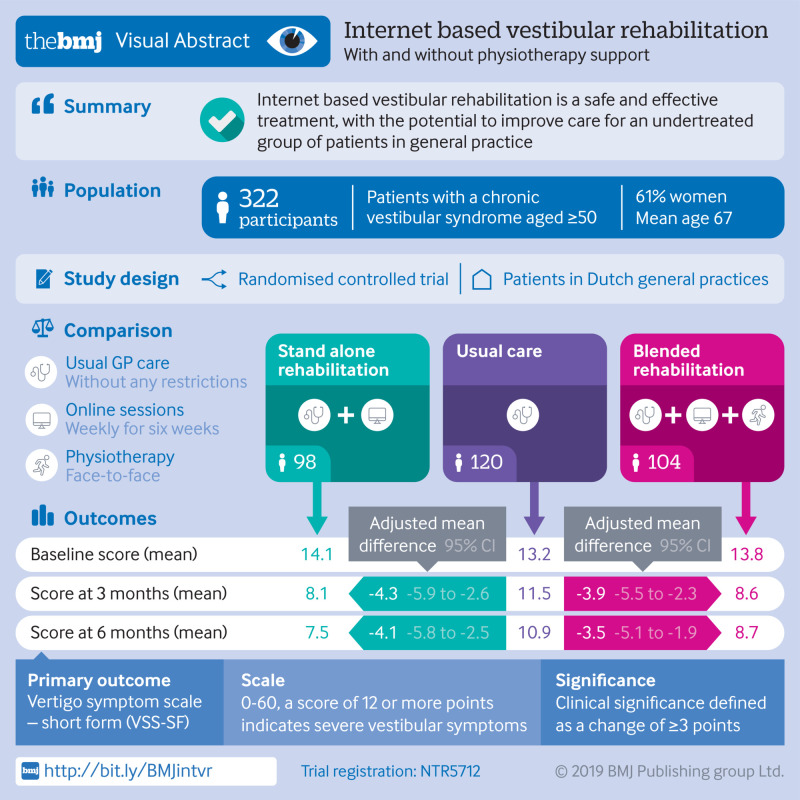
Abstract
Objective
To investigate the clinical effectiveness and safety of stand alone and blended internet based vestibular rehabilitation (VR) in the management of chronic vestibular syndromes in general practice.
Design
Pragmatic, three armed, parallel group, individually randomised controlled trial.
Setting
59 general practices in the Netherlands.
Participants
322 adults aged 50 and older with a chronic vestibular syndrome.
Interventions
Stand alone VR comprising a six week, internet based intervention with weekly online sessions and daily exercises (10-20 minutes a day). In the blended VR group, the same internet based intervention was supplemented by face-to-face physiotherapy support (home visits in weeks 1 and 3). Participants in the usual care group received standard care from a general practitioner, without any restrictions.
Main outcome measures
The primary outcome was vestibular symptoms after six months as measured by the vertigo symptom scale-short form (VSS-SF range 0-60, clinically relevant difference ≥3 points). Secondary outcomes were dizziness related impairment, anxiety, depressive symptoms, subjective improvement of vestibular symptoms after three and six months, and adverse events.
Results
In the intention-to-treat analysis, participants in the stand alone and blended VR groups had lower VSS-SF scores at six months than participants in the usual care group (adjusted mean difference −4.1 points, 95% confidence interval −5.8 to −2.5; and −3.5 points, −5.1 to −1.9, respectively). Similar differences in VSS-SF scores were seen at three months follow-up. Participants in the stand alone and blended VR groups also experienced less dizziness related impairment, less anxiety, and more subjective improvement of vestibular symptoms at three and six months. No serious adverse events related to online VR occurred during the trial.
Conclusion
Stand alone and blended internet based VR are clinically effective and safe interventions to treat adults aged 50 and older with a chronic vestibular syndrome. Online VR is an easily accessible form of treatment, with the potential to improve care for an undertreated group of patients in general practice.
Trial registration
Netherlands Trial Register NTR5712.
Introduction
Vestibular symptoms such as vertigo and dizziness are common in general practice.1 Each year 5% of the general population experiences vertigo symptoms.2 The prevalence, frequency, and severity of vertigo generally increases with age.2 3 Four out of five people with vertigo report that it severely affects their daily functioning.4 Vertigo also represents a substantial economic burden, as a result of absenteeism, high use of healthcare services, and an increased risk of falling.2 5 More than 80% of patients experiencing vertigo in the Netherlands, United Kingdom, and United States are primarily treated by their general practitioner or primary care doctor and are never referred to a medical specialist for their symptoms.6 7 8
In the International Classification of Vestibular Disorders, developed by the leading international Bárány society for neuro-otology, four types of vestibular symptoms are identified: vertigo, dizziness, vestibulovisual symptoms, and postural symptoms.9 10 No vestibular symptom is considered pathognomonic in its links to underlying vestibular disease, and the same patient often experiences more than one vestibular symptom.10 11 Peripheral vestibular dysfunction due to disorders such as benign paroxysmal positional vertigo, vestibular neuritis, vestibular migraine, or Meniere’s disease is the most important cause of vestibular symptoms.2 Each peripheral vestibular disorder has a distinct initial treatment, and they all have a substantial potential to result in chronic vestibular symptoms.12 When a vestibular disorder damages the peripheral vestibular system, an innate repair mechanism called vestibular compensation is activated that aids functional recovery and decreases vestibular symptoms.13 When vestibular compensation fails, a chronic vestibular syndrome can occur.12 This is defined in the International Classification of Vestibular Disorders as a clinical syndrome of chronic vestibular symptoms, lasting months to years, which includes features suggestive of persistent vestibular system dysfunction. Symptoms in chronic vestibular syndromes can either have a progressively deteriorating course, reflect a stable, yet incomplete recovery after an acute vestibular event, or represent persistent, lingering symptoms between episodic vestibular attacks.10 11
Vestibular rehabilitation (VR), an exercise based treatment that gradually stimulates the vestibular system, can be used to stimulate vestibular compensation.12 Moderate to strong evidence shows that VR is a safe and effective treatment for unilateral peripheral vestibular dysfunction.14 VR is now recommended in US,15 16 UK,17 and Dutch18 clinical guidelines as the preferred treatment for a chronic vestibular syndrome. Despite the scientific evidence for VR, less than 10% of general practitioners in the Netherlands19 and UK20 reported its use. Investigating alternative ways to deliver VR in general practice may stimulate the implementation of VR. Internet interventions are easily accessible and inexpensive and can be tailored to the patient’s needs. The University of Southampton used the content of an effective VR booklet21 22 to develop an online VR intervention.23 In a randomised controlled trial in the UK this fully automated stand alone internet based intervention effectively reduced vestibular symptoms compared with usual care.24 Stand alone internet based interventions are prone to non-adherence and attrition, and therefore online treatment is often combined with face-to-face therapy by a healthcare professional.25 26 27 Thus, combining online VR with physiotherapy support might provide even better results. This guided approach with physiotherapy support, known as blended care, might be especially effective in people with vestibular symptoms, as anxiety is strongly associated with vestibular disorders.28 29 30 31 Physiotherapists are used to reassure and encourage participants doing vestibular exercises and regard the management of anxiety as an important aspect of treatment.16 32 We investigated the clinical effectiveness and safety of stand alone and blended internet based VR versus usual care for adults aged 50 and older with a chronic vestibular syndrome in primary care. We hypothesised that both stand alone and blended internet based VR would result in a clinically relevant decrease in vestibular symptoms compared with usual care after six months.
