Abstract
Mechanical ventilation (MV) is the primary risk factor for the development of ventilator-associated pneumonia (VAP). Besides inducing a pro-inflammatory T-helper (Th)-1 cytokine response, MV also induces an anti-inflammatory Th2 cytokine response, marked by increased IL-4 secretion and reduced bacterial phagocytic capacity of rodent lung macrophages. Since IL-4 is known to downregulate both Th1 and Th17 cytokines, the latter is important in mediating mucosal immunity and combating bacterial and fungal growth, we studied and showed here in a rat model of MV that Th17 cytokines (IL-17A, IL-17F, and IL-22) were significantly upregulated in the lung as a response to different MV strategies currently utilized in clinic. To study whether the increased IL-4 levels are associated with downregulation of the anti-bacterial Th17 cytokines, we subsequently challenged mechanically ventilated rats with an intratracheal inoculation of Pseudomonas aeruginosa (VAP model) and showed a dramatic downregulation of IL-17A, IL-17F, and IL-22, compared to animals receiving the same bacterial burden without MV. For the studied Th1 cytokines (IFNγ, TNFα, IL-6, and IL-1β), only IFNγ showed a significant decrease as a consequence of bacterial infection in mechanically ventilated rats. We further studied IL-17A, the most studied IL-17 family member, in intensive care unit (ICU) pneumonia patients and showed that VAP patients had significantly lower levels of IL-17A in the endotracheal aspirate compared to patients entering ICU with pre-existing pneumonia. These translational data, obtained both in animal models and in humans, suggest that a deficient anti-bacterial Th17 response in the lung during MV is associated with VAP development.
Keywords: mechanical ventilation, ventilator-associated pneumonia, VAP, Pseudomonas aeruginosa, IL-17, IL-17A, IL-22, IFNγ
1. Introduction
Patients receiving mechanical ventilation (MV) for more than 48 h have an approximately 10-fold higher likelihood of developing pneumonia compared to non-ventilated patients in intensive care units (ICUs) [1,2]. MV causes tidal volume (VT)-dependent ventilator-induced lung inflammation. This inflammation is characterized by both activation of proinflammatory TH1 cytokines such as tumor necrosis factor alpha (TNF-α), interleukin 1β (IL-1β), interferon gamma (IFN-γ), and interleukin 6 (IL-6), as well as lung infiltration of innate cells such as neutrophils and macrophages [3,4,5,6,7,8]. We recently showed that even using a so-called “lung-protective” ventilation strategy in rats induces these TH1 cytokines, and at the same time it also induces an anti-inflammatory TH2 type response marked by elevated IL-4 secretion [7]. Interestingly, while infiltration of innate cells, such as neutrophils and macrophages, was also noted, macrophages isolated from bronchoalveolar lavage (BAL) were specifically activated towards anti-inflammatory M2 macrophages, displaying reduced bacterial phagocytic capacity [7]. Mechanically ventilated rats, when challenged with bacterial lung inoculation to mimic a ventilator-associated pneumonia (VAP) model, had a higher lung bacterial burden compared to non-mechanically ventilated animals receiving the same bacterial dose [7,9]. Interestingly, blocking of the IL-4 signaling normalized the lung bacterial burden in the VAP-induced animals [7]. Thus, while it appears that TH2 cytokines could be important in transient immunosuppression in ventilated lungs favoring bacterial and fungal growth, the precise downstream mechanisms remain unknown.
The propagation and regulation of an immune response is driven by a network of regulatory T and effector cells as well as their innate immune counterparts. The interplay between different effector T cells determines the direction of the immune response towards inflammation or its resolution. For instance, TH2 cells and its main cytokine IL-4 are crucial in maintaining the balance between proinflammatory and anti-inflammatory pathways [10]. Similar to the proinflammatory TH1 cytokines, TH17 cytokines such as IL-17A, IL-17F, and IL-22 have a major impact on epithelial cells in various tissues and are important in mediating mucosal immunity, particularly against Gram-negative bacteria and fungal infections, by inducing neutrophil chemotaxis [11,12,13,14].
However, published data on IL-17/IL-22 in MV or VAP are conflicting. One study on ventilated patients showed that TH17 cells were increased both in the peripheral blood and in the lung in VAP patients compared to ventilated patients who did not develop pneumonia [15]. In contrast, another study on ventilated patients showed a reduced number of TH17 cells in lung lavage fluid of VAP patients compared to those who did not develop VAP, suggesting that TH17 cells were protective against VAP [16]. However, IL-17 levels in BAL fluid showed an opposite trend, where IL-17 levels were higher in VAP than non-VAP patients, though it should be noted that IL-17 levels in most of the patients were below the limit of detection of the assay [16]. Similarly, although IL-17 in VAP animal models has not been studied, mice ventilated for one hour with VT 7 mL/kg were reported to have increased IL-22 but decreased IL-17 levels [17].
Here, we aimed to clarify the expression pattern of IL-17-related cytokines as a response to MV and VAP in both rodent models and patients and further questioned whether bacterial-induced stimulation of TH1 and TH17 cytokines could be dampened due to MV.
2. Results
2.1. Mechanical Ventilation Induces VT Dose-Dependent TH17 Cytokine Expression in Lungs of Rats
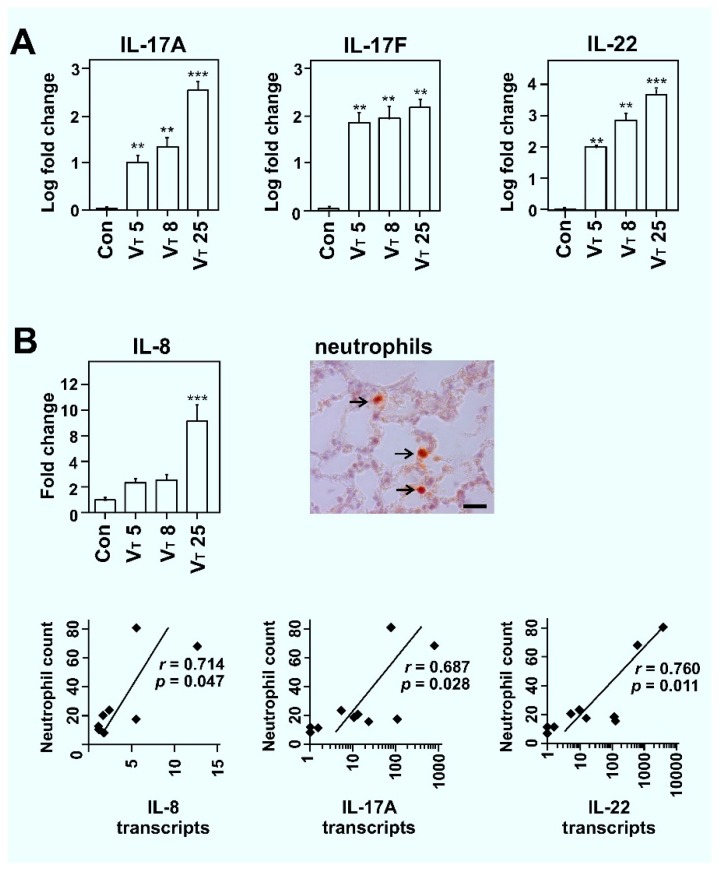
To study whether TH17 cytokine expression is induced by MV, we ventilated rats for 2 h using three different lung-ventilation strategies: (i) A lung protective ventilation setting (avoiding injury by stretching) of VT 5 mL/kg commonly employed in both healthy patients and those suffering from severe forms of lung injury to prevent further damage [18]; (ii) a VT 8 mL/kg ventilation protocol, the highest level considered more or less “not damaging” to lungs by overstretching [6,19]; and (iii) a VT 25 mL/kg ventilation protocol, which causes lung injury and neutrophil influx by itself, and therefore commonly employed to establish acute severe lung injury-like features in rodents [20]. Studying lung transcript levels for TH17 associated cytokines, we observed that IL-17A, IL-17F, and IL-22 transcripts were modestly but significantly elevated in all studied ventilation groups in a volume-dependent manner (p < 0.01, for all; Figure 1A).
