Abstract
CO2 inhalation can provoke panic attacks in humans, and the likelihood is increased in patients with panic disorder. Identifying brain sites involved could provide important mechanistic insight into the illness. In mice, the amygdala has been suggested to promote CO2 responses; however, recent studies in humans with amygdala damage indicate the amygdala is not required for CO2-induced fear and panic and might actually oppose these responses. To clarify the role of the amygdala, we produced lesions in mice paralleling the human lesions, and characterized behavioral responses to CO2. Compared to sham controls, we found that amygdala-lesioned mice froze less to 10% CO2, and unlike shams they also began to jump frenetically. At 20% CO2, controls also exhibited jumping, suggesting it is a normal response to more extreme CO2 concentrations. The effect of amygdala lesions was specific to CO2 as amygdala-lesioned mice did not jump in response to a predator odor or to an auditory conditioned stimulus. In amygdala-lesioned mice, jumping evoked by 10% CO2 was eliminated by co-lesioning the dorsal periaqueductal gray, a structure implicated in panic and escape-related behaviors. Together, these observations suggest a dual role for the amygdala in the CO2 response: promoting CO2-induced freezing, and opposing CO2-induced jumping, which may help explain the exaggerated CO2 responses in humans with amygdala lesions.
Keywords: Carbon dioxide, Amygdala, Periacqueductal gray, Panic
1. Introduction
Faced with a threat, all animals including humans must rapidly assess the severity and imminence of the risk and defend themselves. An inappropriate, ineffective, or maladaptive defensive response can be deadly; thus, choosing an efficacious strategy is critical for survival [1]. Defensive responses exist on a spectrum whereby urgency, severity, and magnitude of the response escalate with increasing threat imminence and intensity. Low intensity or low risk threats generally elicit vigilance and avoidance, whereas moderate intensity or more proximal threats often elicit immobility which likely helps one avoid detection. In contrast, high intensity or unavoidable threats evoke extreme responses including rapid attempts to escape and violent preemptive attacks or counterattacks [2, 3]. These responses are largely conserved across species and are seen in response to a range of threatening stimuli, such as predators [4, 5], shocks [6, 7], and cues previously associated with aversive stimuli [8]. Despite substantial previous work, current knowledge of the circuits and mechanisms underlying the range and distinctions between defensive response remains incomplete.
Carbon dioxide (CO2) has been a valuable experimental tool for studying the defensive response spectrum. As CO2 levels rise, the threat of suffocation becomes increasingly imminent, thus shifting behaviors across the defensive response spectrum. In humans, CO2 inhalation can elicit anxiety, fear and panic [9–11]. These responses can occur in healthy individuals, but occur more frequently in patients with panic disorder [12]. In mice, CO2 inhalation elicits avoidance and freezing [13–16].
Previous work implicated the amygdala in CO2-evoked freezing in mice [13]. This observation was consistent with literature implicating the rodent amygdala in freezing evoked by other aversive stimuli [17–19], as well as with literature implicating the human amygdala in fear responses [20–23]. More recently, the role of the human amygdala in CO2-evoked defensive responses was investigated in subjects with bilateral amygdala damage due to Urbach-Wiethe disease using a 35% CO2 challenge. Contrary to the expectation that amygdala damage would attenuate CO2 responses, individuals with amygdala lesions experienced CO2-evoked panic attacks and an increased likelihood of panicking [24]. These studies suggested that while the amygdala may promote some defensive responses, such as avoidance and freezing, it may also suppress other more extreme defensive responses. We therefore sought to clarify the role of the amygdala in CO2-evoked defensive responses in mice by modeling the amygdala lesions caused by Urbach-Wiethe disease.
2. Materials and methods
2.1. Mice.
Mice were maintained on a congenic C57BL/6J background, with ad libitum access to water and chow (Teklab), and kept on a 12-hour light/dark cycle with experiments performed during the light phase. Both sexes, aged 10—16 weeks were used in the behavioral experiments and all groups were age- and sex-matched. Separate groups of mice were used to test effects of amygdala lesions on CO2-evoked behaviors, TMT-evoked freezing, and fear conditioning. Prior to testing CO2 responses, amygdala-, BNST-, and hippocampus-lesioned mice were tested in the open field test to determine whether the lesions affected locomotor activity. Animal care met National Institutes of Health standards and the University of Iowa Animal Care and Use Committee approved all experiments.
2.2. Electrolytic brain lesions.
Bilateral brain lesions were generated as previously described [14] with a unipolar electrode (an insected pin coated in Epoxylite) stereotactically inserted into the region of interest with the following coordinates relative to bregma or pial surface: amygdala: 1.4 mm posterior, 3.2 mm lateral, and 3.9 mm ventral; bed nucleus of the stria terminalis (BNST): 0.4 mm anterior, 1.0 mm lateral, 4.3 mm ventral; hippocampus: 1.4 mm posterior, 1.0 mm lateral, 1.0 mm ventral; and dorsal periaqueductal gray (dPAG): 4.0 mm posterior, 0.5 mm lateral, 1.5 mm ventral. Current (1 mA anodal DC) was applied for 17 s (amygdala and hippocampus), 10 s (BNST), or 7 s (dPAG). Duration of current application was varied between sites so as to generate lesions of an appropriate size. Unilateral amygdala lesions were made in equal numbers in the left and right amygdalae. Sham surgeries were identical to lesion surgeries, but without current application or electrode insertion. Sham controls were compared to a relatively large group of non-surgery exposed wild-type C57Bl/6J mice (n = 47) freezing and jumping were not statistically different (freezing: p = 0.5736, jumping: p = 0.4546) suggesting sham surgery did not affect 10% CO2-evoked behaviors. In addition, sham controls were compared to a separate group of mice in which the surgeries included electrode insertion but not lesioning; the sham mice also did not differ significantly from this group (Figure S1). One week of surgical recovery preceded behavioral testing. Lesion location and size were assessed postmortem. Five animals were excluded from analyses because their lesions did not hit the structure of interest.
