Abstract
Physiology, fitness and disease phenotypes are complex traits exhibiting continuous variation in natural populations. To understand complex trait gene functions transgenic lines of undefined genetic background are often combined to assess quantitative phenotypes ignoring the impact of genetic polymorphisms. Here, we used inbred wild-type strains of the Drosophila Genetics Reference Panel to assess the phenotypic variation of six physiological and fitness traits, namely, female fecundity, survival and intestinal mitosis upon oral infection, defecation rate and fecal pH upon oral infection, and terminal tracheal cell branching in hypoxia. We found continuous variation in the approximately 150 strains tested for each trait, with extreme values differing by more than four standard deviations for all traits. In addition, we assessed the effects of commonly used Drosophila UAS-RNAi transgenic strains and their backcrossed isogenized counterparts, in the same traits plus baseline intestinal mitosis and tracheal branching in normoxia, in heterozygous conditions, when only half of the genetic background was different among strains. We tested 20 non-isogenic strains (10 KK and 10 GD) from the Vienna Drosophila Resource Center and their isogenized counterparts without Gal4 induction. Survival upon infection and female fecundity exhibited differences in 50% and 40% of the tested isogenic vs. non-isogenic pairs, respectively, whereas all other traits were affected in only 10–25% of the cases. When 11 isogenic and their corresponding non-isogenic UAS-RNAi lines were expressed ubiquitously with Gal4, 4 isogenic vs. non-isogenic pairs exhibited differences in survival to infection. Furthermore, when a single UAS-RNAi line was crossed with the same Gal4 transgene inserted in different genetic backgrounds, the quantitative variations observed were unpredictable on the basis of pure line performance. Thus, irrespective of the trait of interest, the genetic background of commonly used transgenic strains needs to be considered carefully during experimentation.
Keywords: Complex traits, Drosophila, transgenic lines, genetic background, isogenic, RNAi, DGRP, VDRC
Physiological, morphological, biochemical, behavioral, fitness and disease phenotypes exhibit continuous variation among natural populations and are therefore considered complex or quantitative traits. This continuity in variation is attributed to a combination of genetic composition, environmental effects and gene-environment interactions (Mackay 2010). Inbred lines can be used to assess the impact of environmental factors on defined genetic backgrounds to tease out the effect of the genotype from that of the environment. Naturally-occurring phenotypic variation in distinct inbred backgrounds allows the implementation of genome-wide association (GWA) and the identification of key genetic variants impinging on the phenotype under study. The Drosophila Genetics Reference Panel (DGRP) collection corresponds to a set of >200 inbred sequenced fruit fly strains, which facilitate GWA studies (Mackay et al. 2012; Huang et al. 2014). GWAS analyses using the DGRP collection have identified significant genomic associations with a variety of fitness and physiology traits ranging from lifespan and sleep to abdominal pigmentation (Durham et al. 2014; Ivanov et al. 2015; Harbison et al. 2013; Dembeck et al. 2015; Sunaga et al. 2016).
The natural variation of the wild-type genetic background not only affects complex traits, but it often also affects the expressivity and penetrance of different mutations. Genetic background effects have been observed in all model organisms tested, including bacteria, yeast, nematodes, fruit flies, mice and plants (Chandler et al. 2013). For example, mouse inbred strains vary significantly with respect to various complex traits, such as diet-induced obesity (West et al. 1992), atherosclerosis (Nishina et al. 1993) and reproductive potential (Canning et al. 2003; Festing 1998). Also, the obesity and diabetes phenotypes caused by the obese LepOB mutation vary dramatically in three different mouse genetic backgrounds indicating the presence of genetic modifiers (Coleman and Hummel 1973; Stoehr et al. 2000). In C. elegans, Ras mutations produce quantitatively different developmental phenotypes across different genetic backgrounds (Milloz et al. 2008). Conditional essentiality in S. cerevisiae, whereby the loss of function of one gene causes lethality in one genetic background but not another, is basically driven by complex interactions of genetic modifiers (Dowell et al. 2010; Hou et al. 2019). In Drosophila, extensive studies assessing the phenotype of various alleles of the gene sd, which affect wing size, have shown that the same allele is able to generate a continuum of phenotypes when introduced in different wild-type genetic backgrounds (Chari and Dworkin 2013; Dworkin et al. 2009). Genetic background effects potentially led to contradictory results across studies that investigated the role of genes affecting longevity in Drosophila, such as Indy and sir-2, whereby the original papers that implicated the genes in increased longevity (Rogina et al. 2000; Rogina and Helfand 2004) could not be replicated in subsequent studies performed in different wild-type genetic backgrounds (Toivonen et al. 2007; Burnett et al. 2011; Viswanathan and Guarente 2011). In addition, it has been shown that the genetic background can also affect RNAi phenotypes. For example, injection of RNAi constructs in different wild-type genetic backgrounds of Tribolium led to distinct phenotypes, which were attributed not to off-target effects or differences in the function of the RNAi machinery, but to maternal effects (Kitzmann et al. 2013). In conclusion, such phenotypic inconsistencies underscore the importance of controlling for the genetic background in similar studies.
Backcrossing is an established breeding scheme where a characteristic (i.e., a trait, a gene, a locus or a chromosome segment) is introgressed from a donor parent into the genomic background of a recurrent parent (Hospital 2005). Backcrossing and introgression can occur in natural populations and contribute to speciation and evolution (Harrison and Larson 2014; Goulet et al. 2017; Harris and Nielsen 2016), but also they have long been used in breeding improvement programs both in plants and animals (Anamthawat-Jónsson 2001; Cheng et al. 2017; Der Sarkissian et al. 2015; Gootwine 2011; Holt et al. 2004). Backcrossing is particularly useful in dissecting the genetic architecture of quantitative traits, such as aging, because it can isolate a gene or chromosomal locus of interest in different genetic backgrounds (Piper and Partridge 2016). Progeny of successive generations are selected for the characteristic of interest, often facilitated by the use of molecular or visible markers (marker-assisted selection), and subsequently backcrossed to the recurrent parent. As backcrossed generations accumulate, the proportion of the genome of the donor parent tends to zero, except of the part hosting the characteristic of interest. Isogenic (or congenic) strains derive from backcrossing schemes and contain nearly identical genomes; namely, the genome of the recurrent parent except from the small part that is selected to differ (Kooke et al. 2012; Grove et al. 2016; Frisch and Melchinger 2005). Isogenic strains incorporating specific transgenes (with P or other transposable element ends or phage ends) in different genetic backgrounds can be easily generated in Drosophila using phenotypic markers of transgenesis (i.e., eye color, fluorescence) as a tool for selection.
Here, we used Drosophila melanogaster to assess the effects of the genetic background in six different fitness and physiology traits (female fecundity, survival and intestinal stem cell mitosis upon oral bacterial infection, defecation rate and fecal pH upon oral bacterial infection, and tracheal branching upon hypoxia). First, we assessed natural phenotypic variation of these traits using the DGRP collection. Then, we modified the genetic background of UAS-RNAi transgenic lines and compared the acquired phenotypes in isogenic vs. non-isogenic lines in the absence and presence of a Gal4 driver. Finally, we modified the genetic background of a Gal4 driver and assessed its phenotypic consequences. We found that the genetic background affects different traits to different extents and we propose ways to control for such effects in future experiments.
Materials and Methods
Drosophila maintenance
All strains and crosses were maintained on standard agar cornmeal fly food in a 12-hour light-dark cycle in a temperature-controlled incubator (Fitotron) at 25° (unless specified otherwise) with 65% humidity. For the DGRP screening for fecal spot number and pH measurements, prior to infection, female mated flies were aged for 4 days at 25° in bottles changed daily containing fly food supplemented with 50 ug/ml of the broad-range antibiotic Rifampicin, which does not kill PA14, but can eliminate most of the microorganisms present in the intestine of the flies. Rifampicin was also used in screening of the isogenic/non-isogenic pairs (Figure 3) for all assays except branching.
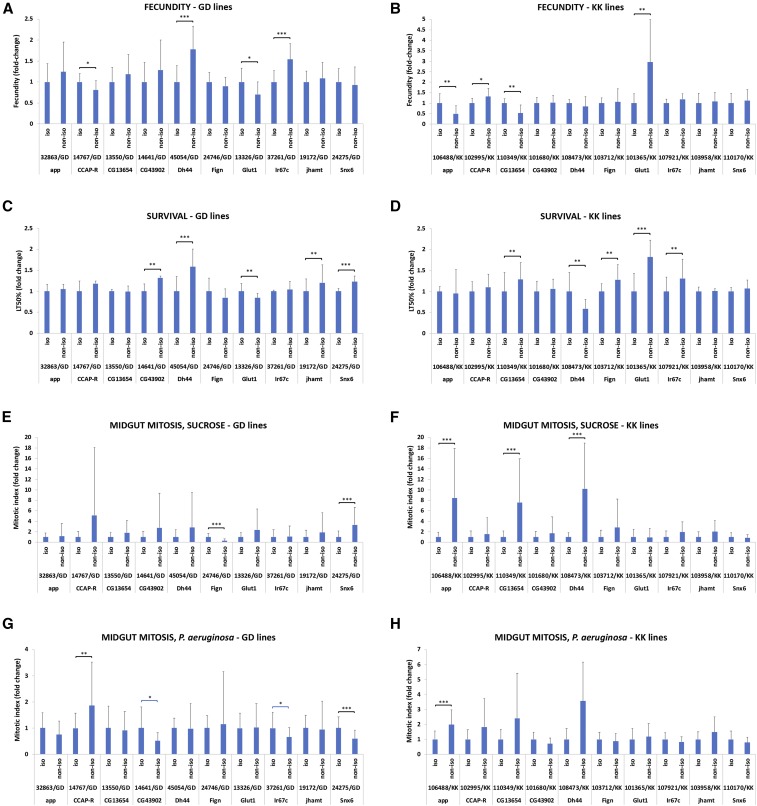
Figure 3.