Figure 1.
Effect of mechanical ventilation on transcript levels of IL-17A, IL-17F, IL-22, and IL-8 and correlations with tissue neutrophils. (A) IL-17A, IL-17F, and IL-22 lung transcripts analyses of spontaneously breathing control (Con), and in tidal volume (VT) 5, VT 8, and VT 25 mL/kg ventilation groups of Wistar rats analyzed after 2 h of mechanical ventilation (MV). Data is presented as averages ± standard error of mean (SEM); *p < 0.05, **p < 0.01; and ***p < 0.001; asterisk above the error bars denotes significance against spontaneously breathing control group; n = 6 animals per group. (B) A similar IL-8 lung transcript analysis and Spearman correlation between lung neutrophil counts (neutrophils were detected with an anti-neutrophil antibody; scale bar, 20 μm; arrow indicates neutrophils) and transcript levels of IL-8, IL-17A, and IL-22 (n = 10 animals per group).
We further studied the transcript levels of IL-8, a potent chemoattractant of neutrophil in lung injury and an essential component of the innate immune system acting as a first line of defense against invading microorganisms and fungi [21,22]. IL-17 can activate the neutrophil/TH17 cell-dependent immune response through different cytokines and chemokines that includes IL-8 [14]. Recent data also suggest that neutrophil production by IL-17 enhances host antibacterial ability [23], and both IL-17 and neutrophils unexpectedly contribute to type 2 immunity [24]. We show here that IL-8 transcripts were non-significantly elevated for VT 5 and VT 8 groups and significantly (approximately nine-fold; p < 0.001) elevated for the VT 25 group (Figure 1B). Furthermore, we studied whether levels of IL-17-related cytokines, or neutrophil chemotactic cytokine IL-8, correlated with neutrophil infiltration in ventilated lungs. For this, a subset of samples was stained using a neutrophil-specific antibody and their numbers estimated as described [7,25]. We previously showed that mechanical ventilation in rats causes a VT-dependent increase in interstitial neutrophils (p < 0.01) [7]. We further show here that lung neutrophils in ventilated lungs correlated well with transcript levels of IL-8 (r = 0.714, p = 0.047), IL-17A (r = 0.687, p = 0.028), and IL-22 (r = 0.760, p = 0.011). These data suggest that increased expression of TH17 cytokines correlated with neutrophil infiltration in ventilated lungs through, or together with, IL-8 (Figure 1B).
2.2. Dampened IL-17 Response after Infection in Mechanically Ventilated Rats
We previously showed that MV induces besides a TH1 response [5,6,7], also a significant TH2 cytokine response [7], which is known to suppress TH1 and TH17 cytokine production [10]. We questioned whether this impacts TH17 and TH1 cytokine expression induced by bacterial infection. For this, we ventilated rats with VT 8 mL/kg for 2 h followed by an intra-tracheal challenge with Pseudomonas aeruginosa (MV+PA group) to mimic VAP. The levels of TH17 and TH1 cytokines of VAP animals were compared with animals that received the same bacterial dose without ventilation (PA group). P. aeruginosa was used as an infectious organism as it is an important cause of hospital-acquired infections including VAP and it is known to cause a strong inflammatory response in lung epithelial cells [26,27,28].
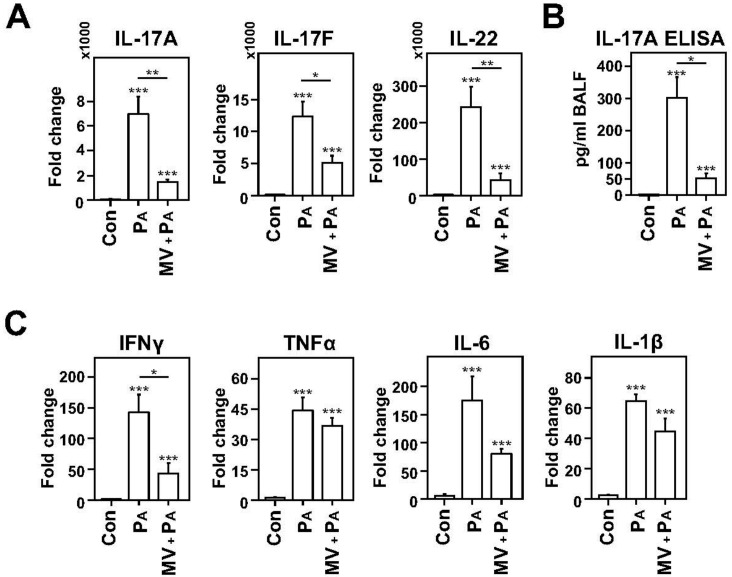
Bacterial challenge with P. aeruginosa of lungs in non-ventilated rats induced an increase in expression of TH17 cytokines (IL-17A, IL-17F, and IL-22; p < 0.001 for all; Figure 2A). However, the same bacterial challenge in mechanically ventilated rats at VT 8 mL/kg (MV+PA group) caused significantly lower transcript levels of IL-17A (four-fold; p < 0.01), IL-17F (two-fold; p < 0.05), and of IL-22 (four-fold; p < 0.01) when compared to the PA group. We further confirmed the reduction in IL-17A at the protein level with an ELISA, where a six-fold decrease in protein expression in the MV+PA (VAP) group was observed compared to the PA group (p < 0.05; Figure 2B). Similarly, bacterial challenge in non-ventilated lungs, as expected, induced an increase in transcript expression of TH1 cytokines (IFNγ, TNFα, IL-6, and IL-1β) compared to the non-infectious control group (p < 0.001 for all; Figure 2B). However, for TH1 cytokines, the MV+PA group showed a reduction only in the transcript levels of IFN-γ compared to the PA group (three-fold, p < 0.05), although declining trends for TNF-α, IL-6, and IL-1β were also noted (Figure 2C). These data suggest that MV leads to a dampening of pro-inflammatory response, especially of the TH17 cytokines, after the bacterial challenge.
