Abstract
Introduction
Data on percutaneous kidney biopsy (KBx) incidence and frequencies of hemorrhagic complications among inpatients are limited.
Methods
Using nationally representative US hospitalization discharge data, we report temporal trends in inpatient KBx rates from 2007 to 2014 and estimate frequencies of, and risk factors for, utilization of packed red blood cell (pRBC) transfusion and renal angiography.
Results
From 2007 to 2014, rates of native KBx among adult inpatients increased from 8.2 to 10.0 per 100,000, while transplant KBx rates declined from 3.6 to 3.1 per 100,000. We studied 35,183 and 14,266 discharge records with native and transplant KBx. We found that 5.7% (95% confidence interval [CI]: 5.3%–6.0%) of inpatients undergoing native KBx and 4.9% (4.2%–5.5%) of those undergoing transplant KBx received a pRBC transfusion within 2 days of biopsy. Similarly, 0.6% (0.5%–0.7%) of inpatients undergoing native KBx and 0.4% (0.2%–0.5%) undergoing transplant KBx received a renal angiogram within 2 days of KBx. For inpatient native KBx, female sex, older age, higher chronic kidney disease stage, acute renal failure, lupus, vasculitis, cirrhosis, multiple myeloma/paraproteinemia, and anemia of chronic disease were independently associated with increased odds of pRBC transfusion; cirrhosis and end-stage renal disease (ESRD) were associated with increased odds, and nephrotic syndrome was associated with decreased odds, of renal angiography.
Conclusions
In this large population-based study of inpatient KBx practices, we demonstrate increasing rates of inpatient native KBx among US adults and provide accurate estimates of the frequencies of, and risk factors for, pRBC transfusion and renal angiography following inpatient KBx.
Keywords: acute kidney injury, glomerular disease, renal biopsy
Percutaneous kidney biopsy (KBx) remains the gold standard test for the diagnosis of intrinsic kidney disease. Numerous studies have demonstrated the role of KBx in establishing a diagnosis, predicting prognosis, and guiding treatment in patients with kidney disease.1, 2 However, KBx is not without risk, with the primary associated complication being hemorrhage. Clinically significant hemorrhage following KBx might necessitate a blood transfusion, angiographic intervention, rarely a nephrectomy, and very rarely can even result in death.3, 4 At present, no formal guidelines exist on when, or in whom, to pursue a KBx: thus, clinicians and patients must carefully weigh the risks and benefits of KBx on an individual patient basis.
Developing a detailed understanding of contemporary KBx utilization practices and accurately quantifying KBx-associated risks can reliably inform clinicians and patients considering this diagnostic procedure. Although several prior studies have examined bleeding complication risks following KBx, there is large variation in absolute bleeding rates across individual studies. For example, post-KBx hemorrhage requiring transfusion has been reported to complicate 0.9% to 9.0% of all KBx: this order of magnitude risk difference is likely explained by study population heterogeneity.5, 6 Efforts to accurately quantify rarer hemorrhagic complications (e.g., the need for angiographic intervention) or to identify independent risk factors for hemorrhagic complications (using multivariable models) have been severely limited by the small sample sizes of individual published studies.7 Accordingly, a nationally representative study with sufficiently large patient numbers to accurately quantify the frequency of, and risk factors for, post-KBx blood transfusion and angiographic intervention would be highly informative.
Herein we report temporal trends in KBx rates and quantify the risk of post-KBx blood transfusion and angiographic intervention among hospitalized adults in the United States from 2007 to 2014, using a large, nationally representative data source.
Methods
Data
The Nationwide Inpatient Sample (NIS), maintained by the Healthcare Cost and Utilization Project at the Agency of Healthcare Research and Quality, is the largest all-payer inpatient care administrative database in the United States. Each year, the NIS collects discharge data on approximately 7 million inpatient stays. All NIS discharge records contain International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes relevant to a patient’s hospital stay. Information on the timing of procedures relative to the day of admission are also provided for each record. By design, the NIS approximates a stratified 20% sample of US hospitals (before 2012), or a stratified 20% sample of US hospital discharges (beginning in 2012, when the sampling scheme of the NIS was altered). Each NIS discharge is assigned a weight so that nationally representative estimates of quantities of interest can be obtained. To generate population-level rates for this study, age-specific census data were obtained from the National Cancer Institute’s Surveillance, Epidemiology and End Results Program.8 To better reflect contemporary practice, we focused on the period after 2007. This approach also enabled us to adjust for chronic kidney disease (CKD) presence and stage in our multivariable models, as stage-specific codes for CKD only came into use in 2005.
Inclusion Criteria
We extracted all discharge records from the NIS, 2007 to 2014, containing a procedure code for KBx (ICD-9-CM procedure code: 55.23) in adult patients (age ≥18).
Exclusion Criteria
Discharge records missing data on age, sex, death, and length of stay were excluded. Records containing ICD-9-CM diagnosis codes for benign and malignant neoplasms of the kidney were also excluded (codes: 223.0, 223.1, 189.0, 189.1, 198.0, V105.2, 209.24, 209.64, 236.91), as KBx performed to evaluate kidney tumors was not the focus of this study.
Trends Over Time
Nationally representative, age-specific estimates of the number of inpatient KBx per year were obtained using the “trend weight” variable provided by the NIS. These weights were specifically designed to allow for valid analysis of time trends, and account for the sampling scheme changes in the NIS over time.9 Standard deviations associated with each yearly estimate were obtained, accounting for the cluster-stratified nature of the NIS sample.10 Age-specific, population-adjusted rates of KBx per 100,000 population were obtained using census data. For each age group, we fit an inverse-variance weighted simple linear model; the P value associated with the slope parameter of the model is reported to assess the statistical significance of observed time trends (vs. a slope of zero, indicating no change over time).
Blood Transfusions and Renal Angiography
We sought to quantify the fraction of patients receiving pRBC transfusions and angiographic interventions (renal arteriography and/or embolization) post-KBx. Prior studies on the timing of hemorrhagic complications following KBx have demonstrated that >90% of such complications occur within 24 hours, and ∼100% occur within 48 hours, of KBx.3, 11 We therefore considered the following outcomes: (i) pRBC transfusion occurring on the same day as KBx or within 2 days of KBx (i.e., on the day of, or the day following biopsy), and (ii) renal arteriography occurring on the same day as KBx or within 2 days of KBx. We identified pRBC transfusions by ICD-9-CM procedure code 99.04 and renal arteriography by code 88.45. Data on the timing of ICD-9-CM–coded procedures relative to the day of admission are provided for each discharge record. These data allowed us to calculate the relative timing of KBx and each of the interventions of interest (in days). We excluded records with missing data on the timing of KBx and/or pRBC transfusion or renal arteriography (where relevant). Records for which KBx occurred before admission were also excluded. In sensitivity analyses, we explored the potential impact of missing data on study findings.
