Abstract
Recombinant tissue plasminogen activator (rt-PA) can be utilized to treat ischemic stroke with safety and effectiveness but limited by a narrow therapeutic window. In the present clinical trial among patients with stroke, we sought to evaluate the potential of fisetin to extend the therapeutic window of rt-PA treatment. Patients with stroke were divided based on their onset-to-treatment time (OTT) and then randomly assigned to receive the rt-PA treatment combined with fisetin or placebo. Primary outcome was evaluated using the National Institutes of Health Stroke scale (NIHSS), and secondary outcome was assessed by serum levels of matrix metalloproteinase (MMP) 2, MMP 9, and C-reactive protein (CRP). Fisetin dramatically improved the treatment outcomes of the patients with stroke in the delayed OTT strata, as revealed by lower NIHSS scores. The beneficial effect of fisetin was likely attributable to reduced levels of MMP-2, MMP-9, and CRP in the serum, as evidenced by strong linear correlations between serum levels of such markers with the NIHSS scores in all enrolled patients. Fisetin may possess the potential to supplement traditional rt-PA treatments among patients with stroke, particularly for those with delayed OTT, and thereby extend the otherwise narrow therapeutic window and improve the treatment outcomes.
Keywords: ischemic stroke, recombinant tissue plasminogen activator (rt-PA), fisetin, C-reactive protein (CRP)
Introduction
Brain ischemic stroke, with an increasing incidence rate, is among the leading causes of motility and morbidity globally.1 Brain damages after ischemic stroke often result in severe short- and/or long-term sensorimotor and neurological dysfunctions, along with pathological injuries such as disruption of the blood–brain barrier (BBB) and cerebral edema. Currently, treatment within 3 hours after the symptoms onset using the recombinant tissue plasminogen activator (rt-PA), a serine protease for the degradation of fibrin clots,2 is widely applied clinically as an effective therapy for acute brain ischemia. Although rt-PA is indeed effective when administered in a timely fashion, delayed treatments beyond the initial 3 hours reportedly yielded less satisfactory outcomes and were more likely to cause adverse responses including intracerebral hemorrhage and hyperperfusion, thereby hindering a broader clinical application of the rt-PA treatment.3,4
During the past several decades, natural compounds derived from fruits, vegetables, or plants have attracted great interest for their therapeutic potential against various diseases. Fisetin is a bioactive flavonoid enriched in fruits and vegetables such as apple, strawberry, persimmon, cucumber, and onion,5,6 and has been recently recognized for its beneficial effect on health and well-being. Fisetin is reported to exert neuroprotective actions.7 For instance, using cultured PC12 cells as a model of permanent focal ischemia, fisetin significantly increased cell survival after hydrogen peroxide challenge.8 In addition, in a mouse stroke models, fisetin attenuated postischemic immune cell infiltration, activation and infarct size,9 and reduced the behavioral deficits following a stroke.10
To further assess the clinical efficacy of fisetin in expanding the therapeutic window of rt-PA treatment against acute brain ischemic stroke, we hereby designed the present clinical trial and treated patients with stroke who were receiving delayed rt-PA with fisetin. The treatment outcomes were examined using clinical scores as well as biochemical parameters and compared to those of the patients with stroke receiving rt-PA combined with placebo.
Methods
Ethical Statements
The current intent-to-treat clinical trial was performed during January 2015 and December 2018 in compliance with the guidelines laid down by the Declaration of Helsinki and received the approval of the Ethics Committee of Cangzhou Central Hospital. All patients (or their family) provided signed written informed consent before enrollment into this study.
Patient Selection
A total of 215 patients admitted into Cangzhou Central Hospital for acute ischemic stroke were initially recruited for this study. Inclusion criteria include (1) clearly defined onset time, (2) deficit(s) measurable on the National Institutes of Health Stroke scale (NIHSS),11 (3) computed tomographic scan of the brain showing no signs of intracranial hemorrhage at the time of the recruitment.
Exclusion criteria include (1) platelet counts below 100 000 per mm3 or glucose levels above 22.2 mmol/L or below 2.7 mmol/L; (2) diastolic blood pressure above 110 mm Hg or systolic blood pressure above 185 mm Hg; (3) seizure at the stroke onset; (4) international normalized ratio above 1.6; (5) minor or rapidly improving symptoms; (6) signs of intracranial, subarachnoid, gastrointestinal, or urinary tract hemorrhage within 21 days; (7) arterial puncture at a noncompressible site within 7 days; (8) received heparin or anticoagulants within 2 days prior to the onset of the stroke; (9) major head surgery or intracranial neurosurgical procedure within 3 months; (10) consumed any supplements containing fisetin within 2 days prior to the stroke; and (11) received aggressive treatments in order to reduce the blood pressure to the aforementioned limits.
