Abstract
The effect of direct oral anticoagulants (DOACs) on laboratory tests dependent on the production of their targets, factor IIa and factor Xa (FXa), is a well-known problem and can cause both false positive and negative results. Therefore, the correct interpretation of tests performed in patients receiving DOACs is necessary to avoid misclassification and subsequent clinical consequences. However, even with significant experience, there are situations where it is not possible to assess the influence of some methods. Particularly important is the situation in the diagnosis of lupus anticoagulants using the dilute Russell viper venom timetest, which is based on direct FXa activation. A very promising solution to this situation is offered by the DOAC laboratory balancing procedure DOAC-Stop. For evaluating the effectiveness of this procedure, 60 (20 apixaban, 20 dabigatran, and 20 rivaroxaban) patients treated with DOACs were enrolled. All patient samples were analyzed for the presence of individual DOAC types and subsequently subjected to the DOAC-Stop procedure.We evaluated its effectiveness by our own high-performance liquid chromatography-coupled tandem mass spectrometrymethod, which simultaneously sets all high-sensitivity DOACs. Unlike coagulation tests based on the determination of the residual effects of DOACs on target enzymes, which is complicated by extensive interindividual variation, this methodology is highly specific and sensitive.The DOAC-Stop procedure eliminated dabigatran from 99.5%, rivaroxaban from 97.9%, and apixaban from 97.1% of participants in our group. Residual amounts did not exceed 2.7 ng/mL for dabigatran, 10.9 ng/mL for rivaroxaban, or 13.03 ng/mL for apixaban, which are safe values that do not affect either screening or special coagulation tests.
Keywords: dabigatran, rivaroxaban, apixaban, DOAC-Stop, HPLC MS/MS, anticoagulants, factor Xa inhibitors, lupus inhibitor
Introduction
Direct oral anticoagulants (DOACs) are targeted coagulation factor inhibitors bringing about improvement in anticoagulation therapy. While the “old anticoagulants,” vitamin K antagonists have many limitations, such as unpredictable anticoagulant effect, the needs for routine monitoring, parenteral administration, awareness of various drug and food interactions, and dose adjustment, DOACs seem to overcome these limitations. Since DOACs are selective inhibitors of coagulation factors,their modes of action are more predictable. Other advantages include fixed doses, no need for routine monitoring, very few drug and food interactions, and mainly oral administration.1
Currently, the following 4DOACs are used in anticoagulation therapy: dabigatran, a direct thrombin inhibitor; rivaroxaban, apixaban, and edoxaban, direct factor Xa (FXa) inhibitors. Their indications include prevention of venous thromboembolism in patients undergoing elective knee and hip surgery, prevention of stroke and systemic embolism in patients with atrial fibrillation, treatment and prevention of recurrent deep vein thrombosis (DVT), and pulmonary embolism (PE).
Even though routine determination of DOACs is not needed, samples of patients treated with DOACs are rather common in our laboratory. One of the adverse effects of DOAC therapy is the false positive or negative results of screening coagulation tests as well as specific tests like thrombophilia screening (eg, antithrombin, protein C, protein S, lupus anticoagulant[LA], activated C protein resistance).2,3 This situation prevents the diagnosis of patients using this type of medication and forces us to concede to one of the following changes, should testing be necessary.
Several strategies were proposed to minimize the impact of residual DOACs on coagulation assays: (1) the use of DOAC insensitive assays, (2) the addition of idarucizumab to the plasma sample (Praxbind, Boehringer Ingelheim, Germany) to specifically neutralize the in vitro activity of dabigatran, or (3) skipping 1(for once-daily fixed-dose regimens) or 2(for twice-daily fixed-dose regimens) DOAC intakes in patients with low thromboembolic risk. However, any interruption of anticoagulation will expose the patient to an increased risk of thrombosis, and residual drug levels may still affect test results.4 Thus, none of these approaches are considered optimal, and a simple way to overcome the problem would be to remove DOAC from the plasma sample without influencing its coagulant properties.
