See Clinical Research on Page 1373
Worldwide, 425 million individuals or 1 in 11 has diabetes, and projections suggest a 48% increase by 2045 to 629 million people, according to the International Diabetes Federation, and whenever projections are updated, the numbers are increased. Treatment of diabetes and chronic kidney disease in diabetes has improved with multiple risk factor control, including blood glucose and blood pressure, including blockade of the renin angiotensin system with significant improvements in outcome.1 US national survey data demonstrated a 28% reduction in risk for end-stage renal disease (ESRD) for the individual patient from 1990 to 2010, but regardless, the number of subjects referred for ESRD treatment increased from 20,000 to 50,000 during this period because of the increasing prevalence of diabetes.2 The increasing population at risk is a major determinant, although also reduced mortality from cardiovascular disease before ESRD, and increased eligibility for treatment of ESRD has contributed to this, but it also reflects a need for better prevention and treatment of diabetic kidney disease (DKD). Approximately 30% to 40% of subjects with diabetes have chronic kidney disease and conversely DKD is the leading cause of ESRD in the Western world. In addition to the risk for ESRD, DKD is associated with a significantly increased risk for cardiovascular disease, including atherosclerotic cardiovascular disease, as well as heart failure.
To stop this epidemic of DKD, prevention is of the utmost importance. Prevention of diabetes would be ideal, but the large and growing population of subjects with diabetes makes it necessary to consider prevention of initiation or progression of DKD in subjects with established diabetes, being it type 1 or type 2. Treatment of overt DKD with risk factor control and blockade of the renin angiotensin system has been recommended for decades, and recent studies suggest that new glucose-lowering agents, such as sodium glucose transporter 2 inhibitors and perhaps glucagon-like peptide 1 receptor agonists, also will be helpful but will not solve the problem.
Early prevention of microalbuminuria has been more difficult, although controlling glucose seems useful, but difficult to do successfully, and quality assurance data show that fewer than 15% are at target for glucose, lipids, blood pressure, and smoking.
Attempts to prevent initiation of microalbuminuria with early renin angiotensin system blockade has given mixed results, with best effect in hypertensive normoalbuminuric type 2 patients. For early prevention to be successful, we need better understanding of the underlying pathophysiology. Studying cohorts of patients at risk for DKD, and identifying biomarkers associated with risk for progression, could provide us with clues to the underlying processes leading from uncomplicated diabetes to DKD. Such markers also could provide targets for intervention or markers used for identification of high-risk subjects in need of intensive risk factor control. Markers also may be useful for monitoring the effect of targeted interventions.
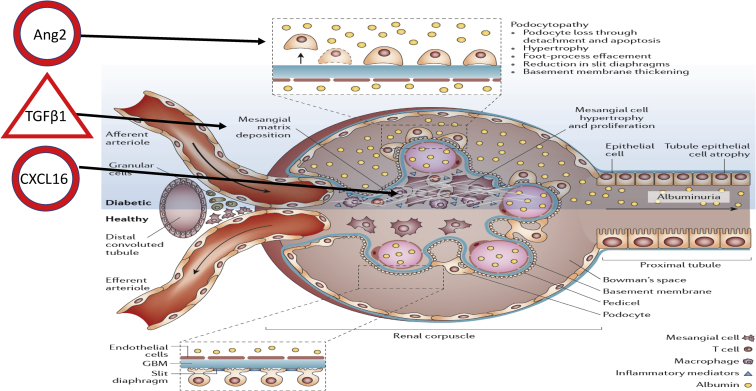
Randomized Olmesartan And Diabetes MicroAlbuminuria Prevention (ROADMAP) was a study of 4447 subjects with type 2 diabetes and normal urinary albumin excretion randomized to the angiotensin II receptor blocker olmesartan or placebo, and demonstrated a small but significant reduction in progression to microalbuminuria, which developed in 8.2% of the patients in the olmesartan group (178 of 2160 patients) and 9.8% in the placebo group (210 of 2139). Patients were followed for an extended period, and the cohort has now been used for a study of biomarkers as predictors of progression to microalbuminuria to teach us about underlying pathophysiology by selection of markers reflecting different potential pathways, including inflammation, fibrosis, endothelial dysfunction, and vascular formation and damage. The markers were studied in a case-control subset of 172 patients progressing to microalbuminuria, with serum samples available before progression, and a control group of 188 matched controls. The study tested 15 markers, and 3 were identified as independent risk factors: CXCL16, angiopoietin 2, and transforming growth factor β1.3 This would suggest that systemic inflammation, extracellular matrix remodeling, and angiogenesis-related processes are important (see Figure 14).