Onset-to-Treatment Time Determination
The onset time of the stroke was first determined by interviewing patients and/or family members present when the symptoms were initially noticed and was later validated by corroborating evidence such as ambulance reports. The possibility of onset during sleep was also carefully examined. If the patient woke up with stroke symptoms, the onset time of the stroke was established as the last time when the individual was known to be awake and/or without any stroke symptoms. The patient would not be included if the stroke onset time could not be determined with certainty. The onset-to-treatment time (OTT) was then calculated as the interval between the stroke onset and the treatment initiation. The OTT between 0 and 3 hours is categorized as normal OTT, and OTT of 3 to 5 hours is categorized as delayed OTT.
Randomization and Treatment
A total of 192 eligible patients eventually participated in the current study and were randomly assigned to different groups using a permuted-block randomization with varying block sizes and stratified according to their OTT. Patients were then treated with rt-PA (“Actilyse”; Boehringer Ingelheim, Germany) in the dose of 0.9 mg/kg body weight, which was administered intravenously with the initial 10% as a bolus and the remaining 90% as a constant infusion over 1 hour; 100 mg of Wax Tree derived fisetin (“Novusetin”, Bioriginal, Anaheim, California) or cellulose as the placebo was mixed with rt-PA and delivered at the same time. After the initial administration, patients received continuous treatment of either 100 mg placebo or 100 mg fisetin daily for a period of 7 days. A daily dose of 100 mg fisetin was chosen based on the Food and Drug Administration model of conversion from an in vivo dose to the safe human equivalent dosage, for this trial was the first intervention study in patients with stroke.12 In the initial treatment as well as the following daily treatments, fisetin and placebo were packed to mask their contents to both the investigator and the patients.
Definition of Treatment Outcome
The NIHSS score was employed to evaluate the neurological deficits of the patients.11 National Institutes of Health Stroke scale, a 0- to 42-point scale, quantifies neurologic deficits in 11 categories, with zero indicating normal function with no sign of neurologic deficit. Scores of 0 to 10 were regarded as favorable outcomes. All evaluations were conducted by an investigator blind to the group assignment.
Enzyme-Linked Immunosorbent Assay
Samples of patients’ blood were harvested before the start of rt-PA treatment as the baseline, and at 1 day and 7 days posttreatment as end point. Samples were immediately centrifuged to isolate the serum, which was stored at −80°C within 1 hour after collection to avoid the degradation. Serum levels of matrix metalloproteinase (MMP) 2, MMP-9, C-reactive protein (CRP), cystatin C, and neuropeptide Y were assessed using Human ELISA kits (Sigma-Aldrich, St. Louis, MO) in accordance to the provided protocols.
Statistical Analysis
Data were expressed as mean (standard deviation). The normality of data distribution was determined using the Kolmogorov-Smirnov goodness-of-fit test. Then 2-tailed Student t test was utilized to examine normally distributed data, and the Mann-Whitney test was utilized to examine non-normally distributed data; P values less than .05 were regarded as indicative of statistical significance. Sample size of treatment groups was determined using Cohen d method.13 Briefly, the mean values of MMP-2, MMP-9, and CRP from all groups were divided by their respective standard deviation to give a series of standardized effect size numbers. The largest number in all calculations was then used for reference to Cohen’s d power table, compared to which our group size was still able to yield sufficient statistical significance and power.
