Abstract
Cell-material interactions are important to tissue engineering. Inspired by the natural topographic structures on the extracellular matrix, a growing number of studies have integrated engineering topography into investigations of cell behavior on biomaterials. Engineering topography has a significant influence on cell behaviors. These cell-topography interactions play an important role in regenerative medicine and tissue engineering. Similarly, cell-topography interactions are important to corneal reconstruction and regeneration. In this review, we primarily summarized the effects of topographic cues on the behaviors of corneal cells, including cell morphology, adhesion, migration, and proliferation. Furthermore, the integration of engineering surface topography into corneal tissue engineering was also discussed.
Keywords: Surface topography, Corneal cells, Cell behaviors, Tissue engineering
Graphical abstract
Highlights
-
•
This article aims to provide insight into the potential applications of topography on corneal reconstruction and regeneration.
-
•
Discussed the relation between surface topography and corneal repair.
-
•
Reviewed the effect of topographic cues on corneal cell behaviors.
-
•
Reviewed the applications of topographic cues in corneal tissue engineering.
-
•
Discussed the underlying mechanism of the topographic effect on cell behaviors.
1. Introduction
Cells sense and interact with their microenvironment, which includes surrounding extracellular matrix (ECM), adjacent cells and various soluble factors [1]. The ECM provides not only physical support but also biochemical and biophysical cues as inputs into cell behaviors and functions [[2], [3], [4]]. Numerous studies have explored the effects of the biochemical cues, such as ECM components, on cell behaviors over the past several decades [5,6]. However, relatively less attention has been paid to the role of biophysical cues, including the surface topography of ECM, in modulating the activities of cells. In fact, ECM exhibits abundant micro- and nanoscale, geometrically defined, three-dimensional physical cues, named topography, which has been proven to be involved in cell behaviors in many ways [[7], [8], [9]]. For example, nanotopographic structures have been found to appear on basement membrane surfaces of virous tissue, including the cornea and the aortic heart valve [[10], [11], [12]]. Guided by an axial, fibrous mat formed in epithelial muscle processes, the migration of nematocytes in hydra is strongly bidirectional [13].
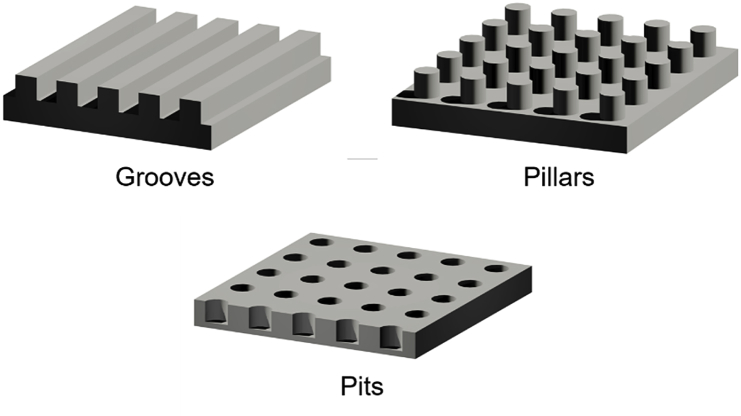
As a part of the microenvironment, the artificial scaffold also interferes with cell behavior in tissue engineering. Therefore, the investigation of cell-topography interactions is of considerable importance to the development of bioactive implants, which also benefits for a deeper understanding of the fundamentals of cell behaviors [14]. In 1912, Harrison first described the phenomenon that the shape and migration of cells cultured on spider web were influenced by the web fibers [15]. Subsequently, a similar phenomenon was observed and named “contact guidance” by Paul Weiss et al. in their studies [16]. As micro- and nanofabrication technologies were introduced in the cell biology field, surfaces textured with various micro- or nanotopographies were also designed and fabricated to evaluate their effects on the activities of cells. The three most commonly studied engineering topographies include microscale or nanoscale patterns of grooves, pillars and pits (Fig. 1), which are also the main point of focus and interest of this paper. Surface topography can influence the morphology, adhesion, proliferation, differentiation and ultrastructure of different cells [[17], [18], [19], [20], [21]]. These findings provide a new strategy to regulate the cell and to develop tissue engineering and regeneration medicine [[21], [22], [23], [24]].
Fig. 1.
Schematic images of representative topography features. Reprinted with permission from John Wiley and Sons Ltd: Angewandte Chemie International Edition, copyright (2009).
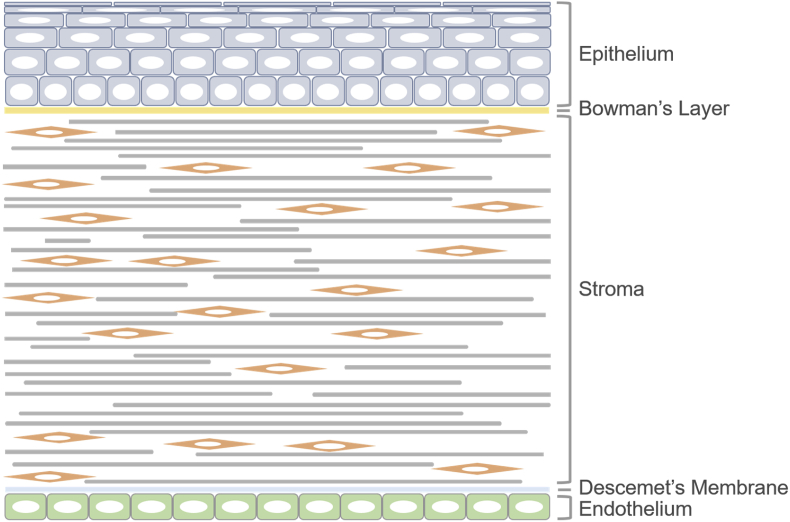
As the outermost layer of the eye, the cornea protects the eye from external damage and functions as an important optical element that transmits incoming light to the interior of eye and provides most of the refractive power of the eye. As shown in Fig. 2, the cornea is composed of five separated layers, three of which are cellular layers (epithelium, stroma and endothelium), with the other two acellular layers serving as the interfaces separating the cellular layers (Bowman's layer and Descemet's membrane). The cornea is exposed and subjected to external damage, and the outcome is the high incidence of visual impairment due to corneal disease worldwide [25,26]. For patients whose corneal clarity has been affected, corneal transplantation is the only option for regaining sight [27]. The allograft is the preferred choice for the transplantation, but its shortfall in supply limits its application. To date, corneal tissue engineering has been considered as an important approach to construct bioequivalents of corneal tissue [28]. The physical and chemical properties of the scaffold material, including the surface topography, are involved in signal transduction. Therefore, it is important to reveal the effects of surface topography on corneal cells behaviors. Over the past 20 years, with the discovery of surface topography regulating the activities of corneal cells, there have been rapidly expanding studies incorporating substrate topography into the construction of corneal substitutes. However, a systematic elaboration on these findings is absent. This article mainly recapitulates developments toward how corneal cells sense and respond to engineering surface topography of substrates and its mechanism. Furthermore, applications of topographic strategy in corneal tissue engineering are also reviewed. This systematic summary may help to elucidate understand the fundamentals of cell-material interactions and shed light on corneal tissue engineering and regeneration medicine.
Fig. 2.
Schematic images of human cornea structure. The corneal consists of five layers. Reprinted with permission from Mary Ann Liebert, Inc. Tissue Engineering Part B: Reviews: copyright (2003).
2. Effect of substratum topography on corneal cells
2.1. Corneal epithelial cells
Corneal epithelium, along with the overlapped tear film that smooths the anterior epithelial surface, provides the eye with the first protection and 70% of the total refractive power. The stratified, non-keratinized squamous epithelium consists of 4–6 layers of corneal epithelial cells (CEpCs) derived from corneal limbal epithelial cells, sourcing from limbal epithelial crypts [29,30]. Self-renewal of corneal epithelium and healing of minor wounds in the corneal epithelium are due to these limbal stem cells as well. It should be noted that the epithelial cell layers do not directly contact with Bowman's layer, but rest on a basement membrane that adheres tightly to Bowman's layer. Previous studies have shown that there are many micro- or nanoscale surface textures on the basement membrane that are involved in many activities of the epithelial cells [31,32]. In corneal epithelium tissue engineering, the design of biomimetic patterned surfaces can help to reconstruct the microenvironment in vivo and benefit corneal tissue regeneration.
Many previous studies have proven that a notably wide range of behaviors of CEpCs are profoundly influenced by engineering topographic features. A list of the studies related to the behaviors of CEpCs on substrates with engineering micro- or nanotopography is presented in Table 1. Similar to other types of cells, when cultured on grooves, CEpCs tend to be aligned and elongated along with the direction of the groove axis in most of cases (Fig. 3). Generally, the percentage of aligned cells is largely influenced by the augmentation of the depth of grooves instead of the width [34,35]. However, the culture environment of cells may affect cell-topography interactions and may alter signal transduction pathways. For example, Teixeira et al. showed that when cultured in Epilife® medium, the CEpCs were mainly aligned parallel to micron-sized grooves but perpendicular to the nanogrooves (Fig. 4), and the ratio of cell perpendicular to the grooves increased with the decease of the pitch from 4000 to 400 nm [36]. The alignment and elongation of cells is often accompanied by morphological changes of some ultrastructures in the cells. Karuri et al. found that the size of the grooves could regulate the size of cytoskeleton fibers for cells aligned along with grooves [37]. Additionally, the width and pitch of the focal adhesion were also found to be significantly influenced by underlying topography [34]. Except for dimensions, the orientations of actin microfilaments and focal adhesions might also become to be parallel or oblique to the grooves with the change of the groove pitch [36]. Meanwhile, surface topography has the ability to alter nuclear alignment as well. According to Raghunathan et al., nuclei were preferentially aligned perpendicular to grooves with smaller pitches, except those on 400 nm, and increasingly parallel to the grooves with increasing pitch [38]. Some nuclei were perpendicular to the grooves while their cell bodies aligned along the grooves, indicating that the orientation and shape of the cell nucleus are mediated by the topographic features but not the cell body. These misalignment of the cell body and nuclei may alternate gene and protein expression, and further work needs to be performed, since variation of nuclei shape has been shown to influence gene and protein expression.
