Abstract
Extensive studies have shown that the MBW complex consisting of three kinds of regulatory proteins, MYB and basic helix–loop–helix (bHLH) transcription factors and a WD40 repeat protein, TRANSPARENT TESTA GLABRA1 (TTG1), acts in concert to promote trichome formation and flavonoid accumulation in Arabidopsis thaliana. TTG1 functions as an essential activator in these two biological processes. However, direct downstream targets of the TTG1-dependent MBW complex have not yet been obtained in the two biological processes at the genome-wide level in A. thaliana. In the present study, we found, through RNA sequencing and quantitative real-time PCR analysis, that a great number of regulatory and structural genes involved in both trichome formation and flavonoid accumulation are significantly downregulated in the young shoots and expanding true leaves of ttg1-13 plants. Post-translational activation of a TTG1-glucocorticoid receptor fusion protein and chromatin immunoprecipitation assays demonstrated that these downregulated genes are directly or indirectly targeted by the TTG1-dependent MBW complex in vivo during trichome formation and flavonoid accumulation. These findings further extend our understanding of the role of TTG1-dependent MBW complex in the regulation of trichome formation and flavonoid accumulation in A. thaliana.
Keywords: MBW complex, TTG1, trichome formation, flavonoid accumulation, Arabidopsis thaliana
1. Introduction
TRANSPARENT TESTA GLABRA1 (TTG1) encodes a WD40 repeat transcription factor that plays pleiotropic roles in the regulation of seed development and postembryonic processes in Arabidopsis thaliana [1,2,3,4,5,6,7,8]. During seed development, it not only promotes the biosynthesis of proanthocyanidins (PAs) [1,2,3,8,9] but also accelerates the production of mucilage and columella [1,8,10,11]. By contrast, it negatively regulates the accumulation of seed storage reserves, including fatty acids and proteins during seed maturation [7,8]. In the postembryonic process, TTG1 functions in root development including root length and hairs [12,13] and the response to abiotic stresses [13,14]. Further, TTG1 also acts as a key transcriptional activator in trichome formation [1,3,12,15] and flavonoid deposition [1,2,3,9,16], and the TTG1-dependent regulatory network in the two biological processes has been extensively studied in A. thaliana.
Trichomes are single-celled and hairy structures that develop into an epidermis of the aerial parts, including leaves, stems, and sepals, in A. thaliana. They are related with water regulation, temperature control, and protection against biotic and abiotic stresses [15,17,18,19]. Trichome formation is activated by the MYB–basic helix–loop–helix (bHLH)–WD40 (MBW) complex, which includes an R2R3-type MYB-related transcription factor (GLABRA1 (GL1) or MYB23), a bHLH protein (GLABRA3 (GL3) or ENHANCER OF GLABRA3 (EGL3)), and the WD40-repeat protein TTG1 [12,15,20,21]. The GL1-GL3/EGL3–TTG1 complex promotes trichome formation through inducing the expression of GLABRA2 (GL2) and some single-repeat R3 MYB transcription factors, and then the induced MYB transcription factors in turn impede the complex formation by competing with GL1 for binding GL3 or EGL3 [22,23,24]. TTG1 directly induces the TRANSPARENT TESTA GLABRA2 (TTG2) expression [25]. The further study indicated that TTG1 represses the activation of the CAPRICE (CPC) promoter by GL1 and GL3, and GL1 suppresses the activation of the TRIPTYCHON promoter by GL3 and TTG1 [26].
Flavonoids, as secondary metabolites, are ubiquitously produced in higher plants and can be categorized into three major classes in A. thaliana, namely, flavonols, PAs, and anthocyanins [9,27,28]. They are involved in feeding and pollination attraction, rhizosphere signaling, auxin movement, nutrient retrieval during senescence, and protection against phytopathogens and sunlight irradiance [29,30,31,32,33,34,35]. Further, flavonoids also serve as the source of beneficial micronutrients for humans, useful for human health and protecting against many diseases [36,37,38,39,40]. The MBW complex, which promotes flavonoid accumulation, consists of an R2R3 MYB gene (PRODUCTION OF ANTHOCYANIN PIGMENT 1 (PAP1), PAP2, MYB113 or MYB114), a bHLH transcription factor (TRANSPARENT TESTA 8 (TT8), GL3 or EGL3), and the WD40-repeat protein TTG1 [41,42,43,44,45]. TTG1 directly activates BANYULS (BAN) expression through TT8 stability in A. thaliana siliques [46].
Such studies as the abovementioned indicate the TTG1-dependent MBW complex acts as a regulatory hub in the modulation of trichome formation and flavonoid accumulation, and TTG1 functions as an essential activator in the two biological processes, in A. thaliana. However, direct downstream targets of the TTG1-dependent MBW complex have so far not yet been obtained at the genome-wide level in A. thaliana.
In the present study, we found that a great number of regulatory and structural genes involved in trichome formation and flavonoid accumulation are significantly downregulated in the young shoots and expanding true leaves of ttg1-13 plants through transcriptome analysis. We demonstrated that these downregulated genes are directly or indirectly targeted by the MBW complex in vivo, using the approaches of the dexamethasone (DEX)-inducible gene expression system and chromatin immunoprecipitation (ChIP), during trichome formation and flavonoid accumulation in A. thaliana.
2. Results
2.1. Genome-Wide Analysis of Downstream Targets Involved in Trichome Formation and Flavonoid Accumulation in ttg1-13 Young Shoots and Expanding True Leaves
The loss-of-function mutant of ttg1-13 (CS67772) as a mixed Columbia/Ler background was obtained by fast neutron mutagenesis from the Arabidopsis Biological Resource Center (ABRC). This mutant was backcrossed twice to the wild type Col-0 to purify its background and eliminate other possible mutations [14], and then used in this study. There are no trichome formation and flavonoid deposition in ttg1-13 young shoots and true leaves (Figure S1, [14]), which is consistent with the previous evidence that TTG1 positively promotes trichome formation and flavonoid accumulation in A. thaliana [1,2,3,9,12,15,16]. Trichomes are commonly present in the surface of stems and leaves, and anthocyanins, the most conspicuous class of flavonoids, are frequently produced in young shoots and expanding leaves [47]. Further, TTG1 is highly expressed in young shoots and expanding true leaves [25,46]. Therefore, to identify downstream targeted genes of the TTG1-dependent MBW complex involved in trichome formation and flavonoid accumulation at the genome-wide level, the tissues of young shoots and expanding true leaves from the wild type and ttg1-13 plants at 20 days after germination (DAG) (Figure S1) were used for the RNA-seq experiment.
The RNA-seq analysis identified 987 differential expressed genes (DEGs) (Tables S1 and S2), of which 732 were expressed at lower levels (‘downregulated’; Table S1) and 255 at higher levels (‘upregulated’; Table S2) in the ttg1-13 young shoots and expanding true leaves. Based on the functional annotations, 14 and 15 of the downregulated genes were found to be involved in trichome formation and flavonoid biosynthesis, respectively, but none of the upregulated genes were involved in the two biological processes (Table 1 and Table 2; Tables S1 and S2). We noted that the number of downregulated genes related to the primary metabolic processes, such as carbohydrates, amino acids and proteins, and lipids, was more than that of the upregulated genes, as was the number of other several biological processes, including cell wall, signaling transduction, oxidation–reduction, and stress/defense response, in the ttg1-13 young shoots and expanding true leaves (Tables S1 and S2). These likely indicated that the TTG1-dependent MBW complex functions as a key transcriptional activator in the regulation of trichome formation, flavonoid biosynthesis, and other major biological processes.
Table 1.
Differentially expressed genes (DEGs) contributing to trichome formation in the young shoots and expanding true leaves of ttg1-13 plants at 20 days after germination. DEGs with |log2 ratios| ≥ 1.00, and only GO Slim IDs with FDR ≤ 0.05, are listed here.
| Differentially Expressed Genes | log2 Ratios | Functions | References |
|---|---|---|---|
| SPL8 (AT1G02065) | −2.36 | Promoting trichome formation | [48] |
| ETC1 (AT1G01380) | −3.73 | Repressing trichome formation | [49] |
| MYC2 (AT1G32640) | −1.27 | Promoting trichome initiation | [50,51,52,53] |
| BLT (AT1G64690) | −2.10 | Promoting trichome branching | [54,55] |
| HDG11 (AT1G73360) | −1.78 | Promoting trichome differentiation | [56] |
| GL2 (AT1G79840) | −6.33 | Promoting trichome differentiation | [22,23,24] |
| TTG2 (AT2G37260) | −7.89 | Promoting trichome formation | [25,57] |
| CPC (AT2G46410) | −4.09 | Repressing trichome formation | [49] |
| MYB106 (AT3G01140) | −2.82 | Promoting trichome differentiation and repressing trichome branching | [58,59] |
| MYB5 (AT3G13540) | −7.07 | Repressing trichome branching | [60,61] |
| TT8 (AT4G09820) | −7.14 | Promoting flavonoid accumulation | [62] |
| SIM (AT5G04470) | −1.94 | Associated with trichome development | [63,64] |
| MYB23 (AT5G40330) | −7.13 | Repressing trichome branching | [60,61] |
| SVB (AT1G56580) | −1.43 | Associated with trichome size and branching | [65] |
Table 2.
