619, doi: 10.3174/ajnr.A1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronkainen A, Hernesniemi J, Puranen M, Niemitukia L, Vanninen R, Ryynanen M, et al. (1997). Familial intracranial aneurysms. [Research Support, Non-U.S. Gov’t], Lancet, 349(9049), 380–384, doi: 10.1016/S0140-6736(97)80009-8. [DOI] [PubMed] [Google Scholar]
- Ronkainen A, Hernesniemi J, & Ryynanen M (1993). Familial subarachnoid hemorrhage in east Finland, 1977-1990. [Review], Neurosurgery, 33(5), 787–796; discussion 796-797. [DOI] [PubMed] [Google Scholar]
- Roos YB, Pals G, Struycken PM, Rinkel GJ, Limburg M, Pronk JC, et al. (2004). Genome-wide linkage in a large Dutch consanguineous family maps a locus for intracranial aneurysms to chromosome 2pl3. [Research Support, Non-U. S. Gov’t], Stroke, 35(10), 2276–2281, doi: 10.1161/01.STR.0000141415.28155.46. [DOI] [PubMed] [Google Scholar]
- Roszer T (2015). Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. [Research Support, Non-U.S. Gov’t Review], Mediators Inflamm, 2015, 816460, doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui YN, Xu Z, Fang X, Menezes MR, Balzeau J, Niu A, et al. (2017). The Intracranial Aneurysm Gene THSD1 Connects Endosome Dynamics to Nascent Focal Adhesion Assembly. Cell Physiol Biochem, 43(6), 2200–2211, doi: 10.1159/000484298. [DOI] [PubMed] [Google Scholar]
- Ruigrok YM, Rinkel GJ, & Wijmenga C (2006). The versican gene and the risk of intracranial aneurysms. [Research Support, Non-U.S. Gov’t], Stroke, 37(9), 2372–2374, doi: 10.1161/01.STR.0000236499.55301.09. [DOI] [PubMed] [Google Scholar]
- Ruigrok YM, Rinkel GJ, Wijmenga C, Kasuya H, Tajima A, Takahashi T, et al. (2009). Association analysis of genes involved in the maintenance of the integrity of the extracellular matrix with intracranial aneurysms in a Japanese cohort. [Meta-Analysis Research Support, Non-U.S. Gov’t], Cerebrovasc Dis, 28(2), 131–134, doi: 10.1159/000223438. [DOI] [PubMed] [Google Scholar]
- Ruigrok YM, Wijmenga C, Rinkel GJ, van’t Slot R, Baas F, Wolfs M, et al. (2008). Genomewide linkage in a large Dutch family with intracranial aneurysms: replication of 2 loci for intracranial aneurysms to chromosome Ip36.11-p36.13 and Xp22.2-p22.32. [Research Support, Non-U.S. Gov’t], Stroke, 39(4), 1096–1102, doi: 10.1161/STROKEAHA.107.495168. [DOI] [PubMed] [Google Scholar]
- Santiago-Sim T, Depalma SR, Ju KL, McDonough B, Seidman CE, Seidman JG, et al. (2009a). Genomewide linkage in a large Caucasian family maps a new locus for intracranial aneurysms to chromosome 13q. [Research Support, Non-U.S. Gov’t], Stroke, 40(3 Suppl), S57–60, doi: 10.1161/STROKEAHA.108.534396. [DOI] [PubMed] [Google Scholar]
- Santiago-Sim T, Fang X, Hennessy ML, Nalbach SV, DePalma SR, Lee MS, et al. (2016). THSD1 (Thrombospondin Type 1 Domain Containing Protein 1) Mutation in the Pathogenesis of Intracranial Aneurysm and Subarachnoid Hemorrhage. Stroke, 47(12), 3005–3013, doi: 10.1161/STROKEAHA.116.014161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Sim T KD (2011). Pathobiology of intracranial aneurysms. [Book Chapter], Youmans Neurological Surgery(Winn HR, ed) 6th ed., 4(Philadelphia, PA: Elsevier, Saunders;), 3747–3755. [Google Scholar]
- Santiago-Sim T, Mathew-Joseph S, Pannu H, Milewicz DM, Seidman CE, Seidman JG, et al. (2009b). Sequencing of TGF-beta pathway genes in familial cases of intracranial aneurysm. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t], Stroke, 40(5), 1604–1611, doi: 10.1161/STROKEAHA.108.540245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro MM, Samuel T, Mitchell T, Reed JC, & Stainier DY (2007). Birc2 (clap1) regulates endothelial cell integrity and blood vessel homeostasis. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t], Nat Genet, 39(11), 1397–1402, doi: 10.1038/ng.2007.8. [DOI] [PubMed] [Google Scholar]
