Abstract
Aims
(1) To develop an analytical method for recovery and quantification of bacteriophage MS2 – as a surrogate for foot and mouth disease virus (FMDV) – from complex porous surfaces, with and without the presence of laboratory-developed agricultural grime; (2) To evaluate, with a 4-log dynamic range, the virucidal activity of common biocides for their ability to decontaminate surfaces, and hence remediate facilities, following a foreign animal disease contamination incident.
Methods and Results
An analytical method was developed and optimized for MS2 recovery from simulated agricultural surfaces. The addition of Dey-Engley neutralizing broth to an extraction buffer improved MS2 viability in liquid extracts, with optimal analytical holding times determined as < 8h to ≤24h, depending on matrix. The recovery of MS2 from surface materials decreased in order: non-porous reference material > grimed porous materials > non-grimed porous materials. In disinfectant testing, two spray applications of pAB were effective against MS2 (≥ 4-log reduction) on all operational-scale materials. 2% citric acid had limited effectiveness, with ≥ 4-log reduction observed on a selected subset of grimed concrete samples.
Conclusions
Decontamination efficacy test results can be affected by surface characteristics, extraction buffer composition, analytical holding time, and surface-specific organism survivability. Efficacy should be evaluated using a test method that reflects the environmental characteristics of the intended application.
Significance and Impact of the Study
The results of this study demonstrate the importance of analytical method verification tests for disinfectant testing prior to application in complex environments.
Keywords: bacteriophage MS2, FMDV surrogate, agricultural surfaces, synthetic agricultural grime, surface decontamination, pH-amended bleach, pAB, citric acid
Introduction
Widespread releases of human and animal biologically infectious agents can be in the form of intentional dissemination (bioterror attack) or natural outbreak (Kortepeter and Parker 1999; US EPA 2011; Calfee et al. 2012; US EPA 2016). Both scenarios present significant challenges regarding qualitative and quantitative analytical methods for determining the extent of the biocontamination and the consequent selection of appropriate decontamination strategies for remediation of contaminated environments (FAO 2001; Julian et al. 2011; US EPA 2016). Decontamination strategies for deactivating and removing infectious agents typically focus on the etiology (viral vs. bacterial) associated with the biocontamination event (FAO 1999). As the identification of the organism and its epidemiology remain a focal point for the selection of appropriate disinfectant(s), other factors that may affect the overall decontamination performance, such as surface characteristics and environmental conditions, are often generalized or not fully accounted for in disinfectant testing (FAO 1999; US EPA 2016). Current standardized decontamination test methods for registration of products under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) require efficacy be demonstrated on reference materials such as glass or steel, and therefore may not accurately predict decontaminant performance in challenging situations (Calfee et al. 2012; Krug et al. 2012; US EPA 2016).
The foot-and-mouth disease virus (FMDV) is classified as one of the most important infectious animal disease agents based on pathogenicity, difficulty in eliminating it from the environment, and the significant economic impact associated with reported outbreaks (FAO 1999; Mummert and Weiss 2017). A comparison of key decontamination parameters such as amplification, persistence, and resistance using a qualitative grade from 0 to 10 (least to most difficult to eliminate from the environment) ranked FMDV at 9 in amplification by the species involved, 7 in environmental persistence (manure at 25°C), and 3 in resistance to inactivation by disinfection (FAO 1999). FMDV is considered a category B infectious agent that is best inactivated with hypochlorites, alkalis, Virkon®, glutaraldehyde, and acids (FAO 1999). FMDV is reported to be relatively resistant to lipophilic disinfectants such as detergents and resistant to other common disinfectants such as quaternary ammonium compounds and phenolics (FAO 1999). The existing disinfectant selection guidelines (FAO 1999) do not provide specific recommendations on decontamination of complex porous surfaces (e.g., wood, concrete) and surfaces with heavy organic loads (e.g., manure, agricultural grime). In fact, there are a limited number of reports on the decontamination efficacies of pathogenic viruses from challenging indoor surfaces (Krug et al. 2011; Krug et al. 2012).
The methods used to detect pathogenic viruses from environmental surfaces remain limited, primarily due to difficulties related to development and application of standardized methods (Dawson et al. 2005; Butot et al. 2007). In laboratory studies, the use of RNA bacteriophages as surrogates for enteroviruses is often a preferred method for disinfection testing (Steinmann 2004; Boudaud et al. 2012). Because it is similar to some enteric viruses, the MS2 bacteriophage (an RNA bacteriophage) is the surrogate most commonly proposed and used to assess the efficacy of virucidal decontaminants (D’Souza and Xiaowei 2010; Boudaud et al. 2012). MS2 has been reported to have a sensitivity (D’Souza et al. 2010; Morin et al. 2015) to selected disinfectants recommended for FMDV decontamination (Krug et al. 2011; Krug et al. 2012). Many methods have been described for detection of MS2 from various inanimate surfaces but few address detections of MS2 from complex environmental matrices (Krug et al. 2011; Krug et al. 2012; Shim et al. 2017). Common challenges of MS2 analytics relate to its moisture- and/or temperature-dependent survivability (Hurst et al. 1980; Krug 2011), and general issues related to optimization of the analytical method’s performance (e.g., poor recovery for certain sampling media, wetting solvent/eluent type, and/or the surface material) (Julian et al. 2011). Therefore, the purpose of this study was to develop a robust analytical procedure for recovering and quantifying MS2 from samples of simulated agricultural surfaces. The method was further deployed in disinfectant efficacy testing on porous and heavily soiled surfaces at both the laboratory and operational scales.
Material and methods
Preparation of test coupons
Laboratory-scale (18-mm diameter) and operational test coupons (35.6 cm × 35.6 cm) were used in the analytical method optimization and disinfectant effectiveness testing. The coupons were manufactured using two commercial materials representative of surfaces found in agricultural animal husbandry settings: pressure-treated plywood (alkaline copper quaternary type D [ACQ-D], 1.91-cm thick; Georgia-Pacific, Atlanta, GA, USA) and general purpose concrete (Quikrete® sand/topping mix; Quikrete Companies, Atlanta, GA, USA). The nonporous reference material was stainless steel (multipurpose stainless steel, 0.91-mm thick, type 304, 2B mill, unpolished; McMaster-Carr, Douglasville, GA, USA). Coupons were sterilized using ethylene oxide, vaporous hydrogen peroxide, or autoclaving. Detailed procedures for fabrication and sterilization of test coupons are described elsewhere (US EPA 2016).