Differentially expressed genes (DEGs) contributing to flavonoid biosynthesis in the young shoots and expanding true leaves of ttg1-13 plants at 20 days after germination. DEGs with |log2 ratios| ≥ 1.00, and only GO Slim IDs with FDR ≤ 0.05, are listed here.
| Differentially Expressed Genes | log2 Ratios | Functions | References |
|---|---|---|---|
| MYC2 (AT1G32640) | −1.27 | Promoting flavonoid accumulation | [50,51,52,53] |
| GL2 (AT1G79840) | −6.33 | Inhibiting anthocyanin accumulation | [66] |
| TTG2 (AT2G37260) | −7.89 | Promoting flavonoid accumulation | [25,57] |
| ANL2 (AT4G00730) | −1.06 | Promoting anthocyanin accumulation | [67] |
| TT8 (AT4G09820) | −7.14 | Promoting flavonoid accumulation | [68,69] |
| PAL4 (AT3G10340) | −3.72 | Converting phenylalanine into trans-cinnamic acid | [70,71] |
| F3’H (AT5G07990) | −4.08 | Converting naringenin and dihydrokaempferol into eriodictyol and dihydroquercetin, respectively | [70,72] |
| DMR6 (AT5G24530) | −9.50 | Converting flavanones into flavones | [73] |
| FLS3 (AT5G63590) | −7.55 | Promoting flavonol accumulation | [74] |
| DFR (AT5G42800) | −10.85 | Converting dihydroflavonols into leucoanthocyanidins | [2,75] |
| ANS (AT4G22880) | −10.68 | Converting leucoanthocyanidins into 3-OH-anthocyanins | [76,77] |
| UGT79B1 (AT5G54060) | −8.29 | Involved in the glycosylation of anthocyanins | [70,78] |
| UGT75C1 (AT4G14090) | −4.09 | Involved in the malonylation of anthocyanins | [79] |
| 5MAT (AT3G29590) | −11.05 | Involved in the accumulation of malonylated anthocyanins | [80] |
| GSTF12 (AT5G17220) | −3.73 | Involved in transport and accumulation of both anthocyanins and proanthocyanidins | [81,82] |
2.2. Validation of Downstream Targets Related to Trichome Formation and Flavonoid Accumulation in ttg1-13 Young Shoots and Expanding True Leaves
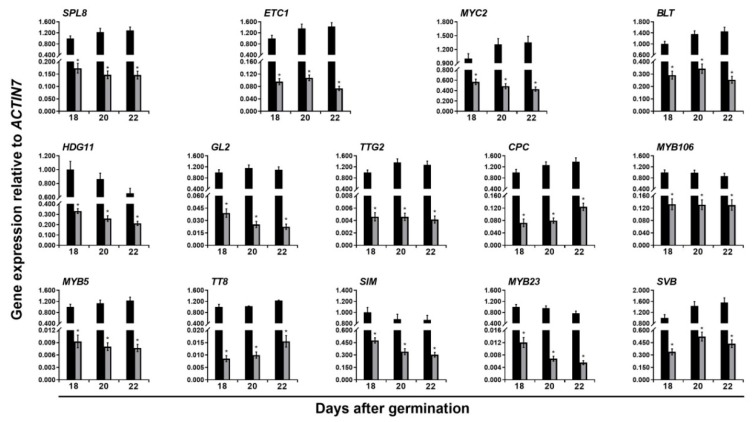
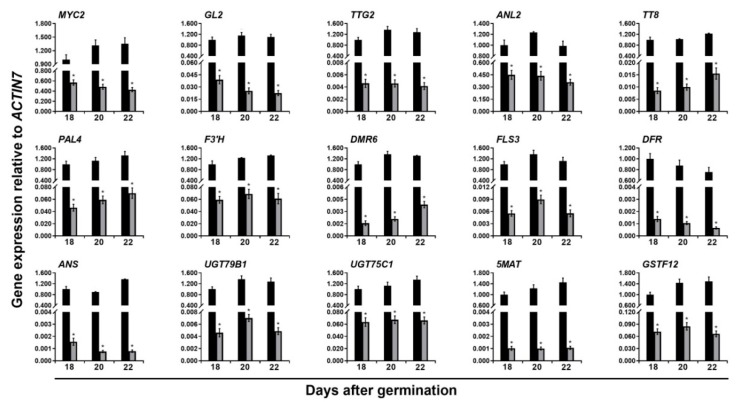
To further confirm the expression of these downregulated genes related to trichome formation and flavonoid accumulation, quantitative real-time PCR (qRT-PCR) was then used to analyze their dynamic expression levels in the ttg1-13 young shoots and expanding true leaves at 18, 20, and 22 DAG. The results showed that the expression levels of all these genes involved in trichome formation and flavonoid accumulation are significantly reduced at 20 DAG, which is highly consistent with the RNA-seq results (Figure 1 and Figure 2; Table 1 and Table 2, Tables S1 and S2). For the trichome-inducing genes, the expression levels of 9 regulatory genes, SQUAMOSA PROMOTER BINDING PROTEIN–LIKE 8 (SPL8), ENHANCER OF TRYAND CPC 1 (ETC1), BRANCHLESS TRICHOMES (BLT), HOMEODOMAIN GLABROUS11 (HDG11), CPC, MYB106, MYB5, SIAMESE (SIM), and MYB23, and 1 structural gene, SMALLER WITH VARIABLE BRANCHES (SVB), were always significantly lower in the ttg1-13 young shoots and expanding true leaves relative to the wild type plants at 18 and 22 DAG (Figure 1; Table 1 and Table S1). On the other hand, the expression levels of 11 genes related to flavonoid biosynthesis were also greatly downregulated in the ttg1-13 young shoots and expanding true leaves at 18 and 22 DAG, which includes 1 regulatory gene, ANTHOCYANINLESS2 (ANL2), and 10 structural genes in the flavonoid biosynthetic pathway, PHENYLALANINE AMMONIA-LYASE4 (PAL4), FLAVONOID 3’-HYDROXYLASE (F3’H), DOWNY MILDEW RESISTANT 6 (DMR6), FLAVONOL SYNTHASE3 (FLS3), DIHYDROFLAVONOL REDUCTASE (DFR), ANTHOCYANIDIN SYNTHASE (ANS), ANTHOCYANIN 3-O-GLUCOSIDE: 2’’-O-XYLOSYLTRANSFERASE (UGT79B1), ANTHOCYANIN 5-O-GLUCOSYLTRANSFERASE (UGT75C1), ANTHOCYANIN 5-O-GLUCOSIDE-6’’-O-MALONYLTRANSFERASE (5MAT), and GLUTATHIONE S-TRANSFERASE F12 (GSTF12) (Figure 2; Table 2 and Table S1). It is notable that 4 regulatory genes involved in both trichome formation and flavonoid accumulation, namely MYC2, GL2, TTG2, and TT8, were dramatically downregulated in the ttg1-13 young shoots and expanding true leaves at 18 and 22 DAG (Figure 1 and Figure 2; Table 1 and Table 2, and Table S1). Previous studies have demonstrated that TTG2, TT8, DFR, F3’H, and UGT79B1 are targets of TTG1 [25,44,69], which is a proof of our successful gene expression analysis in this study. Taken together, these results further indicated that the TTG1-dependent MBW complex promotes trichome formation and flavonoid accumulation by activating a series of regulatory and structural genes involved in trichome development and flavonoid biosynthesis, respectively, in A. thaliana young shoots and expanding true leaves.
Figure 1.
Dynamic expression analysis of differentially expressed genes involved in trichome formation in the young shoots and expanding true leaves between the wild type ( ) and ttg1-13 (
) and ttg1-13 ( ) plants at 18, 20, and 22 days after germination. The house-keeping gene ACTIN7 was used as the internal control. The expression of each gene was first calculated relative to ACTIN7 and then normalized to its expression level at 18 days after germination in the wild type that was set to 1. Asterisks indicate significant differences in gene expression compared with the wild type control (two-tailed paired Student’s t test, p ≤ 0.05). Values are means ± SD (n = 3). Error bars denote SD.
) plants at 18, 20, and 22 days after germination. The house-keeping gene ACTIN7 was used as the internal control. The expression of each gene was first calculated relative to ACTIN7 and then normalized to its expression level at 18 days after germination in the wild type that was set to 1. Asterisks indicate significant differences in gene expression compared with the wild type control (two-tailed paired Student’s t test, p ≤ 0.05). Values are means ± SD (n = 3). Error bars denote SD.
Figure 2.