Isogenic vs. non-isogenic comparisons of twenty UAS-RNAi transgenic lines in the absence of Gal4 for eight traits. (A-B) Fecundity in GD and KK lines. (C-D) Survival upon oral infection with P. aeruginosa in GD and KK lines. (E-F) Midgut mitosis in uninfected conditions in GD and KK lines. (G-H) Midgut mitosis upon oral infection with P. aeruginosa in GD and KK lines. (I-J) Defecation rate upon oral infection with P. aeruginosa in GD and KK lines. (K-L) Fecal pH upon oral infection with P. aeruginosa in GD and KK lines. (M-N) Tracheal branching in normoxia in GD and KK lines. (O-P) Tracheal branching in hypoxia in GD and KK lines. The y axis in all graphs represents the fold change difference between isogenic and non-isogenic strains crossed to our laboratory w1118. In all comparisons the isogenic is set to 1. Significant (via Student’s t-test) pair-wise isogenic/non-isogenic comparisons are indicated with brackets, whereby * 0.01<P ≤ 0.05; ** 0.001<P ≤ 0.01; *** P ≤ 0.001.
Drosophila strains
The Drosophila Genetics Reference Panel (DGRP) collection of wild type inbred sequenced strains (Mackay et al. 2012; Huang et al. 2014) was used in all screens assessing physiological traits (fecundity, survival, intestinal regeneration, fecal motility, fecal spots, tracheal branching). The Vienna Drosophila Research Center (VDRC) UAS-RNAi lines used in this study were the following: 32863/GD and 106488/KK targeting CG42318/app, 45054/GD and 108473/KK targeting CG8348/Dh44, 19172/GD and 103958/KK targeting CG17330/jhamt, 13550/GD and 110349/KK targeting CG13654, 14641/GD and 101680/KK targeting CG43902, 14747/GD and 102995/KK targeting CG33344/CCAP-R, 37261/GD and 107921/KK targeting CG32058/Ir67c, 24275/GD and 110170/KK targeting CG8282/Snx6, 24746/GD and 103712/KK targeting CG3326/Fign, 13326/GD and 101365/KK targeting CG43946/Glut1. Other stocks used in this study were the following (source and/or stock center numbers in parentheses): UAS-apcRNAi (VDRC, #1333), UAS-srcGFP (BDSC, #5432), Dl-Gal4 (Zeng et al. 2010), yw; ey-FLP2/T(2;3)CyO;TM6B,Tb,Hu (BDSC, #8204), Actin5C-Gal4 (BDSC, #25374).
Isogenization of transgenic lines
The VDRC lines, referred to as “non-isogenic”, were backcrossed to the laboratory w1118 strain for at least 6 generations to produce the “isogenic” lines. For the GD lines, the transgenes were homozygosed following the backcrossing based on the intensity of their eye color. For the KK lines, due to the intensity of the red eye color, it was safer to maintain the transgenes in a heterozygous state (unless specified otherwise). Since a fraction of the KK strains has been shown to carry two inserts (Green et al. 2014; Vissers et al. 2016), we tested all of them during the isogenization process for segregation of different eye colors, as well as by PCR (Green et al. 2014); we found that 101680/KK and 103958/KK carried double insertions (data not shown). Nevertheless, we did not observe any bias in iso/non-iso variation between these particular pairs.
Fecundity measurement assays
Female flies were allowed to mate for 2 days at 25°. Subsequently, males are removed from the cultures and females were left to lay eggs for 24 hr in a square bottle with 50 ml of food. Females were flipped to a new bottle for egg-laying every day for the next 3 days. Average fecundity was calculated as the number of progeny produced by the females in the 4 bottles divided by the number of females.
Pseudomonas aeruginosa infection
A 3 ml overnight culture of Pseudomonas aeruginosa (P. aeruginosa) PA14 was diluted 1:100 in LB (Lysogeny Broth) to prepare an overday 3 ml culture. When the culture reached OD600 = 3, it was used to prepare the infection mix (5 ml per vial: 3.5 ml ddH2O, 1 ml 20% sucrose and 0.5 ml PA14 OD600 = 3) or sucrose mix (5 ml per vial control: 4 ml ddH2O and 1 ml 20% sucrose). 5 ml of the infection or control mix was used to soak a cotton ball in a narrow fly vial, which was subsequently plugged with a dry cotton ball. After 4-5 hr starvation in empty vials, the flies were put in the infection vials and incubated at 25° with the cotton plug facing down.
For the fecal spot and pH measurements, flies were fed concentrated bacteria of OD600 = 50 (when the overday culture reached OD600 = 2, it was concentrated 25 times by centrifugation and resuspension of the bacterial pellet in 4% sucrose). Specifically, each feeding vial plugged with a cotton ball contained 5 ml agar gel (3% w/v agar in H2O) on top of which a Whatmann disc with 200 ul of the infection mix was placed. 25 mated starved young female flies were allowed to feed for 15 hr at 25° on infection mix (or 4% sucrose for control) in each vial and subsequently their fecal spots were assessed for numbers and pH.
Survival assays
Young female flies were subjected to intestinal infection with the virulent P. aeruginosa strain PA14. The percentage of dead flies was calculated daily as the (number of dead flies per vial/total number of flies) × 100 until all flies were dead in each vial. LT50% (lethal time 50%), the time when 50% of the flies were dead was used as an indicator of survival for comparisons. For Act5C-Gal4 experiments, the crosses with the UAS-RNAi strains were maintained at 18° to suppress expression of the RNAi. Emerging female flies were allowed to mature and mate at 25° and were subsequently subjected to infection with PA14 at 25°, as described above.
Intestinal regeneration measurement
Female adult flies (4-7 days old) were subjected to oral infection with the pathogenic strain of P. aeruginosa PA14. Only females were assessed in this assay because the level of mitosis in male midguts is low in baseline conditions as well as upon damage and aging (Hudry et al. 2016; Regan et al. 2016). Five days upon infection initiation, intestinal mitosis was measured by staining the intestines with the Rabbit-anti-pH 3 antibody (Millipore) followed by secondary detection with Donkey-anti-Rabbit Alexa555 (Invitrogen), as described previously (Apidianakis et al. 2009). Mitotic cells were counted in whole midguts under the fluorescent Zeiss Axioscope A1. At least 10 midguts per genotype were used for mitotic index calculations per genotype.
Fecal number and pH measurements
Infected female flies were starved in empty vials for 5 hr and then placed in vials with cotton balls impregnated with 5 ml 4% sucrose with 0.5% w/v bromophenol blue (BPB) pH = 7. BPB is a pH-sensitive dye, which is yellow at pH < 3 and turns blue at pH > 4.7. 50 flies were split in 3 vials with BPB and were allowed to feed for 5 hr (vials were placed in the incubator with the cotton plug facing down). 10 flies from each vial were moved to a petri dish containing a sterile cotton ball impregnated with 2.5 ml of the BPB solution and were left to defecate for 20 hr. Then, the flies were removed from the plates and the total number of fecal spots was measured in the 3 plates. After counting the fecal spots, 10 spots from 2 separate locations of each plate (6 samples) were collected in an microtube in 25 ul ddH2O pH = 5.5 and their pH was measured with a fine tip pH meter. All experiments were performed at 25°.
Terminal tracheal branching
Vials containing mixed-sex L3 larvae were capped with net during development to allow quick gas exchange and were subjected to hypoxia (5% O2) for 4 hr in a controlled chamber in the 25° incubator. For control experiments, larvae were maintained in normoxia (21% O2) in the 25° incubator. Late L3 larvae were aligned on a microscope slide (dorsal side up) and heat-killed by placing the slide on a 70° hot plate for 3-5 sec (Ghabrial and Krasnow 2006). Subsequently, and in less than one hour upon heat-killing, the two terminal tracheal cells of the second tracheal metamere were assessed for the number of their terminal branches and photographed under bright-field on a Zeiss Axioscope A1. At least 20 terminal cells were assessed per genotype and per condition.
