Abstract
Background:
Deep venous thrombosis (DVT) is a common complication after stroke. It is easy to identify the patients with symptomatic DVT; however, the tool for asymptomatic high-risk population needs to be further explored. Our aim was to explore the risk factors of acute stroke patients with asymptomatic DVT.
Methods:
We performed a prospective observation study among 452 patients with acute stroke who had a stroke within 14 days. Ultrasound examination of deep veins was repeatedly performed in each patient for DVT every 7 days during his admission. The dynamic rate of DVT in acute stroke was analyzed. Then risk factors were compared between DVT patients and non-DVT patients. The predictive model was explored based on thr cox proportion model.
Results:
Asymptomatic DVT was detected in 52 (11.5%) patients with stroke and 85.9% of thrombi were identified in their distal veins. Patients with longer length of stay (P = .004), more severe stroke (P = 0.001), higher level of D-dimer (P = .003), and higher blood glucose level were associated with higher risk of DVT, while patients with higher triglyceride level (P = .003) were less likely to have DVT, after adjusting age and sex. With the median of D-dimer (0.38 FEU mg/L) as cutoff value. Patients with higher level of D-dimer might have a higher risk of DVT with a significant statistical difference. Also, the severity of stroke differed DVT risk in Kaplan-Meier model. Using cox-proportion hazard regression model, asymptomatic DVT could be predicted (area under the curve 0.852).
Conclusion:
Our data showed that asymptomatic DVT was common in patients with acute stroke and most of thrombosis occurred in distal veins. Combination of clinical manifestation and laboratory results might be helpful predict DVT. DVT prophylaxis should be condisdered in high risk.
Keywords: deep vein thrombosis, asymptomatic, D-dimer, acute stroke
Introduction
Patients with acute stroke might be associated with an increasing risk of deep venous thrombosis (DVT) due to immobility, but most of which are found as asymptomatic.1,2 The need for routine screening of DVT and the benefit of prophylactic therapy in patients with acute stroke is controversial, especially in patients with stroke who are active and with mobility. Prediction tools to identify symptomatic patients with DVT need to be further explored. However, the clinical utility tool in the diagnosis of symptomatic DVT has not been well explored.3,4
The clinical risk factors of reported symptomatic DVT has been well-studying while asymptomatic DVT in patients with acute stroke have been discussed in surprisingly few studies. The only small-sample study specifically investigating DVT in patients with acute stroke showed that 18 of 92 participants were discovered asymptomatic DVT diagnosed in the second week.5 There are changes of DVT prevalence, especially in asymptomatic patients. We investigated the incidence of asymptomatic DVT in patients with acute stroke by ultrasound, as well as establish a predict model in patients with asymptomatic DVT.
Method
Study Design
All patients who were diagnosed as acute stroke and admitted between July 1, 2017, and Jun 30, 2018, were eligible. All clinical data were extracted and recorded from electronic history record system, including demographics, stroke features, and laboratory test. All laboratory tests were performed within 24 hours after admission.
