The prevalence of pediatric chronic kidney disease (CKD) ranges from 15 to 74.7 cases per 1 million children.1 Mortality among children who progress to end-stage kidney disease (ESKD) is 30 to 50 times higher compared to that in the general population.1, 2 Most of the data on pediatric CKD comes from the developed world. The North American Pediatric Renal Collaborative Trials (NAPRTCS) data (n = 4133) show a progression rate from CKD stages II to IV to ESKD of 17% at 1 year and 39% at 3 years, with the median time to ESKD being 4.5 years.3 The NAPRTCS registry and the Chronic Kidney Disease in Children (CKiD) study identified several modifiable (proteinuria, hypertension, anemia, dyslipidemia) and nonmodifiable (age, primary disease, stage of CKD) risk factors for progression of CKD in children.3, 4, 5, 6 The Effect of Strict Blood Pressure Control and Angiotensin Converting Enzyme Inhibition on the Progression of Chronic Renal Failure in Pediatric Patients (ESCAPE) trial showed that reduction of blood pressure in children with CKD to below the 50th centile significantly reduced the rate of progression.7
There is scant literature on the etiology, rate of progression, risk factors for progression, comorbidities, and outcomes in children with CKD from low- to middle-income countries (LMIC). Common problems in LMIC include delay in diagnosis, increased burden of comorbidities, poor access to health care, high financial burden of treatment, and lack of social support.8, 9,S1−S5 There is a large knowledge gap regarding the risk factors for CKD progression under such circumstances.8,S6 Better understanding of modifiable risk factors and early interventions to delay progression to ESKD in LMIC is urgent, as many children are unlikely to gain access to dialysis or transplantation should their kidneys fail.S7 We conducted a prospective cohort study to determine the rate of progression of CKD stages II to IV, to identify the risk factors associated with progression of CKD, and to assess the impact of CKD on quality of life (QoL) in children attending a pediatric CKD clinic in India.
Results
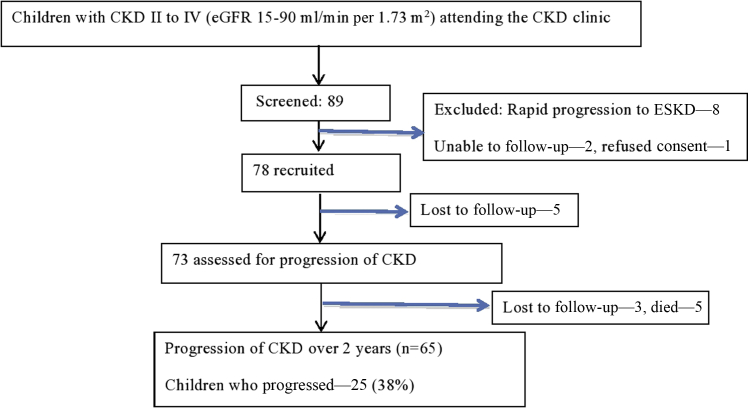
The recruitment and follow-up algorithm is shown in Figure 1. The evaluation at recruitment and follow-up are depicted in Table 1. Demographic details of the cohort (n = 78) at recruitment are described in Table 2. Of the 78 children, 5 were lost to follow-up before their first visit, 3 (4.1%) were lost to follow-up later, and 5 (6.8%) died. A total of 65 children were followed up for 2 to 3 years or until they reached an estimated glomerular filtration rate (eGFR) of <15 ml/min per 1.73 m2. The median GFR, CKD stages, and prevalence of comorbidities at recruitment are described in Table 2.
Figure 1.
Algorithm depicting recruitment and follow-up of the cohort. CKD, chronic kidney disease; ESKD, end-stage kidney disease.
Table 1.
Evaluation of the patient at recruitment and follow-up
| Evaluation | Recruitment | 6 mo | 1-yr follow-up | 18 mo | 2-yr follow-up |
|---|---|---|---|---|---|
| History | Symptoms | a | b | a | b |
| Examination | Height Weight Body mass index Tanner stage Blood pressure |
a | b | a | b |
| eGFR | Creatinine (eGFR) | a | b | a | b |
| Renal | Urine Protein/creatinine ratio Metabolic panelc |
b b |
b b |
||
| Mineral bone disease | Calcium Phosphate Alkaline phosphatase Vitamin D Parathormone |
a |
b b b |
a |
b b b |
| Cardiovascular | Lipid profile Carotid intima-media thickness Echocardiography |
b | b |
eGFR, estimated glomerular filtration rate.