2.3. CO2-evoked behaviors.
CO2-evoked behaviors were assessed as previously described [13, 14, 25]. Briefly, mice were placed in a custom-made Plexigas chamber (20.3 cm wide X 20.3 cm deep X 16.5 cm high) for 10 min. Compressed air, 10% CO2 (21% O2, balanced with N2), or 20% CO2 (21% O2, balanced with N2) was infused into the chamber at a rate of 5 L/minute. A separate group of mice was used for each gas mixture. Squads of sham mice which underwent gas exposures at separate times but under consistent conditions did not differ significantly from each other and were collapsed for analysis. Behavioral testing was videotaped and assessed by a trained observer blinded to condition. Freezing was defined as a lack of movement other than respiration, and jumping was defined as all paws being simultaneously airborne.
2.4. Locomotor testing.
Locomotion was assessed as previously described [14, 26] in an open field chamber (40.6 cm wide 40.6 cm deep 36.8 cm high, San Diego Instruments) in room air for 20 min.
2.5. Auditory cue fear conditioning.
Day 1: mice were placed in video fear conditioning chambers (Med Associates) for a total of 14 min. For the first 3 min, they were allowed to explore, followed by 5 tones (80 dB, 3 kHz, 20 s), co-terminating with a foot shock (1 s, 0.75 mA), with a 100 s inter-trial interval. Day 2: conditioned freezing and jumping were assessed in response to a tone presented continuously during minutes 4—6 of a 10 min trial in a novel context with floor, ceiling, lighting and odor altered from Day 1. Freezing was assessed using VideoFreeze software (Med Associates) and jumping assessed as above.
2.6. TMT-evoked behaviors.
TMT exposure was performed as previously described [27, 28]. Briefly, 6 μl of trimethythiazoline (TMT) (Contech) was pipetted onto a piece of tissue paper in a beaker. Mice, along with the beaker, were placed in a Plexiglas behavior chamber (20 cm wide X 21 cm deep X 17 cm long) for 10 min. Behavioral testing was videotaped and assessed by a trained observer blinded to condition. Freezing and jumping were assessed in the same manner as with CO2 exposure.
2.7. Statistical analysis.
Statistical significance between two groups was tested with the Mann-Whitney test (ordinal variables) or Student’s t-test (continuous variables). Welch’s correction was applied when an F test revealed significantly different variances. ANOVA was used to test statistical significance between more than two groups. Relationships between groups hypothesized a priori were tested by planned contrast testing within the context of the full ANOVA. For all tests, p < 0.05 was considered significant. Values are displayed as mean ± SEM. All statistical tests were performed using Graphpad Prism software.
3. Results
3.1. Amygdala lesions alter CO2-evoked behaviors
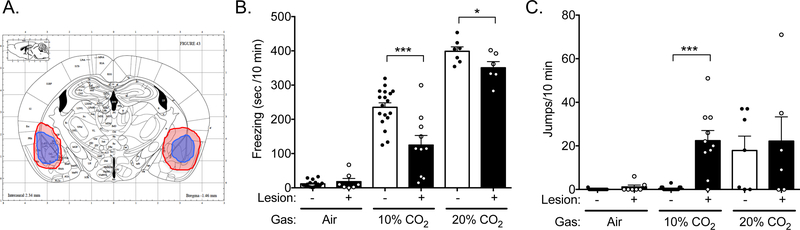
To attempt to reconcile the seemingly contradictory findings that the amygdala inhibits CO2-evoked defensive responses in humans [24], yet promotes CO2-evoked defensive response in mice [13], we sought to approximate in mice the amygdala damage seen in patients with Urbach-Wiethe disease. Therefore, we created bilateral electrolytic lesions in mice. Although electrolytic lesions are less discriminant than other methods, we chose this approach, because the effects are permanent and destroy both amygdala tissue and fibers of passage similar to the destructive lesions in the patients. And like individuals with Urbach-Wiethe disease [24], the mouse lesions affected the basolateral amygdala (BLA) as well as some adjacent structures (Figure 1A, S2A,B). Although notably, the heterogenous lesions generated in mice may not reproduce the lesion heterogenity in human patients, and occurred en bloc unlike the progressive amygdala damage that occurrs over time in the disease. In order to test the effects of these lesions on CO2 responses, we placed mice in an airtight chamber infused with compressed air, 10% CO2, or 20% CO2, as described previously [13, 14, 25, 28]. Consistent with our earlier studies, exposure to 10% or 20% CO2 evoked robust, dose-dependent freezing in sham controls, which was absent in compressed air (Figure 1B). Amygdala-lesioned mice responded quite differently. In 10% CO2, they froze much less than their sham counterparts (Figure 1B). Interestingly, they also displayed additional behaviors: the amygdala-lesioned mice jumped frenetically, often hitting the ceiling of the clear Plexiglas chamber (Figure 1C, Video S1). This jumping was similar to behavior previously reported in response to other aversive stimuli, which has been thought to be analogous to escape or flight [7, 8]. Mice with unilateral amygdala lesions also jumped in 10% CO2 but froze normally (Figure S3A, S3B), suggesting the jumping was not simply unmasked by the absence of freezing. In compressed air, the jumping was largely absent, suggesting it was a response to the CO2 itself, rather to some other aspect of the assay. Sham-lesioned mice rarely jumped in response to 10% CO2. Although a higher CO2 concentration, 20%, evoked jumping in both amygdala-lesion and sham-lesion mice, and to a similar degree (Figure 1C), perhaps due to a ceiling effect. Together these data suggest that jumping represents a more extreme defensive response that can be evoked by high CO2 concentrations. Moreover, these data suggest that amygdala lesions may shift the defensive spectrum and lower the threshold of more extreme CO2-evoked defensive behaviors.