Dysplastic cluster assays
Dysplastic clusters encompassing more than 5 cells were counted in adult Drosophila midguts of flies expressing GFP in intestinal stem cells via Dl-Gal4 > GFP. UAS-srcGFP and Dl-Gal4 were introgressed together in 22 DGRP lines. After six backcrossing generations, the transgenes were maintained in the DGRP backgrounds by selection of GFP expression and were kept in a heterozygous state. Baseline cluster formation was measured in 5-7 days old adult females maintained at 25°. For apcRNAi experiments, the DGRP (Dl-Gal4 > GFP) isogenic strains were crossed to UAS-apcRNAi at 25° and the adult female progeny were aged for 3 days at 25° before dysplastic cluster measurement for the uninduced state (the fact that no increase of cluster number was observed confirmed that apcRNAi was not induced in these conditions). For apcRNAi induction, the adult female progeny were aged for 3 days at 25° before feeding with P. aeruginosa PA14 for 2 days at 29°, where apcRNAi is induced concomitantly with infection, which boosts the regenerative ability of the gut. Dysplastic clusters were measured under a fluorescent Axioscope A.1 microscope (Zeiss) in dissected midguts (Apidianakis et al. 2009) upon fixation and staining with rabbit- or chicken-anti-GFP (Invitrogen) and mouse-anti-Prospero (DSHB) followed by secondary detection with anti-rabbit or anti-chicken Alexa 488 and anti-mouse Alexa 555 (Invitrogen).
Statistical analysis
Normalized z-scores for each DGRP screen were calculated by the formula z=(x-μ)/σ, where x is the mean observed value for each genotype normalized to the set average (each DGRP screen was performed in multiple sets of at least 10-20 genotypes), μ is the normalized population mean and σ is the standard deviation of the population. For hypoxia branching, z-score was calculated from the observed genotype means without normalization of the values, because the genotypes assessed per set were few.
For the isogenic/non-isogenic pair screening, initially each experiment was performed in duplicate and if both times the result had the same trend (at least once significant based on Student’s t-test), the experiment was repeated for a third time. If 2 out of 3 times the result was statistically significant with the same trend, the difference was considered significant (noted with p-values in Figures 3 and 7, * 0.01<P ≤ 0.05; ** 0.001<P ≤ 0.01; *** P ≤ 0.001).
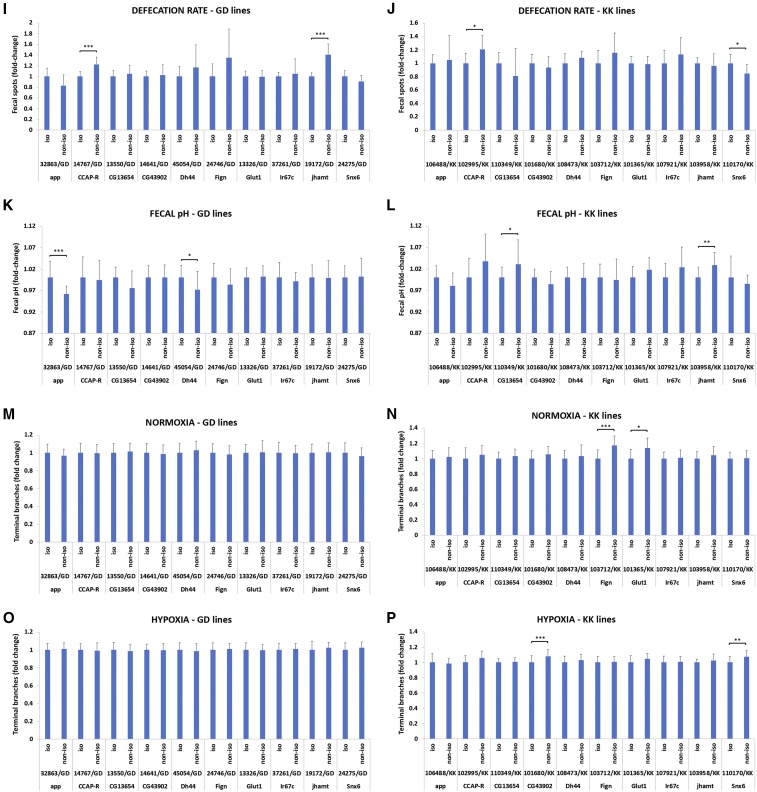
Figure 7.
A modulator of terminal tracheal branching lies on the Y or the 4th chromosome. (A) Crossing scheme used to ensure that larvae assessed for terminal branching carried half of the KK isogenic genotype. Chromosomes X, 2 and 3, but not 4, were always derived from the KK isogenic parent. (B) Isogenic via T(2;3) vs. non-isogenic comparisons for tracheal branching in normoxia. (C) Isogenic via T(2;3) vs. non-isogenic comparisons for tracheal branching in hypoxia. The y axis in (B-C) represents the number of terminal branches per terminal tracheal cell (TTC) in isogenic via T(2;3) and non-isogenic strains crossed to our laboratory w1118. Significant (via Student’s t-test) pair-wise isogenic/non-isogenic comparisons are indicated with brackets, whereby * 0.01<P ≤ 0.05; ** 0.001<P ≤ 0.01; *** P ≤ 0.001.
For survival assays the p-value was calculated with the Kaplan Meier estimator using the log-rank test (MedCalc statistical software). For all other assays, p-values were calculated with the Student’s t-Test in Excel (Office package). For correlation graphs, trendlines and R2 values were calculated in Excel and p-values were calculated by Pearson r value and the number of values correlated.
Data availability
All data necessary to repeat these experiments are included in the manuscript and associated supplementary files. All reagents used in this study are listed in the Reagent Table. Supplemental material available at FigShare: https://doi.org/10.25387/g3.9801638.
Results
The genetic background plays a key role in the phenotype of complex traits
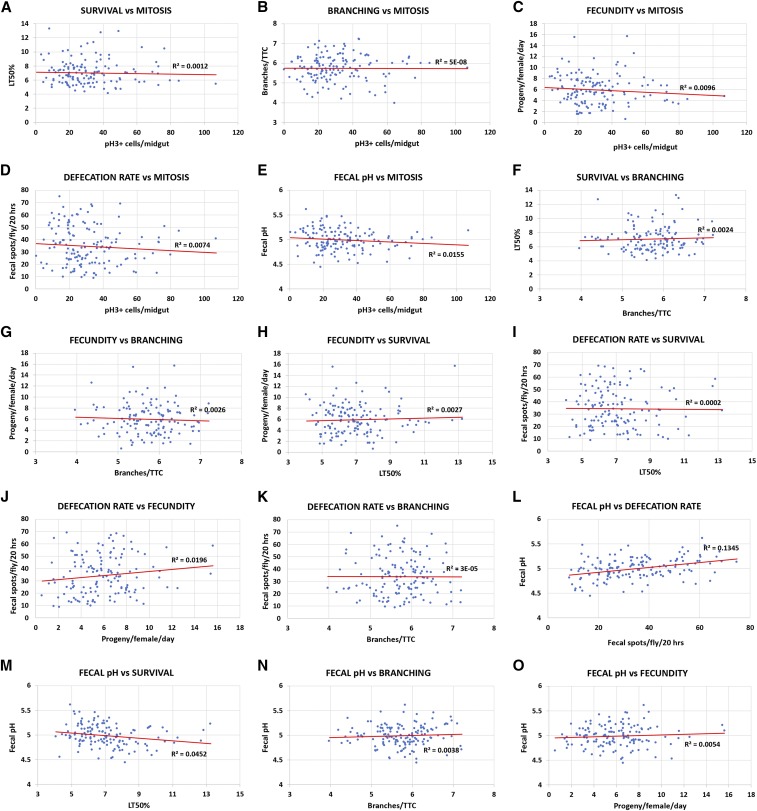
To understand the complexity of various quantitative fitness and physiology traits of interest, we screened the DGRP collection of wild-type inbred isogenic strains (Mackay et al. 2012) for female fecundity, survival upon oral bacterial infection, intestinal physiology upon oral bacterial infection (intestinal stem cell mitosis-mediated regeneration, defecation rate and fecal pH) and cellular response to hypoxia (terminal tracheal branching). All traits were assessed in Drosophila adults, except tracheal branching, which was assessed in third instar larvae. Table S1 includes all the data of the 6 DGRP screens. Z-score analysis underscored the continuity of the observed phenotypes for each trait (Figure 1). The z-score range of the screened DGRP strains, which indicates the phenotypic range, for each screen equaled 5.57, 5.24, 5.43, 4.28, 4.55 and 4.85 for fecundity, survival, intestinal mitosis, defecation rate, fecal pH and branching, respectively. GWA analysis of each screen via the DGRP2 algorithm (http://dgrp2.gnets.ncsu.edu/) identified 36, 44, 95, 32, 18 and 21 variants (single nucleotide polymorphisms: SNPs, insertions and deletions: InDels) associated with fecundity, survival, intestinal mitosis, defecation rate, fecal pH and branching, respectively. To assess if the six traits tested are functionally linked, we performed 15 pair-wise correlation analyses of each trait values for a common set of 128 DGRP strains (Table S1). A weak correlation was found between the defecation rate and fecal pH (R2 = 0.1345, P = 0.1) (Figure 2L) with all other comparisons exhibiting essentially no correlation (R2 < 0.04) (Figure 2A-K, M-O). Thus, no significant correlation was observed between traits (Figure 2), and the six different traits can be considered largely independent.
Figure 1.