Table 1.
Summary of studies on the effect of topography on corneal epithelial cells.
| Feature type | cell type | feature dimensions | Pitch | substrate material | effects on cell behaviors |
|---|---|---|---|---|---|
| wave ordered structure, grooves | primary human CEnCs | 30, 45 and 70 nm ridge width, 200 nm in depth | 60, 90, 140 nm | silicon | cell responding to pitch of 60 nm in a serum-free basal medium and to pitch of 90 nm in epithelial medium, increased percentage of aligned cells with combination of wave ordered structure and grooves with 4000 nm width [33] |
| grooves | human CEnCs | 70, 250, 400, 650, 850, 1900 nm in width, 150 and 600 nm in depth | 400, 800, 1200, 1600, 2000, and 4000 nm | silicon oxide coated silicon | alignment more affected by depth than pitch, larger percentage of aligned cells with serum and increasing depth, alignment of cytoskeletal organization, decreasing width of focal adhesions with decreasing ridge width [34] |
| primary human CEnCs | 75, 150, 265, 400, 550, 700 and 800 nm in depth | 400, 800, 1200, 1600, 2000, and 4000 nm | silicon oxide coated silicon | larger percentage of aligned cells with increasing depth in medium containing serum, parallel orientation to micron-sized grooves but perpendicular orientation to nanogrooves in Epilife® medium [35] | |
| primary human CEnCs |
600 nm in depth | 400, 800, 1200, 1600, 2000, and 4000 nm | silicon oxide coated silicon | parallel orientation to micron-sized grooves but perpendicular orientation to nanogrooves in Epilife® medium with a transition zone, focal adhesions parallel to the grooves in the transition zone but oblique to the grooves on 400 and 4000 nm [36] | |
| SV40 human CEnCs | 400 ± 150 nm in depth | 400, 800, 1,200, 1,600, 2,000, 4,000 nm pitch | silicon | larger cytoskeleton bundles on the top of the ridges parallel to the grooves, while smaller cytoskeleton fibers spanning the grooves and its width and thickness depending on the size of the grooves [37] | |
| primary human CEnCs | 300 nm in depth | 400, 800, 1,200, 1,600, 2,000, 4,000 nm pitch | NOA81 | more cell nuclie aligned perpendicular to the grooves with smaller pitches expect those on 400 nm and increasing number of nuclei parallel to the grooves with increasing pitch in both of medium, different orientation of cell body and its nucleus [38] | |
| SV40 human CEnCs, primary human CEnCs | 176, 344, 511, 664, 863, 1835 nm in width, 400 ± 150 nm in depth | 400, 800, 1200, 1600, 2000, and 4000 nm | silicon oxide coated silicon | enhanced adhesion with smaller pitch [39] | |
| SV40 human CEnCs, primary human CEnCs | 300 nm in depth | 400, 800, 1200, 1600, 2000, and 4000 nm | NOA61 polyurethane | proliferation of primary corneal and SV40-HCEC inhibited with decreasing groove depth [40] | |
| SV40 human CEnCs | N.A. | 400, 800, 1200, 1600, 2000, and 4000 nm | NOA61 polyurethane | migration of single cell along grooves of all pitches, migration of cell colonies out from initially circular zones predominantly along grooves and ridges [41] |
|
| intact bovine epithelial tissue sheets, a confluent culture of epithelial cells | 1 or 5 μm in depth, 1, 2, 5, or 10 μm width |
2,4,10,20 μm | polystyrene | migration of epithelial tissuue and cells enhanced along the grooves and inhibited across the grooves, migration more affected by depth than pitch, migration inhibited with increasing groove depth [42] | |
| fresh bovine corneas | 1 and 5 μm in depth, 1, 2, 5, and 10 μm width | 2,4,10,20 μm | Silicone rubber | healing process of epithelial wound not promoted on 1 μm-deep grooves and impeded on 5 μm deep grooves [43] | |
| Pillars | immortalized human corneal keratinocytes | 15 μm in height, 5 μm in width, | 5, 7, 9, and 11 μm in spacing | Fibronectin- coated PDMS | attenuated proliferation, lower expression of early differentiation marker and higher expression of late and terminal differentiation marker at large pillar patterns [44] |
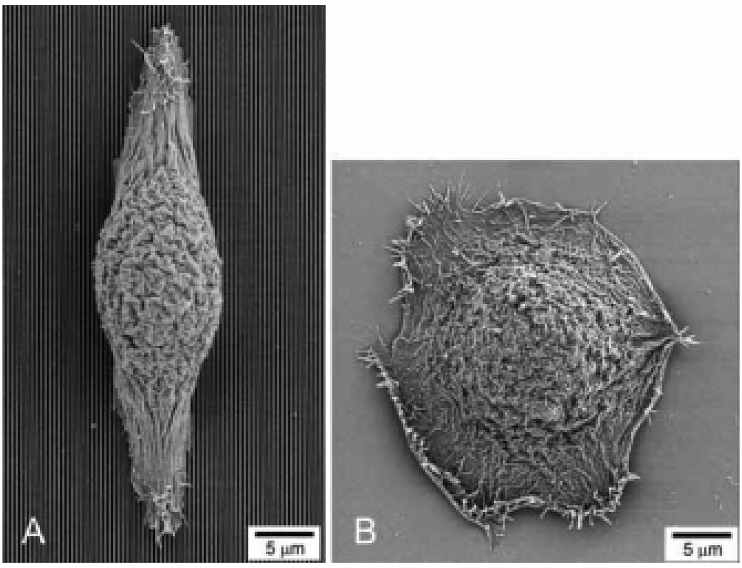
Fig. 3.
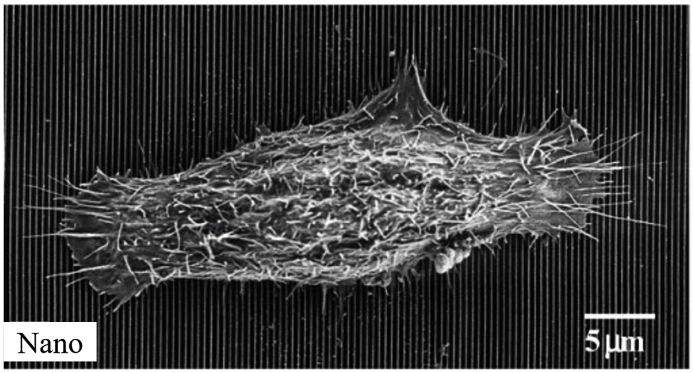
SEM images of human corneal epithelial cells cultured on a patterned silicon oxide substrate (A) and a smooth silicon oxide substrate (B). Reprinted with permission from Elsevier Publishers Ltd: Biomaterials, copyright (2003).
Fig. 4.
Perpendicularly aligned cells on nanopatterned substrates (70 nm wide ridges on a 400 nm pitch). Reprinted with permission from Elsevier Publishers Ltd: Biomaterials, copyright (2006).
For cell adhesion, Karuri et al. found that the silicon substrate with grooves of 400–4000 nm pitch could enhance the tolerance and number of attached human CEpCs under a well-defined fluid shear, and the smaller pitch stimulates tighter adhesion compared to the larger pitch [39]. These observations might be associated with β1-integrins secreted by cells, which preferentially gather at the poles of aligned spindle-shaped cells [37]. The proliferation of CEpCs is also affected by surface topography. In studies of Liliensiek, both SV40-transformed human CEpCs and primary human CEpCs showed decreased proliferation rate for substrates with smaller grooves or groove pitch [40]. Chemically-grooved patterned surfaces also have the abilities to influence adhesion and the proliferation of CEpC. Islam et al. fabricated fibronectin groove patterns on PDMS via microcontact printing on PDMS substrates. The results showed that fibronectin stripes enhanced cell attachment and induced higher mitotic rates of human CEpCs [45].
After injury, the most critical activities of corneal epithelium are the migration and spreading of the cell surrounding the wound and then the proliferation of the cells in the neighboring area [[46], [47], [48]]. Therefore, expediting the migration of the CEpCs may facilitate the healing of the corneal epithelium. A previous study by Diehl et al. showed that individual human CEpCs always migrated only along grooves on patterned substrates in contrast to the random migration on smooth substrates. In addition, the circular colonies of cells extended mainly in the direction of the grooves while extended equally in all directions on smooth surfaces. These conclusions demonstrated that the surface microstructure could regulate the directed migration and migratory direction [41], which corresponded to the conclusion of Evans and his groups [42]. Furthermore, Evans et al. studied the migration of intact corneal epithelial tissue excised from the cornea on microgroove polystyrene substrates with groove/ridge widths of 1, 2, 5 or 10 μm and depths of 1 or 5 μm. These results showed that the outward extension of the epithelial tissue followed the same rules as the corneal tissue formed from a single cell [42]. In addition, the migration rate of epithelial tissue was affected by the size of groove depth. Evans implanted the microgroove silicones into bovine corneas and found that the healing speeds of epithelium on 1-μm-deep grooves were comparable to that on smooth substrate, while the healing of epithelial wounds was inhibited on 5-μm-deep grooves. The results indicate that linear parallel grooves might not be the optimal choice to stimulate healing of epithelial wounds [43]. According to Walczysko, it is an intracellular cAMP pathway that transmits the topographic cues to cells on grooves and accelerates cell migration. However, further research is warranted [49].