Figure 1: Amygdala lesions alter CO2-evoked behaviors.
A) Diagram [58] showing size and location of the largest (red) and smallest (blue) amygdala lesions. B) Amygdala lesions reduce CO2-evoked freezing. ANOVA revealed an interaction between CO2 concentration and lesion status (F(2,57) = 7.382, p = 0.0014, n = 15, 7, 18, 10, 6, 6). Planned contrast testing revealed that amygdala lesions reduce freezing to both 10% (***p = 0.0004) and 20% CO2 (*p = 0.0481). C) Amygdala lesions elicit 10% CO2-evoked jumping. ANOVA revealed an interaction between CO2 concentration and lesion status (F(2,57) = 5.248, p = 0.0081. Planned contrast testing revealed that mice with amygdala lesions exhibit increased jumping in response to 10% (***p < 0.0001) but not 20% (p = 0.9709) CO2, and that sham controls jumped more in response to 20% CO2 than 10% CO2 (p = 0.0066).
Because freezing and jumping both depend on movement, we tested whether the lesions affected general locomotor activity. Amygdala lesions did not alter locomotor activity in an open field test (Figure S4), suggesting that the altered defensive responses evoked by CO2 were not due to baseline differences in locomotion.
3.2. Amygdala-lesioned mice did not jump in response to other aversive stimuli
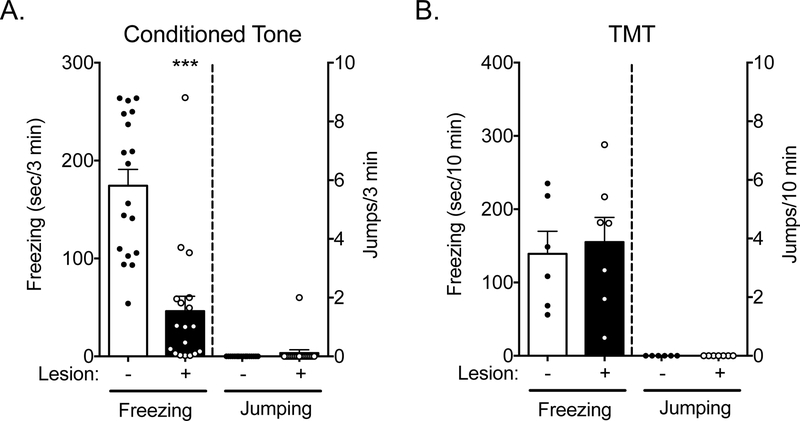
We next sought to determine if amygdala lesions produced a similar shift in the defensive responses to other aversive stimuli. First, we tested the effects of amygdala lesions on auditory fear conditioning. Amygdala lesions are known to impair the acquisition and expression of conditioned freezing behavior [29, 30]. Consistent with others’ observations [31], our amygdala-lesioned mice froze less than their sham counterparts during acquisition (data not shown, sham controls 265.7 ± 20.19, amygdala-lesioned mice 67.06 ± 20.39, t (34) = 6.92, p < 0.0001) and in response to presentation of the conditioned stimulus, an auditory tone that was paired with footshocks, 24 h after training (Figure 2A). However, little or no jumping was observed in response to the conditioned stimulus in either the amygdala-lesioned or sham-lesioned mice (Figure 2A). In another group of mice, we tested the effects of amygdala lesions on the behavioral response to the predator odor trimethythiazoline (TMT). Previous studies have reported that lesion or acute inactivation of the basolateral amygdala does not alter TMT-evoked freezing [32, 33]. Consistent with those observations, we observed no effect of amygdala lesions on TMT-evoked freezing (Figure 2B). In addition, neither amygdala-lesioned mice nor sham controls jumped in response to TMT exposure (Figure 2B). Together, these findings suggest that effects of amygdala lesions on CO2 responses may not generalize to all stimuli that induce freezing.
Figure 2: Amygdala-lesioned mice do not jump to an auditory conditioned stimulus or a predator odor.
A) Amygdala lesions greatly attenuate conditioned freezing to a tone that had been previously paired with footshocks (t(34) = 5.676, ***p < 0.0001, n = 18/group). However, exposure to this tone does not elicit jumping (p > 0.9999). B) Amygdala lesions do not alter freezing in response to the predator odor TMT (t(11) = 0.3492, p = 0.7336, n = 6, 7), nor do they elicit jumping.