Methods
We conducted a pragmatic, three group, parallel arm, individually randomised controlled trial among adults aged 50 and older with a chronic vestibular syndrome. The clinical effectiveness and safety of stand alone and blended internet based VR with physiotherapy support was compared with usual care. A detailed study protocol has been published,33 and the trial was registered in the Netherlands Trial Register before the first patient was recruited. We followed the CONSORT reporting guidelines for non-drug treatment interventions.34
Participants
We recruited participants from 59 general practices in the Netherlands. A search of the electronic medical records identified eligible adults aged 50 and older who had visited their general practitioner with a vestibular symptom in the past two years. The doctor screened the list of potentially eligible participants and excluded those with an identifiable non-vestibular cause of their symptoms, medical contraindications for making the required head movements (eg, severe cervical arthrosis), serious comorbid conditions precluding participation in an exercise programme, or current enrolment in another similar study. Potential participants received information about the trial and a form to express interest. One of the physicians in the research team (VAvV or ORM) checked the eligibility criteria of interested patients by telephone. The inclusion criteria were a good command of the Dutch language, access to the internet and an email account, and persisting vestibular symptoms at time of inclusion that had been present for at least one month and were exacerbated or triggered by head movements. These inclusion criteria allowed us to identify participants with a chronic vestibular syndrome, as defined in the International Classification of Vestibular Disorders10 11 who were suitable to receive online vestibular rehabilitation. The research team physician used a checklist (supplementary appendix 1) to distinguish a chronic vestibular syndrome from an acute or episodic vestibular syndrome.10 11
Interventions
Stand alone internet based VR
VR entails specific exercises for maximising central nervous system compensation for vestibular disease. Additionally, provoking vestibular symptoms in a controlled context constitutes a form of exposure based behaviour therapy.35 36 Booklet based VR is an effective method for reducing vestibular symptoms and dizziness related impairment.21 22 37 These booklets served as the basis for a British online intervention, called Balance Retraining (freely available from https://balance.lifeguidehealth.org/).23 24 35 The development of Balance Retraining employed a person based approach, drawing on an in-depth understanding of intervention target users.38 39 It was built using LifeGuide software (University of Southampton, Southampton, UK). Vertigo Training, the internet based VR intervention we used in this trial, was a Dutch translation of Balance Retraining.
The intervention period lasted six weeks. Vertigo Training consisted of weekly online sessions and daily VR exercises (supplementary appendix 2). In the first session, written instructions and video demonstrations were used to teach participants the six core VR exercises. Participants were asked to perform these exercises twice daily for 10 minutes during the intervention period. Exercises were tailored to the individual by taking into account the participants’ symptoms and balance capabilities. At the start of the intervention, participants performed all exercises sitting down and directly afterwards scored the level of vestibular symptoms associated with each of the six exercises. Vertigo Training scores were used to automatically produce an exercise prescription for the coming week, custom-built to the participant’s vestibular symptoms. Every week participants scored the vestibular symptoms associated with each of the six exercises in a new online session. When an exercise was performed while sitting down and no vestibular symptoms were elicited (anymore), the difficulty level was increased by an instruction to perform that specific exercise while standing or eventually when walking around. Through this personalised approach, participants gradually increased the intensity of their exercises and continued to challenge their vestibular system. Vertigo Training also provided information and advice on coping and symptom control strategies (supplementary appendix 2).37 Muscle relaxation and breathing techniques can decrease psychophysiological arousal, whereas cognitive restructuring and problem solving might lessen anxiety provoked by vestibular symptoms.40 In addition, Vertigo Training included several features to increase engagement. To raise participants’ expectations about the content of Vertigo Training, scientific studies supporting the effectiveness of VR were summarised in plain language. Participants received email messages to remind them to log in to the website each week. Participants also received the standard level of care provided by their own doctor, without restrictions.
Blended internet based VR with physiotherapy support
Participants in the blended VR group received access to the same Vertigo Training intervention as participants in the stand alone VR group. In addition, a trained physiotherapist visited participants in the blended VR group twice at home. These physiotherapy sessions occurred in weeks 1 and 3 of the six week intervention period and lasted for 45 minutes each. During these sessions, the physiotherapist: provided information about the background of vestibular symptoms and VR, elicited and addressed doubts and concerns about vestibular symptoms and VR, taught participants how to use the online intervention, described and took participants through a set of VR exercises, advised on how to anticipate and cope with obstacles to adherence, and provided support and encouraged adherence. The first session was designed to make the participant feel comfortable with the VR exercises, and the second session focused on adherence to treatment. Participants in this group also received the standard level of care provided by their doctor, without restrictions.
Usual care
Participants in the usual care group received the standard level of care provided by their doctor, without restrictions. They had access to any treatment available in primary care or in secondary care after referral. Participants in the usual care group were offered access to stand alone internet based VR after the trial was completed. Participating doctors received written instructions, asking them to diagnose causes of vestibular symptoms and treat identified disorders for all participants according to the guidelines of the Dutch College of General Practitioners.18
Outcomes
Measurements were collected at baseline and at three and six months follow-up. The primary outcome measure was vestibular symptoms as measured by the vertigo symptom scale-short form (VSS-SF)41 42 six months after baseline. The VSS-SF has been used effectively in several VR trials21 22 24 37 and has shown excellent discriminative ability (area under the curve 0.87), high internal consistency (Cronbach’s α 0.90), and high test-retest reliability (intraclass correlation coefficient 0.88).42 The instrument measures the frequency of 15 vestibular symptoms on a scale from 0 (no symptoms) to 4 (symptoms most days) during the past month (total range 0-60 points). Improvement can reflect either fewer or less frequent symptoms. A total score of 12 points or more has been classified as severe vestibular symptoms,22 and a change in score of 3 points or more has been defined as clinically significant.21 22 24 37 A clinically relevant improvement on the VSS-SF can represent either marked improvement on one symptom (eg, vertigo) or some improvement on three symptoms (eg, vertigo, nausea, and unsteadiness). Secondary outcome measures included the dizziness handicap inventory,43 which measures dizziness related impairment; subjective improvement in vestibular symptoms,37 a single dichotomous item that indicated whether participants felt they had or had not improved (ie, worse or the same) compared with baseline; and the patient health questionnaire (PHQ)44 to determine the presence of a panic disorder, generalised anxiety disorder, or major depressive disorder, and to measure the severity of anxiety (generalised anxiety disorder assessment (GAD)-7 subscale)45 and depressive symptoms (PHQ-9 subscale).46 In participants in the stand alone and blended VR groups, we also assessed self reported perceived barriers to adherence with the problematic experiences of therapy scale (PETS).47 At baseline, we asked participants to report demographic characteristics (age, sex, level of education, and living situation), comorbidities, the vestibular diagnosis, frequency and average duration of vestibular symptoms, and time since vestibular diagnosis. Engagement in Vertigo Training was measured by automatically collected data for intervention usage, number of sessions completed, and time spent on each page of the trial website. We reported all serious adverse events that occurred during the trial to the medical ethics committee. The relation of all serious adverse events with vestibular symptoms or the intervention was judged, after contacting the participant or general practitioner, or both.
Randomisation and blinding
Participants who completed the informed consent procedure received an email with a link to the trial website. After participants had filled out the baseline questionnaire, the LifeGuide software allocated them to stand alone VR, blended VR, or usual care. A simple randomisation algorithm stratified participants by severity of vestibular symptoms using a cut-off of 12 or higher on the VSS-SF. The automated randomisation process took place online and was concealed from the research team. Owing to the nature of the trial interventions, it was not possible to blind participants, physiotherapists, and research assistants. The trial statistician (JWRT) remained blinded to allocation until the analyses had been completed.
