ABSTRACT
Background
Nondairy beverages, produced from soy, rice, oat, almond, or coconut, are increasingly being used as alternatives to dairy milk, with the perception that they are healthier and/or more sustainable products than dairy products.
Objective
The aim of this study was to compare the effects of supplementing either bovine milk, soy, or almond-based beverages to young, growing rats fed an intact-protein diet or a diet that had protein substituted with amino acids (AA-diet).
Methods
Three-week-old male Sprague-Dawley rats were randomly assigned to 5 groups (n = 10/group) and fed ad libitum for 4 wk. Two control groups were fed either standard AIN-93G food [20% casein (CN) protein] or AIN-93G with amino acids (AAs) equivalent to CN protein, and water to drink. Three treatment groups were fed AIN-93G AA and supplemented with either bovine ultra-heat treatment (UHT) milk or soy or almond UHT beverages. Rat weight gain and food intakes were recorded. During week 4, body composition was assessed using DEXA to determine lean soft tissue, fat, and bone mass. At trial end, bone biomechanical properties and blood plasma mineral concentrations were measured.
Results
At the end of the trial, animals supplemented with almond beverage were lightest (P > 0.05), with higher plasma calcium concentrations (P > 0.05) and lower bone mineral content (BMC) and bone density (P > 0.05) than animals supplemented with milk or soy beverage. Soy-supplemented animals had similar BMC and bone density compared with milk-supplemented animals, although the soy group gained most weight (P > 0.05) and had the highest fat:lean ratio (P > 0.05) compared with other groups.
Conclusions
In the model tested, supplementing rats with bovine UHT milk and soy UHT beverage provided favorable bone health outcomes. Conversely, almond UHT beverage was not an effective supplement and could be detrimental to bone mineralization and strength outcomes.
Keywords: milk, soy drink, almond drink, amino acid diet, bone mineralization
Introduction
Dietary calcium accounts for 1–2% of adult human body weight with the majority found in bones and teeth (1). The role of dietary calcium in influencing bone mineral mass is well recognized and of equal importance is the maintenance of skeletal mass in later adulthood to prevent age-related bone loss and minimize the risk of fracture (2). Chronic deficiency of calcium, from either inadequate intake or poor intestinal absorption, is one of several important causes of reduced bone mass and osteoporosis (1). Dairy products, which include milk, cheese, and yoghurt, are the richest source of dietary calcium available. Several nondairy foods also contain calcium but in lower amounts. These foods include
tofu, sardines, some nuts (e.g., almonds), sesame seeds, and broccoli.
In Western societies, the consumption of liquid milk has progressively decreased over the last few decades (3). Conversely, substitution of nondairy “milk-like” drinks has risen significantly, with industry growth of 155% between 2012 and 2014 (4). Plant-based drinks, produced from soy, rice, oat, almond, or coconut, are used as alternatives to dairy milk, and are often perceived to be healthier and more sustainable products than dairy products (5). Milk substitutes are manufactured by extracting plant material, such as soy, nut, or rice, into water. The plant materials are then homogenized and thermally treated [using ultra-heat treatment (UHT)] to improve suspension of particles and to increase shelf life. The nutritional content of these plant-based drinks depends on the source, methods of processing, and whether the products are fortified (6). Dairy milk naturally contains minerals such as calcium and potassium, whereas plant-based brands are often fortified (Supplemental Table 1). Macronutrients in plant-based brands can vary substantially (5) (Supplemental Figure 1), whereas dairy milk is standardized for protein and fat content.
Soy beans and soy foods have long been recognized for their low fat and “good-quality” proteins with beneficial health effects (7–9), although not all studies have replicated these observations (10). Soy drink is the most popular soy “food” in the United States and consumption of these products is increasing rapidly (11). Almond drink contains fewer calories than milk or soy drink, and much of the energy (in kilocalories) comes from carbohydrate rather than protein (12). The concentrations of protein in dairy milk and soy drink can be ≤4 times higher than in almond drinks. Substitute milk drinks contain neither cholesterol nor lactose and are often consumed by those who are lactose-intolerant and others who wish to avoid dairy products, including vegans.
Potential detrimental effects of “health-food” milk alternatives have been reported when consumed by children (13–15), with some now labeled as “not suitable” for infants.
The importance of the structure of the food (food matrix) for nutrient uptake is well documented (16). The present study compared UHT bovine milk with 2 UHT plant-based drinks made from soy and almond, as a supplement for growing rats. To compare the impact of the beverages we wanted a model of compromised growth. Previously we had fed AIN-93G [containing intact casein (CN)] and AIN-93G amino acid (AA) [with AA content equivalent to the AIN-93G CN but no intact protein] diets to growing rats and had observed impaired growth and bone mineralization in those animals on the AA diet (unpublished data). We therefore chose these 2 rodent foods as controls in a young, growing rat model to understand the effects of supplementary dietary components on bone development, bone strength, and mineral uptake (17–19). We hypothesized that dairy milk and soy drink, containing similar macronutrients and micronutrients, would provide the necessary proteins and minerals to support growing rats on the AA diet. We hypothesized that the almond drink would provide less benefit for the growing rats than milk and soy drink, owing to its reduced nutritional value (Supplemental Table 1), but would be more beneficial than for animals fed an AA diet and water.