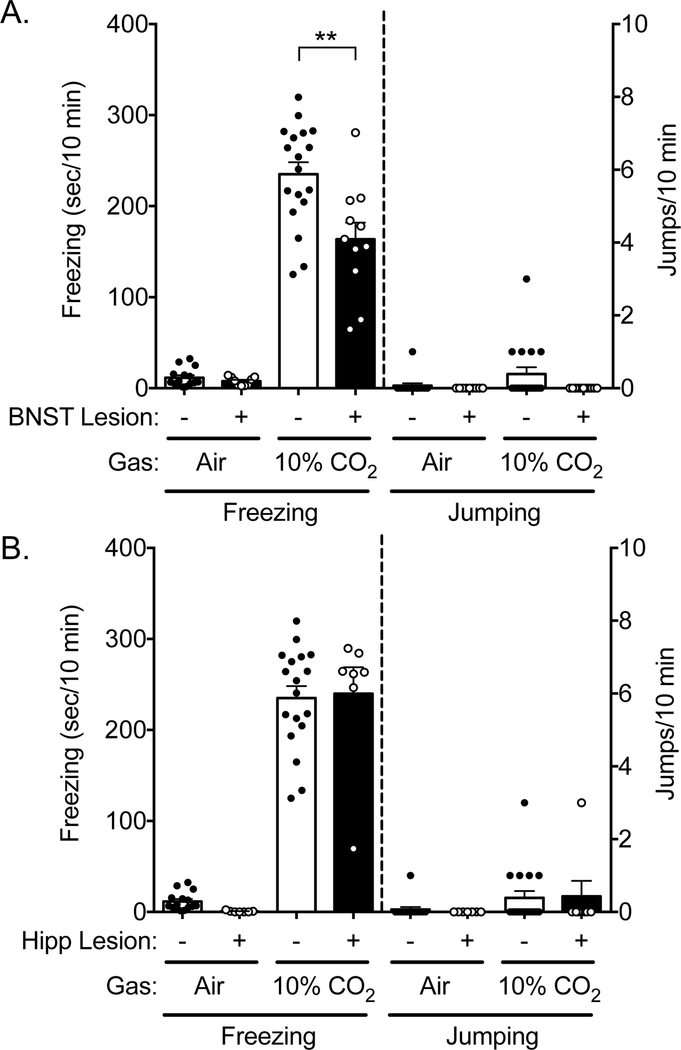
3.3. BNST and hippocampus lesions did not elicit jumping in 10% CO2
Our previous work also implicated the bed nucleus of the stria terminalis (BNST) in behavioral responses to CO2 [14]. Thus, we sought to test whether or not BNST lesions altered CO2-evoked jumping. We tested the response to 10% CO2, as this was the concentration at which amygdala-lesioned mice jumped and their sham counterparts did not. Like amygdala lesions, BNST lesions also attenuated freezing to CO2 (Figure S5A, 3A) [14], a difference not explained by a change in locomotion (Figure S4). However, BNST lesions did not lead to jumping (Figure 3A), suggesting that the circuitry underlying CO2-evoked freezing and jumping behaviors is distinguishable. To further test site specificity, we also assessed the effects of dorsal hippocampal lesions (Figure S5B), and found that they did not affect freezing to 10% CO2, nor did they lead to jumping (Figure 3B), though consistent with previous observations, hippocampus lesions increased overall locomotion [34] (Figure S4). Thus, among the three sites tested, both BNST and amygdala lesions altered CO2-evoked freezing, whereas only amygdala lesions led to jumping in 10% CO2.
Figure 3: BNST and hippocampus lesions do not elicit 10% CO2-evoked jumping.
A) As previously reported [14], BNST lesions reduce 10% CO2-evoked freezing. However, BNST lesions do not elicit 10% CO2-evoked jumping. ANOVA revealed an interaction between CO2 concentration and lesion status (F(1,49) = 7.56, p = 0.0083, n = 15, 9, 18, 11). Planned contrast testing revealed that BNST-lesioned mice freeze less than sham-lesioned mice in 10% CO2 (**p = 0.0032). However, ANOVA revealed no effect of CO2 concentration on jumping (F(1,49) = 1.4, p = 0.2425), no effect of lesion (F(1,49) = 2.797, p = 0.1008), and no interaction (F(1,49) = 1.4, p = 0.2425). B) Hippocampus lesions do not alter 10% CO2-evoked freezing behavior or elicit 10% CO2-evoked jumping. ANOVA revealed an effect of CO2 concentration on freezing (F(1,43) = 253.2, p < 0.0001, n = 15, 7, 18, 7), but no effect of lesion (F(1,43) = 0.0439, p = 0.8350), and no interaction (F(1,43) = 0.5889, p = 0.5889). ANOVA revealed no effect of CO2 on jumping (F(1,43) = 3.14, p = 0.0835), no effect of lesion (F(1,43) = 0.004056, p = 9495), and no interaction (F(1,43) = 0.06299, p = 0.8030).