Sample size
Our sample size calculation was based on the comparison between participants allocated to stand alone VR and those allocated to usual care. We chose this comparison because, based on previous blended internet based research projects,25 26 27 we anticipated that blended VR would be just as effective or more effective than stand alone VR. In a previous VR trial with VSS-SF as the primary outcome measure, booklet based VR alone compared with usual care showed an effect size (Cohen’s d) of 0.45, favouring booklet based VR.21 To be able to detect such an effect, and assuming that stand alone internet based VR would produce the same effect size as booklet based VR, we needed 80 participants in each group for a two sided test with an α of 0.05 and β of 0.20. As attrition from internet based interventions can be substantial,25 we recruited a minimum of 100 participants in each group (300 total sample) to allow for up to 20% loss to follow-up.
Statistical analyses
We used descriptive statistics to compare the baseline characteristics of participants in the three trial arms. For the primary intention-to-treat analysis, participants were analysed according to their randomisation group. The two comparisons were stand alone internet based VR versus usual care and blended internet based VR with physiotherapy support versus usual care. In a secondary per protocol analysis we only included participants assigned to stand alone VR who completed all six online sessions, and we only included participants assigned to blended VR who completed all six online sessions and both physiotherapist visits. We used a linear mixed models analysis for continuous outcome variables, and generalised estimating equation analysis for binary outcome variables. These techniques can account for repeated measures within an individual and are also capable of handling missing data in a longitudinal dataset without the need to perform multiple imputations.48 For each outcome measure we reported a crude and an adjusted analysis. The crude analysis included the group variable (using the usual care group as reference), time, and the interaction between the group variable and time. Furthermore, we adjusted for the baseline values of the outcome, as is customary in randomised controlled trials,49 and for other prespecified potential confounders—that is, age (continuous),50 51 52 53 54 sex (categorical),50 55 level of education (categorical),54 living situation (categorical),54 56 number of chronic diseases (categorical),50 54 56 57 time since diagnosis (categorical),55 56 58 and the presence of a panic disorder, generalised anxiety disorder, or major depressive disorder at baseline (categorical).50 56 57 58 59 Lastly, we conducted a post hoc analysis to assess possible effect modification in the primary intention-to-treat analysis for each potential confounder by using interaction terms in the model. SPSS 22.0 and Stata 14.1 were used for statistical analyses.
Patient and public involvement
Patients played an important role in the development of Vertigo Training. Detailed feedback by patients with vestibular symptoms on the content, usability, and Dutch translation in prototype versions led to some amendments of the online intervention. No patients advised on interpretation of the results nor were they involved in writing the manuscript. A lay summary of the research findings will be distributed to all participants and the results will be disseminated to the relevant patient community.
Results
Participants were recruited between June 2017 and July 2018 from 59 Dutch general practices. Figure 1 shows the patient flow during the trial. At baseline, 322 participants were randomised: 98 to the stand alone VR group, 104 to the blended VR group, and 120 to the usual care group. Follow-up data on the primary outcome was complete at three and six months for 292 (91%) and 286 (89%) of participants, respectively. Table 1 shows the baseline characteristics of participants. The groups were generally well balanced, although the usual care group comprised relatively more men. In the stand alone VR group, 70 participants (71%) completed at least one online session and 47 (48%) completed all six sessions. In the blended VR group, 83 participants (80%) completed at least one online session, 85 (82%) received both physiotherapist visits, and 55 (53%) completed all six sessions and were visited twice by the physiotherapist.
Table 1.
Baseline characteristics of participants assigned to stand alone vestibular rehabilitation (VR), blended VR, or usual care. Values are numbers (percentages) unless stated otherwise
| Characteristics | Stand alone VR (n=98) | Blended VR (n=104) | Usual care (n=120) | Total sample (n=322) |
|---|---|---|---|---|
| Mean (SD) age (years) | 66.7 (9.5) | 67.4 (9.8) | 67.0 (9.4) | 67.0 (9.5) |
| Female | 64 (65) | 69 (66) | 64 (53) | 197 (61) |
| Level of education: | ||||
| Low | 33 (34) | 37 (36) | 36 (30) | 106 (33) |
| Middle | 25 (26) | 31 (30) | 30 (25) | 86 (27) |
| High | 40 (41) | 36 (35) | 54 (45) | 130 (40) |
| Living situation: | ||||
| Alone | 34 (35) | 33 (32) | 35 (29) | 102 (32) |
| With partner | 64 (65) | 71 (68) | 85 (71) | 220 (68) |
| No of chronic diseases*: | ||||
| 0 | 59 (60) | 64 (62) | 63 (53) | 186 (58) |
| 1 | 28 (29) | 32 (31) | 41 (34) | 101 (31) |
| 2 | 8 (8) | 4 (4) | 12 (10) | 24 (7) |
| ≥3 | 3 (3) | 4 (4) | 4 (3) | 11 (3) |
| Time since vestibular diagnosis†: | ||||
| 1-6 months | 15 (15) | 22 (21) | 13 (11) | 50 (16) |
| 6 months to 2 years | 28 (29) | 27 (26) | 39 (33) | 94 (29) |
| 2-10 years | 31 (32) | 44 (42) | 48 (40) | 123 (38) |
| >10 years | 23 (24) | 11 (11) | 18 (15) | 52 (16) |
| Self reported vestibular diagnosis†: | ||||
| No known diagnosis | 67 (68) | 69 (66) | 77 (64) | 213 (66) |
| Benign paroxysmal positional vertigo | 11 (11) | 17 (16) | 22 (18) | 50 (16) |
| Meniere’s disease | 9 (9) | 9 (9) | 10 (8) | 28 (9) |
| Vestibular neuritis | 6 (6) | 4 (4) | 7 (6) | 17 (5) |
| PPPD | 0 (0) | 1 (1) | 0 (0) | 1 (0) |
| Other‡ | 4 (4) | 4 (4) | 2 (2) | 10 (3) |
| Disorders at baseline according to PHQ: | 14 (14) | 16 (15) | 23 (19) | 53 (17) |
| Panic disorder | 2 (2) | 3 (3) | 5 (4) | 10 (3) |
| Generalised anxiety disorder | 12 (12) | 15 (14) | 19 (16) | 46 (14 |
| Major depressive disorder | 5 (5) | 6 (6) | 9 (8) | 20 (6) |
PPPD=persistent postural-perceptual dizziness; PHQ=patient health questionnaire.
Chronic non-specific lung disease, cardiac disease, peripheral arterial disease, stroke, diabetes mellitus, arthritis, and cancer.
Data on this variable was missing for three participants: stand alone VR (n=1), usual care (n=2).
Traumatic brain injury, cerebrovascular accident, Parkinson’s disease, bacterial meningitis, and vestibular organ surgical procedures.
Fig 1.