Figure 2.
Dampening of proinflammatory TH17 and, to a limited extent, TH1 cytokines induced by infection in mechanically ventilated rats. (A) Lung transcript levels of IL-17A, IL-17F, and IL-22 are reduced in mechanically ventilated animals infected with Pseudomonas aeruginosa (MV+PA) compared to non-ventilated, infected animals (PA) euthanized after 24 h. (B) IL-17A protein measured in bronchoalveolar lavage (BAL) fluid was also reduced in MV + PA group compared to the PA group. (C) Similarly, lung transcript levels of TH1 cytokines showed a declining trend for MV+PA group compared to the PA group, but these differences were significant only for IFN-γ. (A–C) Data are presented as averages ± SEM; * p < 0.05, ** p < 0.01; and *** p < 0.001; asterisk above the error bars denotes significance against control group; n = 5–6 animals per group.
2.3. Dampened IL-17A Response in Patients with Ventilator-Associated Pneumonia
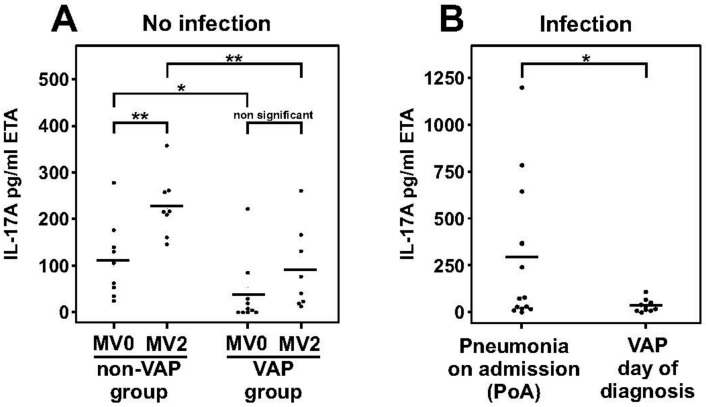
We further studied the impact of MV and of VAP on IL-17 levels in endotracheal aspirate (ETA) samples from mechanically ventilated patients. ETA has an advantage over BAL in allowing an easier and frequent longitudinal sampling from MV patients. Comparing IL-17 levels in ETA samples taken from 19 patients at the start of mechanical ventilation (MV0 time point; 72 pg/mL ± 8.7 standard error of mean (SEM)), we found an increase in IL-17A protein levels after 2 days of MV (MV2 time point; 159 pg/mL ± 10.8 SEM; paired t test p < 0.001). Furthermore, 10 of these patients developed VAP, defined as development of pneumonia after 48 h of mechanical ventilation. Stratifying the patients into VAP (n = 10) and non-VAP (n = 9) groups, we found that IL-17A levels in the VAP group were lower at the start of mechanical ventilation (MV0) compared to the non-VAP group (37 pg/mL ± 13.9 SEM versus 112 pg/mL ± 17.9, respectively; p = 0.044). After two days of mechanical ventilation (MV2), patients who developed VAP continued to show a significantly lower level of IL-17A compared to patients who did not develop VAP (91 pg/mL ± 22.0 SEM versus 227 pg/mL ± 16.6 SEM, respectively; p = 0.003; Figure 3A). The Acute Physiology and Chronic Health Evaluation (APACHE) II scores, a validated measure of severity of critical illness [29], in the VAP and non-VAP groups did not significantly differ (23.4 ± 18 SEM versus 24.8 ± 1.5 SEM, respectively; p = 0.699). In earlier studies neutropenia has been shown to be associated with increased risk of infection and lymphopenia has been linked with development of septic shock [30,31]. However, no significant differences between the VAP and non-VAP groups existed for total white blood cell count at MV0 (12.8 ± 3.34 10E9/L versus 15.3 ± 2.45 10E9/L, respectively; p = 0.552) and at a MV2 timepoint (15.5 ± 1.72 10E9/L versus 13.6 ± 1.25 10E9/L, respectively; p = 0.606). Differential data was also available for all patients for the MV0 timepoint and did not show significant differences between VAP and non-VAP groups for either neutrophils (10.0 ± 0.45 10E9/L versus 13.0 ± 2.19 10E9/L, respectively; p = 0.465) or lymphocytes (1.5 ± 0.22 10E9/L versus 1.3 ± 0.23 10E9/L, respectively; p = 0.682). These clinical data suggest that MV induces a significant increase in IL-17A levels in mechanically ventilated patients and that patients on MV having low levels of IL-17A are associated with a higher likelihood of developing VAP compared to patients on MV with on average higher levels of IL-17A.
Figure 3.
Dampened IL-17A response in ventilator-associated pneumonia (VAP) patients. (A) MV-induced IL-17 protein secretion in endotracheal aspirate (ETA) samples in patients who developed VAP (VAP group) and those that did not (non-VAP group) after 48 h (MV2) compared to the pre-MV timepoint (MV0). The VAP group had lower IL-17A levels than the non-VAP group at both the MV0 and MV2 timepoints. (B) Dampened IL-17A response was observed in VAP patients on the day of diagnosis compared to patients who arrived in the ICU with pneumonia (PoA). Line represents the average, dots are individual values of each patient; * p < 0.05, and ** p < 0.01).
To understand if MV had an influence on IL-17 levels in VAP patients versus those who develop pneumonia without mechanical ventilation, we compared the VAP patient cohort (n = 10) with 13 randomly selected patients who were admitted to the ICU with pre-existing pneumonia (pneumonia on ICU admission; PoA). The PoA patients had an APACHE II score of 33 ± 13.5 SEM, which was not significantly different from that of the VAP patient cohort (23.4 ± 18 SEM; p = 0.106). Although samples (ETA) from PoA patients were taken after MV was initiated, the duration of MV here (2 ± 0.9 SEM MV days) was significantly shorter than the duration of MV before patients developed VAP (4.9 ± 2.8 SEM MV days; p < 0.001). Examining IL-17 levels in VAP patients at the day of VAP development (VAP0 timepoint), we showed that IL-17 levels were nine-fold lower in the VAP group (32 pg/mL ± 6.7 SEM; n = 10) compared to that in the PoA group (292 pg/mL ± 74.8 SEM; n = 13; p = 0.041; Figure 3B). Moreover, the total white blood cell count did not differ between the VAP and PoA groups (p = 0.703; see Table 1), and while neutropenia was an exclusion criterion for the enrolment of VAP patients, one patient was neutropenic in the PoA group. Excluding the latter patient from our analysis did not alter the significant differences observed between the VAP and PoA group (p = 0.041). These data suggest that like rodents, IL-17A levels were also dampened in patients with VAP in comparison with non-ventilator associated pneumonia.
Table 1.