Methods
Animal ethics
All animal experiments were performed in accordance with the guidelines of the New Zealand National Animal Ethics Advisory committee for the use of animals in research, testing, and teaching. All animal manipulations were approved by the Ruakura Animal Ethics Committee (AEC#14346), established under the Animal Protection (code of ethical conduct) Regulations Act, 1987 (New Zealand). Animals were sourced from the Ruakura Small Animal Colony (Hamilton, NZ). A total of 50 healthy male Sprague-Dawley rats were used in the study and housed (3–4 per cage) in specific-pathogen-free conditions in a temperature-controlled room with a 12-h light-dark cycle.
Rodent diets and liquids
Standard AIN-93G rodent food (20) containing CN protein [20% (20)] and a modified version of AIN-93G food containing AAs equivalent to CN (Supplemental Table 2) were purchased from Research Diets Inc. Diets were analyzed by commercial testing to confirm the similarity of protein nitrogen, fat, and mineral content [Assure Quality; Method references: AOAC 942.05, AOAC 930.15, AOAC 988.05, AOAC 954.02, AsureQuality Method (inductively coupled plasma optical emission spectrometry), Supplemental Figure 2].
A range of UHT, unsweetened, unflavored plant-based beverages were selected from a local supermarket and evaluated for energy, protein, fat, and carbohydrate content (Supplemental Figure 1). Bovine UHT milk was used as the reference point for choosing the nearest comparable soy and almond plant-based drink for the feeding trial. Gross composition and mineral concentrations of the supplements chosen for use in the trial were confirmed by commercial testing [Gross composition: Milk Test NZ; ISO (International Organization for Standardization) 1211/IDF 1 2010, ISO 2450/IDF 16 2008 modified, ISO 8968-1/IDF 20-1 2014, Gravimetric ISO 6731/IDF 21 2010, ISO 17997-1/IDF 29-1 2004 (modified), ISO 8969-4/IDF 20-4 2016 (modified); Minerals-Analytica Laboratories Ltd] using inductively coupled plasma mass spectrometry after digestion of an acid (Supplemental Table 1). Sufficient amounts of the same batch number for each product were purchased for the trial. Beverage cartons were chilled to 4°C before opening and open cartons stored at the same temperature. Cartons were mixed well before dividing liquids into aliquots in feeding bottles.
Animal husbandry
Weanling male rats (3 wk of age) were randomly assigned to 5 groups (n = 10/group), to obtain even weight distribution between groups. Two control groups were fed ad libitum either standard AIN-93G CN food and water (CNwater) or modified AIN 93-G AA food and water (AAwater). Three experimental groups were fed ad libitum AIN-93G AA food and either UHT bovine milk (AAmilk), UHT soy beverage (AAsoy), or UHT almond beverage (AAalmond), as their sole liquid. Liquids were replaced daily and food and liquid intakes recorded. Animals remained on their allocated food and liquid diets for 4 wk. Rats were weighed twice per week to monitor health and body weight gain.
DEXA scanning of live animals
During week 4, animal body composition was assessed, using dual energy X-ray absorptiometry (DEXA) (Lunar Hologic) with dedicated animal software, to determine lean soft tissue, fat soft tissue, and bone mineral content (BMC) and bone mineral density (BMD). For this procedure, individual rats were anesthetized using isoflurane in a Perspex chamber to ensure animals were motionless during scanning. They were then transferred to the scanning bed, laid face down, and maintained under light anesthesia using a nose-cone device. Removal of the nose cone resulted in spontaneous recovery. Nose to tail measurements were also recorded.
Sample collection
At the end of the trial, 50 rats were killed by carbon dioxide asphyxiation and cervical dislocation. There were no losses in the course of the experiments. Bloods were collected and allowed to clot at room temperature for 3–4 h, before chilling overnight at 4°C. Bloods were then spun at 9520 × g for 5 min (4°C) and supernatant collected. Plasma was tested for calcium, phosphate, and magnesium concentrations by New Zealand Veterinary Pathology (Hamilton, NZ). Spines and femurs were removed by simple dissection and then stored in PBS solution at −20°C until analyzed.
Postmortem bone mineral analysis
Right femurs and spines were assessed for bone mineralization using DEXA (Hologic Discovery A machine with Apex 3.2 software including small animal software). Before DEXA scanning, frozen right femurs and spines were thawed and dissected to a tissue depth of ∼5 mm. A quality control scan was undertaken at the start and end of each scanning session using a spine phantom according to the manufacturer's guidelines to verify system calibration. Femurs and lumbar spine (LS1–LS4) were individually scanned using a small animal regional high-resolution protocol.
Biomechanical properties of the left femur
Biomechanical testing was carried out as described previously (21). Briefly, length of the thawed left femurs was measured using an electronic caliper, and the wet weight recorded. The midpoint of the femur was marked with a waterproof pen and then placed in a testing jig constructed for a 3-point bending test. The distance between the supporting rods had a fixed length of 12 mm. Load was applied at a constant deformation rate of 50 mm/min. Maximum force (in newtons) and elasticity (in newtons per square millimeter) were measured using a Shimadzu Ezi-test texture analyzer. The maximum force is the load required to break the bone and is thought to reflect the mineral content, as well as the protein component of bone. Measured elasticity reflects the distance in millimeters by which a bone can bend under the applied load without permanent deformation (stiffness).
Statistical analysis
The sample size of 10/group was based on a similar experiment comparing goat and cow milks, where the SD of the main variable (BMC) was 0.025. Power was at 80%, and the significance level was 5% (21).