Results
Patient Characteristics
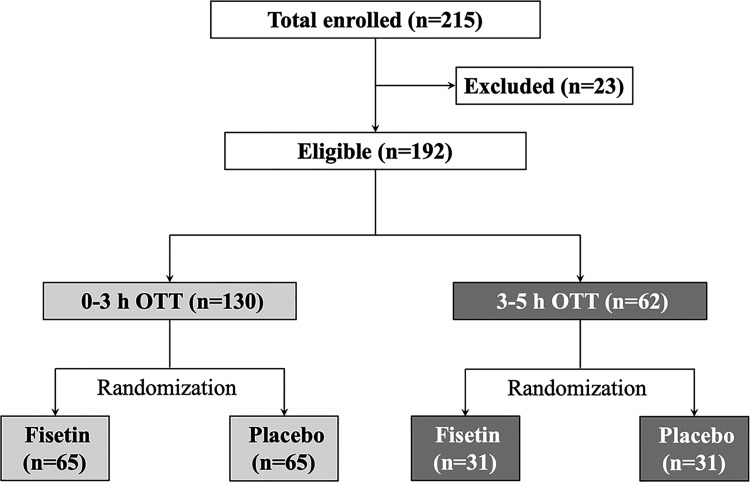
During January 2015 and December 2018, a total of 215 patients were initially enrolled into this trial, among which 23 patients were excluded based on the inclusion and exclusion criteria. The remaining 192 eligible patients, with 130 patients in the 0- to 3-hour OTT strata and 62 in the 3- to 5-hour OTT strata, were assigned into 2 treatment groups in a random manner (Figure 1). Baseline characteristics and medical histories of all patients were listed in Tables 1 and 2. The baseline characteristics as well as preexisting medical conditions prior to the stroke onset were comparable among the 4 OTT strata and treatment groups. Importantly, as listed in Table 2, we did not observe any significant differences in the baseline NIHSS scores or the stoke deficits among 4 OTT strata and treatment groups.
Figure 1.
Flow chart of study design.
Table 1.
Medical History of All Eligible Patients in the Study.
| Medical History | 0- to 3-hour OTT (n = 130) | 3- to 5-hour OTT (n = 62) | ||
|---|---|---|---|---|
| Fisetin (n = 65) | Placebo (n = 65) | Fisetin (n = 31) | Placebo (n = 31) | |
| Hypertension | 46 | 47 | 17 | 19 |
| Transient ischemic attack | 18 | 17 | 11 | 9 |
| Stroke | 11 | 12 | 10 | 8 |
| Myocardial infarction | 18 | 17 | 9 | 10 |
| Atrial fibrillation | 19 | 21 | 8 | 10 |
| Angina pectoris | 12 | 14 | 7 | 9 |
| Preexisting disability | 7 | 5 | 3 | 4 |
| Valvular heart disease | 8 | 6 | 4 | 4 |
| Congestive heart failure | 9 | 11 | 5 | 6 |
| Smoking in previous year | 29 | 26 | 15 | 13 |
| Drinking in previous year | 31 | 28 | 13 | 14 |
Abbreviation: OTT, onset-to-treatment time.
Table 2.
Baseline Characteristics and Deficits of All Eligible Patients in the Study.a
| Baseline Characteristics | 0- to 3-hour OTT (n = 130) | 3- to 5-hour OTT (n = 62) | ||
|---|---|---|---|---|
| Fisetin (n = 65) | Placebo (n = 65) | Fisetin (n = 31) | Placebo (n = 31) | |
| Age, year | 61.9 ± 6.2 | 62.4 ± 7.3 | 62.6 ± 6.1 | 63.1 ± 7.6 |
| Gender (male/female) | 32/33 | 34/31 | 16/15 | 14/17 |
| Weight, kg | 65.7 ± 8.2 | 66.3 ± 7.5 | 67.1 ± 8.4 | 65.4 ± 7.9 |
| Blood pressure, mm Hg | ||||
| Systolic | 151 ± 19 | 153 ± 21 | 152 ± 24 | 156 ± 23 |
| Diastolic | 83 ± 11 | 81 ± 12 | 80 ± 13 | 82 ± 11 |
| Deficits | ||||
| Level of consciousness | 15 | 16 | 15 | 14 |
| Best visual | 29 | 31 | 30 | 28 |
| Best gaze | 28 | 29 | 31 | 29 |
| Sensory | 41 | 38 | 40 | 42 |
| Best language | 39 | 42 | 40 | 37 |
| Extinction and inattention | 28 | 26 | 30 | 29 |
| Ataxia | 4 | 5 | 6 | 4 |
| Dysarthria | 46 | 51 | 49 | 52 |
| Average motor | 39 | 40 | 37 | 42 |
| Median NIHSS (range) | 14 (1-34) | 13 (2-32) | 13 (2-33) | 15 (2-35) |
Abbreviations: NIHSS, National Institutes of Health Stroke scale; OTT, onset-to-treatment time.
a All intergroup differences were statistically insignificant (P > .05).