Preparation and application of simulated agricultural grime
The composition of synthetic agricultural grime was developed based on a scientific literature review and its applicability for use in evaluating the performance of decontamination methods for heavily soiled surfaces (US EPA 2016). Grime was made using commercially available standardized/pure products and biocomponents prepared by the North Carolina State University (NCSU) Animal and Poultry Waste Management Center (APWMC). The raw materials used for the preparation of simulated agricultural grime are given is Table S1 of Supporting Information. Grime manufacturing and application procedures are explained in detail elsewhere (US EPA 2016). The procedure resulted in surface grime burdens of 0.33 ± 0.02 kg m−2 for concrete and 0.25 ± 0.03 kg m−2 for plywood. Based on reported manure and sebum extraction rates for ruminants (O’Kelly and Reich 1981), these values can be considered representative of heavily soiled surfaces in animal facilities (US EPA 2016).
Target organism and inoculation of coupons
Bacteriophage MS2 (ATCC® 15597-B1™, American Type Culture Collection, Manassas, VA, USA) was propagated in Escherichia coli host cells (ATCC® 15597™ Escherichia coli strain C3000) and analyzed using the two-step enrichment double-agar overlay method (US EPA 2001). The MS2 target titer (1 × 108 plaque forming units [PFU] ml −1) was confirmed before each liquid inoculation. Inoculations were performed using an air-displacement pipette (100-μl per each 18-mm coupon, 20 × 100-μl per operational scale coupon). The MS2-contaminated coupons were dried at room temperature from 10 min (non-grimed porous materials, and stainless-steel inoculation controls) to 2–18 h (grimed materials) before testing.
Optimization of decontaminant neutralizer
Precise and complete neutralization is critical for decontaminant efficacy evaluations. The concentrated Dey–Engley neutralizing broth (hereafter referred to as D–E broth) was prepared by mixing 250 g of D–E broth powdered formulation (BD 281910, BD Diagnostic Systems, Franklin Lakes, NJ, USA, or equivalent) with 250 ml of DI water, and autoclaving at 121°C liquid cycle for 15 minutes. D–E broth was added to PBST extraction buffer (10 mmol l−1 phosphate-buffered saline with Tween 20 [PBST; 138 mmol l−1 NaCl, 2.7 mmol l−1 KCl, 0.05% Tween 20]) at 1:10 D–E broth:PBST ratio (v/v) and evaluated for the efficacy of decontaminant quenching. D–E broth is reported to be suitable for the neutralization of a wide range of disinfectant test samples obtained under environmental sampling applications, including chlorine and iodine formulations, quaternary ammonium compounds, phenolics, formaldehydes, and glutaraldehydes (Hardy Diagnostics 1996; Downes 2001; Remel 2010). As such, D‒E broth was expected to quench both virucidal chemicals used in this study (pAB and 2% citric acid). Neutralization testing was performed on laboratory-scale coupons of grimed materials (concrete and plywood) and in liquid-liquid reaction testing, with the target MS2 concentration of 1 × 108 PFU (log PFU = 8.0). Neutralized test samples were extracted within 2 h post-inoculation and plated within <8 h and 96 h analytical hold time (HT). Details of the experimental design of the initial neutralizer testing are given in Section S2 of Supporting Information (Table S2).
Optimization of hold times and extraction buffer
Due to the enhanced stability and increased recovery observed for D–E neutralized samples, the D–E broth was further evaluated as an extraction enhancer and sample stabilizer/preservative, with up to 96 h extract holding time (experimental details are given in Section S3). Then, a series of laboratory-scale experiments were conducted to evaluate extraction buffer options for MS2 recovery from grimed and non-grimed porous surfaces. Four D–E broth-enriched (1:10, v/v) extraction buffer combinations were evaluated; (1) D–E + deionized water (DI); (2) D–E + phosphate buffered saline with 0.05% Tween20 (PBST); (3) D–E + phosphate buffered saline (PBS); and (4) D–E + tryptic soy broth (TSB). Extraction efficacy testing was performed in conjunction with MS2 surface survivability tests, whereby the stability of MS2 was determined at 10 min, 2 h, and 18 h post-inoculation onto non-grimed and grimed test surfaces. Details of the experimental procedure and test matrix for the optimization of HTs and extraction buffer tests are given in Section S4.
Decontaminants
Two decontaminants with reported efficacy against FMDV (FAO 1999; Krug et al. 2011, Krug et al. 2012) were evaluated against MS2: pH-amended bleach (pAB) and 2% (w/v) citric acid solution. The pAB solution was prepared using 1 part concentrated germicidal bleach (sodium hypochlorite concentration of 5.25–6%) (Clorox Corp., Oakland, CA, USA) combined with 8 parts DI water and 1 part 5% (v/v) acetic acid (ACS grade, Thermo Fisher Scientific, Waltham, MA, USA). The final pH of the pAB solution was 6.5–7.0, with a target free available chlorine (FAC) concentration of 6,000–6,800 ppmv. Due to chlorine gas generation, proper engineering controls and personal protective equipment (PPE) must be used to avoid exposure during work with pAB. The 2% citric acid solution was prepared daily using ≥99.5% purity citric acid (ACS grade, Sigma-Aldrich, St. Louis, MO, USA) dissolved in DI water (final pH approximately 2.0). The concentration of active ingredients was confirmed before each experiment through titration.