In previous studies, the most popular topography applied on epithelial cells is microgrooves or nanogrooves. However, there are a few studies investigating CEpCs on pillar or pit patterns. For example, Eberwein et al. studied the effect of micropillars on corneal keratinocytes [44]. When the space of the pillar increased, the proliferation and metabolic activity appeared to be attenuated, but the differentiation of cells was more prominent. Karuri et al. cultured human CEpCs on substrates with micro- and nanoscale pits and found that cells cultured on nanoscale holes possessed a stellate morphology and higher proliferation rate and adhered more tightly compared to those on microscale features or planar substrates [50]. To date, there is not a universal agreement on the response of CEpCs to micro or nanoscale pillars or pits. However, it is clear that pillar and pit patterns have applications in corneal tissue engineering.
2.2. Corneal stromal cells
The corneal stroma is responsible for approximately 80% of the corneal thickness [51]. The corneal stroma is mainly composed of keratocytes and the ECM they secrete, including collagen and various proteoglycans. Numerous bundles of collagen fibers, named collagen fibrils, are arranged in parallel into collagen lamella. Approximately 200–250 distinct lamellae, together with the proteoglycans and keratocytes sandwiched between lamellae, make up the corneal stroma with fibrils in neighboring lamella crossed at the right angle [[52], [53], [54]]. The proteoglycans are involved in maintaining the space between the fibrils and the hydration of the cornea [55]. As the major cell type of the corneal stroma, keratocytes also play an important role in maintaining the stromal structure. This highly organized structure contributes to the formation of a transparent and mechanically strong cornea.
Corneal stromal cells can change their state activity with the surrounding environment. When in healthy conditions, corneal stroma is populated by quiescent keratocyte. However, after injury, the keratocyte around the wound will initiate apoptosis, and many keratocytes will propagate and transit to an activated phenotype, known as fibroblasts. Some fibroblasts will further express alpha smooth muscle actin (α-SMA) and become myofibroblast under the impact of TGF-β and other soluble factors [56,57]. These opaque cells produce enormous quantities of disorganized ECM in the anterior stroma and finally form a haze in the stroma, resulting loss of corneal transparency [58].
Generally, Cells cultured on the groove pattern and ECM secreted by them usually tend to be parallel to the axis of the grooves, which resemble the architecture of native corneal stroma. Therefore, many researchers investigated the responses of the three types of stromal cells to groove patterns (Table 2).
Table 2.
Summary of studies on the effect of topography on the cell behavior of corneal stroma.
| feature type | cell type | feature dimensions | Pitch | substrate material | effects on cell behaviors |
|---|---|---|---|---|---|
| grooves | human corneal keratocytes | 600 nm in depth | 400, 800, 1200, 1600, 2000, and 4000 nm | silicon oxide coated silicon | more aligned cell on pitches with 800 nm or larger, alignment of focal adhesions and associated stress fibers, fewer stress fibers and focal adhesions on nanopatterns [59] |
| primary rabbit corneal keratocytes, fibroblasts, and myofibroblasts | 300 nm in depth | 400,800,1200,1600,2000,4000 nm | polyurethane | Keratocytes, fibroblasts, and myofibroblasts aligned and elongating to pitches larger than 1000 nm, immobile keratocytes, both fibroblasts and myofibroblasts migrating parallel to grooves with pitches larger than 1000 nm [60] | |
| primary human corneal epithelial cells |
75, 150, 265, 400, 550, 700 and 800 nm depth | 400, 800, 1200, 1600, 2000, and 4000 nm | silicon oxide coated silicon | increasing cell elongated and aligned with increasing groove depth, similar alignment level [35] | |
| human keratocytes | 4.0 μm in depth, 12.5 μm in width | 25 μm | Polycaprolactone | an angle between the second cell layer and the initial layer, upregulated or downregulated expression of mRNA involved in integrins and matrix metalloproteinases [61] | |
| primary human keratocytes | 350 nm in depth, 350, 500, 1000, 2000 and 10000 nm in width | 700, 1000 and 20000 nm in pitch | PDMS, Chitosan | alignment and elongation in grating direction of cells and collagen I, reduced proliferation with decreased width, increased ALDH3 expression on nanogratings [62] | |
| corneal fibroblast cells | 300 nm in depth | 400, 1400, and 4000 nm pitch | Collagen-coated silicon surface | reduced levels of αSMA expression on patterned substrate and highest level on pitch of 1400 nm, enhanced Smad7 mRNA expression [63] |
As expected, the orientation of stromal cells is regulated by the topographic cues. Teixeira et al. studied responses of the human keratocytes to grooved silicon substrate with pitch varying from 400 to 4000 nm [59]. Approximately 70% of human keratocytes were aligned and elongated along the grooves with pitches larger than 800 nm. Meanwhile, the focal adhesions and associated stress fibers in keratocytes were also aligned along the grooves. Similarly, the fibroblasts and myofibroblasts also intended to be parallel to grooves with an acuity of about 1000 nm [60]. Considering the length scale of the grooves, it was reported that increasing groove depth would elicit alignment and elongation of a larger population of corneal fibroblasts, while variations in groove pitch have no significant influence on the alignment and elongation of cells [35]. However, when more than one cell layer forms on the patterned substrate, the orientation of cells of the second layer displays an angle shift from the bottom layer, [61,64], which was similar to the native stromal structure where fibrils in neighboring lamella are crossed at right angle.
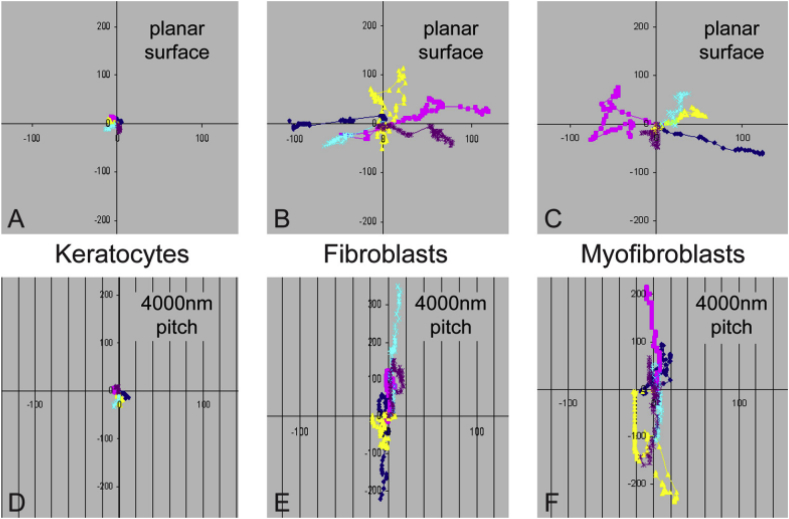
For cell migration on patterned substrates, keratocytes almost remain immobile due to their instinct, whereas trajectories of the fibroblasts and myofibroblasts are often limited to parallel to the grooves (Fig. 5) [60]. The effect of surface pattern on proliferation of the stromal cells was also interrogated. According to Liliensiek et al., corneal fibroblasts displayed a slower proliferation on nanoscale grooves compared to microscale grooves and smooth substrate [40]. The inhibited proliferation rate of primary human keratocytes on grooves with decreased width was also observed by Koo et al., [62], suggesting that overly narrow grooves might impede the proliferation of keratocytes.
Fig. 5.
Migration pattern of primary rabbit corneal keratocytes, fibroblasts, and myofibroblasts cultured on collagen-coated planar and topographically patterned surfaces. Each color represents the movement of a single cell. Reprinted with permission from Association for Research in Vision and Ophthalmology Publishers Ltd: Investigative ophthalmology & visual science, copyright (2010).
The organized alignment of the ECM in stroma is known to be important for corneal transparency. Therefore, if the orientation of collagen could be guided to imitate the structure of native stroma in corneal tissue engineering, the formation of misty cornea in the wound healing process might be inhibited effectively. Stephanie Koo found that collagen I produced by the keratocytes cultured on grooves was aligned with the grooves while randomly distributed on the smooth control [62]. Gene expression of keratocytes has also been shown to be mediated by surface topography. The aldehyde-3-dehydrogenase, a prominent crystalline protein, exhibited higher expression on narrower grooves, while collagen type I, III, V and keratocan were expressed independently on the topographic cues. Additionally, in a study by Then et al., the expression level of genes involved in the production of integrins and matrix metalloproteinases was also enhanced significantly in the presence of the grooves [61]. Hence, both the alignment and expression of ECM can be mediated by the topographic cues.
Furthermore, the differentiation of keratocytes to myofibroblasts is regulated by topographic cues. Keratocytes tend to differentiate into myofibroblasts under the induction of TGF-β in vitro. Myrna et al. found that the transformation to myofibroblasts can be effectively inhibited when keratocytes were cultured on patterned grooves with 1400-nm-wide pitch [63]. Therefore, the surface topography has the potential to inhibit of the TGF-β-induced myofibroblast differentiation and help to impeding the development of fibrosis and corneal haze in the process of wound healing.