The aim of this study was to evaluate the efficiency of a new and simple procedure, DOAC-Stop (Haematex Research, Hornsby, Australia),5 to overcome the effect of all DOACs by detecting the residual activity of DOACs through high-performance liquid chromatography-coupled tandem mass spectrometry (HPLC-MS/MS).6–8 The HPLC-MS/MS is used as a referential method for assessing the suitability of other methods but not for routine determination. It shows high sensitivity, accuracy, and precision, as well as an adequate limit of quantification. This method is very simple, fast, and specific, enabling simultaneous determination of all DOACs. However, the method is suitable only for highly specialized and equipped laboratories.
The second aim of this study was to determine the potential level of DOAC that affects the most sensitive coagulation test, the determination of LA using the dilute Russell viper venom(dRVVT) test.9
Material and Methods
Patients
This study was conducted on a set of blood samples sent to our laboratory from 60 patients treated with DOACs for all approved indications of treatment and prevention of DVT, PE, and stroke. Citrated plasma samples were stored at −80°C before analysis.
Blood Sampling
Blood was collected with a Vacuette needle (Greiner Bio-One, Vienna, Austria) into a vacuum tube with a buffered solution containing sodium citrate at a concentration of 0.109 mol/L(3.2%). The system ensured a blood and anticoagulant mixture at a desired 1:10 ratio. Then the blood was carefully mixed in a test tube, with the tube being gently turned upside down several times and transported to the laboratory. Next, the sample was centrifuged for 10 minutes at 3000g, following which 0.5mL of the upper layer of platelet-poor plasma was aspirated, frozen, and stored at −80°C until analysis was performed. In the analysis of LA, the sample was repeatedly centrifuged under the same conditions. For the actual analysis, the sample was thawed at room temperature.
The DOAC-Stop Procedure
The DOAC levels were determined both before and after the addition of adsorbent tablets DOAC-Stop, according to the manufacturer’s instructions and depending on the available plasma volume. Briefly, a halftablet of DOAC-Stop designed to adsorb DOACs was added to each 0.5mL of plasma. Thereafter, the sample was gently mixed for 5 minutes and centrifuged for 2 minutes at 3000g to precipitate the DOACs with adsorbent. Finally, the supernatant conjectured to be free of DOACs is collected in order to be further analyzed. The composition of DOAC-Stop is Haematex proprietary information. Concentrations of apixaban, dabigatran, edoxaban, and rivaroxaban were also assayed before and after the DOAC-Stopprocedure.
Liquid Chromatography-Coupled Tandem Mass Spectrometry
The plasma sample for HPLC-MS/MS analysis (50 μL) was deproteinized with methanol (180 μL) with the addition of an internal standard (deuterated analogue of dabigatran [DAB-D3], 20 μL, 100 ng/mL). The sample was shaken (5minutes), frozen (60minutes; −80°C), and centrifuged (5minutes; 3000g). The supernatant was transferred into a 350-μLglass vial (12mm × 32mm, fused insert) and analyzed.
The HPLC-MS/MS analysis was performed using the liquid chromatography system UltiMate 3000 RS (Dionex, Sunnyvale, California) coupled with a triple quadrupole 6500 tandem mass spectrometer (AB Sciex, Foster City, California). A Luna Omega C18 Polar column 1.6µm, 2.1 mm×50mm protected by guard column 4 mm ×2mm ID of the same material (Phenomenex, Torrance, California) in normal aqueous phase mode was used for separation. The mobile phase consisted of ammonium formate (25mmol/L, pH 3.5) in water and acetonitrile (MF A: 95:5 and MF B: 5:95, vol/vol). The gradient employed was 0 to 0.5minute: 15% B; 0.5 to 1.0minute: 15% to 100% B; 1.0 to 1.9minutes: 100% B; 1.9 to 2.0minutes: 100% to15% B; 2.0 to 2.7minutes: 15% B. The column was maintained at 50°C and the flow rate at 0.4 mL/min.
