Napoli and Flores preview work from the Jackson laboratory showing that chemotherapy triggers macrophage-like features in tumor cells, which phagocyte other cells to outlast dormancy.
Abstract
Chemotherapy-resistant tumor cells are responsible for poor patient outcome. In this issue, Tonnessen-Murray et al. (2019. J. Cell Biol. https://doi.org/10.1083/jcb.201904051) elegantly show that chemotherapy triggers macrophage-like features in surviving cancer cells, which in turn phagocyte normal and tumor cells alike to outlast dormancy and cause relapse.
The most challenging hurdle faced by cancer therapy is the onset of resistance, which ultimately leads to the patient’s demise. Regardless of the minimal residual disease, chemotherapy-resistant cancer cells can persist up to decades in a state defined as tumor cell dormancy before driving relapse (1). Notably, this quiescent state both facilitates immune evasion, which is one of the most recently recognized hallmarks of cancer cells (2), and lets cancer cells acquire novel mutations necessary for the progression of the disease (3). Accordingly, metastases are believed to derive from disseminated tumor cells that underwent a dormant phase while adapting to the new microenvironmental cues through the accumulation of genetic and epigenetic changes (4). Indeed, in contrast to irreversible cell cycle arrest, the dormant state is temporary and cancer cell proliferation can be reactivated in response to mitogens and other signaling factors present in the microenvironment of the host organ. Although the molecular mechanisms initiating and maintaining cancer cell dormancy are still largely uncharacterized and vary based on the considered tumor type (1), a ubiquitous feature seems to be the increased activity of p38 MAPK over that of ERK1/2, such that the p38 MAPKhigh/ERKlow phenotype is a well-established marker to define the dormant state (5). Another important characteristic of dormant tumor cells that has recently emerged is the up-regulation of p53. This event may appear counterintuitive, since the activation of a tumor suppressor rarely correlates with cancer cell survival and tumor relapse. However, an ever-increasing body of evidence derived from breast cancer patients and murine models shows that breast cancers retaining wild-type p53 are seldomly cured by chemotherapeutic treatments (6, 7). A landmark paper explained this incongruence by demonstrating that mouse adenocarcinomas that either lost or mutated the TP53 gene could not sustain the additional mutations caused by the treatment and regressed due to massive apoptosis (7). Instead, tumors with wild-type p53 try to cope with the increased mutational burden by activating cellular senescence and DNA repair mechanisms (7). It is when these safeguards fail that cancer cells exit dormancy and relapse will eventually occur. What remains unsolved is how these chemotherapy-induced senescent cells (CISCs) manage to survive during dormancy. In this issue, Tonnessen-Murray et al. (8) provide some answers by analyzing the interplay between CISCs and their neighboring cells.
The researchers first labeled p53 wild-type MMTV-Wnt1 breast cancer cells with either a nuclear GFP or a plasma membrane–bound mCherry. These cells were then mixed and transplanted orthotopically to produce mammary adenocarcinomas, which were subsequently treated with doxorubicin to induce the formation of CISCs. By performing confocal microscopy on cross sections of these tumors during regression, the authors made an unexpected discovery: GFP-positive CISCs contained large vesicles surrounding mCherry-positive tumor cells and vice versa. This crucial observation of CISCs engulfing neighboring cells was corroborated by an exhaustive series of in vitro experiments using time-lapse and confocal microscopy showing that both normal and tumor cells are entirely subsumed by CISCs. It is worth noting that this cannibalistic behavior could be triggered by different types of DNA-damaging drugs and, more importantly, by nutlin, a compound that activates p53 without causing genotoxic stress. Although the authors noted this phenotype to be present also in normal cells and in cancer cells expressing mutant p53, thus indicating that it is a property shared by all the senescent cells that survive doxorubicin treatment, the extent of the effect was exacerbated in CISCs derived from cancer cells expressing wild-type p53. These data hence demonstrate a role for p53 in the chemotherapy-induced cell engulfment.
