INTRODUCTION
The importance of the gastrointestinal (GI) tract in human disease was first raised by Hippocrates m more than 2000 years ago. His statement, that “all disease begins in the gut,” however, has only recently garnered significant interest. Although GI dysfunction has been reported to accompany an increasing number of central nervous system (CNS) disorders, there is emerging evidence to suggest that intestinal dysfunction may occur before the CNS manifestations and/or be a contributing factor to CNS disease.
The relationship between the brain and the intestine, termed the “brain–gut axis,” begins during development and persists throughout life. It is what is thought to specifically link the emotional and cognitive centers of the brain with intestinal functions.1–3
This axis regulates homeostatic functions that were classically thought to be exclusively gut-centric or brain-centric. In the intestine, these functions include sensation and motility. In the CNS, these roles evolve around the control of behavior, cognition, and mental health. Thus, when either system is abnormal disease states emerge that can affect both systems.
In this article, we provide an overarching review of what is currently known about the physiology of the brain–gut axis in both health and disease and how these concepts apply to irritable bowel syndrome (IBS), the most researched gut–brain axis condition in neurogastroenterology.
THE PHYSIOLOGY OF BRAIN–GUT AXIS COMMUNICATION
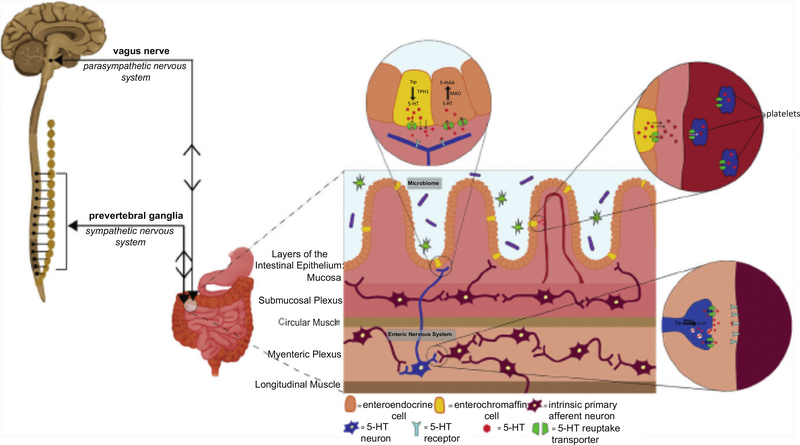
The autonomic nervous system is the portion of the peripheral nervous system that is largely responsible for the body’s reflexive, physiologic control. The autonomic nervous system is divided into the sympathetic, parasympathetic, and enteric nervous systems (ENS). The ENS is the subdivision of the autonomic nervous system that directly controls the GI tract.4 The ENS consists of more than 100 million neurons that are organized into highly complex microcircuits that can function independent from the CNS and spinal cord.5 Although the ENS has the ability to act autonomously, it does not usually do so. ENS communication with the CNS is continuous and bidirectional (Fig. 1). This communication normally occurs through the peripheral nervous system and sympathetic nervous system, via the vagus nerve and prevertebral ganglia, respectively.4
Fig. 1.
Communication between the enteric nervous system (ENS) and central nervous system (CNS, brain and spinal cord) is bidirectional and continuous. The gut and the brain communicate via the parasympathetic nervous system through afferent sensory pathways of the vagus nerve and via the sympathetic nervous system through efferent motor pathways of the prevertebral ganglia. The brain–gut axis is also modulated by the enteric microbiota. The microbiota can modulate the CNS directly via the vagus nerve and/or indirectly by influencing the ENS and by production of metabolites that cross the blood brain barrier. Serotonin (5-HT) is important for brain–gut communication as both a neurotransmitter in the ENS and CNS, as well as a hormone present throughout the circulation. 5-HT is synthesized by tryptophan hydroxylase 1 (TPH1) in enterochromaffin (EC) cells, TPH2 in neurons, and is inactivated after reuptake primarily by the serotonin reuptake transporter (SERT). 5-HIAA, 5-hydroxyindoleacetic acid; 5-HT, 5-hydroxytryptamine; MAO, monoamine oxidase; TPH, tryptophan hydroxylase; Trp, tryptophan.
Abnormalities in brain–gut communication are one way that brain–gut axis disease may arise. There is also evidence to suggest that they may arise from common defects in developmental pathways of the CNS and ENS. In fact, the ENS is described as a second brain because of its many similarities with the CNS. Virtually every class of neurotransmitter found in the CNS is also detected in the ENS.5 Further, the development of the ENS shows numerous parallels with that of the CNS.6 Environmental and/ or genetic influences on CNS development and function might, therefore, also affect these parameters in the ENS. Of these factors, serotonin (5-hydroxytryptamine [5-HT]) has been demonstrated to play important roles in development and function of the CNS and ENS and, as discussed elsewhere in this article, may modulate critical factors contributing to the development of brain–gut axis conditions like IBS.