Patient details.
| MV Av ± SD n = 9 |
VAP Av ± SD n = 10 |
PoA Av ± SD n = 13 |
p-Value (VAP vs. PoA) |
|
|---|---|---|---|---|
| Age (years) | 61.1 ± 12.2 | 60.2 ± 11.6 | 62.6 ± 9.6 | 0.592 |
| Gender (% male) | 63% | 50% | 69% | |
| MV before sampling (days) | 0; 2 | 0; 2; 4.9 ± 2.8 | 1.9 ± 0.9 | 0.0018 |
| Total ICU stay (days) | 20.2 ± 11.2 | 24.8 ± 12.8 | 18.2 ± 13.4 | 0.243 |
| APACHE II at admission | 24.9 ± 6.6 | 23.4 ± 8.8 | 33 ± 13.5 | 0.106 |
| Prognosis (% deceased) | 33% (3/9) | 40% (4/10) | 15% (2/13) | |
| Total white blood cell count * | 13.6 ± 1.25 | 13.7 ± 1.60 | 15.4 ± 2.69 | 0.703 |
MV = mechanical ventilation; VAP = ventilator associated pneumonia; PoA = pneumonia on admission; ICU = intensive care unit; APACHE II = Acute Physiology and Chronic Health Evaluation II; *, Average total white blood cell counts (with SEM) are shown for MV2 timepoint for patients who did not develop infection, VAP0 or day of clinical diagnosis for VAP patients and at the day of admission to the ICU for patients with non-VAP pneumonia (PoA).
3. Discussion
VAP is estimated to occur in 9–27% of all mechanically ventilated patients and contributes to approximately half of all cases of hospital-acquired pneumonia [2,32]. Approximately 50% of all antibiotics administered in ICUs are for treatment of VAP [32]. Improved general management of ventilated patients has somewhat reduced VAP incidence in the last decades, however, the mortality and morbidity associated with VAP has not abated. This is because while there are several risk factors associated with VAP development and pathogenesis, the precise mechanism leading to VAP remains unknown.
MV, obviously the biggest risk factor for VAP, has been demonstrated to cause induction of proinflammatory cytokine expression and innate cell infiltration in a tidal volume-dependent manner [7,9,10,11,12,13,14]. However, proinflammatory cytokines are anti-bacterial and per se do not explain VAP development. Using a rodent VAP model, we recently showed that MV-induced lung inflammation caused elevated IL-4 secretion and M2 macrophage polarization with reduced bacterial phagocytic capacity [7]. This suggested that besides the classically studied TH1 cytokines, other immune pathways are involved in ventilation-induced lung inflammation and in VAP. One of the important functions of IL-4 is in maintaining the balance between proinflammatory and anti-inflammatory pathways by suppressing TH1 and TH17 cytokines [10].
Several studies have shown the importance of IL-17 in various physiological and pathophysiological processes, including host defense against infections, especially to Gram negative and fungal infections [11,13,33,34,35,36]. For instance, mice treated with anti-IL-17A neutralizing antibody showed a reduced bacterial clearance in a P. aeruginosa lung infection model [33]. Similarly, IL-17A overexpression led to increased survival of animals and decreased bacterial burden in Gram-negative pathogen infection models [11,34]. Furthermore, a murine vaccination experiment against P. aeruginosa showed a protective effect due to an increased IL-17 response upon infection [37]. Importantly, hospital-acquired infections with Gram-negative pathogens are a major current challenge in hospitals [38] and many of the VAP-causing Gram-negative pathogens are on the WHO priority list [26]. However, the role of IL-17 cytokine family in VAP and in MV, hitherto, remained largely unknown.
While IL-17A is one of the best studied and most important IL-17 cytokines, another important member of the IL-17/IL-10 family is IL-22. Increasing evidence suggests that together with IL-17, IL-22 is also a key regulator of homeostasis and epithelial barrier function and combats infections [11,13,33,34]. Concerning the cellular sources of IL-17 and IL-22, early studies suggested that both cytokines were almost exclusively co-expressed by TH17 cells, though recent data suggest that IL-17 and IL-22 may also be expressed by natural killer T cells (NKT cells), γδ T cells, and innate lymphoid cells; and interestingly, these cells produce IL-17 more rapidly than do T cells and are especially important in the early response to infection [12,35,36,39,40,41].
Here, we showed that MV in healthy rats activates IL-17A and IL-22 in a VT-dependent manner. This was confirmed by clinical data in MV patients, where two days of mechanical ventilation, in the absence of any infection, led to a significant increase in IL-17A levels in ETA, compared to the pre-ventilation timepoint levels. We also demonstrated an increase in IL-17F, the second-most important member of the IL-17 family, however, this was only noted in rodents. In patients, only three showed IL-17F above the lower limit of detection and these were amongst the group of patients with the highest IL-17A levels (data not shown). While the impact of MV on IL-17 cytokines has not been studied in humans, a study in mice addressing the impact of a high-fat diet on MV-induced inflammation reported that one hour of VT 7 mL/kg ventilation led to increased IL-22 but decreased IL-17 levels [17]. However, the tissue was analyzed 24 h after the end of MV, which may have shown a healing response rather than a direct MV-induced response. Thus, our data suggest that MV-induced lung injury proficiently induces proinflammatory TH17 cytokines, as has been shown previously for TH1 cytokines [5,6,7,8,42].
Interestingly, as indicated earlier, we also previously showed that MV co-induces a significant TH2 cytokine response [7], and mechanically ventilated rat and mouse when followed by Pseudomonas aeruginosa inoculation had worsened disease progression and reduced bacterial clearance from lungs [7]. When IL-4 signaling was blocked in IL-4R knockout mice, the worsened phenotype and increased lung bacterial count observed in rodent VAP models was restored, compared to animals that received the same bacterial dose without prior MV [7,10]. Thus, to further understand how the interplay between TH1/TH2/TH17 cytokines impacts the development of VAP, we studied these cytokines in VAP animals that received MV followed by intratracheal instillation of P. aeruginosa. Spontaneously breathing animals that either received P. aeruginosa or remained non-infected were used as control. In accordance with data that TH1 as well as IL-17A and IL-22 are drastically increased in several lung and other infection models [11,33,34], we first showed that bacterial challenge with P. aeruginosa of lungs in non-ventilated rats induced a significant increase in TH17 cytokines (IL-17A, IL-17F, and IL-22) and TH1 cytokines (IFNγ, TNFα, IL-6, and IL-1β) compared to the non-infected/non-ventilated control group. However, the same bacterial challenge in mechanically ventilated rats (i.e., VAP rats) led to a significantly lower response for all three IL-17 family cytokines studied (IL-17A, IL-17F, and IL-22) as well as for IFNγ, compared to the infected/non-ventilated control group, although declining trends for IL-6 and IL-1β were also observed. These data suggest that MV leads to dampening of TH17 cytokines after a bacterial challenge in mouse models.