For liquid and food consumption we took the amount per cage and divided by the number of animals to get per rat. The 3 cages per treatment were used as replicates for the analysis of intake per rat. Differences in mean water and liquid consumption were analyzed by treatment using Genstat for Windows 17th edition (VSN International). Genstat analysis of weight gain used trial day 0 weight as a covariate. Genstat analysis of DEXA measurements on live animals used end-of-trial body weight as a covariate. For postmortem analyses, bone weight was used as a covariate [which correlates to bone length (R2 = 0.9)] in Genstat analyses. All means of treatment groups are reported with their corresponding SEs of difference. Means were compared using Fisher's unprotected least significant difference test and P values < 0.05 were considered significant.
Results
Comparison of beverages
We first looked at commercially available UHT dairy milk, soy, and almond beverages. Dairy milk is standardized, but soy and almond are not. We observed that locally available UHT dairy milk and soy milks were higher in energy, protein, fat, and mineral content (except for sodium) than were locally available UHT almond beverages. The composition of almond beverage brands was highly variable (Supplemental Figure 1). The almond beverage with the highest energy (kilocalories per 100 mL) was used in the trial, but this contained approximately half the calories of the dairy milk and soy beverages. The calcium contents of the milks used in the trial are as follows: dairy milk, 127 mg/100 mL; soy beverage, 181 mg/100 mL; and almond beverage, 105 mg/100 mL (Supplemental Table 1). Mineral content of AIN-93G CN and AIN-93G AA-CN food was analogous (Supplemental Table 2).
Liquid intake and food intake
Liquid intake (in milliliters) was measured daily and food intake (in grams) weekly. As expected, rats consumed increasing amounts of liquid and food over the course of the trial (Table 1). The AAwater and AAalmond groups consumed significantly less liquid than the AAmilk and AAsoy groups (Table 1). Rats in the AAsoy group drank more but consumed less food than other groups and this was particularly noticeable in week 4 (Table 1).
TABLE 1.
Weekly food and liquid intakes for each group1
| Intake type by group | Week 1 | Week 2 | Week 3 | Week 4 |
|---|---|---|---|---|
| Food, g/rat | ||||
| CNwater | 43.2c | 91.1c | 114.0c | 193.3c |
| AAwater | 37.7b | 73.2b | 102.0b | 180.3c |
| AAmilk | 34.5b | 69.6b | 89.0a | 143.6b |
| AAsoy | 29.1a | 70.6b | 90.5a | 118.4a |
| AAalmond | 30.6a | 59.0a | 82.9a | 150.8b |
| SED | 1.5 | 3.4 | 4.0 | 7.6 |
| Liquid, mL/rat | ||||
| CNwater | 81.5a | 116.4a | 141.2a | 158.7a |
| AAwater | 76.8a | 110.1a | 154.1a | 180.6a |
| AAmilk | 107.0a | 176.8b | 211.1b | 260.6b |
| AAsoy | 188.4b | 259.0c | 319.1c | 451.7c |
| AAalmond | 116.6a | 125.2a | 173.7a,b | 196.9a,b |
| SED | 19.06 | 21.17 | 23.78 | 32 |
Values are means with SEDs for each parameter, n = 10 rats/group. Data were analyzed by ANOVA. a–cLabeled means in a column without a common letter are significantly different (P < 0.05). AA, amino acid; CN, casein; SED, SE of difference.
Energy intake (in kilocalories) per week was estimated by summing the calorie value (in kilocalories) of group food (in grams) and liquid (in milliliters) intakes (Table 2). The CNwater and AAmilk groups consumed similar amounts of kilocalories. Conversely, the AAwater and AAalmond groups consumed 11% and 17% fewer calories than the CNwater group. Surprisingly, the AAalmond group consistently consumed fewer calories than the AAwater group. Equally unexpectedly, the AAsoy group consumed the most calories: 12%, 10%, and 26% more calories than the CNwater, AAmilk, and AAalmond groups, respectively. Total calcium intake (in grams) per group was calculated by group over the trial (Table 3). The AA diet contained 4.8 mg Ca/g food and the calcium of liquids was as follows: milk, 1.27 mg/mL; soy 1.81, mg/mL; and almond, 1.05 mg/mL.
TABLE 2.
Weekly and total calorie intake for each group1
| Calorie intake, kcal | ||||||
|---|---|---|---|---|---|---|
| Group | Week 1 | Week 2 | Week 3 | Week 4 | Total | Difference to CNwater,2 % |
| CNwater | 3057 | 4005 | 4821 | 5414 | 17,297 | — |
| AAwater | 2539 | 3408 | 4363 | 5142 | 15,452 | 11 |
| AAmilk | 2849 | 4180 | 4959 | 5652 | 17,640 | 2 |
| AAsoy | 3013 | 4914 | 5468 | 5909 | 19,304 | 12 |
| AAalmond | 2394 | 3033 | 3977 | 4878 | 14,281 | 17 |
Total energy intake (in kcal) per group was estimated, weekly and for the total trial, by adding calories from food and liquid intakes. n = 10 rats/group. AA, amino acid; CN, casein; kcal, kilocalories.
The percentage difference in kcal between CNwater and all other groups was calculated.
TABLE 3.
