Summary
Uterine endometrial cancer is associated with poor survival outcomes in patients with advanced-stage disease. Here, we developed a three-dimensional cell cultivation method of endometrioid cancer stem-like cells with high aldehyde dehydrogenase (ALDH) activity from clinical specimens. ALDH inhibition synergized with paclitaxel to block cancer proliferation. In the clinical setting, high ALDH1A1 expression was associated with poor survival. A high level of ALDH correlated with an increase of glucose uptake, activation of the glycolytic pathway, and elevation of glucose transporter 1 (GLUT1). Blockade of GLUT1 inhibited characteristics of cancer stem cells. Similarly to ALDH inhibition, GLUT1 inhibition synergized with paclitaxel to block endometrial cancer proliferation. Our data indicated that ALDH-dependent GLUT1 activation and the resulting glycolytic activation are of clinical importance for both prognostic evaluation and therapeutic decision-making in endometrial cancer patients. In addition, the synergistic effects of taxane compounds and ALDH or GLUT1 inhibitors may serve as a new clinical treatment option for endometrial cancer.
Keywords: endometrial cancer, cancer stem cells, in vitro cultivation, patient-derived xenograft tumor, aldehyde dehydrogenase, paclitaxel, drug resistance, glycolysis, glucose transporter, GLUT1
Graphical Abstract
Highlights
-
•
Establishment of patient-derived endometrial cancer stem cells with ALDH activity
-
•
Endometrial cancer stemness depends on ALDH-mediated glycolysis via GLUT1
-
•
High ALDH and GLUT expression is associated with poor outcome in endometrial cancer
-
•
Paclitaxel and ALDH or GLUT inhibitor synergistically suppress endometrial cancer
In this article, Ishiguro and colleagues demonstrated the stable in vitro cultivation of human endometrial cancer stem cells from clinical specimens. Investigation of patient-derived spheroid cells showed that ALDH-mediated glycolysis through GLUT1 was essential for endometrial cancer stem cells. ALDH and GLUT are novel therapeutic targets in endometrial cancer to complement standard taxane treatment.
Introduction
Uterine endometrial cancer is one of the most common gynecological malignancies (Morice et al., 2016). Despite macroscopic complete surgical resection of the cancerous tumor plus adjuvant chemotherapy, high-grade endometrial cancer cells tend to form recurrent metastatic tumors (Nomura et al., 2011, Siegel et al., 2015). Moreover, the 5-year overall survival rate for advanced-stage cancer with distance metastasis is no more than ∼25% (Aoki, 2014). Currently combination drug therapies with taxanes (paclitaxel or docetaxel) and platinum analogs (carboplatin or cisplatin) are used as a first-line chemotherapy for endometrial cancer (Bestvina and Fleming, 2016, Nomura et al., 2011); however, the appropriate chemotherapy regimen for high-risk disease is still controversial (de Boer et al., 2018, Morice et al., 2016). Thus, establishing a better chemotherapeutic strategy is essential for the treatment of advanced endometrial cancer.
Cancer stem cells (CSCs) are a small fraction of cancer cells with central roles in cancer propagation and proliferation among heterogeneous tumors (Lytle et al., 2018) and are thought to contribute to metastatic spread and resistance to chemotherapy and radiotherapy. Previous research has shown that a small population of freshly isolated cells from clinical endometrial cancer tissues has the capacity for clonogenicity in vitro and tumorigenicity in vivo (Hubbard et al., 2009), and that transiently cultured endometrial cells are resistant to cisplatin- and paclitaxel-induced cytotoxicity (Rutella et al., 2009), suggesting the presence of CSC-like cells in endometrial cancers. However, the detailed biology of endometrial CSCs in clinical specimens has not been elucidated, potentially as a result of difficulties in the stable in vitro cultivation of endometrial CSCs isolated from clinical tumors.
Cells with characteristics of CSCs can be expanded in vitro under floating conditions in a unique three-dimensional format called tumor-derived spheroids or tumor spheres (Pastrana et al., 2011, Valent et al., 2012); the spheroid cultivation system may facilitate identification of the biological characteristics of CSCs. Hence, this cultivation method has been established in several types of malignant tumors (Dontu et al., 2003, Lonardo et al., 2011, Ricci-Vitiani et al., 2007, Singh et al., 2003). Previously, we generated stable cancer spheroid cells with CSC characteristics from clinical colorectal and ovarian cancer specimens (Ohata et al., 2012, Ishiguro et al., 2016).
In this study, we aimed to develop a stable culture method for CSC spheroids from clinical endometrial cancer specimens. Our results demonstrated that aldehyde dehydrogenase (ALDH), via enhanced glycolysis through glucose transporter 1 (GLUT1) upregulation, plays an important role in the maintenance of endometrial CSCs. Further investigation revealed the synergistic effects of inhibition of ALDH activity or GLUT1 with taxane treatment on cell proliferation in vitro and tumorigenesis in vivo.
Results
Human Uterine Endometrial Cancer Spheroid Cells Exhibit CSC-like Characteristics
To develop new therapeutic strategies for refractory endometrial cancer, in vitro three-dimensional culture systems from human clinical specimens may provide a useful platform. Hence, we attempted to establish a cultivation method for spheroid cells from human uterine endometrial cancer tumors. Spheroid cells from 8 of 19 high-grade carcinoma samples (42%), and one case of grade 2 endometrioid carcinoma could be expanded under the spheroid culture conditions (Table S1; Figure 1A). Interestingly, the established endometrial cancer spheroid cells were capable of proliferating under floating conditions in the absence of ROCK inhibitors, which were required for proliferation and maintenance of ovarian and colorectal cancer spheroid cells (Ishiguro et al., 2016, Ohata et al., 2012).
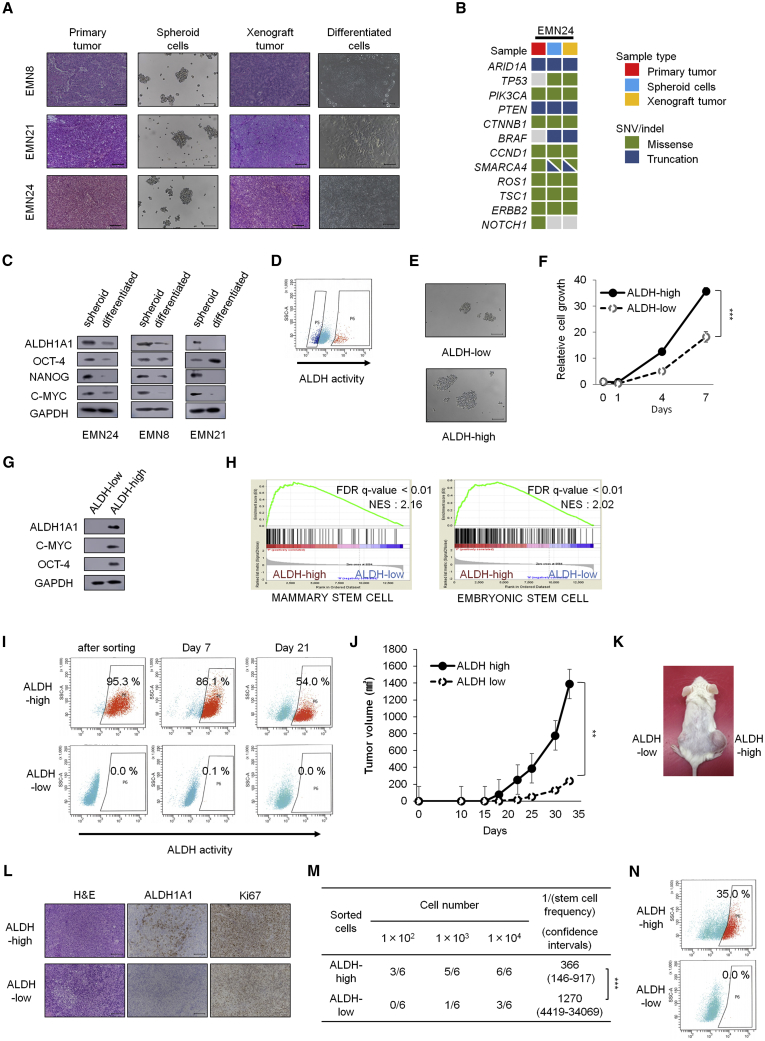
Figure 1.
Spheroid Cells Derived from Human Endometrial Cancer with High ALDH Activity Shows CSC Characteristics and Genetic Background Similar to that of the Primary Tumor
(A) H&E staining of the primary tumor (left), bright-phase image of the indicated spheroids (center left), H&E staining of xenograft tumors (center right), bright-phase image of cells grown under differentiation conditions (right). Scale bars, 100 μm.
(B) Targeted sequencing analyses of the primary tumor, spheroid cells, and spheroid-derived xenograft tumors.
(C) Western blot analyses of the spheroid cells and differentiated cells shown in (A).
(D) FACS analyses of ALDH activity after ALDEFLUOR staining. Left gated population, ALDH-low cells; right gated population, ALDH-high cells.
(E) Bright-phase images of spheroid formation (7 days after in vitro cultivation). Scale bars, 100 μm.
(F) Time course analyses of cell growth in ALDH-high and ALDH-low cells after sorting. n = 4 independent experiments, p < 0.001, Student's t tests.
(G) Western blot analyses of ALDH-high and ALDH-low cells after sorting.
