Abstract
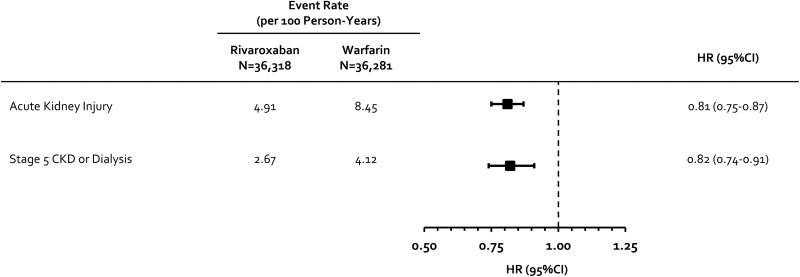
Warfarin has been associated with renovascular calcification and worsening renal function, whereas rivaroxaban may provide a degree of renopreservation by decreasing vascular inflammation. We sought to compare rivaroxaban and warfarin’s impact on renal decline in patients with nonvalvular atrial fibrillation (NVAF) treated in routine practice. Using US MarketScan claims data from January 2012 to December 2017, we identified patients with NVAF newly initiated on rivaroxaban or warfarin with ≥12 months of continuous insurance coverage prior to initiation. Patients with stage 5 chronic kidney disease (CKD) or receiving hemodialysis at baseline were excluded. Outcomes included rates (events/100 person-years) of hospital or emergency department admission for acute kidney injury (AKI) or progression to stage 5 CKD or need for hemodialysis. Differences in baseline covariates between cohorts were adjusted using inverse probability-of-treatment weights based on propensity scores (absolute standardized differences <0.1 achieved for all covariates after adjustment). Patients were followed until an event, anticoagulant discontinuation/switch, insurance disenrollment, or end of data availability. Hazard ratios with 95% confidence intervals (CIs) were estimated using Cox regression. We assessed 36 318 rivaroxaban (19.8% received a dose <20 mg/d) and 36 281 warfarin users. Stages 3 and 4 CKD were present in 5% and 1% of patients at baseline, and proteinuria was present in 2%. Rivaroxaban was associated with a 19% (95% CI = 13%-25%) reduction in the hazard of AKI (rates = 4.91 vs 8.45) and an 18% (95% CI = 9%-26%) reduction in progression to stage 5 CKD or hemodialysis (rates = 2.67 vs 4.12). Rivaroxaban appears associated with lower hazards of undesirable renal end points versus warfarin in patients with NVAF.
Keywords: rivaroxaban, warfarin, oral anticoagulation, kidney function decline, atrial fibrillation
Introduction
The prevalence of both nonvalvular atrial fibrillation (NVAF) and declining renal function increases with advancing age.1 Long-term oral anticoagulation is utilized in most patients with NVAF to prevent thromboembolic stroke, and consequently, oral anticoagulants are frequently used in patients with concomitant NVAF and declining (or poor) renal function.2–5
Clinical trial data suggest vitamin K antagonists (VKAs) and non-vitamin K oral anticoagulants (NOACs) may have differential effects on the rate of renal function decline in patients with NVAF.6–8 While warfarin has been associated with renovascular calcification and postulated to accelerate the worsening of renal function (a phenomenon often referred to as “warfarin-related nephropathy”),9 many NOACs are thought to provide a degree of renopreservation by decreasing protease-activated receptor (PAR)-mediated vascular inflammation.9
In the present study, we sought to compare rivaroxaban and warfarin’s impact on the development of acute kidney injury (AKI) and the composite end point of progression to stage 5 chronic kidney disease (CKD) or the need for hemodialysis in patients with NVAF managed in routine practice.
Materials and Methods
We performed a retrospective claims analysis using US Truven MarketScan data from January 2012 through December 2017. Truven MarketScan combines 2 separate databases, a commercial and a Medicare supplemental database, to cover all age-groups and contains claims from 260 contributing employers, 40 health plans, and government and public organizations representing ∼240 million lives.10 Truven MarketScan captures enrollment records, demographics, International Classification of Diseases, Ninth and Tenth Revision (ICD-9 and ICD-10) diagnosis codes, procedure codes, admission and discharge dates, outpatient medical services data, and prescription dispensing records. All Truven MarketScan data were deidentified and are thus in compliance with the Health Insurance Portability and Accountability Act of 1996 to preserve patient anonymity and confidentiality. Further, this study was determined by our institutional review board to not constitute research involving humans according to 45 US Code of Federal Regulations 46.102(f) and was deemed exempt from board oversight.