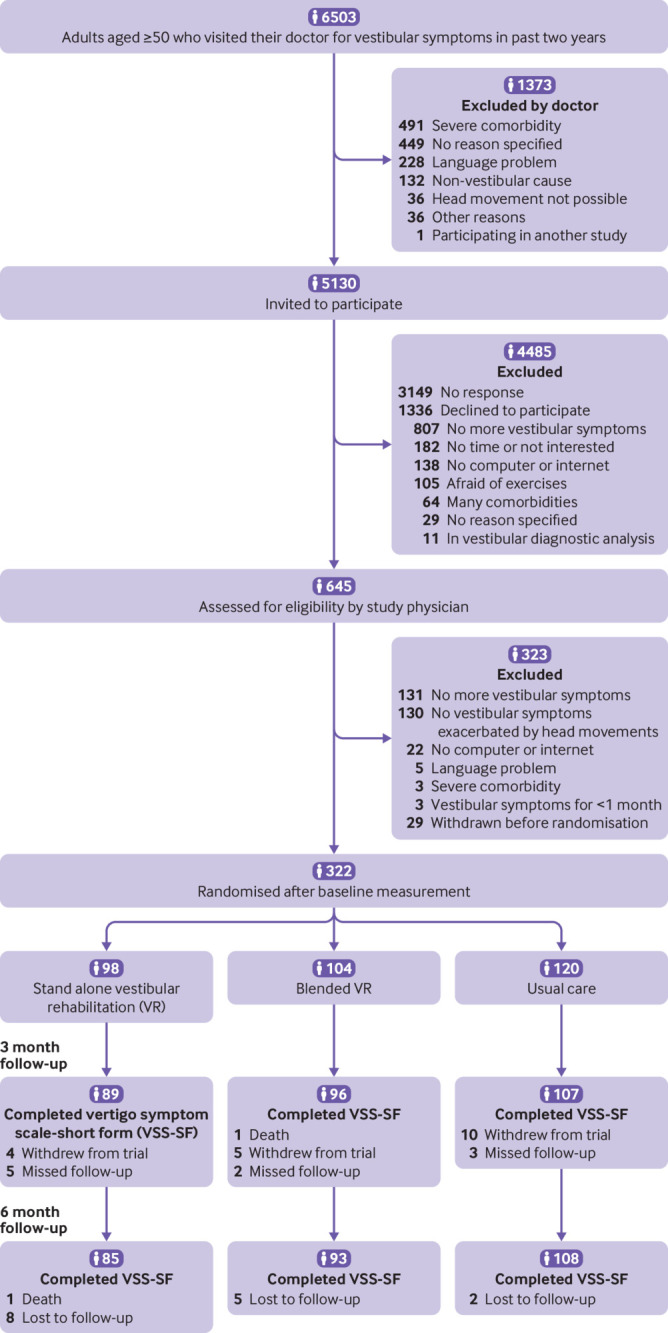
Flow of participants through trial
Primary outcome
In the intention-to-treat analysis, participants at three and six months follow-up in both the stand alone VR group and the blended VR group reported fewer vestibular symptoms than participants in the usual care group (table 2). After controlling for baseline values and prespecified confounders, participants in the stand alone VR group scored 4.3 points lower on the VSS-SF than participants in the usual care group at three months (95% confidence interval −5.9 to −2.6) and 4.1 points lower at six months (−5.8 to −2.5). Participants in the blended VR group scored 3.9 points lower than participants in the usual care group at three months (−5.5 to −2.3) and 3.5 points lower at six months (−5.1 to −1.9). The differences between groups were statistically significant and exceeded the clinically relevant difference of 3 points. In the per protocol analysis, participants in the stand alone VR group reported a 5.4 point lower VSS-SF score at six months compared with participants in the usual care group (95% confidence interval −7.4 to −3.4), and the blended VR group scored 3.5 points lower than the usual care group (−5.5 to −1.6). In a post hoc analysis, no effect modification was found for the primary outcome (supplementary appendix 3).
Table 2.
Comparison of primary outcome between treatment groups
| Primary outcome measure | Mean (SD) score | Crude mean difference (95% CI)* | Adjusted mean difference (95% CI)† | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Stand alone VR | Blended VR | Usual care | Stand alone VR v usual care | Blended VR v usual care | Stand alone VR v usual care | Blended VR v usual care | |||
| VSS-SF (intention to treat)‡: | n=98 | n=104 | n=120 | n=218 | n=224 | n=218 | n=224 | ||
| Baseline | 14.1 (8.9) | 13.8 (8.3) | 13.2 (8.6) | - | - | - | - | ||
| 3 months | 8.1 (7.4) | 8.6 (7.1) | 11.5 (9.9) | −4.2 (−5.9 to −2.5) | −3.9 (−5.5 to −2.2) | −4.3 (−5.9 to −2.6) | −3.9 (−5.5 to −2.3) | ||
| 6 months | 7.5 (7.8) | 8.7 (6.9) | 10.9 (9.3) | −4.0 (−5.8 to −2.3) | −3.5 (−5.1 to −1.8) | −4.1 (−5.8 to −2.5) | −3.5 (−5.1 to −1.9) | ||
| VSS-SF (per protocol)§: | n=47 | n=55 | n=120 | n=167 | n=175 | n=167 | n=175 | ||
| Baseline | 13.2 (6.2) | 15.0 (8.6) | 13.2 (8.6) | - | - | - | - | ||
| 3 months | 6.0 (4.0) | 10.0 (8.0) | 11.5 (9.9) | −6.0 (−8.1 to −4.0) | −3.4 (−5.3 to −1.4) | −6.0 (−8.0 to −3.9) | −3.3 (−5.2 to −1.4) | ||
| 6 months | 5.9 (5.0) | 9.4 (7.4) | 10.9 (9.3) | −5.4 (−7.5 to −3.4) | −3.6 (−5.6 to −1.7) | −5.4 (−7.4 to −3.4) | −3.5 (−5.5 to −1.6) | ||
VR=vestibular rehabilitation; VSS-SF=vertigo symptom scale-short form, range 0-60, clinically relevant difference 3 points.
Adjusted for baseline values and repeated measurements within participants.
Adjusted for baseline values, repeated measurements within participants, age, sex, level of education, living situation, number of chronic diseases, time since vestibular diagnosis, and presence of a panic disorder, generalised anxiety disorder, or major depressive disorder at baseline.
All participants analysed according to allocation.
Only participants in stand alone VR group who completed all six online sessions and participants in blended VR group who completed all six online sessions and both physiotherapist visits were analysed.
Secondary outcomes
Table 3 shows the comparison between the groups on dizziness related impairment, anxiety, depressive symptoms, and subjective improvement of vestibular symptoms. Participants in the stand alone and blended VR groups experienced less impairment from dizziness than participants in the usual care group at three and six months (adjusted mean difference on dizziness handicap inventory at 6 months −4.9 points, 95% confidence interval −8.4 to −1.3, and −4.5 points, −8.0 to −0.9). They also experienced fewer anxiety symptoms at three and six months (adjusted mean difference GAD-7 at 6 months −1.2 points, −2.0 to −0.4, and −1.2 points, −2.0 to −0.4). No significant differences were found in severity of depressive symptoms between the VR groups and the usual care group at three and six months. At six months 46/87 (53%) participants in the stand alone VR group, 48/93 (52%) in the blended VR group, and 43/110 (39%) in the usual care group reported subjective improvement of vestibular symptoms compared with the start of the trial. Over the course of the trial, participants in the stand alone VR group and blended VR group were more likely to experience subjective improvement than participants in the usual care group (adjusted odds ratio stand alone VR versus usual care 2.2, 95% confidence interval 1.2 to 4.1; blended VR versus usual care 2.1, 1.2 to 3.8). No significant differences were found in self reported perceived barriers to adherence between participants in the stand alone VR group and blended VR group (supplementary appendix 4).
Table 3.