(H) Gene set enrichment analyses of gene expression profiles between ALDH-high and ALDH-low cells.
(I) Time course of ALDH activity in vitro after ALDEFLUOR sorting.
(J) Volume (mean ± SEM) of xenograft tumors from 1 × 105 ALDH-low and ALDH-high cells. n = 5 independent experiments, p = 0.002, Student's t tests.
(K) Image of mice on day 33. ALDH-low cells were injected on the left side, and ALDH-high cells were injected on the right side.
(L) H&E staining and immunostaining of xenograft tumors derived from ALDH-high and ALDH-low cells, 33 days after transplantation. Scale bars, 100 μm.
(M) Limiting dilution analysis of ALDH-high cells and ALDH-low cells in vivo on day 23 after subcutaneous injection. p < 0.01.
(N) ALDH activity after ALDEFLUOR staining in cancer cells from ALDH-high and ALDH-low xenograft tumors. Experiments in (D to G) and (I to N) were performed with EMN24 cells.
∗∗ p < 0.01; ∗∗∗p < 0.001. See also Figure S1.
To evaluate the tumorigenicity of these spheroid cells, cells were injected into immunodeficient NOG mice. The generated xenograft tumors were histologically similar to the original human endometrial tumors (Figures 1A and S1A). Immunostaining patterns for PAX8, cytokeratin 7, and p53 were similar between primary and xenograft tumors (Figure S1B). To determine whether spheroid cells and primary tumors were genetically identical, targeted sequencing analysis was performed for 114 cancer-related genes (Table S2). Overall the mutational profile of spheroid cells was identical to that of the original tumor except some mutations observed only in spheroids (Figures 1B and S1C). The mutation profiles of spheroids and xenograft are identical, indicating that xenograft formation did not significantly promote additional oncogenic mutations. Most shared mutations were previously reported as common genomic alterations in endometrial cancer tissues, i.e., PTEN, PIK3CA, ARID1A, and TP53 (The Cancer Genome Atlas Network, 2013, Soumerai et al., 2018).
ALDH Activity Is Related to the CSC Characteristics of Endometrial Cancer Spheroid Cells
Next, we examined the CSC characteristics of these established spheroids. Expression levels of stemness-related markers, including Nanog, c-myc, and ALDH1A1, were higher in the spheroid cells than in differentiated cells (Figures 1A and 1C). In accordance with elevated levels of ALDH1A1, fluorescence-assisted cell sorting (FACS)-sorted spheroid cells with high ALDH activity (ALDH-high cells) could form and expand spheroids more rapidly in vitro than cells with no or low ALDH activity (ALDH-low cells; Figures 1D–1F and S1D–S1F). Western blot analysis showed that ALDH-high cells expressed higher levels of the stemness markers Oct-4, c-myc, and ALDH1A1 than ALDH-low cells (Figures 1G and S1G). Moreover, gene set enrichment analysis (GSEA) demonstrated that ALDH-high cells preferentially expressed stem cell-related genes found in breast cancer (false discovery rate [FDR] q value < 0.01, normalized enrichment score [NES] 2.16) (Pece et al., 2010) and embryonic stem cells (ESCs) (FDR q value < 0.01, NES 2.02) (Wong et al., 2008) (Figure 1H), which may reflect the activation of an ESC-like transcriptional program in endometrial cancer. Induction of stem cell-related genes was previously reported in other types of human cancers, including glioblastoma (grade 4), breast cancer, and lung cancer (Ben-Porath et al., 2008, Wong et al., 2008). Although ALDH-high cells generated both ALDH-high and ALDH-low cells, ALDH-low cells propagated more slowly than ALDH-high cells and did not give rise to ALDH-high cells during in vitro cultivation (Figure 1I). These results indicated that ALDH-high cells had the CSC ability to self-renew and to differentiate into ALDH-low cells. In contrast, high expression levels of CD44 or CD133 were not associated with the capability to form spheres in vitro (Figures S1H–S1K).
Next, we explored the tumorigenic ability of ALDH-high cells in vivo. The FACS-sorted ALDH-high cells generated xenograft tumors more aggressively than ALDH-low cells (Figures 1J–1L and S1L). In vivo serial dilution spheroid cell assays showed that the tumorigenic ability of ALDH-high cells was higher than that of ALDH-low cells (p < 0.0001; Figure 1M). ALDH activity assays and immunohistochemical analyses showed that the xenograft tumors from ALDH-high cells contained both ALDH-high cells and ALDH-low cells, whereas tumors from ALDH-low cells contained only ALDH-low cells (Figures 1L and 1N); these in vivo results were consistent with the in vitro results (Figure 1I). Immunohistochemical analyses also showed that xenograft tumors from ALDH-high cells contained more Ki67-positive cells than tumors from ALDH-low cells (Figure 1L). These results indicated that ALDH-low cells were derived from CSC-like ALDH-high cells.
Inhibition of ALDH Activity Reduces the Propagation of Endometrial Cancer Spheroid Cells
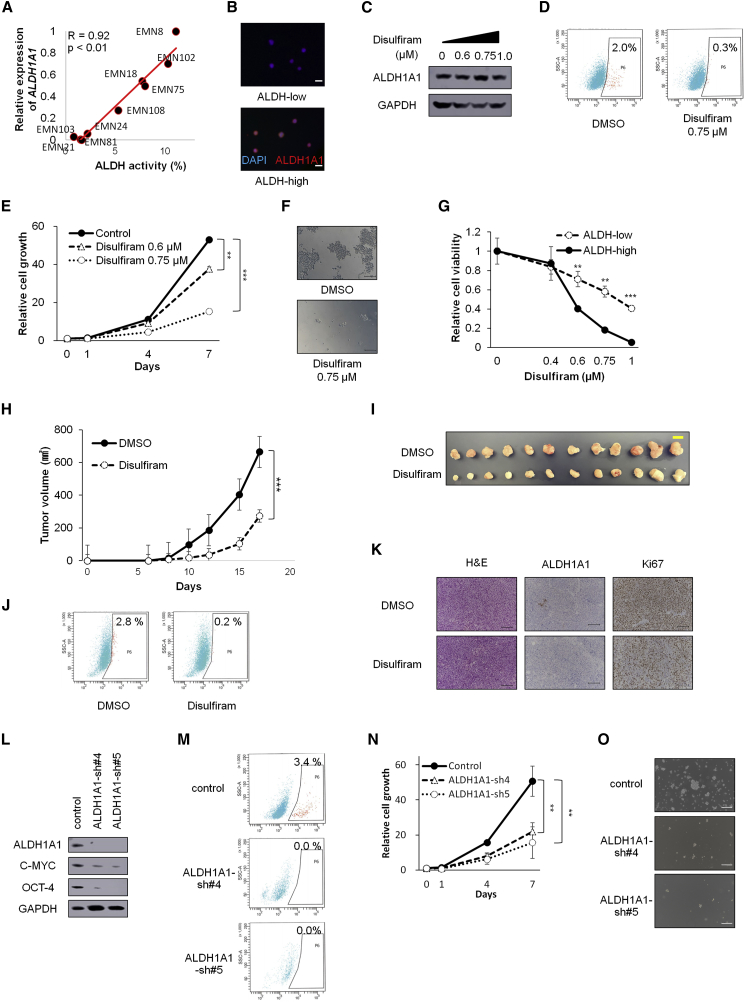
Next, we attempted to determine whether ALDH1A1 or other isoforms of ALDH were responsible for ALDH activity in the endometrial cancer spheroids. The ALDH family is composed of at least 19 functional isoforms with similar catalytic functions (Tomita et al., 2016). Although each of the established spheroid cells had different levels of ALDH activities, qRT-PCR analyses showed that only ALDH1A1 mRNA expression was clearly correlated with ALDH activity (Spearman R value = 0.92, p < 0.01; Figures 2A and S2A). The association between ALDH1A1 expression and ALDH activity was confirmed by western blotting (Figure S2B) and by immunocytochemical analyses, which showed that most ALDH-high cells expressed detectable ALDH1A1 (Figure 2B), although some spheroid cells also expressed higher levels of other ALDH isoforms (Figure S2B).
Figure 2.
Inhibition of ALDH Activity Blocks the Formation and Proliferation of Spheroid Cells (EMN24 cells)
(A) Correlation between ALDH activity and ALDH1A1 mRNA levels on culture day 10.
(B) Immunofluorescence staining with anti-ALDH1A1 antibody and DAPI in ALDH-high and ALDH-low cells after sorting. Scale bars, 20 μm.
(C) Western blot analyses of spheroids cells after disulfiram treatment for 24 h.
(D) FACS analyses of ALDH activity in the presence or absence of disulfiram (24 h after treatment).
(E) Time course of cell growth in spheroid cells treated with the indicated amounts of disulfiram. n = 4 independent experiments, p < 0.001, Student's t test.
(F) Bright-phase images of spheroids (7 days after disulfiram treatment). Scale bars, 100 μm.
(G) Responses of ALDH-high and ALDH-low cells to different concentrations of disulfiram after treatment for 7 days. n = 4 independent experiments, p < 0.001, Student's t test.
(H) Tumor volumes (mean ± SEM) of xenograft tumors from 1 × 105 spheroid cells subcutaneously injected. Disulfiram (40 mg/kg) was intraperitoneally injected into mice in the disulfiram-treatment group, and vehicle (DMSO) was intraperitoneally injected into mice in the control group. n = 12 independent experiments, p < 0.001, Student's t test.
