Abstract
Background:
Identifying reliable predictors of long-term weight loss could lead to improved weight management.
Objective:
To identify predictors.
Design:
The Diabetes Prevention Program (DPP) was a randomized controlled trial that compared weight loss using placebo, intensive lifestyle intervention, or metformin, and its Outcomes Study (DPPOS) observed patients after the masked treatment phase ended.
Setting:
27 DPP/DPPOS clinics.
Participants:
Of the 3234 randomized participants, 1066 lost ≥ 5% of their baseline weight during the first year and have been followed for 15 years.
Measurements:
Treatment assignment, personal characteristics, and weight.
Results:
After 1 year, 289 (28.5%) metformin participants, 640 (62.6%) intensive lifestyle participants, and 137 (13.4%) placebo participants, achieved ≥ 5% weight loss. After the masked treatment phase ended, the mean (95% CI) amount of weight loss relative to baseline that was maintained between years 6 and 15 was 6.2% (5.2, 7.2) for metformin participants, 3.7% (3.1, 4.4) for intensive lifestyle participants, and 2.8% (1.3, 4.4) for placebo participants. Independent predictors of long-term weight loss included greater weight loss during the first year in all study groups, older age and continued use of metformin in the metformin group, older age and not having diabetes or a family history of diabetes in the intensive lifestyle group, and higher baseline fasting plasma glucose in the placebo group.
Limitation:
Post-hoc analysis. Nonrandomized subsets of randomized groups examined after year 1.
Conclusion:
Among those with ≥ 5% 1-year weight loss, the group originally randomized to metformin had the greatest success with weight loss during years 6–15. Older age and the amount of initial weight loss were the most consistent predictors for maintaining long-term weight loss.
Primary Funding Source:
National Institutes of Health.
Trial Registration: ClinicalTrials.gov and .
Keywords: prediabetes, type 2 diabetes, diabetes prevention, impaired glucose tolerance, overweight, obesity, body weight, metformin, lifestyle intervention, weight loss maintenance, sustained weight loss
INTRODUCTION
Weight loss has been central in efforts to prevent or delay type 2 diabetes (T2D) in persons with overweight or obesity, which are strong and potentially modifiable risk factors for T2D. Therefore, prevention of T2D among those with prediabetes is a public health priority. Just as excess body weight is strongly associated with insulin resistance and hyperglycemia, improved glycemia is readily noticeable when individuals with overweight/obesity lose weight; however, the health benefits of weight loss may persist only when the initial weight loss persists over the long-term (1). There is clinical impetus to examine whether baseline demographic, psychosocial, or physiological factors can help to identify more favorable long-term weight loss patterns, especially in the context of available treatments for diabetes prevention.
Randomized controlled trials (RCTs) have demonstrated efficacy of metformin and/or intensive lifestyle intervention (ILS) in preventing or delaying the onset of T2D among overweight/obese persons at high risk for the condition (2, 3). In the Diabetes Prevention Program (DPP), metformin and ILS reduced the risk of diabetes relative to placebo by 31% and 58%, respectively, over an average follow-up period of 2.8 years (2). In the DPP, metformin and ILS were associated with average weight losses over 2.8 years of 2.1 kg and 5.6 kg, respectively vs 0.1 kg with placebo (2); additionally, weight loss was the primary driver of diabetes prevention (4, 5). During long-term follow-up of DPP participants in its Outcomes Study (DPPOS), body weight changes of the participants differed among the treatment groups at 10- and 15-year follow-up (6, 7). After achieving significantly larger initial weight loss compared to the other treatment groups, the ILS group regained ~3.5 kg over time with a net weight loss of ~2 kg relative to baseline after a 10-year follow-up; however, metformin participants achieved and maintained an average ~2.5 kg weight loss while the average weight of placebo participants changed by ≤1 kg during the same period (6).
Furthermore, DPP/DPPOS is the largest and longest clinical trial of metformin for prevention of T2D. In the DPP, weight loss explained 64% of the beneficial effect of metformin on T2D risk (4).
In the current investigation, the primary aims were to compare, among participants who achieved clinically significant 1-year weight loss of ≥5%, differences in long-term weight loss maintenance according to originally randomized intervention group, and to examine baseline factors that predicted success in maintaining weight loss for up to 15 years. We also examined the impact of post-baseline factors such as degree of initial weight loss and adherence to the randomized interventions.
METHODS
The DPP Study (July 1996 to July 2001) was an RCT to compare the efficacy of ILS, metformin, and placebo interventions on development of T2D among 3,234 participants with elevated glucose levels and overweight/obesity. American Diabetes Association (ADA) 1997 diagnostic criteria (8) were used to establish the primary study outcome of diabetes defined by fasting plasma glucose ≥7.0 mmol/L (≥126 mg/dL) measured semiannually or 2-hour plasma glucose of ≥11.1 mmol/L (≥200 mg/dL) after a 75 g oral glucose load measured annually, with confirmation within six weeks. The protocol, baseline characteristics of the sample, and primary outcomes (including all clinical and physiologic variables examined in these analyses) have been described previously (2, 9, 10) along with minor variations in eligibility criteria.