National Institutes of Health Stroke Scale Score of All Eligible Patients 1 Day After Initial Treatment
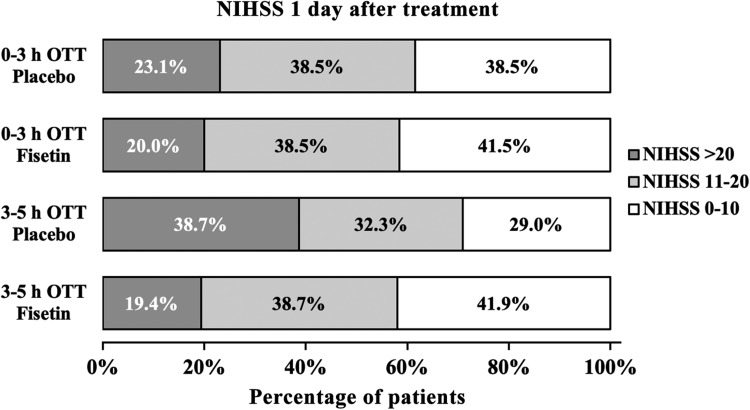
Treatment outcome was assessed using the NIHSS scores as determined by an investigator blind to the group assignment. One day after the initiation of rt-PA treatment, in the 0- to 3-hour OTT strata, distribution of NIHSS scores of patients receiving simultaneous fisetin treatment was statistically indistinguishable from that of patients receiving placebo (Figure 2; 0- to 3-hour OTT/fisetin vs 0- to 3-hour OTT/placebo). In the 3- to 5-hour OTT strata, treatment outcome of patients given placebo was noticeably less satisfactory (Figure 2; 3- to 5-hour OTT/placebo), in comparison with patients from the 0- to 3-hour OTT strata regardless of treatment (Figure 2; 0- to 3-hour OTT/fisetin and 0- to 3-hour OTT/placebo). This finding was in agreement with prior reports on the therapeutic window of rt-PA treatment as ideally within the first 3 hours of stroke onset. However, within this 3- to 5-hour OTT strata, patients receiving fisetin exhibited great improvement as compared to those given placebo (Figure 1; 3- to 5-hour OTT/fisetin vs 3- to 5-hour OTT/placebo).
Figure 2.
NIHSS score of all eligible patients 1 day after initial treatment. Scores of 0 to 10 was considered to indicate a favorable outcome. NIHSS indicates National Institutes of Health Stroke scale; OTT, onset-to-treatment time.
National Institutes of Health Stroke Scale Score of All Eligible Patients 7 Days After Initial Treatment
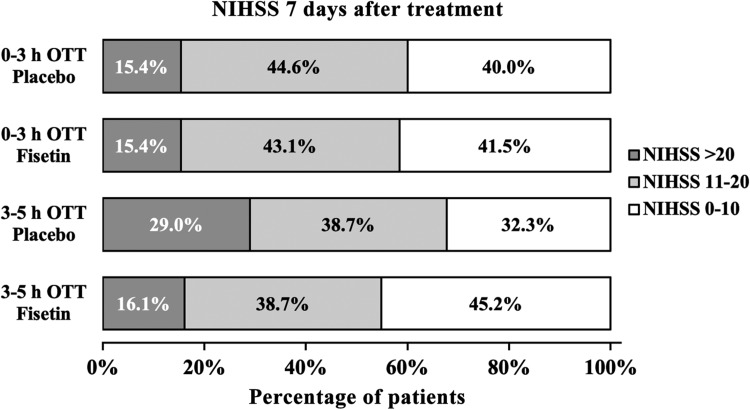
Following the rt-PA treatment on the first day, patients who were initially administered simultaneous fisetin continuously received daily fisetin, while patients initially receiving placebo were continuously given daily placebo, both for 7 days. The NIHSS scores of all patients at the end of 7-day treatment are presented in Figure 3. The distribution of NIHSS scores in different OTT strata and treatment groups exhibited identical trend as on day 1 following the initial rt-PA treatment (Figure 3).
Figure 3.
NIHSS score of all eligible patients 7 days after initial treatment. Scores of 0 to 10 was considered to indicate a favorable outcome. NIHSS indicates National Institutes of Health Stroke scale; OTT, onset-to-treatment time.