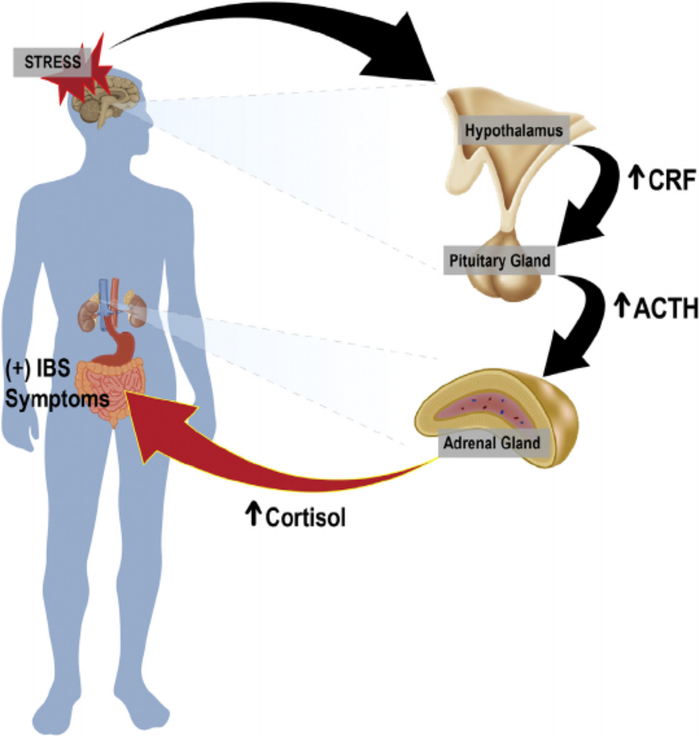
An additional group of mediators that facilitate brain–gut interactions originate from the hypothalamic–pituitary–adrenal (HPA) axis (Fig. 2). The HPA axis is part of the limbic system, which is a brain area predominantly involved in emotional responses. HPA axis activation can be initiated by stressors and/or systemic proinflammatory cytokines that induce secretion of corticotropin-releasing factor (CRF) from the hypothalamus. CRF secretion stimulates adrenocorticotropic hormone secretion from the pituitary gland that, in turn, leads to cortisol release from the adrenal glands. Cortisol is key in the stress response and may play an important role in IBS physiology.7
Fig. 2.
The hypothalamic–pituitary–adrenal (HPA) axis influences the brain–gut axis. Stress can elicit an emotional response and hypothalamic activation. Activation of the hypothalamus induces the secretion of corticotropin-releasing factor (CRF). CRF activates the pituitary gland, and in response, adrenocorticotropic hormone is secreted. Adrenocorticotropic hormone (ACTH) acts on the adrenal gland to induce the secretion of cortisol. The increase in cortisol, a key hormone in stress response, acts on the gut to alter GI tract function, autonomic tone, visceral perception, and behavior, which all relate to irritable bowel syndrome (IBS).
THE BRAIN–GUT AXIS IS MODULATED IN PART BY THE ENTERIC MICROBIOTA
The more than 1 billion organisms that inhabit the intestine influence human ENS and CNS development8,9 and functions, including GI motility,10–12 mood,13 cognition, and learning.14 This microbial population communicates directly with the CNS and ENS by way of the vagus nerve,9,14–17 and/or by the production of active metabolites that directly influence enteric function. The microbiota can also act indirectly on the CNS by the production of metabolites that are carried in the blood and cross the blood–brain barrier.18
Enteric microbial manipulation may be a novel way to establish optimal neurodevelopment; evidence supports the idea that an infant’s initial microbiome is influenced by its mother throughout the perinatal and breastfeeding periods and that it is this initial inoculation and subsequent development in early life that is crucial for healthy neurodevelopment.1,19–21 The initiation of symbiosis during adolescence also seems to be a crucial step for optimal brain development and long-term mental health.1,22,23
SEROTONIN AS A CRITICAL MODULATOR OF THE BRAIN–GUT–MICROBIOME AXIS
There is significant evidence to suggest that serotonin may be an important link in the brain–gut axis. Serotonin and its primary inactivator, the serotonin reuptake transporter, are present in both the CNS and the ENS where they are critical mediators of development and function. During CNS development, serotonin is neurogenic and plays critical roles in cell division, migration, and differentiation.16,17,24 CNS functions modulated by serotonin include mood (eg, depression and anxiety) and cognition.19
Despite its critical roles in CNS development and function, only approximately 3% of the body’s serotonin is located in the CNS. The vast majority of serotonin (approximately 95%) is found in the intestine, where it is a multifunctional signaling molecule during fetal and throughout adult life.25 Approximately 90% of enteric serotonin is located in the intestinal epithelium, in enterochromaffin cells, with the remainder located in neurons of the ENS. Two different isoforms of the same rate-limiting biosynthetic enzyme, tryptophan hydroxylase, are responsible for serotonin production in enterochromaffin cells and the ENS; tryptophan hydroxylase 1 is responsible for serotonin production in enterochromaffin cells and tryptophan hydroxylase 2 is in the ENS. Although it constitutes the minority of enteric serotonin, tryptophan hydroxylase 2-derived serotonin is most critical for ENS development, intestinal motility, permeability, and enteric epithelial growth and differentiation.26–29
There is evidence of interaction between serotonin and the enteric microbiome. Enteric bacteria and bacterial products, such as indigenous spore-forming bacteria and short chain fatty acids, have been demonstrated to modulate serotonin production.12,17
Based on its interactions with the CNS, ENS, and microbiome, serotonin may be a common node connecting the brain–gut–microbiome axis. In accordance with this notion, there are numerous links between the enteric microbiome, serotonin production, and changes in CNS and ENS function, demonstrated in both preclinical and clinical settings.1,30–35
THE BRAIN–GUT AXIS IN CLINICAL DISEASE: FOCUS ON IRRITABLE BOWEL DISEASE