Decontamination and sampling procedures
Laboratory-scale testing was performed using a custom-built bench-scale apparatus consisting of an orifice plate, funnel, and decontamination run-off and rinsate collection tube (50 ml BD Falcon™ conical tube, Thermo Fisher Scientific, Waltham, MA) preloaded with D–E:PBST extraction buffer (US EPA 2016). The decontamination solution was applied using a commercial handheld sprayer (RL FloMaster 56HD; Root Lowell Manufacturing Co., Lowell, MI, USA) triple rinsed with sterile DI water and purged with target decontamination solution before each test. The spray nozzle was held approximately 15 cm from the spray apparatus orifice plate, and each 18-mm coupon was sprayed for 10 s. After a 15-min initial dwell time (DT1), decontaminant was reapplied (10 s spray), and a second dwell time of 15 min (DT2) was rendered so that the total exposure time was 30 min. The decontaminant application was followed by a sterile DI water rinse (5 s spray) of each coupon using the same type of sprayer. Operational scale-decontamination testing was conducted in a 1.22 m (H) × 1.22 m (W) × 1.22 m (D) spray chamber designed to accommodate up to three large (35.56 cm × 35.56 cm) coupons at a time in a horizontal or vertical position (Calfee et al. 2012). During operational-scale testing, decontaminants and water rinses were applied at a flow rate of 1.2 L-min−1 to a set of three vertically assembled coupons using a commercial backpack sprayer (SHURFLO® ProPack™ SR600 rechargeable electric backpack sprayer equipped with Teejet 50800 spray wand; Pentair/SHURFLO Inc., Costa Mesa, CA, USA). During each application, a single spray nozzle was positioned approximately 30 cm from the test coupon surface. The decontaminant spray was applied with a continuous left-to-right-to-left motion onto the set of three test coupons. The following sequence was used: (1) 15 s application of decontaminant per coupon set, with each coupon receiving a 5 s spray, (2) 15 min (DT1) dwell of decontaminant, (3) 15 s reapplication of decontaminant, with each coupon receiving a 5 s spray; and (4) final 15 min (DT2) dwell of decontaminant. After 30 min of total exposure time, a DI water rinse (10 s spray at the rate of approximately 130 L-min−1) was applied to the set of large coupons using a standard garden hose and the same continuous spray motion. Fifteen (15) minutes after completion of the water rinse, surface samples were collected using the modified protocol of the US Centers for Disease Control and Prevention (CDC) (CDC 2010; US EPA 2011; Calfee et al. 2012). After sampling method optimization (described in Section S5), samples were extracted with 20 ml of D–E:PBST (1:10, v/v) using a vortex mixer for 2 min in 10-s intervals. After the extraction, the sample buffer was subjected to a five-stage serial dilution (10−1 to 10−5). The resulting dilutions were plated in triplicate on the same day (<8 h HT post-extraction) using a two-step enrichment (double-overlay agar) method (US EPA 2001) and incubated overnight (minimum of 18 h) at 35°C ± 2°C. After incubation, PFUs were enumerated manually. MS2 recovery efficiency was measured by comparing the recovered bacteriophage (PFU) in each test to the theoretical inoculated amount (MS2 surface loading, SL). The surface loading was calculated based on the pretest inoculum concentration (PFU ml−1) multiplied by the volume of liquid applied to each coupon (ml).
The surface decontamination efficacy was expressed as log10 reduction (LR). The LR was calculated for each decontaminant-material combination by the difference in mean log10 PFU recoveries of positive control (PC) samples (n = 3) and decontaminated test (TC) samples (n = 3 to 5) (US EPA 2016). All sample averages were reported as arithmetic mean ± one (1) standard deviation (STD). Statistical analyses (multi-factor analysis of variance [ANOVA]) were performed using SigmaStat 14.0. All multiple pairwise comparisons for three-way ANOVA were performed using the Holm-Sidak method.
RESULTS
Optimization of MS2 recovery from non-grimed and grimed surfaces
Surface sampling and extraction recovery tests
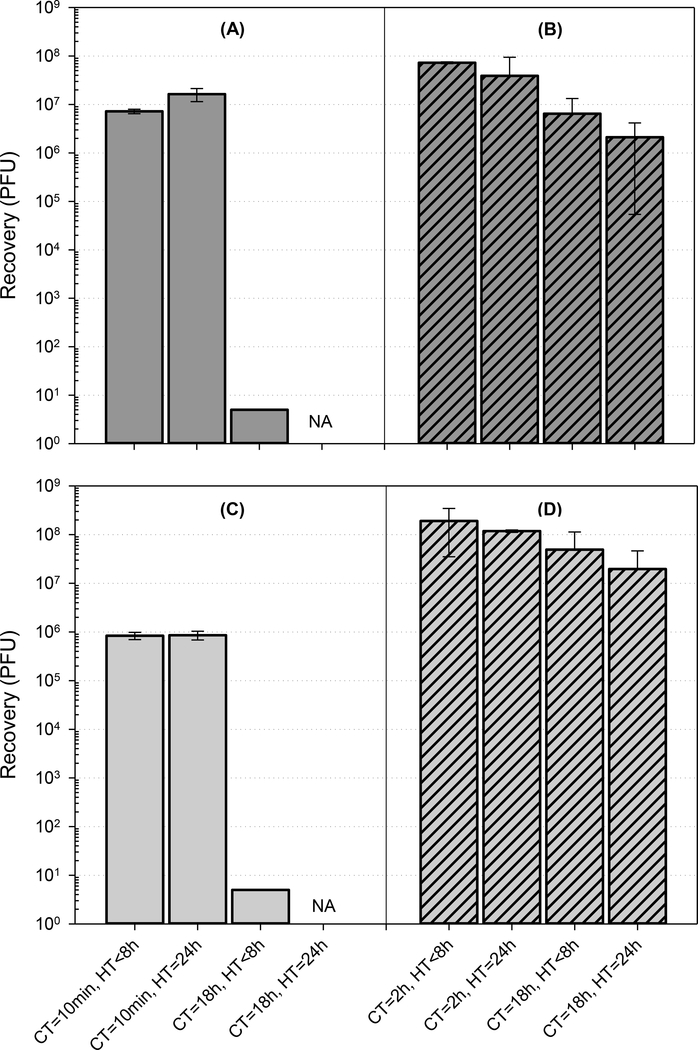
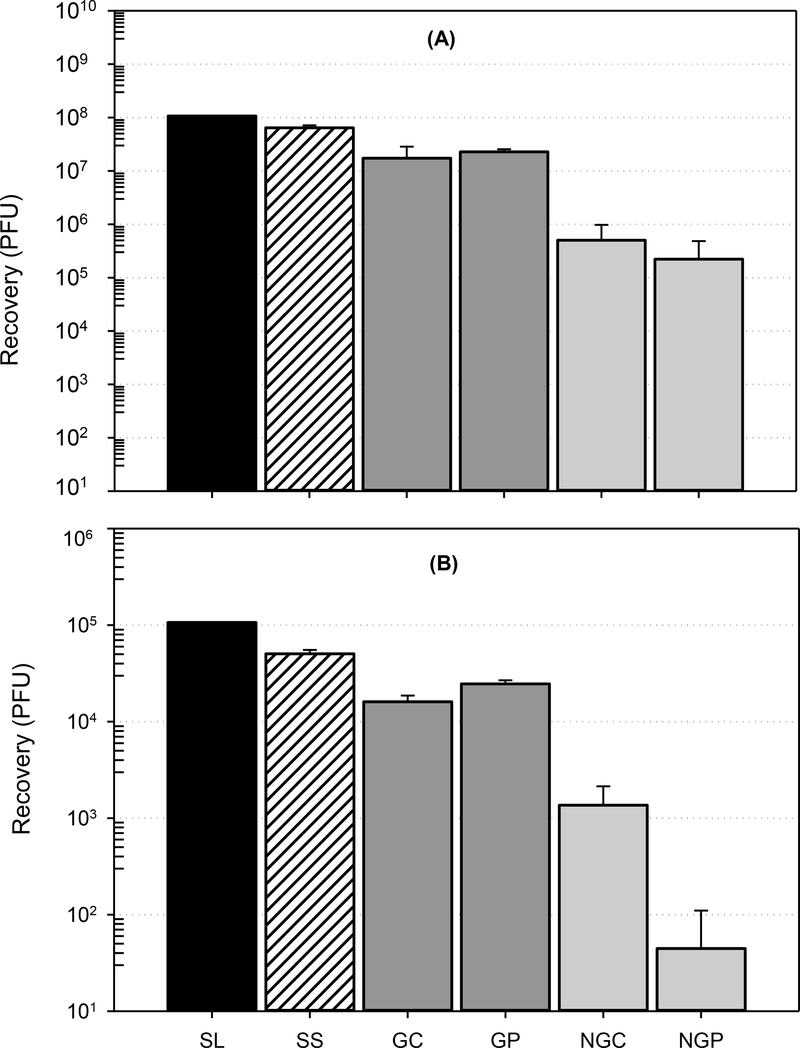