To dissect the molecular mechanisms underlying the observed phenotype, Tonnessen-Murray et al. (8) performed an RNA-sequencing analysis in doxorubicin-treated cancer cells collected at two different time points: before (24 h) and after the CISC-related cell engulfment reaches its peak (8 d). Intriguingly, with respect to untreated cells, the gene set enrichment analysis unveiled the induction of several pathways, including cell cycle arrest, lysosomal signature, and phagocytosis, thus suggesting that cells surviving doxorubicin treatment activate a macrophage-like transcriptional program that allows CISCs to surround neighboring cells, engulf them, and digest them via the lysosomes. In line with this, the authors showed that the use of the lysosomal inhibitor chloroquine increases the number of engulfed cells since it prevents their degradation.
The data described by Tonnessen-Murray et al. (8) provide the long-awaited answer to the metabolic source allowing senescent cancer cells to survive dormancy even for a long period of time (Fig. 1). It would be interesting to verify whether the mechanism of cell engulfment by CISCs can be therapeutically exploited to prevent relapse and prolong patient survival. Indeed, being characterized by cell cycle arrest, the quiescent state makes cancer cells insensitive to commonly used chemotherapeutics, which specifically target highly proliferative cells. Drugs designed against the CISC-related cell engulfment, instead, would provide the perfect strategy to eliminate dormant cancer cells that are responsible for tumor relapse and the onset of metastases. Based on the findings reported by Tonnessen-Murray et al. (8), it is conceivable that such a strategy would be more effective in tumors harboring wild-type p53 than in those expressing mutant p53. It remains to be determined whether the latter case may benefit from a concurrent treatment aiming either to reactivate p53’s tumor suppressive functions or to induce the other proapoptotic members of the p53 family, TAp63 and TAp73. Since mutations in the TP53 gene can be detected in almost half of all human tumors (9), addressing this issue would extend the impact of therapies targeting cell engulfment by CISCs.
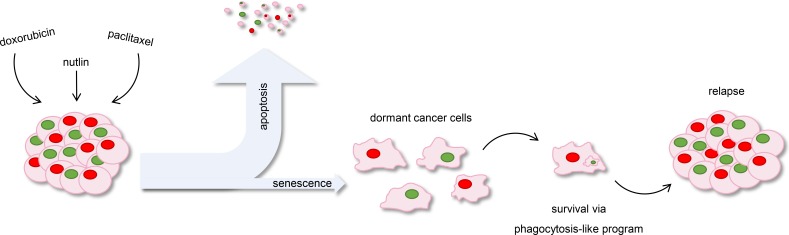
Figure 1.
Chemotherapy-induced senescence leads to cancer cell survival and relapse. Chemotherapeutic drugs and p53-activating compounds cause tumor regression by inducing apoptosis in most cancer cells. A subset of cancer cells, however, is resistant to the treatment and undergoes senescence. This quiescence state endows cancer cells with a macrophage-like program that allows them to engulf neighboring cells and to persist over time until they ultimately drive relapse.
Acknowledgments
E.R. Flores is a National Cancer Institute Outstanding Investigator (R35CA197452), Moffitt Distinguished Scholar, and Scholar of the Leukemia and Lymphoma Society, the Rita Allen Foundation, and the V Foundation for Cancer Research. M. Napoli is a Scholar of the Cancer Prevention Research Institute of Texas-Translational Research in Multidisciplinary Program.
The authors declare no competing financial interests.
References
- 1.Gomis R.R., and Gawrzak S. Mol. Oncol. 2017 doi: 10.1016/j.molonc.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D., and Weinberg R.A. Cell. 2011 doi: 10.1016/j.cell.2011.02.013. [DOI] [Google Scholar]
- 3.Giancotti F.G. Cell. 2013 doi: 10.1016/j.cell.2013.10.029. [DOI] [Google Scholar]
- 4.Sosa M.S., et al. Nat. Rev. Cancer. 2014 doi: 10.1038/nrc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aguirre-Ghiso J.A., et al. 2003. Cancer Res. 63:1684–1695. [PubMed] [Google Scholar]
- 6.Bertheau P., et al. Lancet. 2002 doi: 10.1016/S0140-6736(02)09969-5. [DOI] [Google Scholar]
- 7.Jackson J.G., et al. Cancer Cell. 2012 doi: 10.1016/j.ccr.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tonnessen-Murray C.A., et al. J. Cell Biol. 2019 doi: 10.1083/jcb.201904051. [DOI] [Google Scholar]
- 9.Kandoth C., et al. 2013. Nature. 502:333–339. 10.1038/nature12634 [DOI] [PMC free article] [PubMed] [Google Scholar]