Serum Levels of MMP-2, MMP-9, and CRP
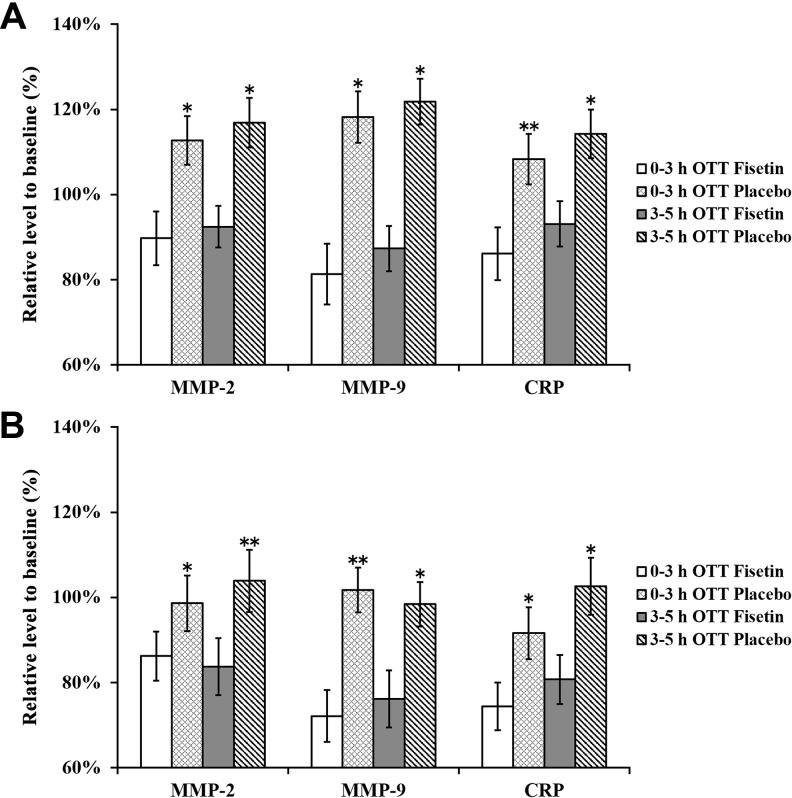
Next, we evaluated the serum levels of MMP-2, MMP-9, and CRP in patients from all groups at baseline, 1 day, and 7 days following the initial rt-PA treatment (Figure 4). We found that 1 day after the initial treatment, serum levels of both MMPs and CRP in both 0- to 3-hour and 3- to 5-hour OTT strata were noticeably reduced in fisetin-treated patients than those treated with placebo (Figure 4A). After the 7-day continuous treatment, serum levels of all 3 markers were significantly lower than those at 1 day after treatment in all patients (Figure 4B). Nevertheless, in both OTT strata, fisetin treatment still markedly reduced the serum levels of MMP-2, MMP-9, and CRP.
Figure 4.
Serum levels of MMP-2, MMP-9, and CRP at (A) 1 day and (B) 7 days after initial treatment. Values were normalized as percentage to baseline values in respective treatment groups and expressed as mean (SD). ∗P < .05 fisetin versus placebo treatment at respective OTT groups. CRP indicates C-reactive protein; MMP, matrix metalloproteinase; NIHSS, National Institutes of Health Stroke scale; OTT, onset-to-treatment time; SD, standard deviation.
Strong Linear Correlations Between NIHSS Scores and Plasma Levels of MMP-2, MMP-9, and CRP
Last but not least, we were curious about the correlations between the NIHSS score improvements and the reduction in MMP and CRP levels in the enrolled patients with stroke. To this end, we combined clinical and laboratory data for all 192 eligible patients throughout the entire study and performed correlation analysis (Table 3). Indeed, strong linear correlations between changes in the NIHSS scores and the serum levels of all 3 factors were observed, clearly indicating that the observed improvements in the NIHSS score were likely attributable to the reduced serum levels of MMP-2, MMP-9, and CRP.
Table 3.
Strong Linear Correlations Between NIHSS Scores and Plasma Levels of MMP-2, MMP-9, and CRP in All Patients.
| NIHSS | P Value | |
|---|---|---|
| MMP-2 | γ = 0.67 | .033 |
| MMP-9 | γ = 0.71 | .028 |
| CRP | γ = 0.65 | .041 |
Abbreviations: CRP, C-reactive protein; MMP, matrix metalloproteinase; NIHSS, National Institutes of Health Stroke scale.