To study whether a similar dampening of the IL-17 response to infection exists in VAP patients, we studied IL-17A in VAP patients on the day of VAP diagnosis compared to patients that were admitted to the ICU with pre-existing pneumonia. Following clinical protocols, the latter group of patients were also started on MV, though the duration of MV in these patients was on average 2.5 days shorter than the VAP patients at the time of ETA collection. Correcting for co-morbidities and the APACHE II scores, and despite the small number of patients in this study, we showed that VAP patients had significantly lower IL-17A levels compared to non-VAP pneumonia patients. Interestingly, a reduction in TH17 cells in VAP patients in comparison to ventilated patients who did not develop pneumonia has been reported in one study [16]. Another study found the opposite [15]. While the precise reasons for this discrepancy is unknown, in acute infections and therefore in absence of an adaptive immune response, the numbers of TH cells should not be expected to change. Recent data also suggest that in response to infection, innate cell sources are more rapid and robust in secreting IL-17 compared to T-cell sources [12,35,36,39,40,41]. Nevertheless, it remains possible that prior adaptive or varied innate cell responses can predispose patients with low levels of IL-17 to develop VAP. In support of this premise, we showed here that, corrected for APACHE II scores (co-morbidities and other covariates), mechanically ventilated patients who eventually developed VAP had significantly lower levels of IL-17A levels in their ETA samples at start of mechanical ventilation compared to those who did not develop VAP. Even after 2 days of MV, IL-17A levels in VAP patients remained significantly lower than in the MV patient group that did not develop VAP, suggesting that low IL-17 levels could be co-contributing to VAP development. These observations are, of course, based on data from a small number of patients and await confirmation in a larger patient population.
Lastly, one of the ways TH17 cytokines combat bacterial infection is by recruiting neutrophils and monocytes to the site of inflammation/infection [12,14,43]. Interestingly, neutrophils themselves have been identified as a source of IL-17 in tissue inflammation and infection [23,41,43,44]. While neutrophil recruitment is also regulated by several chemokines like IL-8, MMP8, MMP9, CXCL1, CXCL2, CXCL5, LTB4 etc., here we show that lung neutrophils in ventilated lungs correlate well with transcript levels of IL-8, IL-17A, and IL-22, suggesting that IL-17 cytokines might have important downstream effector functions in MV-related inflammation [14,21].
To conclude, our translational studies show a distinct involvement of IL-17 associated cytokines during acute MV-induced lung inflammation. Ventilation–volume dependent IL-17A upregulation also associated strongly with neutrophil infiltration in ventilated lungs, a function fulfilled in part by IL-17A, IL-22, and IL-8. Lastly, in concordance with an MV-mediated TH2 response shown in earlier studies [7,16], we showed that MV is associated with a dampened TH17 cytokine response in lungs of infected animals as well as in VAP patients suggesting that this immune incompetence could be one of the mechanisms associated with VAP development.
4. Materials and Methods
4.1. Rat Mechanical Ventilation Model
Animal experiments were conducted according to the guidelines of the Federation of European Laboratory Animal Science Associations and approved by the Ethical Commission for Animal Experimentation of the University of Antwerp (ECD 2010-42 approval date 17/01/2011). Wistar rats, aged 8–10 weeks, weight approximately 320 g, were obtained from Charles River Laboratories (Brussels, Belgium). Up to four rats were simultaneously ventilated with a Servo 900 C ventilator (Siemens, Solna, Sweden) [7]. Briefly, anesthesia was induced by a combination of 100 mg/kg ketamine and 0.5 mg/kg medetomidine. Rats were orotracheally intubated with 14 G angiocatheter (BD–Life Sciences, Ermebodegem, Belgium). Three ventilation protocols were utilized: (i) Ventilation with VT 5 mL/kg (n = 8) for 2 h with 7 cm H2O peak-inspiratory pressure (PIP), 4 cm H2O positive end-expiratory pressure (PEEP) and 80 breaths/min, time inspiratory/expiratory (TI/E) 1:2, and 21% inspired oxygen, (ii) ventilation with VT 8 mL/kg (n = 8) for 2 h with 10 cm H2O PIP, 4 cm H2O PEEP and 60 breaths/min, and (iii) ventilation with VT approximately 25 mL/kg (n = 6) for 2 h with zero PEEP, 26 cm H2O PIP, and 40 breaths/min. Animals were continuously monitored for oxygen saturation (pulse oximetry, MouseSTAT, Kent Scientific, Torrington, CT, USA) and body temperature was maintained at 37 °C (RightTemp, Kent Scientific). After ventilation, animals were euthanized and studied. Spontaneously breathing, non-manipulated animals (n = 10) served as controls.
4.2. Induction of Bacterial Pneumonia in Ventilated Rats
For the MV+PA group, animals (n = 8) ventilated with VT 8 mL/kg for 2 h received an intratracheal dose of 2 × 107 colony forming units of P. aeruginosa ATCC-27853 strain suspended in 500 μL saline immediately after ventilation. As controls, animals (n = 8) received identical bacterial instillation without prior ventilation (PA group). Anesthesia was reversed using 300 μg/kg atipamezole and animals were monitored for clinical signs of pneumonia [7,45] until 24 h post-infection when they were euthanized, tissue collected, and analyzed.
4.3. Patient Samples
VAP and non-pneumonia patients’ samples were obtained from ‘Identification of predictive biomarkers of pneumonia in artificially ventilated patients’ (IBIVAP) study conducted at the Intensive Care Unit of the Antwerp University Hospital. Written consent was obtained from the closest relatives of patients and the study was approved by the Ethics Committee UZA (ECD: 12/12/112, approval date 07/05/2012). The exclusion criteria for enrolment were: Infection other than VAP, prior gastric or esophageal surgery, neutropenia, and expected short duration of ventilation such as after cardiac surgery. VAP was clinically diagnosed in patients ventilated for >48 h, and was based on a new or progressive consolidation on chest radiology and at least two of the following variables: Fever greater than 38 °C, leukocytosis or leukopenia, and purulent secretions [2]. At this point, respiratory samples were taken (endotracheal aspirates obtained through tracheal aspiration or bronchoalveolar lavage (BAL) and/or BAL fluid) and these were cultured to isolate the causative pathogen. A VAP diagnosis was only made when pathogens were cultured and identified in these samples. In total 100 patients were enrolled in the study of which 10 developed VAP of different etiologies. From 90 non-VAP patients, 10 patients who did not develop any form of infection were randomly selected to be studied as a control group. One patient had sepsis and was excluded from further analysis.
Pneumonia on admission (PoA) patients’ samples were obtained from ‘The Advanced understanding of Staphylococcus aureus and Pseudomonas aeruginosa Infections in Europe–Intensive Care Units’ (ASPIRE-ICU) study (NCT02413242—ClinicalTrials.gov) [46]. Although the study focuses on ICU-VAP, in this sub-study, a random selection was made of patients that arrived in the ICU with a pre-existing pneumonia (n = 13).