Dynamic expression analysis of downregulated genes involved in flavonoid biosynthesis in the young shoots and expanding true leaves between the wild type ( ) and (
) and ( ) ttg1-13 plants at 18, 20, and 22 days after germination. The house-keeping gene ACTIN7 was used as the internal control. The expression of each gene was first calculated relative to ACTIN7 and then normalized to its expression level at 18 days after germination in the wild type that was set to 1. Asterisks indicate significant differences in gene expression compared with the wild type control (two-tailed paired Student’s t test, p ≤ 0.05). Values are means ± SD (n = 3). Error bars denote SD.
) ttg1-13 plants at 18, 20, and 22 days after germination. The house-keeping gene ACTIN7 was used as the internal control. The expression of each gene was first calculated relative to ACTIN7 and then normalized to its expression level at 18 days after germination in the wild type that was set to 1. Asterisks indicate significant differences in gene expression compared with the wild type control (two-tailed paired Student’s t test, p ≤ 0.05). Values are means ± SD (n = 3). Error bars denote SD.
2.3. Identification of Direct Downstream Targets Contributing to Trichome Formation and Flavonoid Accumulation Regulated by the TTG1-Dependent MBW Complex in Young Shoots and Expanding True Leaves
To investigate how the TTG1-dependent MBW complex controls the mRNA expression of downstream targeted genes, we created a steroid-inducible version of TTG1 in the background of ttg1-13, in which the TTG1 gene was fused to the rat glucocorticoid receptor (GR) and driven by the 35S promoter. We isolated a ttg1-13 35S:TTG1-GR transgenic line, which fully rescued the phenotypes of ttg1-13 leaves without trichomes and flavonoids after DEX treatment every other day after germination (Figure S2), whereas the mock-treated ttg1-13 35S:TTG1-GR exhibited similar trichome and flavonoid phenotypes in leaves with ttg1-13 (Figures S1 and S2). This indicated that the TTG1-GR fusion protein has a biological function like that of the wild type TTG1 upon steroid induction.
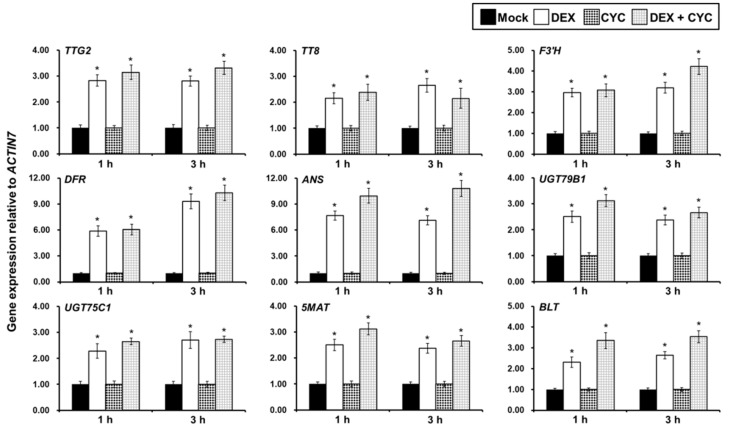
By utilizing the established steroid-inducible activation of TTG1, we further detected whether the expression of these downregulated genes is activated by TTG1 activity (Figure 1 and Figure 2). DEX treatment of ttg1-13 35S:TTG1-GR young shoots and expanding true leaves at 20 DAG for 1 or 3 h resulted in a significant increase in the expression of TTG2, TT8, F3’H, DFR, ANS, UGT79B1, UGT75C1, 5MAT, BLT, ANL2, PAL4, DMR6, FLS3, GSTF12, MYB5, and MYB23, compared with that of the mock-treated controls (Figure 3 and Figure S3). To determine whether 35S:TTG1-GR directly or indirectly represses these genes, we repeated DEX applications to the 35S:TTG1-GR young shoots and expanding true leaves of the ttg1-13 background in the presence of the protein synthesis inhibitor cycloheximide (CYC), because the induction of TTG1-GR activity by DEX does not require protein synthesis. We found that the combined treatment of DEX and CYC for 1 or 3 h only dramatically promoted the expression of TTG2, TT8, F3’H, DFR, ANS, UGT79B1, UGT75C1, 5MAT, and BLT (Figure 3 and Figure S3). These indicated that these 9 genes are immediate targets transcriptionally induced by the TTG1-dependent MBW complex in the young shoots and expanding true leaves (Figure 3), whereas the activation of the other 12 genes in the ttg1-13 mutant is reliable on other intermediate proteins (Figure S3).
Figure 3.
Induced TRANSPARENT TESTA GLABRA1 (TTG1) activity transcriptionally promotes the expression of several genes involved in flavonoid biosynthesis, including TTG2, TT8, F3’H, DFR, ANS, UGT79B1, UGT75C1, and 5MAT, and the gene related to trichome formation BLT in the young shoots and expanding true leaves. The ttg1-13 35S:TTG1-GR young shoots and expanding true leaves at 20 days after germination were mock-treated (Mock) or treated with 10 μM dexamethasone (DEX), 5 μM cycloheximide (CYC), or 10 μM DEX plus 5 μM CYC (DEX + CYC). The expression of these genes was determined after 1 or 3 h of treatment using qRT-PCR analyses. The house-keeping gene ACTIN7 was used as the internal control. The expression level of each gene was first calculated relative to ACTIN7, and its expression levels in both Mock and CYC treatments were set to 1. Asterisks indicate significant differences in gene expression in DEX-treated samples compared with their respective controls (two-tailed paired Student’s t test, p ≤ 0.05). Values are means ± SD (n = 3). Error bars denote SD.
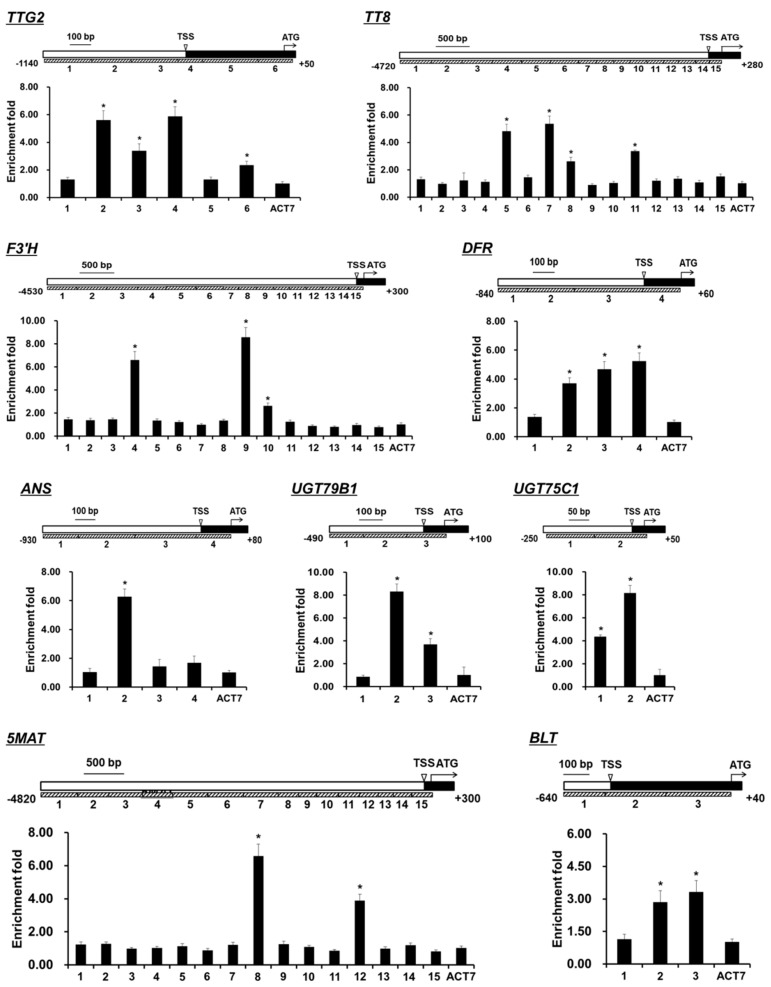
To explore whether the TTG1-dependent MBW complex binds directly to the promoter regions of TTG2, TT8, F3’H, DFR, ANS, UGT79B1, UGT75C1, 5MAT, and BLT to regulate their expressions, the young shoots and expanding true leaves from mock- or DEX-treated ttg1-13 35S:TTG1-GR plants with were used for the ChIP assay. To cover all the possible cis-elements, sufficient pairs of primers were designed in the promoter regions of these 9 genes (Figure 4). The ChIP results showed that TTG1-GR was associated with the promoter regions near fragments 2, 3, and 4 of TTG2, fragments 5, 7, 8, and 11 of TT8, fragments 4, 9, and 10 of F3’H, fragments 2, 3, and 4 of DFR, fragment 2 of ANS, fragments 2 and 3 of UGT79B1, fragments 1 and 2 of UGT75C1, fragments 8 and 12 of 5MAT, and fragments 2 and 3 of BLT (Figure 4). These results collectively suggested that the TTG1-dependent MBW complex binds directly to the loci of TTG2, TT8, F3’H, DFR, ANS, UGT79B1, UGT75C1, 5MAT, and BLT to promote their expression.