This analysis included adult patients who were oral anticoagulant naive during the 12 months before the day of the first qualifying rivaroxaban or warfarin dispensing (index date) and had ≥2 inpatient or outpatient ICD codes in any position for AF without codes suggesting valvular disease. Patients with stage 5 CKD or receiving hemodialysis during the baseline period were excluded as were patients with alternate indications for full-dose anticoagulation.
Differences in baseline characteristics between the rivaroxaban and warfarin cohorts were adjusted for using inverse probability of treatment weighting based on propensity scores.11 Propensity scores (and patient weights) were estimated using generalized boosted models based on 10 000 regression trees using the “TWANG” package (version 1.5) and R statistical software (version 3.4.3, The R Project for Statistical Computing) which implements an automated, nonparametric machine learning method.12 The weights were derived to obtain estimates of the population average treatment effect. Variables entered into the generalized boosted modeling procedure including demographics, comorbidities (including the Elixhauser comorbidity index),13 components of the CHA2DS2-VASc and modified HAS-BLED scores),14,15 and concurrent nonoral anticoagulant medications are provided in the Supplemental Appendix, eTables 1-3, and were identified during the 12-month baseline period. The presence of residual differences in measured covariates following cohort weighting was assessed by calculating absolute standardized differences (a difference <0.1 was considered well balanced for each variable).11
The primary end points of this study were the incidence (events per 100 person-years of follow-up) of hospital or emergency department admission for AKI (≥1 appropriate ICD diagnosis in the primary or non-primary position during a hospitalization or emergency department visit)16 and the composite of progression to stage 5 CKD or need for hemodialysis (≥1 appropriate inpatient or outpatient ICD diagnosis or procedure code in the primary or non-primary position). Secondary end points assessed in this study included traditional NVAF end points including the composite of stroke or systemic embolism (SSE) including ischemic stroke, intracranial hemorrhage, or SSE (each defined by an appropriate inpatient discharge diagnosis code in the primary position). Major bleeding rates were assessed using the validated Cunningham algorithm for detection of bleeding-related hospitalizations.17 Patients were followed until end point occurrence, index oral anticoagulation discontinuation or switch (assuming a 30 day permissible gap), insurance plan disenrollment, or end of claims data availability.
Baseline characteristics were analyzed using descriptive statistics. Categorical data were reported as percentages and continuous data as medians with accompanying 25%, 75% ranges. The incidence rate of each end point of interest was reported as events per 100 person-years. A Cox proportional hazards regression model was fit to compare event rates over time for the weighted rivaroxaban and warfarin cohorts. Since some patients were assigned very low or high weights, we recalibrated their weights by resetting those below or above the 1st and 99th percentile to the corresponding percentile threshold values.11 This recalibration was conducted prior to performing the weighted regression analyses. Cox proportional hazards regression was performed using the “SURVEY” package (version 3.34) in R, and since all baseline variables had an absolute standardized difference <0.1 after IPTW, the only independent variable entered into Cox regression was the indicator of index oral anticoagulation used. A level of significance of P < .05 was prespecified as being significant, unless otherwise noted. The proportional hazard assumption was tested based on Schoenfeld residuals and was found valid for all end points.
We performed subgroup analyses on the basis of age (<70 years or ≥70 years), use of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) at baseline, and those with a CHA2DS2-VASc score of 0-1 (low-risk), 2-3 (moderate-risk) and ≥4 (high-risk). The IPTW process was repeated for each of the subgroup analyses. The presence of a statistical interaction across subgroups was tested using the methods described by Altman and Bland.18 To reduce the chances of obtaining false-positive results (type I error) as a result of our multiple hypothesis testing/subgroup analyses, we utilized a Bonferroni-corrected P value of <.01 to suggest a statistically significant subgroup interaction for the AKI and composite of progression to stage 5 CKD or need for hemodialysis endpoints.19
The report for this analysis was written in order to comply with the Reporting of Studies Conducted using Observational Routinely-Collected Health Data (RECORD) statement.20
Results
In total, we identified 36 318 rivaroxaban (19.8% received a reduced dose) and 36 281 warfarin users eligible for study inclusion (Supplemental Materials, eFigure 1). Baseline covariates were well balanced after IPTW (absolute standardized differences <0.1 for all covariates; Table 1). Median (25%, 75% range) age was 69 (60, 79) years, CHA2DS2-VASc score was 3 (2, 4), duration on-index oral anticoagulant was 141 (54, 355) days, rivaroxaban: 160 (56, 404) days and warfarin: 125 (52, 308) days, and duration of available patient follow-up was 1.8 (0.8, 3.3) years, rivaroxaban: 1.7 (0.7, 3.1) years and warfarin: 1.8 (0.8, 3.4) years. Stages 3 and 4 CKD were present in 5% and 1% of patients at baseline and proteinuria was present in 2%.