Participants were randomly assigned to receive either individually administered ILS (a 16-session lifestyle behavior modification intervention implemented over ~6–8 months with a goal of achieving 7% weight loss through healthy dietary changes and reductions in calories and fat and 150 minutes of moderate-intensity physical activity per week, mostly walking), metformin (850 mg/twice daily), or placebo (11). Both medication interventions were masked (double-blind), but not ILS. At 6 months, those in ILS continued to receive individual and group reinforcement of the behavioral intervention every two months at minimum, regardless of diabetes status, but they were referred to their primary care provider (PCP) for diabetes management. The metformin and placebo groups continued to receive the masked study drug until plasma glucose worsened to ≥7.8 mmol/L (≥140 mg/dL), at which time study medications were discontinued and diabetes management was transferred to the PCP. After an average 3.2-year follow-up, the DPP study protocol was modified (July 2001), participants were informed of the main results, and there was a 1–2 week metformin and placebo washout period to identify whether treatment of fasting glucose accounted for the diabetes risk reduction with metformin (12).
The Bridge Protocol (August 1, 2001 to August 31, 2002) was the 13-month period between the DPP study and the start of the long-term follow-up, DPPOS. Because efficacy was established for ILS compared to metformin and placebo on the cumulative reduction of diabetes incidence during the first 3.2 years, participants in all three randomized groups were offered a group-administered version of the 16-session ILS. The group intervention, similar in content but without individualized problem-solving and behavior change support components, was implemented over a 6-month period and usually administered by the original staff. Rates of attending one or more sessions varied by group (58% in the metformin group, 40% in the ILS group, and 57% in the placebo group) (13).
The DPPOS began in September 2002 and examined 2,779 (89%) of the remaining DPP participants for further diabetes incidence and development of diabetes-related complications. While the DPPOS is ongoing, the current analysis is based on 15-year average follow-up from DPP baseline using annual data collection through 2013 (Appendix Figure 1). A similar proportion of the three randomized DPP intervention groups enrolled in the DPPOS (6). During DPPOS, as in DPP, metformin was provided to the group originally assigned to it, however it was now unmasked. Additionally, when HbA1c reached ≥7%, study metformin was discontinued, and medical care and medication management were transferred to the participant’s PCP. This management often included metformin, but prescribed by the PCP. In the ILS and placebo groups, all diabetes medical care and medication management were provided by the participant’s PCP. Metformin use was recorded at all visits during DPP/DPPOS for all participants. During DPPOS, all three groups were offered educational lifestyle classes 4 times yearly. Twice yearly, the original ILS group was offered 2–4 booster sessions designed to reinforce self-management behaviors for weight and activity. Lifestyle class attendance among the three intervention groups did not exceed 20% for any of the sessions offered during DPPOS (data not shown). For all trial phases, written informed consent was obtained from all participants and studies were approved by each center’s IRB. An independent data safety monitoring board appointed by the study sponsor (NIDDK) oversaw the studies.
Statistical Analysis
Long-term Weight Loss (LTWL) was defined at each annual examination past 1 year as ≥5% weight loss relative to baseline. We used fixed-effects models with the assumption of normally distributed errors to estimate percent weight loss over time (SAS Proc MIXED), and Generalized Estimating Equation (GEE) models to estimate the percentage of participants with LTWL over time (SAS Proc GENMOD), among participants who lost ≥5% weight at DPP Year 1 in the three groups (14). The fixed-effects models used a compound symmetry correlation, and the GEE models used a logit link with an exchangeable correlation structure.
The primary aim was to evaluate baseline and post randomization predictors of maintaining weight loss among those who initially achieved clinically significant weight loss of ≥5% at 1 year after randomization. Potential predictors of LTWL include baseline variables listed in Supplement Table 1 as well as post-randomization measures of percent weight loss at DPP Year 1, meeting the ILS exercise goal at the end of the core 16-session ILS curriculum, meeting the ILS weight loss goal of 7% at the end of the core curriculum, total ILS sessions, taking PCP-prescribed metformin at the annual visit, taking study metformin at the annual visit, and diabetes status as of the visit. Two statistical methods were used to examine the predictors of LTWL. First, we used logistic regression models to evaluate potential predictors of LTWL separately at follow-up years 5, 10 and 15 to specifically evaluate predictors of medium-term, long-term and very long-term weight loss success (SAS Proc LOGISTIC). The second approach used GEE models (15,16) to evaluate potential predictors of LTWL on average across all years of follow-up. GEE models provided an average estimate of the predictor effect on LTWL over the course of the study (SAS Proc GENMOD). These GEE models used a logit link and an independent correlation to assure proper parameter estimation (17). For both approaches, univariate models predicting LTWL were developed separately for each predictor variable. Multivariate models included variables which were significant (p<0.05) predictors of LTWL in the univariate analyses. Final models included only measures that remained significant in the multivariate model. Given the wide variation in the numbers of participants achieving and then maintaining ≥ 5% weight loss among the intervention groups, analyses were performed within treatment arm.
The incidence of diabetes diagnosed at any time throughout DPP and DPPOS was compared for those who did and did not meet the ≥5% weight loss goal at Year 1 using Cox Proportional Hazards models within each treatment group (SAS Proc PHREG). SAS version 9.4 was used for all statistical analyses.