Z-score analysis of the DGRP phenotypes of six complex traits. (A) Female fecundity, (B) Survival upon oral infection with P. aeruginosa, (C) Defecation rate upon oral infection with P. aeruginosa, (D) Fecal pH upon oral infection with P. aeruginosa, (E) Intestinal mitosis upon oral infection with P. aeruginosa, and (F) Terminal tracheal branching in hypoxia.
Figure 2.
Pair-wise correlation analyses of the six DGRP screens. (A) Survival vs. intestinal mitosis upon oral infection with P. aeruginosa. (B) Branching in hypoxia vs. intestinal mitosis upon oral infection with P. aeruginosa. (C) Fecundity vs. intestinal mitosis upon oral infection with P. aeruginosa. (D) Defecation rate upon oral infection with P. aeruginosa vs. intestinal mitosis upon oral infection with P. aeruginosa. (E) Fecal pH upon oral infection with P. aeruginosa vs. intestinal mitosis upon oral infection with P. aeruginosa. (F) Survival upon oral infection with P. aeruginosav vs. branching in hypoxia. (G) Fecundity vs. branching in hypoxia. (H) Fecundity vs. survival upon oral infection with P. aeruginosa. (I) Defecation rate upon oral infection with P. aeruginosa vs. survival upon oral infection with P. aeruginosa. (J) Defecation rate upon oral infection with P. aeruginosa vs. fecundity. (K) Defecation rate upon oral infection with P. aeruginosa vs. branching in hypoxia. (L) Fecal pH upon oral infection with P. aeruginosa vs. defecation rate upon oral infection with P. aeruginosa. (M) Fecal pH upon oral infection with P. aeruginosa vs. survival upon oral infection with P. aeruginosa. (N) Fecal pH upon oral infection with P. aeruginosa vs. branching in hypoxia. (O) Fecal pH upon oral infection with P. aeruginosa vs. fecundity. Trendlines are indicated in red and the R2 values are shown in all graphs.
The genetic background of UAS-RNAi transgenes (without Gal4) affects complex traits to different extents
Since the effect of the homozygous wild-type genotype produces distinct quantitative phenotypes in all traits studied, we devised a way to assess the effects of heterozygosis in the above traits. The use of heterozygotes is necessary in most genetic experiments utilizing the classical genetic tools available in Drosophila, such as the Gal4-UAS system (Brand and Perrimon 1993) and the ever-increasing number of UAS-RNAi lines targeting practically any gene of the fly genome. Initially, to avoid the effect of overexpression by the Gal4, we decided to assess the effect of the genetic background on the UAS-RNAi insertion alone. All UAS-RNAi lines were generated in an isogenic w1118 host strain at the VDRC and the balancer stocks used to map or stabilize insertions were also isogenic to the VDRC w1118 strain. Therefore, and to maintain the insertions as closely as possible to their original genotype, we introgressed the UAS-RNAi insertions of twenty randomly-selected VDRC lines in our laboratory w1118 strain for further experiments. Ten strains from the VDRC KK library, generated via site-specific φχ31 recombination (Markstein et al. 2008), and ten strains from the VDRC GD library (Dietzl et al. 2007), generated via P-element random transposition (Spradling and Rubin 1982), targeting 10 genes were used (one KK and one GD strain per gene). To introgress the VDRC UAS-RNAi insertions in our laboratory w1118 strain, we backcrossed each VDRC KK and GD strain to our laboratory w1118 strain for at least six generations. During backcrossing the fraction of the donor parent (VDRC line) halves after every backcross: 50%, 25%, 12.5% etc., in F1, 1st backcross, 2nd backcross etc., respectively. The average proportion of the recurrent parental nuclear genome after each backcross increases and can be calculated by the formula: (2(b+1) - 1)/2(b+1) or 1 - (1/2)(b+1), where b equals the number of backcrosses assuming an infinite population. Thus, the proportion of the donor genome is given as (1/2)(b+1) (Kooke et al. 2012). Assuming six generations of backcrossing, the recurrent parental nuclear genome (our laboratory w1118) equals 99.21% and the donor genome (VDRC w1118) 0.79%. From now on, we will refer to the original VDRC UAS-RNAi strains as “non-isogenic” and to the introgression lines generated in our laboratory w1118 strain as “isogenic”.
The homozygous non-isogenic (VDRC) and isogenic (VDRC in our w1118 strain) lines were subsequently crossed to w1118, the heterozygous progeny of the crosses were collected and assessed for eight fitness and physiology traits. In addition to the traits studied in the DGRP screening, we also tested intestinal mitosis during homeostasis (without infection) and tracheal branching in normoxia (21% O2). GD and KK non-isogenic and isogenic lines were assessed in pairs and those producing significantly different phenotypes were quantified (Figure 3). In this scheme, the progeny of the non-isogenic cross to our laboratory w1118 contain 50% of our laboratory w1118 genome and 50% of the VDRC w1118 genome, whereas the progeny of the isogenic cross to our laboratory w1118 contain approximately 100% of our laboratory w1118 genome. Therefore, the genome of the progeny of the two crosses differs by up to 50%. Theoretically, since the VDRC lines were generated in a w1118 genetic background, we expected not to find significant differences between the original VDRC strains (non-isogenic) and the introgressed lines (isogenic). Strikingly, we observed different impact of isogenization in the various traits and this was independent of the type of transgene (GD, inserted in a random genomic location; or KK, inserted in a specific genomic location); the most affected being survival and fecundity, with 50% and 40% of the isogenic vs. non-isogenic pairs producing significantly different phenotypes (Figure 3A-D), respectively, and the least affected being tracheal branching in normoxia and hypoxia with only 10% of the isogenic vs. non-isogenic pairs producing significantly different phenotypes (Figure 3M-P).
Specifically, 4 out of 10 GD (2 up, 2 down) and 4 out of 10 KK (2 up, 2 down) isogenic vs. non-isogenic pairs (40% in total) differed in fecundity (Figure 3A-B); 5 out of 10 GD (4 down, 1 up) and 5 out of 10 KK (4 down, 1 up) isogenic vs. non-isogenic pairs (50% in total) differed in survival upon pathogenic oral bacterial infection (Figure 3C-D); 2 out of 10 GD (1 up, 1 down) and 3 out of 10 KK (3 down) isogenic vs. non-isogenic pairs (25% in total) differed in intestinal mitosis in homeostatic conditions (Figure 3E-F); 4 out of 10 GD (1 down, 3 up) and 1 out of 10 KK (1 down) isogenic vs. non-isogenic pairs (25% in total) differed in intestinal mitosis upon oral pathogenic infection (Figure 3G-H); 2 out of 10 GD (2 down) and 2 out of 10 KK (1 up, 1 down) isogenic vs. non-isogenic pairs (20% in total) differed in defecation rate, i.e., number of fecal spots upon bacterial infection (Figure 3I-J); 2 out of 10 GD (2 up) and 2 out of 10 KK (2 down) isogenic vs. non-isogenic pairs (20% in total) differed in fecal pH upon bacterial infection (Figure K-L); 0 out of 10 GD and 2 out of 10 KK (2 down) isogenic vs. non-isogenic pairs (10% in total) differed in tracheal branching in normoxia (Figure 3M-N); and 0 out of 10 GD and 2 out of 10 KK (2 down) isogenic vs. non-isogenic pairs (10% in total) differed in tracheal branching in hypoxia (Figure 3O-P). A summary of these data are presented in Table S2. In conclusion, some complex traits were affected more strongly by the genetic background than others.
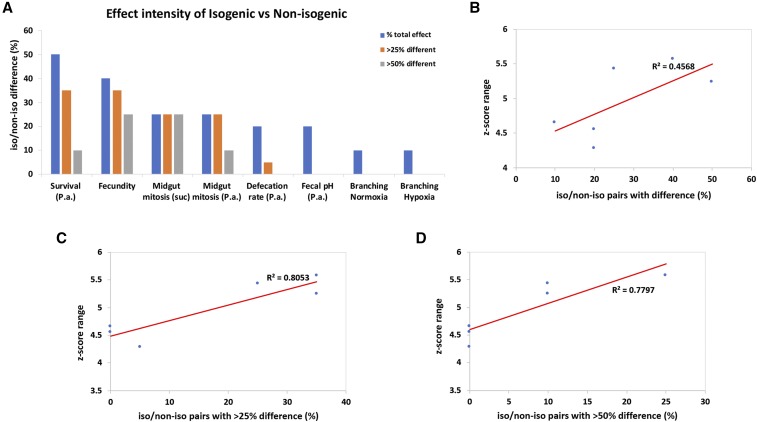
Further analysis of the differences of isogenic vs. non-isogenic pairs in the various assays indicated that, although statistically significant, the phenotype might differ by a small or large percentage, as reflected by the fold-change difference between the genotypes. For example, for the total of 10 isogenic/non-isogenic pairs exhibiting differences in survival upon infection, the percentage of phenotypic difference ranges from 16% (13326/GD iso/non-iso pair) to 81.8% (101365/KK iso/non-iso pair). Since the biggest difference between an isogenic/non-isogenic pair might be considered more important, we assessed the intensity of the observed phenotypic differences by calculating the percentage of the pairs exhibiting differences by more than 25% and more than 50% (Table S3). We noticed that only 10–25% of the iso/non-iso pairs exhibited differences of >50% for fecundity, midgut mitosis and survival to infection, while no pair exhibited differences of >50% for defecation rate, fecal pH and branching in normoxia or hypoxia (Figure 4A). For the six traits that we had performed z-score analysis in wild-type inbred DGRP strains the range of z-scores was between 4.25 and 5.5. We then correlated this range for each trait with the percentage of isogenic vs. non-isogenic pairs exhibiting significant differences, as well as, the percentage of those that exhibited more that 25% and 50% differences (Figure 4B-D). We found a significant correlation between the z-score range of each trait and the fraction of isogenic/non-isogenic pairs exhibiting >25% difference (R2 = 0.8053, P < 0.05; Figure 4C) or >50% difference (R2 = 0.7797, P < 0.05; Figure 4D). Therefore, the higher the phenotypic range measured as the range of z-scores among inbred strains for a particular trait, the more likely UAS-RNAi lines sharing half or more of their DNA to exhibit differences of >25%.
