Abstract
Background:
Right ventricular (RV) dysfunction is frequently observed in patients with aortic stenosis (AS). Nevertheless, assessment of regional RV deformation is yet not performed. The aim of the study was to analyze the impact of moderate and severe AS on global and regional RV function by a multisegmental approach using tissue Doppler imaging (TDI).
Methods:
In 50 patients (Group I – AS [n = 25] and Group II – normal controls [n = 25]), additional echocardiographic views of the RV were prospectively performed. The TDI sample volume was placed in the basal myocardial region of the anterior (RV-anterior), inferior (RV-inferior), and free RV wall (RV-free wall) to assess the following parameters: S'RV, E'RV, and A'RV waves; IVCTRV; IVRTRV; and myocardial performance index (MPIRV).
Results:
In AS patients, left ventricular (LV) mass index, left atrial (LA) volume index, and LV end-diastolic pressure were significantly increased. Moreover, AS patients had higher systolic pulmonary artery pressure (sPAP) and lower values for PV AccT (P < 0.0001), but TAPSE was not different between the two groups (P = 0.062). In AS patients, IVRTRV-anterior, IVRTRV-inferior, and IVRTRV-free wall and MPIRV were statistically increased (P < 0.0001). A significant correlation between IVRTRV (evaluated at all three regions) and the parameters including sPAP, PV AccT, and ELV/e'LV ratio was observed in AS. A strong correlation was observed between IVRTRV-free wall/inferior and AS severity by evaluation of velocities, gradient, and aortic valve area (P < 0.0001).
Conclusions:
The present study reports a correlation between the severity of AS and the increase of IVRTRV and MPIRV. Thus, a distinct analysis of RV performance is important for echocardiographic evaluation of patients with AS.
Keywords: Aortic stenosis, echocardiography, right ventricular function, tissue Doppler imaging
INTRODUCTION
In clinical routine noninvasive imaging of the right ventricle (RV), assessment of function remains challenging because of the peculiar and complex RV morphology.[1,2] However, the role of RV function is well recognized as a determinant of survival and cardiac symptoms in patients with valvular heart diseases. Some studies have demonstrated that RV dysfunction (RVD) is frequent in patients with aortic stenosis (AS) and is associated with poor prognosis.[3] In the current guidelines, there are no specific recommendations for the evaluation of RV function in patients with left-sided valvular heart diseases or even in patients with AS.[4]
Different segmental models of the left ventricle are implemented in the routine clinical assessment of regional left ventricular (LV) wall motion abnormalities. It can be assumed that a segmental model of the RV will also provide complementary informations about the regional function and deformation of the RV. Although a segmental description of the RV has been provided by the American Society of Echocardiography, this method of analysis is not yet established in routine clinical practice.[1]
RV function is commonly evaluated by a rather qualitative approach than a quantitative approach using two-dimensional (2D) transthoracic echocardiography.[5] Fractional area change (FAC) is a systolic index used to assess global RV function, rather than regional, and is calculated by a single RV section leading frequently to errors in measurements. In comparison with conventional 2D echocardiography, tissue Doppler imaging (TDI) might be more sensitive for the detection of subclinical myocardial changes of RV function and might be useful for the evaluation and monitoring of valvular heart diseases.[6] TDI allows the regional assessment of systolic and diastolic myocardial RV velocities and specific time intervals: systolic (S'RV), early diastolic (E'RV), and late atrial diastolic (A'RV) velocities; isovolumic contraction and relaxation times (IVCTRV, IVRTRV); and the RV ejection time (ETRV). However, these parameters have been rarely assessed in the literature and have been evaluated only in the region of the free RV wall in the apical four-chamber view.[7] Regions of the anterior and inferior RV wall and their contribution on RV mechanics are not investigated.
The purpose of the present study was to provide and offer a comprehensive echocardiographic approach for a more detailed evaluation of RV function in routine clinical practice. The present study focuses on the assessment of RV function by a multisegmental approach using TDI in patients with AS in comparison with healthy controls and intends to analyze the impact of moderate and severe AS on global and regional RV function.
METHODS
Study population
Among 190 patients who underwent echocardiographic examinations and screened, patients with sinus rhythm and moderate (aortic valve area [AVA], 0.60–0.85 cm2/m2) or severe AS (AVA, <0.60 cm2/m2) (Group I, n = 25), according to the current recommendations of the European Association of Cardiovascular Imaging (EACVI), and normal LV systolic function (ejection fraction [EF], ≥50%) were included prospectively from June to October 2017.[8] Furthermore, healthy controls (Group II, n = 25), admitted for a routine visit, without evidence of cardiovascular diseases, with completely normal clinical examination and normal structural and functional findings on echocardiography, were included as a control cohort. The following exclusion criteria were considered: incomplete echocardiographic datasets or poor image quality, atrial fibrillation, frequent extrasystoles, right bundle branch block and left bundle branch block, other electrocardiographic conduction abnormalities, coronary artery diseases that had an impact on LV and RV ventricular function, previous myocardial infarction or pulmonary embolism, severe chronic obstructive pulmonary disease, presence of pacemakers or other devices in the RV, concomitant moderate or severe valvular defects, and pericardial effusion [Supplementary Material Figure 1 (220.4KB, tif) ]. Informed consent was obtained from all participants included in the study.