For both IBIVAP and ASPIRE-ICU studies, endotracheal aspirate (ETA) was collected in a standardized manner with a mucus extractor, transported on 4 °C and stored at −80 °C until they were batch processed as described below. IBIVAP samples were collected at the start of mechanical ventilation, on day two of MV and on the first day of VAP diagnosis. PoA patients entered the ICU with a positive diagnosis of pneumonia and were sampled after MV started. Patient details are listed in Table 1.
4.4. Transcript Studies
Complete right rat lung was snap frozen in liquid nitrogen and preserved for RNA extraction. The tissue was homogenized by crushing in liquid nitrogen and RNA was extracted using RNeasy-mini spin columns (Qiagen, Antwerp, Belgium). Integrity and concentrations were estimated using RNA-nanochips on Bio-analyzer (Agilent, Leuven, Belgium). RNA was converted to cDNA (RT2 First Strand Kit, Qiagen, Antwerp, Belgium) and quantitative PCR was performed on Bio-Rad CFX Connect with SsoAdvanced SYBR green supermix (Bio-Rad, Temse, Belgium) utilizing 2 step PCR with cycles of 95 °C for 10 s followed by 60 °C for 30 s. Primer sequences are shown in Table A1. Data were analyzed using the comparative CT method with Actb and Sdha as housekeeping genes as described earlier [7,47,48].
4.5. Neutrophil Quantification
Paraffin-embedded sections from the left lung were prepared and stained with H/E and anti-neutrophil immunohistochemistry (1:10,000, LifeSpan Biosciences LC-348181, Seattle, WA, USA). Number of neutrophils for each group was manually counted on 10 consecutive fields of 200× magnification, as described by us previously [7,25].
4.6. IL-17 Immunoassay
Broncho-alveolar lavage fluid (BALF) samples from rats were collected by gentle injection of 32 mL/kg body weight of cold sterile PBS in the lungs and withdrawal after 10 s of 70% of the injected solution. An IL-17A sandwich ELISA was performed on this BALF according to the instructions of the manufacturer (R&D systems, Abingdon, UK). In short, BALF samples were diluted 1:1 in assay diluent and added to pre-coated wells. Samples were incubated with a biotin conjugated anti-IL-17A antibody and subsequently with a streptavidin-labeled horse radish peroxidase (HRP). Tetramethylbenzidine (TMB) substrate was used to quantify the amount of bound protein by measuring the colorimetric reaction at 450 nm.
Patient ETA samples were mechanically disrupted by blending (30,000 rpm, probe size 8 mm) and diluted 1:1 v/v with Sputolysin (Boehringer Ingelheim, Ingelheim am Rhein, Germany) and incubated for 15 min. Thereafter the mixture was centrifuged at 3000 g and the supernatants aliquoted. Supernatants were measured on an IL-17A and IL-17F MSD human U-plex assay on QuickPlex SQ 120 according to the manufacturer’s instructions (Mesoscale discovery; MSD, Rockville, MD, USA). The lower limit of detection for IL-17A and IL-17F were 5.35 pg/mL and 164 pg/mL respectively.
4.7. Data Analyses and Statistics
Data analyses were performed using SPSS—v.24. Transcript data are presented as average fold-differences with standard errors of the mean. ELISA data are presented as averages of each group with standard errors of the mean. MSD results from patients are represented as means with the individual data points. The Kolmogorov–Smirnov test was utilized for testing normality before testing statistical significance of differences by two-tailed independent or paired t-tests. Values of all significant correlations (p < 0.05) are given with degree of significance indicated (* p < 0.05, ** p < 0.01, and *** p < 0.001).
Abbreviations
| ASPIRE-ICU | The Advanced understanding of Staphylococcus aureus and Pseudomonas aeruginosa Infections in Europe—Intensive Care Units |
| BALF | Bronchoalveolar lavage fluid |
| ETA | Endotracheal aspirate |
| IBIVAP | Identification of predictive biomarkers of pneumonia in artificially ventilated patients |
| MV | Mechanical ventilation |
| PA | Pseudomonas aeruginosa |
| PEEP | Positive end expiratory pressure |
| PIP | Positive inspiratory pressure |
| PoA | Pneumonia on admission |
| VAP | Ventilator-associated pneumonia |
| VT | Tidal volume |
Appendix A
Table A1.
Primer sequences used for this study. ACTB and SDHA were used as housekeeping genes.
| Target | Host | Fw seq | Rv seq |
|---|---|---|---|
| Actb | Rat | GTCGTACCACTGGCATTGTG | CTCTCAGCTGTGGTGGTGAA |
| Sdha | Rat | CTCTTTTGGACCTTGTCGTCTTT | TCTCCAGCATTTGCCTTAATCGG |
| Ifn-γ | Rat | ATTCATGAGCATCGCCAAGTTC | TGACAGCTGGTGAATCACTCTGAT |
| Tnf-α | Rat | CTTCTCATTCCTGCTCGTGG | TGATCTGAGTGTGAGGGTCTG |
| IL-1β | Rat | TGCAGGCTTCGAGATGAAC | GGGATTTTGTCGTTGCTTGTC |
| IL-6 | Rat | AAGCCAGAGTCATTCAGAGC | GTCCTTAGCCACTCCTTCTG |
| IL-17A | Rat | ACTACCTCAACCGTTCCACTTCA | CTTCAGGACCAGGATCTCTTGCT |
| IL-17F | Rat | CCCGGAGACCTCTCAGAAGA | GCCCTACTTTGGGGTTCCTC |
| IL-22 | Rat | TCCAGCAGCCATACATCGTC | GGCTTTGACTCCTCGGAACA |
| IL-10 | Rat | GCTCTTACTGACTGGCATGAG | CGCAGCTCTAGGAGCATGTG |
| IL-8 | Rat | CCCCCATGGTTCAGAAGATTG | TTGTCAGAAGCCAGCGTTCAC |
Author Contributions
Conceptualization, S.K.-S.; Data curation, F.H.R.D.W., B.S.J., K.B., D.M., L.T., C.L., V.V.a., J.B., J.K., A.R., S.M.-K., P.G.J., H.G. and S.K.-S.; Formal analysis, F.H.R.D.W., B.S.J., K.B., and S.K.-S.; Methodology, K.B., D.M., J.K., A.R., S.M.-K., P.G., H.G. and S.K.-S.; Project administration, L.T., C.L., O.A., J.K., A.R., S.M.-K., P.G.J. and H.G.; Supervision, S.K.-S.; Validation, B.S.J. and S.K.-S.; Visualization, F.H.R.D.W.; Writing—original draft, F.H.R.D.W., K.B. and S.K.-S.; Writing—review & editing, B.S.J., D.M., L.T., C.L., V.V.a., J.B., O.A., J.K., A.R., S.M.-K., P.G.J. and H.G.