In a series of preliminary experiments, the D–E:PBST solution was a suitable neutralizing agent for 2% citric acid and pAB disinfectants, with no apparent toxicity to MS2 at the target concentration of 1 × 108 PFU (or a decontamination challenge threshold of 8 logs). The surface test samples that received neutralization treatment had higher recoveries of MS2 than non-treated controls, suggesting that D–E broth can enhance sample stability during wet storage in PBST. Results are given in Section S2 of Supporting Information (Fig. S1). The use of D–E broth as an MS2 extraction enhancer and preservative (with no presence of disinfectant) was consequently tested on grimed materials and showed improved recoveries of MS2 for the same-day extractions – approximately 3–4 logs for both grimed materials for the short (<8 h) and extended (96 h) HTs tested. Results from these preliminary extraction efficacy tests are given in Section S3 of Supporting Information (Fig. S2). After the initial testing, the effect of D–E broth on extraction efficacy was systematically evaluated using various extraction buffers and surface-specific analytical HTs. The buffer that provided the largest MS2 recovery, from both grimed and non-grimed surfaces, was the D–E:PBST solution. The average log PFU ( log PFU) recoveries for samples extracted and plated on the same day (HT <8 h) ranged from 5.9 to 8.1 (Fig. 1, Table S3). The average percent recoveries of the initial MS2 loadings were 90 ± 36% and 3.6 ± 4.0% for grimed and non-grimed materials, respectively. Results for other extraction buffers with comparably high extraction efficacies and no observable negative impacts on MS2 storage stability (D–E:DI water and D–E:TSB) are listed in in Section S4 of Supporting Information (Table S3). D–E broth-enriched PBS medium provided the lowest MS2 recoveries: for HT <8 h, the log PFU recoveries ranged from 0.7 – 3.6 (<1% of the initial surface loading); for a 24 h HT, there was a significant (up to 7.3 logs) reduction in MS2 recovery (data not shown). For comparison, the average difference between samples extracted with D–E:PBST buffer and analyzed within <8 h and 24 h HTs was approximately 0.5 log (Fig. 1, Table S3). Based on this data, D–E:PBST buffer was used for extraction of wipe samples during operational-scale testing. In addition to surface-related decreases in MS2 recovery, a time-dependent low surface survivability effect was observed for all non-grimed porous materials, regardless of extraction buffer used. Up to a 5.8-log decrease in MS2 recovery was observed for overnight (18 h) contact times (CTs) (Fig. 1). This suggests that prolonged drying causes reduced viability of MS2 on porous materials. Conversely, the presence of agricultural grime slowed surface desiccation and allowed for improved surface survivability of MS2. No statistically significant difference (P = 0.069–0.19, one-way ANOVA; α = 0.05) was observed between subsets sampled from grimed surfaces within minutes to hours post-inoculation and after the overnight drying period. Applicability of the optimized analytical method for operational-scale testing was evaluated before decontamination experiments by determining MS2 recoveries from reference and test materials inoculated with various concentrations. Recovery results for the nonporous reference material and grimed surfaces were 7.2–7.8 log PFU (16–60%) for the high-concentration (1 × 108) surface loading and 4.7–4.2 log PFU (15–47%) for the medium-concentration (1 × 105) surface loading (Fig. 2). MS2 recoveries from non-grimed materials were much lower (≤1% of theoretical surface loading; Fig. 2) and showed significant (−99.2 to −99.9%) relative percent differences in MS2 recoveries compared to nonporous reference materials (Table S4).
Figure 1. Recovery of MS2 in D–E-enriched PBST for different types of materials, surface contact times (CTs), and analytical hold times (HTs).
Data are reported as the arithmetic mean of recovered PFU ± 1 standard deviation; spiked concentration of MS2 was at 1 × 108 PFU level. Testing was performed on 18-mm coupons and four types of materials: non-grimed concrete (A), grimed concrete (B), non-grimed plywood (C), and grimed plywood (D). Extraction buffers: D–E broth-enriched PBST (1:10, v/v); MS2 surface contact time from spiking to extraction (CT); analytical holding time from extraction to plating (HT); and not analyzed (NA). The next-day plating (HT = 24 h) was not performed due to the low recovery observed for the same-day HT (<8 h) analyses.
Figure 2. Wipe sampling method optimization for different types of material.
Data are reported as the arithmetic mean of recovered PFU ± 1 standard deviation; spiked concentration of MS2 levels: high-surface loading (A) at 1 × 108 PFU per coupon and medium-surface loading (B) at 1 × 105 PFU per coupon. Testing was performed on 35.56 cm × 35.56 cm coupons and five types of materials: stainless steel (SS [nonporous reference material]), non-grimed concrete (NGC), grimed concrete (GC), non-grimed plywood (NGP), and grimed plywood (GP). Wipe samples were sampled, extracted, and analyzed on the day of the experiment (CT <10 min, HT <8 h).