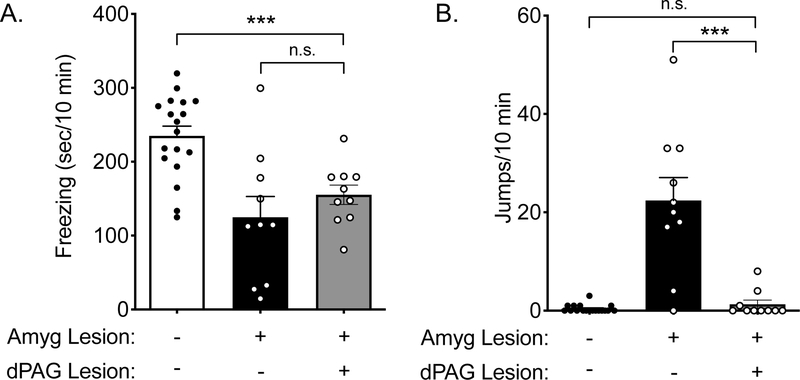
3.4. The dorsal periaqueductal gray (dPAG) is critical for jumping responses to CO2 in amygdala-lesioned mice.
We next sought to determine whether the dPAG might be required for the CO2-evoked jumping in the amygdala lesioned mice. Electrical activation of the dPAG can evoke panic-like attacks in humans [35, 36] and defensive behaviors such as jumping in rodents [37–39]. Recent studies have further probed the role of the dPAG using optogenetics, suggesting dPAG neurons encode escape behavior [7, 40, 41]. To test the role of the dPAG in CO2-induced jumping, we electrolytically lesioned both the amygdala and dPAG (Figure S5C), and compared the behavioral responses to 10% CO2 in these mice to previously tested amygdala-lesioned and sham control mice (Figure 1B, 1C). The mice with both amygdala and dPAG lesions were run under similar conditions, although a potential limitation is that these mice were not run concurrently. Unlike amygdala-lesioned mice who jumped robustly, mice with both amygdala and dPAG lesions rarely jumped in 10% CO2 (Figure 4B), although similar to mice with amygdala lesions alone, freezing was reduced compared to sham controls (p = 0.0005) (Figure 4A). Neither amygdala lesions nor dPAG lesions alone altered general locomotor activity in the open field (Figure S4), suggesting that the reduction in jumping did not arise from altered locomotion. Together, these data implicate the dPAG as a key brain site underlying CO2-evoked jumping in the absence of the amygdala.
Figure 4: dPAG lesions reduce jumping behavior in amygdala-lesioned mice.
10% CO2-evoked behavior of mice with both amygdala and dPAG lesions was compared to that of sham controls and mice with only amygdala lesions (from Figure 1). A) ANOVA revealed a difference in freezing among groups (F(2,35) = 11.35, p = 0.0002, n = 18, 10, 10). Planned contrast testing revealed that freezing in mice with both amygdala and dPAG lesions was less than in sham controls (***p = 0.0005) and similar to mice with only amygdala lesions (p = 0.3445). B) ANOVA revealed a difference in jumping among groups (F(2,35) = 30.00, p < 0.0001). dPAG lesions greatly attenuated the CO2-evoked jumping seen in amygdala-lesioned mice (***p = 0.0005) and mice with both amygdala and dPAG lesions exhibited similar jumping as sham controls (p = 0.6950).
4. Discussion
These results support key roles for the amygdala in CO2-evoked behaviors. Consistent with previous effects of amygdala lesions in humans [24], bilateral amygdala lesions in mice also altered defensive responses to CO2 by decreasing immobility (freezing) and increasing active (jumping) responses (Figure 1B, 1C). Two observations suggest that freezing and jumping are separable, and thus likely distinct phenomena: 1) unilateral amygdala lesions increased jumping without reducing freezing (Figure S3A, S3B) and 2) BNST lesions reduced freezing without precipitating jumping (Figure 3A) [14]. Furthermore, co-lesioning the dPAG dramatically reduced jumping but had no effect on freezing compared to mice with amygdala lesions alone (Figure 4B). Together, these observations suggest a pathway by which the amygdala regulates downstream sites such as the dPAG to generate differential responses to rising CO2 and the impending threat of suffocation.
Humans who have undergone CO2 challenges report symptoms of panic such as intense emotional distress, fear of dying, and shortness of breath [10, 11, 24, 42]. Because we cannot know the subjective experience of a mouse, we cannot equate jumping with panic, however both likely represent species-specific extremes in the defensive response spectrum. Additionally, the shift towards more extreme defensive responses may not be readily revealed by all aversive stimuli. One of the individuals with amygdala damage (patient S.M.) studied in Feinstein et al., 2013, was presented with an array of aversive stimuli including naturalistic predator threats such as snakes and spiders [20] and unlike CO2, these stimuli failed to elicit fear or panic responses. Patient S.M. also failed to exhibit fear conditioning to a loud noise [21]. Similarly, in amygdala-lesioned mice, predator odor and fear conditioning failed to elicit the jumping response observed with CO2 (Figure 2A, 2B). We suspect the differences with CO2 may reflect an ability to more effectively engage the extreme end of the defensive response spectrum. Unlike most external threats, which animals detect through sensory cues (e.g. sight, sound, smell), inhaled CO2 is rapidly absorbed and elevated CO2 concentrations and the associated acidosis can directly stimulate pH sensitive neurons in the brain and elsewhere [13, 14, 43]. However, because many threats induce fight or flight, we speculate that amygdala damage might also enhance defensive responses to other threats in addition to CO2 and it will be particularly important to determine if there are distinctions in the role of the amygdala between external and internal stimuli or between stimuli that induce acidosis and those that do not modulate brain pH. Consistent with a role for the amygdala in modulating other extreme defensive responses, patients with amygdala lesions have been noted to experience spontaneous panic attacks [44], as well as panic symptoms following injection of isoproterenol, a β-adrenergic agonist thought to stimulate cardiorespiratory interoception [45].
A shared limitation of the mouse and human studies is that they do not allow us to pinpoint the specific circuits, cell types, and molecules responsible for the CO2-evoked defensive responses. The amygdala projects to and may regulate numerous downstream sites such as the extended amygdala, hypothalamus, respiratory brainstem, and PAG [46]. Consistent with this regulation, recent studies in humans suggest brainstem chemosensory areas can be inhibited by the amygdala [47–49]. Dysregulation of one or more of these areas may contribute to the observed behavioral effects. Our results suggest the BLA regulates dPAG function to oppose CO2-evoked jumping. The BLA has been suggested to regulate dPAG function through at least two circuits, e.g. via connections to the central amygdala and via the lateral hypothalamus. These structures project directly to the PAG and recent work has identified neuron populations in both the central amygdala and lateral hypothalamus that are involved in active defensive responses to other aversive stimuli [7, 8, 50]. Thus, assessing effects of CO2 on these neurons would be very informative.