- Sathyan S, Koshy LV, Balan S, Easwer HV, Premkumar S, Nair S, et al. (2014). Association of Versican (VCAN) gene polymorphisms rs251124 and rs2287926 (G428D), with intracranial aneurysm. Meta Gene, 2, 651–660, doi: 10.1016/j.mgene.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer DM, Pace LA, Pascale CL, Kutchin AC, O’Neill BE, Starke RM, et al. (2016). Lymphocytes influence intracranial aneurysm formation and rupture: role of extracellular matrix remodeling and phenotypic modulation of vascular smooth muscle cells. J Neuroinflammation, 13(1), 185, doi: 10.1186/s12974-016-0654-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanarini M, Mingrino S, Zuccarello M, & Trincia G (1978). Scanning electron microscopy (s.e.m.) of biopsy specimens of ruptured intracranial saccular aneurysms. Acta Neuropathol, 44(2), 131–134. [DOI] [PubMed] [Google Scholar]
- Schievink WI, Parisi JE, Piepgras DG, & Michels VV (1997). Intracranial aneurysms in Marfan’s syndrome: an autopsy study. Neurosurgery, 41(4), 866–870; discussion 871. [DOI] [PubMed] [Google Scholar]
- Schievink WI, Riedinger M, & Maya MM (2005). Frequency of incidental intracranial aneurysms in neurofibromatosis type 1. [Comparative Study]. Am J Med Genet A, 134A(1), 45–48, doi: 10.1002/ajmg.a.30475. [DOI] [PubMed] [Google Scholar]
- Schievink WI, Schaid DJ, Michels VV, & Piepgras DG (1995). Familial aneurysmal subarachnoid hemorrhage: a community-based study. J Neurosurg, 83(3), 426–429, doi: 10.3171/jns.1995.83.3.0426. [DOI] [PubMed] [Google Scholar]
- Schievink WI, Schaid DJ, Rogers HM, Piepgras DG, & Michels VV (1994). On the inheritance of intracranial aneurysms. [Review]. Stroke, 25(10), 2028–2037. [DOI] [PubMed] [Google Scholar]
- Schurmann C, Gremse F, Jo H, Kiessling F, & Brandes RP (2015). Micro-CT Technique Is Well Suited for Documentation of Remodeling Processes in Murine Carotid Arteries. PLoS One, 10(6), e0130374, doi: 10.1371/journal.pone.0130374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sforza DM, Putman CM, & Cebral JR (2009). Hemodynamics of Cerebral Aneurysms. Annu Rev Fluid Mech, 41, 91–107, doi: 10.1146/annurev.fluid.40.111406.102126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L, Qin X, Liu J, Jian Z, Xiong X, & Liu R (2017). Macrophage Polarization in Cerebral Aneurysm: Perspectives and Potential Targets. J Immunol Res, 2017, 8160589, doi: 10.1155/2017/8160589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen TL, Park AY, Alcaraz A, Peng X, Jang I, Koni P, et al. (2005). Conditional knockout of focal adhesion kinase in endothelial cells reveals its role in angiogenesis and vascular development in late embryogenesis. [Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. J Cell Biol, 169(6), 941–952, doi: 10.1083/jcb.200411155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorelli F, Sela S, Gesualdo L, Chevrel S, Tollet F, Pailler-Mattei C, et al. (2018). Hemodynamic Stress, Inflammation, and Intracranial Aneurysm Development and Rupture: A Systematic Review. World Neurosurg, 115, 234–244, doi: 10.1016/j.wneu.2018.04.143. [DOI] [PubMed] [Google Scholar]