Clinical details and eGFR done 6 monthly.
Investigations repeated at yearly intervals.
Metabolic panel: serum electrolytes, serum bicarbonate.
Table 2.
Demographic characteristics of the cohort
| Demographic characteristics (n = 78) | Number |
|---|---|
| Age, mo, median (IQR) | 108 (69.2, 134.2) |
| Sex (%) | Male, 75% |
| Duration of CKD from diagnosis, mo, median (IQR) | 12 (3−21) |
| Stage of CKD, n (%) | Stage II, 21 (27) Stage III, 26 (33) (IIIa, 14; IIIb, 12) Stage IV, 31 (40) |
| eGFR (ml/min per 1.73 m2) at recruitment, median (IQR) | 34.67 (24.3, 65.4) |
| Incident cases (n = 28, 36%) | 32 (21.5, 55.6) |
| Prevalent cases | 37 (27.2, 68.1) |
| Prematurity/low birthweight | 14 (19) |
| Educational status of parents | 68% Nongraduates |
| Family income <250 USD/mo | 75% |
| Socioeconomic status (Modified Kuppuswamy classification) | 62% Middle class 25.6% Lower class |
| Etiology of CKD | |
| Glomerular disease | 12 (15) |
| Nonglomerular disease | |
| Renal hypoplasia/dysplasia | 20 (25.6) |
| Obstructive uropathy | 12 (15.3) |
| Neurogenic bladder | 13 (16.6) |
| Reflux nephropathy | 5 (6.4) |
| Others | 14 (18) |
| Unknown | 2 (2.5) |
| Comorbidities associated with CKD at recruitment | |
| Proteinuria (n = 73, 93.5%) | UPCr >2, 36 (46) ACEi use, 4 (5) |
| Hypertension (n = 46, 59%) | Treated, 21 (45.6) Uncontrolled BP, 14 (66) |
| Anemia | n = 29 (37.17) |
| Mineral bone disease | Bony deformities, 14 (19) |
| Hypocalcemia, 36 (46.1) | |
| Hyperphosphatemia, 25 (32) | |
| Vitamin D deficiency, 72 (92.3) | |
| Hyperparathyroidism, 44 (56.4) | |
| Cardiovascular | Dyslipidemia, 50 (64) |
| LVH, 34 (44.7) | |
| CIMT, cm, mean 0.05 ± 0.008 SD | |
| Growth | Median height z score, −2.1 (−3.3, −1.16) |
| Short stature, 51 (65.4) | |
| Median BMI z score, −1 (−2, −0.12) | |
| Undernourished, 20 (25.6) | |
| Delayed puberty, 22 (66) | |
| Metabolic acidosis | 59 (75.7) |
ACEi, angiotensin-converting enzyme inhibitor; BMI, body mass index; BP, blood pressure; CIMT, carotid intima-media thickness test; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; LVH, left ventricular hypertrophy; IQR, interquartile range; UPCr, urine protein-to-creatinine ratio.
Numbers in parentheses are percentages unless otherwise noted.
A total of 25 children (38%) experienced progression of CKD within 2 years of follow-up. In all, 12 children had a >50% decline from baseline glomerular filtration rate (GFR), 8 reached a GFR of <15 ml/min per 1.73 m2, 4 were initiated on dialysis, and 1 child underwent renal transplantation.
The median rate of decline in GFR was 3.5 (IQR 1.7, 11.00) ml/yr. The median time to progression of CKD was 22.98 (IQR 20.56, 25.41) months. There was no significant difference in the time to progression between incident and the prevalent cases after recruitment into the study, and therefore further analyses were conducted on pooled data.