Figure 4.
The phenotypic range correlates with the intensity of the difference between isogenic vs. non-isogenic phenotypes. (A) Percentage of isogenic vs. non-isogenic pairs presented with phenotypic differences and subcategories indicating the intensity of the observed differences, more than 25% difference between isogenic/non-isogenic and more than 50% difference between isogenic/non-isogenic. (B) Correlation of z-score range of the 6 traits and the percentage of isogenic/non-isogenic pairs that differed significantly for the same traits. (C) Correlation of z-score range and the fraction of the total isogenic/non-isogenic pairs that differed by more than 25%. (D) Correlation of z-score range and the fraction of the total isogenic/non-isogenic pairs that differed by more than 50%. Trendlines are indicated in red and the R2 values are shown in all graphs.
An alternate way to assess phenotypic variability among inbred lines for different traits measured in different units is coefficient of variation (CV), i.e., variability of the data relative to the mean. We performed CV analysis of the six DGRP screens and found %CV values of 45.7%, 24.8%, 45%, 4.2%, 57.4% and 11.6% for fecundity, survival upon infection, defecation rate, fecal pH, midgut mitosis upon infection and branching upon hypoxia, respectively. Interestingly, when we assessed the association between %CV and the percentage of isogenic/non-isogenic UAS-RNAi pairs with difference, we found no significant correlation. Specifically, we found R2 = 0.07, when all different pairs were included, R2 = 0.3, when only those with >25% difference were assessed and R2 = 0.28, when only the ones with >50% difference were assessed. Thus, the z-score range of the phenotypes of inbred strains, but not the CV, correlates with the observed isogenic/non-isogenic pair differences.
The genetic background of UAS-RNAi transgenes affects survival upon oral infection in the presence of Gal4
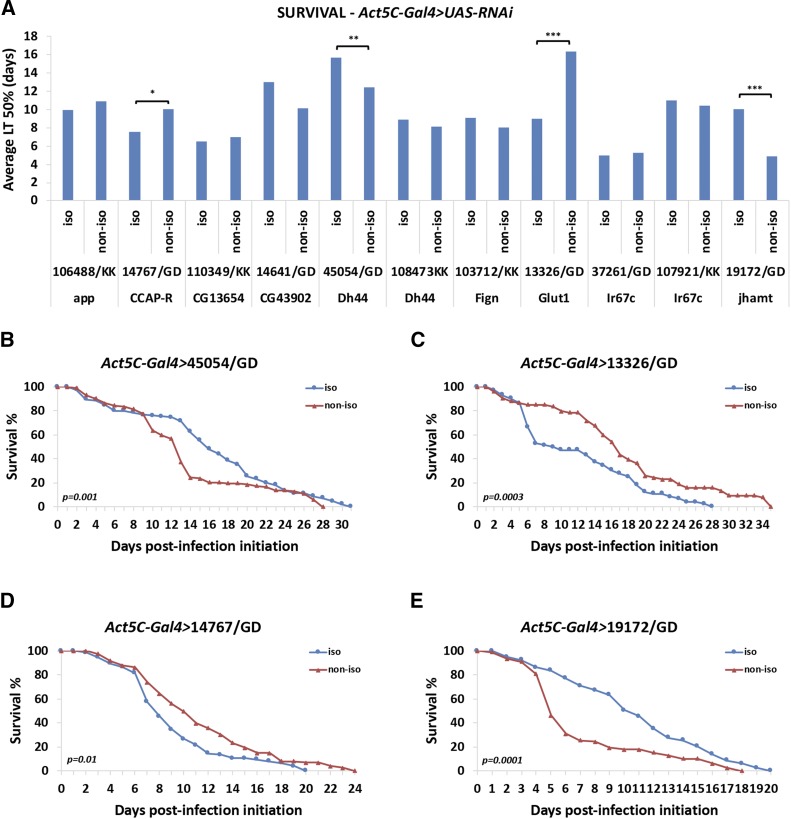
To assess the effect of the genetic background of UAS-RNAi lines in the context of Gal4 activation, we crossed the ubiquitously-expressed Act5C-Gal4 driver with the isogenic (in our laboratory w1118) and non-isogenic (original VDRC) UAS-RNAi lines. We focused on the trait we found being mostly affected by the genetic background, survival upon oral bacterial infection. We assessed survival of P. aeruginosa orally infected flies expressing 11 isogenic vs. their corresponding 11 non-isogenic VDRC UAS-RNAi lines (6 GD and 5 KK iso/non-iso pairs) under the control of the ubiquitous Act5C-Gal4 driver. We found significant differences in survival in 4 out of the 11 iso/non-iso pairs crossed to Act5C-Gal4 (Figure 5A). Interestingly, none of the 5 iso/non-iso KK pairs tested exhibited differential phenotypes, when induced by Act5C-Gal4, although 3 of those exhibited significantly different survival in the absence of Gal4 (Figure 3D). Furthermore, 4 of the 6 iso/non-iso GD pairs tested exhibited significant differences in survival when induced by Act5C-Gal4 (Figure 5B-E). Although 3 of those iso/non-iso GD pairs (45054/GD, 13326/GD, 19172/GD) also affected survival at significantly different levels in the absence of Gal4 (Figure 3C), all 3 behaved the opposite way upon Gal4 induction (e.g., 45054/GD iso exhibited increased and reduced survival compared to 45054/GD non-iso in the presence and absence of Act5C-Gal4, respectively). Thus, activation of the isogenic vs. the non-isogenic UAS-RNAi transgenes via Gal4 leads to differential effects in survival that cannot be predicted by the behavior of the same transgenic lines in uninduced conditions.
Figure 5.
The genetic background affects survival upon Gal4-induction of the transgenic RNAi lines. (A) Average LT50% in days upon P. aeruginosa infection of Act5C-Gal4 > UAS-RNAi isogenic vs. non-isogenic flies. (B-E) Survival curves of the statistically-different isogenic vs. non-isogenic UAS-RNAi driven by Act5C-Gal4. 2-3 independent survival experiments were averaged and p-values were calculated by the Kaplan-Meier method (A-E). Comparisons are always performed between isogenic/non-isogenic genotypes. Brackets indicate significant pair-wise comparisons, whereby * 0.01<P ≤ 0.05; ** 0.001<P ≤ 0.01; *** P ≤ 0.001.