The physiological and behavioral effects of CO2 are thought to be largely mediated by acidosis, thus some of the numerous pH-sensitive molecules expressed in the brain are likely involved [12, 51, 52]. Recent work has implicated two pH-sensitive receptors, acid-sensing ion channel-1A [13, 14] and T cell death-associated gene-8 [15], in CO2-evoked freezing. Future studies will be needed to determine whether these or other pH-sensitive receptors play a role in more extreme defensive responses evoked by CO2, such as jumping.
The observation that defensive responses evoked by CO2 escalated in both mice and humans with amygdala lesions suggests the amygdala plays a critical role in gauging the appropriate magnititude of defensive responses. In life threatening situations, fight or flight responses are adaptive, but when these extreme defensive responses occur in the absence of serious threat, they are maladaptive, as in panic disorder or post-traumatic stress disorder (PTSD). MRI studies of individuals with panic disorder and PTSD have reported changes in the volume and the function of both the amygdala and PAG [53–57]. Combined with these observations, our results support the possibility that amygdala and dPAG dysfunction may lead to exaggerated and maladaptive defensive responses in these illnesses.
Supplementary Material
Acknowledgements:
J.A.W. was supported by the Department of Veterans Affairs (Merit Award), the NIMH (R01MH085724, R01MH113325), and a NARSAD Independent Investigator Award. R.J.T. was supported by NIMH training grant T32MH019113. C.J.K was supported by NINDS training grant T32NS045549. We thank John Freeman, Michael Welsh, Ryan LaLumiere, Nandakumar Narayanan, Rong Fan, Amanda Wunsch, Mackenzie Conlon, Levi Sowers, and the University of Iowa Central Microscopy Facility for their recommendations and assistance.
Abbreviations:
- BNST
bed nucleus of the stria terminalis
- BLA
basolateral amygdala
- CO2
carbon dioxide
- DC
direct current
- dPAG
dorsal periaqueductal gray
- TMT
trimethylthiazoline
Footnotes
Financial Disclosures: The authors declare no competing financial interests.
Data sharing. The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- [1].Blanchard DC, Griebel G, Pobbe R, Blanchard RJ, Risk assessment as an evolved threat detection and analysis process, Neurosci Biobehav Rev 35(4) (2011) 991–8. [DOI] [PubMed] [Google Scholar]
- [2].Perusini JN, Fanselow MS, Neurobehavioral perspectives on the distinction between fear and anxiety, Learn Mem 22(9) (2015) 417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Harrison LA, Ahn C, Adolphs R, Exploring the Structure of Human Defensive Responses from Judgments of Threat Scenarios, PLoS One 10(8) (2015) e0133682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Eilam D, Die hard: a blend of freezing and fleeing as a dynamic defense--implications for the control of defensive behavior, Neurosci Biobehav Rev 29(8) (2005) 1181–91. [DOI] [PubMed] [Google Scholar]
- [5].Dielenberg RA, McGregor IS, Defensive behavior in rats towards predatory odors: a review, Neurosci Biobehav Rev 25(7–8) (2001) 597–609. [DOI] [PubMed] [Google Scholar]
- [6].Low A, Weymar M, Hamm AO, When Threat Is Near, Get Out of Here: Dynamics of Defensive Behavior During Freezing and Active Avoidance, Psychological science 26(11) (2015) 1706–16. [DOI] [PubMed] [Google Scholar]
- [7].Tovote P, Esposito MS, Botta P, Chaudun F, Fadok JP, Markovic M, Wolff SB, Ramakrishnan C, Fenno L, Deisseroth K, Herry C, Arber S, Luthi A, Midbrain circuits for defensive behaviour, Nature 534(7606) (2016) 206–12. [DOI] [PubMed] [Google Scholar]
- [8].Fadok JP, Krabbe S, Markovic M, Courtin J, Xu C, Massi L, Botta P, Bylund K, Muller C, Kovacevic A, Tovote P, Luthi A, A competitive inhibitory circuit for selection of active and passive fear responses, Nature 542(7639) (2017) 96–100. [DOI] [PubMed] [Google Scholar]
- [9].Drury AN, The percentage of carbon dioxide in the alveolar air, and the tolerance to accumulating carbon dioxide in case of co-called “irritable heart”, Heart 7 (1918) 165–173. [Google Scholar]
- [10].Gorman JM, Kent JM, Sullivan GM, Coplan JD, Neuroanatomical hypothesis of panic disorder, revised, Am. J. Psychiatry 157(4) (2000) 493–505. [DOI] [PubMed] [Google Scholar]
- [11].Papp LA, Klein DF, Gorman JM, Carbon dioxide hypersensitivity, hyperventilation, and panic disorder, Am. J. Psychiatry 150(8) (1993) 1149–1157. [DOI] [PubMed] [Google Scholar]
- [12].Wemmie JA, Neurobiology of panic and pH chemosensation in the brain, Dialogues Clin Neurosci 13(4) (2011) 475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA 3rd, Welsh MJ, Wemmie JA, The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior, Cell 139(5) (2009) 1012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Taugher RJ, Lu Y, Wang Y, Kreple CJ, Ghobbeh A, Fan R, Sowers LP, Wemmie JA, The bed nucleus of the stria terminalis is critical for anxiety-related behavior evoked by CO2 and acidosis, J Neurosci 34(31) (2014) 10247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vollmer LL, Ghosal S, McGuire JL, Ahlbrand RL, Li KY, Santin JM, Ratliff-Rang CA, Patrone LG, Rush J, Lewkowich IP, Herman JP, Putnam RW, Sah R, Microglial Acid Sensing Regulates Carbon Dioxide-Evoked Fear, Biol Psychiatry 80(7) (2016) 541–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].McMurray KMJ, Strawn JR, Sah R, Fluoxetine Modulates Spontaneous and Conditioned Behaviors to Carbon Dioxide (CO2) Inhalation and Alters Forebrain-Midbrain Neuronal Activation, Neuroscience 396 (2019) 108–118. [DOI] [PubMed] [Google Scholar]