(I) Images of whole resected tumor xenografts excised on day 17. Scale bar, 10 mm.
(J) ALDH activity of the cancer cells derived from xenograft tumors.
(K) H&E staining and immunostaining of xenograft tumors, 17 days after transplantation. Scale bars, 100 μm.
(L) Western blot analyses after infection with the indicated lentiviruses.
(M) ALDH activity in the infected cells after ALDEFLUOR staining.
(N) Time course of proliferation of infected cells. n = 4 independent experiments, p < 0.01, Student's t test.
(O) Bright-phase images of the infected cells on day 7. Scale bars, 100 μm.
∗∗p < 0.01; ∗∗∗p < 0.001. See also Figure S2.
Consistent with ALDH1A1 expression in spheroids (Figures 2A and S2B), xenograft tumors derived from EMN8 and EMN102 cells showed strong staining of ALDH1A1, whereas those from EMN103 cells showed weak expression of ALDH1A1 (Figure S2C).
We then examined differences in function after treatment with the pan-ALDH-specific inhibitor disulfiram. Although exposure to disulfiram did not significantly change ALDH1A1 expression (EMN24 cells, Figure 2C), disulfiram treatment suppressed ALDH activity (Figure 2D) and spheroid propagation (Figures 2E and 2F). Other spheroid cells (i.e., EMN81 cells) were more sensitive to disulfiram than EMN24 spheroid cells (Figures S2D–S2F), and the surviving cells retained reduced levels of ALDH1A1 expression after treatment (Figure S2G). ALDH-high cells were more sensitive to disulfiram than ALDH-low cells (Figures 2G and S2H).
To further confirm the inhibitory effects of ALDH inhibition, we used three other ALDH inhibitors, diethylaminobenzaldehyde (DEAB), CM037, and NCT501 (a selective inhibitor of ALDH1A1). Again, these ALDH inhibitors caused inhibition of ALDH activity (Figure S2I), reduction of spheroid cells (Figure S2J), and preferential cell death in ALDH-high cells (Figures S2K and S2L). Collectively, these data indicated that ALDH inhibition blocked spheroid cell propagation and spheroid formation via preferential targeting of ALDH-high cells.
Based on the effects of ALDH inhibitors on in vitro spheroid cells, we attempted to examine the effects of ALDH inhibitor treatment on tumor formation in vivo. Because disulfiram has been used in the preclinical and clinical settings (Ishiguro et al., 2016, Nechushtan et al., 2015, Safi et al., 2014, Xu et al., 2017, Yip et al., 2011), we chose disulfiram to investigate the inhibitory effects in vivo. Disulfiram treatment suppressed tumorigenesis in spheroid cells in vivo (Figures 2H and 2I), markedly reduced a fraction of ALDH-high cells in the tumor (Figure 2J), and caused decreased levels of Ki-67 staining, not ALDH1A1 staining (Figure 2K). Thus, inhibition of ALDH activity by disulfiram treatment inhibited spheroid-derived tumor growth in vivo.
Next, we examined the functional effects of ALDH1A1 on cancer stemness, proliferation, and ALDH activity. Inhibition of ALDH1A1 expression by shRNA lentiviral transfer caused reduction of the stemness-related markers c-myc and Oct4 (Figures 2L and S2M) and ALDH activity (Figures 2M and S2N). In addition, ALDH1A1 inhibition blocked spheroid formation and propagation in vitro (Figures 2N, 2O, S2O, and S2P).
Exogenous ALDH Expression Enhances the Stemness Features of Endometrial Cancer
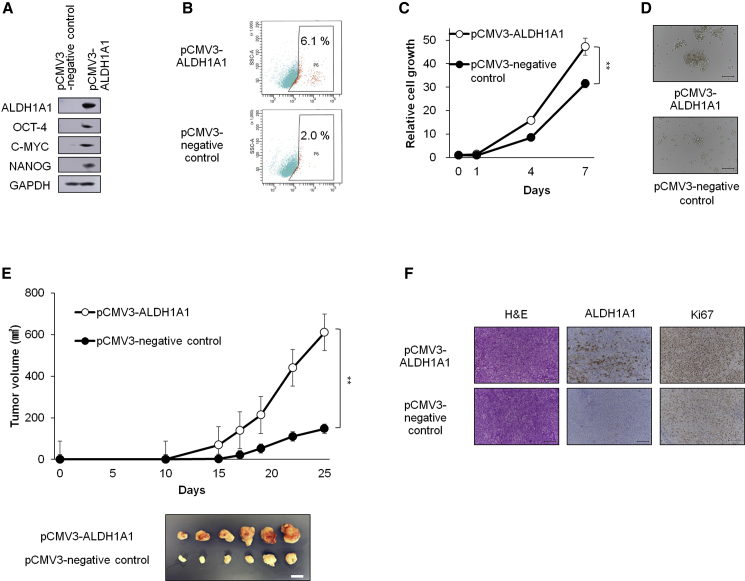
We next examined whether exogenous ALDH1A1 overexpression enhanced the proliferation of endometrial cancer spheroid cells. Introduction of ALDH1A1 via lentivirus-mediated gene transfer into EMN24 cells augmented ALDH activity and expression of stemness-related markers (Figures 3A and 3B). Although ALDH1A1 expression caused a modest increase in the proliferation rate of the spheroids (Figures 3C and 3D), ALDH1A1 expression also resulted in the formation of the transplanted tumors that were four times larger than those formed after transplantation of control cells (Figure 3E). The xenograft tumors generated from the ALDH1A1-introduced spheroid cells contained more Ki67-positive cells than the control of xenograft tumors (Figure 3F). EMN21 cells, which showed the lowest ALDH activity among the established endometrial spheroid cells (Figure 2A), also propagated rapidly in vitro after the introduction of exogenous ALDH1A1 (Figures S2Q–S2T). Collectively, our data indicated that ALDH activity, which was mainly attributed to ALDH1A1 expression, played an essential role in the survival and propagation of endometrial CSCs.
Figure 3.
Exogenous ALDH1A1 Enhances the Formation and Proliferation of Spheroids and Tumorigenicity (EMN24 Cells)
(A) Western blot analyses of spheroid cells after infection with the indicated lentiviruses.
(B) ALDH activity in the infected cells after ALDEFLUOR staining.
(C) Time course of proliferation of infected cells. n = 4 independent experiments, p < 0.01, Student's t test.
(D) Bright-phase images of the infected cells. Scale bars, 100 μm.
(E) Volumes (mean ± SEM) of xenograft tumors from 1 × 105 infected cells. n = 6 independent experiments, p < 0.01, Student's t tests. Images of whole resected tumor xenograft tumors excised on day 25 (bottom). Scale bar, 10 mm.
(F) H&E staining and immunostaining of xenograft tumors, 25 days after transplantation. Scale bars, 100 μm.
∗∗p < 0.01. See also Figure S2.
Spheroid Cells with ALDH Activity Are less Sensitive to Paclitaxel
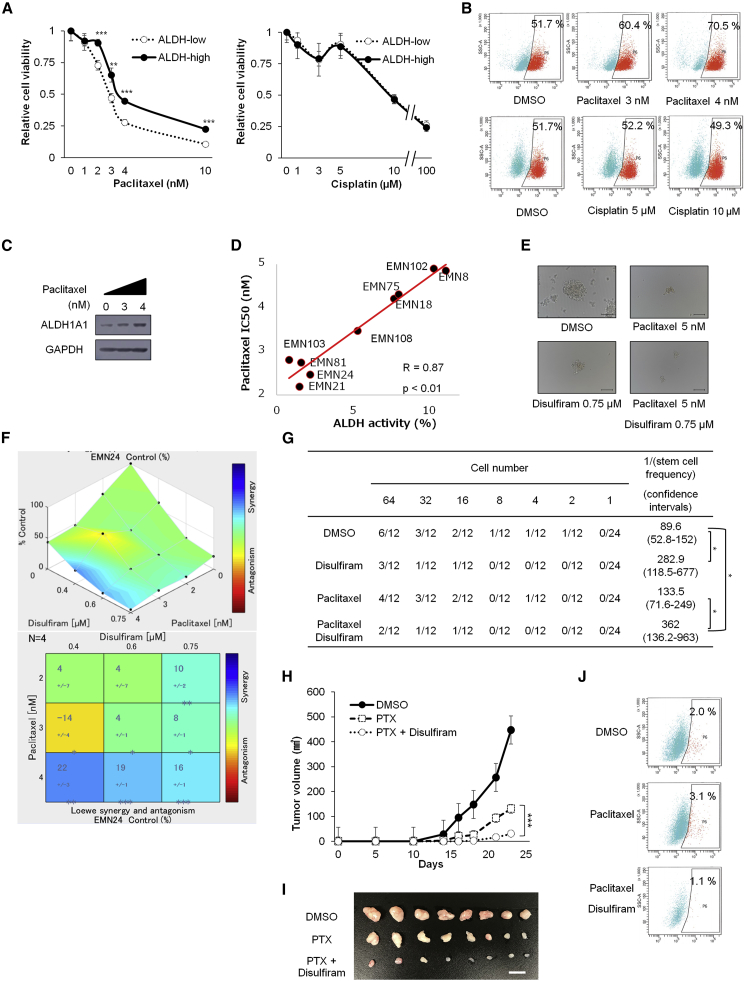
Cancer stem-like cells have been reported to be responsible for drug resistance in many cancers (Hida et al., 2017, House et al., 2017). To evaluate the drug sensitivity of cancer stem-like cells in our endometrial cancer spheroid cells, we examined the sensitivity of ALDH-high cells for paclitaxel or cisplatin, which have been used in the clinical setting as key drugs for the treatment of endometrial cancer (Nomura et al., 2011). Interestingly, although there were no significant differences in the sensitivity of ALDH-high and ALDH-low cells to cisplatin, ALDH-high cells were more resistant to paclitaxel than ALDH-low cells (Figures 4A and S3A).