Role of the Funding Source
The NIH, the primary study sponsor, was represented on the Steering Committee and contributed to study design, implementation, and publication. The funding agency was not represented on the writing group, although all members of the Steering Committee had input into the report’s contents. All authors in the writing group had access to all data.
RESULTS
Participant Disposition
Appendix Figure 2 shows the number of participants randomized to DPP overall and those achieving ≥5% weight loss at Year 1. Among those who achieved ≥5% weight loss at Year 1, the number of participants remaining in DPP/DPPOS at Years 5, 10 and 15 along with the numbers taking study-provided (original metformin group only) or PCP-provided metformin are shown. Participants discontinued DPP and DPPOS visits for a variety of reasons including loss to follow-up, illness, distance, and death.
Characteristics of Participants Achieving ≥5% weight loss at Year 1
Participant characteristics in each of the 3 randomized treatment groups are shown in Appendix Table 1 by DPP Year 1 weight loss of <5% and ≥5%. A total of 289 (28.5%) metformin, 640 (62.6%) ILS, and 137 (13.2%) placebo participants achieved ≥5% weight loss at Year 1. Mean (95% CI) percent weight loss at DPP Year 1 among these participants was 8.9% (8.5, 9.3), 11.0% (10.6, 11.4), and 9.2% (8.3, 10.1), in metformin, ILS, and placebo groups, respectively.
Weight Change Trajectories
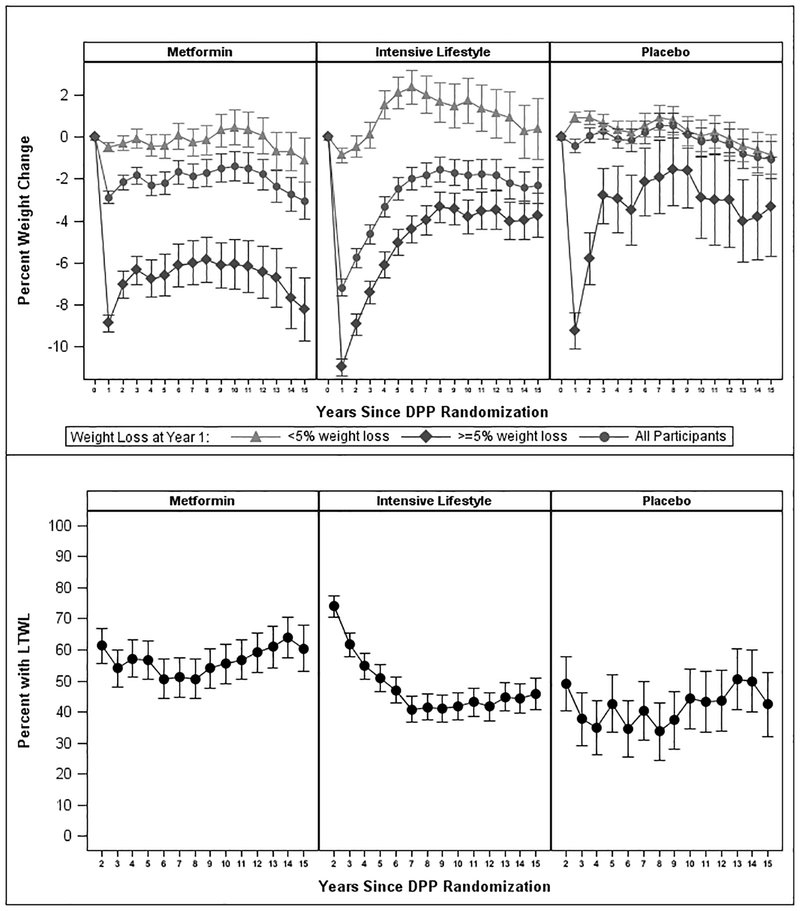
Figure 1A shows the pattern of weight changes by treatment over the 15-year follow-up among all participants and stratified by weight loss (<5%, ≥5%) at DPP Year 1. Among those who lost ≥5% by Year 1, mean percentage weight losses at yearly follow-up visits during Years 2–15 relative to baseline ranged from 5.8% to 8.2% for metformin, 3.4% to 8.9% for ILS, and 1.6% to 5.8% for placebo. The mean (95% CI) weight losses, across the 10-year period of Years 6–15 (after the intensive lifestyle intervention was completed and weight stabilized) relative to baseline, were 6.2% (5.2, 7.2) in the metformin group, 3.7% (3.1, 4.4) in ILS, and 2.8% (1.3, 4.4) in placebo. Although the ILS group had the highest percentage (74%) of participants achieving ≥5% weight loss at Year 2, these figures declined to 51–62% in Years 3–5, and 41–47% in Years 6–15 (Figure 1B). In contrast, the percentages of participants achieving ≥5% weight loss changed from 61% at Year 2 to 54–57% in Years 3–5 and 51–64% in Years 6–15 in the metformin group, and from 49% at Year 2 to 35–43% in Years 3–5 and 35–50% in Years 6–15 in the placebo group. more. The mean (95% CI) percentages of participants with LTWL among those who lost ≥5% by Year 1, across the entire 14-year follow-up were 56.5% (55.5, 57.5), 48.9% (47.9, 49.9) and 41.7% (40.7, 42.7) in metformin, ILS, and placebo groups, respectively; and, the percentages for the Years 6–15 were 56.1% (55.1, 57.1), 43.1% (42.1, 44.1), and 41.9% (39.9, 43.9), respectively.
