Abstract
The worldwide increase in type 2 diabetes (T2D) is becoming a major health concern, thus searching for novel preventive and therapeutic strategies has become urgent. In last decade, the paralogous transcription factors MondoA and carbohydrate response element-binding protein (ChREBP) have been revealed to be central mediators of glucose sensing in multiple metabolic organs. Under normal nutrient conditions, MondoA/ChREBP plays vital roles in maintaining glucose homeostasis. However, under chronic nutrient overload, the dysregulation of MondoA/ChREBP contributes to metabolic disorders, such as insulin resistance (IR) and T2D. In this review, we aim to provide an overview of recent advances in the understanding of MondoA/ChREBP and its roles in T2D development. Specifically, we will briefly summarize the functional similarities and differences between MondoA and ChREBP. Then, we will update the roles of MondoA/ChREBP in four T2D-associated metabolic organs (i.e., the skeletal muscle, liver, adipose tissue, and pancreas) in physiological and pathological conditions. Finally, we will discuss the opportunities and challenges of MondoA/ChREBP as drug targets for anti-diabetes. By doing so, we highlight the potential use of therapies targeting MondoA/ChREBP to counteract T2D and its complications.
Keywords: glucose, MondoA, ChREBP, insulin resistance, type 2 diabetes, metabolism, skeletal muscle, liver, adipose tissue, pancreas
1. Introduction
Type 2 diabetes (T2D) is a chronic metabolic disease which affects more than 370 million people worldwide [1]. It is associated with high incidence of developing several medical complications, including but not limited to cardiovascular disease, kidney disease, some types of cancer, and vision problems [2,3]. T2D is one of the leading causes of global morbidity and mortality due to this [4]. Despite great progress that has been made in the understanding of the pathophysiologic mechanisms of T2D in recent years, the incidence and prevalence of the disease continues to rise globally, and the affected population is estimated to be as high as 570 million by 2040 [1]. T2D thereby places considerable socioeconomic pressures on the individual and huge costs to global health economies in the present and future [5]. The reasons for the escalating epidemic of T2D include being overweight or obese, sedentary lifestyle, and increased consumption of unhealthy diets, as well as refined grains and sugar-sweetened beverages, which are all related to economic globalization and urbanization [2,3].
At the cellular and molecular levels, T2D is characterized by relative insulin deficiency caused by pancreatic β-cell dysfunction and insulin resistance (IR) in target organs [1]. Although the specific mechanisms of T2D pathogenesis are still missing to date, the proposed models are as follows [6,7]. Normally, pancreatic β-cells produce insulin to maintain postprandial blood glucose level through increasing glucose disposal by insulin-sensitive organs (e.g., the skeletal muscle, liver, and adipose tissue), and meanwhile inhibiting hepatic glucose production. However, under conditions of chronic energy overload, ectopic lipid accumulation causes IR in the skeletal muscle and liver, leading to the impairment of glucose uptake and glycogen synthesis but promotion of hepatic de novo lipogenesis (DNL) and hyperlipidemia. In parallel, the overexpansion of white adipose tissue (WAT), especially the visceral adipose tissue (VAT), induces the production of pro-inflammatory adipokines (e.g., monocyte chemoattractant protein-1 (MCP-1) and tumor necrosis factor-α (TNFα)), which subsequently recruit the macrophage infiltration into the WAT, leading to the WAT IR and elevation of lipolysis. Then, the WAT disorder further increases hepatic triglyceride synthesis, hyperlipidemia, and hepatic gluconeogenesis, promoting fasting and postprandial hyperglycemia. In response, pancreatic β-cells initially compensate for IR by hypersecretion of insulin. However, as the disease progresses over time, the β-cell compensation fails due to β-cell apoptosis which is caused by chronic hyperglycemia and its complications, and eventually, T2D ensues.
Even though the modification of lifestyle, including weight loss, increasing physical activity and adopting a healthy diet [8,9], have been proved to be a practical strategy for the management of T2D, the anti-T2D drugs are still strongly needed. Not only patients who are unwilling to change their lifestyle require pharmacological treatment, but the progressive nature of T2D (relentless loss of functional β-cell mass) also constitutes a major need for novel antidiabetic agents that hold potentials of arresting this process. Currently, the available anti-T2D drugs (e.g., metformin) or the strategies of developing new drugs usually aim to increase pancreatic β-cell insulin production or systemic insulin sensitivity. However, so far, only small numbers of gene candidates, e.g., peroxisome proliferator-activated receptor-γ (PPARγ), have been identified and applied in clinical practices [10]. Notably, studies in recent years have shown that the glucose-sensing transcription factor MondoA/carbohydrate response element-binding protein (ChREBP) is emerging as a potential target to treat T2D [11,12,13,14]. To advance our knowledge of MondoA/ChREBP, this review will summarize the recent findings concerning the respective regulation and functions of MondoA and ChREBP in each metabolic organ (i.e., the skeletal muscle, liver, adipose tissue, and pancreas), with an emphasis on its challenges and opportunities in the treatment of T2D.
2. Glucose Sensors: MondoA vs. ChREBP
Glucose is used almost universally as the preferred carbon and energy source [15]. Breakdown of this major cellular nutrient provides both a source of the starting material for the synthesis of all major classes of biomolecules, and also ATP production through glycolysis and oxidative phosphorylation. Given the importance of glucose in metabolism, cells have evolved mechanisms to sense and utilize this crucial fuel in their environment. In mammalian cells, glucose or simple carbohydrates are sensed by several mechanisms, of which the MondoA/ChREBP transcription factor has been revealed to be at the core in linking glucose metabolism to downstream gene transcription [16,17,18]. Hence, derangements in this glucose-sensing pathway in mammalian cells often contribute to pathological conditions such as obesity, fatty liver, and T2D in both animals and humans [17,19,20,21].
2.1. Overview of the Intrinsic Regulation of MondoA/ChREBP Activity
MondoA (also termed as Mlx (Max-like protein x) -interacting protein, MLXIP) and its paralog ChREBP (also known as MondoB or Mlx-interacting protein-like (MLXIPL)) were discovered a decade ago when researchers tried to figure out the molecular mechanisms of intracellular glucose sensing [22,23]. Almost at the same time, Mlx, a binding partner of Mad and Mnt proteins [24,25], was found to be the common heterodimerization partner of both MondoA and ChREBP [23,26]. Later, the MondoA/ChREBP-Mlx complexes were identified as a conserved regulator of glucose metabolism from yeast to mammals [18,27,28,29].
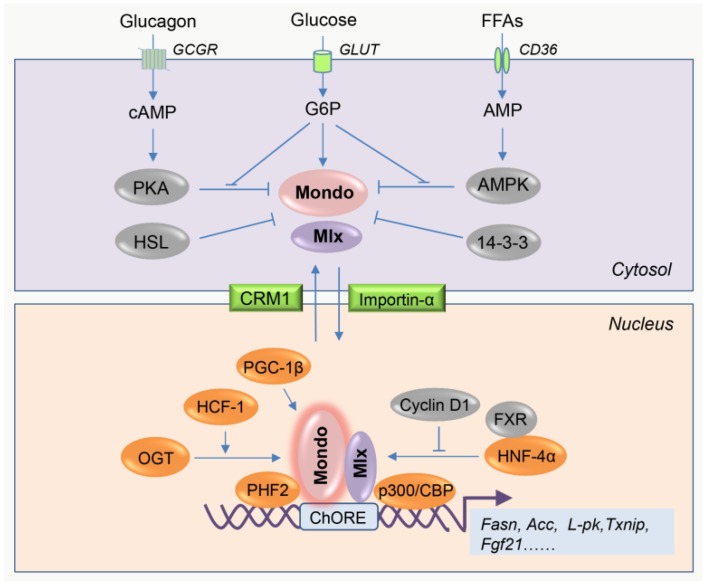
These complexes play actions principally in two steps, including glucose metabolites-induced nuclear translocation and activation of target gene transcription by coordination with cofactors (Figure 1). A detailed representation of the activations has been presented elsewhere [16,17]. Briefly, under fasting conditions, all the components of the complexes are inactive due to their cytoplasmic localization. However, in response to carbohydrate feeding, the increased levels of glucose intermediate metabolites, e.g., glucose-6-phosphate (G6P) [29,30,31] and fructose-2,6-bisphosphate (Fru-2,6-P2) [32,33], might bind a specific recognition motif of MondoA/ChREBP, which may cause an allosteric conformational change of MondoA/ChREBP, that leads to MondoA/ChREBP recruitment of transcriptional partners (i.e., Mlx). Subsequently, MondoA/ChREBP-Mlx complexes translocate into the nucleus, where they bind the conserved carbohydrate response element (ChoRE) motif presented on promoters of the glycolytic (e.g., L-pk [22]), lipogenic (e.g., Acc, Fasn, Scd1, and Elovl6 [28]) and other (e.g., Fgf21 and Txnip [34,35]) genes, and then activate their transcription through coordination with other coregulators.
Figure 1.