Figure 4.
Schematic diagrams illustrate the promoter regions of TTG2, TT8, F3’H, DFR, ANS, UGT79B1, UGT75C1, 5MAT, and BLT, and ChIP analysis indicates the TTG1-dependent MBW complex binding to their promoter regions in in the young shoots and expanding true leaves at 20 days after germination. The transcriptional start site (TSS) and exon are indicated by black boxes, whereas promoter regions are indicated by white boxes. Gray boxes represent the DNA fragments amplified in ChIP analysis for each gene. The enrichment fold of each fragment was calculated first by normalizing the amount of a target DNA fragment against a genomic fragment of EF1aA4 as an internal control and then by normalizing the value for dexamethasone-treated ttg1-13 35S:TTG1-GR against that for mock-treated ttg1-13 35S:TTG1-GR. An ACTIN7 (ACT7) fragment was amplified as a negative control. Asterisks indicate significant differences in comparison with the enrichment of the ACT7 fragment (two-tailed paired Student’s t test, p ≤ 0.05). Values are means ± SD (n = 3). Error bars denote SD.
3. Discussion
Transcriptional regulation is considered to be the essential mechanism controlling the gene expression of metabolic pathways in higher plants. Extensive studies have elucidated the TTG1-dependent MBW complex that controls trichome formation and flavonoid accumulation in A. thaliana. However, its direct targeted genes are still largely unknown in the regulation of trichome formation and flavonoid accumulation in A. thaliana. In this study, we identified a series of new downstream targeted genes for trichome formation and flavonoid accumulation directly or indirectly regulated by the TTG1-dependent MBW complex at the genome-wide level in A. thaliana young shoots and expanding true leaves.
We found that there were 4 downregulated regulatory genes involved in both trichome formation and flavonoid biosynthesis, TTG2, TT8, GL2, and MYC2 (Figure 1 and Figure 2; Table 1 and Table 2), among which the former two genes and the latter two genes were directly and indirectly regulated by the TTG1-dependent MBW complex (Figure 3 and Figure 4, and Figure S3), in the ttg1-13 young shoots and expanding true leaves. The TTG2 protein encoding a WRKY transcription factor plays positive roles in trichome initiation and flavonoid accumulation and is also directly induced by TTG1 [25,57], which is consistent with our results (Figure 3 and Figure 4). The TT8 transcription factor is essential for trichome initiation in the margin of true leaves [62] and positively regulates flavonoid biosynthesis [68,69]. GL2 functions as a transcriptional activator and repressor in trichome initiation [22,23,24] and anthocyanin biosynthesis [66], respectively. The previous study showed that GL2 inhibits anthocyanin biosynthesis by directly repressing the expression of PAP1, PAP2, MYB113, MYB114, and TT8 [66], which theoretically should be increased because of the lower expression of GL2 in the ttg1-13 young shoots and expanding true leaves. However, the TT8 expression was significantly reduced (Figure 1 and Figure 2; Table 1 and Table 2, and Table S1), and the expression of PAP1, PAP2, MYB113, and MYB114 was not altered (Tables S1 and S2). Therefore, it can be deduced that the TTG1-dependent MBW complex promotes TT8 expression probably independent of GL2, or the simulative effect of the TTG1-dependent MBW complex and/or other downregulated regulatory genes is stronger than the inhibitory effect of GL2 on the TT8 expression, in the young shoots and expanding true leaves. Similarly, the simulative effect of the TTG1-dependent MBW complex and/or other downregulated regulatory genes is comparable to the inhibitory effect of GL2 on the expression of PAP1, PAP2, MYB113, and MYB114. These interesting questions need further investigation. Genetic analysis showed that the double mutant of ttg2-1 gl2-1 has more defective trichome development than in either single mutant, and TTG2 positively regulates GL2 [57]. Thus, it is possible that the reduced expression of GL2 is caused by the lower expression of TTG2, which would be in accordance with GL2 being indirectly regulated by the TTG1-dependent MBW complex (Figure S3). The bHLH transcription factor MYC2 as the central mediator of the phytohormone jasmonic acid (JA) signaling positively regulates trichome initiation and flavonoid accumulation [50,51,52,53]. The previous study indicated that all the three phytohormones inclusive of JA, gibberellin A3 (GA3), and cytokinin (6-benzylaminopurine) stimulate trichome initiation not through regulating the TTG1 expression, and whereas both JA and 6-benzylaminopurine promote anthocyanin production, GA3 does not [62]. The expression of JA biosynthetic genes was not altered in the ttg1-13 young shoots and expanding true leaves (Tables S1 and S2), indicating that the TTG1-dependent MBW complex and JA function in an independent manner in inducing the expression of MYC2, thus promoting trichome formation and flavonoid accumulation. Gibberellin 2-β-DIOXYGENASE6 (GA2OX6) encoding a GA 2-oxidase functions in converting bioactive gibberellins and their precursors into inactive forms, thus reducing endogenous bioactive gibberellins [83]. CYTOKININ DEHYDROGENASE4 (CKX4) encodes a cytokinin oxidase that is responsible for the cytokinin accumulation [84]. The expression levels of GA2OX6 and CKX4 were significantly decreased in the ttg1-13 young shoots and expanding true leaves (Table S1), suggesting that the TTG1-dependent MBW complex promotes trichome formation, and flavonoid accumulation might rely on cytokinin, but not gibberellins.
We demonstrated that the TTG1-dependent MBW complex directly promoted the expression of the regulatory gene BLT (Figure 3 and Figure 4) and indirectly activated the expression of the regulatory genes SPL8, ETC1, HDG11, CPC, MYB106, MYB5, SIM, and MYB23, and the structural gene SVB during trichome formation in the young shoots and expanding true leaves (Figure S3). BLT encodes a key regulator of trichome branching, and its mutation results in the formation of branchless trichomes with blunt tips [54,55]. SPL8 positively regulates the trichome number on flower sepals [48]. ETC1 acts in concert with CPC to repress the trichome cell fate in the shoot epidermis [49]. SVB is positively correlated with trichome size, and its mutant exhibits trichome branches of variable length and number [65]. HDG11 encodes a homeodomain leucine zipper transcription factor like GL2 and displays a positive role in trichome differentiation [56]. MYB106 functions as an activator in trichome differentiation [58], and a repressor in trichome branching [59]. Loss of functions of MYB5 and MYB23 display increased numbers of small and two-branched trichomes, respectively [60,61]. Further genetic analysis indicated that MYB5 and MYB23 are partially redundant in repressing trichome branching [60]. MYB23 is directly activated by GL2 during trichome formation [56], suggesting that the TTG1-dependent MBW complex promotes the expression of MYB23 possibly via the direct activation of GL2. SIM encodes a member of plant-specific cyclin-dependent kinase inhibitors, and its mutation results in multicellular trichomes [63,64]. Considering that there is no trichome present in the ttg1-13 young shoots and expanding true leaves (Figure S1), it is plausible to assume that the TTG1-dependent MBW complex promotes trichome formation first by activating the expression of the genes that induce trichome initiation and differentiation, including TTG2, GL2, MYC2, SPL8, HDG11, and MYB106, and then by promoting the other genes that positively regulates branching, size, and cell number, including BLT, MYB5, SIM, MYB23, and SVB, in the young shoots and expanding true leaves. Further, the TTG1-dependent MBW complex might be independent of ETC1 and CPC or might be due to the fact that the negative influence of the two genes is noncompetitive with the positive effect of other genes during trichome formation in the young shoots and expanding true leaves.