Figure 1. Patterns of weight changes over 15 years.
(A) Shown is the observed percent weight loss over 15 years for all participants by treatment group and for the subsets of participants who achieved <5% or ≥5% weight loss at Year 1. Data shown are Mean ± 95% Confidence Intervals (CI). (B) Shown are the observed percentages of participants by treatment group achieving ≥5% weight loss yearly during Years 2–15 among participants who achieved ≥5% weight loss at Year 1. Data shown are Mean ± 95% CI. Sample sizes: Metformin Year 1: 289, Year 5: 247, Year 10: 236. Year 15: 172. Intensive Lifestyle: Year 1: 640, Year 5: 540, Year 10: 491, Year 15: 373. Placebo: Year 1: 137, Year 5: 110, Year 10: 104, Year 15: 92.
Predictors of LTWL
Metformin
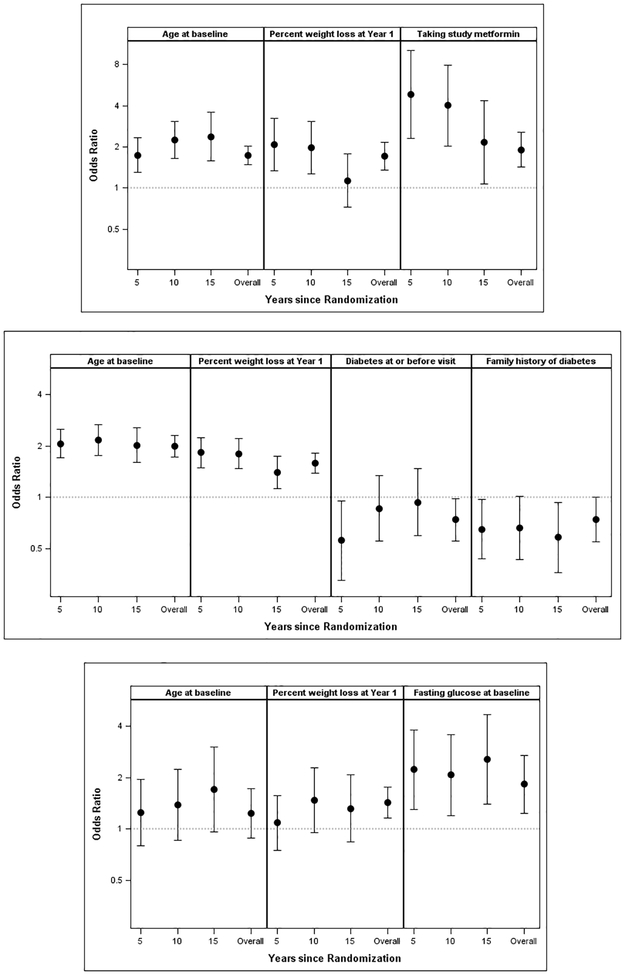
In the metformin group, older baseline age, higher systolic blood pressure, higher 2-hr glucose, greater weight loss at Year 1, and active use of study-metformin at the time of the visit were associated with higher odds of LTWL in the univariate models. In multiply-adjusted logistic and GEE models, for Years 5, 10, 15 and overall, only older age at baseline (per 10 years; odds ratios [ORs] 1.74, 2.25, 2.37 and 1.74, respectively; overall p<0.001), greater Year 1 weight loss (per 5% weight loss; ORs 2.08, 1.97, 1.14 and 1.70, respectively, overall p<0.001), and active use of study-metformin (taking vs not taking metformin; ORs 4.83, 4.02, 2.17 and 1.91; overall p<0.001) independently predicted LTWL (Figure 2A).
Figure 2. Predictors of long-term weight loss.
Metformin (A), Intensive Lifestyle (ILS) (B), and Placebo (C). GEE and Multiple Logistic Regression Models Predicting ≥ 5% Weight Loss overall and at 5, 10, and 15 years (Odds Ratios & 95% CI). Odds ratios are displayed on a log scale. With Metformin (A), older age at randomization (per 10 years), greater % weight loss at Year 1 (per 5%), and active use of study metformin increased odds of long-term weight loss (LTWL). With ILS (B), older age at randomization (per 10 years) and greater % weight loss at Year 1 (per 5%) increased odds of LTWL, and current diabetes status, and a family history of diabetes decreased the odds of LTWL. With Placebo (C), greater % weight loss at Year 1 (per 5%) and higher fasting glucose (per 0.55 mmol/L [10 mg/dl]) were the only predictors of LTWL. ILS = intensive lifestyle; LTWL = long-term weight loss
ILS
In the ILS group, although several measures were significant in univariate models, in multiply-adjusted models, for Years 5, 10, 15 and overall, only older age at baseline (ORs per 10 years; 2.07, 2.17, 2.03 and 2.00, respectively; overall p<0.001) and greater Year 1 weight loss (ORs per 5% weight loss; 1.83. 1.80, 1.40, and 1.59, respectively; overall p<0.001) independently increased the odds of LTWL, while a diagnosis of diabetes during follow-up (ORs for diabetes vs. no diabetes; 0.56, 0.86, 0.94 and 0.74; overall p=0.038) and family history of diabetes (ORs for history vs. no history; 0.65, 0.66, 0.58 and 0.74; overall p=0.051) independently decreased the odds of LTWL (Figure 2B).