Procedures
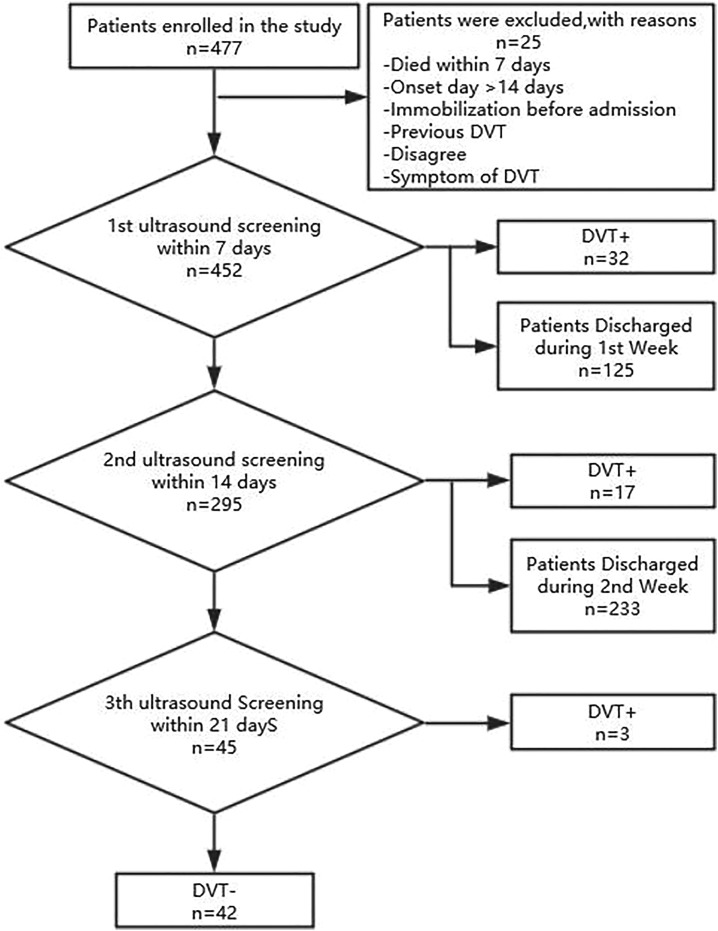
Compression Doppler ultrasound examination of bilateral deep veins (including proximal and distal veins) was performed in admission patient with stroke within the first 7 days. Patients with no DVT would be rescreening every 7 days after admission. All the ultrasound screening was performed by a well-trained ultrasound physicians. The workflow was illustrated in Figure 1. The study was approved by the ethics committees of Huashan Hospital, an affiliated teaching hospital of Fudan University (KY2018-227).
Figure 1.
A workflow of deep venous thrombosis study in acute stroke patients in admission. DVT indicates deep vein thrombosis.
Participants
All patients were eligible if they were diagnosed with acute stroke (ischemic or hemorrhagic) and confirmed by computed tomography or magnetic resonance imaging, age >18 years, and within 14 days after stroke onset. We excluded patients who were admitted 14 days after onset, or died within 7 days, or immobilization before stroke, or who with previous DVT and had symptom of DVT before admission. Participants were enrolled and their data were collection, after the informed written consent has been signed.
None of DVT prophylactic anticoagulant drug was initiated routinely in mobile patients, as there is still a controversy in guidelines. However, in patients with immobile stroke without contraindications, intermittent pneumatic compression in addition to routine care (aspirin and hydration) is used over routine care, which might be of help to reduce the risk of DVT.1
Statistical Analysis
Continuous variables were given as mean (standard deviation) or median (interquartile range [IQR]) for normal and non-normal distribution, respectively. Categorical variables are presented as count and percentage. Comparisons were conducted between groups (with/without DVT) using t test for normal distributed continuous variables. The Mann-Whitney U test was used for non-normally distributed variables. Fisher exact test was used to compare categorical variables between those with and without DVT. Odd ratios were obtained from logistic regression in an adjusted analysis as a measure of association between variables tested and DVT. Only factors with a P ≤ .10 from univariable analysis were included in this analysis. The Kaplan-Meier survival curves were used to examine unadjusted time to DVT risk and a log-rank test was used to evaluate differences between different severities of stroke. Predictions of DVT risk by different level D-dimer were based on Cox-proportional hazard regression models. Further analysis was performed with receiver operating characteristic (ROC) curves to determine the area under the ROC curve (AUC), which was used to identify the optimum cut point. Statistical significance was given a P value less than .05. All statistical analyses were performed using Stata version 15.0 software (StataCorp LP, Texas).