Nonmodifiable Risk Factors for Progression at Baseline
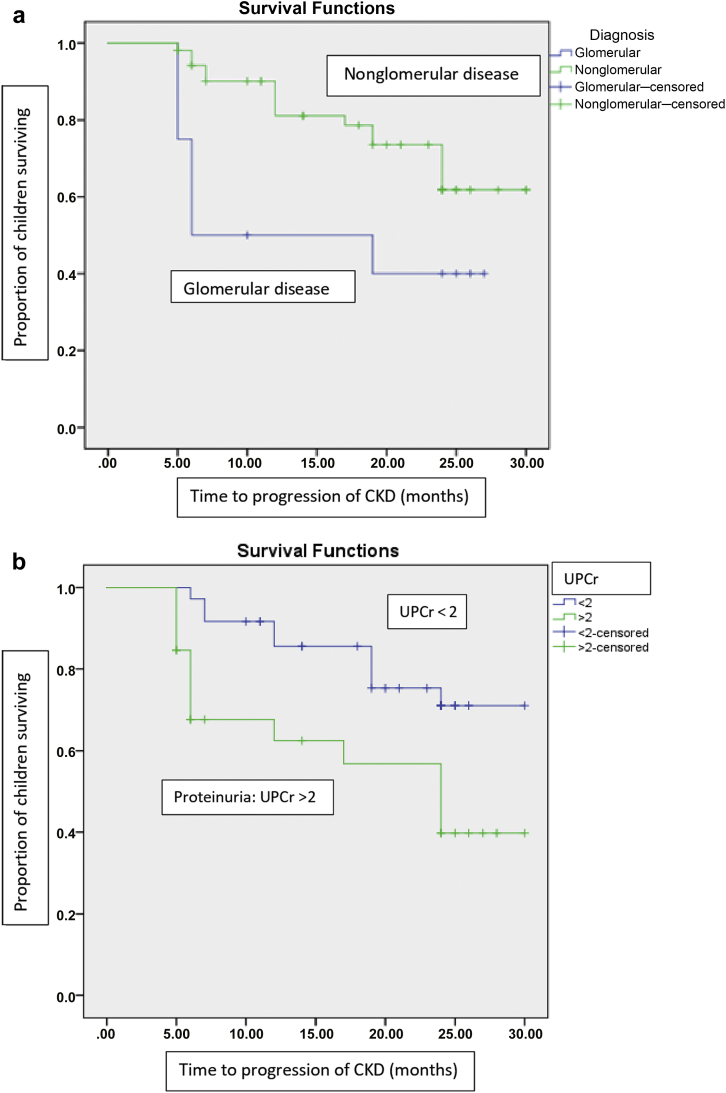
Age at onset of CKD and sex were not associated with progression. The risk of progression was significantly greater in children with glomerular compared with nonglomerular disease (hazard ratio [HR] 2.59, 95% confidence interval [CI] 1.07, 6.27, P = 0.034). The median decline in GFR was 11.36 (IQR 8.7, 12.6) ml/yr, and the mean time to progression of CKD was 15.45 (IQR 9.64, 21.25) months in children with glomerular disease compared to 2.38 ml/yr (IQR 1.1, 4.2) and 24.43 months (IQR 20.56, 25.41) in those with nonglomerular disease (P = 0.007 and P = 0.021 respectively, Figure 2a). An eGFR of <45 ml/min at recruitment was associated with a hazard ratio of 3.3 (95% CI 1.3, 8.4) for progression (P = 0.009).
Figure 2.
(a) The survival analysis showing time to progression of chronic kidney disease (CKD) in children with glomerular disease in comparison to those with nonglomerular disease. Children with glomerular disease had a higher risk of progression when compared to those with nonglomerular disease (hazard ratio 2.59, 95% confidence interval 1.07, 6.27, P = 0.034). (b) Survival analysis comparing time to progression of CKD among children with proteinuria (urine protein-to-creatinine ratio [UPCr] > 2) and those without proteinuria. UPCr > 2 at baseline was associated with the risk of progression of CKD (hazard ratio 3.1, 95% confidence interval 1.33, 7.23, P = 0.009).
Modifiable Risk Factors for Progression at Baseline
Proteinuria was present in 73 children (93%) at recruitment (Table 2). Urine protein-to-creatinine ratio (UPCr) of >2 at baseline was associated with risk of progression of CKD (HR 3.1, 95% CI 1.33, 7.23, P = 0.009) (Figure 2b). Proteinuria was also associated with progression of CKD in children with nonglomerular disease (HR 1.5, 95% CI 1.21, 1.9, P = 0.02). Hypertension was present in 46 of 78 children (59%) at recruitment (Table 2). Hypertension or systolic/diastolic z scores at recruitment were not associated with CKD progression. When adjusted for baseline eGFR, the following parameters were associated with an increased risk of progression: anemia, hypocalcemia, hyperphosphatemia, hyperparathyroidism, and height z score (Table 3).