Table 1.
Baseline Characteristics of Included Patients Before and After Inverse Probability of Treatment Weighting.
| Before IPTW | After IPTW | |||||
|---|---|---|---|---|---|---|
| Rivaroxaban, N = 36 318, % | Warfarin, N = 36 281, % | Absolute Standardized Difference | Rivaroxaban, N = 36 318, % | Warfarin, N = 36 281, % | Absolute Standardized Difference | |
| Demographics | ||||||
| Age, years | ||||||
| 18-49 | 7.5 | 3.7 | 0.16 | 5.7 | 5.4 | 0.01 |
| 50-64 | 40.7 | 28.0 | 0.27 | 34.8 | 34.1 | 0.02 |
| 65-74 | 22.8 | 22.7 | 0.00 | 22.7 | 22.8 | 0.00 |
| 75-79 | 11.6 | 14.9 | 0.10 | 13.6 | 13.4 | 0.01 |
| ≥80 | 17.4 | 30.6 | 0.31 | 23.2 | 24.3 | 0.03 |
| Male sex | 63.6 | 59.4 | 0.09 | 61.6 | 61.0 | 0.01 |
| Past medical history | ||||||
| Acute decompensate heart failure | 1.4 | 2.9 | 0.10 | 2.2 | 2.2 | 0.00 |
| Acute kidney injury | 5.6 | 8.4 | 0.11 | 6.8 | 7.0 | 0.01 |
| Anal fistula | 0.2 | 0.2 | 0.01 | 0.2 | 0.2 | 0.01 |
| Anemia | 10.4 | 14.8 | 0.13 | 12.5 | 12.8 | 0.01 |
| Anxiety | 9.0 | 7.6 | 0.05 | 8.5 | 8.3 | 0.01 |
| Asthma | 7.1 | 6.1 | 0.04 | 6.7 | 6.6 | 0.01 |
| Barrett’s esophagus | 1.2 | 1.0 | 0.02 | 1.1 | 1.1 | 0.00 |
| Gastrointestinal bleeding | 0.8 | 1.2 | 0.04 | 1.0 | 1.0 | 0.01 |
| Genital urinary bleeding | 0.1 | 0.1 | 0.01 | 0.1 | 0.1 | 0.01 |
| Intracranial hemorrhage | 0.1 | 0.1 | 0.02 | 0.1 | 0.1 | 0.00 |
| Ischemic stroke | 5.1 | 9.1 | 0.15 | 7.0 | 7.2 | 0.01 |
| Coronary artery bypass grafting | 7.3 | 10.7 | 0.12 | 9.1 | 9.2 | 0.01 |
| Cancer | 10.4 | 12.0 | 0.05 | 11.0 | 11.2 | 0.01 |
| Carotid stenosis | 6.1 | 8.2 | 0.08 | 7.3 | 7.2 | 0.00 |
| Chronic kidney disease | ||||||
| Stage 3 | 3.7 | 6.3 | 0.12 | 4.9 | 5.1 | 0.01 |
| Stage 4 | 0.5 | 1.8 | 0.13 | 1.0 | 1.2 | 0.02 |
| Chronic obstructive pulmonary disease | 11.0 | 14.8 | 0.11 | 12.7 | 13.1 | 0.01 |
| Coronary artery disease | 2.7 | 3.7 | 0.06 | 3.2 | 3.3 | 0.01 |
| Coagulopathy | 2.4 | 4.3 | 0.11 | 3.3 | 3.4 | 0.01 |
| Crohn disease | 0.8 | 1.2 | 0.04 | 1.0 | 1.1 | 0.01 |
| Dementia | 3.7 | 6.0 | 0.10 | 4.8 | 4.9 | 0.01 |
| Depression | 8.2 | 8.9 | 0.03 | 8.5 | 8.6 | 0.01 |
| Diverticulitis | 7.1 | 6.9 | 0.01 | 7.1 | 7.0 | 0.01 |
| Type 1 diabetes | 5.6 | 7.8 | 0.09 | 6.6 | 6.8 | 0.01 |