Total calcium intake for each group1
| Calcium, g | ||||
|---|---|---|---|---|
| Group | Liquid | Food | Total2 | Difference to CNwater,3 % |
| CNwater | 0 | 21.1 | 21.1 | — |
| AAwater | 0 | 18.9 | 18.9 | −10.4 |
| AAmilk | 8.6 | 16.2 | 24.8 | 17.5 |
| AAsoy | 19.5 | 15.0 | 34.4 | 63.0 |
| AAalmond | 5.7 | 15.5 | 21.2 | 0.5 |
AA, amino acid; CN, casein; kcal, kilocalories.
Total calcium intake (in grams) per group was calculated by group over the trial. CN and AA diet contained 4.8 mg Ca/g food. Calcium of liquids: milk, 1.27 mg/mL; soy, 1.81 mg/mL; almond, 1.05 mg/mL.
The percentage difference in calcium intake between CNwater and all other groups was calculated.
Animal weights
Differences between group kilocalorie intakes were reflected in group body weights. At the start of the trial, group means weights of weanling rats were 47.5 g (range: 34.2–61.6 g) and group means weights were not significantly different (Figure 1; Supplemental Table 3). The CNwater group had similar weight gains to the AAmilk group over the course of the trial. For other groups, weights diverged significantly from the CNwater group by the end of the first week and this continued until trial end. Over this time, the AAwater and AAalmond groups were significantly lighter than the CNwater and AAmilk groups, and the AAalmond group was lighter than the AAwater group. In contrast, the AAsoy group was significantly heavier than all other groups (Supplemental Table 3). In all cases, group kilocalorie intake was highly correlated with group weight over the 4 wk (R2 range: 0.87–0.99).
FIGURE 1.
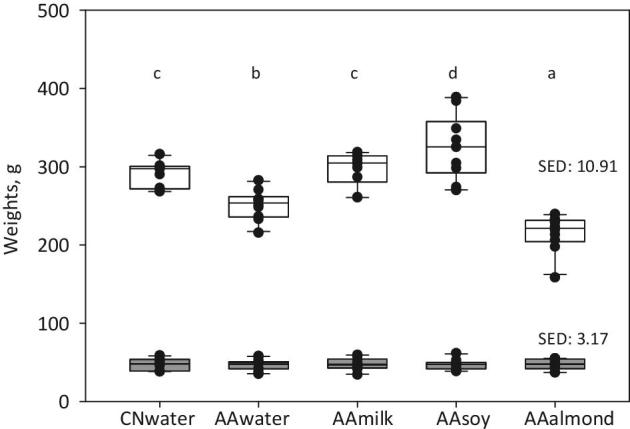
Animal weights at the start and the end of the feeding trial. Individual animal values are shown as black circles; group data are represented as box and whisker plots, with SEDs for each time point. End-of-trial weights were analyzed by ANOVA, using start weight as a covariate, n = 10/group; boxes without a common letter denote a significant difference (P < 0.05). Trial start, grey boxes; trial end, open boxes. AA, amino acid; CN, casein; SED, SE of difference.
Body composition (DEXA) of live animals
At the end of week 4, the fat:lean ratio differed significantly between groups (Figure 2). The AAmilk and AAsoy groups were “fatter” (fat:lean ratio = 0.30 and 0.35, respectively) than the CNwater group (fat:lean ratio = 0.25). Conversely, the AAalmond and AAwater groups had a significantly lower fat:lean ratio (0.21 and 0.19, respectively) than CNwater; however, they were not significantly different to each other. The nose to tail measurements show the AAalmond group was significantly shorter than other groups (Supplemental Table 4).
FIGURE 2.
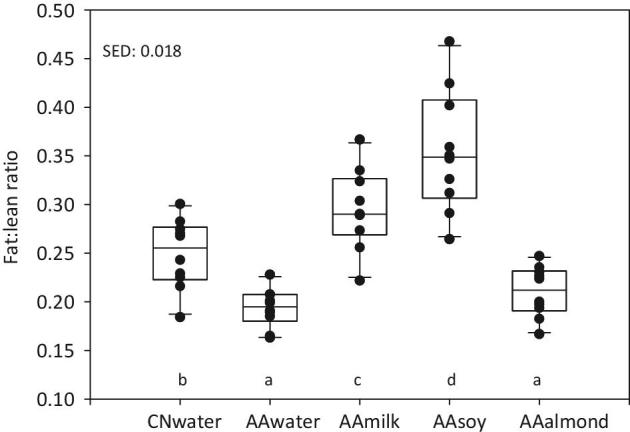
Fat:lean ratios. Body composition was assessed on live animals using DEXA and the fat:lean ratios were calculated for each animal. Individual animal values are shown as black circles; group data are represented as box and whisker plots, with SEDs. Data were analyzed by ANOVA, using start weight as a covariate, n = 10/group; boxes without a common letter denote a significant difference (P < 0.05). AA, amino acid; CN, casein; SED, SE of difference.
Measurement of minerals in plasma
Calcium concentrations were significantly higher in plasma collected from the AAwater and AAalmond groups (3.1 and 3.3 mmol/L, SEM = 0.04 and 0.06, respectively) compared with other groups (2.9 mmol/L, SEM = 0.04 for AAmilk and CNwater and 0.06 for AAsoy). Plasma phosphate concentrations were significantly higher in the AAsoy and AAmilk groups (5 mmol/L, SEM = 0.21 and 0.16, respectively) than in all other groups (CNwater: 4.3 mmol/L, SEM = 0.18; AAwater: 4.1 mmol/L, SEM = 0.18; and AAalmond: 3.9 mmol/L, SEM = 0.13). Plasma magnesium concentrations for the AAalmond group were significantly lower than for the AAwater group (1.0 mmol/L and 1.3 mmol/L, SEM = 0.05, respectively). The AAsoy group had the highest magnesium concentrations (1.6 mmol/L, SEM = 0.05), which was significantly higher than CNwater (1.4 mmol/L, SEM = 0.03), but not AAmilk (1.5 mmol/L, SEM = 0.05) (Supplemental Table 5).