The targeted analytes were measured in scheduled multiple reaction monitoring mode with prolonged dwell times. Both quadrupoles were set at unit resolution. The parameters of the Turbo VTM ion source and gases were as follows: ion spray voltage, +5500V; curtain gas, 35 psi; both ion source gases, 40 psi; and source temperature, 400°C. High-purity nitrogen was used as collision gas (pressure adjusted to “medium settings”), curtain gas, and ion source gas. The compound parameters declustering potential, entrance potential, collision energy, and collision cell exit potential were optimized on previous standards.10 The instrument was controlled by the Analyst version 1.6.2 software.
The analytes were detected and identified according to multiple reaction monitoring transitions and retention times in the MultiQuant version 3.0 software (Sciex, Foster City, California). Dabigatran, rivaroxaban, and its deuterated analogue DAB-D3 (Toronto Research Chemicals Inc, Toronto, Canada) and apixaban (Pfizer Inc, Dublin, Ireland) were dissolved in methanol (LC-MS quality, Sigma, Seelze, Germany) to a final concentration of 1 mg/mLexpressed as free substances. Those stock solutions were then used for the preparation of all other standards. For quantification, a series of calibration standards in methanol were prepared (concentrations 0, 10, 50, 100, and 500 ng/mLof dabigatran, apixaban, and rivaroxaban). The calibration standards were prepared in addition to drug-free plasma from healthy volunteers. All the solutions were stored at −20°C (Table 1).
Table 1.
Multiple reaction monitoring transitions and optimized mass spectrometry parameters for the analyzed compounds.
| Compound (I, II—First and Second MRM Transitions) | MRM Transition (m/z) | Dwell Time (ms) | Declustering Potential (V) | Entrance Potential (V) | Collision Energy (V) | Collision Cell Exit Potential (V) | Limit of Quantitation (ng/mL) |
|---|---|---|---|---|---|---|---|
| Apixaban I | 460.1 → 199.1 | 20 | 196 | 10 | 53 | 8 | 0.22 |
| Apixaban II | 460.1 → 185.1 | 20 | 196 | 10 | 55 | 12 | 1.06 |
| DAB-D3 I | 475.2 → 292.1 | 100 | 146 | 10 | 39 | 18 | - |
| DAB-D3 II | 475.2 → 175.1 | 100 | 146 | 10 | 55 | 10 | - |
| Dabigatran I | 472.1 → 289.0 | 100 | 126 | 10 | 39 | 6 | 0.57 |
| Dabigatran II | 472.1 → 324.1 | 100 | 126 | 10 | 29 | 8 | 1.18 |
| Rivaroxaban I | 436.0 → 144.8 | 160 | 156 | 10 | 33 | 18 | 0.79 |
| Rivaroxaban II | 436.0 → 231.0 | 160 | 156 | 10 | 29 | 20 | 3.38 |
Abbreviations: DAB-D3, deuterated analogue ofdabigatran; MRM, multiple reaction monitoring.
Lupus Anticoagulants
Lupus anticoagulant tests are performed by LAC screen tests (Werfen, Barcelona, Spain). They are improved dRVVT belonging to the group of antiphospholipid antibodies, which are directed against negatively charged phospholipids or complexes between phospholipids and proteins (either β-2-glycoprotein 1 or clotting factors such as prothrombin).
For the purpose of determining the effect of DOAC on dRVVT assays, we performed measurements using standard plasma spiked with a known DOAC amount from 0 to 250 ng/mLto evaluate the effect of DOAC itself on dRVVT.
Statistical Analysis
Statistical analysis was performed using the software package Statistica version 12 (StatSoft, Czech Republic).