- Sima X, Sun H, Zhou P, & You C (2015). A Potential Polymorphism in the Promoter of Let-7 is Associated With an Increased Risk of Intracranial Aneurysm: A Case-Control Study. [Observational Study Research Support, Non-U.S. Gov’t]. Medicine (Baltimore), 94(51), e2267, doi: 10.1097/MD.0000000000002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Liu P, Li Z, Shi Y, Huang J, Li S, et al. (2018). The Effect of Myosin Light Chain Kinase on the Occurrence and Development of Intracranial Aneurysm. Front Cell Neurosci, 12, 416, doi: 10.3389/fncel.2018.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke RM, Chalouhi N, Ding D, Raper DM, McKisic MS, Owens GK, et al. (2014). Vascular smooth muscle cells in cerebral aneurysm pathogenesis. Trans! Stroke Res, 5(3), 338–346, doi: 10.1007/s12975-013-0290-1. [DOI] [PubMed] [Google Scholar]
- Stehbens WE, Delahunt B, & Hilless AD (1989). Early berry aneurysm formation in Marfan’s syndrome. [Case Reports Research Support, Non-U.S. Gov’t]. Surg Neurol, 31(3), 200–202. [DOI] [PubMed] [Google Scholar]
- Sun H, Zhang D, & Zhao J (2008). The interleukin-6 gene −572G>C promoter polymorphism is related to intracranial aneurysms in Chinese Han nationality. Neurosci Lett, 440(1), 1–3, doi: 10.1016/j.neulet.2008.04.077. [DOI] [PubMed] [Google Scholar]
- Suo M, Lin Y, Yu H, Song W, Sun K, Song Y, et al. (2014). Association of Kallikrein gene polymorphisms with sporadic intracranial aneurysms in the Chinese population. [Research Support, Non-U.S. Gov’t]. J Neurosurg, 120(6), 1397–1401, doi: 10.3171/2013.11.JNS131036. [DOI] [PubMed] [Google Scholar]
- Superti-Furga A, Gugler E, Gitzelmann R, & Steinmann B (1988). Ehlers-Danlos syndrome type IV: a multi-exon deletion in one of the two COL3A1 alleles affecting structure, stability, and processing of type III procollagen. [Research Support, Non-U.S. Gov’t]. J Biol Chem, 263(13), 6226–6232. [PubMed] [Google Scholar]
- Tada Y, Kanematsu Y, Kanematsu M, Nuki Y, Liang EI, Wada K, et al. (2011). A mouse model of intracranial aneurysm: technical considerations. [Research Support, N.I.H., Extramural]. Acta Neurochir Suppl, 111, 31–35, doi: 10.1007/978-3-7091-0693-8_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale GM, Wardlaw JM, White PM, Murray G, Teasdale EM, & Easton V (2005). The familial risk of subarachnoid haemorrhage. [Research Support, Non-U.S. Gov’t]. Brain, 128(Pt 7), 1677–1685, doi: 10.1093/brain/awh497. [DOI] [PubMed] [Google Scholar]
- Teo M, Johnson JN, Bell-Stephens TE, Marks MP, Do HM, Dodd RL, et al. (2016). Surgical outcomes of Majewski osteodysplastic primordial dwarfism Type II with intracranial vascular anomalies. [Case Reports]. J Neurosurg Pediatr, 25(6), 717–723, doi: 10.3171/2016.6.PEDS16243. [DOI] [PubMed] [Google Scholar]
- Thompson BG, Brown RD Jr., Amin-Hanjani S, Broderick JP, Cockroft KM, Connolly ES Jr., et al. (2015). Guidelines for the Management of Patients With Unruptured Intracranial Aneurysms: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. [Practice Guideline]. Stroke, 46(8), 2368–2400, doi: 10.1161/STR.0000000000000070. [DOI] [PubMed] [Google Scholar]
- Tromp G, Weinsheimer S, Ronkainen A, & Kuivaniemi H (2014). Molecular basis and genetic predisposition to intracranial aneurysm. [Review]. Ann Med, 46(8), 597–606, doi: 10.3109/07853890.2014.949299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsipouras P, Byers PH, Schwartz RC, Chu ML, Weil D, Pepe G, et al. (1986). Ehlers-Danlos syndrome type IV: cosegregation of the phenotype to a COL3A1 allele of type III procollagen. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.]. Hum Genet, 74(1), 41–46. [DOI] [PubMed] [Google Scholar]
- van den Berg JS, Limburg M, & Hennekam RC (1996). Is Marfan syndrome associated with symptomatic intracranial aneurysms? [Review]. Stroke, 27(1), 10–12. [DOI] [PubMed] [Google Scholar]