On the other hand, we proved that the TTG1-dependent MBW complex directly promoted the expression of the regulatory gene TT8 and the structural genes F3’H, DFR, ANS, UGT79B1, UGT75C1, and 5MAT (Figure 3 and Figure 4), and indirectly activated the expression of the regulatory gene ANL2 and the structural genes PAL4, DMR6, FLS3, and GSTF12 during flavonoid biosynthesis in the young shoots and expanding true leaves (Figure S3). PAL comprises four isoforms of PAL1, PAL2, PAL3, and PAL4 that convert phenylalanine into trans-cinnamic acid, which is the first step in the flavonoid biosynthetic pathway in A. thaliana [70,71]. F3’H encoding a cytochrome P450 monooxygenase catalyzes the formation of eriodictyol and dihydroquercetin from naringenin and dihydrokaempferol, respectively [70,72]. DMR6 is a flavone synthase I enzyme that catalyzes the conversion of the flavanones into flavones [73]. FLS acts as the first committed enzyme for flavonol biosynthesis [85,86], and there are five FLS genes in the A. thaliana genome, among which FLS3 exhibits the FLS activity, promoting flavonol accumulation [74]. DFR catalyzes the formation of leucoanthocyanidins from dihydroflavonols [2,75]. ANS encoding a 2-oxoglutarate-dependent dioxygenase catalyzes the conversion of leucoanthocyanidins into 3-OH-anthocyanins [76,77]. UGT79B1 is involved in the glycosylation of anthocyanins at the C-5 position, and its knockout mutant contains a drastically decreased content of anthocyanins [70,78]. Another UDP-glucose: UGT75C1 is involved in the malonylation of anthocyanins, and its mutation results in the complete loss of anthocyanin 5-O-glucosides [79]. 5MAT is specific for malonyl–CoA and for anthocyanins with 5-O-glucosylation and accelerates the accumulation of malonylated anthocyanins [80]. GSTF12, a member of GST-like proteins, is involved in transport and promotes anthocyanin accumulation [81,82]. ANL2, as a member of homeodomain proteins, positively regulates anthocyanin accumulation in shoot cells [67]. These suggested that the TTG1-dependent MBW complex promotes flavonoid biosynthesis directly or indirectly through a series of regulatory and structural genes, and TTG1-mediated flavonoid biosynthesis might be independent of GL2 or might be due to the fact that the negative influence of GL2 is noncompetitive with the positive effect of other genes, in the young shoots and expanding true leaves.
Previous studies indicated that the TTG1-dependent MBW complex has no obvious effect on MYB5 expression in seeds [60] and directly activates BAN expression in siliques [46]. Here, we found that MYB5 and BAN were indirectly activated and not regulated, respectively, in the ttg1-13 young shoots and expanding true leaves (Figure S3; Tables S1 and S2), indicating that the TTG1-dependent MBW complex regulates gene expression is tissue-dependent during trichome formation and flavonoid accumulation. It is worth noting that 8 and 7 downregulated genes involved in flavonoid accumulation were directly and indirectly regulated by the TTG1-dependent MBW complex, whereas 2 and 11 downregulated genes related to trichome formation were directly and indirectly regulated by the TTG1-dependent MBW complex in the young shoots and expanding true leaves (Figure 3 and Figure 4, and Figure S3; Table 1 and Table 2, Tables S1 and S2), suggesting that the TTG1-dependent MBW complex is likely to be a key direct and indirect transcription factor in regulating flavonoid accumulation and trichome formation, respectively.
In the present study, we report on direct targets of the TTG1-dependent MBW complex, revealing that the TTG1-dependent MBW complex functions primarily or exclusively as a transcriptional direct and indirect activator in the regulation of trichome formation and flavonoid accumulation, respectively, in the young shoots and expanding true leaves of A. thaliana.
4. Materials and Methods
4.1. Plant Material and Growth Condition
The A. thaliana ecotype Columbia (Col-0) was used as the wild type control. The ttg1-13 mutant was utilized in our previous study [14]. The growth condition of A. thaliana plants has been described previously [14].
4.2. Generation of Transgenic Plants
The construct of 35S:TTG1-GR, which was created in our previous study [7], was transformed into the ttg1-13 mutant via floral dip [87]. The ttg1-13 35S:TTG1-GR transgenic plants were selected by Basta on soil and also verified by DNA analysis until T3 homozygous transgenic progeny was generated.
4.3. RNA Sequencing (RNA-seq) and Data Analyses
The samples of young shoots and expanding true leaves used for RNA-seq analysis were carefully harvested from Col-0 and ttg1-13 plants at 20 DAG following the previously reported method [88]. Three independent biological replicates from three different plantings were performed in the RNA-seq experiment. The RNA-seq and data analysis were carried out through the Gene Denovo service (http://www.genedenovo.com/) following the standard protocol (http://www.genedenovo.com/product/41.html): (1) The quality and quantity of the isolated RNA samples were assessed by Nanodrop 2000 Spectrophotometer (Thermo, Wilmington, NC, USA) and Agilent 2100 Bioanalyzer (Agilent, Böblingen, Germany), (2) removement of the possible DNA contamination with DNase I, (3) mRNA enrichment and fragmentation, (4) sequencing adaptor ligation and PCR amplification, and (5) assessment on quality and quantity of the sample library. Finally, the cDNA library products were utilized for sequenced analysis via the Illumina HiSeq™ 2000. Transcript abundance was calculated as RPKM (reads per Kb per million reads) [89]. RPKM values presenting as “0” were artificially set to “0.001” for subsequent analysis. Comparisons of RPKM between treatments (WT (Col-0) vs. ttg1-13) were performed for each Unigene. The DEGs were functionally classified using the biological process category of Arabidopsis Gene Ontology (http://www.geneontology.org). A differential expression analysis of the two treatments was conducted using the DESeq R package (1.10.1). The resulting p-values were adjusted using Benjamini and Hochberg’s approach for controlling the false discovery rate (FDR). The DEGs with |log2 ratios| ≥ 1 and FDR ≤ 0.05 are functionally categorized and listed in Tables S1 and S2.
4.4. Gene Expression Analysis
The tissues of young shoots and expanding true leaves used for gene expression analysis are the same as those in the RNA-seq experiment. They were collected from eight individual plants at 20 DAG, and three independent biological replicates were performed for gene expression analysis. Total RNA extraction and first-strand cDNA synthesis were conducted according to the method previously described [90]. The SYBR Green Master Mix (TaKaRa Bio, Dalian, China) was utilized for qRT-PCR reaction with QuantStudioTM 7 Flex Real-Time PCR System (Life technologies, Carlsbad, CA, USA), and the relative gene expression level was calculated as reported previously [91]. The house-keeping gene ACTIN7 was used as the internal control. Primers used for qRT-PCR analysis are listed in Table S3.
4.5. GR Induction
For the induction of TTG1:GR, young shoots and expanding true leaves were harvested from 8 individual plants at 20 DAG 1 and 3 h after different treatments of mock, DEX, CYC, and DEX plus CYC. The concentrations of DEX and CYC used here were 10 and 5 μM, respectively.
4.6. ChIP Assay
The tissues of young shoots and expanding true leaves at 20 DAG used for ChIP assay are the same as those in the RNA-seq experiment. The ttg1-13 35S:TTG1-GR plants after germination were treated for 10 days with mock and 10 μM DEX every other day, after which they were used for the ChIP experiment. The ChIP assay was carried out as described previously [7]. In brief, 3 g of young shoots and expanding true leaves were fixed with 37% formaldehyde. After nuclear protein–DNA extraction and sonication, immunoprecipitation was conducted using a GR antibody coupled to magnetic beads. The relative enrichment of each fragment was detected by qRT-PCR, and the ChIP experiment was performed in three biological replicates. Primers used for ChIP assay are listed in Table S3.
4.7. Statistical Analysis
Completely randomized block designs were utilized in three biological replicates. Data were analyzed with use of the SPSS statistical package (version 8.0). The two-tailed paired Student’s t-test was used to analyze gene expression. p values ≤ 0.05 indicated a statistically significant difference.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/20/20/5014/s1. Supplementary Figure S1. The schematic diagram shows tissues from the wild type (Col-0) and ttg1-13 plants at 20 days after germination harvested for the RNA-seq experiment. The yellow crosses indicate that the whole leaves, including blades and petioles, were removed completely, whereas the red ellipses indicate that the tissues, including young shoots and expanding true leaves, were collected for RNA-seq analysis. Supplementary Figure S2. Generation of a ttg1-13 35S:TTG1-GR transgenic line containing the biologically active TTG1-GR fusion, and the tissues of young shoots and expanding true leaves indicated by the red ellipses were collected for GR induction and ChIP analyses. Individual seedlings of ttg1-13 35S:TTG1-GR plants were mock-treated (Mock, left) or treated with 10 μM dexamethasone (Dex, right) every other day after germination. Mock plants generate leaves without flavonoids and trichomes as ttg1-13, whereas DEX treatment rescues these phenotypes. Supplementary Figure S3. The genes involved in flavonoid biosynthesis and trichome formation are not immediate targets of transcriptional regulation by TTG1 in the young shoots and expanding true leaves. The ttg1-13 35S:TTG1-GR young shoots and expanding true leaves at 20 days after germination were mock-treated (Mock) or treated with 10 μM dexamethasone (DEX), 5 μM cycloheximide (CYC), or 10 μM DEX plus 5 μM CYC (DEX + CYC). The expression of these genes was determined after 1 or 3 h of treatment using qRT-PCR analyses. The house-keeping gene ACTIN7 was used as the internal control. The expression level of each gene was first calculated relative to ACTIN7, and its expression levels in both Mock and CYC treatments were set to 1. Asterisks indicate significant differences in gene expression in DEX-treated samples compared with their respective controls (two-tailed paired Student’s t test, p ≤ 0.05). Values are means ± SD (n = 3). Error bars denote SD. Supplementary Table S1. A list of genes expressed at lower levels (‘downregulated’) in the young shoots and expanding true leaves of ttg1-13 plants than in wild type rosette leaves at 20 days after germination. Supplementary Table S2. A list of genes expressed at higher levels (‘upregulated’) in the young shoots and expanding true leaves of ttg1-13 plants than in wild type rosette leaves at 20 days after germination. Supplementary Table S3. Primers used in the present study.