Table 3.
Univariate analysis and multivariate analysis of baseline and follow-up risk factors for progression of chronic kidney disease in children
| Univariate analysis | ||
|---|---|---|
| Risk factors | Hazard ratio (95% CI) after adjusting for baseline GFR | P value |
| Nonmodifiable risk factors | ||
| Glomerular disease | 2.59 (1.07, 6.27) | 0.034 |
| eGFR at baseline | 3.3 (1.3, 8.4) | 0.001 |
| Modifiable risk factors | 0.56 | |
| Proteinuria | 3.1 (1.3, 7.2) | 0.009 |
| Hypertension | 1.4 (0.43, 4.9) | 0.56 |
| Anemia | 4.08 (1.8, 9.26) | 0.001 |
| Calcium level | 0.3 (0.19, 0.487) | <0.001 |
| Phosphate level | 2.09 (1.36, 3.22 | 0.001 |
| Parathormone level | 1.004 (1.002, 1.005) | <0.001 |
| Height z score | 0.75 (0.6, 0.9) | 0.006 |
| Metabolic acidosis | 3.05 (0.91, 10.2) | 0.071 |
| Multivariate analysis | ||
| Glomerular disease | 2.7 (1.1, 6.3) | 0.032 |
| eGFR at baseline | 3.39 (1.3, 8.8) | 0.005 |
| Proteinuria (baseline) | 1.04 (1.01, 1.07) | 0.009 |
| Proteinuria on follow-up | 1.4 (1.2, 1.52) | 0.005 |
| Uncontrolled hypertension at last follow-upa | 2.53 (1.01, 6.77) | 0.04 |
| Uncontrolled hypertension at last follow-up + proteinuriaa | 4.2 (1.85. 9.63) | 0.001 |
CI, confidence interval; eGFR, estimated glomerular filtration rate; GFR, glomerular filtration rate.
Numbers in bold indicate statistically significant risk factors.
Uncontrolled hypertension is defined as blood pressure above the 95th centile for age and sex despite medications.
Risk Factors for Progression on Follow-up
Persistent proteinuria (UPCr > 2) was significantly associated with progression of CKD (HR 1.4, 95% CI 1.2, 1.52; P = 0.005). Children with uncontrolled hypertension on follow-up had a higher risk of progression (HR 2.53, 95% CI, 1.01, 6.67) compared to those who had normal blood pressure values or controlled hypertension (P = 0.04). Adherence to antihypertensive medications was documented in 92% of the cohort. The combination of severe proteinuria on follow-up (UPCr > 2) and uncontrolled hypertension at last follow-up was associated with a hazard ratio of 4.2 (95% CI, 1.8, 9.6, P = 0.001) for progression.
On multivariate analysis, glomerular disease, baseline GFR <45 ml/min, UPCR >2, and the combination of uncontrolled hypertension and proteinuria at follow-up were independent risk factors for progression of CKD (Table 3).
Impact of Disease on Quality of Life
The median QoL scores of the child (n = 45) and parents (n = 47) are shown in Table 4.
Table 4.
Quality of life scores of child and parent proxy in the cohort of children with chronic kidney disease
| Score | Child (n = 45 ) | Parent (n = 47 ) |
|---|---|---|
| Physical score | 76.56 (62.37, 82.81) | 75 (42.18, 89.05) |
| Psychosocial score | 71.66 (64.16, 78.74) | 75 (55.8, 86.6) |
| Total score | 72.26 (60.17, 82.05) | 70.47 (50.6, 83.36) |
Data are median (interquartile range).
The total scores of the child and the parent correlated significantly (P = 0.01, r = 0.6). The physical and psychosocial scores were significantly lower in the children with lower socioeconomic status (P = 0.035, r = 0.522). There was no difference in the QOL scores based on etiology or stage of CKD. The parent proxy physical score correlated significantly with the height z score (P = 0.002, r = 0.485), eGFR (P = 0.003, r = 0.475), and parent proxy psychosocial score (P < 0.001, r = 0.796). The parent proxy psychosocial score correlated with the height z score (P = 0.004, r = 0.459), eGFR (P = 0.025, r = 0.367) and psychosocial score of the child (P = 0.001, r = 0.73). The psychosocial score was also significantly lower in patients with lower socioeconomic status (P = 0.032).