The diagnosis of IBS relies on symptom-based criteria and is classified as constipation-predominant IBS or diarrhea-predominant IBS, mixed pattern, or unsubtyped.36 Although individuals are diagnosed according to abdominal pain and bowel patterns, up to 50% of individuals also present with mood, anxiety, and/or somatoform disorders.37,38 Further, it is this subset of individuals who often have a worse prognosis.39
Little is known regarding how and when brain–gut dysfunction originates in IBS. For many, abdominal pain begins in childhood and reflects learned illness behaviors.40 Other childhood instances are triggered by CNS-based precipitants such as trauma, stress, abuse, or maternal neglect.41 Patients with history of early adverse life events not only have increased odds of having IBS, but also experience an increase in disease severity.42 The increased susceptibly may result from brain remodeling; exposure to childhood adversity resulting in alterations in emotional regulation and salience regions in the CNS, as detected by functional MRI (fMRI).43 The CNS, however, is not always the precipitating factor; 50% of patients present after an intestinal trigger (eg, acute gastroenteritis and abdominal surgery).44,45
The gut-brain connection in IBS is further manifest in the abdominal pain sensation that is critical to its diagnosis. Some individuals with IBS demonstrate increased engagement of endogenous pain faciliatory mechanisms and decreased levels of endogenous pain inhibitory mechanism in brain regions associated with visceral afferent processing and emotional arousal, including the left dorsal anterior cingulate gyrus and the bilateral anterior insulae.46 A metaanalysis of adult studies that evaluated brain response to rectal balloon distension by fMRI reported differences between healthy control subjects and patients with IBS in these brain regions47 and a more consistent activation in regions associated with stress and arousal circuits and endogenous pain modulation.48 In a separate study, patients with IBS with a history of abuse reported increased pain and anxiety with rectal distension accompanied by similar fMRI changes.49 Interestingly, the stress and arousal circuit demonstrated in human subjects by fMRI share significant homology with the stress circuit related to CRF-CRF1 receptor signaling in rodents, potentially implicating the HPA axis as a facilitator of brain–gut axis communication.50
Findings in pediatric studies have largely mirrored those in adults.51–53 Children exhibit an increased activation in perceptual brain regions during pain,54 a high rate of psychiatric comorbidities,51 and a significant association between psychiatric comorbidity and worse outcomes.52
As a mediator of gut-brain development and function, serotonin may play important roles in the brain and intestinal manifestations of IBS.55 Serotonin modulates all GI functions implicated in IBS pathology, including motility, secretion, and visceral hypersensitivity.56 Further, alterations in enteric mucosal and blood serotonin signaling have been demonstrated in children with IBS.57 Serotonin regulates GI motility, at least in part, by binding to the serotonin 4 receptor (5-HT4), which is located on both enteric neurons and intestinal epithelial cells.25 Serotonin can affect pain pathways by binding to serotonin 3 (5-HT3) receptors on intrinsic primary afferent neurons.58
Irritable Bowel Syndrome: Therapeutic Targets
Pharmacologic therapies targeted to the brain–gut axis in IBS have focused on the HPA axis, the microbiome and the serotonergic system. Nonpharmacologic holistic therapies are targeted toward diet, neurostimulation, cognitive–behavioral therapy, and hypnosis.
Pharmacologic therapies for irritable bowel syndrome
Serotonergic agents
The physiology involved in the generation of IBS symptoms is thought to include modulation of 5-HT4 and/or 5-HT3.56 By enhancing intestinal secretion, peristalsis, and GI transit, 5-HT4 agonists have been most successful in treating constipation-predominant IBS.25,56 The initial class of 5-HT4 agonists (eg, cisapride) bound to cardiac potassium channels independent of their prokinetic activities, resulting in adverse events, including death. The more recently developed 5-HT4 agonists (eg, prucalopride, velusetrag) demonstrate greater GI specificity and are successful in treating symptoms in constipation-predominant IBS without evidence of cardiac injury.59These newer agonists, however, are either not currently available in the United States or are in clinical trials. The 5HT4 agonists have also been suggested as an effective target for depression.60
The 5-HT3 antagonists decrease intestinal pain sensation and slow transit.56 The selective 5-HT3 antagonist, alosetron, showed efficacy for IBS-D,61 although instances of severe constipation and ischemic colitis62 led to its restriction for women with severe IBS-D who have not responded to conventional therapies.63 Other 5-HT3 antagonists have not been introduced in the United States for similar safety concerns.