Figure 1.
Randomized Olmesartan And Diabetes MicroAlbuminuria Prevention (ROADMAP) biomarkers of microalbuminuria. ROADMAP has identified 3 biomarkers, CXCL16, angiopoietin 2 (Ang2), and transforming growth factor beta 1 (TGFβ1), as markers of progression to microalbuminuria, related to inflammation, endothelial cell/podocyte damage, and deposition of extracellular matrix, as indicated on the figure. Modified with permission from Thomas MC, Brownlee M, Susztak K, et al. Diabetic kidney disease [published correction appears in Nat Rev Dis Primers. 2015;1:15070]. Nat Rev Dis Primers. 2015;1:15018.4 GBM, glomerular basement membrane.
As discussed in their article, both in preclinical and clinical studies, systemic as well as local inflammation has been identified as an important process in the development of renal and cardiovascular complications in diabetes, but it is difficult to know if increased markers of inflammation are the cause of renal disease or are a response to other processes underlying the disease initiation. In the current study, markers were measured once at baseline, before development of microalbuminuria, but trajectories of inflammatory markers were not studied. In a group of normoalbuminuric subjects with type 1 diabetes, repeated measurements of markers of inflammation and endothelial dysfunction for 20 years demonstrated a rise in most markers over time, but inflammation was more pronounced and preceded increases in albuminuria even in the normal range.5
Recently a kidney risk inflammatory signature in circulation was described in a study of 3 diabetes cohorts predicting ESRD but with more advanced DKD, illustrating how markers of inflammation are increased both in relation to initiation and progression of DKD.6 This marker included several tumor necrosis factor–related proteins, including TNFR 1+2, whereas these proteins were not significantly different between cases and controls in the ROADMAP study. Whether the lack of overlap between markers reflects the different platforms used, different stages of disease, or other factors is not clear. For future studies it would be important to standardize platforms across studies and validate findings in multiple cohorts both in terms of setting, stage of disease, and populations, as well as impact of interventions.
In relation to the dream of biomarkers guiding us to useful therapy, it is somewhat discouraging that 1 of the 3 significant markers, transforming growth factor β1, has been targeted unsuccessfully, as discussed in the article, whereas MCP1, although significantly different between cases and controls in the univariate analysis did not make it as a predictor, but has been the target in several studies with some effect on albuminuria. In addition, VAP1, which was not significant between groups, was also recently targeted with success.7
The chemokine CXCL16 was 1 of the 3 biomarkers and at least other chemokines have been targeted with an albuminuria-lowering effect.S1 One of the largest studies targeting inflammation in DKD was the Beacon trial, and with bardoxolone increasing estimated glomerular filtration rate, not affecting albuminuria but stopped because of side effects.8 It may be important that all the clinical intervention studies were performed in early or late established DKD and thus were not preventive studies, and although several studies reduced albuminuria, the effect has generally been modest with approximately 20% reduction in albuminuria. The bardoxolone studies increased estimated glomerular filtration rate but gave side effects before long-term benefits were demonstrated.