Comorbidities and Clinical Syndromes
A secondary aim of our study was to quantify risk factors associated with utilization of pRBC transfusion or renal angiogram following KBx. For each discharge record, we extracted data on the following comorbidities and clinical syndromes, based on ICD-9-CM diagnosis/procedure codes: acute renal failure (acute [nontraumatic] kidney injury) (584.xx), rapidly progressive glomerulonephritis (580.4, 582.4, 583.4), nephrotic syndrome (581.xx), diabetes (250.xx), hypertension (401.xx, 402.xx, 403.xx, 404.xx), systemic lupus erythematosus (710.0, 695.4), vasculitides (446.xx), cirrhosis (571.2, 571.5, 571.6), multiple myeloma and other paraproteinemias (203.0, 273.1, 273.2), amyloidosis (277.3x), and anemia of chronic illness (258.2). Kidney transplant status was determined by a combination of diagnosis and procedure codes (diagnosis codes: V42.0, 996.81; procedure codes: 55.53, 55.61, 55.69). Specific diagnosis codes for CKD were implemented in 2005 (585.1–585.6); therefore, to allow sufficient time for the routine adoption of these codes in discharge records, our analyses focused on the period from 2007 to 2014.
Quantifying Factors Associated With Utilization of pRBC Transfusion and Renal Angiography
We fit univariate logistic regression models to quantify associations between individual-level demographics and clinical syndromes and the interventions of interest (pRBC transfusion and/or angiographic intervention within 2 days of biopsy). Covariates significantly associated with interventions in univariate analyses were included in multivariable models. We incorporated NIS discharge weights and the 2-stage clustered design of the NIS in all regression models.
Additional Analyses
In the Supplementary Material, we performed several sensitivity analyses. We provide (i) data on the rates of pRBC transfusion and renal angiogram as a function of time post-KBx and (ii) data on the rates of pRBC transfusion as function of the number of inpatient native KBx performed at hospitals sampled.
Results
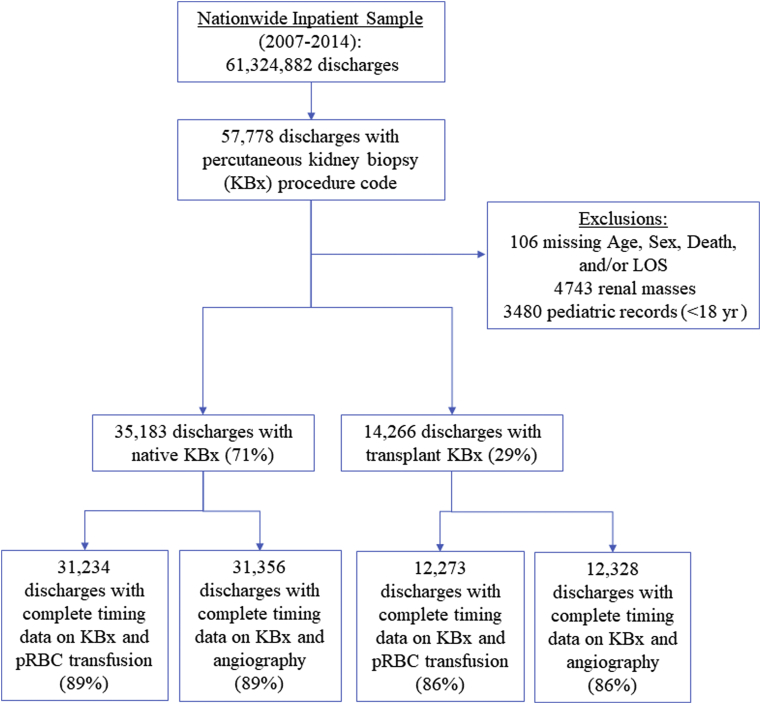
The NIS contains 61,324,882 discharge records from 2007 to 2014, of which 49,449 (0.08%) met our inclusion and exclusion criteria (Figure 1). Of these, 35,183 hospitalizations (71%) included a native KBx and 14,266 (29%) included a transplant KBx. Incorporating the NIS weighting scheme, this corresponds to an estimated 174,475 inpatients undergoing native and 71,081 inpatients undergoing transplant KBx in the United States during the study period, for an average of 21,809 and 8885 per year, respectively.
Figure 1.
Study cohort creation. All discharge records from the Nationwide Inpatient Sample (NIS) from 2007 to 2014 with a procedure code for percutaneous kidney biopsy (KBx) were included; records missing data on patients’ age, sex, mortal status at the time of discharge, and length of stay (LOS) were excluded, along with pediatric patients (aged <18 years). For analyses of packed red blood cell (pRBC) transfusions and renal angiography associated with KBx, we excluded discharge records that were missing data on the timing of KBx and the timing of pRBC transfusion/renal angiogram (where relevant), as well as discharge records in which KBx occurred before admission. Final cohorts represent data with complete information on the timing of KBx and pRBC transfusion/renal angiogram (where relevant).
For each discharge record, we extracted information on demographics and several comorbidities and clinical conditions based on ICD-9-CM discharge diagnoses, Table 1. Significant differences between inpatients undergoing native and transplant biopsies were observed. Among inpatients undergoing native KBx, discharge diagnosis codes for acute renal failure were most prevalent (70%), followed by codes for hypertension (69%), chronic (including end-stage) kidney disease (50%), and diabetes mellitus (28%). Inpatients with ESRD codes at the time of discharge experienced disproportionately higher rates of hemodialysis during their hospitalization (Supplementary Table S1).
Table 1.