Figure 4.
Combination Therapy with Paclitaxel and the ALDH Inhibitor Synergistically Inhibits Endometrial Cancer Cell Progression (EMN24 Cells)
(A) Responses of ALDH-high and ALDH-low cells to different concentrations of cisplatin and paclitaxel after treatment for 7 days. n = 4 independent experiments, Student's t test.
(B) FACS analyses of ALDH activity in ALDH-high cells in the presence or absence of paclitaxel or cisplatin after in vitro treatment for 7 days.
(C) Western blot analyses of ALDH-high cells treated with paclitaxel for 7 days.
(D) Correlation between ALDH activity and paclitaxel half-maximal inhibitory concentration (nM).
(E) Bright-phase images of spheroids after in vitro paclitaxel and disulfiram treatment for 7 days. Scale bars, 100 μm.
(F) Relative cell viability of spheroid cells after culture for 7 days with the indicated concentrations of ALDH inhibitor and paclitaxel. Synergistic interaction was assessed with Combenefit software. n = 4 independent experiments.
(G) In vitro limiting dilution analysis over 14 days culture using spheroid cells exposed to 7 days treatment of disulfiram and/or paclitaxel in vitro.
(H) Volume (mean ± SEM) of xenograft tumors from 1 × 105 spheroid cells. Mice were separated into the vehicle (DMSO)-treated group, paclitaxel-treated group, and paclitaxel + disulfiram-treated group. n = 8 independent experiments, p < 0.001, Student's t test.
(I) Images of whole resected tumor xenografts excised on day 23. Scale bar, 10 mm.
(J) ALDH activity in cancer cells derived from xenograft tumors.
∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. See also Figure S3.
Given the relative resistance of ALDH-high cells against paclitaxel, we examined whether ALDH-high cells preferentially survived following paclitaxel treatment. Treatment of spheroids with paclitaxel, but not with cisplatin, caused the proportion of ALDH-high cells to increase (Figures 4B and S3B). Consistent with the major role of ALDH1A1 in ALDH activity of spheroids, ALDH1A1 increased after exposure to paclitaxel (Figures 4C and S3C). Furthermore, evaluation of the ALDH activity and half-maximal inhibitory concentration of paclitaxel of nine established endometrial cancer spheroids revealed strong correlations (Spearman R value = 0.87, p < 0.01; Figure 4D). These results indicated that an ALDH-high cell population in endometrial spheroid cells was responsible for paclitaxel resistance in endometrial cancer.
Because of the relative resistance of ALDH-high cells to paclitaxel, we examined the synergistic effects of paclitaxel and an ALDH inhibitor. Disulfiram synergistically suppressed spheroid cell proliferation in vitro when used in combination with paclitaxel (Figures 4E–4G and S3D–S3F). Similarly, the combination of paclitaxel and other ALDH inhibitors (DEAB, CM037, and NCT501) suppressed spheroid cell propagation to a greater extent than paclitaxel alone (Figure S3G).
To determine the synergistic effects of paclitaxel and disulfiram in vivo, we treated spheroid cell-transplanted mice with paclitaxel with or without disulfiram. The sizes of tumors observed after combination therapy were approximately one-eighth of those after paclitaxel alone (Figures 4H and 4I). As expected, paclitaxel treatment led to an increase in the population of ALDH-high cells, which was suppressed by additional treatment with disulfiram (Figure 4J). Thus, combination therapy with paclitaxel and the ALDH inhibitor synergistically inhibited endometrial cancer cell progression in in vivo tumors as well as in vitro spheroids.
ALDH1A1 Expression Is Correlated with Poor Prognosis in Patients with Endometrial Cancer
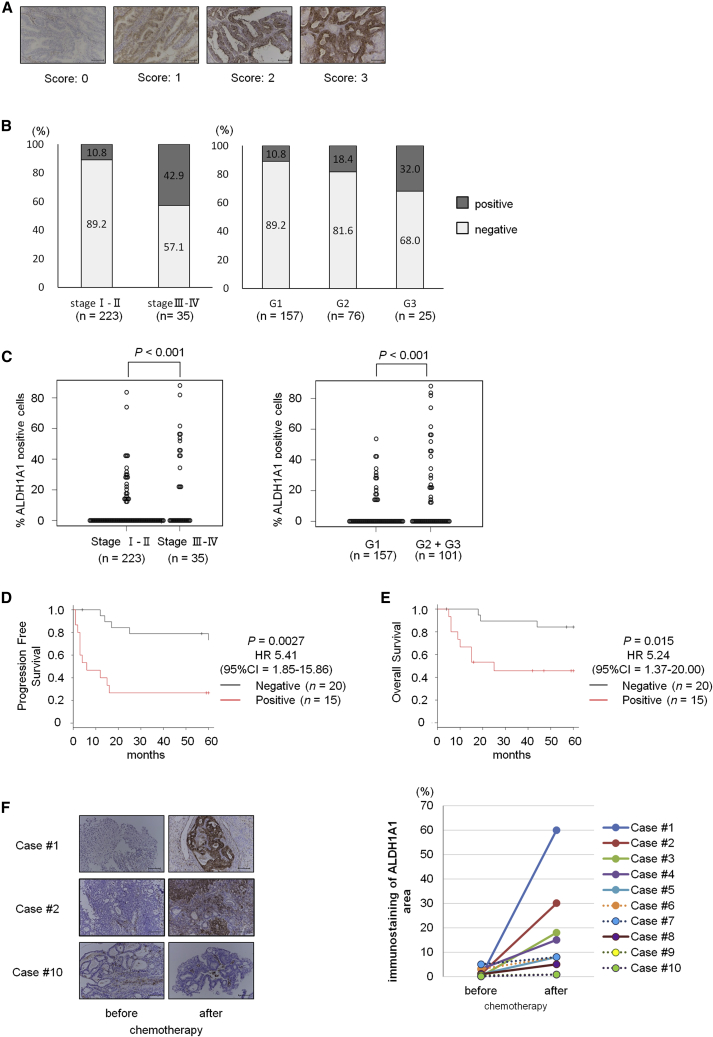
To examine whether ALDH1A1 expression was associated with prognosis in clinical endometrial cancer, we determined the expression of ALDH1A1 in 258 clinical primary uterine endometrial endometrioid carcinoma tumor tissue samples by immunohistochemistry (Figure 5A). The clinicopathological characteristics are shown in Table S3. Immunostaining demonstrated that ALDH1A1 was expressed in more than 40% of tumor samples in patients with advanced-stage disease (stages III and IV), but was expressed only in 10% of patients with early-stage disease (stages I and II; Figure 5B). In addition, significantly higher levels of ALDH1A1 were observed in patients with high-grade endometrioid cancer than in patients with low-grade cancer (Figure 5B). Moreover, a fraction of ALDH1A1-expressed cells was higher in clinical specimens from advanced-stage or high-grade cancer than from early-stage or low-grade cancer (Figure 5C). Kaplan-Meier survival analyses of 35 patients with advanced-stage cancer showed that high ALDH1A1 expression was correlated with survival rate (Figure 5D, p < 0.01 for progression-free survival; Figure 5E, p = 0.015 for overall survival), consistent with previous reports (Huang et al., 2018, Rahadiani et al., 2011). Univariate and multivariate analyses revealed that positive ALDH1A1 staining was an independent prognostic factor of both progression-free and overall survival in patients with advanced-stage endometrial cancer (Tables S4 and S5). These results indicated that ALDH1A1 expression was associated with pathological grade and poor prognosis.
Figure 5.
ALDH1 Expression Is Associated with Advanced Clinical Stage, Poor Prognosis, and Paclitaxel Sensitivity in Human Endometrial Cancer
(A) Representative immunostaining of ALDH1A1 (score 0–3). Scale bars, 100 μm.
(B) Distribution of ALDH1A1 expression during different clinical stages in endometrial cancer, and distribution of ALDH1A1 expression in different histological grades of endometrial cancer (n = 258).
(C) Percentage of ALDH1A1-expressed cancer cells in different clinical stage or histological grades of endometrial cancer (n = 258).
(D and E) Kaplan-Meier analyses of progression-free survival (D) and overall survival (E) in patients with advanced-stage endometrial cancer. The patients were stratified into ALDH1A1-positive (red lines, n = 15) and ALDH1A1-negative (gray lines, n = 20) groups. Most of these patients were treated with taxane-containing chemotherapy as the first-line regimen.
(F) Immunostaining for ALDH1A1 in uterine tumors before and after chemotherapy. Scale bars, 100 μm (left). ALDH1A1 immunostaining area in uterine tumors before and after chemotherapy (right). Cases of ALDH1A1 staining area expanded up to five times are indicated as straight lines (n = 6), and other cases are indicated as dotted lines (n = 4).
See also Table S3.