Antidepressants
Antidepressants (tricyclic antidepressants [TCAs] and selective serotonin reuptake inhibitors) were introduced for IBS management based on the recognition that depression and anxiety were frequent comorbidities.37 Accordingly, studies suggest that these agents relieve visceral pain and stabilize mood.
TCAs are associated with a decrease in IBS symptoms compared with placebo in adults.61 A double-blind, placebo-controlled study in adolescents with IBS showed that patients receiving amitriptyline had an improvement in overall quality of life and were more likely to experience a decrease in abdominal pain and diarrhea.64 Unfortunately, another pediatric double-blind, placebo-controlled, randomized, controlled trial examining amitriptyline efficacy in children with IBS, functional abdominal pain, and functional dyspepsia did not support these findings.65 Further, TCAs have dose-limiting anticholinergic side effects. Selective serotonin reuptake inhibitors have been found in some adult randomized, controlled trials to be associated with a reduction in IBS symptoms compared with placebo with a number needed to treat that is similar to TCAs.66,67 Unfortunately, these findings have not been replicated in children with functional abdominal pain.68
BRAIN–GUT–MICROBIOME AXIS: ROLE OF PROBIOTICS AND DIET
Enteric microbial imbalance has been suggested to underlie the pathophysiology of IBS and comorbid psychiatric conditions.69 Controlled clinical trials on IBS with coexistent mental health conditions report symptom improvement following enteric microbial manipulation.70–72 Microbial manipulation has been attempted by use of probiotics, antibiotics and the low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) diet.
Probiotics
Bacterial species that have been shown to improve, symptom severity in adults and children with IBS include strains of Bifidobacterium and/or Lactobacillus73–79 and VSL#3.80,81 Probiotics may impact pain pathways by influencing brain signaling; Bifidobacterium longum NCC3001 was shown to reduce depressive symptoms in adults with IBS while decreasing responses to negative emotional stimuli in IBS-associated brain areas, including the amygdala and frontolimbic regions.72
Low Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols Diet
A low FODMAP diet may result in decreased microbial fermentation, and decreased production of gas and osmotically active metabolites leading to improvement in bloating, flatulence, and pain.82 Randomized controlled trails in children and adults demonstrate that the low FODMAP diet improves IBS symptoms, regardless of subtype.83,84 Further, this diet is also associated with improvements in health-related quality of life, anxiety, and activity impairment for adults with IBS-D.85
NONPHARMACOLOGIC BRAIN–GUT THERAPIES FOR IRRITABLE BOWEL SYNDROME
Psychological Therapies
In a metaanalysis including 32 adult trials and more than 2000 patients, psychological therapies were more effective than control treatments (placebo, supportive therapy, physician’s “usual management”) for alleviating chronic abdominal pain in adults and children, with the most beneficial outcomes reported for cognitive behavioral therapy, hypnotherapy, multicomponent therapy, and dynamic psychotherapy.86 Cognitive-behavioral therapy, hypnotherapy, and guided imagery are also beneficial in decreasing GI and psychiatric symptoms in pediatric IBS.87 Although the precise mechanisms are largely unknown, psychotherapy may enhance inhibition of hyperactive stress and arousal circuits, thus repairing ineffective cortico–limbic–pontine pain modulation mechanisms observed in individuals with chronic abdominal pain.88
Neuromodulation
The external ear contains branches of 4 cranial nerves (V, VII, IX, and X) that project to brainstem nuclei, particularly the nucleus tractus solitarius, a “relay station” to brain structures involved in autonomic control and pain.89 The percutaneous electrical nerve field stimulator has recently been approved by the US Food and Drug Administration as a noninvasive central pain pathway modulator; percutaneous electrical nerve field stimulator is thought to provide analgesia by providing electrical stimulation of the peripheral cranial neurovascular bundles in the external ear that modulate central pain pathways. In a randomized, controlled study, adolescents with abdominal pain-related functional gastrointestinal disorder who received percutaneous electrical nerve field stimulator experienced a greater reduction in pain with sustained effect.90
Placebo
A high proportion of adults and children with IBS respond to placebo.91,92 It is likely that a multitude of mechanisms contribute to the placebo effects, including expectancy of treatment success93 and involvement of endogenous opioids.94
SUMMARY AND FUTURE DIRECTIONS
Although considerable progress has been made in the understanding of the brain–gut axis in health and disease, the precise mechanisms underlying both normal brain–gut homeostasis and the pathophysiology in conditions like functional gastrointestinal disorders remain incompletely understood. This lack of understanding has limited the development of holistic therapies. Several targets are evolving based on current research; targets for somatostatin, opioid and neurokinin receptors95 present in both the CNS and intestine, as well as CRF1 receptors, that target the HPA axis96 have been developed but most have not been trialed in clinical studies and those that have did not demonstrate efficacy.97,98 Pharmacologic therapies may not be necessary in all cases; the strong association of IBS with childhood trauma may provide a role for preventative psychotherapy in this subset of patients. A more comprehensive understanding of how serotonin affects the interplay between the components of the brain–gut–microbiome axis is needed to decipher novel therapies and is an area of active investigation. It is critical, however, that research focused on brain–gut physiology be undertaken to develop novel therapies for IBS and other brain–gut axis disorders.