The genetic background affects Gal4 transgenes and midgut dysplasia induced by the same UAS-apcRNAi line
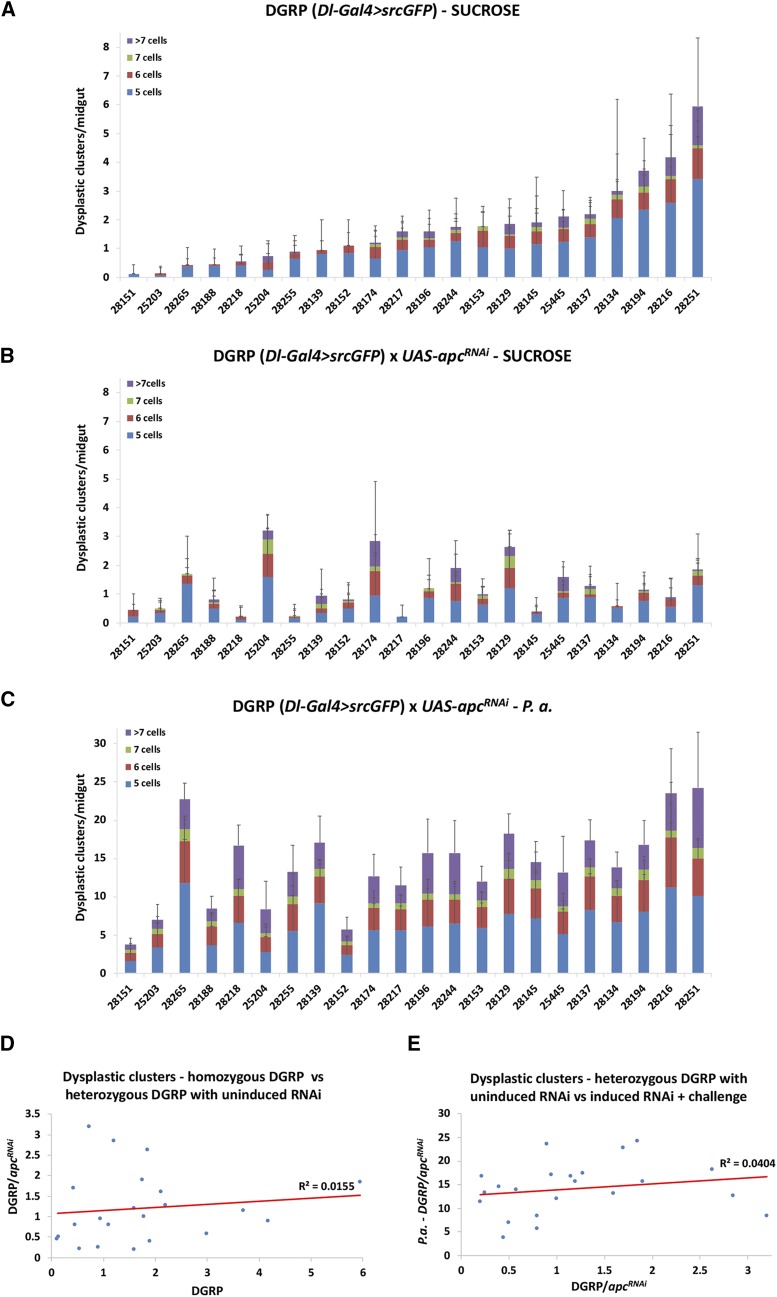
To assess the effect of the genetic background on Gal4 activity per se, we jointly introgressed two transgenes, UAS-srcGFP and Dl-Gal4 (Dl-Gal4 > GFP), in 22 different DGRP lines. Dl-Gal4 > GFP allows the visualization of intestinal stem cells in the adult Drosophila intestine (Zeng et al. 2010). In addition, it provides a way to assess the generation of dysplastic stem cell clusters (Biteau et al. 2008; Apidianakis et al. 2009) upon genetic perturbations (e.g., by driving expression of UAS lines impinging on mitosis and cell differentiation), during aging or bacterial challenge. After six backcrossing generations to individual DGRP strains, we generated flies heterozygous for both the UAS-srcGFP and Dl-Gal4 transgenes in 22 different wild type DGRP genetic backgrounds. We assessed dysplastic cluster formation (clusters of more than 5 Dl-Gal4 > GFP positive cells) in these strains by visualizing the GFP in dissected midguts. We found that there was variability in cluster formation in the different homozygous DGRP genotypes (Figure 6A). To assess the effect of overexpression with Gal4, we used an RNAi line targeting apc (UAS-apcRNAi), which, when induced, promotes intestinal stem cell proliferation (Cordero et al. 2012). The phenotypic range was manifold and comparable among: (i) the 22 extensively inbred DGRP lines bearing a single copy of Dl-Gal4 > GFP (Figure 6A); (ii) the corresponding 22 uninduced UAS-apcRNAi heterozygotes arising from crosses between a single UAS-apcRNAi line with each of the 22 DGRP (Dl-Gal4 > GFP) lines (Figure 6B); and (iii) the corresponding 22 induced and infected UAS-apcRNAi heterozygotes arising from the same crosses as in (ii) (Figure 6C). Strikingly, no pairwise strain correlation was observed between setups (i) and (ii) (Figure 6D) or between setups (ii) and (iii) (Figure 6E). Thus, the genetic background matters even when the same UAS-RNAi line is crossed with Gal4 lines in different genetic backgrounds; and its impact is unpredictable and depends on the specific genetic and experimental setup.
Figure 6.
Gal4 is affected by the genetic background. (A) Dysplastic cluster formation in 22 DGRP lines carrying one copy of the Dl-Gal4 > GFP transgenes. (B) Dysplastic cluster formation in the progeny of 22 DGRP lines carrying one copy of the Dl-Gal4 > GFP transgenes crossed to UAS-apcRNAi in uninduced conditions. (C) Dysplastic cluster formation in 22 DGRP lines carrying one copy of the Dl-Gal4 > GFP transgenes crossed to UAS-apcRNAi in induced conditions plus oral infection with P. aeruginosa. (D) Correlation of homozygous DGRP (Dl-Gal4 > GFP) strains and heterozygous DGRP Dl-Gal4 > GFP/UAS-apcRNAi. (E) Correlation of heterozygous DGRP Dl-Gal4 > GFP/UAS-apcRNAi uninduced and induced with oral P. aeruginosa infection.
A modulator of terminal branching lies on the Y or the 4th chromosome
A strong genetic background effect from the 4th chromosome was observed during assessment of branching in the isogenic/non-isogenic experiment. Specifically, since terminal branching needed to be assessed in larvae instead of adults, and because the KK VDRC lines had a deep red eye color as heterozygotes, initially, we maintained the isogenized KK strains as heterozygotes and we kept selecting subsequent generations by eye color. Because the use of KK heterozygotes would prevent us from being certain for the genotype of the progeny larvae upon crossing to w1118, we used a compound balancer stock to ensure that the genetic composition of the progeny would be the desired (Figure 7A). By pre-crossing the heterozygous isogenic KK lines to yw/Y; T(2;3)/eyFLP2 males, we acquired males with balanced X, 2nd and 3rd chromosomes (but not 4th). When these males were crossed to w1118 females, the genotype of the progeny was selected to be isogenic KK for all chromosomes, except for half of the cases of the Y and 4th chromosome (which were derived from the compound balancer stock). Impressively, we noticed that when these KK [via T(2;3)] isogenic strains were crossed to our laboratory w1118, their progeny differed from those derived from crosses of the non-isogenic strains (original VDRC strains) to our laboratory w1118 in 40% of the cases (4 out of 10 iso/non-iso pairs; 1 down and 3 up in isogenic) in normoxia and in 100% of the cases (10 out of 10 iso/non-iso pairs; all 10 up in isogenic) in hypoxia (Figure 7B-C). Thus, the Y or the 4th chromosome of the compound balancer stock imposed a strong enhancing effect on branching.
Discussion
Genetic background effects have been observed in various model organisms ranging from bacteria to mice and in various fitness, physiology and disease phenotypes (Chandler et al. 2013). In this study, the effects of the genetic background on different quantitative traits were primarily measured in inbred homozygous DGRP strains. Not surprisingly, we found that all traits studied (female fecundity, survival upon pathogenic infection, intestinal mitosis upon pathogenic infection, defecation rate and fecal pH upon pathogenic infection and terminal branching in hypoxia) produced variable phenotypes. Based on the genomic information of the DGRP strains, we have identified genomic variants (SNPs, InDels) with a potential role in these phenotypes. Genetic characterization of these variants will be presented elsewhere.
In addition, we observed significant and variable genetic background effects, when we assessed transgenic UAS-RNAi and Gal4 constructs in different wild type genetic backgrounds. Specifically, although the VDRC UAS-RNAi lines were generated in an isogenic w1118 host strain and, whenever necessary, the balancers used were isogenic to the host strain (Dietzl et al. 2007), when the same UAS-RNAi insertions were introgressed in our laboratory w1118 strain (referred to as “isogenic”), they produced quantitatively different phenotypes in various traits, when compared to the original VDRC lines (referred to as “non-isogenic”). This would mean that either the original lines changed over time or that the two w1118 strains (that of the VDRC and ours) were not the same, because they were maintained in different laboratory environments. We did not observe major differences between the GD and KK types of transgene insertions in terms of the number of strains affected by isogenization, although the first were generated in random genomic locations using P-element transformation, and thus, cannot be controlled for position effects. This indicates that, not only each GD, but also each KK UAS transgene, which is inserted into the same genomic locus, lies in a variable genetic background.
Interestingly, the percentage of isogenic/non-isogenic UAS-RNAi pairs exhibiting differences varied for each trait studied. Survival upon oral infection and female fecundity, were more sensitive to genetic background differences with 50% and 40% of the isogenic/non-isogenic pairs exhibiting significantly different phenotypes. Only 10–25% of the isogenic/non-isogenic pairs exhibited differences of >50% for fecundity, midgut mitosis and survival to infection, whereas no pair exhibited differences of >50% for defecation rate, fecal pH and branching in normoxia or hypoxia (Figure 4A; Table S3). Moreover, using the z-score range of each DGRP screen as a measure of the extent to which each trait varied among the ∼150 inbred strains tested, we found a correlation with the percentage of isogenic/non-isogenic pairs differing for the same trait, but only when the differences were >25% (Figure 4). Thus, the more variable the trait from inbred strain to inbred strain, when measured as the range of z-scores, the more likely UAS-RNAi lines sharing half or more of their DNA to exhibit differences of >25%. Interestingly, CV analysis of the DGRP screens found no correlation with the percentage of isogenic/non-isogenic pairs differing for the same trait. This result probably reflects the fact that the z-score considers the presence of outliers in inbred lines. Outliers might also arise, when assessing transgenes in different genetic backgrounds.