DEXA imaging of bones
DEXA imaging of live animals showed significant differences in BMC and BMD. The AAmilk and AAsoy groups had comparable BMC and BMD (Figure 3; P < 0.05). Whereas the AAmilk and AAsoy groups had similar BMD to the CNwater group, AAsoy had higher BMC than the CNwater group. Both the AAwater group and the AAalmond group had lower BMD and BMC values than the other groups (P < 0.05). Bone mineral analysis of live animals was highly correlated with postmortem spine or femur bone mineral analysis (Supplemental Table 6).
FIGURE 3.
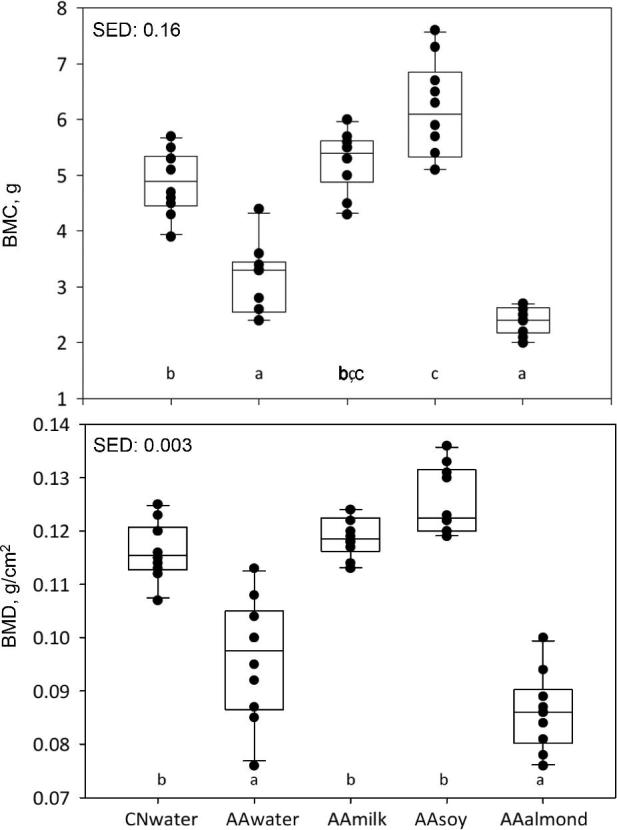
BMC and BMD of live animals measured in week 4 using DEXA. Individual animal values are shown as black circles; group data are represented as box and whisker plots, with SEDs. Data were analyzed by ANOVA, using start weight as a covariate, n = 10/group; boxes without a common letter denote a significant difference (P < 0.05). AA, amino acid; BMC, bone mineral content; BMD, bone mineral density; CN, casein; SED, SE of difference.
Bone biomechanical testing
Size and strength of left femurs collected post mortem were compared between groups, using bone weight as a covariate. Bone weight and length were highly correlated (R2 = 0.9). The CNwater and AAsoy groups had significantly longer bones than the AAwater and AAalmond groups (Table 4). Bone max force (in newtons) and elasticity measures were comparable for the CNwater, AAmilk, and AAsoy groups; in contrast the AAwater and AAalmond groups were significantly different to the other groups (Table 4). The max force required to break the bones (reflecting mineral content) was significantly lower in the AAalmond and AAwater groups. Similarly, elasticity (in newtons per square millimeter), the distance in millimeters by which bone can bend under the applied load without permanent deformation, was significantly different in the AAwater compared with the CNwater, AAmilk, and AAsoy groups. Bones from the AAalmond group were so pliable that measurements could not be recorded.
TABLE 4.
Bone length and strength measurements in rats at the end of the trial1
| Group | Length, mm | Max force, N | Elasticity, N/mm2 |
|---|---|---|---|
| CNwater | 34.11b | 77.84b | 414.9b |
| AAwater | 33.32a | 57.64a | 267.7a |
| AAmilk | 33.89a,b | 80.59b | 542.4b |
| AAsoy | 34.18b | 79.73b | 527.3b |
| AAalmond | 33.30a | 45.03a | No data |
| SED | 0.370 | 7.82 | 69.4 |
Values are means with SEDs for each parameter, n = 10 rats/group. Data were analyzed by ANOVA, using bone weight as a covariate. a,bLabeled means in a column without a common letter are significantly different (P < 0.05). AA, amino acid; CN, casein; SED, SE of difference.
Discussion
The aim of the present study was to compare bovine milk with plant-based alternative products in a young, growing rat model, using bone mineralization and strength as outcome measures. We identified differences in growth rates and body composition between treatment groups.