2.3. Corneal endothelial cells
Underlying corneal stroma is Descemet's membrane, an acellular membrane rich in collagen and glycoproteins secreted by corneal endothelial cells (CEnCs) and functioning as an attachment site for corneal endothelium. The surface of Descemet's membrane has a similar appearance to the corneal basement membrane and consists of a meshwork of fibers and pores [31]. The innermost corneal endothelium is a single layer of endothelial cells. Corneal endothelium is the crucial tissue responsible for keeping stroma relatively deturgesced, which is important to maintain the clarity of cornea [65]. This function is ensured by a pump-leak progress of the endothelium, where water is pumped into stroma at the same rate that it leaks out from stroma, which depends on the osmotic gradient generated by the action of sodium/potassium (Na+/K+) ATPase and sodium/bicarbonate (Na+/HCO3−) cotransporter pumps [66]. CEnCs in adult cornea have little capability to proliferate [67]. As a result, the number of CEnCs decreases with aging and other diseases or damages, such as wounding and trauma, often leading to irreversible corneal endothelial dysfunction.
Research focusing on the interaction between surface topography and CEnCs is relatively less compared to other corneal cells, probably due to CEnCs’ limited proliferative capability and difficulty in maintaining its characteristics in vitro. In addition, in contrast to other CEpCs and keratocytes, the pillar or pit patterns are the most commonly used topographic feature. Muhammada et al. found that both the bovine and human CEnCs on micro- and nanosized pillars and wells could form a confluent endothelial monolayer and exhibited polygonal geometries similar to corneal cells in vivo [[68], [69], [70]]. In addition, a lower coefficient of variation of area that was comparable to the healthy endothelium was found on nanopillars, and the expressions of Na+/K+-ATPase and cell-cell tight junction protein zona occludens-1 were both largely upregulated by the patterns. More importantly, the expansion of human CEnCs could be significantly enhanced by the surface patterns. These results indicated that the formation and function of endothelium in vitro could be significantly improved with patterned substrate, which would benefit therapy of endothelium dysfunction and corneal tissue engineering.
2.4. Influence of topographic cues integrated with other cues
Residing in a complex environment, cells are exposed to a milieu of abundant biophysical and biochemical signals that are simultaneously involved in a diverse set of cellular processes. It is difficult to decouple those signals, which makes it imperative to study the synthetic effect of two or more stimuli on cells. biochemical and electrical cues are two stimuli that are often used to study the effects of surface topography on corneal cells. Some studies incorporated the RGD peptide motif onto microgrooved surfaces to investigate their effect on CEpCs [[71], [72], [73]]. The presence of RGD peptide markedly increased the number of aligned cells on the patterned surface as opposed to the preferential perpendicular alignment on the substrate without RGD peptide. These results demonstrate that the surface chemical composition significantly affects how CEpCs respond to substrate topography. In addition, fibronectin and other ECM proteins, such as collagen, have also been integrated into surface topography, and the responses of corneal cells to the substrate topography are differentially altered, as well [74]. Aside from peptide motifs and ECM proteins, soluble factors were sometimes directly added to the culture medium to observe their regulation to cells. According to Walboomers et al., [75], the TGF-β 1, -2, and -3 were able to weaken the effect of microgrooves on the epithelial tissue and impede the extension of the epithelial tissue to varying degrees, which is contrary to the hypothesis that TGF-β 1 could contribute to enhancing the growth of epithelial cells on substrate materials.
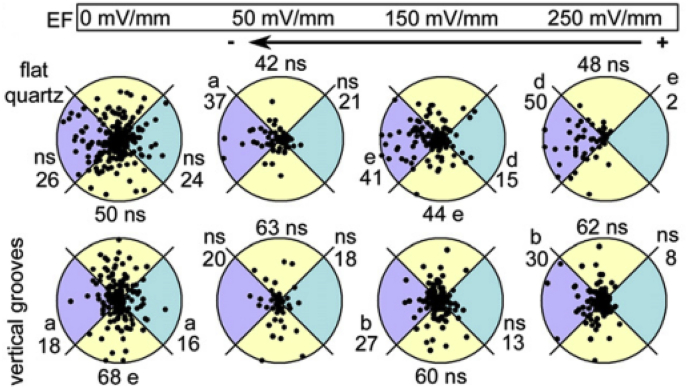
The intrinsic electric field was also found to participate in controlling the rate and direction of migration and division of CEpCs in the cornea wound healing process in vivo [[76], [77], [78]]. Therefore, Rajnicek et al. studied the coeffects of electric field and patterned substrate on the migration direction of CEpCs and found that the migration direction of CEpCs would be changed according to the superimposed direction of the electric field and nanogrooves [79,80]. When the electric field and nanogrooves were superimposed in parallel, cathodal electrotaxis along the nanogrooves of CEpCs was enhanced. However, when the electric field and nanogrooves were superimposed orthogonally, cells were recruited from the guidance of nanogrooves to electrotaxis (Fig. 6). These cooperative effects in controlling cells may occur in vivo incessantly and ubiquitously, and it is vital to explore the effects for cell biology and corneal tissue engineering.
Fig. 6.
Polar plots for cells on flat or vertical grooves. Cells were exposed to an EF for 1 h. Radius 50 μm/h. The numbers indicate the % of cells in each segment. ns = not significant; (a) p < 0.05; (b) p < 0.02; (d) p < 0.002; (e) p < 0.001. Reprinted with permission from Elsevier Publishers Ltd: Development Biology, copyright (2016).
3. Application of topography cues in corneal tissue engineering
Previous studies have revealed that surface topography could modulate corneal cell behaviors, but most early studies were conducted on materials that are not appreciate for implantation, such as silicon and PDMS. Recent studies have begun to investigate the application of topographic cues in corneal tissue engineering and to develop corneal equivalents with patterned surfaces.
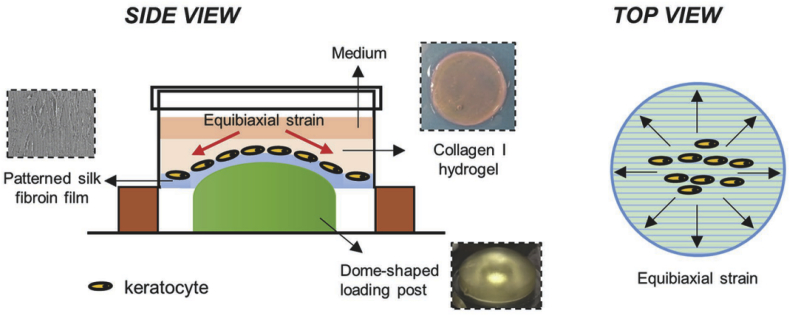
Of natural polymers, collagen and silk fibroin are the most promising and commonly used for corneal tissue engineering [81]. These polymers have different advantages. As the main component of cornea, collagen has well-documented physiochemical and immunological properties comparable with nature tissue [[82], [83], [84], [85], [86]]. Silk fibroin is transparent, mechanically strong and biocompatible [[87], [88], [89], [90], [91]]. When seeded on collagen or silk films with groove patterns, the corneal epithelial cells and stromal cells, as well as the ECM, both tend to elongate with the axis of the grooves [[92], [93], [94]]. For corneal epithelial tissue engineering, Lawrence et al. found that the corneal limbal epithelial cell attachment on the microgooved silk film scaffold displayed a 36–54% increase on average, which corresponded to a more than 2-fold increase in focal adhesion localization when compared to the controls. The migration rate and direction of cells were also directed by the groove pattern. In addition, the cells on microgrooves showed lower expression levels of putative keratocyte differentiation markers and higher levels of putative limbal stem cell markers, suggesting that cells could be in a less differentiated state with the microgrooves [[94], [95], [96]]. This discovery indicated that the patterned silk scaffold has the potential for corneal epithelialization [97]. For corneal reconstruction, scaffolds should have good transparency and machinal properties to meet the demand of corneal transplantation. According to Hasirci et al., the directional keratocytes and secreted ECM on the microgrooved collagen films contribute to transparency, maintaining of collagen films and inhibiting the strength reduction resulting from collagen degradation [92,98]. Therefore, scaffolds with patterned surfaces are a very strong candidates for corneal stromal engineering. For example, Kilic et al. designed a corneal stroma substitute, which comprised a stack of four micropatterned collagen or collagen-elastin-like recombinamer blend layers. The stroma equivalent has a similar structure and transparency with the natural tissue and supports cell attachment and proliferation [99]. Patterned silk films were also used for stroma reconstruction in vitro. Kaplan and his group constructed several types of stroma models using grooved silk films to mimic the natural organization, and they were proven to have potential for corneal tissue engineering [[100], [101], [102], [103]]. In addition, topographic cues could also incorporate with other physical cues to contribute to corneal tissue engineering. For example, Zhang et al. developed a highly biomimetic corneal model combined with topographic and mechanical cues (Fig. 7), which reproduced the native environment and structure [104]. In this model, patterned silk fibroin films could coax keratocytes into forming organized structures and provide ECM with a 3% dome-shaped strain resembling with the native cornea, supporting higher expression of keratocyte and ECM markers.
Fig. 7.