Placebo
In the placebo group, age at baseline did not predict LTWL as it did in the metformin and ILS groups. In univariate and multivariate models, for Years 5, 10, 15 and overall, only fasting glucose (per 0.55 mmol/L [10 mg/dl]; ORs 2.23, 2.08, 2.57 and 1.83, respectively; overall p=0.002) and Year 1 weight loss (per 5%; ORs 1.09, 1.48, 1.32, 1.43, respectively; overall p=0.001) were significant predictors of LTWL (Figure 2C).
Weight Loss and Diabetes Incidence
Over the full 15-year follow-up of participants who achieved <5% vs ≥5% weight loss at Year 1, diabetes incidence rates were 54% vs 41% for metformin (p<0.001), 61% vs 39% for ILS (p<0.001), and 57% vs 48% for placebo (p=0.057) groups.
DISCUSSION
We examined body weight changes over 15 years among overweight or obese subjects at risk for T2D who enrolled in the DPP and continued in the DPPOS, and elucidated characteristics of those achieving LTWL. Our key findings are: 1) although twice as many ILS as metformin group participants achieved ≥5% weight loss at Year 1, those originally assigned to metformin who achieved ≥5% weight loss at Year 1 had greater success in maintaining LTWL; 2) among the numerous characteristics of study participants examined in this exploratory analysis, greater 1-year weight loss predicted LTWL in all treatment groups. Other independent predictors of LTWL were: older age and not having current diabetes or family history of diabetes in the ILS group; older age and current use of metformin in the metformin group; and baseline fasting plasma glucose in the placebo group; 3) cumulative diabetes incidence rates over 15 years were lower among those who achieved ≥5% weight loss than those with <5% weight loss at Year 1.
A recent systematic review and meta-analysis reported an average weight loss of 1.1 kg with metformin used for varying periods (18). In the DPP/DPPOS, the metformin group had an average weight loss of 2.1% after 2 years, and remarkably, the group maintained ~2% weight loss for the next 10 years (19). Taken together, it appears that long-term metformin treatment is associated with an average ~1–2% weight loss when assessed among all of those given the drug. The current investigation builds on this knowledge with the additional observation that approximately 30% with overweight/obesity at baseline achieve ≥5% weight loss over a year with metformin therapy and over a half of this subset of initial responders maintain this weight loss for as long as 15 years.
Among the drugs approved primarily for treatment of obesity, only two have been studied for more than 2 years in RCTs. In the XENDOS trial (20) of 3,305 patients with obesity and diabetes, orlistat was associated with 9.6% weight loss vs 5.6% with placebo, but this difference narrowed to 3.2% vs 1.3% at 4 years at which point significant attrition of the study sample hampered interpretation of weight change and diabetes results. Additionally, long-term adherence to orlistat is poor (21). Liraglutide 3.0 mg daily led to greater weight loss (6.1% vs 1.9%) and lower cumulative diabetes incidence (2% vs 6%) compared to placebo at 3-year follow-up in a large study of individuals with obesity and prediabetes (22). However, the need for daily injections and high cost are key limitations of liraglutide for long-term weight management and T2D prevention. Therefore, other interventions that could promote LTWL are needed. The attractiveness of metformin is that it is recommended as the first-line medication for patients with newly diagnosed T2D by most professional guidelines, including those by the American College of Physicians and the ADA (23, 24). It is inexpensive, generally well-tolerated, and used extensively in primary care.
Metformin has several mechanisms and sites of action in humans. Its beneficial effects include inhibition of gluconeogenesis and consequent decrease in hepatic glucose output and increased insulin sensitivity, increased glucose utilization in the gut and enhanced insulin sensitivity in skeletal muscle (25, 26), and effects on the gut microbiota and the immune system (27). However, the mechanisms that contribute to its effects on body weight are not well understood. Decrease in appetite and food intake have been reported with metformin in several (22, 28–30), but not all (31) studies. Metformin therapy is not known to significantly alter energy expenditure (25, 32). It is well recognized that the human body adapts to weight loss with compensatory neuronal, hormonal, and metabolic changes that promote weight regain (33–36). Whether metformin counters some of these compensatory changes is a subject for further investigation.