Furthermore, we compared the expression levels of ALDH1A1 by immunostaining of paired uterine endometrial tumor samples before and after taxane-containing chemotherapy. Immunostaining analysis showed that, in six of ten patient samples, the ALDH1A1 staining area expanded more than five times after chemotherapy compared with that in matched tumor samples before chemotherapy (Figure 5F), thus further supporting the proposed role of ALDH-positive cells in paclitaxel resistance.
Spheroid Cells with ALDH Activity Show Glycolytic Dependency
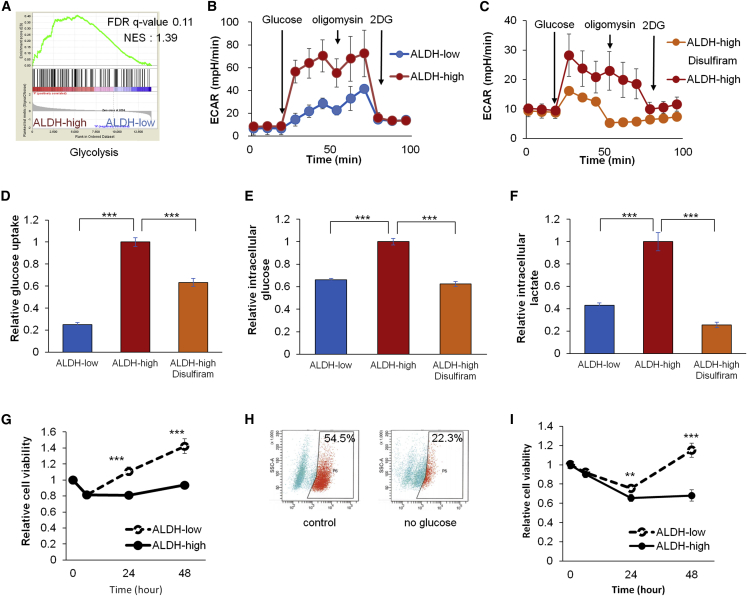
Next, to gain an insight into mechanism by which ALDH function confers for the observed chemoresistance, we searched for biological pathways that were upregulated in cells with high levels of ALDH. GSEA showed the upregulation of glycolysis-related genes (hallmark of glycolysis) in the ALDH-high spheroid cells (Figure 6A). In fact, ALDH-high cells displayed higher levels of the extracellular acidification rate (ECAR) than ALDH-low cells (Figures 6B and S4A) and the treatment with disulfiram reduced ECAR (Figures 6C and S4B), indicating that glycolysis and glycolytic capacity were elevated in ALDH-high cells. In accordance with these results, glucose uptake, intracellular glucose, and lactate level were higher in ALDH-high cells than in ALDH-low cells (Figures 6D–6F and S4C–S4E) and suppressed by disulfiram (Figures 6D–6F and S4C–S4E). These data collectively indicate that spheroid cells with ALDH activity had increased glycolytic activity.
Figure 6.
ALDH-High Endometrial Cancer Cells Depend on Glycolysis for Their Survival and Growth
(A) Gene set enrichment analyses of gene expression profiles between ALDH-high and ALDH-low cells (Hallmark of glycolysis).
(B and C) Time course of ECAR of spheroid cells with the indicated stimulation. The differences between ALDH-high and ALDH-low cells (B), and between ALDH-high cells with vehicle or disulfiram treatment (C). n = 4 independent experiments.
(D–F) Relative glucose uptake (D), intracellular glucose (E), and lactate level (F) of ALDH-low cells, ALDH-high cells, and ALDH-high cells treated with disulfiram. n = 4 independent experiments, p < 0.01, Student's t test.
(G) Time course of the proliferation of ALDH-high and ALDH-low cells under no glucose culture condition. n = 4 independent experiments, Student's t test.
(H) FACS analyses of ALDH activity in ALDH-high cells cultivated under normal or no glucose condition for 2 days.
(I) Time course of the proliferation of ALDH-high and ALDH-low cells under 20μM 2-deoxy-D-glucose (2-DG) treatment. n = 4 independent experiments, Student's t test.
∗∗p < 0.01; ∗∗∗p < 0.001. See also Figure S4.
To examine whether reduced availability of glucose affects cell growth of ALDH-high cells, we cultivated ALDH-high and ALDH-low cells under glucose-free cultivation condition, and found that the reduced levels of glucose led to the relative suppression of cell growth in ALDH-high cells (Figure 6G) as well as the decrease of their proportion (Figure 6H). Similarly, 2-deoxy-D-glucose, an inhibitor of hexokinase, similarly suppressed cell growth of ALDH-high spheroid cells (Figure 6I). Thus, ALDH-high cells were more dependent on glycolytic function than ALDH-low cells for their survival and growth.
Glycolytic Suppression with GLUT1 Inhibition Suppressed Endometrial Cancer Stemness
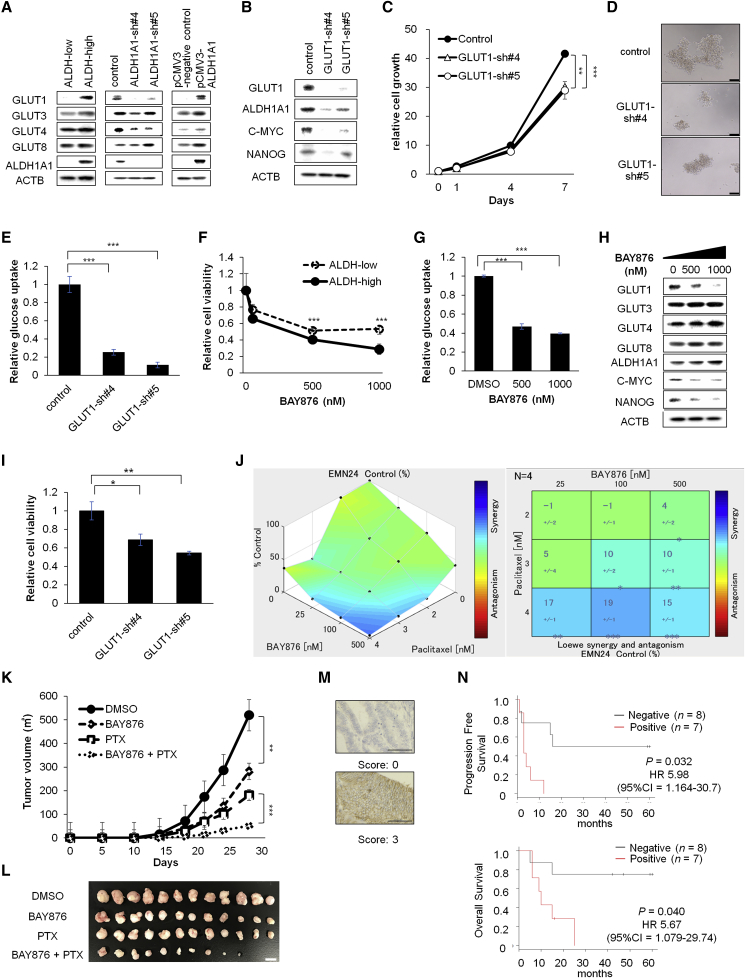
Glucose transporters play an essential role in glucose influx, and 14 members of the glucose transporter (GLUT) family have been reported. Because some of the transporters are overexpressed or dysregulated in cancer cells (Zhao et al., 2013), we evaluated the differences in GLUT expression in ALDH-high and ALDH-low cells. Western blot analysis showed that GLUT1 was specifically expressed at higher levels in ALDH-high cells than in ALDH-low cells (Figures 7A and S4F). ALDH1A1 was responsible for GLUT1 expression because inhibition of ALDH1A1 decreased the expression of GLUT1 and exogenous ALDH1A1 overexpression led to the increase of GLUT1 expression (Figure 7A).
Figure 7.
Combination Therapy with Paclitaxel and the GLUT1 Inhibitor Synergistically Inhibits Endometrial Cancer Cell Progression (EMN24 Cells)
(A and B) Western blot analyses of spheroid cells after indicated ALDH modification (A) and after GLUT1 inhibition (B).
(C) Time course of the proliferation of the infected cells. n = 4 independent experiments, p < 0.01, Student's t test.
(D) Bright-phase images of the infected cells. Scale bars, 100 μm.
(E) Glucose uptake of spheroid cells of the infected cells. n = 4 independent experiments, p < 0.01, Student's t test.
(F and G) Relative cell viability (F) and glucose uptake (G) of spheroid cells after culture for 7 days with the indicated concentration of BAY876. n = 4 independent experiments, p < 0.01, Student's t test.
(H) Western blot analyses of spheroid cells after exposure to the indicated concentration of BAY876.
(I) Relative cell viability of spheroid cells after culture for 7 days with 2 nM paclitaxel. n = 4 independent experiments, p < 0.01, Student's t test.
(J) Relative cell viability of spheroid cells with the indicated concentrations of BAY876 and paclitaxel in vitro. Synergistic interaction was assessed with Combenefit software. n = 4 independent experiments.
(K) Volume (mean ± SEM) of xenograft tumors from 1 × 105 spheroid cells. Mice were separated into the vehicle (DMSO)-treated group, paclitaxel-treated group, BAY876-treated group, and paclitaxel + BAY876-treated group. n = 12 independent experiments, p < 0.001, Student's t test.
(L) Images of whole resected tumor xenografts excised on day 23. Scale bar, 10 mm.