Demographic data and hospitalization outcomes among inpatient adults undergoing percutaneous kidney biopsies, 2007–2014
| Native KBx n = 35,183a | Transplant KBx n = 14,266a | P | |
|---|---|---|---|
| Sex | |||
| Female | 17,058 (48.5) | 8339 (41.5) | |
| Male | 18,125 (51.5) | 5297 (58.5) | <2.2e-16 |
| Age, yr | |||
| 18–39 | 8508 (24.2) | 4187 (29.4) | |
| 40–64 | 15,629 (44.4) | 7946 (55.7) | |
| 65–79 | 8631 (24.5) | 2067 (14.5) | |
| >80 | 2415 (6.9) | 66 (0.5) | 0.0005 |
| Hospital location/teaching status | |||
| Missing | 245 (0.7) | 111 (0.8) | |
| Rural | 1541 (4.4) | 70 (0.5) | |
| Urban nonteaching | 12,385 (35.2) | 866 (6.1) | |
| Urban teaching | 21,012 (59.7) | 13,219 (92.7) | <2.2e-16 |
| Hospital location/geography | |||
| Northeast | 6183 (17.6) | 2609 (18.3) | |
| Midwest | 7675 (21.8) | 3350 (23.5) | |
| South | 14,403 (40.9) | 5430 (38.0) | |
| West | 6922 (19.7) | 2877 (20.2) | 0.0005 |
| Biopsy timing | |||
| Missinga | 3819 (10.9) | 1946 (13.7) | |
| Day 0 (day of admission) | 3641 (11.5) | 3120 (24.5) | |
| Day 1 | 3432 (10.8) | 3249 (25.5) | |
| Day 2 | 3561 (11.2) | 1333 (10.5) | |
| Day 3 + | 20,730 (65.4) | 4618 (36.3) | <2.2e-16 |
| Clinical conditionsb | |||
| Hypertension | 24,230 (68.9) | 11,483 (80.5) | <2.2e-16 |
| Chronic kidney disease | |||
| Absent | 17,695 (50.3) | 7508 (52.6) | |
| Unspecified | 5423 (15.4) | 1484 (10.4) | |
| Stages 1–2 | 459 (1.3) | 40 (0.3) | |
| Stages 3–5 | 6677 (19.0) | 965 (6.8) | |
| ESRD | 4929 (14.0) | 4269 (29.9) | 0.0005 |
| Acute renal failure | 24,757 (70.4) | 9062 (63.5) | <2.2e-16 |
| Diabetes | 9881 (28.1) | 5231 (36.7) | <2.2e-16 |
| Nephrotic syndrome | 5622 (16.0) | 213 (1.5) | <2.2e-16 |
| Lupus | 3936 (11.2) | 622 (4.4) | <2.2e-16 |
| RPGN | 824 (2.3) | 13 (0.1) | <2.2e-16 |
| Vasculitis | 2355 (6.7) | 161 (1.1) | <2.2e-16 |
| Cirrhosis | 782 (2.2) | 107 (0.8) | <2.2e-16 |
| Amyloid | 427 (1.2) | 22 (0.2) | <2.2e-16 |
| Multiple myeloma and other paraproteinemias | 1729 (4.9) | 62 (0.4) | <2.2e-16 |
| Anemia of chronic disease | 8526 (24.2) | 2907 (20.3) | <2.2e-16 |
ESRD, end-stage renal disease; RPGN, rapidly progressing glomerulonephritis.
All values refer to n (%).
Missing records include those in which KBx occurred before admission, which are excluded from analyses of KBx-associated interventions (340 records among native KBx [0.97%] and 414 records among transplant KBx [2.9%]).
Clinical conditions are defined based on International Classification of Diseases, Ninth Revision, Clinical Modification codes at the time of discharge and are not mutually exclusive.
Temporal Trends in Native and Transplant Inpatient KBx Among US Adults, 2007–2014
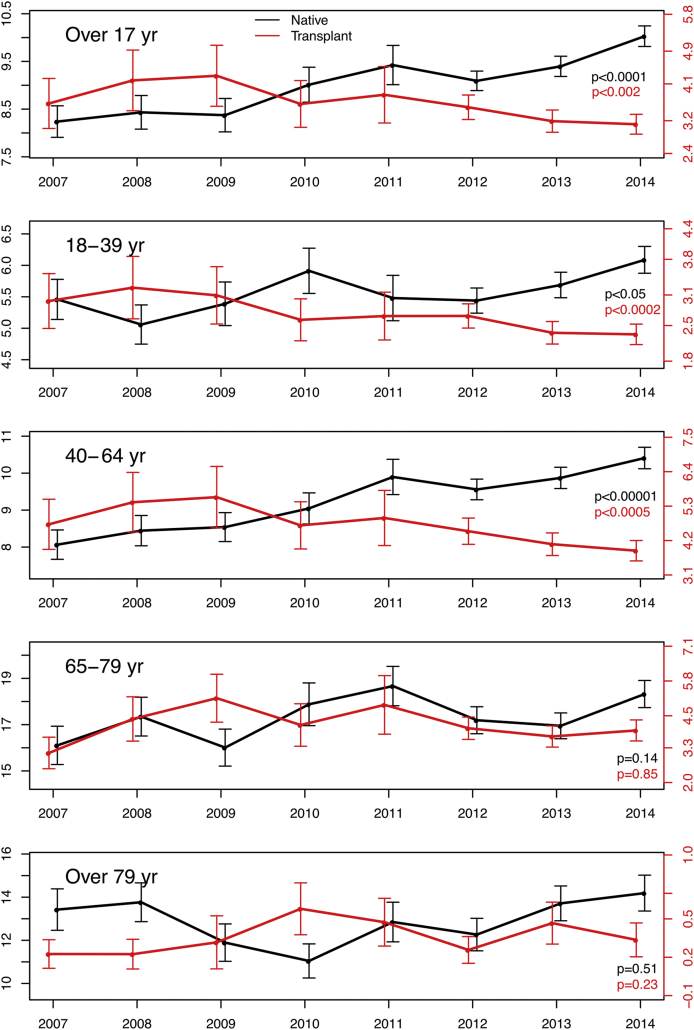
By design, the NIS allows for the estimation of nationally representative rates of hospitalization events. We estimated yearly rates of inpatient native or transplant KBx, adjusting for temporal changes in population size and demographics using the US census data (Figure 2).
Figure 2.
Incidence estimates of native (black) and transplant (red) percutaneous renal biopsies (KBx) among inpatients in the United States, 2007 to 2014. Rates are per 100,000 population. The y-axes on the left of each panel correspond to native KBx (black), and y-axes on the right of each panel correspond to transplant KBx (red). P values for the trend over time are presented in the lower right-hand corner of each panel.
Native KBx rates among adult inpatients in the United States significantly increased over the study period, from an estimated 8.2 per 100,000 adults in 2007 (corresponding to ∼18,720 biopsies per year) to an estimated 10.0 per 100,000 adults in 2014 (∼24,570 biopsies per year), representing a 20% increase (P value for trend < 0.00005; Figure 2). Although inpatient native KBx were more frequently performed among inpatients older than 65 compared with younger adults (per capita), the overall increase in KBx incidence over the period studied appears to be driven by increases in KBx rates among adults aged 18 to 64 years (Figure 2).
In contrast, transplant KBx rates among adult inpatients in the United States declined during the study period, from an estimated 3.6 per 100,000 adults in 2007 (corresponding to ∼8230 biopsies/yr) to 3.1 per 100,000 adults in 2014 (∼7630 biopsies/yr), a decrease of roughly 16% (P value for trend = 0.0016; Figure 2). The overall decline during the study period appears to be driven primarily by a decreasing incidence of transplant inpatient KBx among adults 18 to 64 years old.
Utilization of pRBC Transfusion and Renal Angiogram Associated With Inpatient Native and Transplant KBx, 2007–2014
The NIS does not specifically collect information on hemorrhagic complications following inpatient native and transplant KBx. Without detailed medical records and/or highly specific diagnosis codes, we considered 2 proxies for post-KBx hemorrhagic complications: pRBC transfusion and renal angiogram. For each discharge record, we extracted data on these 2 interventions and their timing relative to KBx. Based on prior studies of the natural history of bleeding complications after KBx,3, 11 we considered pRBC transfusions and renal angiography occurring on the same day as KBx or within 2 days of KBx as biopsy-associated interventions.