Results
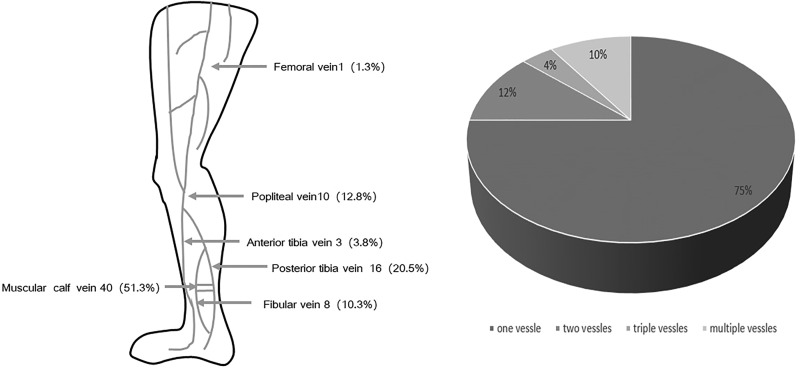
A total of 452 patients with acute stroke were enrolled in our study. Of them, there were 67 patients having hemorrhagic stroke and 385 patients with ischemic stroke. The average age was 63.8 (13.7) years old. Among all the patients, there were 69.2% men, 37.6% were able to mobile with a median National Institution of Health Stroke Scale (NIHSS) 3 (IQR: 2-9). All these patients were admitted within 14 days, mean as 3.1 (3.3) days. Their mean length of stay was 11.0 (6.7) days, 43% of them admitted into an intensive care unit (ICU) with a mean length of ICU stay as 2.0 (5.2) days. Altogether, asymptomatic DVT was detected in 52 (11.5%) patients with acute stroke and 85.9% of thrombi were identified in their distal veins. The most frequently involved vessels are muscular calf veins and posterior tibial vein. More than half of DVT (51.3%) located in muscular calf veins, which might indicate the malfunction of muscular pump (Figure 2). Seven patients were bilateral DVT and all of them are calf vein thrombosis.
Figure 2.
Distribution and probability of asymptomatic DVT in different low limb vessels. The most frequently involved vessels are muscular calf vein and posterior tibia vein, and 25% of patients with DVT have thrombus in more than 1 vessels. DVT indicates deep vein thrombosis.
Compared to patients without DVT, those with DVT had similar ages and onset to admission time. Among patients with DVT, their NIHSS score was higher than those without (median 11.50 [7.25-16.00] vs 3.00 [1.00-8.00], P < .001; Table 1). Patients with DVT stayed longer in hospital and have a higher risk of immobility, as was expected. In terms of stroke features, patients having hemorrhagic stroke were more likely to have a DVT (12/67 vs 35/385, 17.91% vs 5.11%; Table 1). In patients with ischemic stroke, their subtypes were less likely to be lacuna in Oxfordshire Community Stroke Project classification (1/52 vs 41/400, P < .001) and with higher rate of cardioembolism (atrial fibrillation 12/52 vs 26/400, P < .001). In laboratory results, fast blood glucose and level of D-dimer were higher in patients with DVT (Table 2). However, patients with higher level of triglyceride were less likely to have DVT. With each 1 mmol/L triglyceride level increased, there is a 54% (95% confidence interval [CI], 23%-73%, P = .003) relative risk reduction of DVT. Furthermore, we compared with and without asymptomatic DVT in patients with ischemic stroke, the findings were also similar to the general population (Supplementary Tables S1 and S2).
Table 1.