Gal4-induction of the isogenic and non-isogenic UAS-RNAi transgenic strains was used to assess the trait affected the most by the genetic background, survival upon oral infection. Although we were able to assess only 11 isogenic/non-isogenic pairs upon Act5C-Gal4 induction, we found that 4 out of those exhibited significant difference in survival. Interestingly, all pairs presented with differential survival were GD strains. Nevertheless, none of the 3 isogenic/non-isogenic pairs that exhibited differential survival upon infection could be predicted based on their phenotype in uninduced conditions (Figure 5 and Figure 3C). Furthermore, Gal4-dependent phenotypes were also observed, in inbred strains, as well as upon crossing of those to the same UAS-RNAi strain and environmental challenge (Figure 6). Similarly, the observed differences upon induction could not be attributed to the initial differences of the inbred strains. Thus, Gal4/UAS genotypes sharing half of their DNA exhibit differences that cannot be predicted based on prior knowledge on a similar genetic setup.
Moreover, we found a strong effect from the Y or the 4th chromosome in terminal branching, when assessing isogenic genotypes through balancing via a compound balancer strain and a genetic scheme ensuring that X, 2 and 3 chromosomes derived from the KK isogenic, but the Y and the 4th chromosomes were 50% that of the KK isogenic and 50% that of the balancer stock (Figure 7A). Specifically, the Y and 4th chromosomes of the compound balancer increased branching. Since the Y and the 4th chromosomes are small, largely heterochromatic and encompass approximately 20 and 100 genes, respectively (Chang and Larracuente 2019; Riddle and Elgin 2018), it would be interesting to assess if any of these plays a role in terminal branching. Whereas the Y chromosome genes are largely involved in male fertility (Carvalho et al. 2015), Gene Ontology (GO) analysis of the 4th chromosome genes, indicates that three of them (ci, an effector of the Hh pathway, pan, an effector of the Wg pathway, and zyx, a cytoskeletal protein) function in the trachea and zyx is involved in terminal branching morphology and tracheal air filling (Merabet et al. 2005; Renfranz et al. 2010).
Since the genetic toolkit of Drosophila is continuously expanding, we tend to use more and more transgenic lines to understand the function of genes of interest. This is especially true for UAS-RNAi, that allows tissue-specific knockdown, when combined with Gal4 (Mohr and Perrimon 2012; Kaya-Çopur and Schnorrer 2016). Depending on the phenotype we aim to study, we should be aware that the genetic background matters more or less. For example, highly variable (multi-organ or multi-factorial) traits, such as survival and fecundity, are strongly affected by the genetic background, whereas less variable traits, such as tracheal branching, are much less affected. So, is there anything that can be done to ensure that the deviating phenotypes produced in a screen are not because of background effects? Obviously, upon completion of a screen, one would need to follow the general practice of repetition (independent repeats to control for the random variability) and validation of findings with independent genetic tools. For example, when assessing RNAi phenotypes, multiple genetically-different UAS-RNAi lines targeting the same gene must be used to confirm the initial results. Given the results of this study, we propose that isogenization of all transgenic lines used in a study in the same genetic background is ideal and allows direct comparisons of strains. Despite the process being time-consuming, some researchers go even further to recommend using more than one wild-type background for isogenization. This is feasible and desirable, when one’s study focuses on a particular gene. Accordingly, one should know one’s assay and the range of phenotypic differences expected. For a large-scale screen, the phenotypic range can be evident through outliers, i.e., the largest minus the smallest z-score among all strains tested. But also, a small-scale pilot experiment assessing experiment-to-experiment and individual-to-individual variation can provide essential information of the phenotypic range. In our experiments, for example, terminal tracheal branching has a narrow phenotypic range and, thus, smaller phenotypic differences might be significant.
The common practice in quantitative biomedical studies - where, for instance, the number of mice used or the number of humans recruited is of essence - a statistical power analysis is considered mandatory to ensure that the minimum number of individuals is used or recruited. While less of a concern in terms of bioethics, quantitative trait assessment in model organisms, such as D. melanogaster and C. elegans, may follow the common practice or independent repetition and validation. But to estimate the number of hits, i.e., minimizing the false positives and the false negatives of a screen, one could use trait variability as a guide to deploy a higher cut-off of difference as significant for highly-variable traits, as opposed to less variable traits.
Acknowledgments
The authors would like to thank Maria Glykainou, Patapios Patapiou and Styliani Irakleous for help with DGRP screening and Androniki Giakoumi for help with Act5C-Gal4 survival assays. We also thank the VDRC and BDSC for the UAS-RNAi and DGRP fly strains used in this study, respectively, the DSHB for antibodies, and Steven Hou for the Dl-Gal4 stock. Work in the Pitsouli and Apidianakis laboratories was supported by the Marie Curie GIG FP7 - People and the Fondation Santé.
Footnotes
Supplemental material available at FigShare: https://doi.org/10.25387/g3.9801638.
Communicating editor: J. Tennessen
Literature Cited
- Anamthawat-Jónsson K., 2001. Molecular cytogenetics of introgressive hybridization in plants. Methods Cell Sci. 23: 139–148. 10.1023/A:1013182724179 [DOI] [PubMed] [Google Scholar]
- Apidianakis Y., Pitsouli C., Perrimon N., and Rahme L., 2009. Synergy between bacterial infection and genetic predisposition in intestinal dysplasia. Proc. Natl. Acad. Sci. USA 106: 20883–20888. 10.1073/pnas.0911797106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biteau B., Hochmuth C. E., and Jasper H., 2008. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell 3: 442–455. 10.1016/j.stem.2008.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A. H., and Perrimon N., 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- Burnett C., Valentini S., Cabreiro F., Goss M., Somogyvári M. et al. , 2011. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature 477: 482–485. 10.1038/nature10296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning J., Takai Y., and Tilly J. L., 2003. Evidence for genetic modifiers of ovarian follicular endowment and development from studies of five inbred mouse strains. Endocrinology 144: 9–12. 10.1210/en.2002-220988 [DOI] [PubMed] [Google Scholar]
- Carvalho A. B., Vicoso B., Russo C. A., Swenor B., and Clark A. G., 2015. Birth of a new gene on the Y chromosome of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 112: 12450–12455. 10.1073/pnas.1516543112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler C. H., Chari S., and Dworkin I., 2013. Does your gene need a background check? How genetic background impacts the analysis of mutations, genes, and evolution. Trends Genet. 29: 358–366. 10.1016/j.tig.2013.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. H., and Larracuente A. M., 2019. Heterochromatin-Enriched Assemblies Reveal the Sequence and Organization of the Drosophila melanogaster Y Chromosome. Genetics 211: 333–348. 10.1534/genetics.118.301765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chari S., and Dworkin I., 2013. The conditional nature of genetic interactions: the consequences of wild-type backgrounds on mutational interactions in a genome-wide modifier screen. PLoS Genet. 9: e1003661 10.1371/journal.pgen.1003661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A., Ismail I., Osman M., Hashim H., and Mohd Zainual N. S., 2017. Rapid and targeted introgression of fgr gene through marker-assisted backcrossing in rice (Oryza sativa L.). Genome 60: 1045–1050. 10.1139/gen-2017-0100 [DOI] [PubMed] [Google Scholar]
- Coleman D. L., and Hummel K. P., 1973. The influence of genetic background on the expression of the obese (Ob) gene in the mouse. Diabetologia 9: 287–293. 10.1007/BF01221856 [DOI] [PubMed] [Google Scholar]
- Cordero J. B., Stefanatos R. K., Myant K., Vidal M., and Sansom O. J., 2012. Non-autonomous crosstalk between the Jak/Stat and Egfr pathways mediates Apc1-driven intestinal stem cell hyperplasia in the Drosophila adult midgut. Development 139: 4524–4535. 10.1242/dev.078261 [DOI] [PubMed] [Google Scholar]
- Dembeck L. M., Huang W., Magwire M. M., Lawrence F., Lyman R. F. et al. , 2015. Genetic Architecture of Abdominal Pigmentation in Drosophila melanogaster. PLoS Genet. 11: e1005163 10.1371/journal.pgen.1005163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der Sarkissian C., Ermini L., Schubert M., Yang M. A., Librado P. et al. , 2015. Evolutionary Genomics and Conservation of the Endangered Przewalski’s Horse. Curr. Biol. 25: 2577–2583. 10.1016/j.cub.2015.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K. C., Barinova Y. et al. , 2007. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: 151–156. 10.1038/nature05954 [DOI] [PubMed] [Google Scholar]
- Dowell R. D., Ryan O., Jansen A., Cheung D., Agarwala S. et al. , 2010. Genotype to phenotype: a complex problem. Science 328: 469 10.1126/science.1189015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham M. F., Magwire M. M., Stone E. A., and Leips J., 2014. Genome-wide analysis in Drosophila reveals age-specific effects of SNPs on fitness traits. Nat. Commun. 5: 4338 10.1038/ncomms5338 [DOI] [PubMed] [Google Scholar]
- Dworkin I., Kennerly E., Tack D., Hutchinson J., Brown J. et al. , 2009. Genomic consequences of background effects on scalloped mutant expressivity in the wing of Drosophila melanogaster. Genetics 181: 1065–1076. 10.1534/genetics.108.096453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festing, M. F. W., 1998 Inbred strains of mice. Mouse Genomics Informatics. The Jackson Laboratory (http://www.informatics.jax.org/inbred_strains/mouse/STRAINS.shtml). Bar Harbor, Maine.