(M) Representative immunostaining of GLUT1. Scale bars, 100 μm.
(N) Kaplan-Meier analyses of progression-free survival (upper) and overall survival (lower) in patients with advanced-stage ALDH-positive endometrial cancer. The patients were stratified into GLUT1-high (red lines, n = 7) and GLUT1-low (gray lines, n = 8) groups.
∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. See also Figure S4.
To examine whether GLUT1 mediates enhanced cell growth and stem-like property that is conferred by ALDH, we inhibited GLUT1 expression using shRNA lentiviral transfer (Figures 7B and S4G). After inhibition of GLUT1, repression of spheroid cell growth and glucose uptake were observed (Figures 7C–7E and S4H–S4J). In accordance, treatment with BAY876, a specific GLUT1 inhibitor, preferentially suppressed the cell viability and glucose uptake in ALDH-high cells (Figures 7F, 7G, S4K, and S4L). Inhibition of GLUT1 also decreased the expression of stemness markers, including Nanog, and c-myc (Figures 7B, 7H, S4G, and S4M). These data indicated that glucose uptake via GLUT1 is functionally important for the survival of ALDH-high endometrial CSCs.
Remarkably, inhibition of GLUT1 by RNAi or BAY876 sensitized spheroid cells to paclitaxel and synergistically suppressed the spheroid cell propagation in vitro (Figures 7I, 7J, S4N, and S4O) and tumor propagation in vivo (Figures 7K and 7L), thus phenocopying the effect of combination treatment of paclitaxel and disulfiram (Figures 4F, 4H, and S3E). These data indicate that GLUT1 mediates ALDH function to confer stem-like properties and paclitaxel resistance to the endometrial cancer spheroids.
Finally, to evaluate whether GLUT1 expression was associated with poor clinical outcome, we examined GLUT1 expression in clinical endometrial cancer tissue specimens by immunohistochemical staining (Figure 7M). We focused on advanced-stage cancer cases with high ALDH1A1 expression (n = 15), which were linked to poor prognosis (Figure 5). Kaplan-Meier survival analyses showed that high GLUT1 expression correlated with reduced survival rate (Figure 7N; p = 0.032 for progression-free survival; p = 0.04 for overall survival), suggesting that GLUT1 expression was associated with poor prognosis in advanced-stage endometrial cancer.
Discussion
To develop novel clinical therapeutic strategies for refractory cancer, particularly for targeting CSCs, in vitro cultivation models can be invaluable tools. The floating spheroid culture method, which has been widely used for CSC research, retains characteristics of original tumors (Ishiguro et al., 2017). While short-term cultivation methods of three-dimensional cells from endometrial cancer tumor specimens (Girda et al., 2017, Kiyohara et al., 2016), and three-dimensional endometrial cancer cells after primary attachment cultivation in the presence of serum (Ding et al., 2017), have been reported previously, we demonstrated, for the first time, that endometrial cancer spheroids with CSC characteristics could be stably propagated in vitro. Notably, we observed that spheroid cells could be preferentially established from high-grade tumors. This is advantageous for clinical application because high-grade endometrial cancer with poor prognosis is associated with clinically relevant cells with malignant traits (Morice et al., 2016).
ALDH activity has been shown to be a specific marker of normal stem cells (Tomita et al., 2016) and various types of CSCs including endometrial cancer (Ginestier et al., 2007, Ishiguro et al., 2016, Kiyohara et al., 2017, Regan et al., 2017, van der Zee et al., 2015). It is not clear whether the functional importance can be expanded for other types of cancer, although the functional importance of ALDH activity has been shown for some cancer cells (Ishiguro et al., 2016, Liu et al., 2012a).
In this study, we demonstrated that ALDH-high endometrial cancer spheroid cells satisfied various criteria of CSCs. Functional analyses in endometrial spheroids showed that ALDH activity or ALDH1A1 expression promoted the proliferation and survival of endometrial CSCs. In addition, immunohistochemical studies of the clinical tumor showed that high levels of ALDH1A1 expression were associated with poor prognosis and advanced histological tumor grade in patients with endometrial cancer. These results suggested that ALDH1A1 expression facilitated the proliferation of endometrial CSCs, leading to a more aggressive and undifferentiated state in clinical tumors during disease progression (Lytle et al., 2018).
Resistance to current chemotherapy is one of the characteristics of CSCs, and chemotherapy preferentially selects CSC populations (Mueller et al., 2009, Lee et al., 2011, Hirst et al., 2018, Croker and Allan, 2012). Our data obtained from in vitro spheroids and clinical specimens showed that paclitaxel selectively increased the proportion of ALDH-high cells, indicating that ALDH-high endometrial CSCs were resistant to paclitaxel. These observations led us to examine the synergistic effects of paclitaxel and ALDH inhibitors, and we found that the combination therapy strongly suppressed the proliferation of endometrial cancer cells. Interestingly, increased levels of ALDH activity were observed in breast and ovarian cancer cells after paclitaxel exposure (Hirst et al., 2018, Park et al., 2015), and similar combination therapy was shown to be applicable in the treatment of taxane-resistant breast and ovarian cancer cells (Januchowski et al., 2016, Liu et al., 2013, Wu et al., 2019).
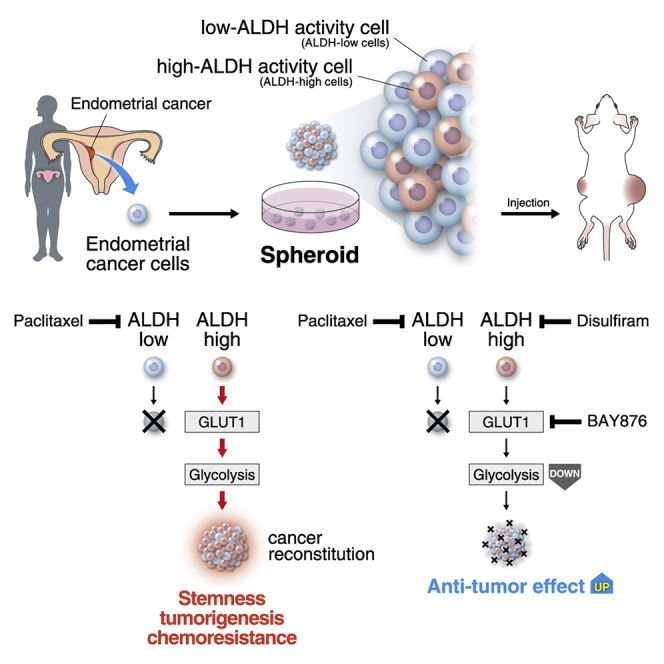
It has been reported that, in many types of cancer, CSCs depend on enhanced glycolysis for their proliferation (Luo and Wicha, 2019, Liu et al., 2012b). Our data indicate that GLUT1 is a crucial downstream effector of ALDH, thus linking ALDH activation and glycolytic pathway in endometrial CSCs. It was previously shown that GLUT1 overexpression is commonly associated with cancer metabolism (Ancey et al., 2018). In addition, GLUT1 is essential for the self-renewal and tumor-initiating capacity of glioma CSCs (Shibuya et al., 2015), and GLUT inhibition and anticancer drug cooperated to suppress other types of cancer (Liu et al., 2012b). Hence, the ALDH-GLUT1 cascade may serve as a crucial pathway for the maintenance of CSCs and chemoresistance (Figure S4P). Because spheroids presumably maintain characteristics similar to those of the original tumors, we expect that endometrial spheroids and spheroid-derived xenografts may have applications in personalized medicine in the future. To preclinically identify patients who may benefit from treatment with ALDH or GLUT inhibitors, our cultivation method may be useful for screening of clinical cases showing high levels of ALDH and hence respond to anti-ALDH or GLUT therapy in combination with taxane-derived compounds.
Experimental Procedures
Tumor-Derived Spheroid Culture
All procedures were performed using protocols approved by the Ethics Committee of Niigata University and the National Cancer Center. Informed consent was obtained from all patients. Endometrial cancer cells from tumor samples were grown on ultra-low-attachment culture dishes (Corning, NY, USA) in StemPro hESC SFM (Gibco) supplemented with 8 ng/mL basic fibroblast growth factor (Invitrogen, Carlsbad, CA) and penicillin/streptomycin (37°C, 5% CO2).
Animal Experiments
For cell transplantation assays in spheroid cells, the spheroids were dissociated into single cells with Accumax, suspended in 50 μL medium containing 50% Matrigel (BD Biosciences), and used for subcutaneous injection with a 27-G needle into the flanks of NOG (NOD/Shi-scid IL-2Rγnull) mice (Central Institute for Experimental Animals, Kawasaki, Japan). All mouse procedures were approved by the Animal Care and Use Committees of Niigata University and performed in accordance with institutional policies.
Lentivirus-Mediated Short Hairpin RNA Transduction
Lentivirus plasmid expressing ALDH1A1 short hairpin RNAs (shRNAs) (sh4, TRCN0000276460; sh5, TRCN0000276461), GLUT1 shRNAs (sh4, TRCN0000424030; sh5, TRCN0000423590), and control shRNA were purchased from Sigma. The pCMV3-ALDH1A1 plasmid (HG11388-UT) and pCMV3 control vector were purchased from Sino Biological. Preparation of virus-containing supernatants and virus infection were performed as previously described (Ishiguro et al., 2016). Cells infected with the viruses were selected in the presence of 2 μg/mL puromycin or 100 μg/mL hygromycin.