Funding
This research was funded by the Flemish Institute for Sciences and Technology (IWT-SBO), grant number 140746, and University of Antwerp-GOA grant number s30729. KB (IWT-SB111664), B’SJ (FWO-SB151525), VVA (FWO-1S93418N) have been PhD fellows of IWT/FWO. This research was also supported by the Innovative Medicines Initiative Joint Undertaking under grant agreements COMBACT-MAGNET and COMBACT-CARE (no. 115523 and 115737) resources, which are composed of financial contribution from the European Union Seventh Framework Program (FP7/2007–2013) and EFPIA companies in kind contribution. The APC was funded by these COMBACTE resources.
Conflicts of Interest
O.A. and A.R. are employees of AstraZeneca. The other authors declare no conflict of interest.
References
- 1.American Thoracic Society. Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 2.Jorens P.G. Sticking to an Old Definition of Ventilator-Associated Pneumonia Is Not Old-Fashioned. Respir. Care. 2016;61:390–392. doi: 10.4187/respcare.04736. [DOI] [PubMed] [Google Scholar]
- 3.Parker J.C., Hernandez L.A., Peevy K.J. Mechanisms of ventilator-induced lung injury. Crit. Care Med. 1993;21:131–143. doi: 10.1097/00003246-199301000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Gajic O., Dara S.I., Mendez J.L., Adesanya A.O., Festic E., Caples S.M., Rana R., St Sauver J.L., Lymp J.F., Afessa B., et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit. Care Med. 2004;32:1817–1824. doi: 10.1097/01.CCM.0000133019.52531.30. [DOI] [PubMed] [Google Scholar]
- 5.Tremblay L., Valenza F., Ribeiro S.P., Li J., Slutsky A.S. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J. Clin. Investig. 1997;99:944–952. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serpa Neto A., Cardoso S.O., Manetta J.A., Pereira V.G., Esposito D.C., Pasqualucci Mde O., Damasceno M.C., Schultz M.J. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: A meta-analysis. JAMA: J. Am. Med. Assoc. 2012;308:1651–1659. doi: 10.1001/jama.2012.13730. [DOI] [PubMed] [Google Scholar]
- 7.Bielen K., Jongers B., Boddaert J., Lammens C., Jorens P.G., Malhotra-Kumar S., Goossens H., Kumar-Singh S. Mechanical ventilation induces IL-4 secretion in lungs and reduces the phagocytic capacity of lung macrophages. J. Infect. Dis. 2018;217:1645–1655. doi: 10.1093/infdis/jix573. [DOI] [PubMed] [Google Scholar]
- 8.Wienhold S.M., Macri M., Nouailles G., Dietert K., Gurtner C., Gruber A.D., Heimesaat M.M., Lienau J., Schumacher F., Kleuser B., et al. Ventilator-induced lung injury is aggravated by antibiotic mediated microbiota depletion in mice. Crit. Care. 2018;22:282. doi: 10.1186/s13054-018-2213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin C.Y., Zhang H., Cheng K.C., Slutsky A.S. Mechanical ventilation may increase susceptibility to the development of bacteremia. Crit. Care Med. 2003;31:1429–1434. doi: 10.1097/01.CCM.0000063449.58029.81. [DOI] [PubMed] [Google Scholar]
- 10.Galli S.J., Borregaard N., Wynn T.A. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat. Immunol. 2011;12:1035–1044. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu X., Shao B., Wang R., Zhou S., Tang Z., Lu W., Xiong S. Role of Interleukin-17 in defense against pseudomonas aeruginosa infection in lungs. Int. J. Clin. Exp. Med. 2014;7:809–816. [PMC free article] [PubMed] [Google Scholar]
- 12.Aujla S.J., Chan Y.R., Zheng M., Fei M., Askew D.J., Pociask D.A., Reinhart T.A., McAllister F., Edeal J., Gaus K., et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat. Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eyerich S., Eyerich K., Cavani A., Schmidt-Weber C. IL-17 and IL-22: Siblings, not twins. Trends Immunol. 2010;31:354–361. doi: 10.1016/j.it.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Laan M., Cui Z.H., Hoshino H., Lotvall J., Sjostrand M., Gruenert D.C., Skoogh B.E., Linden A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J. Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- 15.Liu Y., Sun J.K., Qi X., Chen Y.M., Li J., Chen S.Y., Liu H. Expression and Significance of Th17 and Treg Cells in Pulmonary Infections with Gram-Negative Bacteria. Immunol. Investig. 2017;46:730–741. doi: 10.1080/08820139.2017.1360338. [DOI] [PubMed] [Google Scholar]
- 16.Orlov M., Dmyterko V., Wurfel M.M., Mikacenic C. Th17 cells are associated with protection from ventilator associated pneumonia. PLoS ONE. 2017;12:e0182966. doi: 10.1371/journal.pone.0182966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Souza A.B.F., Chirico M.T.T., Cartelle C.T., de Paula Costa G., Talvani A., Cangussu S.D., de Menezes R.C.A., Bezerra F.S. High-Fat Diet Increases HMGB1 Expression and Promotes Lung Inflammation in Mice Subjected to Mechanical Ventilation. Oxidative Med. Cell. Longev. 2018;2018:7457054. doi: 10.1155/2018/7457054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acute Respiratory Distress Syndrome Network. Brower R.G., Matthay M.A., Morris A., Schoenfeld D., Thompson B.T., Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 19.Sutherasan Y., Vargas M., Pelosi P. Protective mechanical ventilation in the non-injured lung: Review and meta-analysis. Crit. Care. 2014;18:211. doi: 10.1186/cc13778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menendez C., Martinez-Caro L., Moreno L., Nin N., Moral-Sanz J., Morales D., Cogolludo A., Esteban A., Lorente J.A., Perez-Vizcaino F. Pulmonary vascular dysfunction induced by high tidal volume mechanical ventilation. Crit. Care Med. 2013;41:e149–e155. doi: 10.1097/CCM.0b013e318287ef4a. [DOI] [PubMed] [Google Scholar]
- 21.Jorens P.G., Van Damme J., De Backer W., Bossaert L., De Jongh R.F., Herman A.G., Rampart M. Interleukin 8 (IL-8) in the bronchoalveolar lavage fluid from patients with the adult respiratory distress syndrome (ARDS) and patients at risk for ARDS. Cytokine. 1992;4:592–597. doi: 10.1016/1043-4666(92)90025-M. [DOI] [PubMed] [Google Scholar]
- 22.Borregaard N. Neutrophils, from marrow to microbes. Immunity. 2010;33:657–670. doi: 10.1016/j.immuni.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Ferretti S., Bonneau O., Dubois G.R., Jones C.E., Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J. Immunol. 2003;170:2106–2112. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]