Regulation of MondoA/carbohydrate response element-binding protein (ChREBP) (Mondo) activity by nutrients and associated regulatory proteins. The activity of MondoA/ChREBP is dependent on its subcellular localization. Inactive state (upper panel): Under fasting conditions, intracellular cAMP and AMP activates protein kinase A (PKA) and AMP-activated protein kinase (AMPK) upon elevated glucagon release and fatty acids uptake, respectively. Then, the activated PKA and AMPK phosphorylate MondoA/ChREBP at different residues, consequently inhibiting the heterodimerization with Mlx and subsequent nuclear translocation. Besides, cytosolic protein 14-3-3 interacts with Mondo proteins and also blocks Mondo nuclear import. Particularly, in adipocytes, hormone-sensitive lipase (HSL) physically binds ChREBP-α and retains ChREBP-α in the cytosol, also limiting ChREBP activity. When under high glucose conditions, the intracellular glucose is phosphorylated into glucose-6-phosphate (G6P), a substrate which can be further used for producing other metabolites, such as xylulose 5-phosphate (Xu5P) and fructose-2,6-bisphosphate (Fru-2,6-P2). Thereafter, G6P and its certain derivatives induce a conformational change of Mondo, followed by the formation of Mondo-Mlx complexes, which then translocate to the nucleus through nuclear shuttling factor importin-α. Active state (lower panel): In the nucleus of hepatocytes, hepatocyte nuclear factor (HNF)-4α and peroxisome proliferator-activated receptor-γ coactivator (PGC)-1β physically interact with ChREBP and enhance its transcriptional activity. In contrast, cyclin D1 and farnesoid X receptor (FXR) inhibit ChREBP activity through suppression of HNF-4α function and dissociation of ChREBP from the p300/CREB-binding protein (CBP) transcriptional complex, respectively. Besides, host cell factor (HCF)-1 binds ChREBP and then recruits O-linked N-acetylglucosamine transferase (OGT) to ChREBP, stimulating ChREBP O-GlcNAcylation and activation. In parallel, HCF-1 also recruits PHD finger protein (PHF)2 for epigenetic activation of lipogenic gene promoters, further promoting ChREBP-dependent gene transcription. The arrow means the activation or promotion of the function, and the T-bar means the inhibition of the function.
So far, several types of regulators have been identified to be involved in the activation or inhibition of MondoA/ChREBP-Mlx complexe formation or activities (Figure 1). They include post-translational modification enzymes (e.g., protein kinase A (PKA) [36,37], AMP-activated protein kinase (AMPK) [38,39], and O-linked N-acetylglucosamine (O-GlcNAc) transferase (OGT) [40]), nuclear shuttling factors (e.g., 14-3-3 protein [41,42], chromosomal region maintenance 1 (CRM1) [43], and importins [44]), nuclear receptors (e.g., hepatocyte nuclear factor (HNF)-4α [45] and farnesoid X receptor (FXR) [46]), transcription cofactors (e.g., peroxisome proliferator-activated receptor-γ coactivator (PGC)-1β [47]), epigenetic enzymes (e.g., p300/CREB-binding protein (CBP) [48] and PHD finger protein 2 (PHF2) [49]), accessory proteins (e.g., host cell factor (HCF)-1 [50] and cyclin D1 [51]), and even lipases (e.g., hormone sensitive lipase (HSL) [52]). These proteins are implicated in the regulation of all aspects of MondoA/ChREBP-Mlx complexes, including nucleo-cytosolic trafficking, DNA binding ability, transactivation activity, message RNA transcription, and protein stability. Their detailed functions are summarized by other authors [17,53].
2.2. Differences between MondoA and ChREBP
Despite the overall structural and functional similarities between MondoA and ChREBP (reviewed in other works [16,17]), there are differences (Table 1). First, despite both transcription factors have widespread expression, under normal physiological conditions, MondoA expression is most abundant in skeletal muscle where it mainly regulates the glycolytic pathway [23,54], while ChREBP expression predominates in the liver and adipose tissue (with moderate expression in the kidney, skeletal muscle, and small intestine) and primarily regulates lipogenesis [55,56], indicating MondoA-Mlx and ChREBP-Mlx may represent glucose sensors in different organs. Of note, the differences in downstream target genes are not simply due to differences in their expression patterns, but also due to the refined structural differences as each interacts with specific promoters [57]. Second, under basal conditions, MondoA locates to the outer membrane of the mitochondria [54], while ChREBP remains in the cytosol [37], indicating they play different roles under glucose-deprived conditions. Although no evidence is reported to date, the mitochondria-localized MondoA is probably involved in the regulation of mitochondria morphogenesis or respiration activity, which could be explored in future studies. Third, it has been reported that PKA signaling inhibits the activities of both MondoA and ChREBP through phosphorylation, but interestingly the phosphorylation sites are not conserved between them [58,59], suggesting their post-translational modifications are different. Last but not least, ChREBP has two isoforms, i.e., ChREBP-α (the full-length ChREBP) and recently identified ChREBP-β (a constitutively activated isoform lacking the low glucose inhibitory domain) [60]. In contrast, only the full-length isoform of MondoA is reported to date. Whether MondoA also has a constitutive activated isoform is still waiting to be confirmed. It is noteworthy that, during the last decade, ChREBP has gained more attention than MondoA in the fields, thus it is possible that as more understanding of MondoA develops in the future, the more differences between MondoA and ChREBP will be revealed.
Table 1.
Summary of characteristic differences between MondoA and ChREBP.
| Characteristic | MondoA | ChREBP |
|---|---|---|
| Other names | MLXIP | MLXIPL, MondoB, WBSCR14 |
| Coding gene location (Homo sapiens) |
chromosome 12q24.31 |
chromosome 7q11.23 |
| Isoforms | MondoA | ChREBP-α and ChREBP-β |
| Protein weight (Homo sapiens) |
919 AA | 852 AA and 675AA |
| Primary enriched tissues | skeletal muscle | liver, adipose tissue |
| Basal subcellular localization | outer mitochondrial membrane | cytosol |
| Major downstream pathways | glycolysis | lipogenesis |
3. Muscle MondoA: A Negative Regulator of Insulin Sensitivity
Skeletal muscle represents about 40% of body mass, but accounts for 80% of glucose uptake in the body, therefore it plays a fundamental role in the regulation of whole-body glucose homeostasis [61]. Accordingly, enhanced skeletal muscle metabolism or exercise can reduce the incidence of metabolic syndrome, hepatic steatosis, and T2D, whereas skeletal muscle dysfunction or physical disability could contribute to hepatic steatosis and T2D [62]. Therefore, a better understanding of the molecular mechanisms that regulate muscle energy homeostasis may reveal new strategies for combating metabolic diseases. Recently, studies have revealed that MondoA is an important negative regulator of muscle insulin sensitivity, which enables MondoA as a potential therapeutic target for IR and T2D [11,63]. The relevant data are summarized in Figure 2 and Table 2.
Figure 2.
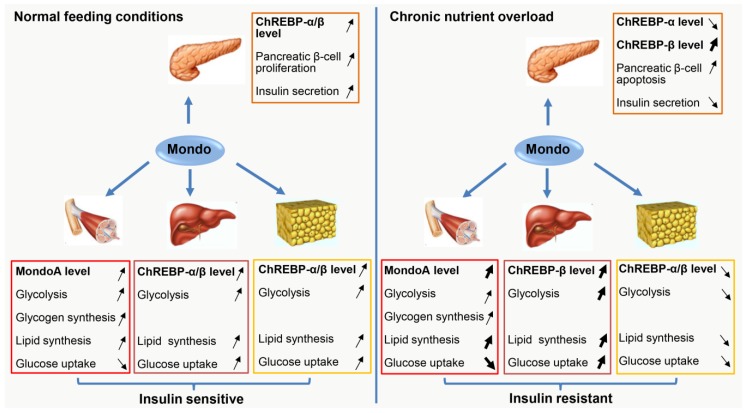
The roles of MondoA/ChREBP in metabolic tissues under normal feeding conditions and chronic nutrient overload. In response to normal carbohydrate diet (left panel), MondoA/ChREBP expression is induced in all metabolic tissues (including the pancreas, skeletal muscle, liver, and adipose tissue), which contributes to adaptive pancreatic β-cell proliferation and increased insulin production, and meanwhile, which also promotes glucose disposal by the muscle, liver, and adipose tissue through enhancing glycolysis, lipid synthesis, as well as glycogen synthesis. All these metabolic alterations guarantee whole-body insulin sensitivity and glucose homeostasis. However, when the body is exposed to chronic nutrient overload (right panel), the response of MondoA/ChREBP expression is not unified among the metabolic tissues. Specifically, in pancreatic β-cells, ChREBP-β expression is strongly induced, while ChREBP-α expression is downregulated, totally leading to lipo-glucotoxicity to β-cells, which causes β-cell apoptosis and reduced insulin secretion. In skeletal muscle, MondoA is persistently-activated, resulting in inhibition of glucose uptake, but promotion of lipid synthesis. Interestingly, in the liver, only ChREBP-β expression is dramatically induced, which then directly increases hepatic lipid synthesis and indirectly enhances glucose uptake. Strikingly, in adipose tissue, both ChREBP-α and ChREBP-β expression is downregulated, leading to reduced lipid synthesis and glucose uptake. Overall, the sum of this metabolic remodeling causes insulin resistance (IR). The arrow pointing up means an increase in the level or activity, the arrow pointing down means a decrease in the level or activity, and the bold arrow means the range of increase or decrease are enhanced. The cartoons used in this figure are adapted from the internet.
Table 2.
Summary of the reported tissue- and context-dependent roles of MondoA/ChREBP in mice insulin sensitivity.
| Mouse Models | Context | Body Weight |
Fat Mass | Hepatic Steatosis |
Insulin Sensitivity |
Reference |
|---|---|---|---|---|---|---|
| MondoA global knockout |
Standard diet | = | ND | ND | = | [64] |
| High-fat diet | NA | NA | ||||
| MondoA muscle-specific knockout | Standard diet | = | ND | ND | = | [11] |
| High-fat diet | = | ND | ND |
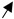
|
[11] | |
| ChREBP global knockout | Standard diet | = |