- Frisch M., and Melchinger A. E., 2005. Selection theory for marker-assisted backcrossing. Genetics 170: 909–917. 10.1534/genetics.104.035451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghabrial A. S., and Krasnow M. A., 2006. Social interactions among epithelial cells during tracheal branching morphogenesis. Nature 441: 746–749. 10.1038/nature04829 [DOI] [PubMed] [Google Scholar]
- Gootwine E., 2011. Mini review: breeding Awassi and Assaf sheep for diverse management conditions. Trop. Anim. Health Prod. 43: 1289–1296. 10.1007/s11250-011-9852-y [DOI] [PubMed] [Google Scholar]
- Goulet B. E., Roda F., and Hopkins R., 2017. Hybridization in Plants: Old Ideas, New Techniques. Plant Physiol. 173: 65–78. 10.1104/pp.16.01340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green E. W., Fedele G., Giorgini F., and Kyriacou C. P., 2014. A Drosophila RNAi collection is subject to dominant phenotypic effects. Nat. Methods 11: 222–223. 10.1038/nmeth.2856 [DOI] [PubMed] [Google Scholar]
- Grove E., Eckardt S., and McLaughlin K. J., 2016. High-Speed Mouse Backcrossing Through the Female Germ Line. PLoS One 11: e0166822 10.1371/journal.pone.0166822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison S. T., McCoy L. J., and Mackay T. F., 2013. Genome-wide association study of sleep in Drosophila melanogaster. BMC Genomics 14: 281 10.1186/1471-2164-14-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K., and Nielsen R., 2016. The Genetic Cost of Neanderthal Introgression. Genetics 203: 881–891. 10.1534/genetics.116.186890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R. G., and Larson E. L., 2014. Hybridization, Introgression, and the Nature of Species Boundaries. J. Hered. 105: 795–809. 10.1093/jhered/esu033 [DOI] [PubMed] [Google Scholar]
- Holt M., Nicholas F. W., James J. W., Moran C., and Martin I. C., 2004. Development of a highly fecund inbred strain of mice. Mamm. Genome 15: 951–959. 10.1007/s00335-004-3030-8 [DOI] [PubMed] [Google Scholar]
- Hospital F., 2005. Selection in backcross programmes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360: 1503–1511. 10.1098/rstb.2005.1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J., Tan G., Fink G. R., Andrews B. J., and Boone C., 2019. Complex modifier landscape underlying genetic background effects. Proc. Natl. Acad. Sci. USA 116: 5045–5054. 10.1073/pnas.1820915116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Massouras A., Inoue Y., Peiffer J., Ràmia M. et al. , 2014. Natural variation in genome architecture among 205 Drosophila melanogaster Genetic Reference Panel lines. Genome Res. 24: 1193–1208. 10.1101/gr.171546.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudry B., Khadayate S., and Miguel-Aliaga I., 2016. The sexual identity of adult intestinal stem cells controls organ size and plasticity. Nature 530: 344–348. 10.1038/nature16953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov D. K., Escott-Price V., Ziehm M., Magwire M. M., Mackay T. F. et al. , 2015. Longevity GWAS Using the Drosophila Genetic Reference Panel. J. Gerontol. A Biol. Sci. Med. Sci. 70: 1470–1478. 10.1093/gerona/glv047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya-Çopur A., and Schnorrer F., 2016. A Guide to Genome-Wide In Vivo RNAi Applications in Drosophila. Methods Mol. Biol. 1478: 117–143. 10.1007/978-1-4939-6371-3_6 [DOI] [PubMed] [Google Scholar]
- Kitzmann P., Schwirz J., Schmitt-Engel C., and Bucher G., 2013. RNAi phenotypes are influenced by the genetic background of the injected strain. BMC Genomics 14: 5 10.1186/1471-2164-14-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooke R., Wijnker E., and Keurentjes J. J., 2012. Backcross populations and near isogenic lines. Methods Mol. Biol. 871: 3–16. 10.1007/978-1-61779-785-9_1 [DOI] [PubMed] [Google Scholar]
- Mackay T. F., 2010. Mutations and quantitative genetic variation: lessons from Drosophila. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365: 1229–1239. 10.1098/rstb.2009.0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay T. F., Richards S., Stone E. A., Barbadilla A., Ayroles J. F. et al. , 2012. The Drosophila melanogaster Genetic Reference Panel. Nature 482: 173–178. 10.1038/nature10811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markstein M., Pitsouli C., Villalta C., Celniker S. E., and Perrimon N., 2008. Exploiting position effects and the gypsy retrovirus insulator to engineer precisely expressed transgenes. Nat. Genet. 40: 476–483. 10.1038/ng.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merabet S., Hombria J. C., Hu N., Pradel J., and Graba Y., 2005. Hox-controlled reorganisation of intrasegmental patterning cues underlies Drosophila posterior spiracle organogenesis. Development 132: 3093–3102. 10.1242/dev.01889 [DOI] [PubMed] [Google Scholar]
- Milloz J., Duveau F., Nuez I., and Félix M. A., 2008. Intraspecific evolution of the intercellular signaling network underlying a robust developmental system. Genes Dev. 22: 3064–3075. 10.1101/gad.495308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr S. E., and Perrimon N., 2012. RNAi screening: new approaches, understandings, and organisms. Wiley Interdiscip. Rev. RNA 3: 145–158. 10.1002/wrna.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishina P. M., Wang J., Toyofuku W., Kuypers F. A., Ishida B. Y. et al. , 1993. Atherosclerosis and plasma and liver lipids in nine inbred strains of mice. Lipids 28: 599–605. 10.1007/BF02536053 [DOI] [PubMed] [Google Scholar]
- Piper M. D., and Partridge L., 2016. Protocols to study aging in Drosophila. Methods Mol. Biol. 1478: 291–302. 10.1007/978-1-4939-6371-3_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan J. C., Khericha M., Dobson A. J., Bolukbasi E., Rattanavirotkul N. et al. , 2016. Sex difference in pathology of the ageing gut mediates the greater response of female lifespan to dietary restriction. eLife 5: e10956 10.7554/eLife.10956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfranz P. J., Blankman E., and Beckerle M. C., 2010. The cytoskeletal regulator zyxin is required for viability in Drosophila melanogaster. Anat. Rec. (Hoboken) 293: 1455–1469. 10.1002/ar.21193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle N. C., and Elgin S. C. R., 2018. The Drosophila Dot Chromosome: Where Genes Flourish Amidst Repeats. Genetics 210: 757–772. 10.1534/genetics.118.301146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogina B., Reenan R. A., Nilsen S. P., and Helfand S. L., 2000. Extended life-span conferred by cotransporter gene mutations in Drosophila. Science 290: 2137–2140. 10.1126/science.290.5499.2137 [DOI] [PubMed] [Google Scholar]
- Rogina B., and Helfand S. L., 2004. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc. Natl. Acad. Sci. USA 101: 15998–16003. 10.1073/pnas.0404184101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., and Rubin G. M., 1982. Transposition of cloned P elements into Drosophila germ line chromosomes. Science 218: 341–347. 10.1126/science.6289435 [DOI] [PubMed] [Google Scholar]
- Stoehr J. P., Nadler S. T., Schueler K. L., Rabaglia M. E., Yandell B. S. et al. , 2000. Genetic obesity unmasks nonlinear interactions between murine type 2 diabetes susceptibility loci. Diabetes 49: 1946–1954. 10.2337/diabetes.49.11.1946 [DOI] [PubMed] [Google Scholar]
- Sunaga S., Akiyama N., Miyagi R., and Takahashi A., 2016. Factors underlying natural variation in body pigmentation of Drosophila melanogaster. Genes Genet. Syst. 91: 127–137. 10.1266/ggs.15-00061 [DOI] [PubMed] [Google Scholar]
- Toivonen J. M., Walker G. A., Martinez-Diaz P., Bjedov I., Driege Y. et al. , 2007. No influence of Indy on lifespan in Drosophila after correction for genetic and cytoplasmic background effects. PLoS Genet. 3: e95 10.1371/journal.pgen.0030095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers J. H. A., Manning S. A., Kulkarni A., and Harvey K. F., 2016. A Drosophila RNAi library modulates Hippo pathway-dependent tissue growth. Nat. Commun. 7: 10368 10.1038/ncomms10368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan M., and Guarente L., 2011. Regulation of Caenorhabditis elegans lifespan by sir-2.1 transgenes. Nature 477: E1–E2. 10.1038/nature10440 [DOI] [PubMed] [Google Scholar]
- West D. B., Boozer C. N., Moody D. L., and Atkinson R. L., 1992. Dietary obesity in nine inbred mouse strains. Am. J. Physiol. 262: R1025–R1032. [DOI] [PubMed] [Google Scholar]
- Zeng X., Chauhan C., and Hou S. X., 2010. Characterization of midgut stem cell- and enteroblast-specific Gal4 lines in Drosophila. Genesis 48: 607–611. 10.1002/dvg.20661 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data necessary to repeat these experiments are included in the manuscript and associated supplementary files. All reagents used in this study are listed in the Reagent Table. Supplemental material available at FigShare: https://doi.org/10.25387/g3.9801638.