Decontamination tests
The results of laboratory-scale decontamination testing (Table 1) indicate that pAB is an effective decontaminant for MS2. The average decontamination efficacy was >6 LR (at DT=30 min) on both grimed and non-grimed concrete. However, a limited efficacy was observed for plywood test matrices (2.4–3.7 LR for non-grimed and grimed plywood, respectively). For laboratory-scale experiments that used an exposure to 2% citric acid, the MS2 surface reduction after 30 min exposure was lower than pAB for all conditions tested (0.08–3.5 LR; Table 1). The composite liquid waste (combined coupon runoff and rinsate) samples collected during laboratory-scale testing showed that MS2 was detected in 25% and 100% of total samples for pAB and 2% citric acid, respectively. An appreciable wash-off of MS2 (3.7–7.2 log PFU; Table 1) suggests that the 2% citric acid formulation did not exhibit a strong virucidal effect during the controlled 30 min decontaminant surface dwell time.
Table 1:
Laboratory-scale decontamination testing results
| Decontaminant | Application method | Material type | Coupon surface | Non-decontaminated positive control coupons (PFU) | Decontaminated test coupons (PFU) | Liquid Waste (PFU) | Log10 reduction (LR) |
|---|---|---|---|---|---|---|---|
| Average ± STD | Average ± STD | Average ± STD | Average ± STD | ||||
| pAB | Handheld sprayer | Concrete | Non-grimed | 6.77 ± 2.68 × 106 | ND | ND | 7.1 ± 0.12 |
| Grimed | 2.99 ± 2.59 × 107 | 2.83 ± 6.34 × 105 | ND | 6.4 ± 1.3 | |||
| Treated plywood | Non-grimed | 1.37 ± 0.80 × 108 | 4.54 ± 1.46 × 105 | 6.05 ± 8.17 × 101 | 2.4 ± 0.19 | ||
| Grimed | 4.91 ± 7.36 × 107 | 8.57 ± 9.86 × 105 | ND | 3.7 ± 1.7 | |||
| 2% citric acid | Concrete | Non-grimed | 3.68 ± 1.24 × 107 | 1.39 ± 0.79 × 107 | 4.82 ± 2.87 × 103 | 0.46 ± 0.15 | |
| Grimed | 0.617 ± 1.03 × 108 | 4.99 ± 4.21 × 106 | 1.44 ± 1.50 × 107 | 1.1 ± 1.1 | |||
| Treated plywood | Non-grimed | 6.21 ± 1.12 × 107 | 3.52 ± 3.83 × 104 | 1.32 ± 1.62 × 104 | 3.5 ± 0.25 | ||
| Grimed | 6.35 ± 8.05 × 107 | 7.88 ± 6.96 × 107 | 5.89 ± 9.72 × 104 | 0.080 ± 0.56 |
LR - log reduction, ND - non-detect, pAB - pH-amended bleach, STD - standard deviation.
The results of operational-scale testing confirmed the virucidal activity of pAB, with full surface decontamination (>6 LR; no recoverable PFUs detected; Table 2) observed for both grimed materials tested. For non-grimed materials, no recoverable PFUs were detected for concrete (>4.7 LR). Similar decontamination efficacy (4.8 LR) was achieved for non-grimed plywood (Table 2). Because of the low efficacy observed during laboratory-scale testing, 2% citric acid was only applied in limited operational-scale test configurations (non-grimed concrete and grimed concrete). The results of operational-scale tests confirmed limited effectiveness of 2% citric acid for MS2 disinfection on complex surfaces (0.20 – 4.3 LR; Table 2). The analysis of MS2 transfers to coupon runoff or rinsate showed a 100% non-detect rate for liquid waste collected during pAB decontamination. But significant MS2 wash off was observed for tests with 2% citric acid (Table 2), where MS2 was detected in 75% of liquid samples.
Table 2:
Operational-scale decontamination testing results
| Decontaminant | Application method | Material type | Coupon surface | Non-decontaminated positive control coupons (PFU) |
Decontaminated test coupons (PFU) |
Liquid waste (PFU) | Log10 reduction (LR) | |
|---|---|---|---|---|---|---|---|---|
| Rinsate | Runoff | |||||||
| Average ± STD | Average ± STD | Averagea | Averagea | Average ± STD | ||||
| pAB | Backpack sprayer | Concrete | Non-grimed | 2.46 ± 0.66 × 104 | ND | ND | ND | 4.7 ± 0.06 |
| Grimed | 1.54 ± 0.27 × 106 | ND | ND | ND | 6.2 ± 0.04 | |||
| Treated plywood | Non-grimed | 3.64 ± 0.00 × 106 | 9.78 ± 4.44 × 101 | ND | ND | 4.8 ± 0.33 | ||
| Grimed | 4.70 ± 0.047 × 106 | ND | ND | ND | 7.0 ± 0.00 | |||
| 2% Citric acid | Concrete | Non-grimed | 6.20 ± 6.74 × 103 | 2.89 ± 1.98 × 103 | 2.20 × 103 | 2.42 × 103 | 0.20 ± 0.36 | |
| Grimed | 8.36 ± 3.26 × 105 | 1.15 ± 1.06 × 102 | ND | 4.50 × 102 | 4.3 ± 0.35 | |||
LR - log reduction ND - non-detect, pAB - pH-amended bleach, STD - standard deviation.
Composite sample collected from the entire test set of five coupons.