Baseline Characteristics of Patients.a
| DVT, n = 52 | No DVT, n = 400 | P Value | |
|---|---|---|---|
| Age, years, median (IQR) | 70.50 (61.00-78.50) | 64 (56-72) | .005 |
| Gender, males, n (%) | 30 (57.7) | 283 (70.8) | .055 |
| BMI, median (IQR) | 24.22 (21.45-26.33) | 24.22 (22.15-26.35) | .875 |
| Onset to admission, days, median (IQR) | 2.00 (1.00-4.00) | 2.00 (1.00-5.00) | .462 |
| LoS, days, median (IQR) | 14.50 (10.00-21.75) | 9.00 (7.00-12.00) | <.001 |
| LoS in the ICU, days, median (IQR) | 0.00 (0.00-9.00) | 0.00 (0.00-2.00) | .001 |
| NIHSS, median (IQR) | 11.50 (7.25-16.00) | 3.00 (1.00-8.00) | <.001 |
| Stroke subtype | <.001 | ||
| Intracranial hemorrhage, n (%) | 17 (32.7) | 50 (12.5) | |
| Ischemic stroke, n (%) | 35 (67.3) | 350 (87.5) | |
| Baseline characteristics | |||
| Systolic blood pressure, mm Hg, median (IQR) | 154 (136.5-165.75) | 151 (133-166) | .839 |
| Diastolic blood pressure, mm Hg, median (IQR) | 83 (75.25-90.00) | 82 (75-92) | .704 |
| Mobility | <.001 | ||
| Aid, n (%) | 42 (80.8) | 126 (31.5) | |
| Partly aid, n (%) | 9 (17.3) | 191 (47.8) | |
| No aid, n (%) | 1 (1.9) | 83 (20.8) | |
| OCSP | <.001 | ||
| Lacuna, n (%) | 1 (1.9) | 41 (10.3) | |
| Partial anterior circulation, n (%) | 30 (57.7) | 213 (53.3) | |
| Posterior circulation, n (%) | 10 (19.2) | 117 (29.3) | |
| Total anterior circulation, n (%) | 11 (21.2) | 29 (7.3) | |
| Risk factors | |||
| AF, n (%) | 12 (23.1) | 26 (6.5) | <.001 |
| Diabetes mellitus, n (%) | 11 (21.2) | 113 (28.3) | .281 |
| Hypertension, n (%) | 33 (63.5) | 269 (67.3) | .585 |
| Smoking, n (%) | 20 (38.5) | 189 (47.3) | .232 |
| Anticoagulants | 3 (5.8) | 16 (4.0) | .682 |
Abbreviations: AF, atrial fibrillation; BMI, body mass index; DVT, deep vein thrombosis; ICU, intensive care unit; IQR, interquartile range; LoS, length of stay; NIHSS, National Institute of Health Stroke Scale; OCSP, Oxfordshire Community Stroke Project.
a N = 452; we measured mobility if the patients could mobilize to the toilet with or without the help of another person.
Table 2.
Laboratory Predictors of Patients.a
| DVT, n = 52 | No DVT, n = 400 | P Value | |
|---|---|---|---|
| HCT, %, median (IQR) | 39.85 (35.25-43.98) | 41.10 (38.50-44.50) | .025 |
| BG, mmol/L, median (IQR) | 7.25 (5.63-9.08) | 5.80 (5.10-7.28) | <.001 |
| HbA1c,%, median (IQR) | 5.95 (5.63-6.88) | 6.00 (5.60-7.10) | .902 |
| D-Dimer, FEU mg/L, median (IQR) | 1.70 (0.25-6.12) | 0.35 (0.19-0.83) | <.001 |
| TC, mmol/L, median (IQR) | 4.48 (3.58-5.07) | 4.23 (3.49-4.91) | .178 |
| TAG, mmol/L, median (IQR) | 1.10 (0.76-1.44) | 1.35 (1.00-1.92) | .002 |
| HDL, mmol/L, median (IQR) | 1.14 (0.94-1.39) | 1.04 (0.86-1.25) | .049 |
| LDL, mmol/L, median (IQR) | 2.79 (2.04-3.53) | 2.53 (1.88-3.21) | .175 |
Abbreviations: BG, blood glucose; DVT, deep vein thrombosis; HbA1c, hemoglobin A1c; HCT, hematocrit; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; TAG, triglyceride; TC, total cholesterol.
a N = 452.