| Type 2 diabetes | 27.4 | 32.0 | 0.10 | 29.2 | 29.7 | 0.01 |
| Ethanol abuse | 2.1 | 1.7 | 0.03 | 2.0 | 1.8 | 0.01 |
| Falls | 5.4 | 4.9 | 0.02 | 5.2 | 5.3 | 0.00 |
| Gastroesophageal reflux disease | 13.4 | 11.4 | 0.06 | 12.5 | 12.2 | 0.01 |
| Hemorrhoids | 3.8 | 3.6 | 0.02 | 3.7 | 3.7 | 0.00 |
| Heart failure | 19.6 | 27.5 | 0.19 | 23.1 | 23.7 | 0.01 |
| Hypertension | 73.6 | 74.1 | 0.01 | 74.1 | 74.0 | 0.00 |
| Hypothyroidism | 14.2 | 14.4 | 0.01 | 14.6 | 14.3 | 0.01 |
| Joint pain or stiffness | 34.0 | 34.8 | 0.02 | 35.0 | 34.8 | 0.00 |
| Liver dysfunction | 3.7 | 3.5 | 0.01 | 3.6 | 3.7 | 0.01 |
| Myocardial infarction | 5.3 | 8.3 | 0.12 | 6.7 | 6.8 | 0.01 |
| Osteoarthritis | 21.1 | 21.8 | 0.02 | 22.3 | 22.0 | 0.01 |
| Obesity | 18.2 | 13.2 | 0.14 | 15.7 | 15.5 | 0.01 |
| Other kidney disease | 0.1 | 0.2 | 0.03 | 0.1 | 0.2 | 0.01 |
| Proteinuria | 2.0 | 2.1 | 0.01 | 2.0 | 2.1 | 0.01 |
| Peripheral artery disease | 5.8 | 8.1 | 0.09 | 6.8 | 7.1 | 0.01 |
| Percutaneous coronary intervention | 2.9 | 3.3 | 0.02 | 3.2 | 3.1 | 0.00 |
| Psychosis | 2.3 | 3.4 | 0.06 | 2.6 | 2.9 | 0.01 |
| Rheumatoid arthritis | 15.8 | 15.5 | 0.01 | 16.1 | 15.8 | 0.01 |
| Sleep apnea | 16.7 | 13.1 | 0.10 | 15.1 | 14.8 | 0.01 |
| Smoker | 6.2 | 5.5 | 0.03 | 5.9 | 6.0 | 0.00 |
| Ulcerative colitis | 0.5 | 0.6 | 0.01 | 0.6 | 0.6 | 0.00 |
| Upper gastrointestinal testing | 5.2 | 5.7 | 0.02 | 5.5 | 5.5 | 0.00 |
| Medications | ||||||
| Alpha-glucosidase inhibitors | 0.1 | 0.1 | 0.02 | 0.1 | 0.1 | 0.01 |
| Amiodarone | 4.3 | 4.2 | 0.00 | 4.4 | 4.3 | 0.00 |
| ACE-I or ARB | 52.8 | 53.1 | 0.01 | 53.1 | 53.1 | 0.00 |
| Aspirin | 1.8 | 1.6 | 0.02 | 1.7 | 1.7 | 0.00 |
| Beta-blockers | 56.3 | 54.4 | 0.04 | 55.6 | 55.3 | 0.01 |
| Cyclooxygenase-2 inhibitors | 2.9 | 2.8 | 0.01 | 2.9 | 2.9 | 0.00 |
| Dihydropyridine calcium channel blockers | 22.1 | 23.3 | 0.03 | 22.8 | 22.9 | 0.00 |
| Digoxin | 5.5 | 6.9 | 0.06 | 6.1 | 6.3 | 0.01 |
| Diltiazem | 12.3 | 11.0 | 0.04 | 11.9 | 11.6 | 0.01 |
| Dipeptidyl peptidase-4 inhibitors | 3.0 | 3.1 | 0.00 | 3.2 | 3.0 | 0.01 |
| Dronedarone | 2.6 | 1.5 | 0.08 | 2.1 | 2.1 | 0.00 |
| Glucagon-like peptide-1 analogues | 1.4 | 1.0 | 0.04 | 1.3 | 1.2 | 0.01 |
| Histamine-2 receptor antagonists | 3.6 | 4.0 | 0.02 | 3.8 | 3.9 | 0.01 |