Author Contributions
Conceived the project and designed the experiment plans: Z.W., Y.C., and M.C.; Performed the experiments: Z.W., Y.C., C.Z., and D.L.; Analyzed the data and wrote the article: Z.W., Y.C., X.G., S.Z., and M.C.
Funding
The work was supported by National Natural Science Foundation of China (Grant nos. 31971974 and 31501336), Shaanxi Youth Science and Technology New Star (Grant no. 2018KJXX-041), General Agricultural Project of Shaanxi Province (2019NY-016), Programme of Introducing Talents of Innovative Discipline to Universities (Project 111) from the State Administration of Foreign Experts Affairs (#B18042) “Crop breeding for disease resistance and genetic improvement”, and Young Elite Scientists Sponsorship Program by CAST (Grant no. 2016QNRC001).
Conflicts of Interest
The authors declare no conflict of interest. Besides, the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Koornneef M. The complex syndrome of ttg mutants. Arabidopsis Inf. Serv. 1981;18:45–51. [Google Scholar]
- 2.Shirley B.W., Kubasek W.L., Storz G., Bruggemann E., Koornneef M., Ausubel F.M., Goodman H.M. Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. Plant J. 1995;8:659–671. doi: 10.1046/j.1365-313X.1995.08050659.x. [DOI] [PubMed] [Google Scholar]
- 3.Walker A.R., Davison P.A., Bolognesi-Winfield A.C., James C.M., Srinivasan N., Blundell T.L., Esch J.J., Marks M.D., Gray J.C. The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. Plant Cell. 1999;11:1337–1350. doi: 10.1105/tpc.11.7.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debeaujon I., Leon-Kloosterziel K.M., Koornneef M. Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol. 2000;122:403–413. doi: 10.1104/pp.122.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Western T.L., Burn J., Tan W.L., Skinner D.J., Martin-McCaffrey L., Moffatt B.A., Haughn G.W. Isolation and characterization of mutants defective in seed coat mucilage secretory cell development in Arabidopsis. Plant Physiol. 2001;127:998–1011. doi: 10.1104/pp.010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuchiya Y., Nambara E., Naito S., McCourt P. The FUS3 transcription factor functions through the epidermal regulator TTG1 during embryogenesis in Arabidopsis. Plant J. 2004;37:73–81. doi: 10.1046/j.1365-313X.2003.01939.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen M., Zhang B., Li C., Kulaveerasingam H., Chew F.T., Yu H. TRANSPARENT TESTA GLABRA1 regulates the accumulation of seed storage reserves in Arabidopsis. Plant Physiol. 2015;169:391–402. doi: 10.1104/pp.15.00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C., Zhang B., Chen B., Ji L., Hao Y. Site-specific phosphorylation of TRANSPARENT TESTA GLABRA1 mediates carbon partitioning in Arabidopsis seeds. Nat. Commun. 2018;9:571. doi: 10.1038/s41467-018-03013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lepiniec L., Debeaujon I., Routaboul J.M., Baudry A., Pourcel L., Nesi N., Caboche M. Genetics and biochemistry of seed flavonoids. Ann. Rev. Plant Biol. 2006;57:405–430. doi: 10.1146/annurev.arplant.57.032905.105252. [DOI] [PubMed] [Google Scholar]
- 10.Windsor J.B., Symonds V.V., Mendenhall J., Lloyd A.M. Arabidopsis seed coat development: Morphological differentiation of the outer integument. Plant J. 2000;22:483–493. doi: 10.1046/j.1365-313x.2000.00756.x. [DOI] [PubMed] [Google Scholar]
- 11.Penfield S., Meissner R.C., Shoue D.A., Carpita N.C., Bevan M.W. MYB61 is required for mucilage deposition and extrusion in the Arabidopsis seed coat. Plant Cell. 2001;13:2777–2791. doi: 10.1105/tpc.010265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tominaga-Wada R., Ishida T., Wada T. New insights into the mechanism of development of Arabidopsis root hairs and trichomes. Int. Rev. Cell Mol. Bio. 2011;286:67–106. doi: 10.1016/B978-0-12-385859-7.00002-1. [DOI] [PubMed] [Google Scholar]
- 13.Hoai Nguyen N., Jun Hyeok K., Woo Young H., Ngoc Trinh N., Suk-Whan H., Hojoung L. TTG1-mediated flavonols biosynthesis alleviates root growth inhibition in response to ABA. Plant Cell Rep. 2013;32:503–514. doi: 10.1007/s00299-012-1382-1. [DOI] [PubMed] [Google Scholar]
- 14.Liu K., Qi S., Li D., Jin C., Gao C., Duan S., Feng B., Chen M. TRANSPARENT TESTA GLABRA 1 ubiquitously regulates plant growth and development from Arabidopsis to foxtail millet (Setaria italica) Plant Sci. 2016;254:60–69. doi: 10.1016/j.plantsci.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Serna L., Martin C. Trichomes: Different regulatory networks lead to convergent structures. Trends Plant Sci. 2006;11:274–280. doi: 10.1016/j.tplants.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Broun P. Transcriptional control of flavonoid biosynthesis: A complex network of conserved regulators involved in multiple aspects of differentiation in Arabidopsis. Curr. Opin. Plant Biol. 2005;8:272–279. doi: 10.1016/j.pbi.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Traw M.B., Bergelson J. Interactive effects of jasmonic acid, salicylic acid, and gibberellin on induction of trichomes in Arabidopsis. Plant Physiol. 2003;133:1367–1375. doi: 10.1104/pp.103.027086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mauricio R. Ontogenetics of QTL: The genetic architecture of trichome density over time in Arabidopsis thaliana. Genetica. 2005;123:75–85. doi: 10.1007/s10709-002-2714-9. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y., Jing S.X., Luo S.H., Li S.H. Non-volatile natural products in plant glandular trichomes: Chemistry, biological activities and biosynthesis. Nat. prod. Rep. 2019;17:626–665. doi: 10.1039/C8NP00077H. [DOI] [PubMed] [Google Scholar]
- 20.Ramsay N.A., Glover B.J. MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci. 2005;10:63–70. doi: 10.1016/j.tplants.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 21.Schellmann S., Hulskamp M., Uhrig J. Epidermal pattern formation in the root and shoot of Arabidopsis. Biochem. Soc. T. 2007;35:146–148. doi: 10.1042/BST0350146. [DOI] [PubMed] [Google Scholar]
- 22.Ishida T., Kurata T., Okada K., Wada T. A genetic regulatory network in the development of trichomes and root hairs. Annu. Rev. Plant Biol. 2008;59:365–386. doi: 10.1146/annurev.arplant.59.032607.092949. [DOI] [PubMed] [Google Scholar]
- 23.Pesch M., Hülskamp M. One, two, three models for trichome patterning in Arabidopsis? Curr. Opin. Plant Biol. 2009;12:587–592. doi: 10.1016/j.pbi.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Wang S.C., Chen J.G. Regulation of cell fate determination by single-repeat R3 MYB transcription factors in Arabidopsis. Front Plant Sci. 2014;5:133. doi: 10.3389/fpls.2014.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao M., Morohashi K., Hatlestad G., Grotewold E., Lloyd A. The TTG1-bHLH-MYB complex controls trichome cell fate and patterning through direct targeting of regulatory loci. Development. 2008;135:1991–1999. doi: 10.1242/dev.016873. [DOI] [PubMed] [Google Scholar]
- 26.Pesch M., Schultheiss I., Klopffleisch K., Uhrig J.F., Koegl M., Clemen C.S., Simon R., Weidtkamp-Peters S., Hulskamp M. TRANSPARENT TESTA GLABRA1 and GLABRA1 Compete for Binding to GLABRA3 in Arabidopsis. Plant Physiol. 2015;168:584–597. doi: 10.1104/pp.15.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nesi N., Jond C., Debeaujon I., Caboche M., Lepiniec L. The Arabidopsis TT2 gene encodes an R2R3 MYB domain protein that acts as a key determinant for proanthocyanidin accumulation in developing seed. Plant Cell. 2001;13:2099–2114. doi: 10.1105/TPC.010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang B.P., Schrader A. TRANSPARENT TESTA GLABRA 1-dependent regulation of flavonoid biosynthesis. Plants. 2017;6:E65. doi: 10.3390/plants6040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mol J., Grotewold E., Koes R. How genes paint flowers and seeds. Trends Plant Sci. 1998;3:212–217. doi: 10.1016/S1360-1385(98)01242-4. [DOI] [Google Scholar]