- 24.Allen J.E., Sutherland T.E., Ruckerl D. IL-17 and neutrophils: Unexpected players in the type 2 immune response. Curr. Opin. Immunol. 2015;34:99–106. doi: 10.1016/j.coi.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Kumar-Singh S., Pirici D., McGowan E., Serneels S., Ceuterick C., Hardy J., Duff K., Dickson D., Van Broeckhoven C. Dense-core plaques in Tg2576 and PSAPP mouse models of Alzheimer’s disease are centered on vessel walls. Am. J. Pathol. 2005;167:527–543. doi: 10.1016/S0002-9440(10)62995-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tacconelli E., Carrara E., Savoldi A., Harbarth S., Mendelson M., Monnet D.L., Pulcini C., Kahlmeter G., Kluytmans J., Carmeli Y., et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 27.Massion P.P., Inoue H., Richman-Eisenstat J., Grunberger D., Jorens P.G., Housset B., Pittet J.F., Wiener-Kronish J.P., Nadel J.A. Novel Pseudomonas product stimulates interleukin-8 production in airway epithelial cells in vitro. J. Clin. Investig. 1994;93:26–32. doi: 10.1172/JCI116954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grgurich P.E., Hudcova J., Lei Y., Sarwar A., Craven D.E. Management and prevention of ventilator-associated pneumonia caused by multidrug-resistant pathogens. Expert. Rev. Respir. Med. 2012;6:533–555. doi: 10.1586/ers.12.45. [DOI] [PubMed] [Google Scholar]
- 29.Knaus W.A., Draper E.A., Wagner D.P., Zimmerman J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985;13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Andersen C.L., Tesfa D., Siersma V.D., Sandholdt H., Hasselbalch H., Bjerrum O.W., Felding P., Lind B., Olivarius Nde F., Palmblad J. Prevalence and clinical significance of neutropenia discovered in routine complete blood cell counts: A longitudinal study. J. Intern. Med. 2016;279:566–575. doi: 10.1111/joim.12467. [DOI] [PubMed] [Google Scholar]
- 31.Aydogdu M., Gursel G. Predictive factors for septic shock in patients with ventilator-associated pneumonia. South. Med. J. 2008;101:1222–1226. doi: 10.1097/SMJ.0b013e3181827891. [DOI] [PubMed] [Google Scholar]
- 32.Hunter J.D. Ventilator associated pneumonia. Bmj. 2012;344:e3325. doi: 10.1136/bmj.e3325. [DOI] [PubMed] [Google Scholar]
- 33.Liu J., Feng Y., Yang K., Li Q., Ye L., Han L., Wan H. Early production of IL-17 protects against acute pulmonary Pseudomonas aeruginosa infection in mice. FEMS Immunol. Med. Microbiol. 2011;61:179–188. doi: 10.1111/j.1574-695X.2010.00764.x. [DOI] [PubMed] [Google Scholar]
- 34.Ye P., Garvey P.B., Zhang P., Nelson S., Bagby G., Summer W.R., Schwarzenberger P., Shellito J.E., Kolls J.K. Interleukin-17 and Lung Host Defense againstKlebsiella pneumoniae Infection. Am. J. Respir. Cell Mol. Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 35.Cua D.J., Tato C.M. Innate IL-17-producing cells: The sentinels of the immune system. Nat. Rev. Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 36.Isailovic N., Daigo K., Mantovani A., Selmi C. Interleukin-17 and innate immunity in infections and chronic inflammation. J. Autoimmun. 2015;60:1–11. doi: 10.1016/j.jaut.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Baker S.M., Pociask D., Clements J.D., McLachlan J.B., Morici L.A. Intradermal vaccination with a Pseudomonas aeruginosa vaccine adjuvanted with a mutant bacterial ADP-ribosylating enterotoxin protects against acute pneumonia. Vaccine. 2019;37:808–816. doi: 10.1016/j.vaccine.2018.12.053. [DOI] [PubMed] [Google Scholar]
- 38.Tacconelli E., Cataldo M.A., Dancer S.J., De Angelis G., Falcone M., Frank U., Kahlmeter G., Pan A., Petrosillo N., Rodriguez-Bano J., et al. ESCMID guidelines for the management of the infection control measures to reduce transmission of multidrug-resistant Gram-negative bacteria in hospitalized patients. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2014;20(Suppl. 1):1–55. doi: 10.1111/1469-0691.12427. [DOI] [PubMed] [Google Scholar]
- 39.Sutton C.E., Mielke L.A., Mills K.H. IL-17-producing gammadelta T cells and innate lymphoid cells. Eur. J. Immunol. 2012;42:2221–2231. doi: 10.1002/eji.201242569. [DOI] [PubMed] [Google Scholar]
- 40.Coquet J.M., Chakravarti S., Kyparissoudis K., McNab F.W., Pitt L.A., McKenzie B.S., Berzins S.P., Smyth M.J., Godfrey D.I. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1- NKT cell population. Proc. Natl. Acad. Sci. USA. 2008;105:11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nieuwenhuis E.E., Matsumoto T., Exley M., Schleipman R.A., Glickman J., Bailey D.T., Corazza N., Colgan S.P., Onderdonk A.B., Blumberg R.S. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat. Med. 2002;8:588–593. doi: 10.1038/nm0602-588. [DOI] [PubMed] [Google Scholar]
- 42.Tsay T.B., Jiang Y.Z., Hsu C.M., Chen L.W. Pseudomonas aeruginosa colonization enhances ventilator-associated pneumonia-induced lung injury. Respir. Res. 2016;17:101. doi: 10.1186/s12931-016-0417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujie H., Niu K., Ohba M., Tomioka Y., Kitazawa H., Nagashima K., Ohrui T., Numasaki M. A distinct regulatory role of Th17 cytokines IL-17A and IL-17F in chemokine secretion from lung microvascular endothelial cells. Inflammation. 2012;35:1119–1131. doi: 10.1007/s10753-011-9419-0. [DOI] [PubMed] [Google Scholar]
- 44.Hoshino A., Nagao T., Nagi-Miura N., Ohno N., Yasuhara M., Yamamoto K., Nakayama T., Suzuki K. MPO-ANCA induces IL-17 production by activated neutrophils in vitro via classical complement pathway-dependent manner. J. Autoimmun. 2008;31:79–89. doi: 10.1016/j.jaut.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Bielen K., ’s Jongers B., Malhotra-Kumar S., Jorens P.G., Goossens H., Kumar-Singh S. Animal models of hospital-acquired pneumonia: Current practices and future perspectives. Ann. Transl. Med. 2017;5:132. doi: 10.21037/atm.2017.03.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paling F.P., Troeman D.P.R., Wolkewitz M., Kalyani R., Prins D.R., Weber S., Lammens C., Timbermont L., Goossens H., Malhotra-Kumar S., et al. Rationale and design of ASPIRE-ICU: A prospective cohort study on the incidence and predictors of Staphylococcus aureus and Pseudomonas aeruginosa pneumonia in the ICU. BMC Infect. Dis. 2017;17:643. doi: 10.1186/s12879-017-2739-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 48.Bielen K., ’s Jongers B., Boddaert J., Raju T.K., Lammens C., Malhotra-Kumar S., Jorens P.G., Goossens H., Kumar-Singh S. Biofilm-Induced Type 2 Innate Immunity in a Cystic Fibrosis Model of Pseudomonas aeruginosa. Front Cell Infect Microbiol. 2017;7:274. doi: 10.3389/fcimb.2017.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]