Discussion
Despite the fact that rt-PA is effective in treating brain ischemic stroke, cautious considerations are needed during the clinical usage of rt-PA, for instance its relatively narrow OTT window of 3 hours.2 There have been reports on serious side effects of delayed rt-PA, including hyperperfusion leading to degradation of extracellular matrix and disruption of the BBB,14–17 as well as hemorrhagic transformation leading to elevated incidence rate of symptomatic intracerebral hemorrhage.18 Although in some parts of Europe, expanded treatment window of 4.5 hours is regarded safe and officially approved, percentage of patients exhibiting improvement when treated within 3 hours is twice as many as that when treated within the 3 to 4.5 hour extended window.4 These aforementioned adverse effects have greatly limited the clinical efficacy and safety of rt-PA treatment. Therefore, alternative treatment such as stent retriever thrombectomy was occasionally employed to replace rt-PA treatment, on patients with prolonged OTT in particular. Stent retriever thrombectomy could ameliorate poststroke disability and improve the rate of functional independence, even when applied with OTT as long as 8 hours.19 Nonetheless, as an invasive procedure, thrombectomy raises other complications and risks including anesthetic adverse effects or clot reforming that would necessitate subsequent procedures and is therefore poorly tolerated in older patients or individuals with chronic health problems such as bleeding or infection.
Our findings in the present clinical study were promising. In patients in the 3- to 5-hour OTT strata, overall NIHSS scores were significantly improved compared to the placebo-treated controls in just 1 day after the initial rt-PA and fisetin treatment. More importantly, this treatment outcome was nearly indistinguishable from that of patients with 0- to 3-hour OTT, indicating that supplement of fisetin in rt-PA reperfusion could significantly broaden the effective OTT to at least 5 hours. In addition to the initial rt-PA/fisetin treatment, patients also received daily fisetin treatment for 7 consecutive days. Treatment outcome of all patients exhibited gradual improvement, as they recover from the acute stroke attack. However, detailed analysis of their NIHSS scores revealed the prolonged therapeutic effects of fisetin. We observed that fisetin treatment for 7 days could further promote the recovery of patients of the 3- to 5-hour OTT strata, to levels similar to the patients of the 0- to 3-hour OTT strata. This finding indicates that besides the role as a supplement in the initial rt-PA treatment, fisetin could also benefit the functional recovery after stroke, in agreement with the reported neuroprotective activities of fisetin in several prior studies.9,10
Another interesting finding in our current study is the reduced serum levels of MMP-2 and MMP-9 in patients receiving fisetin treatments. These results are also in line with previous reports, where fisetin suppressed the expression of both MMPs in human cancer cells20,21 and experimental mice.22 The intriguing observation is the relation between levels of MMPs with treatment outcome of rt-PA, for we found strong linear correlation between the reduction in serum levels of MMPs and improved NIHSS scores indicating more ideal treatment outcomes. This correlation strongly supported that the positive effect on stroke recovery was likely mediated by MMPs, which was inhibited by fisetin in both the initial rt-PA and the 7-day follow-up administrations. Consistent with our findings, both MMP-2 and MMP-9 were implicated in ischemic attacks, via degradation of collagen IV and laminin in the basement membrane to cause consequent disruption of the BBB23,24 and cerebral hemorrhage.25 In clinical usage of rt-PA, patients with upregulated MMP-9 levels face higher risk of parenchymal hemorrhage.26 Additionally, symptoms as a result of the ischemic attack could be attenuated by excessive t-PA, which increases MMP-9.27 In the present study, we found that treatment with fisetin greatly downregulated serum levels of both MMP-2 and MMP-9, which showed strong correlation with, and likely contributed to, the improved treatment outcomes in all patients regardless of their OTT strata.
On the other hand, we also observed significantly reduced serum level of CRP following fisetin treatment. Increase in the serum CRP level is reportedly associated with the development of ischemic attack and hemorrhagic stroke, as well as disease outcome.28,29 However, not all studies confirmed that elevated routine serum CRP affects the outcome in patients receiving intravenous rt-PA for acute stroke.30 Importantly, CRP is one of the most widely used predictive biomarker of stroke outcome in clinical practice.31–33 Studies reporting that fisetin could reduce CRP have been previously documented. For example, in diabetic mouse model, fisetin was able to lower methylglyoxal-dependent protein glycation and inhibit expression of serum CRP.34 Moreover, in patients with colorectal cancer, fisetin supplementation could significantly reduce plasma levels of interleukin 8 and CRP.35 Our current study serves yet another instance, first of its kind among patients with stroke, demonstrating the activity of fisetin in antagonizing serum CRP levels. One limitation of the study is that possible coexistence of infection or inhospital infectious complications was not analyzed, which could potentially affect serum CRP level and should be considered in similar future studies.