| Helicobacter pylori treatment | 0.4 | 0.5 | 0.01 | 0.5 | 0.5 | 0.01 |
| Hypnotics | 6.3 | 5.8 | 0.02 | 6.2 | 6.1 | 0.01 |
| Insulin | 5.3 | 6.9 | 0.07 | 6.0 | 6.2 | 0.01 |
| Loop diuretics | 14.2 | 20.7 | 0.17 | 17.2 | 17.7 | 0.01 |
| Metformin | 15.2 | 14.8 | 0.01 | 15.1 | 14.9 | 0.01 |
| Nonsteroidal anti-inflammatory drugs | 20.4 | 16.9 | 0.09 | 18.9 | 18.6 | 0.01 |
| Other anti-arrhythmic agents | 11.4 | 7.0 | 0.15 | 9.4 | 9.2 | 0.01 |
| Other lipid drugs | 9.1 | 9.0 | 0.00 | 9.3 | 9.0 | 0.01 |
| Other antidepressants | 7.0 | 7.5 | 0.02 | 7.2 | 7.4 | 0.01 |
| P2Y12 platelet inhibitors | 9.7 | 11.0 | 0.05 | 10.5 | 10.5 | 0.00 |
| Proton pump inhibitors | 22.7 | 22.0 | 0.02 | 22.6 | 22.4 | 0.00 |
| Sodium-glucose cotransporter-2 Inhibitors | 0.6 | 0.2 | 0.07 | 0.4 | 0.4 | 0.01 |
| SSRI or SNRI | 13.5 | 13.6 | 0.00 | 13.6 | 13.6 | 0.00 |
| Statins | 49.4 | 50.8 | 0.03 | 50.1 | 50.1 | 0.00 |
| Sulfonylureas or glinides | 7.3 | 9.7 | 0.09 | 8.3 | 8.6 | 0.01 |
| Systemic corticosteroids | 20.7 | 18.8 | 0.05 | 20.0 | 19.8 | 0.01 |
| Thiazides | 27.7 | 27.8 | 0.00 | 27.7 | 27.7 | 0.00 |
| Thiazolidinediones | 1.9 | 2.5 | 0.05 | 2.2 | 2.2 | 0.00 |
| Warfarin inhibitors | 64.4 | 63.2 | 0.03 | 64.1 | 64.0 | 0.00 |
| Warfarin inducers | 27.1 | 27.1 | 0.00 | 27.3 | 27.4 | 0.00 |
| Verapamil | 2.0 | 2.1 | 0.01 | 0.02 | 0.02 | 0.00 |
Abbreviations: ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor
Upon Cox regression analysis, rivaroxaban was associated with a 19% (95% confidence interval [CI] = 13%-25%) reduction in the hazard of AKI and an 18% (95% CI = 9%-26%) reduction in progression to stage 5 CKD or hemodialysis compared to warfarin (Figure 1, Supplemental Materials, eFigures 2 and 3).
Figure 1.
Incidence and hazard ratios for the comparison of rivaroxaban and warfarin for renal end points. CKD indicates chronic kidney disease; CI, confidence interval; HR, hazard ratio.
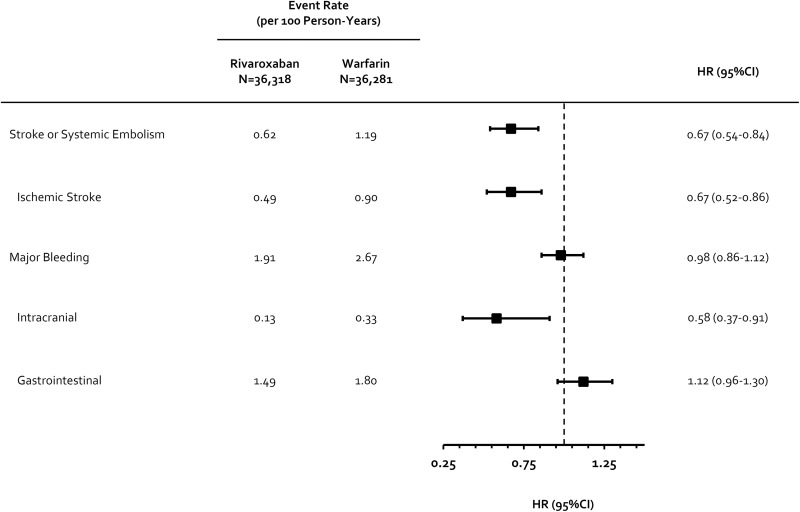
Rivaroxaban was also associated with significant reductions in SSE (hazard ratio [HR] = 0.67) as well as ischemic (HR = 0.67) and intracranial hemorrhage (HR = 0.58) alone. Overall, no significant difference in Cunningham major bleeding was observed between rivaroxaban and warfarin users (HR = 0.98; Figure 2).