|
= |

|
[55] |
| Standard diet in ob/ob mice background |

|

|

|
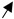
|
[65] | |
| ChREBP liver-specific knockout | Standard diet | = |

|
= |

|
[66] |
| High-fat diet | = | = | = |

|
[66] | |
| High-carbohydrate diet |

|

|

|

|
[66] | |
| ChREBP liver-specific overexpression | Standard diet | = |

|
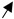
|
= | [67] |
| High-fat diet | = |

|
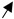
|
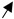
|
[67] | |
| ChREBP AT-specific knockout | Standard diet | = | = |

|

|
[12] |
| High-fat diet | = | = | = |

|
[12] | |
| ChREBP AT-specific overexpression | Standard diet |

|

|
= | = | [68] |
| High-fat diet |

|

|

|
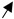
|
[68] | |
| ChREBP pancreatic β cell-specific overexpression | Standard diet |

|
ND | ND |

|
[69] |
| High-fat diet | NA | NA | ||||
Note: “=” means not changed. ND = not detected. NA = not available. The arrow pointing up means an increase in the level. The arrow pointing down means a decrease in the level.
3.1. Role of MondoA in Muscle Glucose Metabolism
As mentioned previously, MondoA is predominantly expressed in skeletal muscle, but its specific roles in skeletal muscle were not clear until recently. By adapting human skeletal myotube as an in vitro model, Ahn et al. have found very recently that MondoA activity in skeletal muscle is physiologically regulated by glucose or fructose level [11]. Specifically, glucose deprivation inhibits, in contrast glucose refeeding activates, the activity of MondoA. In addition, global RNA-seq analysis has further revealed that MondoA activates a wide range of genes, which are involved in lipid metabolism (e.g., acyl-CoA synthetase long chain family member 1 (ACSL1)), elongation of very long chain fatty acids protein 5 (ELOVL5), stearoyl-CoA desaturase (SCD)), glycogen synthesis (e.g., phosphoprotein phosphatase 1 regulatory subunit 3A (PPP1R3A)), and hexosamine biosynthetic pathway (e.g., glutamine-fructose-6-phosphate transaminase (GFPT)-1/2), but strikingly inhibits the expression of genes implicated in insulin signaling pathway (e.g., thioredoxin-interacting protein (TXNIP) and arrestin domain–containing 4 (ARRDC4)) in human skeletal myotubes [11]. Consistent with the RNA-seq results, knockdown of MondoA in human skeletal myotubes inhibits oleate loading-induced triglycerides (TG) accumulation, but enhances 2-deoxyglucose uptake [63]. Notably, the increased glucose uptake is also observed in Mlx-knockdown myoblasts [70], further confirming the inhibitory effects of MondoA on glucose uptake in skeletal muscle. Since the intact insulin signaling is crucial for the insulin-stimulated glucose uptake, and meanwhile, the intracellular glucose level determines MondoA activity, thus it seems that there exists a MondoA-mediated negative feedback mechanism by which MondoA functions as a gatekeeper to maintain glucose homeostasis in skeletal muscle.
3.2. Role of Muscle MondoA in IR Development
However, under conditions of chronic nutrient overload, the MondoA-mediated negative feedback mechanism is crashed, leading to persistently activated MondoA, which results in a vicious cycle of muscle lipid accumulation and IR. In this setting, MondoA actually acts as a molecular driver for the pathogenesis of IR. In support, selective loss of MondoA in mice muscle improves high-fat diet (HFD)-induced glucose tolerance and IR through reducing muscle lipid accumulation, and improving muscle insulin signaling as well as glucose uptake [11]. Besides, given the global MondoA-deficient mice show enhanced exercise capacity [64], it is possible that the mice with muscle-specific MondoA deficiency may also improve physical activity, which probably contributes to the improvement of insulin sensitivity as well. Inaddition, mice administrated with a small molecule, SBI-993, which is identified as an inhibitor of MondoA, also exhibit reduced muscle lipid accumulation, as well as improved muscle insulin signaling and systemic glucose tolerance upon HFD feeding [63]. However, it is noteworthy that SBI-993 may also inhibit hepatic ChREBP activity, as the occupation of ChREBP on liver target genes is reduced after SBI-993 treatment [63]. Thus, MondoA-specific inhibitors are needed to further verify the therapeutic potential of MondoA. Nevertheless, the present studies have strongly suggested that the inhibition of MondoA-Mlx transcriptional activity may provide an attractive strategy for the development of anti-diabetic therapeutics.
4. Hepatic ChREBP: More Protector than Killer for Insulin Sensitivity
The liver plays a central role in the maintenance of systemic glucose homeostasis in response to different energy status. [71]. Under fasting conditions, liver produces glucose through glycogenolysis and gluconeogenesis to supply the energy for the needs of other organs, e.g., the brain [72]. After feeding, liver stores extra energy in the form of glycogen [72]. In addition, liver is also the principal organ responsible for the conversion of excess dietary carbohydrate into TG through glycolysis and DNL, which is then mobilized and exported to adipose tissue for long-term storage [73]. To maintain the energy balance, these physiological processes in the liver are precisely regulated under normal physiological conditions. However, under conditions of chronic energy excess, the dysregulation of energy metabolism results in the accumulation of TG in the liver, leading to the pathogenesis of nonalcoholic fatty liver disease (NAFLD), IR and T2D [74,75].
ChREBP is most highly expressed in the liver and has emerged as a principal insulin-independent hepatic lipogenic determinant during the last decade [28,55]. A growing number of evidence has shown that ChREBP is involved in the pathogenesis of hepatic steatosis and IR, but the current records are still controversial about the roles of hepatic ChREBP in IR development (reviewed in [76,77]). The relevant data are also summarized in Figure 2 and Table 2.
4.1. Promotion of IR Development by Hepatic ChREBP
Hepatic ChREBP expression is highly regulated by nutritional states. Under physiological conditions, the hepatic expression of both ChREBP-α and ChREBP-β is low in fasting state, but it can be significantly induced (especially for ChREBP-β isoform) when refeeding with carbohydrate-rich diet [78]. However, under pathological conditions, the hepatic ChREBP expression is continuously upregulated. For example, compared with the lean controls, the expression of hepatic ChREBP-β (but not ChREBP-α) is dramatically increased in obese humans, which is further elevated in patients with T2D regardless of under fasting or refeeding conditions [78,79,80]. Similar expression patterns are also observed in the livers of HFD-induced obese mice and genetically induced obese ob/ob mice [14]. These results indicate a functional relevance of ChREBP in promoting the development of IR and T2D.
In support of this speculation, so far, the most direct evidence is derived from two early studies performed in the ob/ob mouse model, where globally or liver-selectively deficiency of ChREBP decreases hepatic lipogenesis and restores hepatic and peripheral insulin sensitivity [14,65]. Consistently, indirect inhibition of ChREBP transcriptional activity by hepatic overexpression of dominant negative Mlx also decreases hepatic steatosis and improves glucose tolerance in diabetes-prone aging C57BL6J mice [81]. Furthermore, the similar phenotypes are also recently observed in mice where hepatic ChREBP expression is suppressed due to deletion of ChREBP upstream regulator retinol saturase (RetSat) or zinc finger and BTB domain-containing protein 20 (ZBTB20) [82,83]. Therefore, it is persuasive that hepatic ChREBP acts as a molecular driver for IR development at least in mice with leptin deficiency.
4.2. Inhibition of IR Development by Hepatic ChREBP
Interestingly, recent studies have indicated that ChREBP is beneficial for hepatic insulin sensitivity and whole-body glucose homeostasis. Benhamed et al. have found that mice with liver-specific ChREBP overexpression develop obvious hepatic steatosis, but surprisingly protect against the systemic IR regardless of feeding with normal diet or HFD [67]. Consistently, in patients with nonalcoholic steatohepatitis (NASH), ChREBP expression is positively correlated with the degree of hepatic steatosis, but inversely related to IR [67]. One early explanation for ChREBP-mediated IR improvement is that hepatic upregulation of ChREBP induces the expression of SCD1, which remodels the lipid partitioning in the liver, including raising beneficial lipid species (e.g., monounsaturated fatty acids (MUFAs)) and reducing deleterious lipid species (e.g., saturated fatty acids (SFAs)) [67]. In addition, recent studies have provided an alternative mechanism in which ChREBP directly activates the expression of fibroblast growth factor 21 (FGF21) [34,84,85], an important hepatokine that plays a beneficial role in whole-body metabolic regulation [86,87]. Thus, it seems that the mechanisms for the improvement of IR by ChREBP overexpression is complex. Besides, in agreement with the ChREBP overexpression studies, liver-specific ChREBP knockout mice show dampened hepatic insulin sensitivity, elevated hepatic glucose production, and impaired systemic glucose tolerance under various feeding conditions, including normal chow and HFD [66]. Moreover, the global ChREBP deficient mice also exhibit IR and glucose intolerance, albeit multiple tissues involvement [55]. Thereby, hepatic ChREBP indeed protects against IR development at least under the above-mentioned conditions.
Overall, the role of hepatic ChREBP in IR development seems to be context-dependent, but under most conditions, ChREBP is more likely protective rather than detrimental in maintaining insulin sensitivity. However, given the fact that overexpression of ChREBP also causes hepatic steatosis, which impairs the insulin sensitivity, thus activation of ChREBP may be not a good choice for the improvement of hepatic insulin sensitivity.