Echocardiography
In all patients, transthoracic echocardiography (TTE) was performed in accordance with the ASE and ESC/EACVI recommendations.[8] TTE was performed with a Vivid E95 system with a M5S phased array probe (GE Healthcare Vingmed Ultrasound AS, Horten, Norway), and all parameters were analyzed offline using the EchoPac software (version 12.0.1; GE Healthcare Vingmed Ultrasound AS, Horten, Norway) by two expert operators blinded to clinical data. LV systolic function was characterized by LV volume and LV EF analysis using the modified Simpson's rule in the apical two- and four-chamber view. The assessment of LV mass index and LV hypertrophy was performed. LV diastolic function was characterized by the assessment of peak E-wave velocity, peak A-wave velocity, mitral valve DT, and ELV/e'LV ratio.[9] LV dimensions and LV wall thickness were assessed by M-Mode measurements. LV mass was measured with M-Mode echocardiography as LV mass (g) = 0.8 (1.04 [([LVEDD + IVSd + PWd]3− LVEDD3)]) +0.6, where IVSd is interventricular septum thickness at end-diastole, LVEDD is LV end-diastolic diameter, and PWd is posterior wall thickness at end-diastole. For the assessment of early diastolic filling (E), the pulsed-wave Doppler sample volume was positioned at the tip of the tenting area of the mitral valve in the apical long-axis view. The mean e' was assessed in the basal inferoseptal and lateral LV region in the apical four-chamber view using TDI. Left atrial (LA) volume index was assessed by biplane LA planimetry in the apical two- and four-chamber view.[10,11] AS severity was evaluated by the assessment of maximum/mean velocities and maximum/mean gradient of the aortic valve (AV) and by effective AVA, calculated by the continuity equation. LV ventricular outflow tract (LVOT) diameter was measured in the long-axis view in systole, and LVOT velocities were assessed by pulsed-wave Doppler echocardiography in the apical long-axis view.
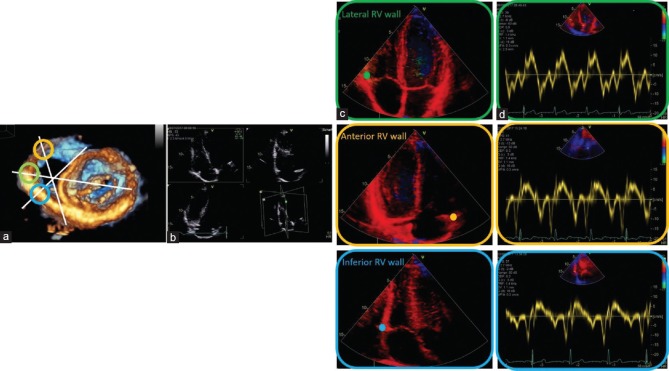
The following RV parameters were assessed by conventional 2D and Doppler echocardiography: basal and mid-RV diameters in the apical four-chamber view, proximal and distal RV outflow tract (RVOT) diameters and RVOT flow velocities, and pulmonary artery acceleration time (PV AccT) 7 by pulsed-wave Doppler echocardiography in the parasternal short-axis view, tricuspid annular plane systolic excursion (TAPSE), maximum (TR PGmax), and velocities (Vmax TR) of the tricuspid regurgitant flow in the apical four-chamber view. The systolic pulmonary artery pressure (sPAP) was estimated by tricuspid regurgitation velocity. The pressure gradient RV to the right atrium was calculated as 4V2 and according to Bernoulli's equation, where V is the peak velocity of the jet. The sPAP was estimated using the equation PASP = 4V2+ RA pressure. The right arterial pressure (RAP) was derived from the inferior vena cava diameter and degree of respiratory collapse. Particular attention was devoted to the assessment of the RV views. Beyond conventional standardized RV views, reported by Rudski et al.,[1] additional RV views were documented that permitted a regional assessment of the different RV walls. The RV inflow tract (RVIT) was centralized within the sector by tilting the apical long-axis view of the LV into the RVIT to document the anterior RV regions. By 60° clockwise rotation, the inferior RV regions were documented, followed by a documentation of the free RV wall in the conventional four-chamber view, which is often labeled as lateral RV wall [Figure 1]. Using pulsed-wave Doppler TDI, Doppler sample volume was placed at the anterior, inferior, and lateral RV myocardium near the tricuspid annulus. S'RV, E'RV, and A'RV waves; IVRTRV; IVCTRV; and ETRV were assessed from each region. Finally, myocardial performance index (MPIRV) was calculated by the following: (IVCTRV + IVRTRV)/ETRV. In addition, TDI parameters measured at the three points of the RV myocardium near the tricuspid annulus were compared between Group I and II to assess whether or not regional contribution to RV function of anterior, inferior, and lateral RV wall was different. Some patients, initially enrolled according the inclusion criteria, were afterward excluded by analysis because the visualization of the three RV walls and respective TDI parameters was challenging. We included only the patients with excellent echocardiographic windows and good quality of images.