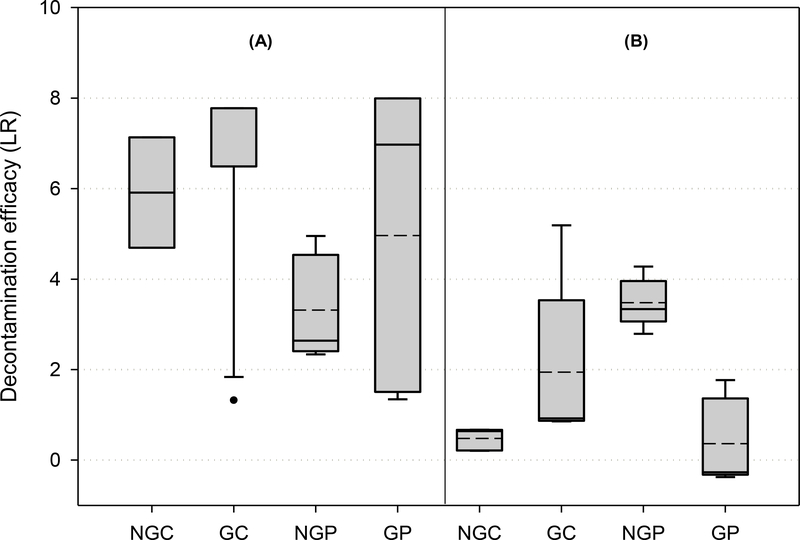
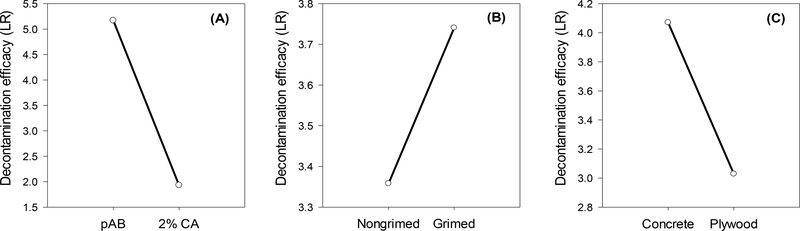
A comparison of the laboratory and operational-scale decontamination efficacy results is shown in Figure 3. The results illustrate MS2 susceptibility to decontaminants tested on different grimed and non-grimed materials. The pAB preparation showed stronger decontamination activity against MS2, with an average >4 log reduction – which is considered effective for anti-viricidal treatments according to EPA FIFRA requirements – observed for three out of four surface materials tested, while the average effectiveness of 2% citric acid was < 4LR (Fig. 3). The main effects of all independent experimental variables on LR are shown in Fig. 4. Three-way ANOVA results of the combined laboratory- and operational-scale LR values showed that in addition to independent experimental factors (material and decontaminant type, Table 3), there is a statistically significant interaction between decontaminant-material-grime test variables (P = <0.001, α = 0.05; Table 3). This indicates that any single experimental factor effect is not consistent at all combinations of the two other factors; and, therefore, an unambiguous interpretation of the main effects is not possible. Additional multi-factor analysis of three-way interactions between experimental variables showed that the decontaminant-material interaction is statistically significant when agricultural grime is present (P = <0.001). There is not a statistically significant three-way interaction when no grime is present (P = 0.346), which means the difference in the LR values among the different types of non-grimed materials is not great enough to exclude the possibility that the difference is caused by random sampling variability of materials without organic loading.
Figure 3. Decontamination efficacy from the laboratory- and operational-scale tests.
Data are reported as the median surface LR ±1 standard deviation with 5th and 95th data percentile from all samples tested for each type of material: non-grimed concrete (NGC), grimed concrete (GC), non-grimed plywood (NGP), grimed plywood (GP). pH-amended bleach (A) and 2% citric acid (B) decontamination solutions delivered via two spray applications with a 30-min total processing time, followed by a water-rinse step. The surface challenge was at 1 × 108 PFU level. The arithmetic mean values are represented by dashed lines.
Figure 4. Main effects of independent experimental factors on decontamination efficacy.
Main effects are reported as the least square mean (LSM) for: decontaminant type (A): pH-amended bleach (pAB) vs. 2% citric acid (2% CA), levels of surface grime (B), and type of surface material (C).
Table 3:
Three-way ANOVA results showing statistical significance of individual experimental factors and multi-factor interactions averaged over type of material and level of grime
| Source of Variation | DF | SS | MS | F-value | P-value |
|---|---|---|---|---|---|
| Decontaminant | 1 | 157.068 | 157.068 | 56.541 | <0.001 |
| Material | 1 | 16.261 | 16.261 | 5.854 | 0.019 |
| Grime | 1 | 2.192 | 2.192 | 0.789 | 0.378 |
| Decontaminant × Material | 1 | 15.612 | 15.612 | 5.62 | 0.021 |
| Decontaminant × Grime | 1 | 8.017 | 8.017 | 2.886 | 0.095 |
| Material × Grime | 1 | 18.662 | 18.662 | 6.718 | 0.012 |
| Decontaminant × Material × Grime | 1 | 40.841 | 40.841 | 14.702 | <0.001 |
| Residual | 57 | 158.343 | 2.778 | ||
| Total | 64 | 444.267 | 6.942 |
DF- degrees of freedom; SS - sum-of-squares; MS - mean square
DISCUSSION
A careful selection and validation of the analytical method are critical for accurate evaluations of the efficacy of decontamination chemicals and approaches. The characteristics of a contaminated matrix can be an important factor when selecting appropriate analytical techniques for recovering and quantifying viruses on surfaces. A meta-analysis of a large literature search of MS2 sampling methods, combined with a laboratory study that used plastic and stainless steel (Julian et al. 2011), suggests that the positivity rate of bacteriophage MS2 is primarily affected by the type of sampling device for both culture-based (plaque assay) and nonculture-based (quantitative reverse transcription-polymerase chain reaction [qRT-PCR]) detections. Laboratory study results for four sampling tools and four types of wetting solvents (Julian et al. 2011) showed viable virus recoveries that varied widely across implement-wetting solvent combinations (average <10–39% of theoretical surface loading [SL] for culture-based MS2; CT= 45 min). In the current study, the optimized method yielded stainless steel recoveries of 60% and 47% for high (1 × 108 PFU) and medium (1 × 105 PFU) surface MS2 concentration targets, respectively. Recoveries for the grimed and non-grimed surfaces tested in this study varied widely, ranging from 15% to 23% and from .04% to 1.3% of theoretical SL, respectively. The drastically lower MS2 recoveries for grime-free surfaces can be attributed to the synergistic effect of lower sampling efficacy and possible reduced viability of MS2 on porous materials. The low MS2 recovery from porous surfaces is likely due to bacteriophage transport into the material, possibly associated with physicochemical effects on the virus attachment properties (Bales et al. 1993). The lower survivability could be associated with multiple factors – hygroscopicity of the surface, pH conditions, and surface-dependent adsorption and surface affinity of the virus (Hurst et al. 1980, Shim et al. 2017). In this study, the porous characteristics of the plywood and concrete coupons caused accelerated (<10 min) drying of MS2 liquid inoculum droplets, likely leading to relatively rapid desiccation of the target organism. Desiccation-related surface concentration losses have been reported for enteric viruses (Abad et al. 1994). Up to a 4.3 surface log reduction for porous and nonporous surfaces (in the absence or presence of fecal material) after <5 h drying has been observed, with virus survivability generally improved when dried on non-porous fomites under humid conditions. (Abad et al. 1994). The presence of heavy grime improved the MS2 survivability in this study, suggesting that the tolerance of viruses to environmental desiccation can be improved in the presence of lipid and nutrient-rich organic loading. Additionally, hydrophobic organic loadings can reportedly retard MS2 transport into porous substrates (Bales et al. 1993), thereby increasing the surface fraction of viable viruses present on grimed materials. Experimental variances not related to physicochemical characteristics of the sample surface (e.g., wipe sampling efficacy, material-specific efficacy of the plaque assay method) can also contribute to reductions in MS2 fractions recovered from dry/porous surfaces. Further research is needed to optimize the methodology (sampling tool, wetting solvent) for sampling MS2 from porous surfaces.