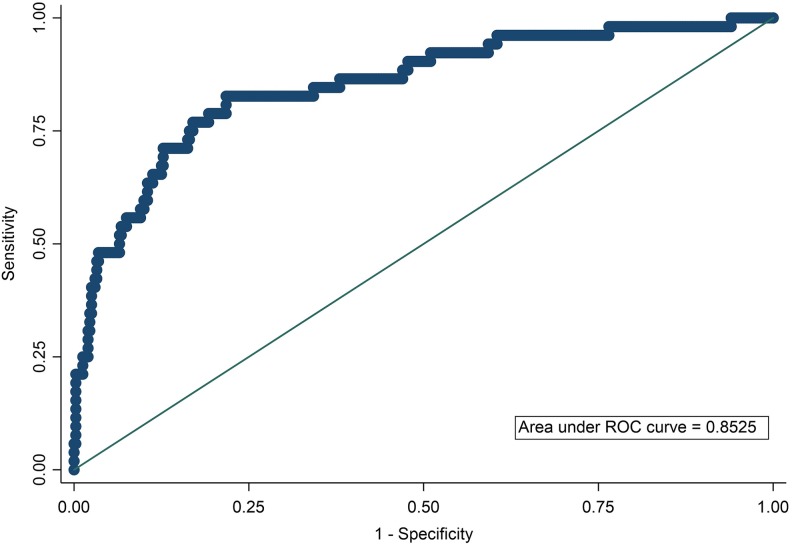
In multivariable regression model, patients with longer length of stay, more severe stroke, higher level of D-dimer, and higher blood glucose were associated with high risk of DVT, while higher triglyceride level was associated with less risk of DVT, after adjusting age and sex (Table 3). To build up a predictive model of asymptomatic DVT based on multivariable regression model, the ROC curve was performed (Figure 3). The corresponding AUC assessed was 0.85 with specificity and sensitivity. The quartile of estimated probabilities was measure by Hosmer-Lemeshow goodness of fitness (P = .76).
Table 3.
Adjusted Odds of Association of DVT.a
| Outcome | Adjusted OR (95% CI) | P Valueb |
|---|---|---|
| Age | 1.01 (0.98-1.03) | .686 |
| Gender | 1.32 (0.63-2.76) | .460 |
| LoS | 1.06 (1.02-1.11) | .004 |
| NIHSS | 1.10 (1.04-1.16) | .001 |
| Mobility | .866 | |
| Partly aid | 0.89 (0.40-1.95) | .77 |
| Aid | 1.14 (0.45-2.86) | .78 |
| OCSP | ||
| Partial anterior circulation | 8.48 (0.32-225.70) | .202 |
| Posterior circulation | 6.59 (0.23-189.04) | .271 |
| Total anterior circulation | 5.99 (0.19-184.52) | .306 |
| AF | 1.74 (0.62-4.88) | .289 |
| BG, mmol/L, median (IQR) | 1.22 (1.08-1.39) | .002 |
| D-Dimer, FEU mg/L, median(IQR) | 1.07 (1.02-1.12) | .003 |
| TAG, mmol/L, median (IQR) | 0.46 (0.27-0.77) | .003 |
Abbreviations: AF, atrial fibrillation; BG, blood glucose; CI, confidence interval; DVT, deep vein thrombosis; IQR, interquartile range; LoS, length of stay; NIHSS, National Institute of Health Stroke Scale; OCSP, Oxfordshire Community Stroke Project; OR, odds ratio; TAG, triglyceride.
a We measured mobile if the patients could mobilize to the toilet with or without the help of another person.
b Adjusted for age, LoS, NIHSS, mobile, OCSP, AF, BG, D-dimer, and TAG.
Figure 3.
The area under receiver operating characteristics (ROC). The area under ROC showed that the predictive model including age, gender, D-dimer, severity of stroke, glucose, and triglyceride had a high specificity and sensitivity to predict asymptomatic deep vein thrombosis in patients with acute stroke. The log D-dimer as D-dimer transformation was entered into this model. We also used National Institute of Stroke Scale to measure the severity of stroke.