Schematic for the biomimetic 3D corneal model of Zhang et al. using patterned (600 grooves mm−1) silk fibroin films, collagen I hydrogel, and 3% dome-shaped strain. Reprinted with permission from John Wiley and Sons Publishers Ltd: Advanced Healthcare Materials, copyright (2016).
Aside from natural polymers, some promising synthetic polymers were also used for tissue engineering scaffolds combined with patterned surfaces which had a significant influence on corneal cells. Zorlutuna and his group produced patterned films of P(L/DL)LA-PHBV blends used for tissue engineering of the epithelial layers of the cornea [105,106]. As the results showed, the patterned corneal tissue engineering scaffold allowed the alignment of the CEpCs. In addition, the CEpCs formed epithelial multilayers with a higher proliferation rate compared to the flat control. The patterned scaffold also showed an increase in strength, suggesting that the scaffold can be considered as a choice for corneal tissue engineering in future studies. Yanez-Soto et al. also developed a topographically patterned poly(ethylene glycol) diacrylate hydrogel scaffold, and the scaffold showed its ability to accelerate epithelial coverage and formation of epithelium [107]. These studies demonstrate the potential application of topographic cues in improving corneal epithelium wound healing once again.
Cell sheet transplantation is an engineering tissue approach in which a cell sheet is constructed on a carrier in vitro and then transplanted into the body. Engineering topography was also incorporated into the development of the carrier used for cell sheet transplantation. For example, Nara et al. developed a chemically grooved thermoresponsive gelatin–poly(N-isopropylacrylamide) (gelatin–PolyNIPA) substrate via direct-write assembly, which could provide contact guidance for keratocytes. The metabolic activity and vinculin expression of keratocytes on the patterned matrix were also enhanced, indicating the improvement of cytocompability with micropatterning [108]. Apart from corneal stroma regeneration, cell sheet transplantation is also one of the main tissue replacement strategies for endothelium regeneration. As mentioned earlier, substrates with patterned surfaces benefited from the formation of the endothelial layer. Rizwan et al. innovated an endothelium carrier with nanopillars using sequentially crosslinked bioactive gelatin methacrylate [109]. In their study, after human CEnCs were seeded, the patterned carrier showed the ability to improve cell density and zona-occludin-1 expression and to homogenize cell size in vitro (Fig. 8). The implantability testing demonstrated that the films have sufficient mechanical strength to bear the implantation process and that the human CEnC monolayer tightly adhered to the films. According to Teo et al., micropillars could even induce a higher density of microvilli on the CEnC layer, which was similar to native corneal endothelium [70]. Therefore, the carrier with a patterned surface could serve as a good carrier for corneal endothelial tissue engineering.
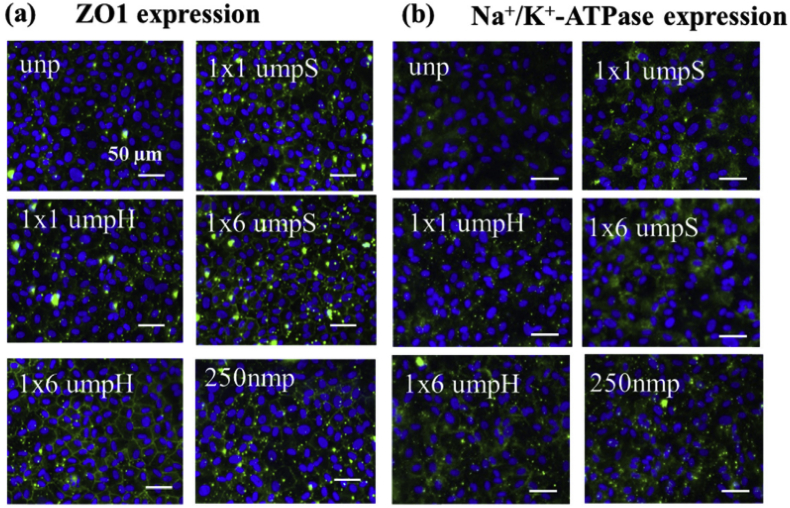
Fig. 8.
Effect of topographic cues on (a) the expression of tight junctional protein Zona Occludens 1 (b) The expression of Na+/K+-ATPase pumps of primary human corneal endothelial cells. Reprinted with permission from Elsevier Publishers Ltd: Biomaterials, copyright (2017).
Some reports proposed a new approach named self-assembly for corneal stroma reconstruction, the general principle of which is to induce corneal stroma cells to secrete ECM to form stroma tissue in vitro [110]. Under the contact guidance of grooves, the ECM could form a parallel arrangement. According to Guillemette, with a grooved (styrene)–(ethylene/butylene)–(styrene) substrate, the self-assembled stroma has a three-dimensional architecture with orthogonally-oriented collagen fibrils, resembling with native tissues. The transparency of the formed tissue was also notably improved (Fig. 9) [64]. Similar multilayered structures were also obtained in the self-assembled stroma tissue on a patterned silk film by Wu et al. [111] Funderburgh and his group transplanted this kind of self-assembled stroma formed on a patterned surface into mouse corneal stromal pockets and found that the engineered corneal stromal tissues became transparent, and parallel human corneal stromal matrix molecules were still expressed [112]. These results demonstrated that the surface topography could induce cells to follow a physiologically consistent orientation mimicking the structure of the native tissue in vitro and in vivo. Therefore, surface micropatterning could be a powerful tool to reconstruct corneal tissue used for treating corneal blindness.
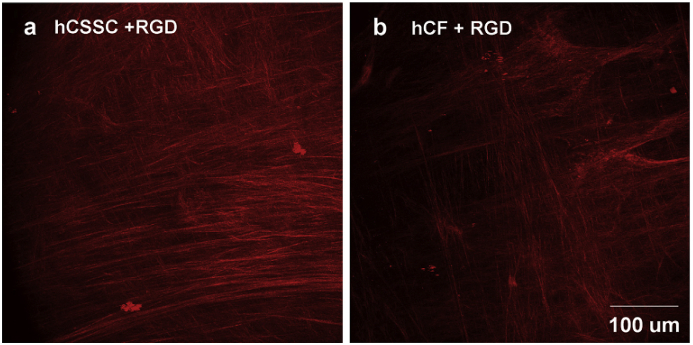
Fig. 9.
ECM with a highly compact with orthogonal orientation secreted by human corneal stromal stem cells (a) and human corneal fibroblasts (b) on the patterned silk substrate with surface-coupled RGD. Reprinted with permission from Elsevier Publishers Ltd: Biomaterials, copyright (2014).
4. Conclusion and future development
Surface topography displays a powerful ability to control and regulate the behaviors of corneal cells, including morphology, migration, proliferation, differentiation and ECM secretion. Cellular responses to topographic cues may vary with the incorporation of other stimuli, such as mechanical and electrical stimuli. Corneal tissue substitutes with topological decorations are benefit for corneal regeneration.
Although a large body of research has produced valuable achievement, there are still several challenges to overcome in this field. The mechanisms by which surface topography is used to control the cells are still unknown and need to be further explored and elucidated. It is also necessary to develop a theory describing the relationship between substrate topographies and cell responses. In addition, the majority of pattern features, such as grooves, are too simple to mimic the intricate topography of ECM. Substrates with more biomimetic patterned surfaces should be fabricated to reproduce the nature architecture in vivo, which requires more attempts on high-precision methods to fabricate controlled surface topography. Furthermore, tissue-engineered corneas with patterned surfaces were merely utilized in vitro experiments. It would be crucial to identify the effect of topography on the corneal healing process.
Overall, surface topography offers us a new strategy to mimic the in vivo environment for corneal cells as closely as possible and may bright us a step closer to understand the interaction of cells with microenvironments. These discoveries may facilitate the development of regenerative medicine.
Acknowledgements
The authors gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (51273072, 51603074, 31971261), the Natural Science Foundation of Guangdong Province (2017A030313294), The National Key Research and Development Program of China (2017YFC1105004), the Guangdong Scientific and Technological Project (2014B090907004, 201508020123) and Medical Scientific Research Foundation of Guangdong Province (A2018169).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
HuiChang Gao, Email: mchcgao@scut.edu.cn.
Li Ren, Email: psliren@scut.edu.cn.