Figure 2.
Incidence and hazard ratios for the comparison of rivaroxaban and warfarin on secondary endpoints. CI indicates confidence interval; HR, hazard ratio.
Subgroup Analyses
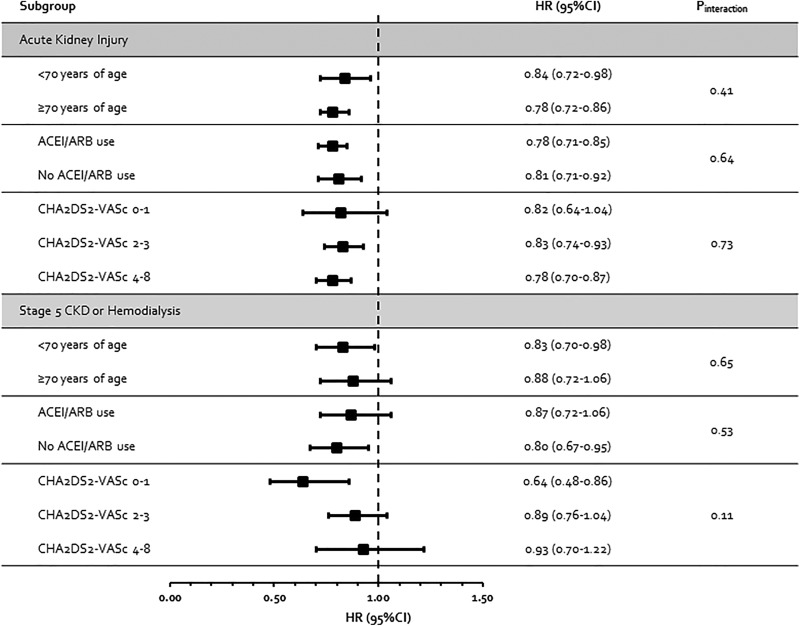
The results for AKI and progression to stage 5 CKD or hemodialysis showed associations that were consistent with the base-case analyses (no statistical interactions were observed, P interaction ≥ 0.11 for all subgroup analyses) in patients <70 or ≥70 years of age; in patients with prior ACEI/ARB use and in those without; and according to baseline CHA2DS2-VASc score (Figure 3).
Figure 3.
Results of subgroup analyses. ACEI indicates angiotensin-converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin receptor blocker; CKD, chronic kidney disease; CI, confidence interval; HR, hazard ratio.
Discussion
In this large US claims database analysis evaluating patients having NVAF without stage 5 CKD or receiving dialysis at baseline, rivaroxaban use was associated with significantly lower risks of undesirable renal end points versus warfarin. This included a 19% relative reduction in the hazard of developing AKI (absolute risk of 2.1%) and an 18% reduction in the progression to stage 5 CKD or need for dialysis (absolute risk of 0.9%). These findings were consistent across all identified subgroups. Results of secondary end point analyses demonstrated effectiveness and safety results that are generally consistent with ROCKET AF4 and earlier real-world analyses of rivaroxaban versus warfarin in patients with NVAF.21 This included significant reductions in SSE as well as ischemic and intracranial hemorrhage in rivaroxaban compared to warfarin users, without a difference in overall major bleeding risk between the cohorts.
It has been shown that VKAs can decrease carboxylation of the matrix G1a protein, an important vitamin K–dependent inhibitor of medial and intimal vascular calcification.9,22 It is also possible that oral factor Xa (FXa) inhibitors exert a renopreserving effect by decreasing PAR-mediated inflammation by inhibition of FXa activity.9 Thus, it is unclear whether the more rapid rate of renal decline seen with VKAs compared to NOACs6–8 is due to a detrimental effect of VKAs (eg, due to promotion of vascular calcification), renoprervation effects of NOACs, or some combination of both.