Discussion
This prospective longitudinal study from a tertiary care hospital in an LMIC shows that children with CKD present late and have a rapid rate of progression. As in most pediatric CKD studies,S8,S9 hypodysplasia, obstructive uropathy, and reflux nephropathy together accounted for about 50% of cases. Glomerular disease and baseline eGFR were significant nonmodifiable risk factors for progression of CKD. Proteinuria, uncontrolled hypertension, and the combination of these were significant modifiable risk factors for progression. Socioeconomic status, eGFR, and height z scores were significantly associated with lower QoL scores.
Very little has been reported on outcomes of children with CKD in LMIC.S6 The median eGFR in our cohort was lower than that observed in CKiD and the Italkid project, 34.7 versus 44 and 41.7 ml/min per 1.73 m2, respectively.S8,S10 It is of concern that one-third of the patients in our cohort were newly diagnosed with CKD (stage IIIb) at the time of recruitment. This reflects the delay in diagnosis of CKD in our population, which is common in practice in LMIC. A similar low baseline eGFR of 33 ml/min per 1.73 m2 was reported from Brazil.S11
The rate of progression of CKD in our cohort was faster than that reported in other studies.4,S12 The median rate of decline in GFR was 3.5 ml/yr, almost twice that in the CKiD study (1.8 ml/yr).S12 A comparable rate of decline in GFR of 5.8 ml/yr was reported from the Latin American Registry.S13 The mean time to progression of CKD in our cohort was 1.8 years. The median time to ESKD was 4.5 years in the NAPRTCS registry4 and 8.2 years in Brazil.S14 In Japan, 12.5% of predialysis children with CKD progressed to ESKD in 1.49 years.S15 The higher rate of progression and shorter time to progression of CKD in our cohort may be multifactorial, including the fact that our children were diagnosed at later stages of CKD and may have received insufficient treatment related to costs and to health literacy of parents. Variability in time to CKD progression may also reflect nonuniform definitions used across studies. The similar progression rate in our incident and prevalent patients is concerning; however, time from diagnosis was not known in prevalent patients, and the numbers may have been too small to detect a true difference.
The major nonmodifiable risk factors for progression in our cohort were glomerular disease as the etiology of CKD and a lower baseline GFR. This is consistent with findings from the CKiD and other studies,7,8,S12,S14 which reported baseline GFR as a significant risk factor for progression, and the NAPRTCS 2010, which showed that primary disease and stage of CKD were significant risk factors for progression of CKD.3
Proteinuria was present in children with both glomerular and nonglomerular disease, and the degree of proteinuria was 4 times higher than that reported in the CKiD cohort.6,S16 The severity of proteinuria likely reflects disease severity, delay in diagnosis of CKD, and lower prevalence of angiotensin-converting enzyme inhibitor use in our population. Only 5% of our patients received angiotensin-converting enzyme inhibitors compared to 50% in the CKiD study,S16 and therefore subgroup analysis was not possible. Proteinuria at recruitment and follow-up were significant predictors of progression on multivariate analysis and after correction for baseline GFR, as reported by others.S14,S15 Proteinuria adjusted for GFR was significantly associated with reduced time to reach ESKD in children with both glomerular and nonglomerular disease in the CKiD study.5 The Ital-Kid project showed that children with a P/Cr of <0.9 had a slower decline of renal function and a better renal survival at 5 years.S10
The prevalence of hypertension in our study was comparable with that reported in the CKiD cohort.5,S8 In contrast to the CKiD study, hypertension at recruitment was not a significant risk factor for progression of CKD in our study. However, on follow-up, children with uncontrolled hypertension had a higher risk of progression of CKD. The ESCAPE trial showed that intensive control of blood pressure was associated with a reduced risk of progression of CKD, emphasizing the importance of blood pressure control in pediatric CKD.7
Anemia was found to be a significant predictor of progression on univariate but not multivariate analysis in our cohort. In contrast, data from the CKiD study found that anemia was a significant predictor of progression in children with nonglomerular disease, as did the NAPRTCS overall.3, 5 Similarly, low calcium, high phosphate, and hyperparathyroidism were significantly associated with progression on univariate but not multivariate analysis, likely reflecting associations with severity of renal dysfunction. This is also in contrast to the NAPRTCS data, which found that inorganic phosphorus of >5.5 mg/dl and calcium of <9.5 mg/dl were associated with a higher risk of progression.3 The lack of significance of these parameters in our cohort may reflect the relatively small participant number.