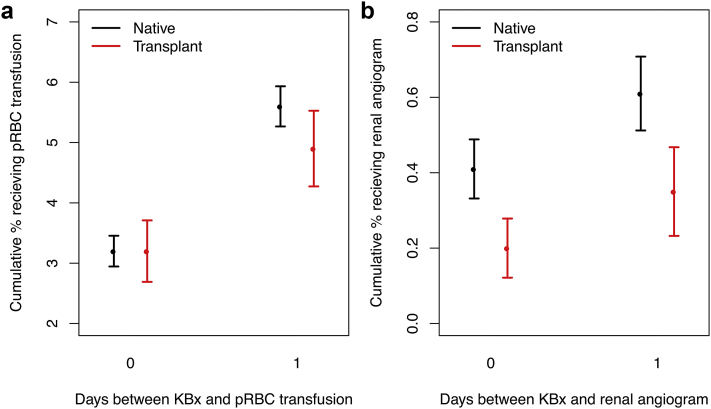
After excluding discharge records lacking information on the timing of KBx or pRBC transfusion (if this occurred), a total of 31,234 (89%) records with native KBx and 12,273 (86%) with transplant KBx remained (Figure 1). Among inpatients undergoing native KBx, 3.23% (95% CI: 2.95%–3.46%) received a pRBC transfusion on the same day as KBx, and 5.66% (95% CI: 5.33%–6.00%) received a pRBC transfusion within 2 days of KBx. Among inpatients undergoing transplant KBx, proportions receiving a pRBC transfusion were generally similar, with 3.22% (95% CI: 2.71%–3.73%) receiving a pRBC transfusion on the same day as KBx, and 4.85% (95% CI: 4.22%–5.48%) receiving a pRBC transfusion within 2 days of KBx (Figure 3). Although point estimates of transfusion frequencies after transplant KBx were generally lower than after native KBx, statistically significant differences were not observed (Figure 3).
Figure 3.
Rates of packed red blood cell (pRBC) transfusion and renal angiogram as a function of time from biopsy (days) among inpatients undergoing native kidney biopsy (KBx; black) and transplant KBx (red). (a, b) Cumulative proportions of patients receiving pRBC transfusion or renal angiogram as a function of days following KBx; only the first 2 days post-intervention are depicted here. Interventions occurring on the same day as KBx are considered “day 0” estimates.
We further explored whether pRBC transfusion rates post-KBx varied according to demographics, comorbidities, or clinical presentations, considering pRBC transfusion within 2 days of KBx as the outcome of interest (Tables 2 and 3). Among native KBx, a significantly increased odds of pRBC transfusion was associated with female sex, older age, higher stages of CKD/ESRD, acute renal failure, vasculitis, cirrhosis, multiple myeloma and other paraproteinemias, systemic lupus erythematosus, and anemia of chronic disease, even after extensive covariate adjustment (adjusted odds ratios: 1.29–1.89; Table 2). We did not observe statistically significant differences in transfusion frequencies by hospital location/teaching status, or among patients with amyloidosis (Table 2). We further did not find evidence of significant variation in pRBC transfusion rates when stratified by the average number of KBx performed at each hospital annually (Supplementary Table S2). Among inpatients undergoing transplant KBx, significantly increased odds of pRBC transfusion were associated with female sex, ESRD, and anemia of chronic disease (adjusted odds ratios: 1.44–1.91; Table 3).
Table 2.
Factors associated with the utilization of pRBC transfusion and renal angiogram within 2 days of native KBx among inpatient adults, 2007–2014
| Covariate | Na (%) | pRBC transfusion within 2 days of KBx |
Renal angiography within 2 days of KBx |
|||||
|---|---|---|---|---|---|---|---|---|
| Crude rate % (SD) | uORb (95% CI) | aORc (95% CI) | Na (%) | Crude rate % (SD) | uORb (95% CI) | aORc (95% CI) | ||
| Total | 31,234 (100) | 5.65 (0.17) | - | - | 31,356 (100) | 0.61 (0.046) | – | – |
| Sex | ||||||||
| Female | 15,227 (48.8) | 6.50 (0.22) | 1.37 (1.24–1.51) | 1.37 (1.24–1.51) | 15,287 (48.8) | 0.69 (0.071) | 1.29 (0.96–1.73) | – |
| Age, yr | ||||||||
| 18–39 | 7706 (24.7) | 4.70 (0.25) | ref. | ref. | 7733 (24.7) | 0.56 (0.084) | ref. | – |
| 40–64 | 13,836 (44.3) | 5.86 (0.23) | 1.26 (1.12–1.43) | 1.23 (1.08–1.40) | 13,889 (44.3) | 0.70 (0.073) | 1.25 (0.89–1.78) | – |
| 65–79 | 7605 (24.3) | 5.90 (0.29) | 1.27 (1.10–1.47) | 1.22 (1.04–1.42) | 7638 (24.4) | 0.52 (0.083) | 0.94 (0.61–1.43) | – |
| 80+ | 2087 (6.7) | 6.80 (0.56) | 1.48 (1.21–1.81) | 1.45 (1.17–1.79) | 2096 (6.7) | 0.53 (0.159) | 0.95 (0.49–1.84) | – |
| Hypertension | 21,641 (69.3) | 5.73 (0.19) | 1.06 (0.95–1.17) | – | 21,722 (69.3) | 0.66 (0.056) | 1.31 (0.95–1.80) | – |
| CKD | ||||||||
| Absent/stages 1–2d | 16,018 (51.3) | 4.57 (0.18) | ref. | ref. | 16,077 (51.3) | 0.43 (0.050) | ref. | ref. |
| Stages 3–5e | 10,802 (34.6) | 6.51 (0.27) | 1.45 (1.30–1.62) | 1.36 (1.21–1.52) | 10,843 (34.6) | 0.73 (0.084) | 1.68 (1.24–2.28) | 1.40 (0.88–2.23) |
| ESRD | 4414 (14.1) | 7.43 (0.42) | 1.67 (1.46–1.92) | 1.51 (1.31–1.75) | 4436 (14.2) | 0.96 (0.15) | 2.23 (1.53–3.26) | 1.73 (1.00–2.97) |
| Acute renal failure | 21,894 (70.1) | 6.21 (0.19) | 1.47 (1.30–1.65) | 1.30 (1.15–1.47) | 21,995 (70.1) | 0.64 (0.054) | 1.21 (0.88–1.67) | – |
| Diabetes | 8857 (28.4) | 6.11 (0.28) | 1.13 (1.01–1.25) | 1.08 (0.97–1.21) | 8897 (28.4) | 0.67 (0.085) | 1.14 (0.85–1.53) | – |
| Nephrotic syndrome | 5062 (16.2) | 4.67 (0.30) | 0.79 (0.69–0.91) | 0.89 (0.77–1.02) | 5079 (16.2) | 0.35 (0.083) | 0.53 (0.33–0.87) | 0.55 (0.34–0.91) |