- [17].Anglada-Figueroa D, Quirk GJ, Lesions of the basal amygdala block expression of conditioned fear but not extinction, J Neurosci 25(42) (2005) 9680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Goosens KA, Maren S, Contextual and auditory fear conditioning are mediated by the lateral, basal, and central amygdaloid nuclei in rats, Learn Mem 8(3) (2001) 148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Phelps EA, LeDoux JE, Contributions of the amygdala to emotion processing: from animal models to human behavior, Neuron 48(2) (2005) 175–87. [DOI] [PubMed] [Google Scholar]
- [20].Feinstein JS, Adolphs R, Damasio A, Tranel D, The human amygdala and the induction and experience of fear, Current biology : CB 21(1) (2011) 34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR, Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans, Science 269(5227) (1995) 1115–1118. [DOI] [PubMed] [Google Scholar]
- [22].Becker B, Mihov Y, Scheele D, Kendrick K, Feinstein J, Matusch A, Aydin M, Reich H, Urbach H, Oros-Peusquens A, Shah N, Kunz W, Schlaepfer T, Zilles K, Maier W, Hurlemann R, Fear processing and social networking in the absence of a functional amygdala., Biological psychiatry 72(1) (2012) 70–7. [DOI] [PubMed] [Google Scholar]
- [23].Adolphs R, Neural systems for recognizing emotion, Current opinion in neurobiology 12(2) (2002) 169–77. [DOI] [PubMed] [Google Scholar]
- [24].Feinstein JS, Buzza C, Hurlemann R, Follmer RL, Dahdaleh NS, Coryell WH, Welsh MJ, Tranel D, Wemmie JA, Fear and panic in humans with bilateral amygdala damage, Nat Neurosci 16(3) (2013) 270–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Price MP, Gong H, Parsons MG, Kundert JR, Reznikov LR, Bernardinelli L, Chaloner K, Buchanan GF, Wemmie JA, Richerson GB, Cassell MD, Welsh MJ, Localization and behaviors in null mice suggest that ASIC1 and ASIC2 modulate responses to aversive stimuli, Genes Brain Behav 13(2) (2014) 179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Coryell M, Ziemann AE, Westmoreland PJ, Haenfler JM, Kurjakovic Z, Zha XM, Price M, Schnizler MK, Wemmie J, Targeting ASIC1a reduces innate fear and alters neuronal activity in the fear circuit, Biol. Psychiatry 62(10) (2007) 1140–1148. [DOI] [PubMed] [Google Scholar]
- [27].Taugher RJ, Ghobbeh A, Sowers LP, Fan R, Wemmie JA, ASIC1A in the bed nucleus of the stria terminalis mediates TMT-evoked freezing, Front Neurosci 9 (2015) 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Taugher RJ, Lu Y, Fan R, Ghobbeh A, Kreple CJ, Faraci FM, Wemmie JA, ASIC1A in neurons is critical for fear-related behaviors, Genes Brain Behav 16(8) (2017) 745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kim JJ, Jung MW, Neural circuits and mechanisms involved in Pavlovian fear conditioning: a critical review, Neurosci Biobehav Rev 30(2) (2006) 188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Johansen JP, Cain CK, Ostroff LE, LeDoux JE, Molecular mechanisms of fear learning and memory, Cell 147(3) (2011) 509–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Phillips RG, LeDoux JE, Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning, Behav Neurosci 106(2) (1992) 274–85. [DOI] [PubMed] [Google Scholar]
- [32].Wallace KJ, Rosen JB, Neurotoxic lesions of the lateral nucleus of the amygdala decrease conditioned fear but not unconditioned fear of a predator odor: comparison with electrolytic lesions, J Neurosci 21(10) (2001) 3619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fendt M, Endres T, Apfelbach R, Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces, J Neurosci 23(1) (2003) 23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rossi-Arnaud C, Ammassari-Teule M, Modifications of open field and novelty behaviours by hippocampal and amygdaloid lesions in two inbred strains of mice: Lack of strain x lesion interactions, Behavioural processes 27(3) (1992) 155–64. [DOI] [PubMed] [Google Scholar]
- [35].Young RF, Brain and spinal stimulation: how and to whom!, Clin Neurosurg 35 (1989) 429–47. [PubMed] [Google Scholar]
- [36].Nashold BS Jr., Wilson WP, Slaughter DG, Sensations evoked by stimulation in the midbrain of man, J Neurosurg 30(1) (1969) 14–24. [DOI] [PubMed] [Google Scholar]
- [37].Vargas LC, Schenberg LC, Long-term effects of clomipramine and fluoxetine on dorsal periaqueductal grey-evoked innate defensive behaviours of the rat, Psychopharmacology (Berl) 155(3) (2001) 260–8. [DOI] [PubMed] [Google Scholar]