Among those who achieved ≥5% weight loss by DPP Year 1, in each of the originally randomized treatment groups, the degree of weight loss achieved at Year 1 predicted LTWL. Numerous studies have reported that initial weight loss in first 3–6 months predicts weight loss at 1–2 years with lifestyle interventions as well as pharmacotherapy (1, 37–40). Our findings that initial weight loss and older age are predictive of LTWL and that the weight loss is largely associated with favorable diabetes and other health outcomes are consistent with prior observations in DPP/DPPOS and other studies (5, 41–43). Findings of numerous physiological, neurohormonal, and feeding studies that examined differences between older and younger individuals suggest that aging is associated with impaired ability to accurately regulate energy balance via adjustments in energy intake (44–49). It is unclear whether LTWL over periods as long as 15 years is associated with better or worse health outcomes overall, especially among individuals in their seventh and eighth decades of life.
Importantly, despite significant weight regain, those within the ILS group that lost ≥ 5% at Year 1, still maintained an average weight loss of 3.7% during Years 6–15 relative to baseline. From a population health perspective, this finding gains further importance with increasing dissemination and implementation of DPP-modeled lifestyle programs (50) and reimbursement for such interventions (51, 52). Indeed, the decision by Medicare to reimburse DPP-lifestyle interventions, when implemented by recognized providers using standardized outcome measures, has strong potential to add to the research on long-term treatment responsivity.
The strengths of the current investigation include a long follow-up period of 15 years in the originally randomized study group, high retention (7), and large sample sizes for the metformin and lifestyle arms. There are several limitations. While the DPP was a randomized controlled trial, our sub-analysis, examining those with ≥5% weight loss by DPP Year 1, was a secondary analysis. Our study sample, although very well represented by racial and ethnic minorities, had few individuals who were unemployed or had low income (10), and thus may have limited generalizability. Although retention was high, some participants discontinued study participation or died over the 15-year follow-up. However, the observed effects were consistent at 5, 10, and 15 years, as well as on the average across the 15-year follow-up. Other possible predictors of LTWL were not measured in the DPP/DPPOS. Whereas we opted for ≥5% weight loss to define initial weight loss as well as LTWL since this degree and duration of weight loss confers meaningful health benefits (1), some might apply a different threshold (53). Whether the results of our analysis among persons at high risk for diabetes would transfer to those beginning metformin as treatment of T2D is unknown. Lastly, the LTWL success with metformin we report in this paper is only for the 28.5% of participants who had achieved ≥5% weight loss at 1 year. However, this is a sizeable percent considering that millions of individuals worldwide are treated with metformin as the first-line drug for T2D. It is also important to recognize that participants in the metformin group remained on the drug for up to 15 years whereas for the ILS group, the intervention was administered at high intensity in the first 3 years, but at a much lower intensity in the later years, and though booster therapy sessions were offered, attendance was poor, reflecting the difficulty of implementing ILS beyond 1–3 years.
In conclusion, we found that older age and greater 1-year weight loss predicted LTWL over the next 14 years among DPP/DPPOS participants who achieved ≥5% weight loss at 1 year. Numerous other variables explored as potential predictors were not consistently associated with LTWL. Also, among participants with significant initial weight loss, those originally randomized to metformin had greater success in maintaining LTWL than those randomized to ILS with longer follow-up, especially during Years 6–15. Future investigations should focus on whether metformin could be a useful intervention for LTWL after initial weight loss with lifestyle interventions, antiobesity drugs or devices, or bariatric surgery.
Supplementary Material
Grant support and acknowledgements:
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number U01 DK048489. During the DPP and DPPOS, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health provided funding to the clinical centers and the Coordinating Center for the design and conduct of the study, and collection, management, analysis, and interpretation of the data (U01 DK048489). The Southwestern American Indian Centers were supported directly by the NIDDK, including its Intramural Research Program, and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources, and the Department of Veterans Affairs supported data collection at many of the clinical centers. Funding was also provided by the National Institute of Child Health and Human Development, the National Institute on Aging, the National Eye Institute, the National Heart Lung and Blood Institute, the National Cancer Institute, the Office of Research on Women’s Health, the National Institute on Minority Health and Health Disparities, the Centers for Disease Control and Prevention, and the American Diabetes Association. Bristol-Myers Squibb and Parke-Davis provided additional funding and material support during the DPP, Lipha (Merck-Sante) provided medication and LifeScan Inc. donated materials during the DPP and DPPOS. This research was also supported, in part, by the intramural research program of the NIDDK. LifeScan Inc., Health O Meter, Hoechst Marion Roussel, Inc., Merck-Medco Managed Care, Inc., Merck and Co., Nike Sports Marketing, Slim Fast Foods Co., and Quaker Oats Co. donated materials, equipment, or medicines for concomitant conditions. McKesson BioServices Corp., Matthews Media Group, Inc., and the Henry M. Jackson Foundation provided support services under subcontract with the Coordinating Center. The opinions expressed are those of the investigators and do not necessarily reflect the views of the funding agencies. A complete list of Centers, investigators, and staff can be found in the Appendix. The Research Group gratefully acknowledges the commitment and dedication of the participants of the DPP and DPPOS.