| Lupus | 3617 (11.6) | 6.78 (0.43) | 1.25 (1.09–1.44) | 1.42 (1.22–1.66) | 3632 (11.6) | 0.64 (0.13) | 1.06 (0.70–1.61) | – |
| RPGN | 717 (2.3) | 7.87 (1.01) | 1.44 (1.09–1.90) | 1.32 (0.99–1.75) | 728 (2.3) | 0.27 (0.194) | 0.44 (0.11–1.79) | – |
| Vasculitis | 2090 (6.7) | 7.21 (0.58) | 1.33 (1.12–1.57) | 1.31 (1.09–1.54) | 2104 (6.7) | 0.63 (0.17) | 1.03 (0.59–1.81) | – |
| Cirrhosis | 710 (2.3) | 9.78 (1.1) | 1.84 (1.44–2.36) | 1.93 (1.50–2.48) | 714 (2.3) | 1.78 (0.52) | 3.10 (1.71–5.61) | 3.16 (1.75–5.69) |
| Amyloid | 372 (1.2) | 4.50 (1.0) | 0.79 (0.48–1.27) | – | 373 (1.2) | 0.26 (0.26) | 0.43 (0.06–3.06) | – |
| Multiple myeloma + paraproteinemias | 1530 (4.9) | 8.34 (0.79) | 1.57 (1.28–1.93) | 1.48 (1.19–1.82) | 1540 (4.9) | 0.97 (0.25) | 1.64 (0.98–2.77) | – |
| Anemia of chronic disease | 7766 (24.9) | 7.50 (0.32) | 1.53 (1.38–1.70) | 1.32 (1.18–1.47) | 7804 (24.9) | 0.82 (0.101) | 1.51 (1.10–2.07) | 1.27 (0.92–1.77) |
| Location/teachingf | ||||||||
| Rural | 1309 (4.2) | 6.38 (1.00) | ref. | – | 1313 (4.2) | 0.29 (0.15) | ref. | – |
| Urban, nonteaching | 11,230 (36.2) | 5.83 (0.27) | 0.91 (0.65–1.28) | – | 11,274 (36.2) | 0.44 (0.061) | 1.52 (0.54–4.29) | – |
| Urban, teaching | 18,465 (59.6) | 5.49 (0.21) | 0.85 (0.61–1.20) | – | 18,537 (59.6) | 0.73 (0.066) | 2.53 (0.92–6.99) | – |
–, not included; CI, confidence interval; CKD, chronic kidney disease; ESRD, end-stage renal disease; KBx, kidney biopsy; OR, odds ratio; pRBC, packed red blood cell; RPGN, rapidly progressing glomerulonephritis.
Significant covariates in univariate and multivariable analyses are bolded.
N are numbers of unweighted discharges captured in the NIS from 2007–2014, with complete information on timing of KBx and the outcome of interest.
uORs are unadjusted (univariate) odds ratios.
aORs are adjusted (multivariable) odds ratios. All variables significant in univariate analyses were included in the multivariable model.
Includes patients without CKD codes (n = 15,597) and patients with CKD stages 1–2 (n = 421).
CKD stages 3–5 include discharge records with CKD-unspecified codes.
Data on hospital location/teaching status were missing in 230 discharge records, which were excluded from univariate analysis.
Table 3.
Factors associated with the utilization of pRBC transfusion and renal angiogram within 2 days of transplant KBx among inpatient adults, 2007–2014
| Covariate | Na (%) | pRBC transfusion within 2 days of KBx |
Renal angiography within 2 days of KBx |
|||||
|---|---|---|---|---|---|---|---|---|
| Crude rate % (SD) | uORb (95% CI) | aORc (95% CI) | Na (%) | Crude rate % (SD) | uORb (95% CI) | aORc (95% CI) | ||
| Total | 12,273 (100) | 4.86 (0.32) | – | – | 12,328 (100) | 0.35 (0.061) | – | – |
| Sex | ||||||||
| Female | 5083 (41.4) | 5.85 (0.47) | 1.44 (1.21–1.70) | 1.41 (1.19–1.67) | 5114 (41.5) | 0.35 (0.084) | 1.00 (0.57–1.76) | – |
| Age, yr | ||||||||
| 18–39 | 3552 (28.9) | 4.40 (0.37) | ref. | – | 3568 (28.9) | 0.19 (0.072) | ref. | – |
| 40–64 | 6814 (55.5) | 4.89 (0.39) | 1.12 (0.92–1.36) | – | 6847 (55.5) | 0.41 (0.083) | 2.12 (0.97–4.63) | – |
| 65–79 | 1849 (15.1) | 5.48 (0.62) | 1.26 (0.98–1.62) | – | 1854 (15.0) | 0.42 (0.149) | 2.16 (0.78–5.98) | – |
| 80+ | 58 (0.5) | 8.59 (3.63) | 2.04 (0.81–5.14) | – | 59 (0.5) | 1.53 (1.531) | 8.07 (0.95–68.52) | – |
| Hypertension | 9933 (80.9) | 4.82 (0.32) | 0.97 (0.79–1.18) | – | 9977 (80.9) | 0.38 (0.069) | 1.84 (0.74–4.59) | – |
| CKD | ||||||||
| Absent/stages 1–2d | 6435 (52.4) | 4.17 (0.33) | ref. | ref. | 6459 (52.4) | 0.34 (0.077) | ref. | – |
| Stages 3–5e | 2153 (17.5) | 4.66 (0.48) | 1.12 (0.89–1.41) | 0.77 (0.55–1.08) | 2161 (17.5) | 0.32 (0.158) | 0.95 (0.32–2.79) | – |
| ESRD | 3685 (30.0) | 6.16 (0.56) | 1.51 (1.24–1.83) | 1.05 (0.77–1.44) | 3708 (30.1) | 0.40 (0.109) | 1.19 (0.61–2.31) | – |
| Diabetes | 4561 (37.2) | 4.83 (0.45) | 0.99 (0.82–1.20) | – | 4572 (37.1) | 0.43 (0.106) | 1.42 (0.81–2.51) | – |
| Acute renal failure | 7799 (63.6) | 4.72 (0.32) | 0.92 (0.78–1.10) | – | 7839 (63.6) | 0.38 (0.073) | 1.22 (0.65–2.27) | – |
| Anemia of chronic disease | 2507 (20.4) | 7.60 (0.67) | 1.91 (1.56–2.32) | 1.79 (1.47–2.19) | 2531 (20.5) | 0.41 (0.137) | 1.20 (0.56–2.59) | – |
| Location/teachingf | ||||||||
| Rural | 69 (0.6) | 1.57 (1.66) | ref. | – | 69 (0.6) | 0.00 (0.00) | – | – |
| Urban, nonteaching | 790 (6.5) | 4.82 (0.84) | 3.18 (0.37–26.99) | – | 792 (6.5) | 0.49 (0.251) | ref. | – |
| Urban, teaching | 11,310 (92.9) | 4.91 (0.34) | 3.24 (0.39–26.83) | – | 11,363 (93.0) | 0.33 (0.061) | 0.66 (0.23–1.90)g | – |
–, not included; CI, confidence interval; CKD, chronic kidney disease; ESRD, end-stage renal disease; KBx, kidney biopsy; OR, odds ratio; pRBC, packed red blood cell.