The analytical method applied to bench-scale disinfectant testing was an effective tool for the selection of decontaminants for follow-on operational decontamination testing, with pH-amended bleach being the preferred disinfectant for inactivation of MS2 from complex matrices (with and without heavy agricultural grime). The high virucidal activity of pAB (LR >6) observed for operational-scale grimed materials could be due to lower porosity and material demand of hydrophobic grimed surfaces, but the material-decontaminant reactivity was not specifically studied in this work. The pAB decontamination efficacy observed for operational-scale, non-grimed porous materials was similar to or higher than that previously reported for diluted bleach decontamination of FMDV on wood surfaces, with average surface reduction rates of 2.95–3.77 logs for 1,000 ppm and 2,000 ppm sodium hypochlorite (DT = 30 min), respectively. The 2% citric acid reported as a suitable decontaminant for FMDV (FAO 1999; Krug et al. 2012), was not observed to be effective against MS2 in this study. Data showed that 2% citric acid provided a low decontamination efficacy with a concurrent high transport of viable MS2 to liquid effluents. These differences may be related to the reported low-pH sensitivity of FMDV (rapid capsid dissociation at pH <6.5) (Grubman and Baxt 2004). Such low-pH sensitivity has not been previously described for MS2, with some reports suggesting that MS2 survival rates in aqueous solutions are higher under acidic versus alkaline conditions, and that MS2 – as well as other selected RNA F-specific coliphages – can only be used as viral indicators within an optimal survivability pH range of 6 to 9 and at temperatures of <25°C (Feng et al. 2003).
In summary, a systematic analytical method development performed in this study enhanced the MS2 (FMDV surrogate) recovery from complex surfaces.
The following analytical steps were considered critical for improving MS2 recovery from challenging substrates: (1) addition of nutrient-rich wide-spectrum neutralizer (D–E broth) to surfactant-containing (PBST) extraction buffer, and (2) selection of surface-dependent HTs from extraction to analysis (<8 h for lower-titer samples collected from porous surfaces or liquid effluent samples, ≤24 h for high-titer samples from nonporous and/or heavily grimed substrates), (3) for laboratory testing of disinfectants reduction of MS2 contact/residence time on porous surfaces is recommended to avoid adsorption or possible desiccation of the target organism. The analytical method applies to decontamination testing under conditions that are representative of situations that may be encountered during an emergency response to a viral contamination incident and can be modified for testing of other complex environmental surfaces and virucidal chemicals.
Supplementary Material
Figure S1. Recovery of MS2 from the simulated surface and liquid samples containing 2% citric acid and pAB neutralized with D–E broth.
Figure S2. Recovery of MS2 from grimed materials extracted with PBST and PBST with D–E at analytical hold times of <8 h and 96 h.
Table S1. Structural components of synthetic agricultural grime.
Table S2. Test design of initial neutralization testing.
Table S3. MS2 concentration vs. contact time and analytical hold time in concrete and plywood samples extracted with various D–E-enriched buffers.
Table S4. Results of wipe sampling optimization testing for high and medium surface loading of MS2.
Acknowledgments
This research was initiated following the identification of knowledge gaps by the US Department of Homeland Security’s Agricultural Defense Branch-led subcommittee on Foreign Animal Disease Threats, Decon, Disposal, and Depopulation Working Group, which is co-chaired by the US Environmental Protection Agency (US EPA) and the US Department of Agriculture (USDA). Funding support by the US Department of Homeland Security (DHS) under IA HSHQPM-13-X-00099 to complete this effort is greatly appreciated. This effort was directed by a principal investigator from the US EPA Office of Research and Development’s (ORD’s) National Homeland Research Center (NHSRC), using the support of a project team that consisted of staff from the US EPA, DHS, and USDA. Additionally, the authors thank Dr. J. Mark Rice and Dr. C. Mike Williams of the North Carolina State University Animal and Poultry Waste Management Center (APWMC) for providing useful comments on the preparation of synthetic agricultural grime and for preparation of standardized manure material.
Disclaimer
The US EPA, through its Office of Research and Development’s National Homeland Security Research Center, funded and managed this investigation through contract EP-C-09-027, work assignment (4-6)-52 with Arcadis US, Inc., and contract EP-C-15-008 with Jacobs Technology, Inc. This manuscript has been peer and administratively reviewed and has been approved for publication. The views expressed here are the authors’ and do not necessarily represent the policies or views of the US EPA, USDA, or DHS. No official endorsement should be inferred. This report includes photographs of commercially available products. The photographs are included for purposes of illustration only and are not intended to imply that US EPA approves or endorses the product or its manufacturer. US EPA does not endorse the purchase or sale of any commercial products or services.
Footnotes
Conflict of Interest
No conflict of interest declared.