5. Adipose ChREBP: A Master Regulator of Systemic Insulin Sensitivity
Adipose tissues (ATs) play an important role in regulating whole-body energy and glucose homeostasis [88]. There are two principal types of ATs, namely white adipose tissue (WAT) and brown adipose tissue (BAT). The WAT stores extra energy from the diets in the form of TG, which can be mobilized as free acids to meet the energy demand of other tissues in states of fasting or exercise. In addition, WAT also secretes diverse adipokines and lipokines in response to different metabolic stress, and functions as an important endocrine organ [89]. In contrast, BAT is specialized for energy expenditure, which burns lipids for heat production as a defense against cold and obesity [90]. With the global increase in the prevalence of obesity, ATs have attracted much attention concerning its role in the development of IR and T2D. In various causes of IR, the impaired DNL in ATs has emerged as an important player in recent years [91,92]. ChREBP, as a major determinant for adipose DNL, is therefore considered to be a promising target to reverse IR [60,93]. The relevant data are summarized in Figure 2 and Table 2.
5.1. The Disturbed Expression of Adipose ChREBP during IR Development
Opposite with that in the liver, ChREBP expression in ATs is often impaired under various detrimental conditions. Specifically, the short-term HFD, which causes IR, reduces DNL and ChREBP expression in both WAT and BAT [56,94]. That occurs even earlier than the impairment of phosphorylation of AKT (also known as protein kinase B, PKB), a key step during the pathogenesis of IR [94]. The similar results are also observed in WATs of the ob/ob mice as young as seven-weeks old [94]. Therefore, it is more likely that the repressed expression of adipose ChREBP might be the cause but not the consequence of the IR development. Consistent with animal studies, in adolescents with prediabetes or early T2D, the subcutaneous adipose tissue (SAT) expresses lower amounts of ChREBP-α/β compared with that in the healthy controls [95]. Besides, in adult obese subjects either with or without T2D, ChREBP-α is also lower-expressed in the SAT, but for the ChREBP-β level, it is still uncertain due to its very low expression [80]. Interestingly, in the visceral adipose tissue (VAT), only the ChREBP-β expression is downregulated in obese individuals, implying existence of a tissue-specific regulation of ChREBP-α/β expression [80]. Moreover, ChREBP-β expression in VAT is positively correlated with the VAT DNL, but reversely related to homeostasis model assessment of insulin resistance (HOMA-IR) and liver steatosis in adult subjects [80]. Thereby, the expression levels of ChREBP in ATs could be used as an early predictor for IR development.
5.2. Maintenance of Systemic Insulin Sensitivity by WAT ChREBP
Consistent with the ChREBP expression during the pathogenesis of IR, the gain/loss-of-function studies in animals have further demonstrated that WAT ChREBP preserves the systemic insulin sensitivity. Specifically, mice with fat-specific loss of ChREBP show whole-body IR and WAT inflammation regardless of the lean or obese state [12]. Consistently, global ChREBP knockout mice also exhibit impaired insulin sensitivity [55]. At the molecular level, ChREBP deficiency in adipocytes alters fatty acid composition and lowers the abundance of the newly-identified palmitic acid esters of hydroxy stearic acids (PAHSAs) in WATs [12]. PAHSAs are synthesized through the process of WAT DNL and function as an insulin-sensitizing fatty acid through multiple mechanisms, including stimulating glucose uptake and lowering inflammation in ATs, and also including inducing the secretion of intestinal glucagon-like peptide-1 (GLP-1) and pancreatic insulin [96]. Because of that, supplementation of 9-PAHSA, the most abundant PAHSA isomer in WATs and serum, restores insulin sensitivity and reverses WAT inflammation in fat-specific ChREBP deficient mice [12]. In line with the loss-of-function studies, transgenic overexpression of constitutively active ChREBP (ChREBP-CA) in mice ATs improves insulin sensitivity and glucose tolerance under western diet conditions [68]. Since the levels of PAHSAs are not measured in these transgenic mice, it is still uncertain about the contributions of PAHSAs in the improvement of insulin sensitivity of these mice. In addition to regulating the DNL pathway, it is noteworthy that ChREBP also has other functions in WATs, such as promoting adipocytes differentiation [97] and white adipocyte browning [98], which are both known to positively regulate systemic insulin sensitivity [99,100]. Therefore, WAT ChREBP improves systemic insulin sensitivity probably through multiple mechanisms.
Consistent with the beneficial effects of ChREBP on systemic insulin sensitivity, genetic manipulations of ChREBP upstream factors or binding partners in ATs also regulate mice insulin sensitivity. Glucose transporter 4 (GLUT4) is responsible for the glucose entering into the adipocytes, while glucose metabolites can activate ChREBP, therefore, alterations in adipose GLUT4 expression have profound effects on systemic insulin sensitivity through regulation of ChREBP-α-mediated expression of ChREBP-β [60]. Besides, HSL has been recently identified to be a direct binding partner of ChREBP in adipocytes [52]. The physical interaction between ChREBP-α and HSL keeps ChREBP-α to stay in the cytosol, which blocks ChREBP-α translocation into the nucleus to induce the expression of ChREBP-β [52]. Thus, genetic loss of HSL in mouse ATs can release ChREBP-α, leading to enhanced mice insulin sensitivity, albeit the mice concurrently develop hepatic steatosis [52,101]. Overall, these studies strongly suggest that the WAT ChREBP is a master regulator of systemic insulin sensitivity.
5.3. BAT ChREBP is Dispensable for Systemic Insulin Sensitivity
Although there are no documents directly depicting BAT ChREBP function in the regulation of systemic insulin sensitivity to date, it is already known that BAT DNL seemingly has limited effects on whole-body insulin sensitivity. Abolition of BAT DNL by the specific loss of fatty acid synthase (FASN, the key rate-limiting enzyme in DNL) in brown adipocytes do not alter mice glucose tolerance regardless of feeding with normal chow or HFD [94]. In particularly, loss of AKT2 selectively in BAT impairs the expression and activity of ChREBP, leading to BAT lipodystrophy as expected, but leaves insulin sensitivity and glucose tolerance intact [56]. Together, these results suggest that BAT ChREBP seems to be not essential for the regulation of systemic insulin sensitivity, but more direct evidence is still needed to this end.
Collectively, ChREBP in ATs, particularly in WATs, plays an important role in the maintenance of systemic insulin sensitivity. Since the expression of ChREBP, especially the ChREBP-β isoform, is often downregulated in conditions predisposing to diabetes, therefore, restoration of ChREBP expression or activity in WATs might be an important therapeutic strategy to treat T2D and its complications.
6. Pancreatic ChREBP: A Double-Edged Sword for Insulin Production
Pancreatic β-cells play an important role in maintaining levels of circulating glucose constant through the production of insulin in response to nutritional states [102]. The production of insulin is tightly regulated by physiological cues. Usually, feeding activates, while fasting blocks, insulin production via regulation of β-cell proliferation and insulin gene transcription [102]. However, under conditions of chronic overnutrition, the resulting hyperglycemia causes progressive and deleterious effects on β-cells, leading to reduced insulin production and subsequent β-cells failure, a hallmark of T2D [103]. Thus, understanding the molecular mechanisms responsible for the regulation of β-cells growth and failure is necessary for developing new drugs to cure T2D. In recent years, ChREBP has been revealed to be implicated in both β-cell proliferation and apoptosis [13,69,104], enabling it as a potential target for treating T2D. The relevant data are also summarized in Figure 2 and Table 2.
6.1. Role of Pancreatic ChREBP in β-cell Adaptive Proliferation
Both ChREBP-α and ChREBP-β are expressed in pancreatic β-cells, and their levels/activities are induced by high glucose concentration [13,104]. Additionally, using gain/loss-of-function studies, ChREBP-α/β has been revealed to be both necessary and sufficient for glucose-induced β-cell adaptive proliferation in vitro [13,104]. Specifically, adenoviral overexpression of wild-type ChREBP-α in rat or human β-cells augments glucose-stimulated β-cell proliferation, and meanwhile increases the expression of cell cycle accelerators, including cyclin D2, cyclin A, cyclin E, and cyclin-dependent kinase (CDK) 4/6 [13]. Reversely, depletion of total ChREBP or ChREBP-β alone by small interfering RNA (siRNA) blocks glucose-induced β-cell proliferation and expression of cyclin A and cyclin E [13,104]. The proposed mechanisms include at least two waves of transcriptional cascades [105]. Glucose initiates the first wave of transcription during which activated-ChREBP-α is recruited to a tissue-specific element in the ChREBP-β promoter to activate the expression of ChREBP-β, which then further enhances β-cell glucose and lipid metabolism [104,105]. Thereafter, the expression of cell-cycle genes are induced in the second wave, where ChREBP activates several cell cycle drivers, including nuclear receptor retinoic acid receptor-related orphan receptor-γ (ROR-γ), to promote β-cell proliferation [105]. Besides, more recently, nuclear factor erythroid 2–related factor 2 (NRF2) has been also revealed to be implicated in ChREBP-mediated β-cell proliferation [106]. ChREBP-α activation of NRF2 in β-cells enhances the antioxidant and mitochondrial biogenic programs, which accelerate β-cell proliferation through supplying sufficient energy [106]. Overall, these findings suggest that ChREBP is a central regulator in glucose-induced β-cell adaptive proliferation and highlight ChREBP as a potential therapeutic target for β-cell regeneration in patients with T2D. However, to date the relevant in vivo studies are still missing and should be warranted in the future.