Comparison of secondary outcomes between treatment groups
| Secondary outcome measures | Treatment group | Crude mean difference (95% CI)* | Adjusted mean difference (95% CI)† | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Stand alone VR | Blended VR | Usual care | Stand alone VR v usual care | Blended VR v usual care | Stand alone VR v usual care | Blended VR v usual care | |||
| Mean (SD) score | |||||||||
| DHI: | |||||||||
| Baseline | 34.8 (18.5) | 36.0 (21.9) | 35.8 (19.9) | - | - | - | - | ||
| 3 months | 24.4 (20.8) | 26.4 (20.6) | 29.2 (21.1) | −4.5 (−8.1 to −0.8) | −3.9 (−7.5 to −0.3) | −4.6 (−8.2 to −1.1) | −3.9 (−7.4 to −0.4) | ||
| 6 months | 21.5 (20.4) | 25.4 (21.6) | 27.6 (21.5) | −4.7 (−8.4 to −1.1) | −4.4 (−8.0 to −0.8) | −4.9 (−8.4 to −1.3) | −4.5 (−8.0 to −0.9) | ||
| GAD-7: | |||||||||
| Baseline | 3.7 (4.6) | 4.0 (4.3) | 4.5 (4.8) | - | - | - | - | ||
| 3 months | 3.1 (3.8) | 2.9 (3.4) | 4.5 (5.0) | −1.0 (−1.9 to −0.1) | −1.3 (−2.2 to −0.5) | −1.1 (−1.9 to −0.3) | −1.4 (−2.2 to −0.6) | ||
| 6 months | 2.6 (3.3) | 2.7 (3.5) | 4.2 (5.0) | −1.1 (−1.9 to −0.2) | −1.1 (−2.0 to −0.3) | −1.2 (−2.0 to −0.4) | −1.2 (−2.0 to −0.4) | ||
| PHQ-9: | |||||||||
| Baseline | 4.5 (4.7) | 5.4 (4.5) | 5.9 (5.4) | - | - | - | - | ||
| 3 months | 4.2 (4.2) | 4.3 (4.2) | 5.3 (5.0) | −0.3 (−1.3 to 0.6) | −0.9 (−1.8 to 0.0) | −0.5 (−1.4 to 0.4) | −0.9 (−1.8 to 0.0) | ||
| 6 months | 3.5 (4.3) | 4.0 (3.7) | 4.9 (5.1) | −0.5 (−1.4 to 0.5) | −0.8 (−1.7 to 0.2) | −0.6 (−1.5 to 0.3) | −0.8 (−1.7 to 0.1) | ||
| No (%) of patients reporting improvement | |||||||||
| Subjective improvement: | |||||||||
| 3 months | 49/91 (54) | 54/97 (56) | 39/107 (36) | 2.1 (1.2 to 3.6)*‡§ | 2.2 (1.2 to 3.8)*‡§ | 2.2 (1.2 to 4.1)†‡¶ | 2.1 (1.2 to 3.8)†‡¶ | ||
| 6 months | 46/87 (53) | 48/93 (52) | 43/110 (39) | ||||||
VR=vestibular rehabilitation; DHI=dizziness handicap inventory, range 0-100; GAD-7= generalised anxiety disorder assessment (GAD)-7 subscale, a measure for severity of anxiety symptoms, range 0-21; PHQ-9=patient health questionnaire-9, a measure for severity of depressive symptoms, range 0-27; subjective improvement = dichotomous evaluation of vestibular symptoms compared to baseline measurement: improved or not improved (ie, worse or the same).
All analyses reported in table were performed on an intention-to-treat basis, with participants analysed according to allocation.
Adjusted for baseline values and repeated measurements within participants.
Adjusted for baseline values, repeated measurements within participants, age, sex, level of education, living situation, number of chronic disease, time since vestibular diagnosis, and presence of a panic disorder, generalised anxiety disorder, or major depressive disorder at baseline.
Comparison of overall effect over six months follow-up.
Odds ratios (95% CI).
Adjusted odds ratios (95% CI).
Serious adverse events
A total of 16 serious adverse events were reported during the trial—two participants died (one in the stand alone VR group and one in blended VR group), five were admitted to an intensive care unit (four in the stand alone VR group and one in the usual care group), and nine were admitted to hospital to a non-intensive care unit (three in the stand alone VR group, two in the blended VR group, and four in the usual care group). None of the serious adverse events were judged to be related to vestibular symptoms or the treatment interventions.
Discussion
This three armed randomised controlled trial provides strong evidence for the effectiveness of online vestibular rehabilitation (VR) in general practice for adults with a chronic vestibular syndrome. After three and six months, participants in both the stand alone internet based VR group and the internet based VR group with physiotherapy support (blended VR) experienced significantly fewer vestibular symptoms than participants in the usual care group. They also experienced less dizziness related impairment, less anxiety, and more subjective improvement of vestibular symptoms. In the intention-to-treat-analysis the effects of stand alone VR and blended VR were comparable. In a per protocol analysis that only included participants who adhered fully to the intervention, the treatment effects of stand alone VR were notably better than in the intention-to-treat-analysis. No serious adverse events related to stand alone or blended VR occurred during the trial. In this trial, stand alone and blended VR showed significantly better effects than usual care at three and six months on both the primary outcome measure and three out of four secondary outcome measures, which points to the robustness of these findings.
Comparison with other studies
The positive effects of VR seen in this trial are in line with the results of previous VR randomised controlled trials in general practice.21 22 24 60 Internet based VR was previously investigated in only one randomised controlled trial in general practice.24 The stand alone intervention of Balance Retraining, the English version of our online intervention Vertigo Training, was associated with a significant reduction in vestibular symptoms and dizziness related impairment compared with usual care in British adults aged 50 and older.24 The adjusted mean difference at six months in the vertigo symptom scale-short form (VSS-SF) score in that trial was 2.3 points (95% confidence interval 0.4 to 4.1), favouring the online intervention. Our trial confirms the effectiveness of stand alone internet based VR in general practice. The larger difference we found in our stand alone VR group (4.1 points, 95% confidence interval 2.5 to 5.8) might be explained by better adherence to treatment (71% completed one session or more versus 61%) and a higher follow-up rate (87% v 70%). Our trial assessed the value of guidance in internet based VR. Compared with usual care, blended VR was not different from stand alone VR in our trial in terms of effectiveness, self reported perceived barriers to adherence, and actual adherence to the online intervention. In treating patients with stress, anxiety, and depression, blended online interventions have shown benefits over stand alone internet based interventions.61 62 63 However, a review that examined blended internet based interventions in chronic somatic disorders showed inconsistent effects.64 The optimal quantity (number of face-to-face sessions) and quality of guidance (type of therapist) in internet based interventions are still unclear and might depend on the condition.65 The added value of support by a healthcare professional in chronic vestibular syndromes might be less than in anxiety or depressive disorders. In stand alone VR, participants received automated emails to promote adherence, and treatment was tailored to the participant’s vestibular symptoms, which might have been enough to encourage them to continue. Further research is needed to determine the added value of guidance in internet based VR. A qualitative interview study and a prediction study, announced in the published study protocol,33 are still in progress. Interviews with participants who received blended VR will provide insights into participants’ experiences, and a prediction rule might help to determine which type of patient will benefit most from therapeutic guidance.
Strengths and limitations of this study
Our study has several strengths. The trial was well powered, follow-up rates were high, and participants displayed good adherence to treatment interventions. The primary outcome measure we used, the VSS-SF, is an established patient reported outcome measure42 66 that has been used in several VR trials.21 22 24 60 The pragmatic design of this trial with relatively broad inclusion criteria and no restrictions in usual care, allows general practitioners to directly apply the trial results in daily practice.
Our trial also has some limitations. Firstly, only a small percentage (<10%) of patients who were invited to participate enrolled in the trial, which might represent a selection bias. Of the 5130 invited participants, 3149 (61%) did not respond to our invitation. The low uptake may partly result from inviting patients who visited their doctor for vestibular symptoms in the past two years. Because 807 patients (60%) who declined to participate (see fig 1) reported that they no longer experienced vestibular symptoms, this might also be true for a large proportion of non-responders. Participation was declined by 138 patients (10%) because they had no access to a computer or the internet. When we compared our trial sample with vestibular subgroup data from representative Dutch general practice studies on dizziness,8 54 our participants were more often men and the age group 85 and older was underrepresented. Patients motivated to treat their vestibular symptoms with exercises might have been more likely to participate in the trial, which should be taken into account for implementation in daily general practice.