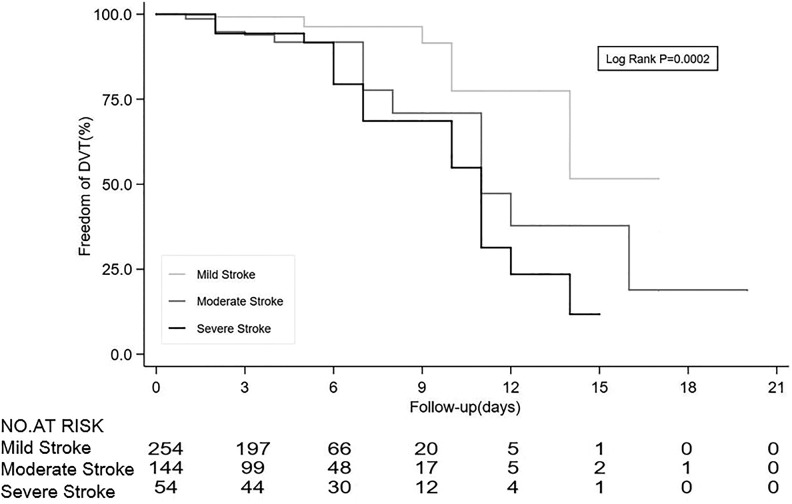
To stratified the risk factors, those with NIHSS 5 to 14 points had 2.5 times of risk (95% CI, 1.05-6.00, P = .039), and those with >14 points had 4.18 times of risk (95% CI, 1.53-11.39, P = .005), compared to those with NIHSS score 0 to 4. There was no significant interaction between the severity of stroke and the length of stay (0.99, 95% CI, 0.99-1.00, interaction P = .067). Therefore, the severity of stroke was independently associated with the risk of DVT, and each point of NIHSS increases 11% (95% CI, 5%-31%) relative risk of asymptomatic DVT. In order to further explore relationship between the severity stroke and DVT risk, Kaplan-Meier curve with risk table demonstrated that patients with mild stroke had the lower risk of asymptomatic DVT (Figure 4).
Figure 4.
Kaplan-Meier curves revealed that patients with mild stroke was associated with lower risk of asymptomatic DVT. Kaplan-Meier curve for time to DVT risk by severity of stroke was measured by the National Institution of Health Stroke Scale (NIHSS). Mild stroke was defined as NIHSS 0 to 4. Moderate stroke was defined as NIHSS 5 to 14. Severe stroke was defined as NIHSS >14. DVT indicates deep vein thrombosis.
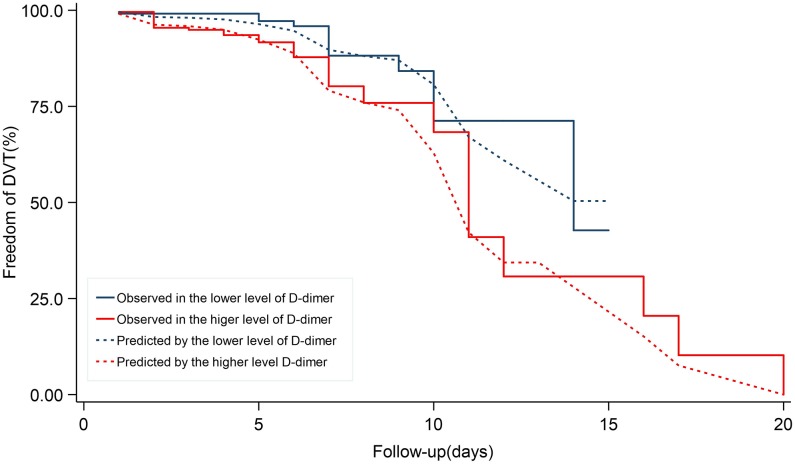
With the median of D-dimer (0.38 FEU mg/L) as cutoff value, patients with higher level of D-dimer might have a higher risk of DVT with a significant statistical difference. The cox-proportional hazard model showed that the higher level of D-dimer was related to the higher risk of DVT (Figure 5).
Figure 5.
Cox-proportional hazard model showed lower level of D-dimer was associated with an increasing mortality rate. Cox-proportional hazard model of DVT risk by levels of D-dimer. The cutoff of D-dimer was 0.38 FEU mg/L. DVT indicates deep vein thrombosis.
Discussion
In our study, asymptomatic DVT was found commonly (11.5%) in patients with acute stroke within 14 days after onset. More than half of DVTs located in distal deep vein. Patients with severe stroke, higher level of D-dimer, higher blood glucose, and longer length of stay were more likely to develop asymptomatic DVT. We performed a practical clinical tool to predict DVT in hospital stroke patients.