References
- 1.Yao X., Peng R., Ding J. Cell-material interactions revealed via material techniques of surface patterning. Adv. Mater. 2013;25:5257–5286. doi: 10.1002/adma.201301762. [DOI] [PubMed] [Google Scholar]
- 2.Hynes R.O. Extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu A.P., Chaudhuri O., Parekh S.H. New advances in probing cell-extracellular matrix interactions. Integr Biol. 2017;9:383–405. doi: 10.1039/c6ib00251j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kyburz K.A., Anseth K.S. Synthetic mimics of the extracellular matrix: how simple is complex enough? Ann. Biomed. Eng. 2015;43:489–500. doi: 10.1007/s10439-015-1297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito J.T., Lourenco J.D., Righetti R.F., Tiberio I., Prado C.M., Lopes F. Extracellular matrix component remodeling in respiratory diseases: what has been found in clinical and experimental studies? Cells. 2019;8:25. doi: 10.3390/cells8040342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meran L., Baulies A., Li V.S.W. Intestinal stem cell niche: the extracellular matrix and cellular components. Stem Cell. Int. 2017:11. doi: 10.1155/2017/7970385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikkhah M., Edalat F., Manoucheri S., Khademhosseini A. Engineering microscale topographies to control the cell-substrate interface. Biomaterials. 2012;33:5230–5246. doi: 10.1016/j.biomaterials.2012.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bettinger C.J., Langer R., Borenstein J.T. Engineering substrate topography at the micro- and nanoscale to control cell function. Angew Chem. Int. Ed. Engl. 2009;48:5406–5415. doi: 10.1002/anie.200805179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simitzi C., Karali K., Ranella A., Stratakis E. Controlling the outgrowth and functions of neural stem cells: the effect of surface topography. ChemPhysChem. 2018;19:1143–1163. doi: 10.1002/cphc.201701175. [DOI] [PubMed] [Google Scholar]
- 10.Brody S., Anilkumar T., Liliensiek S., Last J.A., Murphy C.J., Pandit A. Characterizing nanoscale topography of the aortic heart valve basement membrane for tissue engineering heart valve scaffold design. Tissue Eng. 2006;12:413–421. doi: 10.1089/ten.2006.12.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shirato I., Tomino Y., Koide H., Sakai T. Fine structure of the glomerular basement membrane of the rat kidney visualized by high-resolution scanning electron microscopy. Cell Tissue Res. 1991;266:1–10. doi: 10.1007/BF00678705. [DOI] [PubMed] [Google Scholar]
- 12.Abrams G.A., Goodman S.L., Nealey P.F., Franco M., Murphy C.J. Nanoscale topography of the basement membrane underlying the corneal epithelium of the rhesus macaque. Cell Tissue Res. 2000;299:39–46. doi: 10.1007/s004419900074. [DOI] [PubMed] [Google Scholar]
- 13.Campbell R.D., Marcum B.A. Nematocyte migration in hydra: evidence for contact guidance in vivo. J. Cell Sci. 1980;41:33–51. doi: 10.1242/jcs.41.1.33. [DOI] [PubMed] [Google Scholar]
- 14.Gui N., Xu W., Myers D.E., Shukla R., Tang H.P., Qian M. The effect of ordered and partially ordered surface topography on bone cell responses: a review. Biomater Sci. 2018;6:250–264. doi: 10.1039/c7bm01016h. [DOI] [PubMed] [Google Scholar]
- 15.Harrison R.G. The cultivation of tissues in extraneous media as a method of morpho-genetic study. Anat. Rec. 1912;6:181–193. [Google Scholar]
- 16.Weiss P. The problem of specificity in growth and development. Yale J. Biol. Med. 1947;19:235–278. [PMC free article] [PubMed] [Google Scholar]
- 17.Charest J.L., Garcia A.J., King W.P. Myoblast alignment and differentiation on cell culture substrates with microscale topography and model chemistries. Biomaterials. 2007;28:2202–2210. doi: 10.1016/j.biomaterials.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 18.Leclech C., Renner M., Villard C., Metin C. Topographical cues control the morphology and dynamics of migrating cortical interneurons. Biomaterials. 2019;214:14. doi: 10.1016/j.biomaterials.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Zijl S., Vasilevich A.S., Viswanathan P., Helling A.L., Beijer N.R.M., Walko G. Micro-scaled topographies direct differentiation of human epidermal stem cells. Acta Biomater. 2019;84:133–145. doi: 10.1016/j.actbio.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Q.L., Elkhooly T.A., Liu X.J., Zhang R.R., Yang X., Shen Z.J. Effects of hierarchical micro/nano-topographies on the morphology, proliferation and differentiation of osteoblast-like cells. Colloids Surfaces B Biointerfaces. 2016;145:37–45. doi: 10.1016/j.colsurfb.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 21.Li M.J., Fu X.L., Gao H.C., Ji Y.R., Li J., Wang Y.J. Regulation of an osteon-like concentric microgrooved surface on osteogenesis and osteoclastogenesis. Biomaterials. 2019;216:11. doi: 10.1016/j.biomaterials.2019.119269. [DOI] [PubMed] [Google Scholar]
- 22.Wise S.G., Byrom M.J., Waterhouse A., Bannon P.G., Ng M.K.C., Weiss A.S. A multilayered synthetic human elastin/polycaprolactone hybrid vascular graft with tailored mechanical properties. Acta Biomater. 2011;7:295–303. doi: 10.1016/j.actbio.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Yim E.K.F., Pang S.W., Leong K.W. Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp. Cell Res. 2007;313:1820–1829. doi: 10.1016/j.yexcr.2007.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abadi P., Garbern J.C., Behzadi S., Hill M.J., Tresback J.S., Heydari T. Engineering of mature human induced pluripotent stem cell-derived cardiomyocytes using substrates with multiscale topography. Adv. Funct. Mater. 2018;28:11. [Google Scholar]
- 25.Oliva M.S., Schottman T., Gulati M. Turning the tide of corneal blindness. Indian J. Ophthalmol. 2012;60:423–427. doi: 10.4103/0301-4738.100540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burton M.J. Corneal blindness: prevention, treatment and rehabilitation. Community Eye Health. 2009;22:33. [PMC free article] [PubMed] [Google Scholar]
- 27.Garg P., Krishna P.V., Stratis A., Gopinathan U. The value of corneal transplantation in reducing blindness. Eye. 2005;19:1106–1114. doi: 10.1038/sj.eye.6701968. [DOI] [PubMed] [Google Scholar]
- 28.Ghezzi C.E., Rnjak-Kovacina J., Kaplan D.L. Corneal tissue engineering: recent advances and future perspectives. Tissue Eng. B Rev. 2015;21:278–287. doi: 10.1089/ten.teb.2014.0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dua H.S., Azuara-Blanco A. Limbal stem cells of the corneal epithelium. Surv. Ophthalmol. 2000;44:415–425. doi: 10.1016/s0039-6257(00)00109-0. [DOI] [PubMed] [Google Scholar]
- 30.Dua H.S., Shanmuganathan V.A., Powell-Richards A.O., Tighe P.J., Joseph A. Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. Br. J. Ophthalmol. 2005;89:529–532. doi: 10.1136/bjo.2004.049742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abrams G.A., Schaus S.S., Goodman S.L., Nealey P.F., Murphy C.J. Nanoscale topography of the corneal epithelial basement membrane and descemet's membrane of the human. Cornea. 2000;19:57–64. doi: 10.1097/00003226-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z.G., Huang A.J., Pflugfelder S.C. Evaluation of corneal thickness and topography in normal eyes using the orbscan corneal topography system. Br. J. Ophthalmol. 1999;83:774–778. doi: 10.1136/bjo.83.7.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tocce E.J., Smirnov V.K., Kibalov D.S., Liliensiek S.J., Murphy C.J., Nealey P.F. The ability of corneal epithelial cells to recognize high aspect ratio nanostructures. Biomaterials. 2010;31:4064–4072. doi: 10.1016/j.biomaterials.2010.01.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teixeira A.I., Abrams G.A., Bertics P.J., Murphy C.J., Nealey P.F. Epithelial contact guidance on well-defined micro- and nanostructured substrates. J. Cell Sci. 2003;116:1881–1892. doi: 10.1242/jcs.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fraser S.A., Ting Y.H., Mallon K.S., Wendt A.E., Murphy C.J., Nealey P.F. Sub-micron and nanoscale feature depth modulates alignment of stromal fibroblasts and corneal epithelial cells in serum‐rich and serum‐free media. J. Biomed. Mater. Res. A. 2008;86A:725–735. doi: 10.1002/jbm.a.31519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teixeira A.I., McKie G.A., Foley J.D., Berticsc P.J., Nealey P.F., Murphy C.J. The effect of environmental factors on the response of human corneal epithelial cells to nanoscale substrate topography. Biomaterials. 2006;27:3945–3954. doi: 10.1016/j.biomaterials.2006.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karuri N.W., Nealey P.F., Murphy C.J., Albrecht R.M. Structural organization of the cytoskeleton in sv40 human corneal epithelial cells cultured on nano- and microscale grooves. Scanning. 2008;30:405–413. doi: 10.1002/sca.20123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raghunathan V.K., McKee C.T., Tocce E.J., Nealey P.F., Russell P., Murphy C.J. Nuclear and cellular alignment of primary corneal epithelial cells on topography. J. Biomed. Mater. Res. A. 2013;101A:1069–1079. doi: 10.1002/jbm.a.34417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karuri N.W., Liliensiek S., Teixeira A.I., Abrams G., Campbell S., Nealey P.F. Biological length scale topography enhances cell-substratum adhesion of human corneal epithelial cells. J. Cell Sci. 2004;117:3153–3164. doi: 10.1242/jcs.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liliensiek S.J., Campbell S., Nealey P.F., Murphy C.J. The scale of substratum topographic features modulates proliferation of corneal epithelial cells and corneal fibroblasts. J. Biomed. Mater. Res. A. 2006;79A:185–192. doi: 10.1002/jbm.a.30744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diehl K.A., Foley J.D., Nealey P.F., Murphy C.J. Nanoscale topography modulates corneal epithelial cell migration. J. Biomed. Mater. Res. A. 2005;75A:603–611. doi: 10.1002/jbm.a.30467. [DOI] [PubMed] [Google Scholar]