6.2. Role of Pancreatic ChREBP in β-Cell Failure
Strikingly, ChREBP has also been revealed to be a key contributor to pancreatic β-cell dysfunction under conditions of chronic caloric excess. Firstly, the pancreatic ChREBP is activated in conditions susceptible to T2D. Specifically, the total protein levels of ChREBP, particularly the nucleus-localized ChREBP, are significantly increased in human diabetic pancreatic islets compared with non-diabetic controls [69,107]. Similarly, in the mice model of diabetes, including NOD mice and ob/ob mice (which represents type 1 and type 2 diabetes mouse model, respectively), the ChREBP-β mRNA level is dramatically induced compared with the lean mice or non-diabetic controls [108]. But interestingly, the ChREBP-α mRNA level is downregulated under the same conditions, which may act as a negative feedback mechanism to limit the excessive glucose-induced gene expression in β-cells [108].
Secondly, the activation of pancreatic ChREBP is more likely the cause for the development of T2D. As happened in prolonged hyperglycemia in diabetes, overexpression of ChREBP-CA via viral vectors for too much or too long, causes β-cell apoptosis in vitro [69]. Consistently, β-cell-specific overexpression of ChREBP-CA in mice is sufficient to induce diabetic phenotypes, including β-cell apoptosis and hyperglycemia [69]. At the molecular level, the persistently activated-ChREBP increases β-cell lipid synthesis, and meanwhile decreases mitochondrial fatty acid β-oxidation (through inducing the expression of glycolic and lipogenic genes [69], but inhibiting the expression of peroxisome proliferator-activated receptor α (PPARα) [109], respectively), resultantly leading to increase of intracellular lipid accumulation and ultimately causing lipotoxicity to β-cells. In parallel, the activated ChREBP also induces the expression of TXNIP [110], a critical factor involved in glucose-induced inflammation, oxidative stress as well as apoptosis in β-cells [111,112], thus causing glucotoxicity to β-cells. Therefore, the lipotoxicity and glucotoxicity (also termed as lipo-glucotoxicity) together ultimately lead to β-cell failure [69]. In support, inhibition or deletion of TXNIP in mouse β-cells protects against β-cell apoptosis and diabetes [111]. Meanwhile, the increase of TXNIP expression by selective loss of mechanistic target of rapamycin (mTOR) in mouse β-cells exacerbates the development of diabetes [107]. However, the above speculations are not verified in β-cell-specific ChREBP deficient mice, which should be tested in the future.
Collectively, pancreatic ChREBP plays dual roles in the pathogenesis of T2D. In response to the acute high glucose stimulation, pancreatic ChREBP protects against hyperglycemia through promoting β-cell proliferation. However, under conditions of chronic nutrient overload, the continuously activated ChREBP results in β-cell apoptosis, which exacerbates T2D development. Thereby, developing strategies to precise regulation of ChREBP expression and/or activity in pancreatic β-cells may represent a therapeutic approach in the treatment of T2D.
7. Conclusions and Perspectives
In summary, recent studies have revealed that glucose-sensing transcription factor MondoA/ChREBP plays a fundamental role in the control of systemic glucose metabolism and insulin sensitivity via a context- and tissue-dependent manner. Under normal physiological conditions, MondoA/ChREBP acts as a key player in each metabolic tissue to synergistically maintain the whole-body glucose homeostasis and energy metabolism. However, under conditions of chronic energy surplus, the dysregulated expression and/or activity of MondoA/ChREBP in metabolic tissues often results in a common consequence, namely IR, a prerequisite for the development of T2D. Therefore, targeting MondoA/ChREBP might offer new approaches to combat T2D and its complications in humans.
Based on the functional characteristic of MondoA/ChREBP, so far, at least two strategies can be adopted to develop drugs to prevent T2D. One aim is to develop MondoA/ChREBP-specific inhibitors. This strategy is supported by one recent study in which SBI-993, an inhibitor of MondoA and probably also ChREBP, was given to mice and alleviated HFD-induced mice IR [63]. However, the challenge of this strategy is that the crystal structures of the two proteins (or at least of the glucose sensing module (GSM)) are still unknown. Thus, elucidating the structural basis of the GSM will be required in the future. In addition, given the fact that the inhibition of MondoA/ChREBP in the muscle and pancreas has opposing effects with that in the WAT and the liver, thereby the effectiveness of this strategy might be weakened due to the functional disparities among the different metabolic tissues. Moreover, MondoA and ChREBP are widely expressed, and their functions have not been clearly examined in other tissues, e.g., the brain, kidney, and intestine. Therefore, the systemic evaluation of the consequences of MondoA/ChREBP inhibition will be necessary before the inhibitors are applied to clinical practices.
To avoid the shortcomings of the first strategy, the second strategy focuses on the tissue-specific MondoA/ChREBP targets or effectors which have potentials to serve as the drug leads. One such example is that the insulin-sensitizing fatty acids PAHSAs, which are synthesized by ChREBP-mediated DNL in ATs, have been recently shown with great therapeutic potentials to treating T2D [96]. Thus, this strategy seems to be more promising than the first one, but currently it is still hard to follow due to the lack of the detailed molecular atlas in each metabolic tissue. Hence, more mechanistic studies are still required in the future, especially including the ones in which new technologies, such as mass spectrometry and lipidomics [113], are adopted to deepen our understanding of the downstream events of MondoA/ChREBP.
Acknowledgments
We wish to thank Qionghua Gao for her helpful suggestions and comments to the manuscript. Also, we would like to thank two anonymous reviewers for critical review of the manuscript. We apologize for being unable to cite all original studies due to the space limitation.
Abbreviations
| ACSL1 | acyl-CoA synthetase long chain family member 1 |
| AMPK | AMP-activated protein kinase |
| ARRDC4 | arrestin domain–containing 4 |
| AT | adipose tissue |
| BAT | brown adipose tissue |
| CBP | CREB-binding protein |
| ChoRE | carbohydrate response element |
| ChREBP | carbohydrate response element-binding protein |
| ChREBP-CA | constitutively active form of ChREBP |
| CRM1 | chromosomal region maintenance 1 |
| CDK4/6 | cyclin-dependent kinase (CDK) 4/6 |
| DNL | de novo lipogenesis |
| ELOVL5 | elongation of very long chain fatty acids protein 5 |
| FASN | fatty acid synthase |
| FGF21 | fibroblast growth factor 21 |
| Fru-2,6-P2 | fructose-2,6-bisphosphate |
| FXR | farnesoid X receptor |
| G6P | glucose-6-phosphate |
| GCGR | glucagon receptor |
| GFPT1/2 | glutamine-fructose-6-phosphate transaminase 1/2 |
| GLP-1 | glucagon-like peptide-1 |
| GLUT | glucose transporter |
| GSM | glucose sensing module |
| HCF-1 | host cell factor-1 |
| HFD | high-fat diet |
| HNF-4α | hepatocyte nuclear factor-4α |
| HOMA-IR | homeostasis model assessment of insulin resistance |
| HSL | hormone-sensitive lipase |
| IR | insulin resistance |
| MCP-1 | monocyte chemoattractant protein-1 |
| Mlx | Max-like protein x |
| MLXIP | Mlx-interacting protein |
| MLXIPL | Mlx-interacting protein-like |
| mTOR | mechanistic target of rapamycin |
| MUFAs | monounsaturated fatty acids |
| NAFLD | nonalcoholic fatty liver disease |
| NASH | nonalcoholic steatohepatitis |
| NRF2 | nuclear factor erythroid 2-related factor 2 |
| OGT | O-linked N-acetylglucosamine transferase |
| PAHSAs | palmitic acid esters of hydroxy stearic acids |
| PGC-1β | peroxisome proliferator-activated receptor-γ coactivator-1β |
| PHF2 | PHD finger protein 2 |
| PKA | protein kinase A |
| PPARα | peroxisome proliferator-activated receptor-α |
| PPARγ | peroxisome proliferator-activated receptor-γ |
| PPP1R3A | phosphoprotein phosphatase 1 regulatory subunit 3A |
| RetSat | retinol saturase |
| RORγ | retinoic acid receptor-related orphan receptor-γ |
| SAT | subcutaneous adipose tissue |
| SCD | stearoyl-CoA desaturase |
| SFAs | saturated fatty acids |
| TG | triglycerides |
| TNFα | tumor necrosis factor-α |
| TXNIP | thioredoxin-interacting protein |
| VAT | visceral adipose tissue |
| WAT | white adipose tissue |
| Xu5P | xylulose 5-phosphate |
| T2D | type 2 diabetes |
| ZBTB20 | zinc finger and BTB domain-containing protein 20 |
Author Contributions
Z.S. wrote the manuscript and designed the figures. H.Y., L.Z., and F.Y. commented and edited the manuscript.
Funding
This work was supported by the Starting Research Fund for Advanced Talents of Guangxi University (A3340051005), Guangxi Science and Technology Base and Talents Project (AD18281085), and Training Project of High-Level Professional and Technical Talents of Guangxi University.
Conflicts of Interest
The authors have no conflicts of interest to declare. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Chatterjee S., Khunti K., Davies M.J. Type 2 diabetes. Lancet. 2017;389:2239–2251. doi: 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- 2.Zheng Y., Ley S.H., Hu F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018;14:88. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- 3.Wu Y., Ding Y., Tanaka Y., Zhang W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int. J. Med. Sci. 2014;11:1185. doi: 10.7150/ijms.10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tancredi M., Rosengren A., Svensson A.-M., Kosiborod M., Pivodic A., Gudbjörnsdottir S., Wedel H., Clements M., Dahlqvist S., Lind M. Excess mortality among persons with type 2 diabetes. N. Engl. J. Med. 2015;373:1720–1732. doi: 10.1056/NEJMoa1504347. [DOI] [PubMed] [Google Scholar]