- van der Voet M, Olson JM, Kuivaniemi H, Dudek DM, Skunca M, Ronkainen A, et al. (2004). Intracranial aneurysms in Finnish families: confirmation of linkage and refinement of the interval to chromosome 19q13.3. [Research Support, U.S. Gov’t, P.H.S.]. Am J Hum Genet, 74(3), 564–571, doi: 10.1086/382285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanakker OM, Hemelsoet D, & De Paepe A (2011). Hereditary connective tissue diseases in young adult stroke: a comprehensive synthesis. Stroke Res Treat, 2011, 712903, doi: 10.4061/2011/712903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlaan DJ, Dube MP, St-Onge J, Noreau A, Roussel J, Satge N, et al. (2006). A new locus for autosomal dominant intracranial aneurysm, ANIB4, maps to chromosome 5p15.2-14.3. [Letter Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. J Med Genet, 43(6), e31, doi: 10.1136/jmg.2005.033209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo AH, Swaroop A, Liu Y, Norris ZG, & Shavit JA (2013). Loss of fibrinogen in zebrafish results in symptoms consistent with human hypofibrinogenemia. [Research Support, Non-U.S. Gov’t]. PLoS One, 8(9), e74682, doi: 10.1371/journal.pone.0074682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Liu Z, Ren J, Morgan S, Assa C, & Liu B (2015). Receptor-interacting protein kinase 3 contributes to abdominal aortic aneurysms via smooth muscle cell necrosis and inflammation. Circ Res, 116(4), 600–611, doi: 10.1161/CIRCRESAHA.116.304899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Emeto TI, Lee J, Marshman L, Moran C, Seto SW, et al. (2015). Mouse models of intracranial aneurysm. Brain Pathol, 25(3), 237–247, doi: 10.1111/bpa.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinsheimer S, Goddard KA, Parrado AR, Lu Q, Sinha M, Lebedeva ER, et al. (2007). Association of kallikrein gene polymorphisms with intracranial aneurysms. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Stroke, 38(10), 2670–2676, doi: 10.1161/STROKEAHA.107.486225. [DOI] [PubMed] [Google Scholar]
- Wiebers DO, Piepgras DG, Meyer FB, Kallmes DF, Meissner I, Atkinson JL, et al. (2004). Pathogenesis, natural history, and treatment of unruptured intracranial aneurysms. [Comparative Study Congress]. Mayo Clin Proc, 79(12), 1572–1583, doi: 10.4065/79.12.1572. [DOI] [PubMed] [Google Scholar]
- Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr., Piepgras DG, et al. (2003). Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. [Multicenter Study Research Support, U.S. Gov’t, P.H.S.]. Lancet, 362(9378), 103–110. [DOI] [PubMed] [Google Scholar]
- Willems M, Genevieve D, Borck G, Baumann C, Baujat G, Bieth E, et al. (2010). Molecular analysis of pericentrin gene (PCNT) in a series of 24 Seckel/microcephalic osteodysplastic primordial dwarfism type II (MOPD II) families. J Med Genet, 47(12), 797–802, doi: 10.1136/jmg.2009.067298. [DOI] [PubMed] [Google Scholar]
- Williams LN, & Brown RD Jr. (2013). Management of unruptured intracranial aneurysms. Neurol Clin Pract, 3(2), 99–108, doi: 10.1212/CPJ.0b013e31828d9f6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak MA, Modzelewska K, Kwong L, & Keely PJ (2004). Focal adhesion regulation of cell behavior. Biochim Biophys Acta, 1692(2-3), 103–119, doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Wu Q, Zhang J, Koh W, Yu Q, Zhu X, Amsterdam A, et al. (2015). Talin1 is required for cardiac Z-disk stabilization and endothelial integrity in zebrafish. FASEB J, 29(12), 4989–5005, doi: 10.1096/fj.15-273409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Li Z, Shi Y, Chen L, Tan H, Wang Z, et al. (2017). Exome Sequencing Identifies LOXL2 Mutation as a Cause of Familial Intracranial Aneurysm. World Neurosurg, doi: 10.1016/j.wneu.2017.10.094. [DOI] [PubMed] [Google Scholar]