- 42.Dalton B.A., Walboomers X.F., Dziegielewski M., Evans M.D.M., Taylor S., Jansen J.A. Modulation of epithelial tissue and cell migration by microgrooves. J. Biomed. Mater. Res. 2001;56:195–207. doi: 10.1002/1097-4636(200108)56:2<195::aid-jbm1084>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 43.Evans M.D.M., McFarland G.A., Taylor S., Walboomers X.F. The response of healing corneal epithelium to grooved polymer surfaces. Biomaterials. 2005;26:1703–1711. doi: 10.1016/j.biomaterials.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 44.Eberwein P., Steinberg T., Schulz S., Zimmermann D., Accardi R., Beck D. Expression of keratinocyte biomarkers is governed by environmental biomechanics. Eur. J. Cell Biol. 2011;90:1029–1040. doi: 10.1016/j.ejcb.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 45.Islam M.M., Cepla V., He C.L., Edin J., Rakickas T., Kobuch K. Functional fabrication of recombinant human collagen-phosphorylcholine hydrogels for regenerative medicine applications. Acta Biomater. 2015;12:70–80. doi: 10.1016/j.actbio.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 46.Lu L., Reinach P.S., Kao W.W.Y. Corneal epithelial wound healing. Exp. Biol. Med. 2001;226:653–664. doi: 10.1177/153537020222600711. [DOI] [PubMed] [Google Scholar]
- 47.Dua H.S., Gomes J.A., Singh A. Corneal epithelial wound healing. Br. J. Ophthalmol. 1994;78:401–408. doi: 10.1136/bjo.78.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki K., Saito J., Yanai R., Yamada N., Chikama T., Seki K. Cell-matrix, and cell-cell interactions during corneal epithelial wound healing. Prog. Retin. Eye Res. 2003;22:113–133. doi: 10.1016/s1350-9462(02)00042-3. [DOI] [PubMed] [Google Scholar]
- 49.Walczysko P., Rajnicek A.M., Collinson J.M. Contact-mediated control of radial migration of corneal epithelial cells. Mol. Vis. 2016;22:990–1004. [PMC free article] [PubMed] [Google Scholar]
- 50.Karuri N.W., Porri T.J., Albrecht R.M., Murphy C.J., Nealey P.F. Nano- and microscale holes modulate cell-substrate adhesion, cytoskeletal organization, and -beta 1 integrin localization in sv40 human corneal epithelial cells. IEEE Trans. NanoBioscience. 2006;5:273–280. doi: 10.1109/tnb.2006.886570. [DOI] [PubMed] [Google Scholar]
- 51.DelMonte D.W., Kim T. Anatomy and physiology of the cornea. J. Cataract Refract. Surg. 2011;37:588–598. doi: 10.1016/j.jcrs.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 52.Maurice D.M. The structure and transparency of the cornea. J. Physiol. 1957;136:263–286. doi: 10.1113/jphysiol.1957.sp005758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meek K.M., Boote C. The organization of collagen in the corneal stroma. Exp. Eye Res. 2004;78:503–512. doi: 10.1016/j.exer.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 54.Müller L.J., Pels E., Schurmans L.R.H.M., Vrensen G.F.J.M. A new three-dimensional model of the organization of proteoglycans and collagen fibrils in the human corneal stroma. Exp. Eye Res. 2004;78:493–501. doi: 10.1016/s0014-4835(03)00206-9. [DOI] [PubMed] [Google Scholar]
- 55.Holmes D.F., Gilpin C.J., Baldock C., Ziese U., Koster A.J., Kadler K.E. Corneal collagen fibril structure in three dimensions: structural insights into fibril assembly, mechanical properties, and tissue organization. Proc. Natl. Acad. Sci. U. S. A. 2001;98:7307–7312. doi: 10.1073/pnas.111150598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fini M.E. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog. Retin. Eye Res. 1999;18:529–551. doi: 10.1016/s1350-9462(98)00033-0. [DOI] [PubMed] [Google Scholar]
- 57.West-Mays J.A., Dwivedi D.J. The keratocyte: corneal stromal cell with variable repair phenotypes. Int. J. Biochem. Cell Biol. 2006;38:1625–1631. doi: 10.1016/j.biocel.2006.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Torricelli A.A.M., Santhanam A., Wu J.H., Singh V., Wilson S.E. The corneal fibrosis response to epithelial-stromal injury. Exp. Eye Res. 2016;142:110–118. doi: 10.1016/j.exer.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teixeira A.I., Nealey P.F., Murphy C.J. Responses of human keratocytes to micro- and nanostructured substrates. J. Biomed. Mater. Res. 2004;71A:369–376. doi: 10.1002/jbm.a.30089. [DOI] [PubMed] [Google Scholar]
- 60.Pot S.A., Liliensiek S.J., Myrna K.E., Bentley E., Jester J.V., Nealey P.F. Nanoscale topography-induced modulation of fundamental cell behaviors of rabbit corneal keratocytes, fibroblasts, and myofibroblasts. Investig. Ophthalmol. Vis. Sci. 2010;51:1373–1381. doi: 10.1167/iovs.09-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Then K.Y., Yang Y., Ahearne M., El Haj A.J. Effect of microtopographical cues on human keratocyte orientation and gene expression. Curr. Eye Res. 2011;36:88–93. doi: 10.3109/02713683.2010.512407. [DOI] [PubMed] [Google Scholar]
- 62.Koo S., Ahn S.J., Zhang H., Wang J.C., Yim E.K.F. Human corneal keratocyte response to micro- and nano-gratings on chitosan and pdms. Cell. Mol. Bioeng. 2011;4:399–410. [Google Scholar]
- 63.Myrna K.E., Mendonsa R., Russell P., Pot S.A., Liliensiek S.J., Jester J.V. Substratum topography modulates corneal fibroblast to myofibroblast transformation. Investig. Ophthalmol. Vis. Sci. 2012;53:811. doi: 10.1167/iovs.11-7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guillemette M.D., Cui B., Roy E., Gauvin R., Giasson C.J., Esch M.B. Surface topography induces 3d self-orientation of cells and extracellular matrix resulting in improved tissue function. Integr Biol (Camb). 2009;1:196–204. doi: 10.1039/b820208g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Geroski D.H., Matsuda M., Yee R.W., Edelhauser H.F. Pump function of the human corneal endothelium. Effects of age and cornea guttata. Ophthalmology. 1985;92:759–763. doi: 10.1016/s0161-6420(85)33973-8. [DOI] [PubMed] [Google Scholar]
- 66.Srinivas S.P. Dynamic regulation of barrier integrity of the corneal endothelium. Optom. Vis. Sci. 2010;87:E239–E254. doi: 10.1097/OPX.0b013e3181d39464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joyce N. Proliferative capacity of the corneal endothelium. Prog. Retin. Eye Res. 2003;22:359–389. doi: 10.1016/s1350-9462(02)00065-4. [DOI] [PubMed] [Google Scholar]
- 68.Muhammad R., Peh G.S.L., Adnan K., Law J.B.K., Mehta J.S., Yim E.K.F. Micro- and nano-topography to enhance proliferation and sustain functional markers of donor-derived primary human corneal endothelial cells. Acta Biomater. 2015;19:138–148. doi: 10.1016/j.actbio.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 69.Koo S., Muhammad R., Peh G.S.L., Mehta J.S., Yim E.K.F. Micro- and nanotopography with extracellular matrix coating modulate human corneal endothelial cell behavior. Acta Biomater. 2014;10:1975–1984. doi: 10.1016/j.actbio.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 70.Teo B.K.K., Goh K.J., Ng Z.J., Koo S., Yim E.K.F. Functional reconstruction of corneal endothelium using nanotopography for tissue-engineering applications. Acta Biomater. 2012;8:2941–2952. doi: 10.1016/j.actbio.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 71.Tocce E.J., Liliensiek S.J., Broderick A.H., Jiang Y., Murphy K.C., Murphy C.J. The influence of biomimetic topographical features and the extracellular matrix peptide rgd on human corneal epithelial contact guidance. Acta Biomater. 2013;9:5040–5051. doi: 10.1016/j.actbio.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilson M.J., Jiang Y., Yanez-Soto B., Liliensiek S., Murphy W.L., Nealey P.F. Arrays of topographically and peptide-functionalized hydrogels for analysis of biomimetic extracellular matrix properties. J Vac Sci Technol B Nanotechnol Microelectron. 2012;30:6F903. doi: 10.1116/1.4762842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yanez-Soto B., Liliensiek S.J., Murphy C.J., Nealey P.F. Biochemically and topographically engineered poly(ethylene glycol) diacrylate hydrogels with biomimetic characteristics as substrates for human corneal epithelial cells. J. Biomed. Mater. Res. A. 2013;101:1184–1194. doi: 10.1002/jbm.a.34412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Raghunathan V., McKee C., Cheung W., Naik R., Nealey P.F., Russell P. Influence of extracellular matrix proteins and substratum topography on corneal epithelial cell alignment and migration. Tissue Eng. A. 2013;19:1713–1722. doi: 10.1089/ten.tea.2012.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walboomers X.F., Dalton B.A., Evans M.D., Steele J.G., Jansen J.A. Transforming growth factor-beta 1, 2, and 3 can inhibit epithelial tissue outgrowth on smooth and microgrooved substrates. J. Biomed. Mater. Res. 2002;60:445–451. doi: 10.1002/jbm.1290. [DOI] [PubMed] [Google Scholar]