Results
Demonstrating the Absorbing Effect of DOAC-Stop in HPLC-MS/MS
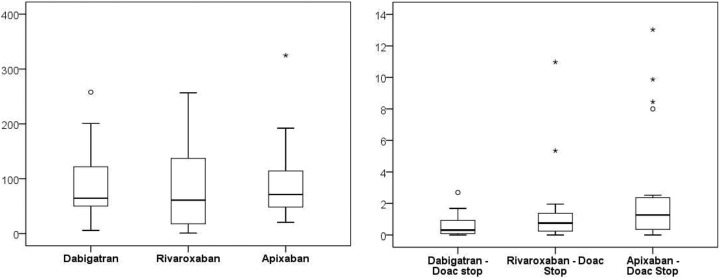
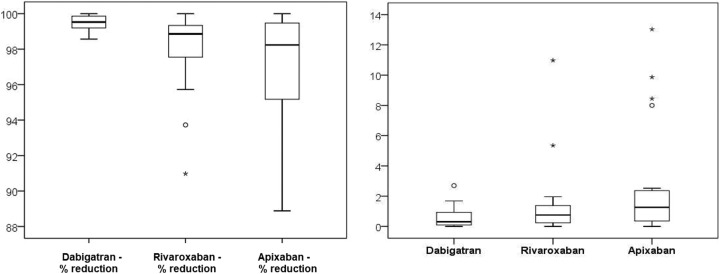
In the first step, we verified the effectiveness of the DOAC-Stop procedure in 20 samples of DOAC-treated patients; the samples were divided into 2aliquots, one of which was subjected to the DOAC-Stop procedure, and then both samples were prepared for HPLC-MS/MS testing (see Table 2 and Figures 1 and 2).
Table 2.
Determination of residual level anticoagulant drug.
| Mean | SD | Median | Minimum | Maximum | |
|---|---|---|---|---|---|
| Dabigatran | 88.98 | 64.55 | 64.46 | 5.70 | 257.76 |
| Dabigatran—DOAC-Stop | 0.60 | 0.73 | 0.31 | 0.00 | 2.70 |
| Dabigatran—% reduction | 99.5 | 0.4 | 99.5 | 98.6 | 100.0 |
| Rivaroxaban | 79.38 | 72.32 | 61.01 | 0.94 | 256.68 |
| Rivaroxaban—DOAC-Stop | 1.46 | 2.53 | 0.75 | 0.00 | 10.97 |
| Rivaroxaban—% reduction | 97.9 | 2.3 | 98.9 | 91.0 | 100.0 |
| Apixaban | 92.47 | 69.88 | 71.23 | 20.51 | 324.73 |
| Apixaban—DOAC-Stop | 2.72 | 3.83 | 1.26 | 0.00 | 13.03 |
| Apixaban—% reduction | 97.1 | 3.4 | 98.2 | 88.9 | 100.0 |
Abbreviations: DOAC, direct oral anticoagulant; SD, standard deviation.
Figure 1.
Statistic assesment of original and eliminated levels of DOACs.
Figure 2.
Statistical assesment of procedure effectiveness.
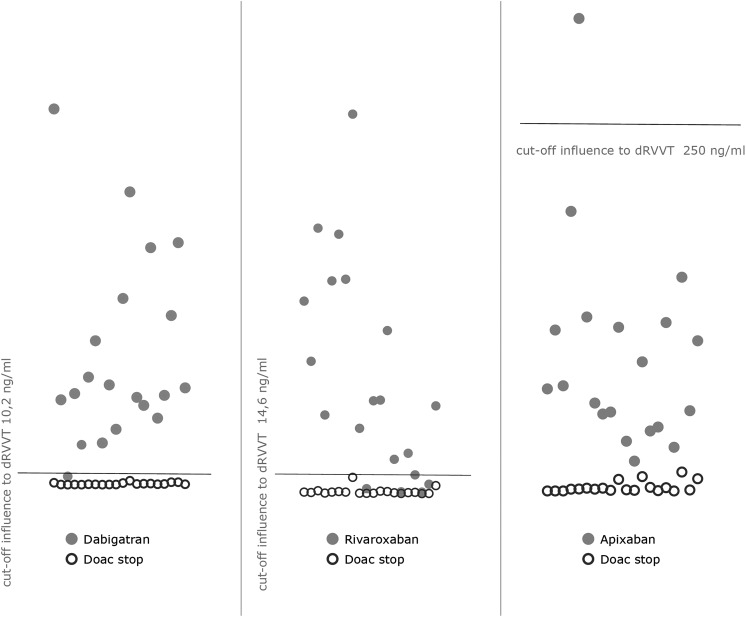
Subsequently, we evaluated these results against the cutoff values for dRVVT assay affection obtained by spiking standard plasma with known DOAC amounts and measuring dRVVT ratio (Figure 3). Residual amounts did not exceed 2.7 ng/mLfor dabigatran, 10.9 ng/mLfor rivaroxaban, or 13.03 ng/mLfor apixaban, which are safe values that do not affect either screening or special coagulation tests, as the most sensitive coagulation test, dRVVT, is affected starting at levels of 10 ng/mLfor dabigatran, 14.2 ng/mLfor rivaroxaban, and 250 ng/mLfor apixaban.