Quality of life correlated significantly with short stature, eGFR, and socioeconomic status. The CKiD study showed that older children and those with a longer duration of CKD had higher scores in the physical, social, and emotional domains, but significantly lower school domain scores.S17 Short stature was significantly associated with poor scores in the physical function domain.S18
Our study has important strengths and some limitations. A major strength of this study is its prospective longitudinal nature, and the study is, to our knowledge, one of the few studies from an LMIC setting. Although this is a single-center study with a relatively small sample, our geographic catchment area is large and is representative of children with CKD in our region. Despite not reaching the target sample size, the pooled analyses revealed highly plausible findings that reflect the day-to-day reality in our clinic, including late presentation and loss to follow-up. Given the small numbers, subgroup analysis of comorbidities associated with CKD and other factors such as low birthweight, prematurity, medication use, and diet could not be analyzed. Owing to resource limitations, we could not measure albumin-to-creatinine ratio. However, with a significant association between proteinuria and progression, a spot urine protein-to-creatinine ratio remains a valid and affordable target for treatment in our cohort. Better data on the burden of hypertension using ambulatory blood pressure measurement would have been helpful; however, this was not routinely available in our setting. The significant association with blood pressure as measured with CKD progression also provides a reproducible target for intervention.
Conclusion
This prospective, longitudinal study shows that the profile of children with CKD stages II to IV in an LMIC is different from those in developed countries. The lower median baseline GFR suggests delay in diagnosis of CKD. The rate of progression of CKD was high, particularly in those with glomerular disease and associated with proteinuria and uncontrolled blood pressure, suggesting that the risk factors associated with progression of CKD must be identified early and treated adequately. The QoL analysis emphasizes the impact of CKD on affected children and highlights the role of socioeconomic status, which influences the ability to seek and to use medical care. There is an urgent need to increase awareness of pediatric CKD in LMICs to facilitate early diagnosis and intervention.
Disclosure
All the authors declared no competing interests.
Acknowledgments
This study was supported by the Clinical Research Program of the International Society of Nephrology.
Footnotes
Supplementary Material
References
- 1.Harambat J., Van Stralen K.J., Kim J.J., Tizard E.J. Epidemiology of chronic kidney disease in children. Pediatr Nephrol. 2012;27:363–373. doi: 10.1007/s00467-011-1939-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitsnefes M.M., Laskin B.L., Dahhou M. Mortality risk among children initially treated with dialysis for end-stage kidney disease, 1990–2010. JAMA. 2013;309:1921–1929. doi: 10.1001/jama.2013.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staples A.O., Greenbaum L.A., Smith J.M. Association between clinical risk factors and progression of chronic kidney disease in children. Clin J Am Soc Nephrol. 2010;5:2172–2179. doi: 10.2215/CJN.07851109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitsnefes M. Hypertension and progression of chronic renal insufficiency in children: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) J Am Soc Nephrol. 2003;14:2618–2622. doi: 10.1097/01.asn.0000089565.04535.4b. [DOI] [PubMed] [Google Scholar]
- 5.Warady B.A., Abraham A.G., Schwartz G.J. Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: the Chronic Kidney Disease in Children (CKiD) cohort. Am J Kidney Dis. 2015;65:878–888. doi: 10.1053/j.ajkd.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong C.S., Pierce C.B., Cole S.R. Association of proteinuria with race, cause of chronic kidney disease, and glomerular filtration rate in the Chronic Kidney Disease in Children study. Clin J Am Soc Nephrol. 2009;4:812–819. doi: 10.2215/CJN.01780408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wühl E., Trivelli A., Picca S. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;17361:1639–1650. doi: 10.1056/NEJMoa0902066. [DOI] [PubMed] [Google Scholar]
- 8.Kamath N., Iyengar A.A. Chronic Kidney Disease (CKD): An observational study of etiology, severity and burden of comorbidities. Indian J Pediatr. 2017;84:822–825. doi: 10.1007/s12098-017-2413-2. [DOI] [PubMed] [Google Scholar]
- 9.Jha V. Current status of chronic kidney disease care in Southeast Asia. Semin Nephrol. 2009;29:487–496. doi: 10.1016/j.semnephrol.2009.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.