Secondly, we chose to include participants with a chronic vestibular syndrome and not a specified vestibular disorder. The effectiveness for VR was first shown in selected populations with various vestibular disorders, including benign paroxysmal positional vertigo, vestibular neuritis, Meniere’s disease, and unilateral and bilateral vestibulopathy.14 37 67 68 This formed the basis for later VR trials that applied a syndrome based approach to include patients who experienced symptoms related to these disorders.21 22 24 These studies showed that VR can be effective when applied in patients with a syndrome diagnosis, which is important for general practice where a final vestibular diagnosis might not be available. In our trial, 213 participants (66%) reported that they had never received a diagnosis for a specific vestibular disorder. Previous studies in general practice have shown that general practitioners record a symptom diagnosis in one third of patients who present with vertigo or dizziness.8 54 As we endorse the importance of a specific vestibular diagnosis in general practice, all participating doctors received written instructions on how to diagnose and treat vestibular symptoms according to the Dutch guidelines,18 and participants received usual care without restrictions. Nevertheless, the absence of a specific vestibular diagnosis should not be a reason to withhold VR from patients when a chronic vestibular syndrome is present. We acknowledge that the inclusion of adults with chronic vestibular syndromes instead of specified vestibular disorder diagnoses entailed a higher risk of diagnostic uncertainty. However, we believe that VR is a low risk treatment, which according to the International Classification of Vestibular Disorders requires a lower level of diagnostic certainty.10 As more than 80% of patients with vestibular symptoms are treated by general practitioners without referral to secondary care,6 7 8 using a syndrome diagnosis over a disorder diagnosis might increase the uptake of VR because it is more applicable to daily general practice.
Thirdly, we compared stand alone VR and blended VR with usual care, the last being an inactive comparator group. In pragmatic trials it is common for usual care to be used as a control group to maximise applicability of the results of the trial in daily practice.69 Nevertheless, not providing any experimental treatment to participants in the usual care group could have affected the results. Unfortunately, it was not possible to blind participants to this exercise based treatment. Therefore, the difference in treatment effects between the intervention groups and usual care group might be partly explained by including an inactive control group. In people with Meniere’s disease and chronic vestibular symptoms, booklet based VR has been compared with both an inactive control group and an active control group where participants received a booklet on how to control their symptoms.37 Compared with the inactive control group in this trial, both vestibular symptoms (VSS-SF) and impairment due to dizziness (dizziness handicap inventory) decreased in the VR group, and only impairment due to dizziness decreased in the active control group. Also, previous trials have shown that improvement in subjective, patient reported outcomes after VR are accompanied by improvements in objective measures of balance function.14 22 70 Therefore it is unlikely that the findings of our study are explained solely by providing any form of experimental treatment.
Fourthly, the absence of serious adverse events related to online VR indicates this treatment is safe to use in general practice, but harms may have been underreported. Participants were asked about adverse reactions at the three and six month follow-up and were encouraged to report these directly to the trial team. Since contact with the trial team during the trial was minimal, participants might have forgotten to mention some harmful side effects. Because our results are consistent with previous VR studies, where serious adverse events were also absent,14 24 online VR can probably be considered a safe form of treatment.
Conclusions and implications for research and practice
Internet based VR is a safe and effective treatment for adults aged 50 and older with a chronic vestibular syndrome. Although further research is needed to determine if certain participants might benefit more from either stand alone or blended VR, this trial shows that both forms of internet based VR can reduce vestibular symptoms. By providing general practitioners with an easily accessible, low cost form of treatment, online VR has the potential to substantially improve care for a largely undertreated group of patients with a chronic vestibular syndrome in general practice.
What is already known on this topic
In chronic vestibular syndromes, patients experience vestibular symptoms (vertigo, dizziness, vestibulovisual symptoms, postural symptoms) with features suggestive of persistent vestibular system dysfunction for months to years
Vestibular rehabilitation (VR) is a form of exercise therapy designed to optimise the process of vestibular compensation, which is disrupted in patients with a chronic vestibular syndrome
Although considered the preferred treatment for chronic vestibular syndromes, VR is still largely underused in general practice
What this study adds
In adults aged 50 and older with a chronic vestibular syndrome in general practice, internet based VR, both with and without physiotherapy support, resulted in a clinically relevant decrease in vestibular symptoms compared with usual care at six months
Acknowledgments
We thank Welmoed Kreb, Claudia van Doorenmaalen, and Marlieke de Vos for help with data collection.
Web extra.
Extra material supplied by authors
Supplementary information: supplementary appendixes and tables
Contributors: ORM obtained the funding and coordinated the study. ORM, VAvV, JCvdW, HEvdH, and LY designed the study. RE and LY advised on the development of the vertigo training intervention. VAvV collected the data. VAvV and JWRT analysed the data. VAvV wrote the first substantial draft of the article and is the guarantor. ORM, JCvdW, RE, LY, HEvdH, and JWRT critically revised the manuscript. All authors read and approved the final manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the Netherlands Organisation for Health Research and Development (ZonMw) for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years, no other relationships or activities that could appear to have influenced the submitted work.
Funding: This study was funded by the Netherlands Organisation for Health Research and Development (ZonMw) (programme Quality in health care; grant No 839110015). The sponsors did not participate in the study design; data collection, analysis, and interpretation; or the preparation or submission of this report.
Ethical approval: The study protocol was approved by the medical ethics committee of the VU University Medical Center. All participants included in the study provided written informed consent.
Data sharing: Deidentified individual participant data and data analysis plan available from the corresponding author on reasonable request.