KEY POINTS.
The brain–gut axis is a complex, bidirectional network consisting of reflex loops that ensure homeostatic control of gastrointestinal function and is impacted by the enteric microbiome.
Serotonin is a critical mediator of the gut–brain development, which impacts the enteric microbiome, and may be a common node linking the brain–gut–microbiome axis.
Irritable bowel syndrome is diagnosed by gastrointestinal symptomatology, and is often accompanied by mood, anxiety and/or somatoform disorders.
Therapies targeting serotonin receptors have been shown to be useful in some cases of irritable bowel syndrome.
Novel approaches to irritable bowel syndrome and other brain–gut axis conditions should consider holistic treatment of the central nervous system and the intestine and may involve modulation of serotonergic signaling.
Acknowledgments
Disclosure Statement: Support was provided by NIH RO1 NS015547-34. Department of Defense PR160365 NIH K08 DK093786 for Kara Gross Margolis.
Abbreviations
- CNS
Central nervous system
- CRF
Corticotropin-releasing factor
- ENS
Enteric nervous system
- fMRI
Functional MRI
- FODMAP
Fermentable oligosaccharides, disaccharides, monosaccharides and polyols
- GI
Gastrointestinal
- HPA
Hypothalamic–pituitary–adrenal
- 5-HT3
Serotonin 4
- 5-HT4
Serotonin 4
- IBS
Irritable bowel syndrome
- IBS-D
Diarrhea-predominant irritable bowel syndrome
- TCAs
Tricyclic antidepressants
REFERENCES
- 1.Borre YE, O’Keeffe GW, Clarke G, et al. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med 2014;20:509–18. [DOI] [PubMed] [Google Scholar]
- 2.Carabotti M, Scirocco A, Maselli MA, et al. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 2015;28:203–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Furness JB, Callaghan BP, Rivera LR, et al. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol 2014;817:39–71. [DOI] [PubMed] [Google Scholar]
- 4.Costa M, Brookes SJ, Hennig GW. Anatomy and physiology of the enteric nervous system. Gut 2000;47(Suppl 4):iv15–9 [discussion: iv26]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gershon MD. Developmental determinants of the independence and complexity of the enteric nervous system. Trends Neurosci 2010;33:446–56. [DOI] [PubMed] [Google Scholar]
- 6.Brummelte S, Mc Glanaghy E, Bonnin A, et al. Developmental changes in serotonin signaling: implications for early brain function, behavior and adaptation. Neuroscience 2017;342:212–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang L The role of stress on physiologic responses and clinical symptoms in irritable bowel syndrome. Gastroenterology 2011;140:761–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obata Y, Pachnis V. The effect of microbiota and the immune system on the development and organization of the enteric nervous system. Gastroenterology 2016;151(5):836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe 2015;17:565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Y, Yu YB. Intestinal microbiota and chronic constipation. Springerplus 2016; 5:1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quigley EM, Spiller RC. Constipation and the microbiome: lumen versus mucosa! Gastroenterology 2016;150:300–3. [DOI] [PubMed] [Google Scholar]
- 12.Reigstad CS, Salmonson CE, Rainey JF 3rd, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J 2015;29:1395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yarandi SS, Peterson DA, Treisman GJ, et al. Modulatory effects of gut microbiota on the central nervous system: how gut could play a role in neuropsychiatric health and diseases. J Neurogastroenterol Motil 2016;22:201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gareau MG. Microbiota-gut-brain axis and cognitive function. Adv Exp Med Biol 2014;817:357–71. [DOI] [PubMed] [Google Scholar]
- 15.Forsythe P, Bienenstock J, Kunze WA. Vagal pathways for microbiome-brain-gut axis communication. Adv Exp Med Biol 2014;817:115–33. [DOI] [PubMed] [Google Scholar]
- 16.Mayer EA, Knight R, Mazmanian SK, et al. Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci 2014;34:15490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015;161:264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith PA. The tantalizing links between gut microbes and the brain. Nature 2015; 526:312–4. [DOI] [PubMed] [Google Scholar]
- 19.Francino MP. Early development of the gut microbiota and immune health. Pathogens 2014;3:769–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thum C, Cookson AL, Otter DE, et al. Can nutritional modulation of maternal intestinal microbiota influence the development of the infant gastrointestinal tract? J Nutr 2012;142:1921–8. [DOI] [PubMed] [Google Scholar]
- 21.Al-Asmakh M, Anuar F, Zadjali F, et al. Gut microbial communities modulating brain development and function. Gut Microbes 2012;3:366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McVey Neufeld KA, Luczynski P, Seira Oriach C, et al. What’s bugging your teen?-The microbiota and adolescent mental health. Neurosci Biobehav Rev 2016;70: 300–12. [DOI] [PubMed] [Google Scholar]