In conclusion, the present randomized double-blind and placebo-controlled trial has provided the first clinical evidence on the role of fisetin as a supplement in rt-PA reperfusion therapy against acute brain ischemic stroke. Fisetin, when administered simultaneously with rt-PA, extended the otherwise narrow effective window of rt-PA treatment and dramatically improved the treatment outcome of this widely used stroke therapy.
Footnotes
Authors’ Note: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Limin Wang  https://orcid.org/0000-0001-5239-9170
https://orcid.org/0000-0001-5239-9170
References
- 1. Feigin VL, Forouzanfar MH, Krishnamurthi R. et al. Global and regional burden of stroke during 1990-2010: findings from the global burden of disease study 2010. Lancet. 2014;383(9913):245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eissa A, Krass I, Bajorek BV. Optimizing the management of acute ischaemic stroke: a review of the utilization of intravenous recombinant tissue plasminogen activator (tPA). J Clin Pharm Ther. 2012;37(6):620–629. [DOI] [PubMed] [Google Scholar]
- 3. Saver JL. Improving reperfusion therapy for acute ischaemic stroke. J Thromb Haemost. 2011;9(suppl 1):333–343. [DOI] [PubMed] [Google Scholar]
- 4. Cheng NT, Kim AS. Intravenous thrombolysis for acute ischemic stroke within 3 hours versus between 3 and 4.5 hours of symptom onset. Neurohospitalist. 2015;5(3):101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khan N, Syed DN, Ahmad N, Mukhtar H. Fisetin: a dietary antioxidant for health promotion. Antioxid Redox Signal. 2013;19(2):151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R, Kinae N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr. 2000;130(9):2243–2250. [DOI] [PubMed] [Google Scholar]
- 7. Maher P. How fisetin reduces the impact of age and disease on CNS function. Front Biosci (Schol Ed). 2015;7(1):58–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dajas F, Rivera F, Blasina F. et al. Cell culture protection and in vivo neuroprotective capacity of flavonoids. Neurotox Res. 2003;5(6):425–432. [DOI] [PubMed] [Google Scholar]
- 9. Gelderblom M, Leypoldt F, Lewerenz J. et al. The flavonoid fisetin attenuates postischemic immune cell infiltration, activation and infarct size after transient cerebral middle artery occlusion in mice. J Cereb Blood Flow Metab. 2012;32(5):835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maher P, Salgado KF, Zivin JA, Lapchak PA. A novel approach to screening for new neuroprotective compounds for the treatment of stroke. Brain Res. 2007;1173:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lyden P, Brott T, Tilley B. et al. Improved reliability of the NIH Stroke Scale using video training. NINDS TPA Stroke Study Group. Stroke. 1994;25(11):2220–2226. [DOI] [PubMed] [Google Scholar]
- 12. Hansen AR, Cook N, Ricci MS. et al. Choice of starting dose for biopharmaceuticals in First-in-Human Phase I Cancer Clinical trials. Oncologist. 2015;20(6):653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed Hillsdale, NJ: L Erlbaum Associates; 1988. [Google Scholar]
- 14. Rosenberg GA, Yang Y. Vasogenic edema due to tight junction disruption by matrix metalloproteinases in cerebral ischemia. Neurosurg Focus. 2007;22(5):E4. [DOI] [PubMed] [Google Scholar]
- 15. Adibhatla RM, Hatcher JF. Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies. CNS Neurol Disord Drug Targets. 2008;7(3):243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamashita T, Kamiya T, Deguchi K. et al. Dissociation and protection of the neurovascular unit after thrombolysis and reperfusion in ischemic rat brain. J Cereb Blood Flow Metab. 2009;29(4):715–725. [DOI] [PubMed] [Google Scholar]
- 17. Zhang JB, Ding ZY, Yang Y. et al. Thrombolysis with alteplase for acute ischemic stroke patients with atrial fibrillation. Neurol Res. 2010;32(4):353–358. [DOI] [PubMed] [Google Scholar]
- 18. Wang X, Tsuji K, Lee SR. et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke. 2004;35(11 suppl 1):2726–2730. [DOI] [PubMed] [Google Scholar]