There are several studies focusing on patients with acute ischemic within 7 days.6,7 As was known, D-dimer was significantly high in patients with all types of thrombosis.8 Our result was consistent to that of previous studies as a biomarker of embolism, serum of D-dimer indicated higher risk of DVT in our study that has been also be found in previous studies.5,9,10 Moreover, we explored the cutoff value in asymptomatic patients and AUC showed it was with good predictable.
The association between the severity of stroke and DVT has been well established, which were consistent to our findings.7,9,11 Despite this, we confirmed as well that there was not a significant interaction between the severity of stroke and the length of stay on effects of DVT. It could also be caused by relatively minor symptoms in our patients, which brought the selection bias. Patients with severe stroke, higher level of D-dimer, higher blood glucose, and longer length of stay were more likely to develop asymptomatic DVT.
Immobility was known as one of major risk factors for DVT in previous studies,12,13 although the guidelines indicated the benefit of DVT prevention in patients with acute stroke was unclear.1 However, immobility was not associated with DVT occurrence in multivariable regression model in our study. We found that 10 patients, who were able to mobile, still had an asymptomatic DVT. Interestingly, we also found the level of triglyceride was associated with a protective effect of DVT occurrence. Although the level of triglyceride was not related to symptomatic DVT after knee surgery in previous study.14 The difference of these findings in triglyceride and mobile patients could be related to the potentially different mechanisms of etiology of thrombosis. The strategy of DVT prophylactic needs to be further explored in high-risk patients.
Body mass index (BMI) in those with DVT was higher than those without in participants from King’s College Hospital in the United Kingdom (28.6 [4.6] vs 26.1 [4.9], P = .05),5 while there was no significant difference in our study. Compared with the participants from the United Kingdom, participants from our study owns much lower BMI values (DVT: 24.22 [21.45-26.33] vs no DVT: 24.22 [22.15-26.35]). As it has come to be known, the triad consists of stasis, vessel damage, and hypercoagulability is used to describe the etiology and assess the risk of thrombosis, especially of DVT.15,16 Our logistic regression model showed patients with NIHSS score >5, D-dimer ≥0.38 FEU mg/L, higher glucose, and lower triglyceride were associated with higher risk of asymptomatic DVT.
In our predictive model, NIHSS was firstly been described. Compared to mild stroke, moderate stroke gave 80.8% sensitivity and 61% specificity, while severe stroke gave 34.62% sensitivity and 91.00% specificity. D-Dimer, at cut point of 0.38 FEU mg/L, gave 73.1% sensitivity and 53.1% specificity.
Our study showed that asymptomatic DVT was found commonly in patients with acute stroke within 14 days after onset. Patients with severe stroke, higher level of D-dimer, higher blood glucose, and longer length of stay were more likely to develop asymptomatic DVT. A practical clinical tool to predict DVT in in-house patients with stroke might be helpful to identify high-risk population, which needs to further validation.
Supplemental Material
Supplementary_Table_1 for Clinical Risk Factors of Asymptomatic Deep Venous Thrombosis in Patients With Acute Stroke by Yi Wang, Yu Shi, Yi Dong, Qiang Dong, Ting Ye and Kun Fang in Clinical and Applied Thrombosis/Hemostasis
Supplemental Material
Supplementary_Table2 for Clinical Risk Factors of Asymptomatic Deep Venous Thrombosis in Patients With Acute Stroke by Yi Wang, Yu Shi, Yi Dong, Qiang Dong, Ting Ye and Kun Fang in Clinical and Applied Thrombosis/Hemostasis
Footnotes
Authors’ Note: Yi Wang and Yu Shi contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Nursing Foundation from 2018 Shanghai Nursing Association Excellent Young Talents Breeding Program (No. 20160001). The National Natural Science Foundation of China (81870915), Grant from the Science and Technology Commission of Shanghai Municipality (2017ZZ01014) and Shanghai Municipal Commission of Health and Family Planning, Key developing disciplines (2015ZB0301).