The animal model
We used 2 rodent feeds with similar composition, except the control AIN-93G CN feed contained intact CN proteins whereas the AIN-93G AA feed contained AAs equivalent to the CN proteins but no intact protein. The CNwater group represented healthy growing rats fed a replete diet and was the baseline control. The AAwater group represented an incomplete diet. In this study, we showed that the AAwater group was noticeably smaller and lighter than the CNwater group, but otherwise appeared healthy. However, compared with the CNwater group the AAwater group had significantly lower BMD and BMC, weight and length of the femurs were reduced, and less force was required to break them. The model showed significant differences for bone health outcomes between these 2 groups.
Effects of treatment liquids on bone health
Bone is a living tissue, ∼70% of which consists of calcium phosphate. Calcium from the diet is absorbed from the gut and is essential for attaining peak bone mass during adolescence and for prevention of osteoporosis. Calcium within dairy products can exert positive effects on bone acquisition and maintenance (22). Deficiency in dietary calcium may result in the upregulation of parathyroid hormone resulting in increased bone resorption to maintain circulating calcium concentrations (23). In the present study, we showed that the AAalmond and AAwater groups had significantly raised serum calcium concentrations compared with other groups. This could suggest mineral loss from the bone to benefit systemic mineral homeostasis. However, the regulation of calcium for homeostasis is complex, and there is insufficient evidence to draw firm conclusions. It is concerning that, despite the AAalmond group ingesting higher volumes of almond drink than the AAwater group, and despite the almond drink containing 105 mg Ca/mL, BMD, BMC, and bone strength were significantly lower in the AAalmond group than in the AAwater group. This suggests that the AAalmond group could not fully utilize the calcium supplied in their diet.
As a nut, almonds contain high concentrations of oxalates (range: 431–490 mg/100 g nut) (24). Uptake of soluble oxalates through the intestine is associated with formation of kidney stones; however, simultaneous ingestion of minerals with oxalate-containing foods can reduce soluble oxalate absorption by up to half (ratio of 1.5 mineral to 1 oxalate) (25). In addition, the absorbability of the minerals is similarly reduced (26, 27). If we consider that almond drinks contain an estimated 2% almonds [8–10 mg oxalate in a 100-mL drink (24)], and the 100-mL almond drink contains 105 mg Ca and 5 mg Mg, we have a ratio of 110 mg Ca + Mg to 10 mg estimated oxalate, so it is difficult to believe there is sufficient oxalate to bind all available calcium from both the almond drink and the AIN-93G AA food. The calculated total calcium intake for the AAalmond group was comparable with that for the CNwater control group (Table 3). It is apparent from the bone outcomes, weight, and body composition that animals on the AA almond regimes were compromised, even in comparison with the AAwater group, which had lower total calcium intake than the AAalmond group. However, further conclusions cannot be drawn without further investigation. We compared supplementation of milk with soy and almond beverages in rats offered a diet previously observed by us to affect bone mineralization. It is important to note that whereas the concentrations of minerals in the soy and milk products were comparable, the almond beverage was lower in all the measured components, except sodium. We, therefore, would expect to see a difference in outcomes between the 2 plant-based drinks. On the other hand, adequate mineral supply was available in the food offered to the animals.
It has been shown that supplementation using different calcium forms can affect bioavailability as well as affect the bone health outcomes for people (23). The calcium in the milk (28), soy (29), and almond drinks used in this study was calcium carbonate so this was not considered a factor in our observations. BMD and BMC measures for the AAsoy group were higher than for the AAmilk group. However, there was a higher volume intake for the AAmilk group than for the AAsoy group, which may account for the differences. Interestingly, BMD and BMC for the CNwater and AAmilk groups were not significantly different. This is perhaps not surprising because much of the calcium in milk is found in the CN micelle (30), and CN is the main component of the AIN-93G CN food. Interestingly, despite the calcium amounts in the AIN-93G CN and AA food being balanced, BMC and BMD for the AAwater group were significantly lower than for the CNwater group. This suggests that the matrix in which calcium is delivered has a substantial impact on bioavailability in the body. This observation was supported by the bone strength data.
Appetite and self-regulation
The dogma that accumulation of body fat reflects positive energy balance is vastly oversimplified (i.e., energy consumed is greater than energy expended through metabolism, thermogenesis, and physical activity). Satiety and control of food intake is a complex system of environmental, social, and hedonistic influences. The gut–brain axis is the holistic control center that links peripheral and central systems in order to control energy homeostasis (31, 32). We have previously observed that rats fed ad libitum, with no restriction on intake, have some self-regulation of calorie intake (data not shown). Also, it has been demonstrated that whey protein and soy protein have equal satiating and thermic effects in humans and that fluid bovine milk, when compared with other drinks, can reduce postmeal glycemia in humans (33). The concentration, ratio, and type of protein are known to be important for appetite and glycemic control (34, 35). In this study, macronutrients and mineral content of the milk and soy beverage were similar, but not in the case of the almond beverage. Total calories consumed over the trial, based on food weight and liquid volume, were highest for the AAsoy group and this was reflected in a significantly higher fat:lean ratio than for all other groups. Interestingly, the AAmilk group had a significantly different fat:lean ratio than the CNwater group despite ingesting similar calories. The AAalmond group consumed fewer calories than all other groups, even compared with the AAwater group. The liquid intake of AAalmond and AAwater groups was similar, but despite the AAalmond group receiving more calories from the liquid overall, they still consumed fewer calories owing to lower food intake. Furthermore, the AAmilk and AAalmond groups ate similar amounts of food even though AAmilk consumed more liquid calories. These observations suggest there was a component in the almond beverage that inhibited appetite signals. This supports the idea that the matrix of the food itself has an impact on self-regulation and appetite signals in addition to individual components in the food.