- [38].Vianna DM, Graeff FG, Brandao ML, Landeira-Fernandez J, Defensive freezing evoked by electrical stimulation of the periaqueductal gray: comparison between dorsolateral and ventrolateral regions, Neuroreport 12(18) (2001) 4109–12. [DOI] [PubMed] [Google Scholar]
- [39].Bittencourt AS, Carobrez AP, Zamprogno LP, Tufik S, Schenberg LC, Organization of single components of defensive behaviors within distinct columns of periaqueductal gray matter of the rat: role of N-methyl-D-aspartic acid glutamate receptors, Neuroscience 125(1) (2004) 71–89. [DOI] [PubMed] [Google Scholar]
- [40].Evans DA, Stempel AV, Vale R, Ruehle S, Lefler Y, Branco T, A synaptic threshold mechanism for computing escape decisions, Nature 558(7711) (2018) 590–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Deng H, Xiao X, Wang Z, Periaqueductal Gray Neuronal Activities Underlie Different Aspects of Defensive Behaviors, J Neurosci 36(29) (2016) 7580–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Leibold NK, van den Hove DL, Viechtbauer W, Buchanan GF, Goossens L, Lange I, Knuts I, Lesch KP, Steinbusch HW, Schruers KR, CO2 exposure as translational cross-species experimental model for panic, Translational psychiatry 6(9) (2016) e885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kumar P, Prabhakar NR, Peripheral chemoreceptors: function and plasticity of the carotid body, Comprehensive Physiology 2(1) (2012) 141–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wiest G, Lehner-Baumgartner E, Baumgartner C, Panic attacks in an individual with bilateral selective lesions of the amygdala, Arch Neurol 63(12) (2006) 1798–801. [DOI] [PubMed] [Google Scholar]
- [45].Khalsa SS, Feinstein JS, Li W, Feusner JD, Adolphs R, Hurlemann R, Panic Anxiety in Humans with Bilateral Amygdala Lesions: Pharmacological Induction via Cardiorespiratory Interoceptive Pathways, J Neurosci 36(12) (2016) 3559–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Maren S, Neurobiology of Pavlovian fear conditioning, Annu Rev Neurosci 24 (2001) 897–931. [DOI] [PubMed] [Google Scholar]
- [47].Dlouhy BJ, Gehlbach BK, Kreple CJ, Kawasaki H, Oya H, Buzza C, Granner MA, Welsh MJ, Howard MA, Wemmie JA, Richerson GB, Breathing Inhibited When Seizures Spread to the Amygdala and upon Amygdala Stimulation, J Neurosci 35(28) (2015) 10281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Nobis WP, Schuele S, Templer JW, Zhou G, Lane G, Rosenow JM, Zelano C, Amygdala-stimulation-induced apnea is attention and nasal-breathing dependent, Ann Neurol 83(3) (2018) 460–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lacuey N, Zonjy B, Londono L, Lhatoo SD, Amygdala and hippocampus are symptomatogenic zones for central apneic seizures, Neurology 88(7) (2017) 701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Li Y, Zeng J, Zhang J, Yue C, Zhong W, Liu Z, Feng Q, Luo M, Hypothalamic Circuits for Predation and Evasion, Neuron 97(4) (2018) 911–924.e5. [DOI] [PubMed] [Google Scholar]
- [51].McGuire J, Herman JP, Ghosal S, Eaton K, Sallee FR, Sah R, Acid-sensing by the T cell death-associated gene 8 (TDAG8) receptor cloned from rat brain, Biochemical and Biophysical Research Communications, 2009, pp. 420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kumar NN, Velic A, Soliz J, Shi Y, Li K, Wang S, Weaver JL, Sen J, Abbott SB, Lazarenko RM, Ludwig MG, Perez-Reyes E, Mohebbi N, Bettoni C, Gassmann M, Suply T, Seuwen K, Guyenet PG, Wagner CA, Bayliss DA, PHYSIOLOGY. Regulation of breathing by CO(2) requires the proton-activated receptor GPR4 in retrotrapezoid nucleus neurons, Science 348(6240) (2015) 1255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ahmed-Leitao F, Spies G, van den Heuvel L, Seedat S, Hippocampal and amygdala volumes in adults with posttraumatic stress disorder secondary to childhood abuse or maltreatment: A systematic review, Psychiatry research. Neuroimaging 256 (2016) 33–43. [DOI] [PubMed] [Google Scholar]
- [54].Fujiwara A, Yoshida T, Otsuka T, Hayano F, Asami T, Narita H, Nakamura M, Inoue T, Hirayasu Y, Midbrain volume increase in patients with panic disorder, Psychiatry Clin Neurosci 65(4) (2011) 365–73. [DOI] [PubMed] [Google Scholar]
- [55].Sakai Y, Kumano H, Nishikawa M, Sakano Y, Kaiya H, Imabayashi E, Ohnishi T, Matsuda H, Yasuda A, Sato A, Diksic M, Kuboki T, Cerebral glucose metabolism associated with a fear network in panic disorder, Neuroreport 16(9) (2005) 927–31. [DOI] [PubMed] [Google Scholar]
- [56].Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL, A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder, Arch Gen Psychiatry 62(3) (2005) 273–81. [DOI] [PubMed] [Google Scholar]
- [57].Harricharan S, Rabellino D, Frewen PA, Densmore M, Theberge J, McKinnon MC, Schore AN, Lanius RA, fMRI functional connectivity of the periaqueductal gray in PTSD and its dissociative subtype, Brain and behavior 6(12) (2016) e00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Paxinos G, Franklin KBJ, The Mouse Brain in Steriotaxic Coordinates, Second ed., Academic Press, San Diego, 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.