- 5.Seuring T., Archangelidi O., Suhrcke M. The economic costs of type 2 diabetes: A global systematic review. Pharmacoeconomics. 2015;33:811–831. doi: 10.1007/s40273-015-0268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samuel V.T., Shulman G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016;126:12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Goblan A.S., Al-Alfi M.A., Khan M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes. Metab. Syndr. Obes. 2014;7:587. doi: 10.2147/DMSO.S67400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ley S.H., Hamdy O., Mohan V., Hu F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet. 2014;383:1999–2007. doi: 10.1016/S0140-6736(14)60613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connelly J., Kirk A., Masthoff J., MacRury S. The use of technology to promote physical activity in type 2 diabetes management: A systematic review. Diabet. Med. 2013;30:1420–1432. doi: 10.1111/dme.12289. [DOI] [PubMed] [Google Scholar]
- 10.Kerru N., Singh-Pillay A., Awolade P., Singh P. Current anti-diabetic agents and their molecular targets: A review. Eur. J. Med. Chem. 2018;152:436–488. doi: 10.1016/j.ejmech.2018.04.061. [DOI] [PubMed] [Google Scholar]
- 11.Ahn B., Wan S., Jaiswal N., Vega R.B., Ayer D.E., Titchenell P.M., Han X., Won K.J., Kelly D.P. MondoA drives muscle lipid accumulation and insulin resistance. JCI Insight. 2019;5:129119. doi: 10.1172/jci.insight.129119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vijayakumar A., Aryal P., Wen J., Syed I., Vazirani R.P., Moraes-Vieira P.M., Camporez J.P., Gallop M.R., Perry R.J., Peroni O.D. Absence of carbohydrate response element binding protein in adipocytes causes systemic insulin resistance and impairs glucose transport. Cell Rep. 2017;21:1021–1035. doi: 10.1016/j.celrep.2017.09.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metukuri M.R., Zhang P., Basantani M.K., Chin C., Stamateris R.E., Alonso L.C., Takane K.K., Gramignoli R., Strom S.C., O’Doherty R.M. ChREBP mediates glucose-stimulated pancreatic β-cell proliferation. Diabetes. 2012;61:2004–2015. doi: 10.2337/db11-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dentin R., Benhamed F., Hainault I., Fauveau V., Foufelle F., Dyck J.R., Girard J., Postic C. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes. 2006;55:2159–2170. doi: 10.2337/db06-0200. [DOI] [PubMed] [Google Scholar]
- 15.Towle H.C. Glucose as a regulator of eukaryotic gene transcription. Trends Endocrinol. Metab. 2005;16:489–494. doi: 10.1016/j.tem.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Havula E., Hietakangas V. Glucose sensing by ChREBP/MondoA–Mlx transcription factors. Semin. Cell Dev. Biol. 2012;23:640–647. doi: 10.1016/j.semcdb.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Richards P., Ourabah S., Montagne J., Burnol A.-F., Postic C., Guilmeau S. MondoA/ChREBP: The usual suspects of transcriptional glucose sensing; implication in pathophysiology. Metabolism. 2017;70:133–151. doi: 10.1016/j.metabol.2017.01.033. [DOI] [PubMed] [Google Scholar]
- 18.Havula E., Hietakangas V. Sugar sensing by ChREBP/Mondo-Mlx—New insight into downstream regulatory networks and integration of nutrient-derived signals. Curr. Opin. Cell Biol. 2018;51:89–96. doi: 10.1016/j.ceb.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Ghasemi A., Aghajani H., Fallah S., Assadi M., Seifi M. C771G (His241Gln) polymorphism of MLXIPL gene, TG levels and coronary artery disease: A case control study. Anatol. J. Cardiol. 2015;15:8. doi: 10.5152/akd.2014.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radovica I., Fridmanis D., Silamikelis I., Nikitina-Zake L., Klovins J. Association between CETP, MLXIPL, and TOMM40 polymorphisms and serum lipid levels in a latvian population. Meta. Gene. 2014;2:565–578. doi: 10.1016/j.mgene.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stagi S., Lapi E., Cecchi C., Chiarelli F., D’Avanzo M.G., Seminara S., de Martino M. Williams-beuren syndrome is a genetic disorder associated with impaired glucose tolerance and diabetes in childhood and adolescence: New insights from a longitudinal study. Horm. Res. Paediatr. 2014;82:38–43. doi: 10.1159/000360476. [DOI] [PubMed] [Google Scholar]
- 22.Yamashita H., Takenoshita M., Sakurai M., Bruick R.K., Henzel W.J., Shillinglaw W., Arnot D., Uyeda K. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc. Natl. Acad. Sci. USA. 2001;98:9116–9121. doi: 10.1073/pnas.161284298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Billin A.N., Eilers A.L., Coulter K.L., Logan J.S., Ayer D.E. MondoA, a novel basic helix-loop-helix-leucine zipper transcriptional activator that constitutes a positive branch of a max-like network. Mol. Cell. Biol. 2000;20:8845–8854. doi: 10.1128/MCB.20.23.8845-8854.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Billin A.N., Eilers A.L., Queva C., Ayer D.E. Mlx, a novel Max-like BHLHZip protein that interacts with the Max network of transcription factors. J. Biol. Chem. 1999;274:36344–36350. doi: 10.1074/jbc.274.51.36344. [DOI] [PubMed] [Google Scholar]
- 25.Meroni G., Cairo S., Merla G., Messali S., Brent R., Ballabio A., Reymond A. Mlx, a new Max-like BHLHZip family member: The center stage of a novel transcription factors regulatory pathway? Oncogene. 2000;19:3266. doi: 10.1038/sj.onc.1203634. [DOI] [PubMed] [Google Scholar]
- 26.Stoeckman A.K., Ma L., Towle H.C. Mlx is the functional heteromeric partner of the carbohydrate response element-binding protein in glucose regulation of lipogenic enzyme genes. J. Biol. Chem. 2004;279:15662–15669. doi: 10.1074/jbc.M311301200. [DOI] [PubMed] [Google Scholar]
- 27.Havula E., Teesalu M., Hyötyläinen T., Seppälä H., Hasygar K., Auvinen P., Orešič M., Sandmann T., Hietakangas V. Mondo/ChREBP-Mlx-regulated transcriptional network is essential for dietary sugar tolerance in drosophila. PLoS Genet. 2013;9:e1003438. doi: 10.1371/journal.pgen.1003438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma L., Robinson L.N., Towle H.C. ChREBP·Mlx is the principal mediator of glucose-induced gene expression in the liver. J. Biol. Chem. 2006;281:28721–28730. doi: 10.1074/jbc.M601576200. [DOI] [PubMed] [Google Scholar]
- 29.Stoltzman C.A., Peterson C.W., Breen K.T., Muoio D.M., Billin A.N., Ayer D.E. Glucose sensing by MondoA: Mlx complexes: A role for hexokinases and direct regulation of thioredoxin-interacting protein expression. Proc. Natl. Acad. Sci. USA. 2008;105:6912–6917. doi: 10.1073/pnas.0712199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li M.V., Chen W., Harmancey R.N., Nuotio-Antar A.M., Imamura M., Saha P., Taegtmeyer H., Chan L. Glucose-6-phosphate mediates activation of the carbohydrate responsive binding protein (ChREBP) Biochem. Biophys. Res. Commun. 2010;395:395–400. doi: 10.1016/j.bbrc.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dentin R., Tomas-Cobos L., Foufelle F., Leopold J., Girard J., Postic C., Ferré P. Glucose 6-phosphate, rather than xylulose 5-phosphate, is required for the activation of ChREBP in response to glucose in the liver. J. Hepatol. 2012;56:199–209. doi: 10.1016/j.jhep.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Arden C., Tudhope S.J., Petrie J.L., Al-Oanzi Z.H., Cullen K.S., Lange A.J., Towle H.C., Agius L. Fructose 2, 6-bisphosphate is essential for glucose-regulated gene transcription of glucose-6-phosphatase and other ChREBP target genes in hepatocytes. Biochem. J. 2012;443:111–123. doi: 10.1042/BJ20111280. [DOI] [PubMed] [Google Scholar]
- 33.Petrie J.L., Al-Oanzi Z.H., Arden C., Tudhope S.J., Mann J., Kieswich J., Yaqoob M.M., Towle H.C., Agius L. Glucose induces protein targeting to glycogen in hepatocytes by fructose 2, 6-bisphosphate-mediated recruitment of MondoA to the promoter. Mol. Cell. Biol. 2013;33:725–738. doi: 10.1128/MCB.01576-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iizuka K., Takeda J., Horikawa Y. Glucose induces FGF21 mRNA expression through ChREBP activation in rat hepatocytes. FEBS Lett. 2009;583:2882–2886. doi: 10.1016/j.febslet.2009.07.053. [DOI] [PubMed] [Google Scholar]
- 35.Cha-Molstad H., Saxena G., Chen J., Shalev A. Glucose-stimulated expression of Txnip is mediated by carbohydrate response element-binding protein, p300, and histone H4 acetylation in pancreatic beta cells. J. Biol. Chem. 2009;284:16898–16905. doi: 10.1074/jbc.M109.010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burke S.J., Collier J.J., Scott D.K. Camp opposes the glucose-mediated induction of the L-PK gene by preventing the recruitment of a complex containing ChREBP, HNF4alpha, and CBP. FASEB J. 2009;23:2855–2865. doi: 10.1096/fj.08-126631. [DOI] [PubMed] [Google Scholar]
- 37.Kawaguchi T., Takenoshita M., Kabashima T., Uyeda K. Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/dephosphorylation of the carbohydrate response element binding protein. Proc. Natl. Acad. Sci. USA. 2001;98:13710–13715. doi: 10.1073/pnas.231370798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato S., Jung H., Nakagawa T., Pawlosky R., Takeshima T., Lee W.-R., Sakiyama H., Laxman S., Wynn R.M., Tu B.P. Metabolite regulation of nuclear localization of carbohydrate-response element-binding protein (ChREBP) role of AMP as an allosteric inhibitor. J. Biol. Chem. 2016;291:10515–10527. doi: 10.1074/jbc.M115.708982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawaguchi T., Osatomi K., Yamashita H., Kabashima T., Uyeda K. Mechanism for fatty acid “sparing” effect on glucose-induced transcription regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. J. Biol. Chem. 2002;277:3829–3835. doi: 10.1074/jbc.M107895200. [DOI] [PubMed] [Google Scholar]