- 30.Feild T.S., Lee D.W., Holbrook N.M. Why leaves turn red in autumn. The role of anthocyanins in senescing leaves of red-osier dogwood. Plant Physiol. 2001;127:566–574. doi: 10.1104/pp.010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winkel-Shirley B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002;5:218–223. doi: 10.1016/S1369-5266(02)00256-X. [DOI] [PubMed] [Google Scholar]
- 32.Bradshaw H.D., Schemske D.W. Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature. 2003;426:176–178. doi: 10.1038/nature02106. [DOI] [PubMed] [Google Scholar]
- 33.Iwashina T. The flavonoids occurring in plants, and their functions and activities to other organisms. Plant Cell Physiol. 2003;44:S6. [Google Scholar]
- 34.Hassan S., Mathesius U. The role of flavonoids in root–rhizosphere signalling: Opportunities and challenges for improving plant–microbe interactions. J. Exp. Bot. 2012;63:3429–3444. doi: 10.1093/jxb/err430. [DOI] [PubMed] [Google Scholar]
- 35.Carletti G., Nervo G., Cattivelli L. Flavonoids and Melanins: A Common Strategy across Two Kingdoms. Int. J. Biol. Sci. 2014;10:1159–1170. doi: 10.7150/ijbs.9672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaziano M., Riceevans C. Epidemiology of flavonoids and coronary heart disease. Int. Congress Symp. Ser. R. Soc. Med. 2000;17:53–61. [Google Scholar]
- 37.Lee E.R., Kang G.H., Cho S.G. Effect of flavonoids on human health: Old subjects but new challenges. Recent Pat. Biotechnol. 2007;1:139–150. doi: 10.2174/187220807780809445. [DOI] [PubMed] [Google Scholar]
- 38.Jadeja R.N., Devkar R.V. Chapter 47–Polyphenols and Flavonoids in Controlling Non-Alcoholic Steatohepatitis. Polyphenols Hum. Health Dis. 2014;1:615–623. [Google Scholar]
- 39.Owaga E.E., Elbakkoush A., Sakhile K.S.M., Nyang’Inja R.A. Nutritional management of mental disorders: Potential role of dietary flavonoids and vitamin E. Food Public Health. 2014;4:104–109. [Google Scholar]
- 40.Prieto-Domínguez N., García-Mediavilla M.V., Campos S.S., Mauriz J.L., González-Gallego J. Autophagy as a Molecular Target of Flavonoids Underlying their Protective Effects in Human Disease. Curr. Med. Chem. 2017;25:814–838. doi: 10.2174/0929867324666170918125155. [DOI] [PubMed] [Google Scholar]
- 41.Borevitz J.O., Xia Y., Blount J., Dixon R.A., Lamb C. Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell. 2000;12:2383–2393. doi: 10.1105/tpc.12.12.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Payne C., Zhang F. Am, GL3 encodes a bHLH protein that regulates trichome development in arabidopsis through interaction with GL1 and TTG1. Genetics. 2000;156:1349–1362. doi: 10.1093/genetics/156.3.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang F., Gonzalez A.M., Payne C.T., Lloyd A. A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development. 2003;130:4859. doi: 10.1242/dev.00681. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez A., Zhao M., Leavitt J.M., Lloyd A.M. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 2008;53:814–827. doi: 10.1111/j.1365-313X.2007.03373.x. [DOI] [PubMed] [Google Scholar]
- 45.Zimmermann I.M., Heim M.A., Weisshaar B., Uhrig J.F. Comprehensive identification of Arabidopsis thaliana MYB transcription factors interacting with R/B-like BHLH proteins. Plant J. 2010;40:22–34. doi: 10.1111/j.1365-313X.2004.02183.x. [DOI] [PubMed] [Google Scholar]
- 46.Baudry A., Heim M.A., Dubreucq B., Caboche M., Weisshaar B., Lepiniec L. TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J. 2004;39:366–380. doi: 10.1111/j.1365-313X.2004.02138.x. [DOI] [PubMed] [Google Scholar]
- 47.Lee D.W. Anthocyanins in leaves: Distribution, phylogeny and development. Adv. Bot. Res. 2002;37:37–53. [Google Scholar]
- 48.Unte U.S., Sorensen A.M., Pesaresi P., Gandikota M., Leister D., Saedler H., Huijser P. SPL8, an SBP-Box gene that affects pollen sac development in Arabidopsis. Plant Cell. 2003;15:1009–1019. doi: 10.1105/tpc.010678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kirik V., Simon M., Huelskamp M., Schiefelbein J. The ENHANCER OF TRY AND CPC1 gene acts redundantly with TRIPTYCHON and CAPRICE in trichome and root hair cell patterning in Arabidopsis. Dev Biol. 2004;268:506–513. doi: 10.1016/j.ydbio.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 50.Kazan K., Manners J.M. MYC2: The master in Action. Mol. Plant. 2013;6:686–703. doi: 10.1093/mp/sss128. [DOI] [PubMed] [Google Scholar]
- 51.Goossens J., Mertens J., Goossens A. Role and functioning of bHLH transcription factors in jasmonate signalling. J. Exp. Bot. 2017;68:1333–1347. doi: 10.1093/jxb/erw440. [DOI] [PubMed] [Google Scholar]
- 52.Dombrecht B., Xue G.P., Sprague S.J., Kirkegaard J.A., Ross J.J., Reid J.B., Fitt G.P., Sewelam N., Schenk P.M., Manners J.M., et al. MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell. 2007;19:2225–2245. doi: 10.1105/tpc.106.048017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chini A., Gimenez-Ibanez S., Goossens A., Solano R. Redundancy and specificity in jasmonate signalling. Curr. Opin. Plant Biol. 2016;33:147–156. doi: 10.1016/j.pbi.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 54.Marks M.D., Wenger J.P., Gilding E., Jilk R., Dixon R.A. Transcriptome analysis of Arabidopsis wild-type and gl3-sst sim trichomes identifies four additional genes required for trichome development. Mol. Plant. 2009;2:803–822. doi: 10.1093/mp/ssp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kasili R., Huang C.C., Walker J.D., Simmons L.A., Zhou J., Faulk C., Hulskamp M., Larkin J.C. BRANCHLESS TRICHOMES links cell shape and cell cycle control in Arabidopsis trichomes. Development. 2011;138:2379–2388. doi: 10.1242/dev.058982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khosla A., Paper J.M., Boehler A.P., Bradley A.M., Neumann T.R., Schrick K. HD-Zip Proteins GL2 and HDG11 Have Redundant Functions in Arabidopsis Trichomes, and GL2 Activates a Positive Feedback Loop via MYB23. Plant Cell. 2014;26:2184–2200. doi: 10.1105/tpc.113.120360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ishida T., Hattori S., Sano R., Inoue K., Shirano Y., Hayashi H., Shibata D., Sato S., Kato T., Tabata S., et al. Arabidopsis TRANSPARENT TESTA GLABRA2 is directly regulated by R2R3 MYB transcription factors and is involved in regulation of GLABRA2 transcription in epidermal differentiation. Plant Cell. 2007;19:2531–2543. doi: 10.1105/tpc.107.052274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glover B.J., Perez-Rodriguez M., Martin C. Development of several epidermal cell types can be specified by the same MYB-related plant transcription factor. Development. 1998;125:3497–3508. doi: 10.1242/dev.125.17.3497. [DOI] [PubMed] [Google Scholar]
- 59.Jakoby M.J., Falkenhan D., Mader M.T., Brininstool G., Wischnitzki E., Platz N., Hudson A., Lskamp M.H.R., Larkin J., Schnittger A. Transcriptional Profiling of Mature Arabidopsis Trichomes Reveals That NOECK Encodes the MIXTA-Like Transcriptional Regulator MYB106. Plant Physiol. 2008;148:1583–1602. doi: 10.1104/pp.108.126979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li S.F., Milliken O.N., Pham H., Seyit R., Napoli R., Preston J., Koltunow A.M., Parisha R.W. The Arabidopsis MYB5 Transcription Factor Regulates Mucilage Synthesis, Seed Coat Development, and Trichome Morphogenesis. Plant Cell. 2009;21:72–89. doi: 10.1105/tpc.108.063503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kirik V., Lee M.M., Wester K., Herrmann U., Zheng Z.G., Oppenheimer D., Schiefelbein J., Hulskamp M. Functional diversification of MYB23 and GL1 genes in trichome morphogenesis and initiation. Development. 2005;132:1477–1485. doi: 10.1242/dev.01708. [DOI] [PubMed] [Google Scholar]