Conclusions
Illness, food allergy, or senescence can result in a diet that falls short of the RDAs. If a diet fails to maintain growth and repair, then supplementation may be required. Our model is based on our earlier observation that growth and bone mineralization in growing rats were compromised in animals fed the AA diet. We therefore considered it a suitable model to explore the effect of beverage supplementation. The model tested the fitness of the chosen supplements (dairy and nondairy drinks) to recover bone and body composition in a situation where the diet had been compromised. Our data suggest that in young, growing rats, milk and soy beverages can provide the necessary elements missing from the AA-based food; conversely, the almond beverage was not able to do this and may even have been detrimental. Interestingly, the AAalmond group took in similar amounts of calcium to the CNwater group, and higher amounts of calcium than the AAwater group, but this was not reflected in the BMD or BMC. There are increasing numbers of people choosing diets that exclude complete groups of foods, either for social or moral reasons, or for weight control. If traditional foods are being replaced, it is desirable to do this with nutritionally equivalent products to ensure dietary needs are met. It is not enough to measure a single constituent, such as calcium, and assume it will guarantee sufficient delivery to the required area. We need to advance our understanding of the synergies between product ingredients and how they work together to nourish the body and ensure a healthy future.
Supplementary Material
Acknowledgements
We acknowledge Daralyn Hurford, Bobby Smith, Ric Broadhurst, and Anne Broomfield for their expertise with animal husbandry and laboratory analyses, and Harold Henderson for his expert assistance with the statistical analysis. The authors’ responsibilities were as follows—JAC and AJH: designed the study, with OAMW contributing to the discussion; JAC, OAMW, and MHV: performed the experimental work and data analysis; JAC: wrote the manuscript; AJH, MCK, and MHV: reviewed and edited the manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by the Ministry of Business, Innovation and Employment, New Zealand, through AgResearch's Strategic Science Investment Fund.
Author disclosures: JAC, OAMW, MCK, MHV, and AJH, no conflicts of interest.
There were no travel expenses, gifts, or honoraria provided to the authors related to this work. No product was donated by the manufacturers.
Supplemental Figures 1 and 2 and Supplemental Tables 1–6 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: AA, amino acid; BMC, bone mineral content; BMD, bone mineral density; CN, casein; DEXA, dual energy X-ray absorptiometry; IDF, International Dairy Federation; ISO, International Organization for Standardization; UHT, ultra-heat treatment.
References
- 1. Cashman K. Calcium intake, calcium bioavailability and bone health. Br J Nutr 2002;87(Suppl 2):S169–77. [DOI] [PubMed] [Google Scholar]
- 2. Caroli A, Poli A, Ricotta D, Banfi G, Cocchi D. Invited review: dairy intake and bone health: a viewpoint from the state of the art. J Dairy Sci 2011;94:5249–62. [DOI] [PubMed] [Google Scholar]
- 3. Watson. Alternative beverages -trends to watch [Internet] 2017[cited 9 November, 2017]. Available from: https://www.dairyreporter.com/News/Retail-Shopper-Insights/Packaged-Facts-lists-dairy-alternative-beverage-trends-to-watch.
- 4. Shooter A. Forget cows, we're getting milk from oats and almonds: non-dairy market has grown 155% in just two years [Internet] 2014[cited 12 September, 2018]. Available from: http://www.dailymail.co.uk/femail/food/article-2709071/Forget-cows-getting-milk-oats-almonds-Non-dairy-market-grown-155-just-two-years.html.
- 5. Singhal S, Baker RD, Baker SS. A comparison of the nutritional value of cow's milk and non-dairy beverages. J Pediatr Gastroenterol Nutr 2017;64(5):799–805. [DOI] [PubMed] [Google Scholar]
- 6. Makinen OE, Wanhalinna V, Zannini E, Arendt EK. Foods for special dietary needs: non-dairy plant-based milk substitutes and fermented dairy-type products. Crit Rev Food Sci Nutr 2016;56(3):339–49. [DOI] [PubMed] [Google Scholar]
- 7. Barbalho S, Farinazzi-Machado F. Soybean: food or remedy? In: El-Shemy PH, editor. Soybean and nutrition. London: InTech; 2011. p. 411–32. [Google Scholar]
- 8. Meyer BJ, Larkin T, Owen A, Asthmeimer L, Tapsell L, Howe P. Limited lipid-lowering effects of regular consumption of whole soybean foods. Ann Nutr Metab 2004;48(2):67–78. [DOI] [PubMed] [Google Scholar]
- 9. Woodside JV, Morton MS. Are soy-milk products viable alternatives to cow's milk? In: Wilson T, editor. Beverages in nutrition and health. Temple, NJ: Humana Press; 2004. p. 223–34. [Google Scholar]
- 10. Ramdath D, Padhi E, Sarfaraz S, Renwick S. Beyond the cholesterol-lowering effect of soy protein: a review of the effects of dietary soy and its constituents on risk factors for cardiovascular disease. Nutrients 2017;9:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu ZS, Chang SKC. Nutritional profile and physicochemical properties of commercial soymilk. J Food Process Preserv 2013;37(5):651–61. [Google Scholar]