Disclosures: EMV has received research support from the National Institutes of Health (NIH); she is an advisor to the Pennsylvania Pharmacists’ Association with payments made to her employer, University of Pittsburgh, and has received speaking honoraria from Innovative Wellness Solutions and American Diabetes Association. EJB has received research support from the Department of Veterans Affairs, and has been provided travel support by the International Diabetes Federation. XP has served on the scientific advisory boards of Novo Nordisk and Zafgen. PWF has received research funding from Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk, Sanofi Aventis, and Servier; he is a consultant for and has stock options in Zoe Global. KMG has received research support from the NIH, AstraZeneca, and BioKier; he is an advisor to AstraZeneca with payments made to his employer, Pennington Biomedical Research Center, and has received travel expenses for attending investigator meetings from AstraZeneca, and speaking honorarium from the American Diabetes Association. JWA, SLE, WCK, RRK, DD, PS, and CHD reported no conflicts of interest.
Footnotes
Members of the Diabetes Prevention Program Research Group are listed in the Appendix.
Reproducible Research Statement: Study protocol is available at: https://dppos.bsc.gwu.edu/web/dppos/dppos. Statistical code: Available from Sharon Edelstein at dppmail@bsc.gwu.edu. Data set: Available via the NIDDK Data Repository at https://repository.niddk.nih.gov/studies/dpp/ and https://repository.niddk.nih.gov/studies/dppos/.
Requests for Single Reprints: Diabetes Prevention Program Research Group, Diabetes Prevention Program Coordinating Center, The Biostatistics Center, George Washington University, Rockville, MD, 20852. Email: dppmail@bsc.gwu.edu
References:
- 1.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(Suppl 2):S102–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–50. [DOI] [PubMed] [Google Scholar]
- 4.Lachin JM, Christophi CA, Edelstein SL, Ehrmann DA, Hamman RF, Kahn SE, et al. Factors associated with diabetes onset during metformin versus placebo therapy in the diabetes prevention program. Diabetes. 2007;56(4):1153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):167786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3(11):86675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183–97. [DOI] [PubMed] [Google Scholar]
- 9.Diabetes Prevention Program Research Group. The Diabetes Prevention Program: Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22(4):62334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diabetes Prevention Program Research Group. The Diabetes Prevention Program: baseline characteristics of the randomized cohort. Diabetes Care. 2000;23(11):1619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diabetes Prevention Program Research Group. The diabetes prevention program (DPP): Description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diabetes Prevention Program Research Group. Effects of withdrawal from metformin on the development of diabetes in the Diabetes Prevention Program. Diabetes Care. 2003;26(4):97780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venditti EM, Bray GA, Carrion-Petersen ML, Delahanty LM, Edelstein SL, Hamman RF, et al. First versus repeat treatment with a lifestyle intervention program: attendance and weight loss outcomes. Int J Obes (Lond). 2008;32(10):1537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diggle PJ, Liang K-Y, Zeger SL. Analysis of longitudinal data. New York: Oxford University Press; 1994. [Google Scholar]
- 15.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuious outcomes. Biometrics. 1986;42(1):121–30. [PubMed] [Google Scholar]
- 16.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a Generalized Estimating Equation approach. Biometrics. 1988;44(4):1049–60. [PubMed] [Google Scholar]
- 17.Lalonde TL, Nguyen AQ, Yin J, Irimata K, Wilson JR. Modeling correlated binary outcomes with time-dependent covariates. J Data Sci. 2013;11:715–38. [Google Scholar]
- 18.Domecq JP, Prutsky G, Leppin A, Sonbol MB, Altayar O, Undavalli C, et al. Drugs commonly associated with weight change: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(2):363–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35(4):731–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155–61. [DOI] [PubMed] [Google Scholar]
- 21.Padwal R, Kezouh A, Levine M, Etminan M. Long-term persistence with orlistat and sibutramine in a population-based cohort. Int J Obes (Lond). 2007;31(10):1567–70. [DOI] [PubMed] [Google Scholar]
- 22.le Roux CW, Astrup A, Fujioka K, Greenway F, Lau DC, Van Gaal L, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017;389(10077):1399–409. [DOI] [PubMed] [Google Scholar]
- 23.Qaseem A, Humphrey LL, Sweet DE, Starkey M, Shekelle P, Clinical Guidelines Committee of the American College of Physicians. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2012;156(3):218–31. [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S73–S85. [DOI] [PubMed] [Google Scholar]
- 25.Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in noninsulin-dependent diabetes mellitus. N Engl J Med. 1995;333(9):550–4. [DOI] [PubMed] [Google Scholar]
- 26.Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollak M The effects of metformin on gut microbiota and the immune system as research frontiers. Diabetologia. 2017;60(9):1662–7. [DOI] [PubMed] [Google Scholar]