Renal outcomes of NOACs compared to warfarin have been assessed through secondary analysis of phase III randomized controlled trials6–8 and in a prior claims database analysis.23 An analysis of ROCKET AF data6 found that while creatinine clearance decreased in both rivaroxaban and warfarin patients over 21 months of follow-up, the rate of decline was slower with rivaroxaban compared to warfarin (−3.5 vs −4.3 mL/min, P < .0001). A similar analysis of ARISITOTLE data7 also found a decline in the estimated glomerular filtration rate (eGFR) over 12 months with both apixaban and warfarin; however, in this analysis, the decline in eGFR was shown to be more pronounced with apixaban than warfarin (−1.42 vs −0.92 mL/minute, P = .01). Yao and colleagues23 performed an analysis of real-world data using the integrated US Optum Labs claims database and electronic health record to assess the impact of NOACs versus warfarin on the renal end points of ≥30% decline in eGFR, doubling in serum creatinine, and development of AKI or kidney failure (eGFR<15 mL, need for dialysis or transplant). Among patients having NVAF with both claims and available serum creatinine data, rivaroxaban was found to be associated with significantly lower risk of 3 of the 4 renal outcomes assessed (HR range of 0.46-0.73) versus warfarin (not kidney failure, HR = 0.63, 95%CI = 0.35-1.15), whereas apixaban did not significantly reduce the risk of any of these outcomes (HR range of 0.80-1.02). Taken together, these studies may suggest that not all oral FXa inhibitors are renoprotective.
This study has limitations worth discussion. First, we did not have access to serum creatinine values in the Truven MarketScan data set utilized for this study.10 Thus, we were unable to assess end points such as >30% increase in eGFR or a doubling in serum creatinine. While these are reasonable renal end points to evaluate, they are surrogate in nature. To assess these serum creatinine-based end points, Yao et al22 had to limit inclusion to the 13.5% of insurance enrollees with available laboratory values, likely decreasing the external validity of their study since patients having NVAF with multiple serum creatinine levels are those likely experiencing more severe renal decline. Second, regardless of the sophistication of the methodology and the number of covariates used in developing propensity scores, residual confounding cannot be fully excluded due to the possibility of confounding from unobserved or unmeasured covariates.24 Of note, we implemented a machine learning method that only requires the researcher to specify which pretreatment covariates need be balanced between the treatment cohorts. The TWANG algorithm,12 and not the researcher, then determines the most appropriate main effects, interactions, and higher order terms that make up the optimal propensity score model. Third, international normalized ratio (INR) data were not available in our data set.10 Of note, prior studies suggest that even in patients with mean INR values <2 or between 2.0 and 3.0, warfarin remained associated with detrimental renal outcomes.23 Consequently, it is unlikely the availability of INR data for adjustment in our analysis would have substantially impacted our overall conclusions. Finally, data on out-of-hospital mortality are not available in the Truven MarketScan database, preventing us from evaluating the outcome of all-cause or cardiovascular mortality.
Conclusion
Rivaroxaban appears associated with lower risks of AKI or progression to stage 5 CKD or need for dialysis versus warfarin in patients with NVAF. The ongoing multicenter FXa-Inhibition in RENal Patients With Non-valvular Atrial Fibrillation–Observational Registry (XARENO)25 will be able to provide a more rigorous assessment of the impact of VKAs and rivaroxaban on decline in in renal function, specifically, eGFR.
Supplemental Material
Supplemental_Materials_(1) for Rivaroxaban’s Impact on Renal Decline in Patients With Nonvalvular Atrial Fibrillation: A US MarketScan Claims Database Analysis by Craig I. Coleman, Reinhold Kreutz, Nitesh Sood, Thomas J. Bunz, Anna-Katharina Meinecke, Daniel Eriksson and William L. Baker in Clinical and Applied Thrombosis/Hemostasis
Footnotes
Authors’ Note: CIC, AKM, and DE contributed to research idea and study design; CIC, AKM, and DE contributed to data acquisition; CIC, RK, TJB, NS, AKM, DE, and WLB contributed to data analysis/interpretation; CIC and WLB contributed to statistical analysis; and CIC contributed to supervision or mentorship. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. This study was determined by our institutional review board to not constitute research involving human subjects according to 45 CFR 46.102(f) and was deemed exempt from board oversight.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Daniel Eriksson and Anne-Katherina Meinecke are employees of Bayer AG.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Craig I. Coleman has received grant funding and consultancy honorarium from Bayer AG, Janssen Scientific Affairs LLC, and Boehringer Ingelheim Pharmaceuticals Inc. Dr Kreutz has received consultancy fees from Bayer, Berlin-Chemie Menarini, and Servier Laboratories Ltd and speaker’s honoraria from Bayer, Berlin-Chemie Menarini, and Daiichi Sankyo. This study was funded by Bayer AG, Berlin, Germany. The funders had no role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