- 12. Vanga SK, Raghavan V. How well do plant based alternatives fare nutritionally compared to cow's milk? J Food Sci Technol 2018;55(1):10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carvalho N, Kenney R, Carrington P, Hall D. Severe nutritional deficiencies in toddlers resulting from health food milk alternatives. Paediatrics 2001;107(4):e46. [DOI] [PubMed] [Google Scholar]
- 14. Ellis D, Lieb J. Hyperoxaluria and genitourinary disorders in children ingesting almond milk products. J Paediatr 2015;167(5):1155–8. [DOI] [PubMed] [Google Scholar]
- 15. Morency M-E, Birken CS, Lebovic G, Chen Y, L'Abbe M, Lee GJ; TARGet Kids! Collaboration. Association between noncow milk beverage consumption and childhood height. Am J Clin Nutr 2017;106(2):597–602. [DOI] [PubMed] [Google Scholar]
- 16. Millward D, Layman D, Tome D, Schaafsma G. Protein quality assessment: impact of expanding understanding of protein and amino acid needs for optimal health. Am J Clin Nutr 2008;87(5):1576S–81S. [DOI] [PubMed] [Google Scholar]
- 17. Hunt J, Hunt C, Zito C, Idso J, Johnson L. Calcium requirements of growing rats based on bone mass, structure, or biomechanical strength are similar. J Nutr 2008;138(8):1462–8. [DOI] [PubMed] [Google Scholar]
- 18. Masarwi M, Gabet Y, Dolkart O, Brosh T, Shamir R, Philip M, Gat-Yablonski G. Skeletal effect of casein and whey protein intake during catch-up growth in young male Sprague-Dawley rats. Br J Nutr 2016;116(1):59–69. [DOI] [PubMed] [Google Scholar]
- 19. Viguet-Carrina S, Hopplera M, Membrez Scalfo F, Vuichoud J, Vigo M, Offord EA, Ammann P. Peak bone strength is influenced by calcium intake in growing rats. Bone 2014;68:85–91. [DOI] [PubMed] [Google Scholar]
- 20. Reeves PG, Nielsen FH, Fahey GCJ. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr 1993;123(11):1939–51. [DOI] [PubMed] [Google Scholar]
- 21. Hodgkinson AJ, Wallace O, Kruger M, Prosser CG. Effect of the dietary delivery matrix on vitamin D3 bioavailability and bone mineralisation in vitamin-D3-deficient growing male rats. Br J Nutr 2018;119:143–52. [DOI] [PubMed] [Google Scholar]
- 22. Bonjour JP. Calcium and phosphate: a duet of ions playing for bone health. J Am Coll Nutr 2011;30(5 suppl 1):438S–48S. [DOI] [PubMed] [Google Scholar]
- 23. Shankar K, Sakthibalan M, Raizada P, Jain R. A randomized open-label clinical study comparing the efficacy, safety, and bioavailability of calcium lysinate with calcium carbonate and calcium citrate malate in osteopenia patients. J Orthop Case Rep 2018;8(4):15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Massey L. Food oxalate: factors affecting measurement, biological variation, and bioavailability. J Am Diet Assoc 2007;107:1191–4. [DOI] [PubMed] [Google Scholar]
- 25. Liebman M, Costa G. Effects of calcium and magnesium on urinary oxalate excretion after oxalate loads. J Urol 2000;163(5):1565–9. [PubMed] [Google Scholar]
- 26. Guéguen L, Pointillart A. The bioavailability of dietary calcium. J Am Coll Nutr 2000;19(2 Suppl):119S–36S. [DOI] [PubMed] [Google Scholar]
- 27. Heaney RP, Weaver CM, Recker RR. Calcium absorbability from spinach. Am J Clin Nutr 1988;47(4):707–9. [DOI] [PubMed] [Google Scholar]
- 28. Holt C, SS H, Hukins DWL. Structure of bovine milk calcium phosphate determined by X-ray absorption spectroscopy. Biochim Biophys Acta 1982;719:299–303. [DOI] [PubMed] [Google Scholar]
- 29. Heaney RP, Dowell MS, Rafferty K, Bierman J. Bioavailability of the calcium in fortified soy imitation milk, with some observations on method. Am J Clin Nutr 2000;71(5):1166–9. [DOI] [PubMed] [Google Scholar]
- 30. Holt C, Carver JA, Ecroyd H, Thorn DC. Invited review: caseins and the casein micelle: their biological functions, structures, and behavior in foods. J Dairy Sci 2013;96(10):6127–46. [DOI] [PubMed] [Google Scholar]
- 31. Hussain S, Bloom S. The regulation of food intake by the gut-brain axis: implications for obesity. Int J Obes (Lond) 2013;37:625–33. [DOI] [PubMed] [Google Scholar]
- 32. Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 2000;404:661–71. [DOI] [PubMed] [Google Scholar]
- 33. Melson CE, Nepocatych S, Madzima TA. The effects of whey and soy liquid breakfast on appetite response, energy metabolism, and subsequent energy intake. Nutrition 2019;61:179–86. [DOI] [PubMed] [Google Scholar]
- 34. Boirie Y, Dangin M, Gachon P, Vasson M-P, Maubois J-L, Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci U S A 1997;94(26):14930–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Panahi S, Luhovyy BL, Liu TT, Akhavan T, Khoury DE, Goff HD, Anderson GH. Energy and macronutrient content of familiar beverages interact with pre-meal intervals to determine later food intake, appetite and glycemic response in young adults. Appetite 2013;60:154–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