- 23.Goyal MS, Venkatesh S, Milbrandt J, et al. Feeding the brain and nurturing the mind: linking nutrition and the gut microbiota to brain development. Proc Natl Acad Sci U S A 2015;112:14105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montiel-Castro AJ, Gonzalez-Cervantes RM, Bravo-Ruiseco G, et al. The microbiota-gut-brain axis: neurobehavioral correlates, health and sociality. Front Integr Neurosci 2013;7:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes 2013;20:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross ER, Gershon MD, Margolis KG, et al. Neuronal serotonin regulates growth of the intestinal mucosa in mice. Gastroenterology 2012;143:408–17.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z, Chalazonitis A, Huang YY, et al. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci 2011;31:8998–9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Margolis KG, Li Z, Stevanovic K, et al. Serotonin transporter variant drives preventable gastrointestinal abnormalities in development and function. J Clin Invest 2016;126:2221–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Margolis KG, Stevanovic K, Li Z, et al. Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut 2014;63: 928–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jenkins TA, Nguyen JC, Polglaze KE, et al. Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients 2016; 8(1) [pii:E56]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kennedy PJ, Cryan JF, Dinan TG, et al. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017;112(Pt B):399–412. [DOI] [PubMed] [Google Scholar]
- 32.Mittal R, Debs LH, Patel AP, et al. Neurotransmitters: the critical modulators regulating gut-brain axis. J Cell Physiol 2017;232(9):2359–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris G, Berk M, Carvalho A, et al. The role of the microbial metabolites including tryptophan catabolites and short chain fatty acids in the pathophysiology of immune-inflammatory and neuroimmune disease. Mol Neurobiol 2017; 54(6):4432–51. [DOI] [PubMed] [Google Scholar]
- 34.O’Mahony SM, Clarke G, Borre YE, et al. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res 2015;277:32–48. [DOI] [PubMed] [Google Scholar]
- 35.Moloney RD, Johnson AC, O’Mahony SM, et al. Stress and the microbiota-gut-brain axis in visceral pain: relevance to irritable bowel syndrome. CNS Neurosci Ther 2016;22:102–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hyams JS, Di Lorenzo C, Saps M, et al. Functional disorders: children and adolescents. Gastroenterology 2016;150:1456–68. [DOI] [PubMed] [Google Scholar]
- 37.Van Oudenhove L, Levy RL, Crowell MD, et al. Biopsychosocial aspects of functional gastrointestinal disorders: how central and environmental processes contribute to the development and expression of functional gastrointestinal disorders. Gastroenterology 2016;150:1355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fond G, Loundou A, Hamdani N, et al. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci 2014;264:651–60. [DOI] [PubMed] [Google Scholar]
- 39.Lackner JM, Brasel AM, Quigley BM, et al. The ties that bind: perceived social support, stress, and IBS in severely affected patients. Neurogastroenterol Motil 2010;22:893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayer EA, Tillisch K. The brain-gut axis in abdominal pain syndromes. Annu Rev Med 2011;62:381–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halland M, Almazar A, Lee R, et al. A case-control study of childhood trauma in the development of irritable bowel syndrome. Neurogastroenterol Motil 2014;26: 990–8. [DOI] [PubMed] [Google Scholar]
- 42.Park SH, Videlock EJ, Shih W, et al. Adverse childhood experiences are associated with irritable bowel syndrome and gastrointestinal symptom severity. Neurogastroenterol Motil 2016;28:1252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta A, Mayer EA, Acosta JR, et al. Early adverse life events are associated with altered brain network architecture in a sex- dependent manner. Neurobiol Stress 2017;7:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sykes MA, Blanchard EB, Lackner J, et al. Psychopathology in irritable bowel syndrome: support for a psychophysiological model. J Behav Med 2003;26:361–72. [DOI] [PubMed] [Google Scholar]
- 45.Rosen JM, Adams PN, Saps M. Umbilical hernia repair increases the rate of functional gastrointestinal disorders in children. J Pediatr 2013;163:1065–8. [DOI] [PubMed] [Google Scholar]
- 46.Wilder-Smith CH, Schindler D, Lovblad K, et al. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut 2004;53:1595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology 2011;140:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phillips ML, Gregory LJ, Cullen S, et al. The effect of negative emotional context on neural and behavioural responses to oesophageal stimulation. Brain 2003; 126:669–84. [DOI] [PubMed] [Google Scholar]