Western Blot Analyses
Cells were lysed in RIPA buffer (50 mM Tris [pH 8.0], 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and 1 mM EDTA) supplemented with protease inhibitors (Roche) and used for Western blot analysis, as described previously (Ishiguro et al., 2016), with primary antibodies (Table S6).
Statistical Analyses
For statistical analyses of spheroid cell experiments, Welch t tests or Student's t tests were performed based on the results of F tests. Differences with p values of less than 0.05 were considered significant. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Statistical analyses of clinical samples were performed using EZR (Kanda, 2013). Univariate survival analysis was performed using the Kaplan-Meier method, and the significance of difference between groups was examined using log rank tests. Multivariate survival analysis was carried out using the Cox proportional hazards regression model. Hazard ratios and 95% confidence intervals were calculated with Cox's proportional hazards regression model. Variables with p values of less than 0.05 on univariate regression analysis were examined in multivariate regression analysis.
Additional materials and methods can be retrieved in Supplemental Experimental Procedures.
Author Contributions
Conception and design, Y.M., K.Yamawaki, T.I., and T.E. Development of methodology, Y.M., K.Yamawaki, T.I., A.S., and H.O. Acquisition of data, Y.M., K.Yamawaki, T.I., and H.U. Analysis and interpretation of data, Y.M., K.Yamawaki, T.I., K.Yoshihara, Y.Y., and T.M. Writing, review, and/or revision of the manuscript, Y.M., K.Yamawaki, T.I., K.Yoshihara, H.U., A.S., H.O., K.O., and T.E. Study supervision, K.O. and T.E.
Acknowledgments
We thank Ryo Tamura, Kazuaki Suda, Kentaro Sugino, Manako Yamaguchi, Nozomi Yachida, Anna Ishida, Nobumichi Nishikawa, and Masayuki Sekine (Department of Obstetrics and Gynecology, Niigata University Graduate School of Medical and Dental Sciences), Ippei Shimizu (Department of Cardiovascular Biology and Medicine, Niigata University Graduate School of Medical and Dental Sciences), and Hiroaki Sakai (National Cancer Center Research Institute) for scientific advice and technical assistance. They disclose no potential conflicts of interest. This research was supported by Grants-in-Aid for Young Scientists (B) (grant no. 15K20132) and Scientific Research (C) (grant no. 18K09250) to T.I. from the Japan Society for the Promotion of Science and the Tsukada Grant for Niigata University Medical Research to T.I. This work was partially supported by the National Cancer Center Research and Development Funds (29-A-2).
Published: September 26, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2019.08.015.
Contributor Information
Tatsuya Ishiguro, Email: tishigur@med.niigata-u.ac.jp.
Takayuki Enomoto, Email: enomoto@med.niigata-u.ac.jp.
Supplemental Information
References
- Ancey P.B., Contat C., Meylan E. Glucose transporters in cancer––from tumor cells to the tumor microenvironment. FEBS J. 2018;285:2926–2943. doi: 10.1111/febs.14577. [DOI] [PubMed] [Google Scholar]
- Aoki D. Annual report of Gynecologic Oncology Committee, Japan Society of Obstetrics and Gynecology, 2013. J. Obstet. Gynaecol. Res. 2014;40:338–348. doi: 10.1111/jog.12360. [DOI] [PubMed] [Google Scholar]
- Ben-Porath I., Thomson M.W., Carey V.J., Ge R., Bell G.W., Regev A., Weinberg R.A. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat. Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestvina C.M., Fleming G.F. Chemotherapy for endometrial cancer in adjuvant and advanced disease settings. Oncologist. 2016;21:1250–1259. doi: 10.1634/theoncologist.2016-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croker A.K., Allan A.L. Inhibition of aldehyde dehydrogenase (ALDH) activity reduces chemotherapy and radiation resistance of stem-like ALDHhiCD44(+) human breast cancer cells. Breast Cancer Res. Treat. 2012;133:75–87. doi: 10.1007/s10549-011-1692-y. [DOI] [PubMed] [Google Scholar]
- de Boer S.M., Powell M.E., Mileshkin L., Katsaros D., Bessette P., Haie-Meder C., Ottevanger P.B., Ledermann J.A., Khaw P., Colombo A. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:295–309. doi: 10.1016/S1470-2045(18)30079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding D.C., Liu H.W., Chang Y.H., Chu T.Y. Expression of CD133 in endometrial cancer cells and its implications. J. Cancer. 2017;8:2142–2153. doi: 10.7150/jca.18869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontu G., Abdallah W.M., Foley J.M., Jackson K.W., Clarke M.F., Kawamura M.J., Wicha M.S. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginestier C., Hur M.H., Charafe-Jauffret E., Monville F., Dutcher J., Brown M., Jacquemier J., Viens P., Kleer C.G., Liu S. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girda E., Huang E.C., Leiserowitz G.S., Smith L.H. The use of endometrial cancer patient-derived organoid culture for drug sensitivity testing is feasible. Int. J. Gynecol. Cancer. 2017;27:1701–1707. doi: 10.1097/IGC.0000000000001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hida K., Maishi N., Akiyama K., Ohmura-Kakutani H., Torii C., Ohga N., Osawa T., Kikuchi H., Morimoto H., Morimoto M. Tumor endothelial cells with high aldehyde dehydrogenase activity show drug resistance. Cancer Sci. 2017;108:2195–2203. doi: 10.1111/cas.13388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J., Pathak H.B., Hyter S., Pessetto Z.Y., Ly T., Graw S., Koestler D.C., Krieg A.J., Roby K.F., Godwin A.K. Licofelone enhances the efficacy of paclitaxel in ovarian cancer by reversing drug resistance and tumor stem-like properties. Cancer Res. 2018;78:4370–4385. doi: 10.1158/0008-5472.CAN-17-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House C.D., Jordan E., Hernandez L., Ozaki M., James J.M., Kim M., Kruhlak M.J., Batchelor E., Elloumi F., Cam M.C. NFkappaB promotes ovarian tumorigenesis via classical pathways that support proliferative cancer cells and alternative pathways that support ALDH(+) cancer stem-like cells. Cancer Res. 2017;77:6927–6940. doi: 10.1158/0008-5472.CAN-17-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.H., Wang Y.C., Chou Y.C., Yu M.H., Chao T.K. The combination of aldehyde dehydrogenase 1 (ALDH1) and CD44 is associated with poor outcomes in endometrial cancer. PLoS One. 2018;13:e0206685. doi: 10.1371/journal.pone.0206685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S.A., Friel A.M., Kumar B., Zhang L., Rueda B.R., Gargett C.E. Evidence for cancer stem cells in human endometrial carcinoma. Cancer Res. 2009;69:8241–8248. doi: 10.1158/0008-5472.CAN-08-4808. [DOI] [PubMed] [Google Scholar]
- Ishiguro T., Sato A., Ohata H., Ikarashi Y., Takahashi R.U., Ochiya T., Yoshida M., Tsuda H., Onda T., Kato T. Establishment and characterization of an in vitro model of ovarian cancer stem-like cells with an enhanced proliferative capacity. Cancer Res. 2016;76:150–160. doi: 10.1158/0008-5472.CAN-15-0361. [DOI] [PubMed] [Google Scholar]
- Ishiguro T., Ohata H., Sato A., Yamawaki K., Enomoto T., Okamoto K. Tumor-derived spheroids: relevance to cancer stem cells and clinical applications. Cancer Sci. 2017;108:283–289. doi: 10.1111/cas.13155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Januchowski R., Wojtowicz K., Sterzyska K., Sosiska P., Andrzejewska M., Zawierucha P., Nowicki M., Zabel M. Inhibition of ALDH1A1 activity decreases expression of drug transporters and reduces chemotherapy resistance in ovarian cancer cell lines. Int. J. Biochem. Cell Biol. 2016;78:248–259. doi: 10.1016/j.biocel.2016.07.017. [DOI] [PubMed] [Google Scholar]
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyohara Y., Yoshino K., Kubota S., Okuyama H., Endo H., Ueda Y., Kimura T., Kimura T., Kamiura S., Inoue M. Drug screening and grouping by sensitivity with a panel of primary cultured cancer spheroids derived from endometrial cancer. Cancer Sci. 2016;107:452–460. doi: 10.1111/cas.12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyohara M.H., Dillard C., Tsui J., Kim S.R., Lu J., Sachdev D., Goodglick L., Tong M., Torous V.F., Aryasomayajula C. EMP2 is a novel therapeutic target for endometrial cancer stem cells. Oncogene. 2017;36:5793–5807. doi: 10.1038/onc.2017.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.K., Castilho A., Cheung V.C., Tang K.H., Ma S., Ng I.O. CD24(+) liver tumor-initiating cells drive self-renewal and tumor initiation through STAT3-mediated NANOG regulation. Cell Stem Cell. 2011;9:50–63. doi: 10.1016/j.stem.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Liu P., Brown S., Goktug T., Channathodiyil P., Kannappan V., Hugnot J.P., Guichet P.O., Bian X., Armesilla A.L., Darling J.L. Cytotoxic effect of disulfiram/copper on human glioblastoma cell lines and ALDH-positive cancer-stem-like cells. Br. J. Cancer. 2012;107:1488–1497. doi: 10.1038/bjc.2012.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Cao Y., Zhang W., Bergmeier S., Qian Y., Akbar H., Colvin R., Ding J., Tong L., Wu S. A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol. Cancer Ther. 2012;11:1672–1682. doi: 10.1158/1535-7163.MCT-12-0131. [DOI] [PubMed] [Google Scholar]