Significant covariates in univariate and multivariable analyses are bolded.
N are numbers of unweighted discharges captured in the NIS from 2007–2014, with complete information on timing of KBx and outcome of interest.
uORs are unadjusted (univariate) odds ratios.
aORs are adjusted odds ratios. All variables significant on univariate analysis were included in the multivariable model.
Includes patients without CKD codes (n = 6400) and patients with CKD stages 1–2 (n = 35).
CKD stages 3–5 include discharge records with CKD-unspecified codes.
Data on hospital location/teaching status were missing in 104 discharge records, which were excluded from the univariate analysis.
No renal angiograms occurred among transplant KBx patients in rural hospitals; univariate ORs comparing renal angiography in urban teaching hospitals compared with urban nonteaching hospitals are provided.
A total of 31,356 (89%) and 12,328 (86%) discharge records with native and transplant KBx, respectively, contained complete information on the relative timing of KBx and renal angiography (Figure 1). Among inpatients undergoing native KBx, 0.41% (95% CI: 0.33%–0.49%) received a renal angiogram on the same day as KBx, and 0.61% (95% CI: 0.51%–0.71%) received a renal angiogram within 2 days of KBx. Among inpatients undergoing transplant KBx, 0.20% (95% CI: 0.12%–0.28%) received a renal angiogram on the same day as KBx, and 0.36% (95% CI: 0.24%–0.48%) received a renal angiogram within 2 days of KBx (Figure 3). The proportion of patients undergoing renal angiography was significantly lower for posttransplant KBx compared with post-native KBx at all time points considered (P < 0.0005; Figure 3). Examining clinical conditions associated with the utilization of renal angiography among inpatients undergoing native KBx, adjusted analyses demonstrated increased odds among patients with ESRD and cirrhosis (adjusted odds ratios: 1.73–3.16; Table 2), and decreased odds among patients with nephrotic syndrome (adjusted odds ratio: 0.55; Table 2). No statistically significant differences were observed in the utilization of renal angiography after transplant KBx among the demographic groups and clinical conditions considered (Table 3).
In supplementary analyses, we present more detailed data on the timing of pRBC transfusion/renal angiogram relative to inpatient KBx in the study cohort (Supplementary Table S3). We also provide additional information regarding discharge records with missing data on the timing of procedures (Supplementary Tables S4 and S5), and substantiate the validity of the analyses limited to complete discharge records presented.12 Finally, we provide data on inpatient mortality within 2 days of KBx (Supplementary Table S6).
Discussion
In this study, we used a nationally representative database on US hospitalization discharges to examine temporal trends in rates of inpatient percutaneous KBx from 2007 to 2014 and to quantify the proportion of patients receiving a blood transfusion or renal angiogram post-KBx. We studied 35,183 discharge records of native KBx and 14,266 discharge records of transplant KBx, representing the largest analysis of US KBx practice patterns and outcomes to date by far.
To our knowledge, ours is the first study to report temporal trends in rates of KBx among adult inpatients at the US population level. We found that rates of inpatient native KBx increased by approximately 20% from 2007 to 2014, from 8.2 to 10.0 per 100,000 adults. Although a true increase in kidney disease incidence cannot be excluded, we hypothesize that the increase in incidence reflects wider recognition of the utility of KBx in the diagnosis and management of intrinsic kidney disease.1, 2 Rates of inpatient transplant KBx, in contrast, declined by approximately 16%, from 3.6 to 3.1 per 100,000 adults over the study period, despite relatively stable rates of kidney transplantation in the United States over this time period.13 Again, a true decrease in kidney transplant complications requiring investigation by KBx cannot be excluded. However, we hypothesize that this trend likely reflects an increased incidence of transplant KBx occurring in the outpatient setting, which would not be captured by the NIS. Overall, our findings suggest that KBx continues to play an important role in the investigation and management of kidney disease, emphasizing an ongoing need for adequate training of nephrologists and radiologists in KBx procurement, as well as for nephropathologists in KBx interpretation.14
Our study also provides accurate and nationally representative estimates of the frequencies of pRBC transfusion and renal angiography following inpatient KBx. We found that 3.2% (95% CI: 3.0%–3.5%) and 5.7% (95% CI: 5.3–6.0%) of adult inpatients undergoing native KBx and 3.2% (95% CI: 2.7%–3.7%) and 4.9% (95% CI: 4.2%–5.5%) of adult inpatients undergoing transplant KBx received a pRBC transfusion on the same day or within 2 days, respectively, of KBx. Although reported rates of pRBC transfusion associated with KBx vary widely across studies, 2 relatively large studies,5, 15 and a systematic review and meta-analysis,4 estimated a post-KBx pRBC transfusion rate of ∼1.0%. Our significantly higher estimates likely reflect our inpatient study population, that might be more prone to complications or more likely to receive pRBC transfusions for anemia associated with other systemic illnesses. Indeed, recent studies of KBx-associated complications among inpatients with acute kidney injury found post-KBx pRBC transfusion rates of 8.0% to 11.3%.6, 11, 16
We estimate that 0.4% (95% CI: 0.3%–0.5%) and 0.6% (95% CI: 0.5%–0.7%) of inpatients undergoing native KBx and 0.2% (95% CI: 0.1%–0.3%) and 0.4% (95% CI: 0.2%–0.5%) of inpatients undergoing transplant KBx receive a renal angiogram on the same day or within 2 days, respectively, of KBx. Published rates of angiographic intervention following KBx are even more sparse and variable than for pRBC transfusion, although generally range from 0.4% to 2.0% in native KBx,11, 17, 18 and ∼0.2% in transplant KBx.18 Accurate estimation of rates of angiographic intervention is extremely challenging because of the relative rarity of this intervention; the sample size assembled within NIS allows for far more precise estimation of this rare intervention than estimates in the published literature.