Figure 3.
Graph of residual DOAC activity (empty points) related to dRVVT test cutoff. DOAC indicates direct oral anticoagulant; dRVVT, dilute Russell viper venom test.
Discussion
There are several papers describing the elimination of the effects of DOACs in both basic coagulation tests,11,12 specific determinations of individual coagulation factors,13 and their inhibitors and global coagulation tests.14–16 The elimination is reported by influencing the level of analytes themselves or the results of individual tests. However, there are no studies comparing the levels of individual DOACs before and after such a procedure. The reason is very simple: the inadequate sensitivity of functional methodologies in determining the goals of anticoagulant treatment, be it factor IIa (FIIa) or FXa.
In our study, the HPLC-MS/MS technique was used with our own method for simultaneously determining all 3DOACs currently on the market with limits of quantification of 0.57 ng/mLfor dabigatran, 0.79 ng/mLfor rivaroxaban, and 0.22 ng/mLfor apixaban. These values reliably guarantee the detection of residual DOAC activity after the DOAC-Stop procedure.
Based on the measured data, we can reliably confirm the data found by other authors, which, however, could not be verified, regarding the reliability of the DOAC-Stop procedure in the elimination of the effect of DOACs on both basic and special coagulation tests. The readings after elimination did not exceed 2.7 ng/mL of residual activity for dabigatran or 10.97 or 13.03 ng/mLfor rivaroxaban and apixaban, respectively. These are very low values undetectable by standard functional tests based on the detection of FIIa or FXa. The limit of detection is 50 ng/mLfor dabigatran functional assays,17 and 20 ng/mLeach for rivaroxaban and apixaban.18
If data on the potential impact of the tests are seen via the ability to influence the most sensitive coagulation assay, dRVVT, false cutoff values corresponding to a ratio of 1.2 are caused by DOAC levels from 10 to 250 ng/mL.
Conclusion
Direct oral anticoagulant-Stop is a procedure that can be implemented in all coagulation tests where DOAC treatment is affected, as confirmed in our study by the validation of the most affected test for the determination of LA using dVVT. The effectiveness has been confirmed for all currently used “new anticoagulants.” The procedure is simple, accessible, technically easy, and can be used in all laboratories.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by the student project IGA_LF_2019_001 of the Palacký University and MH CZ—DRO (FNOl, 00098892).