- Liu P., Kumar I.S., Brown S., Kannappan V., Tawari P.E., Tang J.Z., Jiang W., Armesilla A.L., Darling J.L., Wang W. Disulfiram targets cancer stem-like cells and reverses resistance and cross-resistance in acquired paclitaxel-resistant triple-negative breast cancer cells. Br. J. Cancer. 2013;109:1876–1885. doi: 10.1038/bjc.2013.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonardo E., Hermann P.C., Mueller M.T., Huber S., Balic A., Miranda-Lorenzo I., Zagorac S., Alcala S., Rodriguez-Arabaolaza I., Ramirez J.C. Nodal/Activin signaling drives self-renewal and tumorigenicity of pancreatic cancer stem cells and provides a target for combined drug therapy. Cell Stem Cell. 2011;9:433–446. doi: 10.1016/j.stem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Luo M., Wicha M.S. Targeting cancer stem cell redox metabolism to enhance therapy responses. Semin. Radiat. Oncol. 2019;29:42–54. doi: 10.1016/j.semradonc.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytle N.K., Barber A.G., Reya T. Stem cell fate in cancer growth, progression and therapy resistance. Nat. Rev. Cancer. 2018;18:669–680. doi: 10.1038/s41568-018-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morice P., Leary A., Creutzberg C., Abu-Rustum N., Darai E. Endometrial cancer. Lancet. 2016;387:1094–1108. doi: 10.1016/S0140-6736(15)00130-0. [DOI] [PubMed] [Google Scholar]
- Mueller M.T., Hermann P.C., Witthauer J., Rubio-Viqueira B., Leicht S.F., Huber S., Ellwart J.W., Mustafa M., Bartenstein P., D'Haese J.G. Combined targeted treatment to eliminate tumorigenic cancer stem cells in human pancreatic cancer. Gastroenterology. 2009;137:1102–1113. doi: 10.1053/j.gastro.2009.05.053. [DOI] [PubMed] [Google Scholar]
- Nechushtan H., Hamamreh Y., Nidal S., Gotfried M., Baron A., Shalev Y.I., Nisman B., Peretz T., Peylan-Ramu N. A phase IIb trial assessing the addition of disulfiram to chemotherapy for the treatment of metastatic non-small cell lung cancer. Oncologist. 2015;20:366–367. doi: 10.1634/theoncologist.2014-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura H., Aoki D., Takahashi F., Katsumata N., Watanabe Y., Konishi I., Jobo T., Hatae M., Hiura M., Yaegashi N. Randomized phase II study comparing docetaxel plus cisplatin, docetaxel plus carboplatin, and paclitaxel plus carboplatin in patients with advanced or recurrent endometrial carcinoma: a Japanese Gynecologic Oncology Group study (JGOG2041) Ann. Oncol. 2011;22:636–642. doi: 10.1093/annonc/mdq401. [DOI] [PubMed] [Google Scholar]
- Ohata H., Ishiguro T., Aihara Y., Sato A., Sakai H., Sekine S., Taniguchi H., Akasu T., Fujita S., Nakagama H. Induction of the stem-like cell regulator CD44 by Rho kinase inhibition contributes to the maintenance of colon cancer-initiating cells. Cancer Res. 2012;72:5101–5110. doi: 10.1158/0008-5472.CAN-11-3812. [DOI] [PubMed] [Google Scholar]
- Park S.Y., Kim M.J., Park S.A., Kim J.S., Min K.N., Kim D.K., Lim W., Nam J.S., Sheen Y.Y. Combinatorial TGF-beta attenuation with paclitaxel inhibits the epithelial-to-mesenchymal transition and breast cancer stem-like cells. Oncotarget. 2015;6:37526–37543. doi: 10.18632/oncotarget.6063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrana E., Silva-Vargas V., Doetsch F. Eyes wide open: a critical review of sphere-formation as an assay for stem cells. Cell Stem Cell. 2011;8:486–498. doi: 10.1016/j.stem.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pece S., Tosoni D., Confalonieri S., Mazzarol G., Vecchi M., Ronzoni S., Bernard L., Viale G., Pelicci P.G., Di Fiore P.P. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell. 2010;140:62–73. doi: 10.1016/j.cell.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Rahadiani N., Ikeda J., Mamat S., Matsuzaki S., Ueda Y., Umehara R., Tian T., Wang Y., Enomoto T., Kimura T. Expression of aldehyde dehydrogenase 1 (ALDH1) in endometrioid adenocarcinoma and its clinical implications. Cancer Sci. 2011;102:903–908. doi: 10.1111/j.1349-7006.2011.01864.x. [DOI] [PubMed] [Google Scholar]
- Regan J.L., Schumacher D., Staudte S., Steffen A., Haybaeck J., Keilholz U., Schweiger C., Golob-Schwarzl N., Mumberg D., Henderson D. Non-canonical Hedgehog signaling is a positive regulator of the WNT pathway and is required for the survival of colon cancer stem cells. Cell Rep. 2017;21:2813–2828. doi: 10.1016/j.celrep.2017.11.025. [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani L., Lombardi D.G., Pilozzi E., Biffoni M., Todaro M., Peschle C., De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- Rutella S., Bonanno G., Procoli A., Mariotti A., Corallo M., Prisco M.G., Eramo A., Napoletano C., Gallo D., Perillo A. Cells with characteristics of cancer stem/progenitor cells express the CD133 antigen in human endometrial tumors. Clin. Cancer Res. 2009;15:4299–4311. doi: 10.1158/1078-0432.CCR-08-1883. [DOI] [PubMed] [Google Scholar]
- Safi R., Nelson E.R., Chitneni S.K., Franz K.J., George D.J., Zalutsky M.R., McDonnell D.P. Copper signaling axis as a target for prostate cancer therapeutics. Cancer Res. 2014;74:5819–5831. doi: 10.1158/0008-5472.CAN-13-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya K., Okada M., Suzuki S., Seino M., Seino S., Takeda H., Kitanaka C. Targeting the facilitative glucose transporter GLUT1 inhibits the self-renewal and tumor-initiating capacity of cancer stem cells. Oncotarget. 2015;6:651–661. doi: 10.18632/oncotarget.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2015. CA Cancer J. Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- Singh S.K., Clarke I.D., Terasaki M., Bonn V.E., Hawkins C., Squire J., Dirks P.B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- Soumerai T.E., Donoghue M.T.A., Bandlamudi C., Srinivasan P., Chang M.T., Zamarin D., Cadoo K.A., Grisham R.N., O'Cearbhaill R.E., Tew W.P. Clinical utility of prospective molecular characterization in advanced endometrial cancer. Clin. Cancer Res. 2018;24:5939–5947. doi: 10.1158/1078-0432.CCR-18-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cancer Genome Atlas Network Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita H., Tanaka K., Tanaka T., Hara A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget. 2016;7:11018–11032. doi: 10.18632/oncotarget.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valent P., Bonnet D., De Maria R., Lapidot T., Copland M., Melo J.V., Chomienne C., Ishikawa F., Schuringa J.J., Stassi G. Cancer stem cell definitions and terminology: the devil is in the details. Nat. Rev. Cancer. 2012;12:767–775. doi: 10.1038/nrc3368. [DOI] [PubMed] [Google Scholar]
- van der Zee M., Sacchetti A., Cansoy M., Joosten R., Teeuwssen M., Heijmans-Antonissen C., Ewing-Graham P.C., Burger C.W., Blok L.J., Fodde R. IL6/JAK1/STAT3 signaling blockade in endometrial cancer affects the ALDHhi/CD126+ stem-like component and reduces tumor burden. Cancer Res. 2015;75:3608–3622. doi: 10.1158/0008-5472.CAN-14-2498. [DOI] [PubMed] [Google Scholar]
- Wong D.J., Liu H., Ridky T.W., Cassarino D., Segal E., Chang H.Y. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell. 2008;2:333–344. doi: 10.1016/j.stem.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Meng F., Dong L., Block C.J., Mitchell A.V., Wu J., Jang H., Chen W., Polin L., Yang Q. Disulfiram and BKM120 in combination with chemotherapy impede tumor progression and delay tumor recurrence in tumor initiating cell-rich TNBC. Sci. Rep. 2019;9:236. doi: 10.1038/s41598-018-35619-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B., Wang S., Li R., Chen K., He L., Deng M., Kannappan V., Zha J., Dong H., Wang W. Disulfiram/copper selectively eradicates AML leukemia stem cells in vitro and in vivo by simultaneous induction of ROS-JNK and inhibition of NF-kappaB and Nrf2. Cell Death Dis. 2017;8:e2797. doi: 10.1038/cddis.2017.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip N.C., Fombon I.S., Liu P., Brown S., Kannappan V., Armesilla A.L., Xu B., Cassidy J., Darling J.L., Wang W. Disulfiram modulated ROS-MAPK and NFkappaB pathways and targeted breast cancer cells with cancer stem cell-like properties. Br. J. Cancer. 2011;104:1564–1574. doi: 10.1038/bjc.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Butler E.B., Tan M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013;4:e532. doi: 10.1038/cddis.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.