The large sample size of inpatient KBx considered here also allowed for careful covariate adjustment and identification of demographics and clinical conditions associated with increased utilization of pRBC transfusion or renal angiogram. Prior work has identified the following risk factors associated with major hemorrhagic complications post-KBx: older age (older than 60 years), female sex, low glomerular filtration rate, elevated serum creatinine, acute kidney injury, systolic hypertension, and smaller clinical center size.4, 5, 7, 11 Earlier literature also has suggested that patients with amyloidosis do not experience an elevated risk of hemorrhagic complications post-KBx.19, 20 Among inpatients undergoing native KBx, we too found evidence that older age, female sex, poorer kidney function (as represented by more advanced stages of CKD/ESRD), and acute renal failure were associated with an increased odds of pRBC transfusion post-KBx. And like prior studies, we did not find evidence of increased transfusion or renal angiography utilization among inpatients with amyloidosis. Accordingly, our data confirm that many previously identified risk factors for post-biopsy hemorrhage also hold true at the larger US population level, even after adjustment for potentially confounding factors. In contrast to Tøndel et al.,5 we did not find evidence that pRBC transfusion frequencies were lower in urban academic medical centers. In fact, we found that the proportion of patients receiving renal angiography post-KBx was significantly higher in urban academic medical centers, likely related to a more complex case mix of patients undergoing inpatient KBx and/or the wider availability of proceduralists, including interventional radiologists, at these centers. Furthermore, in a sensitivity analysis, we did not observe significant variation in pRBC transfusion rates after inpatient native KBx, when stratified by the average number of KBx performed at each hospital annually.
Of note, our study highlights that inpatients with nephrotic syndrome experience lower rates of pRBC transfusion and renal angiogram after native KBx. To our knowledge, this finding has not been reported in the literature. We speculate that the lower rates observed may be attributable to hypercoagulability often seen in patients with nephrotic syndrome.21 Likewise, the finding that women experience increased risks of pRBC transfusion after KBx is intriguing and has been reported in prior studies.4, 11 Further work is necessary to determine the underlying etiology of this increased risk, but potential differences in anatomy, vascular reactivity, platelet activity, and/or coagulation may mediate the risk of post-KBx bleeding in women.22
A recent review of kidney biopsy practices highlighted patient populations warranting special consideration and for whom data on the safety of KBx is severely limited. These include elderly or very elderly patients, patients with hepatic failure, and patients with monoclonal gammopathies and paraprotein-related diseases.23 We have helped fill in several of these evidence gaps. We identified increased odds for pRBC transfusion among elderly/very elderly patients, patients with cirrhosis, and patients with multiple myeloma and other paraproteinemias. Patients with cirrhosis also experienced increased odds of renal angiogram after native KBx. Finally, our study also highlights marginally higher post-KBx pRBC transfusion frequencies among patients with lupus or vasculitis. The increased utilization of pRBC transfusion in these specific groups of patients might in part relate to concomitant anemia and thrombocytopenia, which are known to occur frequently in patients with cirrhosis and paraproteinemias.24, 25 It is important to note, however, that the absolute differences in frequencies of pRBC transfusion or renal angiogram were relatively modest across all subgroups studied, and thus the presence of one or more of these risk factors should not necessarily preclude the performance of a KBx if it is otherwise indicated.
Careful interpretation of the data presented herein is necessary. Although the NIS provides a valuable resource for obtaining accurate estimates of the utilization of pRBC transfusion and renal angiography after KBx, data on the whether these interventions truly target hemorrhage were not available. Indeed, pre-biopsy anemia is a significant risk factor for the receipt of pRBC transfusion after KBx, even in the absence of clinically evident bleeding.26 Furthermore, many of the risk factors for pRBC transfusion within 2 days of KBx identified in our study (e.g., older age, female sex, cirrhosis, ESRD, and others) are strongly associated with anemia, suggesting that some proportion of pRBC transfusions after KBx are targeted at correcting anemia rather than treating overt hemorrhage. We suspect that utilization of renal angiogram after KBx is more strongly linked to clinically evident bleeding, but, nonetheless, data on the indications for renal angiography were not available.
Several other limitations of our study should be mentioned. First, as our analyses were based on ICD-9-CM coded discharge records, we lacked some relevant clinical information, including blood pressure and laboratory measurements (e.g., serum creatinine or hemoglobin), as well as information on biopsy operator (nephrologist or radiologist), needle-gauge, and the indication for and diagnostic outcome of the biopsy. The exact timing of pRBC transfusion and renal angiogram relative to biopsy (in hours) was unavailable, as timing of procedures in the NIS is available only at the level of days relative to admission. However, unlike 2 smaller studies using the NIS data,11, 27 we deliberately report the frequencies of pRBC transfusion and renal angiogram as a function of days from KBx, and hypothesize that those occurring on the day of or day following KBx are more likely to be attributable to the KBx procedure. We further demonstrate that failure to consider the timing between KBx and the interventions of interest (i.e., including interventions that occurred before, or many days after, KBx) substantially overestimates the frequency of these biopsy-associated interventions, underscoring the importance of accurate statistical analysis of this large publicly available dataset. Despite this substantial improvement on prior studies, we acknowledge that our approach might still overestimate pRBC transfusion and renal angiogram associated with KBx for the following reasons: (i) transfusions/renal angiography occurring before KBx, but on the same day, would be included in our estimates under our study design. and (ii) patients who obtain a KBx in an outpatient setting and experience a bleeding complication requiring hospitalization might be included in our study cohort as well. Finally, in studying variation in the utilization of pRBC transfusion and renal angiogram post-KBx across demographic groups and clinical conditions, we relied on ICD-9-CM codes to identify selected groups of patients, and patients might have been misclassified based on differing coding practices. The potential for misclassification might be especially pronounced in transplanted patients, who may carry a diagnosis of ESRD from before transplantation despite having a functioning allograft. Reassuringly, the prevalence of diabetes (28%), systemic lupus erythematosus (11%), and vasculitis (7%) in our study cohort, identified by ICD-9-CM codes, is broadly consistent with prevalence reported in published biopsy series.28, 29 Furthermore, patients with ESRD codes at discharge had disproportionately higher rates of inpatient hemodialysis, supporting the validity of ESRD codes.
Despite the aforementioned limitations, our study has several strengths. In particular, it represents the largest and most comprehensive analysis of US inpatient KBx practice patterns and potential risks. Our data confirm the general consensus that KBx is associated with an acceptable level of risk in most patients, even among inpatients who might be acutely unwell or among those with additional risk factors for bleeding complications. Our estimates of the frequencies of pRBC transfusion and renal angiogram, and factors associated with their utilization, are derived from real world patient populations and real world practice: thus, we consider our findings to be directly applicable to clinical practice, particularly with regard to counseling inpatients being considered for KBx regarding their expected risks.
Disclosure
All the authors declared no competing interests.