References
- 1. Adcock DM, Gosselin R. Direct oral anticoagulants (DOACs) in the laboratory: 2015 review. Thromb Res. 2015;136(1):7–12. (PMID: 25981138). [DOI] [PubMed] [Google Scholar]
- 2. Favaloro EJ, Lippi G. Interference of direct oral anticoagulants in haemostasis assays: high potential for diagnostic false positives and false negatives. Blood Transfus. 2017;15(6):491–494. (PMID: 28287385). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tripodi A. The laboratory and the new oral anticoagulants. Clin Chem. 2013;59(2):353–362. (PMID: 23043068). [DOI] [PubMed] [Google Scholar]
- 4. Francart SJ, Hawes EM, Deal AM, et al. Performance of coagulation tests in patients on therapeutic doses of rivaroxaban. Thromb Haemost. 2014;111(6):1133–1140. (PMID: 24401946). [DOI] [PubMed] [Google Scholar]
- 5. Delavenne X, Moracchini J, Laporte S, Mismetti P, Basset T. UPLC MS/MS assay for routine quantification of dabigatran—a direct thrombin inhibitor—in human plasma. J Pharm Biomed Anal. 2012;58:152–156. (PMID: 21996066). [DOI] [PubMed] [Google Scholar]
- 6. Delavenne X, Mismetti P, Basset T. Rapid determination of apixaban concentration in human plasma by liquid chromatography/tandem mass spectrometry: application to pharmacokinetic study. J Pharm Biomed Anal. 2013;(78-790):150–153. (PMID: 23499913). [DOI] [PubMed] [Google Scholar]
- 7. Antovic JP, Skeppholm M, Eintrei J, et al. Evaluation of coagulation assays versus LC-MS/MS for determinations of dabigatran concentrations in plasma. EurJ Clin Pharmacol. 2013;69(11):1875–1881. (PMID: 23784008). [DOI] [PubMed] [Google Scholar]
- 8. Nouman EG, Al-Ghobashy MA, Lotfy HM. Development and validation of LC–MSMS assay for the determination of the prodrug dabigatran etexilate and its active metabolites in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;989:37–45. (PMID: 25797721). [DOI] [PubMed] [Google Scholar]
- 9. Hoxha A, Banzato A, Ruffatti A, Pengo V. Detection of lupus anticoagulant in the era of direct oral anticoagulants. Autoimmun Rev. 2017;16(2):173–178.(PMID: 27988438). [DOI] [PubMed] [Google Scholar]
- 10. Flieder T, Weiser M, Eller T, et al. Interference of DOACs in different DRVVT assays for diagnosis of lupus anticoagulants. Thromb Res. 2018;165:101–106.(PMID: 29627719). [DOI] [PubMed] [Google Scholar]
- 11. Slavik L, Lukes J, Friedecky D, et al. Multianalyte determination of NOACs using LC-MS/MS and comparison with functional coagulation assays. Clin Lab. 2018;64(10):1611–1621.(PMID: 30336535). [DOI] [PubMed] [Google Scholar]
- 12. Platton S, Hunt C. Influence of DOAC-Stop on coagulation assays in samples from patients on rivaroxaban or apixaban. Int J Lab Hematol. 2019;41(2):227–233 (PMID: 30468572). [DOI] [PubMed] [Google Scholar]
- 13. Exner T, Ahuja M, Ellwood L. Effect of an activated charcoal product (DOAC-Stop™) intended for extracting DOACs on various other APTT-prolonging anticoagulants. Clin Chem Lab Med. 2019;57(5):690–696.(PMID: 30427777). [DOI] [PubMed] [Google Scholar]
- 14. Ząbczyk M, Kopytek M, Natorska J, Undas A. The effect of DOAC-stop on lupus anticoagulant testing in plasma samples of venous thromboembolism patients receiving direct oral anticoagulants. Clin Chem Lab Med. 2019;57(9):1374–1381. pii: /j/cclm.ahead-of-print/cclm-2018-1197/cclm-2018-1197.xml (PMID: 30763261). [DOI] [PubMed] [Google Scholar]
- 15. Kopatz WF, Brinkman HJM, Meijers JCM. Use of DOAC-Stop for elimination of anticoagulants in the thrombin generation assay. Thromb Res. 2018;170:97–101.(PMID: 30149286). [DOI] [PubMed] [Google Scholar]
- 16. Exner T, Favresse J, Lessire S, Douxfils J, Mullier F. Clotting test results correlate better with DOAC concentrations when expressed as a “correction ratio” results before/after extraction with the DOAC-stop reagent. Thromb Res. 2019;179:69–72.(PMID: 31096112). [DOI] [PubMed] [Google Scholar]
- 17. Stangier J, Feuring M. Using the HEMOCLOT direct thrombin inhibitor assay to determine plasma concentrations of dabigatran. Blood Coagul Fibrinolysis. 2012;23(2):138–143.(PMID: 22227958). [DOI] [PubMed] [Google Scholar]
- 18. Cuker A, Siegal DM, Crowther MA, Garcia DA. Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J Am Coll Cardiol. 2014;64(11):1128–1139.(PMID: 25212648). [DOI] [PMC free article] [PubMed] [Google Scholar]