ORCID iD: Ting Ye  https://orcid.org/0000-0001-7979-8470
https://orcid.org/0000-0001-7979-8470
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Correction to: 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(6):e233–e234. [DOI] [PubMed] [Google Scholar]
- 2. Sandercock PA, Counsell C, Kane EJ. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev. 2015;(3):CD000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dennis M, Caso V, Kappelle LJ, Pavlovic A, Sandercock P; European Stroke Organisation. Stroke organisation (ESO) guidelines for prophylaxis for venous thromboembolism in immobile patients with acute ischaemic stroke. Eur Stroke J. 2016;1(1):6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whiteley WN, Adams HP, Jr, Bath PM. et al. Targeted use of heparin, heparinoids, or low-molecular-weight heparin to improve outcome after acute ischaemic stroke: an individual patient data meta-analysis of randomised controlled trials. Lancet Neurol. 2013;12(6):539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balogun IO, Roberts LN, Patel R, Pathansali R, Kalra L, Arya R. Clinical and laboratory predictors of deep vein thrombosis after acute stroke. Thromb Res. 2016;142:33–39. [DOI] [PubMed] [Google Scholar]
- 6. Bembenek JP, Karlinski M, Kobayashi A, Czlonkowska A. Deep venous thrombosis in acute stroke patients. Clin Appl Thromb Hemost. 2012;18(3):258–264. [DOI] [PubMed] [Google Scholar]
- 7. Bembenek J, Karlinski M, Kobayashi A, Czlonkowska A. Early stroke-related deep venous thrombosis: risk factors and influence on outcome. J Thromb Thrombolysis. 2011;32(1):96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wada H, Kobayashi T, Abe Y. et al. Elevated levels of soluble fibrin or D-dimer indicate high risk of thrombosis. J Thromb Haemost. 2006;4(6):1253–1258. [DOI] [PubMed] [Google Scholar]
- 9. Kong XL, Zhang X, Zhang SJ, Zhang L. Plasma level of D-dimer is an independent diagnostic biomarker for deep venous thrombosis in patients with ischemic stroke. Curr Neurovasc Res. 2016;13(2):100–106. [DOI] [PubMed] [Google Scholar]
- 10. McLendon K, Attia M. Deep Venous Thrombosis (DVT), Risk Factors. Treasure Island, FL: StatPearls; 2019. [PubMed] [Google Scholar]
- 11. Li Z, Liu L, Wang Y. et al. Factors impact the adherence rate of prophylaxis for deep venous thrombosis in acute ischaemic stroke patients: an analysis of the China National Stroke Registry. Neurol Res. 2015;37(5):427–433. [DOI] [PubMed] [Google Scholar]
- 12. Rolston JD, Han SJ, Bloch O, Parsa AT. What clinical factors predict the incidence of deep venous thrombosis and pulmonary embolism in neurosurgical patients? J Neurosurg. 2014;121(4):908–918. [DOI] [PubMed] [Google Scholar]
- 13. Vine HS, Hillman B, Hessel SJ. Deep venous thrombosis: predictive value of signs and symptoms. AJR Am J Roentgenol. 1981;136(1): 167–171. [DOI] [PubMed] [Google Scholar]
- 14. Kang J, Jiang X, Wu B. Analysis of risk factors for lower-limb deep venous thrombosis in old patients after knee arthroplasty. Chin Med J (Engl). 2015;128(10):1358–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kumar DR, Hanlin E, Glurich I, Mazza JJ, Yale SH. Virchow’s contribution to the understanding of thrombosis and cellular biology. Clin Med Res. 2010;8(3-4):168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Malone PC, Agutter PS. The aetiology of deep venous thrombosis. QJM. 2006;99(9):581–593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_Table_1 for Clinical Risk Factors of Asymptomatic Deep Venous Thrombosis in Patients With Acute Stroke by Yi Wang, Yu Shi, Yi Dong, Qiang Dong, Ting Ye and Kun Fang in Clinical and Applied Thrombosis/Hemostasis
Supplementary_Table2 for Clinical Risk Factors of Asymptomatic Deep Venous Thrombosis in Patients With Acute Stroke by Yi Wang, Yu Shi, Yi Dong, Qiang Dong, Ting Ye and Kun Fang in Clinical and Applied Thrombosis/Hemostasis