Acknowledgments
We are grateful to Chirag Parikh, MD, PhD, at The Johns Hopkins University School of Medicine for helpful discussion regarding the Moledina et al. study.11
Author Contributions
Conceived and designed the experiments/analysis: VC, MOS, GMC, NK. Performed the experiments: VC. Analyzed the data: VC. Contributed reagents/materials/analysis tools: VC, MOS, GMC, NK. Wrote the paper: VC, MOS, GMC, NK.
Footnotes
Supplementary Material
References
- 1.Dhaun N., Bellamy C.O., Cattran D.C., Kluth D.C. Utility of renal biopsy in the clinical management of renal disease. Kidney Int. 2014;85:1039–1048. doi: 10.1038/ki.2013.512. [DOI] [PubMed] [Google Scholar]
- 2.D’Agati V.D., Mengel M. The rise of renal pathology in nephrology: structure illuminates function. Am J Kidney Dis. 2013;61:1016–1025. doi: 10.1053/j.ajkd.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Whittier W.L., Korbet S.M. Timing of complications in percutaneous renal biopsy. J Am Soc Nephrol. 2004;15:142–147. doi: 10.1097/01.asn.0000102472.37947.14. [DOI] [PubMed] [Google Scholar]
- 4.Corapi K.M., Chen J.L.T., Balk E.M., Gordon C.E. Bleeding complications of native kidney biopsy: a systematic review and meta-analysis. Am J Kidney Dis. 2012;60:62–73. doi: 10.1053/j.ajkd.2012.02.330. [DOI] [PubMed] [Google Scholar]
- 5.Tøndel C., Vikse B.E., Bostad L., Svarstad E. Safety and complications of percutaneous kidney biopsies in 715 children and 8573 adults in Norway 1988–2010. Clin J Am Soc Nephrol. 2012;7:1591–1597. doi: 10.2215/CJN.02150212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simard-Meilleur M.-C., Troyanov S., Roy L. Risk factors and timing of native kidney biopsy complications. Nephron Extra. 2014;4:42–49. doi: 10.1159/000360087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manno C., Strippoli G.F.M., Arnesano L. Predictors of bleeding complications in percutaneous ultrasound-guided renal biopsy. Kidney Int. 2004;66:1570–1577. doi: 10.1111/j.1523-1755.2004.00922.x. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Population Data—SEER Population Data. SEER. https://seer.cancer.gov/popdata/index.html Available at:
- 9.NIS Database Documentation. https://www.hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp Available at:
- 10.Dumouchel W.H., Duncan G.J. Using sample survey weights in multiple regression analyses of stratified samples. J Am Stat Assoc. 1983;78:535–543. [Google Scholar]
- 11.Moledina D.G., Luciano R.L., Kukova L. Kidney biopsy-related complications in hospitalized patients with acute kidney disease. Clin J Am Soc Nephrol. 2018;13:1633–1640. doi: 10.2215/CJN.04910418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubin D.B. Inference and missing data. Biometrika. 1976;63:581–592. [Google Scholar]
- 13.v2 CH6 Transplantation. https://www.usrds.org/2018/view/v2_06.aspx Available at:
- 14.Yuan C.M., Nee R., Little D.J. Nephrology Education Research and Development Consortium (NERDC): Survey of Kidney Biopsy Clinical Practice and Training in the United States. Clin J Am Soc Nephrol. 2018;13:718–725. doi: 10.2215/CJN.13471217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atwell T.D., Spanbauer J.C., McMenomy B.P. The timing and presentation of major hemorrhage after 18,947 image-guided percutaneous biopsies. AJR Am J Roentgenol. 2012;205:190–195. doi: 10.2214/AJR.14.13002. [DOI] [PubMed] [Google Scholar]
- 16.Korbet S.M., Gashti C.N., Evans J.K., Whittier W.L. Risk of percutaneous renal biopsy of native kidneys in the evaluation of acute kidney injury. Clin Kidney J. 2018;11:610–615. doi: 10.1093/ckj/sfy048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lees J.S., McQuarrie E.P., Mordi N. Risk factors for bleeding complications after nephrologist-performed native renal biopsy. Clin Kidney J. 2017;10:573–577. doi: 10.1093/ckj/sfx012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whittier W.L., Gashti C., Saltzberg S., Korbet S. Comparison of native and transplant kidney biopsies: diagnostic yield and complications. Clin Kidney J. 2018;11:616–622. doi: 10.1093/ckj/sfy051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soares S.M., Fervenza F.C., Lager D.J. Bleeding complications after transcutaneous kidney biopsy in patients with systemic amyloidosis: single-center experience in 101 patients. Am J Kidney Dis. 2008;52:1079–1083. doi: 10.1053/j.ajkd.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Fish R., Pinney J., Jain P. The incidence of major hemorrhagic complications after renal biopsies in patients with monoclonal gammopathies. Clin J Am Soc Nephrol. 2010;5:1977–1980. doi: 10.2215/CJN.00650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gigante A., Barbano B., Sardo L. Hypercoagulability and nephrotic syndrome. Curr Vasc Pharmacol. 2014;12:512–517. doi: 10.2174/157016111203140518172048. [DOI] [PubMed] [Google Scholar]
- 22.Roy-O’Reilly M., McCullough L.D. Sex differences in stroke: the contribution of coagulation. Exp Neurol. 2014;259:16–27. doi: 10.1016/j.expneurol.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hogan J.J., Mocanu M., Berns J.S. The native kidney biopsy: update and evidence for best practice. Clin J Am Soc Nephrol. 2016;11:354–362. doi: 10.2215/CJN.05750515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tripodi A. Liver disease and hemostatic (dys)function. Semin Thromb Hemost. 2015;41:462–467. doi: 10.1055/s-0035-1550440. [DOI] [PubMed] [Google Scholar]
- 25.Glaspy J.A. Hemostatic abnormalities in multiple myeloma and related disorders. Hematol Oncol Clin North Am. 1992;6:1301–1314. [PubMed] [Google Scholar]
- 26.Whittier W.L., Sayeed K., Korbet S.M. Clinical factors influencing the decision to transfuse after percutaneous native kidney biopsy. Clin Kidney J. 2016;9:102–107. doi: 10.1093/ckj/sfv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al Turk A.A., Estiverne C., Agrawal P.R., Michaud J.M. Trends and outcomes of the use of percutaneous native kidney biopsy in the United States: 5-year data analysis of the nationwide inpatient sample. Clin Kidney J. 2018;11:330–336. doi: 10.1093/ckj/sfx102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma S.G., Bomback A.S., Radhakrishnan J. The modern spectrum of renal biopsy findings in patients with diabetes. Clin J Am Soc Nephrol. 2013;8:1718–1724. doi: 10.2215/CJN.02510213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Shaughnessy M.M., Hogan S.L., Poulton C.J. Temporal and demographic trends in glomerular disease epidemiology in the southeastern United States, 1986–2015. Clin J Am Soc Nephrol. 2017;12:614–623. doi: 10.2215/CJN.10871016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.