- 28.Paolisso G, Amato L, Eccellente R, Gambardella A, Tagliamonte MR, Varricchio G, et al. Effect of metformin on food intake in obese subjects. Eur J Clin Invest. 1998;28(6):441–6. [DOI] [PubMed] [Google Scholar]
- 29.Schultes B, Oltmanns KM, Kern W, Fehm HL, Born J, Peters A. Modulation of hunger by plasma glucose and metformin. J Clin Endocrinol Metab. 2003;88(3):1133–41. [DOI] [PubMed] [Google Scholar]
- 30.Seifarth C, Schehler B, Schneider HJ. Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Exp Clin Endocrinol Diabetes. 2013;121(1):27–31. [DOI] [PubMed] [Google Scholar]
- 31.Out M, Miedema I, Jager-Wittenaar H, van der Schans C, Krijnen W, Lehert P, et al. Metformin-associated prevention of weight gain in insulin-treated type 2 diabetic patients cannot be explained by decreased energy intake: A Post hoc Analysis of a Randomized Placebo-Controlled 4.3 year Trial. Diabetes Obes Metab. 2018;20(1):219–23. [DOI] [PubMed] [Google Scholar]
- 32.Leslie P, Jung RT, Isles TE, Baty J. Energy expenditure in non-insulin dependent diabetic subjects on metformin or sulphonylurea therapy. Clin Sci (Lond). 1987;73(1):41–5. [DOI] [PubMed] [Google Scholar]
- 33.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332(10):621–8. [DOI] [PubMed] [Google Scholar]
- 34.Rosenbaum M, Hirsch J, Murphy E, Leibel RL. Effects of changes in body weight on carbohydrate metabolism, catecholamine excretion, and thyroid function. Am J Clin Nutr. 2000;71(6):1421–32. [DOI] [PubMed] [Google Scholar]
- 35.Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–604. [DOI] [PubMed] [Google Scholar]
- 36.Hall KD, Sanghvi A, Göbel B. Proportional Feedback Control of Energy Intake During Obesity Pharmacotherapy. Obesity. 2017;25(12):2088–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elfhag K, Rössner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev 2005;6(1):67–85. [DOI] [PubMed] [Google Scholar]
- 38.Wing RR, Hamman RF, Bray GA, Delahanty L, Edelstein SL, Hill JO, et al. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004;12(9):1426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Unick JL, Pellegrini CA, Demos KE, Dorfman L. Initial weight loss response as an indicator for providing early rescue efforts to improve long-term treatment outcomes. Curr Diab Rep. 2017;17(9):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gadde KM, Apolzan JW, Berthoud H-R. Pharmacotherapy for Patients with Obesity. Clin Chem 2018;64(1):118–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delahanty LM, Meigs JB, Hayden D, Williamson DA, Nathan DM. Psychological and behavioral correlates of baseline BMI in the diabetes prevention program (DPP). Diabetes Care. 2002;25(11):1992–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Svetkey LP, Clark JM, Funk K, Corsino L, Batch BC, Hollis JF, et al. Greater weight loss with increasing age in the weight loss maintenance trial. Obesity. 2014;22(1):39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Funk LM, Grubber JM, McVay MA, Olsen MK, Yancy WS, Voils CI. Patient predictors of weight loss following a behavioral weight management intervention among US Veterans with severe obesity. Eat Weight Disord. 2018;23:587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roberts SB, Fuss P, Heyman MB, Evans WJ, Tsay R, Rasmussen H, et al. Control of food intake in older men. JAMA. 1994;272:1601–6. [DOI] [PubMed] [Google Scholar]
- 45.Moriguti JC, Das SK, Saltzman E, Corrales A, McCrory MA, Greenberg AS, et al. Effects of a 6-week hypocaloroic diet on changes in body composition , hunger and subsequent weight gain in healthy young and older adults. J Gerontol A Biol Sci Med Sci. 2000;55(12):B580–7. [DOI] [PubMed] [Google Scholar]
- 46.Morley JE. Anorexia or aging: physiologic and pathologic. Am J Clin Nutr. 1997;66:760–73. [DOI] [PubMed] [Google Scholar]
- 47.Blanton CA, Horwitz BA, McDonald RB. Neurochemical alterations during aging-related anorexia. Proc Soc Exp Biol Med. 1999;221:153–65. [DOI] [PubMed] [Google Scholar]
- 48.Das SK, Moriguti JC, McCrory MA, Saltzman E, Mosunic C, Greenberg AS, et al. An underfeeding study in healthy men and women provides further evidence of impaired regulation of energy expenditure in old age. J Nutr. 2001;131:1833–8. [DOI] [PubMed] [Google Scholar]
- 49.Kaneda T, Makino S, Nishiyama M, Asaba K, Hashimoto K. Differential neuropeptide responses to starvation with ageing. J Neuroendocrinol. 2001;13:1066–75. [DOI] [PubMed] [Google Scholar]
- 50.Ely EK, Gruss SM, Luman ET, Gregg EW, Ali MK, Nhim K, et al. A national effort to prevent type 2 diabetes: participant-level evaluation of CDC’s National Diabetes Prevention Program. Diabetes Care. 2017;40(10):1331–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pagoto S, Pbert L, Emmons K, Society of Behavioral Medicine Public Policy Leadership Group. The Society of Behavioral Medicine position statement on the CMS decision memo on intensive behavior therapy for obesity. Transl Behav Med. 2012;2(4):381–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williamson DA. Fifty Years of Behavioral/Lifestyle Interventions for Overweight and Obesity: Where Have We Been and Where Are We Going? Obesity. 2017;25(11):1867–75. [DOI] [PubMed] [Google Scholar]
- 53.Berger SE, Huggins GS, McCaffery JM, Lichtenstein AH. Comparison among criteria to define successful weight-loss maintainers and regainers in the Action for Health in Diabetes (Look AHEAD) and Diabetes Prevention Program trials. Am J Clin Nutr. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.