- 40.Guinez C., Filhoulaud G., Rayah-Benhamed F., Marmier S., Dubuquoy C., Dentin R., Moldes M., Burnol A.-F., Yang X., Lefebvre T. O-GlcNAcylation increases ChREBP protein content and transcriptional activity in the liver. Diabetes. 2011;60:1399–1413. doi: 10.2337/db10-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merla G., Howald C., Antonarakis S.E., Reymond A. The subcellular localization of the ChoRE-binding protein, encoded by the Williams–Beuren syndrome critical region gene 14, is regulated by 14-3-3. Hum. Mol. Genet. 2004;13:1505–1514. doi: 10.1093/hmg/ddh163. [DOI] [PubMed] [Google Scholar]
- 42.Sakiyama H., Wynn R.M., Lee W.-R., Fukasawa M., Mizuguchi H., Gardner K.H., Repa J.J., Uyeda K. Regulation of nuclear import/export of carbohydrate response element-binding protein (ChREBP) interaction of an alpha-helix of ChREBP with the 14-3-3 proteins and regulation by phosphorylation. J. Biol. Chem. 2008;283:24899–24908. doi: 10.1074/jbc.M804308200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eilers A.L., Sundwall E., Lin M., Sullivan A.A., Ayer D.E. A novel heterodimerization domain, CRM1, and 14-3-3 control subcellular localization of the MondoA-Mlx heterocomplex. Mol. Cell. Biol. 2002;22:8514–8526. doi: 10.1128/MCB.22.24.8514-8526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ge Q., Nakagawa T., Wynn R.M., Chook Y.M., Miller B.C., Uyeda K. Importin-alpha protein binding to a nuclear localization signal of carbohydrate response element-binding protein (ChREBP) J. Biol. Chem. 2011;286:28119–28127. doi: 10.1074/jbc.M111.237016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meng J., Feng M., Dong W., Zhu Y., Li Y., Zhang P., Wu L., Li M., Lu Y., Chen H. Identification of HNF-4α as a key transcription factor to promote ChREBP expression in response to glucose. Sci. Rep. 2016;6:23944. doi: 10.1038/srep23944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caron S., Samanez C.H., Dehondt H., Ploton M., Briand O., Lien F., Dorchies E., Dumont J., Postic C., Cariou B. Farnesoid X receptor inhibits the transcriptional activity of carbohydrate response element binding protein in human hepatocytes. Mol. Cell. Biol. 2013;33:2202–2211. doi: 10.1128/MCB.01004-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chambers K.T., Chen Z., Lai L., Leone T.C., Towle H.C., Kralli A., Crawford P.A., Finck B.N. PGC-1β and ChREBP partner to cooperatively regulate hepatic lipogenesis in a glucose concentration-dependent manner. Mol. Metab. 2013;2:194–204. doi: 10.1016/j.molmet.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bricambert J., Miranda J., Benhamed F., Girard J., Postic C., Dentin R. Salt-inducible kinase 2 links transcriptional coactivator p300 phosphorylation to the prevention of ChREBP-dependent hepatic steatosis in mice. J. Clin. Investig. 2010;120:4316–4331. doi: 10.1172/JCI41624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bricambert J., Alves-Guerra M.-C., Esteves P., Prip-Buus C., Bertrand-Michel J., Guillou H., Chang C.J., Vander Wal M.N., Canonne-Hergaux F., Mathurin P. The histone demethylase Phf2 acts as a molecular checkpoint to prevent NAFLD progression during obesity. Nat. Commun. 2018;9:2092. doi: 10.1038/s41467-018-04361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lane E.A., Choi D.W., Garcia-Haro L., Levine Z.G., Tedoldi M., Walker S., Danial N.N. HCF-1 regulates de novo lipogenesis through a nutrient-sensitive complex with ChREBP. Mol. Cell. 2019;75:357–371. doi: 10.1016/j.molcel.2019.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanse E.A., Mashek D.G., Becker J.R., Solmonson A.D., Mullany L.K., Mashek M.T., Towle H.C., Chau A.T., Albrecht J.H. Cyclin D1 inhibits hepatic lipogenesis via repression of carbohydrate response element binding protein and hepatocyte nuclear factor 4α. Cell Cycle. 2012;11:2681–2690. doi: 10.4161/cc.21019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morigny P., Houssier M., Mairal A., Ghilain C., Mouisel E., Benhamed F., Masri B., Recazens E., Denechaud P.-D., Tavernier G. Interaction between hormone-sensitive lipase and ChREBP in fat cells controls insulin sensitivity. Nat. Metab. 2019;1:133. doi: 10.1038/s42255-018-0007-6. [DOI] [PubMed] [Google Scholar]
- 53.Postic C., Ortega-Prieto P. Carbohydrate sensing through the transcription factor ChREBP. Front. Genet. 2019;10:472. doi: 10.3389/fgene.2019.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sans C.L., Satterwhite D.J., Stoltzman C.A., Breen K.T., Ayer D.E. MondoA-Mlx heterodimers are candidate sensors of cellular energy status: Mitochondrial localization and direct regulation of glycolysis. Mol. Cell. Biol. 2006;26:4863–4871. doi: 10.1128/MCB.00657-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iizuka K., Bruick R.K., Liang G., Horton J.D., Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc. Natl. Acad. Sci. USA. 2004;101:7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanchez-Gurmaches J., Tang Y., Jespersen N.Z., Wallace M., Calejman C.M., Gujja S., Li H., Edwards Y.J., Wolfrum C., Metallo C.M. Brown fat AKT2 is a cold-induced kinase that stimulates ChREBP-mediated de novo lipogenesis to optimize fuel storage and thermogenesis. Cell Metab. 2018;27:195–209. doi: 10.1016/j.cmet.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma L., Tsatsos N.G., Towle H.C. Direct role of ChREBP.Mlx in regulating hepatic glucose-responsive genes. J. Biol. Chem. 2005;280:12019–12027. doi: 10.1074/jbc.M413063200. [DOI] [PubMed] [Google Scholar]
- 58.Tsatsos N.G., Davies M.N., O’Callaghan B.L., Towle H.C. Identification and function of phosphorylation in the glucose-regulated transcription factor ChREBP. Biochem. J. 2008;411:261–270. doi: 10.1042/BJ20071156. [DOI] [PubMed] [Google Scholar]
- 59.Uyeda K., Repa J.J. Carbohydrate response element binding protein, ChREBP, a transcription factor coupling hepatic glucose utilization and lipid synthesis. Cell Metab. 2006;4:107–110. doi: 10.1016/j.cmet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 60.Herman M.A., Peroni O.D., Villoria J., Schon M.R., Abumrad N.A., Bluher M., Klein S., Kahn B.B. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature. 2012;484:333–338. doi: 10.1038/nature10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Amoasii L., Holland W., Sanchez-Ortiz E., Baskin K.K., Pearson M., Burgess S.C., Nelson B.R., Bassel-Duby R., Olson E.N. A MED13-dependent skeletal muscle gene program controls systemic glucose homeostasis and hepatic metabolism. Genes Dev. 2016;30:434–446. doi: 10.1101/gad.273128.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aune D., Norat T., Leitzmann M., Tonstad S., Vatten L.J. Physical activity and the risk of type 2 diabetes: A systematic review and dose–response meta-analysis. Eur. J. Epidemiol. 2015;30:529–542. doi: 10.1007/s10654-015-0056-z. [DOI] [PubMed] [Google Scholar]
- 63.Ahn B., Soundarapandian M.M., Sessions H., Peddibhotla S., Roth G.P., Li J.L., Sugarman E., Koo A., Malany S., Wang M., et al. MondoA coordinately regulates skeletal myocyte lipid homeostasis and insulin signaling. J. Clin. Investig. 2016;126:3567–3579. doi: 10.1172/JCI87382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Imamura M., Chang B.H.-J., Kohjima M., Li M., Hwang B., Taegtmeyer H., Harris R.A., Chan L. MondoA deficiency enhances sprint performance in mice. Biochem. J. 2014;464:35–48. doi: 10.1042/BJ20140530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iizuka K., Miller B., Uyeda K. Deficiency of carbohydrate-activated transcription factor ChREBP prevents obesity and improves plasma glucose control in leptin-deficient (ob/ob) mice. Am. J. Physiol. Endocrinol. Metab. 2006;291:E358–E364. doi: 10.1152/ajpendo.00027.2006. [DOI] [PubMed] [Google Scholar]
- 66.Jois T., Chen W., Howard V., Harvey R., Youngs K., Thalmann C., Saha P., Chan L., Cowley M.A., Sleeman M.W. Deletion of hepatic carbohydrate response element binding protein (ChREBP) impairs glucose homeostasis and hepatic insulin sensitivity in mice. Mol. Metab. 2017;6:1381–1394. doi: 10.1016/j.molmet.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benhamed F., Denechaud P.-D., Lemoine M., Robichon C., Moldes M., Bertrand-Michel J., Ratziu V., Serfaty L., Housset C., Capeau J. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J. Clin. Investig. 2012;122:2176–2194. doi: 10.1172/JCI41636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nuotio-Antar A.M., Poungvarin N., Li M., Schupp M., Mohammad M., Gerard S., Zou F., Chan L. FABP4-Cre mediated expression of constitutively active ChREBP protects against obesity, fatty liver, and insulin resistance. Endocrinology. 2015;156:4020–4032. doi: 10.1210/en.2015-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Poungvarin N., Lee J., Yechoor V., Li M., Assavapokee T., Suksaranjit P., Thepsongwajja J., Saha P., Oka K., Chan L. Carbohydrate response element-binding protein (ChREBP) plays a pivotal role in beta cell glucotoxicity. Diabetologia. 2012;55:1783–1796. doi: 10.1007/s00125-012-2506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hunt L.C., Xu B., Finkelstein D., Fan Y., Carroll P.A., Cheng P.F., Eisenman R.N., Demontis F. The glucose-sensing transcription factor MLX promotes myogenesis via myokine signaling. Genes Dev. 2015;29:2475–2489. doi: 10.1101/gad.267419.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rui L. Energy metabolism in the liver. Compr. Physiol. 2011;4:177–197. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nordlie R.C., Foster J.D., Lange A.J. Regulation of glucose production by the liver. Annu. Rev. Nutr. 1999;19:379–406. doi: 10.1146/annurev.nutr.19.1.379. [DOI] [PubMed] [Google Scholar]
- 73.Saponaro C., Gaggini M., Carli F., Gastaldelli A. The subtle balance between lipolysis and lipogenesis: A critical point in metabolic homeostasis. Nutrients. 2015;7:9453–9474. doi: 10.3390/nu7115475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Perry R.J., Samuel V.T., Petersen K.F., Shulman G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Birkenfeld A.L., Shulman G.I. Nonalcoholic fatty liver disease, hepatic insulin resistance, and type 2 diabetes. Hepatology. 2014;59:713–723. doi: 10.1002/hep.26672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abdul-Wahed A., Guilmeau S., Postic C. Sweet sixteenth for ChREBP: Established roles and future goals. Cell Metab. 2017;26:324–341. doi: 10.1016/j.cmet.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 77.Iizuka K. The role of carbohydrate response element–binding protein in the development of liver diseases. In: Watson R.R., Preedy V.R., editors. Dietary Interventions in Liver Disease. Elsevier; Amsterdam, The Netherlands: 2019. pp. 263–274. [Google Scholar]