- 49.Ringel Y, Drossman DA, Leserman JL, et al. Effect of abuse history on pain reports and brain responses to aversive visceral stimulation: an FMRI study. Gastroenterology 2008;134:396–404. [DOI] [PubMed] [Google Scholar]
- 50.Mayer EA, Bradesi S, Chang L, et al. Functional GI disorders: from animal models to drug development. Gut 2008;57:384–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.lovino P, Tremolaterra F, Boccia G, et al. Irritable bowel syndrome in childhood: visceral hypersensitivity and psychosocial aspects. Neurogastroenterol Motil 2009;21:940–e74. [DOI] [PubMed] [Google Scholar]
- 52.Di Lorenzo C, Youssef NN, Sigurdsson L, et al. Visceral hyperalgesia in children with functional abdominal pain. J Pediatr 2001;139:838–43. [DOI] [PubMed] [Google Scholar]
- 53.Van Ginkel R, Voskuijl WP, Benninga MA, et al. Alterations in rectal sensitivity and motility in childhood irritable bowel syndrome. Gastroenterology 2001;120:31–8. [DOI] [PubMed] [Google Scholar]
- 54.Huang JS, Terrones L, Simmons AN, et al. Pilot study of functional magnetic resonance imaging responses to somatic pain stimuli in youth with functional and inflammatory gastrointestinal disease. J Pediatr Gastroenterol Nutr 2016;63:500–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 2007;132:397–414. [DOI] [PubMed] [Google Scholar]
- 56.Mawe GM, Hoffman JM. Serotonin signalling in the gut–functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 2013;10:473–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Faure C, Patey N, Gauthier C, et al. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology 2010;139:249–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gershon MD. Review article: serotonin receptors and transporters – roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther 2004; 20(Suppl 7):3–14. [DOI] [PubMed] [Google Scholar]
- 59.Jadallah KA, Kullab SM, Sanders DS. Constipation-predominant irritable bowel syndrome: a review of current and emerging drug therapies. World J Gastroenterol 2014;20:8898–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Samuels BA, Mendez-David I, Faye C, et al. Serotonin 1A and serotonin 4 receptors: essential mediators of the neurogenic and behavioral actions of antidepressants. Neuroscientist 2016;22:26–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ford AC, Moayyedi P Lacy BE, et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol 2014;109(Suppl 1):S2–26 [quiz: S27]. [DOI] [PubMed] [Google Scholar]
- 62.Chang L, Chey WD, Harris L, et al. Incidence of ischemic colitis and serious complications of constipation among patients using alosetron: systematic review of clinical trials and post-marketing surveillance data. Am J Gastroenterol 2006; 101:1069–79. [DOI] [PubMed] [Google Scholar]
- 63.Tong K, Nicandro JP, Shringarpure R, et al. A 9-year evaluation of temporal trends in alosetron postmarketing safety under the risk management program. Ther Adv Gastroenterol 2013;6:344–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bahar RJ, Collins BS, Steinmetz B, et al. Double-blind placebo-controlled trial of amitriptyline for the treatment of irritable bowel syndrome in adolescents. J Pediatr 2008;152:685–9. [DOI] [PubMed] [Google Scholar]
- 65.Saps M, Youssef N, Miranda A, et al. Multicenter, randomized, placebo-controlled trial of amitriptyline in children with functional gastrointestinal disorders. Gastroenterology 2009;137:1261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tack J, Broekaert D, Fischler B, et al. A controlled crossover study of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome. Gut 2006;55: 1095–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vahedi H, Merat S, Rashidioon A, et al. The effect of fluoxetine in patients with pain and constipation-predominant irritable bowel syndrome: a double-blind randomized-controlled study. Aliment Pharmacol Ther 2005;22:381–5. [DOI] [PubMed] [Google Scholar]
- 68.Roohafza H, Pourmoghaddas Z, Saneian H, et al. Citalopram for pediatric functional abdominal pain: a randomized, placebo-controlled trial. Neurogastroenterol Motil 2014;26:1642–50. [DOI] [PubMed] [Google Scholar]
- 69.Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol 2009;6:306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut 2010;59:325–32. [DOI] [PubMed] [Google Scholar]
- 71.Pirbaglou M, Katz J, de Souza RJ, et al. Probiotic supplementation can positively affect anxiety and depressive symptoms: a systematic review of randomized controlled trials. Nutr Res 2016;36:889–98. [DOI] [PubMed] [Google Scholar]
- 72.Pinto-Sanchez MI, Hall GB, Ghajar K, et al. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology 2017;153:448–59.e8. [DOI] [PubMed] [Google Scholar]
- 73.O’Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and Bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology 2005;128:541–51. [DOI] [PubMed] [Google Scholar]
- 74.Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol 2006;101:1581–90. [DOI] [PubMed] [Google Scholar]
- 75.Gawronska A, Dziechciarz P, Horvath A, et al. A randomized double-blind placebo-controlled trial of Lactobacillus GG for abdominal pain disorders in children. Aliment Pharmacol Ther 2007;25:177–84. [DOI] [PubMed] [Google Scholar]