- 76.Chiang M., Robinson K.R., Vanable J.W., Jr. Electrical fields in the vicinity of epithelial wounds in the isolated bovine eye. Exp. Eye Res. 1992;54:999–1003. doi: 10.1016/0014-4835(92)90164-n. [DOI] [PubMed] [Google Scholar]
- 77.Song B., Zhao M., Forrester J.V., McCaig C.D. Electrical cues regulate the orientation and frequency of cell division and the rate of wound healing in vivo. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13577–13582. doi: 10.1073/pnas.202235299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Song B., Zhao M., Forrester J., McCaig C. Nerve regeneration and wound healing are stimulated and directed by an endogenous electrical field in vivo. J. Cell Sci. 2004;117:4681–4690. doi: 10.1242/jcs.01341. [DOI] [PubMed] [Google Scholar]
- 79.Rajnicek A.M., Foubister L.E., McCaig C.D. Prioritising guidance cues: directional migration induced by substratum contours and electrical gradients is controlled by a rho/cdc42 switch. Dev. Biol. 2007;312:448–460. doi: 10.1016/j.ydbio.2007.09.051. [DOI] [PubMed] [Google Scholar]
- 80.Rajnicek A.M., Foubister L.E., McCaig C.D. Alignment of corneal and lens epithelial cells by co-operative effects of substratum topography and dc electric fields. Biomaterials. 2008;29:2082–2095. doi: 10.1016/j.biomaterials.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 81.Chen Z., You J.J., Liu X., Cooper S., Hodge C., Sutton G. Biomaterials for corneal bioengineering. Biomed. Mater. 2018;13:27. doi: 10.1088/1748-605X/aa92d2. [DOI] [PubMed] [Google Scholar]
- 82.Lee C.H., Singla A., Lee Y. Biomedical applications of collagen. Int. J. Pharm. 2001;221:1–22. doi: 10.1016/s0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- 83.Glowacki J., Mizuno S. Collagen scaffolds for tissue engineering. Biopolymers. 2008;89:338–344. doi: 10.1002/bip.20871. [DOI] [PubMed] [Google Scholar]
- 84.Lynn A.K., Yannas I.V., Bonfield W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. B Appl. Biomater. 2004;71B:343–354. doi: 10.1002/jbm.b.30096. [DOI] [PubMed] [Google Scholar]
- 85.Parenteau-Bareil R., Gauvin R., Berthod F. Collagen-based biomaterials for tissue engineering applications. Materials. 2010;3:1863–1887. [Google Scholar]
- 86.Duan X., Sheardown H. Dendrimer crosslinked collagen as a corneal tissue engineering scaffold: mechanical properties and corneal epithelial cell interactions. Biomaterials. 2006;27:4608–4617. doi: 10.1016/j.biomaterials.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 87.Shao Z.Z., Vollrath F. Materials: surprising strength of silkworm silk. Nature. 2002;418:741. doi: 10.1038/418741a. [DOI] [PubMed] [Google Scholar]
- 88.Meinel L., Hofmann S., Karageorgiou V., Kirker-Head C., McCool J., Gronowicz G. The inflammatory responses to silk films in vitro and in vivo. Biomaterials. 2005;26:147–155. doi: 10.1016/j.biomaterials.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 89.Panilaitis B., Altman G.H., Chen J.S., Jin H.J., Karageorgiou V., Kaplan D.L. Macrophage responses to silk. Biomaterials. 2003;24:3079–3085. doi: 10.1016/s0142-9612(03)00158-3. [DOI] [PubMed] [Google Scholar]
- 90.Altman G.H., Diaz F., Jakuba C., Calabro T., Horan R.L., Chen J.S. Silk-based biomaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 91.Vepari C., Kaplan D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007;32:991–1007. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vrana E., Builles N., Hindie M., Damour O., Aydinli A., Hasirci V. Contact guidance enhances the quality of a tissue engineered corneal stroma. J. Biomed. Mater. Res. A. 2008;84A:454–463. doi: 10.1002/jbm.a.31442. [DOI] [PubMed] [Google Scholar]
- 93.Gil E.S., Park S.-H., Marchant J., Omenetto F., Kaplan D.L. Response of human corneal fibroblasts on silk film surface patterns. Macromol. Biosci. 2010;10:664–673. doi: 10.1002/mabi.200900452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lawrence B.D., Pan Z., Liu A., Kaplan D.L., Rosenblatt M.I. Human corneal limbal epithelial cell response to varying silk film geometric topography in vitro. Acta Biomater. 2012;8:3732–3743. doi: 10.1016/j.actbio.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lawrence B.D., Pan Z., Rosenblatt M.I. Silk film topography directs collective epithelial cell migration. PLoS One. 2012;7:12. doi: 10.1371/journal.pone.0050190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kang K.B., Lawrence B.D., Gao X.R., Guaiquil V.H., Liu A.H., Rosenblatt M.I. The effect of micro- and nanoscale surface topographies on silk on human corneal limbal epithelial cell differentiation. Sci. Rep. 2019;9:8. doi: 10.1038/s41598-018-37804-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jia L., Ghezzi C.E., Kaplan D.L. Optimization of silk films as substrate for functional corneal epithelium growth. J. Biomed. Mater. Res. B Appl. Biomater. 2016;104:431–441. doi: 10.1002/jbm.b.33408. [DOI] [PubMed] [Google Scholar]
- 98.Vrana N.E., Elsheikh A., Builles N., Damour O., Hasirci V. Effect of human corneal keratocytes and retinal pigment epithelial cells on the mechanical properties of micropatterned collagen films. Biomaterials. 2007;28:4303–4310. doi: 10.1016/j.biomaterials.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 99.Kilic C., Girotti A., Rodriguez-Cabello J.C., Hasirci V. A collagen-based corneal stroma substitute with micro-designed architecture. Biomater Sci. 2014;2:318–329. doi: 10.1039/c3bm60194c. [DOI] [PubMed] [Google Scholar]
- 100.Lawrence B.D., Marchant J.K., Pindrus M.A., Omenetto F.G., Kaplan D.L. Silk film biomaterials for cornea tissue engineering. Biomaterials. 2009;30:1299–1308. doi: 10.1016/j.biomaterials.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gosselin E.A., Torregrosa T., Ghezzi C.E., Mendelsohn A.C., Gomes R., Funderburgh J.L. Multi-layered silk film coculture system for human corneal epithelial and stromal stem cells. J. Tissue Eng. Regenerat. Med. 2018;12:285–295. doi: 10.1002/term.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ghezzi C.E., Marelli B., Omenetto F.G., Funderburgh J.L., Kaplan D.L. 3d functional corneal stromal tissue equivalent based on corneal stromal stem cells and multi-layered silk film architecture. PLoS One. 2017;12:18. doi: 10.1371/journal.pone.0169504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gil E.S., Mandal B.B., Park S.H., Marchant J.K., Omenetto F.G., Kaplan D.L. Helicoidal multi-lamellar features of rgd-functionalized silk biomaterials for corneal tissue engineering. Biomaterials. 2010;31:8953–8963. doi: 10.1016/j.biomaterials.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang W., Chen J.L., Backman L.J., Malm A.D., Danielson P. Surface topography and mechanical strain promote keratocyte phenotype and extracellular matrix formation in a biomimetic 3d corneal model. Adv. Healthc. Mater. 2017;6:11. doi: 10.1002/adhm.201601238. [DOI] [PubMed] [Google Scholar]
- 105.Zorlutuna P., Builles N., Damour O., Elsheikh A., Hasirci V. Influence of keratocytes and retinal pigment epithelial cells on the mechanical properties of polyester-based tissue engineering micropatterned films. Biomaterials. 2007;28:3489–3496. doi: 10.1016/j.biomaterials.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 106.Zorlutuna P., Tezcaner A., Kiyat I., Aydinli A., Hasirci V. Cornea engineering on polyester carriers. J. Biomed. Mater. Res. A. 2006;79A:104–113. doi: 10.1002/jbm.a.30772. [DOI] [PubMed] [Google Scholar]
- 107.Yanez-Soto B., Liliensiek S.J., Gasiorowski J.Z., Murphy C.J., Nealey P.F. The influence of substrate topography on the migration of corneal epithelial wound borders. Biomaterials. 2013;34:9244–9251. doi: 10.1016/j.biomaterials.2013.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nara S., Chameettachal S., Midha S., Singh H., Tandon R., Mohanty S. Strategies for faster detachment of corneal cell sheet using micropatterned thermoresponsive matrices. J. Mater. Chem. B. 2015;3:4155–4169. doi: 10.1039/c5tb00350d. [DOI] [PubMed] [Google Scholar]
- 109.Rizwan M., Peh G.S.L., Ang H.P., Lwin N.C., Adnan K., Mehta J.S. Sequentially-crosslinked bioactive hydrogels as nano-patterned substrates with customizable stiffness and degradation for corneal tissue engineering applications. Biomaterials. 2017;120:139–154. doi: 10.1016/j.biomaterials.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 110.Matthyssen S., Van den Bogerd B., Ni Dhubhghaill S., Koppen C., Zakaria N. Corneal regeneration: a review of stromal replacements. Acta Biomater. 2018;69:31–41. doi: 10.1016/j.actbio.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 111.Wu J., Rnjak-Kovacina J., Du Y., Funderburgh M.L., Kaplan D.L., Funderburgh J.L. Corneal stromal bioequivalents secreted on patterned silk substrates. Biomaterials. 2014;35:3744–3755. doi: 10.1016/j.biomaterials.2013.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Syed-Picard F.N., Du Y.Q., Hertsenberg A.J., Palchesko R., Funderburgh M.L., Feinberg A.W. Scaffold-free tissue engineering of functional corneal stromal tissue. J. Tissue Eng. Regenerat. Med. 2018;12:59–69. doi: 10.1002/term.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]