The manuscript’s guarantor (VAvV) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
References
- 1. Bösner S, Schwarm S, Grevenrath P, et al. Prevalence, aetiologies and prognosis of the symptom dizziness in primary care - a systematic review. BMC Fam Pract 2018;19:33. 10.1186/s12875-017-0695-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neuhauser HK. The epidemiology of dizziness and vertigo. Handb Clin Neurol 2016;137:67-82. 10.1016/B978-0-444-63437-5.00005-4 [DOI] [PubMed] [Google Scholar]
- 3. Jönsson R, Sixt E, Landahl S, Rosenhall U. Prevalence of dizziness and vertigo in an urban elderly population. J Vestib Res 2004;14:47-52. [PubMed] [Google Scholar]
- 4. Neuhauser HK, Lempert T. Vertigo: epidemiologic aspects. Semin Neurol 2009;29:473-81. 10.1055/s-0029-1241043 [DOI] [PubMed] [Google Scholar]
- 5. Saber Tehrani AS, Coughlan D, Hsieh YH, et al. Rising annual costs of dizziness presentations to U.S. emergency departments. Acad Emerg Med 2013;20:689-96. 10.1111/acem.12168 [DOI] [PubMed] [Google Scholar]
- 6. Sloane PD, Dallara J, Roach C, Bailey KE, Mitchell M, McNutt R. Management of dizziness in primary care. J Am Board Fam Pract 1994;7:1-8. [PubMed] [Google Scholar]
- 7. Hanley K, O’ Dowd T. Symptoms of vertigo in general practice: a prospective study of diagnosis. Br J Gen Pract 2002;52:809-12. [PMC free article] [PubMed] [Google Scholar]
- 8. Stam H, Harting T, Sluijs Mv, et al. Usual care and management of fall risk increasing drugs in older dizzy patients in Dutch general practice. Scand J Prim Health Care 2016;34:165-71. 10.3109/02813432.2016.1160634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bisdorff A, Von Brevern M, Lempert T, Newman-Toker DE. Classification of vestibular symptoms: towards an international classification of vestibular disorders. J Vestib Res 2009;19:1-13. [DOI] [PubMed] [Google Scholar]
- 10. Bisdorff AR, Staab JP, Newman-Toker DE. Overview of the International Classification of Vestibular Disorders [vii.]. Neurol Clin 2015;33:541-50, vii. 10.1016/j.ncl.2015.04.010 [DOI] [PubMed] [Google Scholar]
- 11. Bisdorff A. Vestibular symptoms and history taking. Handb Clin Neurol 2016;137:83-90. 10.1016/B978-0-444-63437-5.00006-6 [DOI] [PubMed] [Google Scholar]
- 12. van Vugt VA, van der Horst HE, Payne RA, Maarsingh OR. Chronic vertigo: treat with exercise, not drugs. BMJ 2017;358:j3727. 10.1136/bmj.j3727 [DOI] [PubMed] [Google Scholar]
- 13. Lacour M, Helmchen C, Vidal PP. Vestibular compensation: the neuro-otologist’s best friend. J Neurol 2016;263(Suppl 1):S54-64. 10.1007/s00415-015-7903-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McDonnell MN, Hillier SL. Vestibular rehabilitation for unilateral peripheral vestibular dysfunction. Cochrane Database Syst Rev 2015;1:CD005397. 10.1002/14651858.CD005397.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharyya N, Gubbels SP, Schwartz SR, et al. Clinical practice guideline: benign paroxysmal positional vertigo (Update). Otolaryngol Head Neck Surg. 2017;156(3_suppl):S1-S47. [DOI] [PubMed]
- 16. Hall CD, Herdman SJ, Whitney SL, et al. Vestibular rehabilitation for peripheral vestibular hypofunction: an evidence-based clinical practice guideline: from the American Physical Therapy Association Neurology Section. J Neurol Phys Ther 2016;40:124-55. 10.1097/NPT.0000000000000120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Institute for Health and Care Excellence (NICE) Vestibular neuronitis. NICE, 2011 https://cks.nice.org.uk/vestibular-neuronitis
- 18.Summary of the Dutch College of General Practitioners’ practice guideline ‘Dizziness’ (In Dutch). Utrecht: Dutch College of General Practitioner, 2017
- 19. van Vugt VA, Diaz Nerio PM, van der Wouden JC, van der Horst HE, Maarsingh OR. Use of canalith repositioning manoeuvres and vestibular rehabilitation: a GP survey. Scand J Prim Health Care 2017;35:19-26. 10.1080/02813432.2017.1288683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jayarajan V, Rajenderkumar D. A survey of dizziness management in General Practice. J Laryngol Otol 2003;117:599-604. 10.1258/002221503768199915 [DOI] [PubMed] [Google Scholar]
- 21. Yardley L, Barker F, Muller I, et al. Clinical and cost effectiveness of booklet based vestibular rehabilitation for chronic dizziness in primary care: single blind, parallel group, pragmatic, randomised controlled trial. BMJ 2012;344:e2237. 10.1136/bmj.e2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yardley L, Donovan-Hall M, Smith HE, Walsh BM, Mullee M, Bronstein AM. Effectiveness of primary care-based vestibular rehabilitation for chronic dizziness. Ann Intern Med 2004;141:598-605. 10.7326/0003-4819-141-8-200410190-00007 [DOI] [PubMed] [Google Scholar]
- 23. Essery R, Kirby S, Geraghty AW, et al. The development of Balance Retraining: an online intervention for dizziness in adults aged 50 years and older. Am J Audiol 2015;24:276-9. 10.1044/2015_AJA-14-0081 [DOI] [PubMed] [Google Scholar]
- 24. Geraghty AWA, Essery R, Kirby S, et al. Internet-based vestibular rehabilitation for older adults with chronic dizziness: a randomized controlled trial in primary care. Ann Fam Med 2017;15:209-16. 10.1370/afm.2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Ballegooijen W, Cuijpers P, van Straten A, et al. Adherence to Internet-based and face-to-face cognitive behavioural therapy for depression: a meta-analysis. PLoS One 2014;9:e100674. 10.1371/journal.pone.0100674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wilhelmsen M, Lillevoll K, Risør MB, et al. Motivation to persist with internet-based cognitive behavioural treatment using blended care: a qualitative study. BMC Psychiatry 2013;13:296. 10.1186/1471-244X-13-296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van der Vaart R, Witting M, Riper H, Kooistra L, Bohlmeijer ET, van Gemert-Pijnen LJ. Blending online therapy into regular face-to-face therapy for depression: content, ratio and preconditions according to patients and therapists using a Delphi study. BMC Psychiatry 2014;14:355. 10.1186/s12888-014-0355-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. MacDowell SG, Wellons R, Bissell A, Knecht L, Naquin C, Karpinski A. The impact of symptoms of anxiety and depression on subjective and objective outcome measures in individuals with vestibular disorders. J Vestib Res 2018;27:295-303. 10.3233/VES-170627 [DOI] [PubMed] [Google Scholar]
- 29. Bigelow RT, Semenov YR, du Lac S, Hoffman HJ, Agrawal Y. Vestibular vertigo and comorbid cognitive and psychiatric impairment: the 2008 National Health Interview Survey. J Neurol Neurosurg Psychiatry 2016;87:367-72. 10.1136/jnnp-2015-310319 [DOI] [PubMed] [Google Scholar]
- 30. Herdman SJ, Hall CD, Delaune W. Variables associated with outcome in patients with unilateral vestibular hypofunction. Neurorehabil Neural Repair 2012;26:151-62. 10.1177/1545968311407514 [DOI] [PubMed] [Google Scholar]
- 31. Lacour M, Bernard-Demanze L. Interaction between vestibular compensation mechanisms and vestibular rehabilitation therapy: 10 recommendations for optimal functional recovery. Front Neurol 2015;5:285. 10.3389/fneur.2014.00285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Walker A, Kantaris X, Chambers M. Understanding therapeutic approaches to anxiety in vestibular rehabilitation: a qualitative study of specialist physiotherapists in the UK. Disabil Rehabil 2018;40:829-35. 10.1080/09638288.2016.1277393 [DOI] [PubMed] [Google Scholar]
- 33. van Vugt VA, van der Wouden JC, Bosmans JE, et al. Guided and unguided internet-based vestibular rehabilitation versus usual care for dizzy adults of 50 years and older: a protocol for a three-armed randomised trial. BMJ Open 2017;7:e015479. 10.1136/bmjopen-2016-015479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boutron I, Altman DG, Moher D, Schulz KF, Ravaud P, CONSORT NPT Group CONSORT statement for randomized trials of nonpharmacologic treatments: a 2017 update and a CONSORT extension for nonpharmacologic trial abstracts. Ann Intern Med 2017;167:40-7. 10.7326/M17-0046 [DOI] [PubMed] [Google Scholar]