Pentoxifylline is a methylxanthine derivate and nonspecific phosphodiesterase inhibitor with anti-inflammatory, antiproliferative, and antifibrotic actions in experimental studies. A clinical study that successfully targeted inflammation in DKD with pentoxifylline demonstrated a smaller decline in estimated glomerular filtration rate, which after 2 years had decreased by a mean ± SEM of 2.1 ± 0.4 ml/min per 1.73 m2 in the pentoxifylline group compared with 6.5 ± 0.4 ml/min per 1.73 m2 in the control group, with a between-group difference of 4.3 ml/min per 1.73 m2 (95% confidence interval 3.1 to 5.5 ml/min per 1.73 m2; P < 0.001) in favor of pentoxifylline. The study was open label and did not investigate progression to ESRD or doubling of creatinine, for which it was not powered, which may explain why it is not applied.S2
Whether prevention studies targeting inflammation would be successful is not known, and it may be important to select normoalbuminuric individuals with a high risk, based on clinical risk factors or biomarkers. As an example of a study applying risk markers for identification for intervention, the PRIORITY study in normoalbuminuric type 2 diabetes applied the urinary proteome–based risk factor CKD273 to identify high-risk subjects for progression to DKD, and were subsequently targeted with intervention (spironolactone or placebo), thus the study targeted fibrosis, not inflammation, but illustrates enrichment of high-risk candidates.9
When added to the clinical characteristics, the 3 biomarkers were able to increase receiver operating characteristic area under the curve from 0.638 to 0.760, which was highly significant. It is, however, important to recall that the effect of clinical characteristics may be difficult to interpret in a study in which the cases and controls were very carefully matched for multiple risk factors at baseline, and the area under the curve in a case-control study with almost 50% progressors and 50% controls cannot be applied in a clinical setting, as in the ROADMAP study only 8% to 10% were progressors, thus false-positives and false-negatives would be very different if the markers were used to identify progressors
In conclusion, the substudy from ROADMAP has provided new signs leading us in the direction of early DKD. The future will tell if they will be useful as risk markers or also as identified targets.
Disclosure
PR is on the Steering Committee of the following clinical trials: CADA-DIA (Bayer), Fidelio (Bayer), Figaro (Bayer), DAPA-CKD (AstraZeneca), and FLOW (Novo Nordisk); Steno Diabetes Center Copenhagen has received fees for consultancy and/or speaking from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, Eli Lilly, Mundi, Novo Nordisk, and Sanofi Aventis. FP has served as a consultant, on advisory boards, and as an educator for AstraZeneca, Novo Nordisk, Sanofi, Mundipharma, Merck Sharp & Dohme, Boehringer Ingelheim, Novartis, and Amgen; and has received research grants to his institution from Novo Nordisk, Amgen, and AstraZeneca. The other author declared no competing interests.
Footnotes
Supplementary References.
Supplementary Material
References
- 1.Andresdottir G., Jensen M.L., Carstensen B. Improved survival and renal prognosis of patients with type 2 diabetes and nephropathy with improved control of risk factors. Diabetes Care. 2014;37:1660–1667. doi: 10.2337/dc13-2036. [DOI] [PubMed] [Google Scholar]
- 2.Gregg E.W., Li Y., Wang J. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370:1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 3.Scurt F.G., Menne J., Brandt S. Systemic inflammation precedes microalbuminuria in diabetes. Kidney Int Rep. 2019;4:1373–1386. doi: 10.1016/j.ekir.2019.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas M.C., Brownlee M., Susztak K. Diabetic kidney disease [published correction appears in Nat Rev Dis Primers. 2015;1:15070] Nat Rev Dis Primers. 2015;1:15018. doi: 10.1038/nrdp.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferreira I., Hovind P., Schalkwijk C.G. Biomarkers of inflammation and endothelial dysfunction as predictors of pulse pressure and incident hypertension in type 1 diabetes: a 20 year life-course study in an inception cohort. Diabetologia. 2018;61:231–241. doi: 10.1007/s00125-017-4470-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niewczas M.A., Pavkov M.E., Skupien J. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med. 2019;25:805–813. doi: 10.1038/s41591-019-0415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Zeeuw D., Renfurm R.W., Bakris G. Efficacy of a novel inhibitor of vascular adhesion protein-1 in reducing albuminuria in patients with diabetic kidney disease (ALBUM): a randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2018;6:925–933. doi: 10.1016/S2213-8587(18)30289-4. [DOI] [PubMed] [Google Scholar]
- 8.de Zeeuw D., Akizawa T., Audhya P. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med. 2013;369:2492–2503. doi: 10.1056/NEJMoa1306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindhardt M., Persson F., Currie G. Proteomic prediction and Renin angiotensin aldosterone system Inhibition prevention Of early diabetic nephRopathy in TYpe 2 diabetic patients with normoalbuminuria (PRIORITY): essential study design and rationale of a randomised clinical multicentre trial. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-010310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.