- 78.Stamatikos A.D., Da Silva R.P., Lewis J.T., Douglas D.N., Kneteman N.M., Jacobs R.L., Paton C.M. Tissue specific effects of dietary carbohydrates and obesity on ChREBPα and ChREBPβ expression. Lipids. 2016;51:95–104. doi: 10.1007/s11745-015-4090-0. [DOI] [PubMed] [Google Scholar]
- 79.del Pozo C.H., Vesperinas-García G., Rubio M.-Á., Corripio-Sánchez R., Torres-García A.J., Obregon M.-J., Calvo R.M. ChREBP expression in the liver, adipose tissue and differentiated preadipocytes in human obesity. BBA Mol. Cell Biol. 2011;1811:1194–1200. doi: 10.1016/j.bbalip.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 80.Eissing L., Scherer T., Tödter K., Knippschild U., Greve J.W., Buurman W.A., Pinnschmidt H.O., Rensen S.S., Wolf A.M., Bartelt A. De novo lipogenesis in human fat and liver is linked to ChREBP-β and metabolic health. Nat. Commun. 2013;4:1528. doi: 10.1038/ncomms2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Iizuka K., Takeda J., Horikawa Y. Hepatic overexpression of dominant negative Mlx improves metabolic profile in diabetes-prone C57BL/6J mice. Biochem. Biophys. Res. Commun. 2009;379:499–504. doi: 10.1016/j.bbrc.2008.12.100. [DOI] [PubMed] [Google Scholar]
- 82.Heidenreich S., Witte N., Weber P., Goehring I., Tolkachov A., von Loeffelholz C., Döcke S., Bauer M., Stockmann M., Pfeiffer A.F. Retinol saturase coordinates liver metabolism by regulating ChREBP activity. Nat. Commun. 2017;8:384. doi: 10.1038/s41467-017-00430-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu G., Zhou L., Zhang H., Chen R., Zhang Y., Li L., Lu J.Y., Jiang H., Liu D., Qi S., et al. Regulation of hepatic lipogenesis by the zinc finger protein Zbtb20. Nat. Commun. 2017;8:14824. doi: 10.1038/ncomms14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim M., Doridot L., Cunniff J.C., Parker T.S., Levine D.M., Hellerstein M.K., Hudgins L.C., Maratos-Flier E., Herman M.A. A critical role for ChREBP-mediated FGF21 secretion in hepatic fructose metabolism. Mol. Metab. 2017;6:14–21. doi: 10.1016/j.molmet.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iroz A., Montagner A., Benhamed F., Levavasseur F., Polizzi A., Anthony E., Régnier M., Fouché E., Lukowicz C., Cauzac M. A specific ChREBP and PPARα cross-talk is required for the glucose-mediated FGF21 response. Cell Rep. 2017;21:403–416. doi: 10.1016/j.celrep.2017.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tezze C., Romanello V., Sandri M. FGF21 as modulator of metabolism in health and disease. Front. Physiol. 2019;10:419. doi: 10.3389/fphys.2019.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fisher F.M., Maratos-Flier E. Understanding the physiology of FGF21. Annu. Rev. Physiol. 2016;78:223–241. doi: 10.1146/annurev-physiol-021115-105339. [DOI] [PubMed] [Google Scholar]
- 88.Choe S.S., Huh J.Y., Hwang I.J., Kim J.I., Kim J.B. Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Front. Endocrinol. 2016;7:30. doi: 10.3389/fendo.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Booth A., Magnuson A., Fouts J., Foster M.T. Adipose tissue: An endocrine organ playing a role in metabolic regulation. Horm. Mol. Biol. Clin. Investig. 2016;26:25–42. doi: 10.1515/hmbci-2015-0073. [DOI] [PubMed] [Google Scholar]
- 90.Townsend K.L., Tseng Y.H. Brown fat fuel utilization and thermogenesis. Trends Endocrinol. Metab. 2014;25:168–177. doi: 10.1016/j.tem.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ameer F., Scandiuzzi L., Hasnain S., Kalbacher H., Zaidi N. De novo lipogenesis in health and disease. Metabolism. 2014;63:895–902. doi: 10.1016/j.metabol.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 92.Smith U., Kahn B.B. Adipose tissue regulates insulin sensitivity: Role of adipogenesis, de novo lipogenesis and novel lipids. J. Intern. Med. 2016;280:465–475. doi: 10.1111/joim.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Song Z., Xiaoli A., Yang F. Regulation and metabolic significance of de novo lipogenesis in adipose tissues. Nutrients. 2018;10:1383. doi: 10.3390/nu10101383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guilherme A., Pedersen D.J., Henchey E., Henriques F.S., Danai L.V., Shen Y., Yenilmez B., Jung D., Kim J.K., Lodhi I.J. Adipocyte lipid synthesis coupled to neuronal control of thermogenic programming. Mol. Metab. 2017;6:781–796. doi: 10.1016/j.molmet.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kursawe R., Caprio S., Giannini C., Narayan D., Lin A., D’Adamo E., Shaw M., Pierpont B., Cushman S.W., Shulman G.I. Decreased transcription of ChREBP-α/β isoforms in abdominal subcutaneous adipose tissue of obese adolescents with prediabetes or early type 2 diabetes: Associations with insulin resistance and hyperglycemia. Diabetes. 2013;62:837–844. doi: 10.2337/db12-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yore M.M., Syed I., Moraes-Vieira P.M., Zhang T., Herman M.A., Homan E.A., Patel R.T., Lee J., Chen S., Peroni O.D. Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell. 2014;159:318–332. doi: 10.1016/j.cell.2014.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Witte N., Muenzner M., Rietscher J., Knauer M., Heidenreich S., Nuotio-Antar A.M., Graef F.A., Fedders R., Tolkachov A., Goehring I. The glucose sensor ChREBP links de novo lipogenesis to PPARγ activity and adipocyte differentiation. Endocrinology. 2015;156:4008–4019. doi: 10.1210/EN.2015-1209. [DOI] [PubMed] [Google Scholar]
- 98.Katz L.S., Xu S., Ge K., Scott D.K., Gershengorn M.C. T3 and glucose coordinately stimulate ChREBP-mediated Ucp1 expression in brown adipocytes from male mice. Endocrinology. 2017;159:557–569. doi: 10.1210/en.2017-00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gustafson B., Hedjazifar S., Gogg S., Hammarstedt A., Smith U. Insulin resistance and impaired adipogenesis. Trends Endocrinol. Metab. 2015;26:193–200. doi: 10.1016/j.tem.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 100.Kaisanlahti A., Glumoff T. Browning of white fat: Agents and implications for beige adipose tissue to type 2 diabetes. J. Physiol. Biochem. 2019;75:1–10. doi: 10.1007/s13105-018-0658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xia B., Cai G.H., Yang H., Wang S.P., Mitchell G.A., Wu J.W. Adipose tissue deficiency of hormone-sensitive lipase causes fatty liver in mice. PLoS Genet. 2017;13:e1007110. doi: 10.1371/journal.pgen.1007110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Roder P.V., Wu B.B., Liu Y.X., Han W.P. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 2016;48:e219. doi: 10.1038/emm.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Honka H., Hannukainen J.C., Tarkia M., Karlsson H., Saunavaara V., Salminen P., Soinio M., Mikkola K., Kudomi N., Oikonen V. Pancreatic metabolism, blood flow, and β-cell function in obese humans. J. Clin. Endocrinol. Metab. 2014;99:E981–E990. doi: 10.1210/jc.2013-4369. [DOI] [PubMed] [Google Scholar]
- 104.Zhang P., Kumar A., Katz L.S., Li L., Paulynice M., Herman M.A., Scott D.K. Induction of the ChREBPβ isoform is essential for glucose-stimulated β-cell proliferation. Diabetes. 2015;64:4158–4170. doi: 10.2337/db15-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schmidt S.F., Madsen J.G.S., Frafjord K.Ø., la Cour Poulsen L., Salö S., Boergesen M., Loft A., Larsen B.D., Madsen M.S., Holst J.J. Integrative genomics outlines a biphasic glucose response and a ChREBP-RORγ axis regulating proliferation in β cells. Cell Rep. 2016;16:2359–2372. doi: 10.1016/j.celrep.2016.07.063. [DOI] [PubMed] [Google Scholar]
- 106.Kumar A., Katz L.S., Schulz A.M., Kim M., Honig L.B., Li L., Davenport B., Homann D., Garcia-Ocaña A., Herman M.A. Activation of Nrf2 is required for normal and ChREBPα-augmented glucose-stimulated β-cell proliferation. Diabetes. 2018;67:1561–1575. doi: 10.2337/db17-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chau G.C., Im D.U., Kang T.M., Bae J.M., Kim W., Pyo S., Moon E.-Y., Um S.H. mTOR controls ChREBP transcriptional activity and pancreatic β cell survival under diabetic stress. J. Cell Biol. 2017;216:2091–2105. doi: 10.1083/jcb.201701085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jing G., Chen J., Xu G., Shalev A. Islet ChREBP-β is increased in diabetes and controls ChREBP-α and glucose-induced gene expression via a negative feedback loop. Mol. Metab. 2016;5:1208–1215. doi: 10.1016/j.molmet.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boergesen M., la Cour Poulsen L., Schmidt S.F., Frigerio F., Maechler P., Mandrup S. ChREBP mediates glucose repression of peroxisome proliferator-activated receptor α expression in pancreatic β-cells. J. Biol. Chem. 2011;286:13214–13225. doi: 10.1074/jbc.M110.215467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Minn A.H., Hafele C., Shalev A. Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces β-cell apoptosis. Endocrinology. 2005;146:2397–2405. doi: 10.1210/en.2004-1378. [DOI] [PubMed] [Google Scholar]
- 111.Chen J., Saxena G., Mungrue I.N., Lusis A.J., Shalev A. Thioredoxin-interacting protein: A critical link between glucose toxicity and β-cell apoptosis. Diabetes. 2008;57:938–944. doi: 10.2337/db07-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou R., Tardivel A., Thorens B., Choi I., Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010;11:136. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 113.Saurina J., Sentellas S. Liquid chromatography coupled to mass spectrometry for metabolite profiling in the field of drug discovery. Expert Opin. Drug Dis. 2019;14:469–483. doi: 10.1080/17460441.2019.1582638. [DOI] [PubMed] [Google Scholar]