- 62.Maes L., Inze D., Goossens A. Functional specialization of the TRANSPARENT TESTA GLABRA1 network allows differential hormonal control of laminal and marginal trichome initiation in Arabidopsis rosette leaves. Plant Physiol. 2008;148:1453–1464. doi: 10.1104/pp.108.125385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kasili R., Walker J.D., Simmons L.A., Zhou J., De Veylder L., Larkin J.C. SIAMESE cooperates with the CDH1-like protein CCS52A1 to establish endoreplication in Arabidopsis thaliana trichomes. Genetics. 2010;185:257–268. doi: 10.1534/genetics.109.113274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Churchman M.L., Brown M.L., Kato N., Kirik V., Hulskamp M., Inze D., De Veylder L., Walker J.D., Zheng Z., Oppenheimer D.G., et al. SIAMESE, a plant-specific cell cycle regulator, controls endoreplication onset in Arabidopsis thaliana. Plant Cell. 2006;18:3145–3157. doi: 10.1105/tpc.106.044834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oxley D., Ktistakis N., Farmaki T. Differential isolation and identification of PI(3)P and PI(3,5)P-2 binding proteins from Arabidopsis thaliana using an agarose-phosphatidylinositol-phosphate affinity chromatography. J. Proteomics. 2013;91:580–594. doi: 10.1016/j.jprot.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 66.Wang X.Y., Wang X.L., Hu Q.N., Dai X.M., Tian H.N., Zheng K.J., Wang X.P., Mao T.L., Chen J.G., Wang S.C. Characterization of an activation-tagged mutant uncovers a role of GLABRA2 in anthocyanin biosynthesis in Arabidopsis. Plant J. 2015;83:300–311. doi: 10.1111/tpj.12887. [DOI] [PubMed] [Google Scholar]
- 67.Kubo H., Peeters A.J.M., Aarts M.G.M., Pereira A., Koornneef M. ANTHOCYANINLESS2, a homeobox gene affecting anthocyanin distribution and root development in Arabidopsis. Plant Cell. 1999;11:1217–1226. doi: 10.1105/tpc.11.7.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nesi N., Debeaujon I., Jond C., Pelletier G., Caboche M., Lepiniec L. The TT8 gene encodes a basic helix-loop-helix domain protein required for expression of DFR and BAN genes in Arabidopsis siliques. Plant Cell. 2000;12:1863–1878. doi: 10.1105/tpc.12.10.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baudry A., Caboche M., Lepiniec L. TT8 controls its own expression in a feedback regulation involving TTG1 and homologous MYB and bHLH factors, allowing a strong and cell-specific accumulation of flavonoids in Arabidopsis thaliana. Plant J. 2010;46:768–779. doi: 10.1111/j.1365-313X.2006.02733.x. [DOI] [PubMed] [Google Scholar]
- 70.Saito K., Yonekura-Sakakibara K., Nakabayashi R., Higashi Y., Yamazaki M., Tohge T., Fernie A.R. The flavonoid biosynthetic pathway in Arabidopsis: Structural and genetic diversity. Plant Physiol. Biochem. 2013;72:21–34. doi: 10.1016/j.plaphy.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 71.Ohl S., Hedrick S.A., Chory J., Lamb C.J. Functional properties of a phenylalanine ammonia-lyase promoter from Arabidopsis. Plant Cell. 1990;2:837–848. doi: 10.1105/tpc.2.9.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schoenbohm C., Martens S., Eder C., Forkmann G., Weisshaar B. Identification of the Arabidopsis thaliana flavonoid 3 ‘-hydroxylase gene and functional expression of the encoded P450 enzyme. Biol. Chem. 2000;381:749–753. doi: 10.1515/BC.2000.095. [DOI] [PubMed] [Google Scholar]
- 73.Ferreyra M.L.F., Emiliani J., Rodriguez E.J., Campos-Bermudez V.A., Grotewold E., Casati P. The Identification of Maize and Arabidopsis Type I FLAVONE SYNTHASEs Links Flavones with Hormones and Biotic Interactions. Plant Physiol. 2015;169:1090–1107. doi: 10.1104/pp.15.00515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Preuss A., Stracke R., Weisshaar B., Hillebrecht A., Matern U., Martens S. Arabidopsis thaliana expresses a second functional flavonol synthase. Febs Lett. 2009;583:1981–1986. doi: 10.1016/j.febslet.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 75.Shirley B.W., Hanley S., Goodman H.M. Effects of Ionizing-Radiation on a Plant Genome - Analysis of 2 Arabidopsis Transparent-Testa Mutations. Plant Cell. 1992;4:333–347. doi: 10.1105/tpc.4.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakajima J., Tanaka Y., Yamazaki M., Saito K. Reaction mechanism from leucoanthocyanidin to anthocyanidin 3-glucoside, a key reaction for coloring in anthocyanin biosynthesis. J. Biol. Chem. 2001;276:25797–25803. doi: 10.1074/jbc.M100744200. [DOI] [PubMed] [Google Scholar]
- 77.Nakajima J., Sato Y., Hoshino T., Yamazaki M., Saito K. Mechanistic study on the oxidation of anthocyanidin synthase by quantum mechanical calculation. J. Biol. Chem. 2006;281:21387–21398. doi: 10.1074/jbc.M600303200. [DOI] [PubMed] [Google Scholar]
- 78.Yonekura-Sakakibara K., Fukushima A., Nakabayashi R., Hanada K., Matsuda F., Sugawara S., Inoue E., Kuromori T., Ito T., Shinozaki K., et al. Two glycosyltransferases involved in anthocyanin modification delineated by transcriptome independent component analysis in Arabidopsis thaliana. Plant J. 2012;69:154–167. doi: 10.1111/j.1365-313X.2011.04779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tohge T., Nishiyama Y., Hirai M.Y., Yano M., Nakajima J., Awazuhara M., Inoue E., Takahashi H., Goodenowe D.B., Kitayama M., et al. Functional genomics by integrated analysis of metabolome and transcriptome of Arabidopsis plants over-expressing an MYB transcription factor. Plant J. 2005;42:218–235. doi: 10.1111/j.1365-313X.2005.02371.x. [DOI] [PubMed] [Google Scholar]
- 80.D’Auria J.C., Reichelt M., Luck K., Svatos A., Gershenzon J. Identification and characterization of the BAHD acyltransferase malonyl CoA: Anthocyanidin 5-O-glucoside-6’’-O-malonyltransferase (At5MAT) in Arabidopsis thaliana. FEBS Lett. 2007;581:872–878. doi: 10.1016/j.febslet.2007.01.060. [DOI] [PubMed] [Google Scholar]
- 81.Kitamura S., Shikazono N., Tanaka A. TRANSPARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis. Plant J. 2004;37:104–114. doi: 10.1046/j.1365-313X.2003.01943.x. [DOI] [PubMed] [Google Scholar]
- 82.Sun Y., Li H., Huang J.R. Arabidopsis TT19 functions as a carrier to transport anthocyanin from the cytosol to tonoplasts. Mol. Plant. 2012;5:387–400. doi: 10.1093/mp/ssr110. [DOI] [PubMed] [Google Scholar]
- 83.Wiesen L.B., Bender R.L., Paradis T., Larson A., Perera M., Nikolau B.J., Olszewski N.E., Carter C.J. A role for GIBBERELLIN 2-OXIDASE6 and gibberellins in regulating nectar production. Mol. Plant. 2016;9:753–756. doi: 10.1016/j.molp.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 84.Lehotai N., Feigl G., Koos A., Molnar A., Ordog A., Peto A., Erdei L., Kolbert Z. Nitric oxide-cytokinin interplay influences selenite sensitivity in Arabidopsis. Plant Cell Rep. 2016;35:2181–2195. doi: 10.1007/s00299-016-2028-5. [DOI] [PubMed] [Google Scholar]
- 85.Pelletier M.K., Murrell J.R., Shirley B.W. Characterization of flavonol synthase and leucoanthocyanidin dioxygenase genes in Arabidopsis—Further evidence for differential regulation of ‘‘early’’ and ‘‘late’’ genes. Plant Physiol. 1997;113:1437–1445. doi: 10.1104/pp.113.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prescott A.G., Stamford N.P.J., Wheeler G., Firmin J.L. In vitro properties of a recombinant flavonol synthase from Arabidopsis thaliana. Phytochemistry. 2002;60:589–593. doi: 10.1016/S0031-9422(02)00155-3. [DOI] [PubMed] [Google Scholar]
- 87.Clough S.J., Bent A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 88.Gao C.H., Li D., Jin C.Y., Duan S.W., Qi S.H., Liu K.G., Wang H.C., Ma H.L., Hai J.B., Chen M.X. Genome-wide identification of GLABRA3 downstream genes for anthocyanin biosynthesis and trichome formation in Arabidopsis. Biochem. Bioph. Res. Co. 2017;485:360–365. doi: 10.1016/j.bbrc.2017.02.074. [DOI] [PubMed] [Google Scholar]
- 89.Mortazavi A., Williams B.A., Mccue K., Schaeffer L., Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 90.Duan S.W., Wang J.J., Gao C.H., Jin C.Y., Li D., Peng D.S., Du G.M., Li Y.Q., Chen M.X. Functional characterization of a heterologously expressed Brassica napus WRKY41-1 transcription factor in regulating anthocyanin biosynthesis in Arabidopsis thaliana. Plant Sci. 2018;268:47–53. doi: 10.1016/j.plantsci.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 91.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.