- 76.Francavilla R, Miniello V, Magista AM, et al. A randomized controlled trial of Lactobacillus GG in children with functional abdominal pain. Pediatrics 2010;126: e1445–52. [DOI] [PubMed] [Google Scholar]
- 77.Horvath A, Dziechciarz P, Szajewska H. Meta-analysis: Lactobacillus rhamnosus GG for abdominal pain-related functional gastrointestinal disorders in childhood. Aliment Pharmacol Ther 2011;33:1302–10. [DOI] [PubMed] [Google Scholar]
- 78.Giannetti E, Maglione M, Alessandrella A, et al. A mixture of 3 Bifidobacteria decreases abdominal pain and improves the quality of life in children with irritable bowel syndrome: a multicenter, randomized, double-blind, placebo-controlled, crossover trial. J Clin Gastroenterol 2017;51:e5–10. [DOI] [PubMed] [Google Scholar]
- 79.Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life—a double-blind, placebo-controlled study. Aliment Pharmacol Ther 2011;33:1123–32. [DOI] [PubMed] [Google Scholar]
- 80.Yoon JS, Sohn W, Lee OY, et al. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol 2014;29:52–9. [DOI] [PubMed] [Google Scholar]
- 81.Guandalini S, Magazzu G, Chiaro A, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr 2010; 51:24–30. [DOI] [PubMed] [Google Scholar]
- 82.Staudacher HM, Whelan K. The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. Gut 2017;66:1517–27. [DOI] [PubMed] [Google Scholar]
- 83.Halmos EP, Power VA, Shepherd SJ, et al. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology 2014;146:67–75.e5. [DOI] [PubMed] [Google Scholar]
- 84.Chumpitazi BP, Cope JL, Hollister EB, et al. Randomised clinical trial: gut microbiome biomarkers are associated with clinical response to a low FODMAP diet in children with the irritable bowel syndrome. Aliment Pharmacol Ther 2015;42: 418–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eswaran S, Chey WD, Jackson K, et al. A diet low in fermentable Oligo-, Di-, and Monosaccharides and Polyols improves quality of life and reduces activity impairment in patients with irritable bowel syndrome and diarrhea. Clin Gastroenterol Hepatol 2017;15:1890–9.e3. [DOI] [PubMed] [Google Scholar]
- 86.Ford AC, Quigley EM, Lacy BE, et al. Effect of antidepressants and psychological therapies, including hypnotherapy, in irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol 2014;109:1350–65 [quiz: 1366]. [DOI] [PubMed] [Google Scholar]
- 87.Rutten JM, Vlieger AM, Frankenhuis C, et al. Gut-directed hypnotherapy in children with irritable bowel syndrome or functional abdominal pain (syndrome): a randomized controlled trial on self exercises at home using CD versus individual therapy by qualified therapists. BMC Pediatr 2014;14:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lackner JM, Lou Coad M, Mertz HR, et al. Cognitive therapy for irritable bowel syndrome is associated with reduced limbic activity, GI symptoms, and anxiety. Behav Res Ther 2006;44:621–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Frangos E, Ellrich J, Komisaruk BR. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul 2015;8:624–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kovacic K, Hainsworth K, Sood M, et al. Neurostimulation for abdominal pain-related functional gastrointestinal disorders in adolescents: a randomised, double-blind, sham-controlled trial. Lancet Gastroenterol Hepatol 2017;2:727–37. [DOI] [PubMed] [Google Scholar]
- 91.Camilleri M, Di Lorenzo C. Brain-gut axis: from basic understanding to treatment of IBS and related disorders. J Pediatr Gastroenterol Nutr 2012;54:446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kaptchuk TJ, Friedlander E, Kelley JM, et al. Placebos without deception: a randomized controlled trial in irritable bowel syndrome. PLoS One 2010;5:e15591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Benedetti F, Pollo A, Lopiano L, et al. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J Neurosci 2003;23:4315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Finniss DG, Benedetti F. Mechanisms of the placebo response and their impact on clinical trials and clinical practice. Pain 2005;114:3–6. [DOI] [PubMed] [Google Scholar]
- 95.Bradesi S, Tillisch K, Mayer E. Emerging drugs for irritable bowel syndrome. Expert Opin Emerg Drugs 2006;11:293–313. [DOI] [PubMed] [Google Scholar]
- 96.Mayer EA, Tillisch K, Bradesi S. Review article: modulation of the brain-gut axis as a therapeutic approach in gastrointestinal disease. Aliment Pharmacol Ther 2006;24:919–33. [DOI] [PubMed] [Google Scholar]
- 97.Martinez V, Tache Y. CRF1 receptors as a therapeutic target for irritable bowel syndrome. Curr Pharm Des 2006;12:4071–88. [DOI] [PubMed] [Google Scholar]
- 98.Sweetser S, Camilleri M, Linker Nord SJ, et al. Do corticotropin releasing factor-1 receptors influence colonic transit and bowel function in women with irritable bowel syndrome? Am J Physiol Gastrointest Liver Physiol 2009;296:G1299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]