ORCID iD: Craig I. Coleman  https://orcid.org/0000-0003-4868-7158
https://orcid.org/0000-0003-4868-7158
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Benjamin EJ, Virani SS, Callaway CW, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492. [DOI] [PubMed] [Google Scholar]
- 2. Granger CB, Alexander JH, McMurray JJ, et al. ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–992. [DOI] [PubMed] [Google Scholar]
- 3. Connolly SJ, Ezekowitz MD, Yusuf S, et al. RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. [DOI] [PubMed] [Google Scholar]
- 4. Patel MR, Mahaffey KW, Garg J, et al. ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. [DOI] [PubMed] [Google Scholar]
- 5. Giugliano RP, Ruff CT, Braunwald E, et al. ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–3104. [DOI] [PubMed] [Google Scholar]
- 6. Fordyce CB, Hellkamp AS, Lokhnygina Y, et al. ROCKET AF Steering Committee and Investigators. On-treatment outcomes in patients with worsening renal function with rivaroxaban compared with warfarin: insights from ROCKET AF. Circulation. 2016;134(1):37–47. [DOI] [PubMed] [Google Scholar]
- 7. Hijazi Z, Hohnloser SH, Andersson U, et al. Efficacy and safety of apixaban compared with warfarin in patients with atrial fibrillation in relation to renal function over time: insights from the ARISTOTLE randomized clinical trial. JAMA Cardiol. 2016;1(4):451–460. [DOI] [PubMed] [Google Scholar]
- 8. Böhm M, Ezekowitz MD, Connolly SJ, et al. Changes in renal function in patients with atrial fibrillation: an analysis from the RE-LY trial. J Am Coll Cardiol. 2015;65(23):2481–2493. [DOI] [PubMed] [Google Scholar]
- 9. van Gorp RH, Schurgers LJ. New insights into the pros and cons of the clinical use of vitamin k antagonists (VKAs) versus direct oral anticoagulants (DOACs). Nutrients. 2015;7(11):9538–9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hansen L. The Truven Health MarketScan Databases for life sciences researchers. 2017. https://truvenhealth.com/Portals/0/Assets/2017-MarketScan-Databases-Life-Sciences-Researchers-WP.pdf. Accessed May 16, 2018.
- 11. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32(19):3388–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55(7):698–705. [DOI] [PubMed] [Google Scholar]
- 14. Kirchhof P, Benussi S, Kotecha D, et al. ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962. [DOI] [PubMed] [Google Scholar]
- 15. January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74(1):104–132. [DOI] [PubMed] [Google Scholar]
- 16. Hwang YJ, Shariff SZ, Gandhi S, et al. Validity of the International Classification of Diseases, Tenth Revision code for acute kidney injury in elderly patients at presentation to the emergency department and at hospital admission. BMJ Open. 2012;2(6):e001821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf. 2011;20(6):560–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326(7382):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Society Ser B. 1995;57(1):289–300. [Google Scholar]
- 20. Benchimol EI, Smeeth L, Guttmann A, et al. RECORD Working Committee. The REporting of studies conducted using observational routinely-collected health data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coleman CI, Antz M, Bowrin K, et al. Real-world evidence of stroke prevention in patients with nonvalvular atrial fibrillation in the United States: the REVISIT-US study. Curr Med Res Opin. 2016;32(12):2047–2053. [DOI] [PubMed] [Google Scholar]
- 22. Rennenberg RJ, van Varik BJ, Schurgers LJ, et al. Chronic coumarin treatment is associated with increased extracoronary arterial calcification in humans. Blood. 2010;115(24):5121–5123. [DOI] [PubMed] [Google Scholar]
- 23. Yao X, Tangri N, Gersh BJ, et al. Renal outcomes in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2017;70(21):2621–2632. [DOI] [PubMed] [Google Scholar]
- 24. Gandhi SK, Salmon W, Kong SX, Zhao SZ. Administrative databases and outcomes assessment: an overview of issues and potential utility. J Manag Care Spec Pharm. 1999;5(3):215–222. [Google Scholar]
- 25. ClinicalTrials.gov. Factor XA - Inhibition in RENal Patients With Non-valvular Atrial Fibrillation - Observational Registry (XARENO). NCT02663076. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental_Materials_(1) for Rivaroxaban’s Impact on Renal Decline in Patients With Nonvalvular Atrial Fibrillation: A US MarketScan Claims Database Analysis by Craig I. Coleman, Reinhold Kreutz, Nitesh Sood, Thomas J. Bunz, Anna-Katharina Meinecke, Daniel Eriksson and William L. Baker in Clinical and Applied Thrombosis/Hemostasis