- Xu Z, Rui YN, Balzeau J, Menezes MR, Niu A, Hagan JP, et al. (2017). Highly efficient one-step scarless protein tagging by type IIS restriction endonuclease-mediated precision cloning. [Research Support, N.I.H., Extramural]. Biochem Biophys Res Commun, 490(1), 8–16, doi: 10.1016/j.bbrc.2017.05.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Rui YN, Hagan JP, & Kim DH (2018). Precision Tagging: A Novel Seamless Protein Tagging by Combinational Use of Type II and Type IIS Restriction Endonucleases. Bio Protoc, 8(3), doi: 10.21769/BioProtoc.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Utsunomiya M, Inoue K, Nozaki K, Inoue S, Takenaka K, et al. (2004). Genome-wide scan for Japanese familial intracranial aneurysms: linkage to several chromosomal regions. [Research Support, Non-U.S. Gov’t]. Circulation, 110(24), 3727–3733, doi: 10.1161/01.CIR.0000143077.23367.18. [DOI] [PubMed] [Google Scholar]
- Yan J, Hitomi T, Takenaka K, Kato M, Kobayashi H, Okuda H, et al. (2015). Genetic study of intracranial aneurysms. [Research Support, Non-U.S. Gov’t]. Stroke, 46(3), 620–626, doi: 10.1161/STROKEAHA.114.007286. [DOI] [PubMed] [Google Scholar]
- Yang X, Li J, Fang Y, Zhang Z, Jin D, Chen X, et al. (2018). Rho Guanine Nucleotide Exchange Factor ARHGEF17 Is a Risk Gene for Intracranial Aneurysms. Circ Genom Precis Med, 11(7), e002099, doi: 10.1161/CIRCGEN.117.002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuno K, Bilguvar K, Bijlenga P, Low SK, Krischek B, Auburger G, et al. (2010). Genome-wide association study of intracranial aneurysm identifies three new risk loci. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. Nat Genet, 42(5), 420–425, doi: 10.1038/ng.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharia BE, Hickman ZL, Grobelny BT, DeRosa P, Kotchetkov I, Ducruet AF, et al. (2010). Epidemiology of aneurysmal subarachnoid hemorrhage. [Review]. Neurosurg Clin N Am, 21(2), 221–233, doi: 10.1016/j.nec.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Zhang G, Tu Y, Feng W, Huang L, Li M, & Qi S (2011). Association of interleukin-6-572G/C gene polymorphisms in the Cantonese population with intracranial aneurysms. J Neurol Sci, 306(1-2), 94–97, doi: 10.1016/j.jns.2011.03.036. [DOI] [PubMed] [Google Scholar]
- Zhang LT, Wei FJ, Zhao Y, Zhang Z, Dong WT, Jin ZN, et al. (2015). Intracranial aneurysm risk factor genes: relationship with intracranial aneurysm risk in a Chinese Han population. Genet Mol Res, 14(2), 6865–6878, doi: 10.4238/2015.June.18.30. [DOI] [PubMed] [Google Scholar]
- Zheng S, Su A, Sun H, & You C (2013). The association between interleukin-6 gene polymorphisms and intracranial aneurysms: a meta-analysis. [Meta-Analysis]. Hum Immunol, 74(12), 1679–1683, doi: 10.1016/j.humimm.2013.08.274. [DOI] [PubMed] [Google Scholar]
- Zholdybayeva EV, Medetov YZ, Aitkulova AM, Makhambetov YT, Akshulakov SK, Kaliyev AB, et al. (2018). Genetic Risk Factors for Intracranial Aneurysm in the Kazakh Population. J Mol Neurosci, 66(1), 135–145, doi: 10.1007/s12031-018-1134-y. [DOI] [PubMed] [Google Scholar]
- Zhou S, Ambalavanan A, Rochefort D, Xie P, Bourassa CV, Hince P, et al. (2016). RNF213 Is Associated with Intracranial Aneurysms in the French-Canadian Population. Am J Hum Genet, 99(5), 1072–1085, doi: 10.1016/j.ajhg.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Dion PA, & Rouleau GA (2018). Genetics of Intracranial Aneurysms. [Review]. Stroke, 49(3), 780–787, doi: 10.1161/STROKEAHA.117.018152. [DOI] [PubMed] [Google Scholar]