- 19. Jovin TG, Chamorro A, Cobo E. et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296–2306. [DOI] [PubMed] [Google Scholar]
- 20. Liao YC, Shih YW, Chao CH, Lee XY, Chiang TA. Involvement of the ERK signaling pathway in fisetin reduces invasion and migration in the human lung cancer cell line A549. J Agric Food Chem. 2009;57()19):8933–8941. [DOI] [PubMed] [Google Scholar]
- 21. Chien CS, Shen KH, Huang JS, Ko SC, Shih YW. Antimetastatic potential of fisetin involves inactivation of the PI3K/Akt and JNK signaling pathways with downregulation of MMP-2/9 expressions in prostate cancer PC-3 cells. Mol Cell Biochem. 2010;333(1-2):169–180. [DOI] [PubMed] [Google Scholar]
- 22. Pal HC, Diamond AC, Strickland LR. et al. Fisetin, a dietary flavonoid, augments the anti-invasive and anti-metastatic potential of sorafenib in melanoma. Oncotarget. 2016;7(2):1227–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gasche Y, Fujimura M, Morita-Fujimura Y. et al. Early appearance of activated matrix metalloproteinase-9 after focal cerebral ischemia in mice: a possible role in blood-brain barrier dysfunction. J Cereb Blood Flow Metab. 1999;19(9):1020–1028. [DOI] [PubMed] [Google Scholar]
- 24. Gidday JM, Gasche YG, Copin JC. et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol Heart Circ Physiol. 2005;289(2):H558–H568. [DOI] [PubMed] [Google Scholar]
- 25. Liu R, Liu Q, He S, Simpkins JW, Yangr SH. Combination therapy of 17beta-estradiol and recombinant tissue plasminogen activator for experimental ischemic stroke. J Pharmacol Exp Ther. 2010;332(3):1006–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Montaner J, Molina CA, Monasterio J. et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003;107(4):598–603. [DOI] [PubMed] [Google Scholar]
- 27. Morancho A, Rosell A, Garcia-Bonilla L, Montaner J. Metalloproteinase and stroke infarct size: role for anti-inflammatory treatment? Ann N Y Acad Sci. 2010;1207(1):123–133. [DOI] [PubMed] [Google Scholar]
- 28. Tsai NW, Lee LH, Huang CR. et al. The association of statin therapy and high-sensitivity C-reactive protein level for predicting clinical outcome in acute non-cardioembolic ischemic stroke. Clin Chim Acta. 2012;413(23-24):1861–1865. [DOI] [PubMed] [Google Scholar]
- 29. Roudbary SA, Saadat F, Forghanparast K, Sohrabnejad R. Serum C-reactive protein level as a biomarker for differentiation of ischemic from hemorrhagic stroke. Acta Med Iran. 2011;49(3):149–152. [PubMed] [Google Scholar]
- 30. Karlinski M, Bembenek J, Grabska K. et al. Routine serum C-reactive protein and stroke outcome after intravenous thrombolysis. Acta Neurol Scand. 2014;130(5):305–311. [DOI] [PubMed] [Google Scholar]
- 31. Yu H, Huang Y, Chen X. et al. High-sensitivity C-reactive protein in stroke patients—the importance in consideration of influence of multiple factors in the predictability for disease severity and death. J Clin Neurosci. 2017;36:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Everett BM, Kurth T, Buring JE, Ridker PM. The relative strength of C-reactive protein and lipid levels as determinants of ischemic stroke compared with coronary heart disease in women. J Am Coll Cardiol. 2006;48(11):2235–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Makita S, Nakamura M, Satoh K. et al. Serum C-reactive protein levels can be used to predict future ischemic stroke and mortality in Japanese men from the general population. Atherosclerosis. 2009;204(1):234–238. [DOI] [PubMed] [Google Scholar]
- 34. Maher P, Dargusch R, Ehren JL, Okada S, Sharma K, Schubert D. Fisetin lowers methylglyoxal dependent protein glycation and limits the complications of diabetes. PLoS One. 2011;6(6):e21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Farsad-Naeimi A, Alizadeh M, Esfahani A, Darvish Aminabad E. Effect of fisetin supplementation on inflammatory factors and matrix metalloproteinase enzymes in colorectal cancer patients. Food Func. 2018;9(4):2025–2031. [DOI] [PubMed] [Google Scholar]