- 35. Geraghty AW, Kirby S, Essery R, et al. Internet-based vestibular rehabilitation for adults aged 50 years and over: a protocol for a randomised controlled trial. BMJ Open 2014;4:e005871. 10.1136/bmjopen-2014-005871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brandt T. Management of vestibular disorders. J Neurol 2000;247:491-9. 10.1007/s004150070146 [DOI] [PubMed] [Google Scholar]
- 37. Yardley L, Kirby S. Evaluation of booklet-based self-management of symptoms in Ménière disease: a randomized controlled trial. Psychosom Med 2006;68:762-9. 10.1097/01.psy.0000232269.17906.92 [DOI] [PubMed] [Google Scholar]
- 38. Yardley L, Morrison L, Bradbury K, Muller I. The person-based approach to intervention development: application to digital health-related behavior change interventions. J Med Internet Res 2015;17:e30. 10.2196/jmir.4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Essery R, Kirby S, Geraghty AWA, Yardley L. Older adults’ experiences of internet-based vestibular rehabilitation for dizziness: A longitudinal study. Psychol Health 2017;32:1327-47. 10.1080/08870446.2017.1310861 [DOI] [PubMed] [Google Scholar]
- 40. Meli A, Zimatore G, Badaracco C, De Angelis E, Tufarelli D. Effects of vestibular rehabilitation therapy on emotional aspects in chronic vestibular patients. J Psychosom Res 2007;63:185-90. 10.1016/j.jpsychores.2007.02.007 [DOI] [PubMed] [Google Scholar]
- 41. Yardley L, Masson E, Verschuur C, Haacke N, Luxon L. Symptoms, anxiety and handicap in dizzy patients: development of the vertigo symptom scale. J Psychosom Res 1992;36:731-41. 10.1016/0022-3999(92)90131-K [DOI] [PubMed] [Google Scholar]
- 42. Wilhelmsen K, Strand LI, Nordahl SHG, Eide GE, Ljunggren AE. Psychometric properties of the Vertigo symptom scale - Short form. BMC Ear Nose Throat Disord 2008;8:2. 10.1186/1472-6815-8-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jacobson GP, Newman CW. The development of the Dizziness Handicap Inventory. Arch Otolaryngol Head Neck Surg 1990;116:424-7. 10.1001/archotol.1990.01870040046011 [DOI] [PubMed] [Google Scholar]
- 44. Spitzer RL, Kroenke K, Williams JB. Patient Health Questionnaire. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. JAMA 1999;282:1737-44. 10.1001/jama.282.18.1737 [DOI] [PubMed] [Google Scholar]
- 45. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092-7. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 46. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kirby S, Donovan-Hall M, Yardley L. Measuring barriers to adherence: validation of the Problematic Experiences of Therapy Scale. Disabil Rehabil 2014;36:1924-9. 10.3109/09638288.2013.876106 [DOI] [PubMed] [Google Scholar]
- 48. Twisk J, de Boer M, de Vente W, Heymans M. Multiple imputation of missing values was not necessary before performing a longitudinal mixed-model analysis. J Clin Epidemiol 2013;66:1022-8. 10.1016/j.jclinepi.2013.03.017 [DOI] [PubMed] [Google Scholar]
- 49. J T, L B, T H, J R, M W, M H. Different ways to estimate treatment effects in randomised controlled trials. Contemp Clin Trials Commun 2018;10:80-5. 10.1016/j.conctc.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gassmann KG, Rupprecht R, IZG Study Group Dizziness in an older community dwelling population: a multifactorial syndrome. J Nutr Health Aging 2009;13:278-82. 10.1007/s12603-009-0073-2 [DOI] [PubMed] [Google Scholar]
- 51. Stam H, Maarsingh OR, Heymans MW, van Weert HCPM, van der Wouden JC, van der Horst HE. Predicting an unfavorable course of dizziness in older patients. Ann Fam Med 2018;16:428-35. 10.1370/afm.2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Olsson Möller U, Midlöv P, Kristensson J, Ekdahl C, Berglund J, Jakobsson U. Prevalence and predictors of falls and dizziness in people younger and older than 80 years of age--a longitudinal cohort study. Arch Gerontol Geriatr 2013;56:160-8. 10.1016/j.archger.2012.08.013 [DOI] [PubMed] [Google Scholar]
- 53. Sloane PD, Coeytaux RR, Beck RS, Dallara J. Dizziness: state of the science. Ann Intern Med 2001;134:823-32. 10.7326/0003-4819-134-9_Part_2-200105011-00005 [DOI] [PubMed] [Google Scholar]
- 54. Maarsingh OR, Dros J, Schellevis FG, van Weert HC, Bindels PJ, Horst HE. Dizziness reported by elderly patients in family practice: prevalence, incidence, and clinical characteristics. BMC Fam Pract 2010;11:2. 10.1186/1471-2296-11-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wilhelmsen K, Ljunggren AE, Goplen F, Eide GE, Nordahl SH. Long-term symptoms in dizzy patients examined in a university clinic. BMC Ear Nose Throat Disord 2009;9:2. 10.1186/1472-6815-9-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maarsingh OR, Stam H, van de Ven PM, van Schoor NM, Ridd MJ, van der Wouden JC. Predictors of dizziness in older persons: a 10-year prospective cohort study in the community. BMC Geriatr 2014;14:133. 10.1186/1471-2318-14-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tinetti ME, Williams CS, Gill TM. Dizziness among older adults: a possible geriatric syndrome. Ann Intern Med 2000;132:337-44. 10.7326/0003-4819-132-5-200003070-00002 [DOI] [PubMed] [Google Scholar]
- 58. Dros J, Maarsingh OR, Beem L, et al. Functional prognosis of dizziness in older adults in primary care: a prospective cohort study. J Am Geriatr Soc 2012;60:2263-9. 10.1111/jgs.12031 [DOI] [PubMed] [Google Scholar]
- 59. de Moraes SA, Soares WJ, Ferriolli E, Perracini MR. Prevalence and correlates of dizziness in community-dwelling older people: a cross sectional population based study. BMC Geriatr 2013;13:4. 10.1186/1471-2318-13-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yardley L, Beech S, Zander L, Evans T, Weinman J. A randomized controlled trial of exercise therapy for dizziness and vertigo in primary care. Br J Gen Pract 1998;48:1136-40. [PMC free article] [PubMed] [Google Scholar]
- 61. Heber E, Ebert DD, Lehr D, et al. The benefit of web- and computer-based interventions for stress: a systematic review and meta-analysis. J Med Internet Res 2017;19:e32. 10.2196/jmir.5774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Olthuis JV, Watt MC, Bailey K, Hayden JA, Stewart SH. Therapist-supported Internet cognitive behavioural therapy for anxiety disorders in adults. Cochrane Database Syst Rev 2016;3:CD011565. 10.1002/14651858.CD011565.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Richards D, Richardson T. Computer-based psychological treatments for depression: a systematic review and meta-analysis. Clin Psychol Rev 2012;32:329-42. 10.1016/j.cpr.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 64. Kloek C, Bossen D, de Bakker DH, Veenhof C, Dekker J. Blended interventions to change behavior in patients with chronic somatic disorders: systematic review. J Med Internet Res 2017;19:e418. 10.2196/jmir.8108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Andersson G. Internet-delivered psychological treatments. Annu Rev Clin Psychol 2016;12:157-79. 10.1146/annurev-clinpsy-021815-093006 [DOI] [PubMed] [Google Scholar]
- 66. Fong E, Li C, Aslakson R, Agrawal Y. Systematic review of patient-reported outcome measures in clinical vestibular research. Arch Phys Med Rehabil 2015;96:357-65. 10.1016/j.apmr.2014.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dunlap PM, Holmberg JM, Whitney SL. Vestibular rehabilitation: advances in peripheral and central vestibular disorders. Curr Opin Neurol 2019;32:137-44. 10.1097/WCO.0000000000000632 [DOI] [PubMed] [Google Scholar]
- 68. Porciuncula F, Johnson CC, Glickman LB. The effect of vestibular rehabilitation on adults with bilateral vestibular hypofunction: a systematic review. J Vestib Res 2012;22:283-98. [DOI] [PubMed] [Google Scholar]
- 69. Zwarenstein M, Treweek S, Gagnier JJ, et al. CONSORT group. Pragmatic Trials in Healthcare (Practihc) group Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008;337:a2390. 10.1136/bmj.a2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Strupp M, Arbusow V, Maag KP, Gall C, Brandt T. Vestibular exercises improve central vestibulospinal compensation after vestibular neuritis. Neurology 1998;51:838-44. 10.1212/WNL.51.3.838 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: supplementary appendixes and tables