Supporting Information
Additional Supporting Information is available in the online version of this article:
References
- Abad FX, Pinto RM and Bosch A (1994) Survival of enteric viruses on environmental fomites. Appl Environ Microbiol 60, 3704–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASTM (2001) Standard Guide for Testing Cleaning Performance of Products Intended for Use on Resilient Flooring and Washable Walls. ASTM International: West Conshohocken, PA. [Google Scholar]
- Baron PA, Estill CF, Beard JK, Hein MJ and Larsen L (2007) Bacterial endospore inactivation caused by outgassing of vaporous hydrogen peroxide from polymethyl methacrylate (Plexiglas). Lett Appl Microbiol 45, 485–490. [DOI] [PubMed] [Google Scholar]
- Bales CR, Shimin L, Maguire M, Moyasar K, T.Y. and Gerba C (1993) MS2 and poliovirus transport in porous media: Hydrophobic effects and chemical perturbations. Water Resour Res. 29, 957–963. [Google Scholar]
- Boudaud N, Machinal C, David F, Fréval-Le Bourdonnec A, Jossent J, Bakanga F, Arnal C, Jaffrezic MP, Oberti S and Gantzer C (2012) Removal of MS2, Qβ and GA bacteriophages during drinking water treatment at pilot scale. Water Res 46, 2651–2664. [DOI] [PubMed] [Google Scholar]
- Butot S, Putallaz T and Sánchez G (2007) Procedure for rapid concentration and detection of enteric viruses from berries and vegetables. Appl Environ Microbiol 73, 186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfee MW, Ryan SP, Wood JP, Mickelsen L, Kempter C, Miller L, Colby M, Touati A, Clayton M, Griffin-Gatchalian N, McDonald S and Delafield R (2012) Laboratory evaluation of large-scale decontamination approaches. J Appl Microbiol 112, 874–882. [DOI] [PubMed] [Google Scholar]
- CDC (2012) Surface sampling procedures for Bacillus anthracis spores from smooth, non-porous surfaces. Available at: https://www.cdc.gov/niosh/topics/emres/surface-sampling-bacillus-anthracis.html. Accessed January 30, 2018.
- D’Souza DH, and Su X (2010) Efficacy of chemical treatments against murine norovirus, feline calicivirus, and MS2 bacteriophage foodborne pathogens and disease. Foodborne Path Dis 7, 319–326. [DOI] [PubMed] [Google Scholar]
- Dawson DJ, Paish A, Staffell LM, Seymour IJ, and Appleton H (2005) Survival of viruses on fresh produce, using MS2 as a surrogate for norovirus. J Appl Microbiol 98, 203–209. [DOI] [PubMed] [Google Scholar]
- Downes FP, ed. (2001) Compendium of Methods for the Microbiological Examination of Foods, 4th ed. American Public Health Association: Washington, DC. [Google Scholar]
- Food and Animal Organization (FAO) (2001) Manual on Procedures for Disease Eradication by Stamping Out. Food and Agriculture Organization of the United Nations: Rome; ISBN 92–5-104585–2. [Google Scholar]
- FAO (1999) Manual on the Preparation of National Animal Disease Emergency Preparedness Plans. Food and Agriculture Organization of the United Nations: Rome; ISBN 92–5-104290-X. [Google Scholar]
- Feng YY, Ong SL, Hu JY, Tan XL and Ng WJ (2003) Effects of pH and temperature on the survival of coliphages MS2 and Qbeta. J Ind Microbiol Biot 30, 549–552. [DOI] [PubMed] [Google Scholar]
- Grubman MJ and Baxt B (2004) Foot-and-mouth disease. Clin Microbiol Rev 17, 465–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy Diagnostics (1996) D/E Neutralizing Broth. Instruction for Use. Available at: https://catalog.hardydiagnostics.com/cp_prod/Content/hugo/DENeutralizingBroth.htm Accessed Jan. 30, 2018. [Google Scholar]
- Hurst CJ, Gerba CP and Cech I (1980) Effects of environmental variables and soil characteristics on virus survival in soil. Appl Environ Microbiol, 40, 1067–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian TR, Tamayo FJ, Leckie JO and Boehm AB (2011) Comparison of surface sampling methods for virus recovery from fomites. Appl Environ Microbiol, 77, 6918–6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortepeter MG and Parker GW (1999) Potential biological weapons threats. Emerg Infect Dis 5, 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug PW, Lee LJ, Eslami AC, Larson CR and Rodriguez L (2011) Chemical disinfection of high-consequence transboundary animal disease viruses on nonporous surfaces. Biologicals 39, 231–235. [DOI] [PubMed] [Google Scholar]
- Krug PW, Larson CR, Eslami AC and Rodriguez L (2012) Disinfection of foot-and-mouth disease and African swine fever viruses with citric acid and sodium hypochlorite on birch wood carriers. Vet Microbiol 156, 96–101. [DOI] [PubMed] [Google Scholar]
- Morin T, Martin H, Soumet C, Fresnel R, Lamaudière S, Le Sauvage AL, Deleurme K and Maris P (2015) Comparison of the virucidal efficacy of peracetic acid, potassium monopersulphate, and sodium hypochlorite on bacteriophages P001 and MS2. J Appl Environ Microbiol 119, 655–665. [DOI] [PubMed] [Google Scholar]
- Mummert A and Weiss H (2017) Controlling viral outbreaks: quantitative strategies. PLoS ONE 12, e0171199 10.1371/journal.pone.0171199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Kelly JC and Reich HP (1981) Sebum output and water metabolism in different genotypes of cattle in hot environments. J Therm Biol 6, 97–101. [Google Scholar]
- Shim J, Stewart DS, Nikolov AD, Wasan DT, Wang R, Yan R, and Shieh YC (2017) Differential MS2 Interaction with Food Contact Surfaces Determined by Atomic Force Microscopy and Virus Recovery. Appl Environ Microbiol 83, 1881–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann J (2004) Surrogate viruses for testing virucidal efficacy of chemical disinfectants. J Hosp Infect 56, S49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA (2001) Method 1601: Male-specific (F+) and Somatic Coliphage in Water by Two-step Enrichment Procedure. EPA 821-R-01–030. US Environmental Protection Agency: Washington, DC. [Google Scholar]
- US EPA (2011) Effectiveness of Physical and Chemical Cleaning and Disinfection Methods for Removing, Reducing or Inactivating Agricultural Biological Threat Agents. EPA/600/R-11/092, 2011. US Environmental Protection Agency: Washington, DC. [Google Scholar]
- US EPA (2016) Effectiveness of Spray-Based Decontamination Methods for Spores and Viruses on Heavily Soiled Surfaces. EPA/600/R-16/162. US Environmental Protection Agency: Washington, DC. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Recovery of MS2 from the simulated surface and liquid samples containing 2% citric acid and pAB neutralized with D–E broth.
Figure S2. Recovery of MS2 from grimed materials extracted with PBST and PBST with D–E at analytical hold times of <8 h and 96 h.
Table S1. Structural components of synthetic agricultural grime.
Table S2. Test design of initial neutralization testing.
Table S3. MS2 concentration vs. contact time and analytical hold time in concrete and plywood samples extracted with various D–E-enriched buffers.
Table S4. Results of wipe sampling optimization testing for high and medium surface loading of MS2.