Figure 1.
Transthoracic three-dimensional echocardiography, documenting the en-face short-axis view to the mitral valve from the LV cavity, is used only for demonstrative purposes. In white, the cutting planes of RV are labeled. The green circle indicates the lateral RV wall, the yellow circle the anterior RV wall, and the blue circle the inferior RV wall (a). Triplane imaging of the RV was obtained, focusing on RV using the apical four-chamber view as the primary scan plane (b). Complementary views of RV using TDI in addition to the conventional standardized RV views were recorded (c). The sample volume of pulsed-wave TDI was placed on the RV lateral, anterior, and inferior wall at the lateral, anterior, and inferior tricuspid annulus (in green, yellow, and blue, respectively). Tissue velocities were obtained from the three RV walls, respectively (d). LV = Left ventricular, RV = Right ventricle, TDI = Tissue Doppler imaging
Statistical analysis
Continuous variables were reported as mean ± standard deviation, whereas categorical variables were expressed as numbers and percentages. Quantitative variables with a nonparametric distribution between the groups were compared by Mann–Whitney U-test. Categorical variables were compared with Chi-square statistics. Kruskal–Wallis test was performed to compare RV TDI parameters in correspondence with the anterior, inferior, and lateral RV walls, in the control group and AS group. Correlations were examined by Spearman's correlation model for nonparametric data. Statistical significance was set at a level of P < 0.05. Statistical analysis was done using the SPSS version 25.0 software package (SPSS Inc., Chicago, Illinois, USA).
Reproducibility study
All echocardiographic images were digitally recorded and reviewed only if they had a good quality. Interobserver variability for TDI parameters was assessed in ten randomly selected studies and was calculated as the ratio (expressed as a percentage) of the difference between the values obtained by each observer (expressed as an absolute value) divided by the mean of the two values and as intraclass correlation coefficients. Intraobserver variability was calculated by a similar approach.
RESULTS
The baseline characteristics of the study population are presented in Table 1. In AS patients, Vmax was 4.1 ± 0.7 m/s, AVmeanGrad 38.1 ± 12.5 mmHg, and AVA index (AVA-I) 0.4 ± 0.1 cm2/m2. Twenty AS patients had AVA-I <0.6 cm2/m2, suggesting a severe grade of AS, whereas five patients showed moderate AS with AVA-I 0.6–0.85 cm2/m2, according to the current cutoffs. LV mass index (164 ± 45 g/m2vs. 119 ± 31), LA volume index (64 ± 18 mL/m2vs. 46 ± 10), and ELV/e'LV ratio (16.2 ± 5.5 vs. 7.4 ± 1.2) were significantly higher (P < 0.0001) in comparison with the control group. No differences were found between the two groups in terms of LV volumes and LV EF.
Table 1.
Demographic and echocardiographic data of the study population
| Parameter | Control group (n=25) | AS group (n=25) | P |
|---|---|---|---|
| General characteristics | |||
| Age (years) | 54±12 | 71±12 | <0.0001 |
| Male, n (%) | 20 (80) | 18 (72) | 0.508 |
| BSA (m2) | 1.9±0.1 | 1.8±0.1 | 0.404 |
| M-mode | |||
| IVSd (cm) | 1±0.2 | 1.4±0.3 | <0.0001 |
| LVIDd (cm) | 4.8±0.3 | 4.6±0.8 | 0.465 |
| LVPWd (cm) | 1±0.2 | 1.3±0.2 | <0.0001 |
| IVSs (cm) | 1.4±0.2 | 1.7±0.3 | <0.0001 |
| LVIDs (cm) | 3±0.3 | 3±0.7 | 0.571 |
| LVPWs (cm) | 1.6±0.2 | 1.9±0.2 | 0.003 |
| LVmass/BSA (g/m2) | 119±31 | 164±45 | <0.0001 |
| 2D measurements | |||
| LVEDV (ml) | 109±21 | 104.4±32.6 | 0.304 |
| LVESV (ml) | 38±8.5 | 37.1±15.9 | 0.443 |
| LVEF (%) | 65±4 | 65±5 | 0.984 |
| LA volume (ml) | 46±10 | 64±18 | <0.0001 |
| Diastolic function | |||
| MV E max (m/s) | 0.7±0.1 | 0.8±0.2 | 0.620 |
| MV Dec time (ms) | 170±26 | 185.4±83.5 | 0.377 |
| MV A max (m/s) | 0.6±0.1 | 0.9±0.3 | <0.0001 |
| E LV/e’ LV ratio | 7.4±1.2 | 16.2±5.5 | <0.0001 |
| LVOT measurements | |||
| LVOT V mean (m/s) | 0.7±0.1 | 0.6±0.1 | <0.0001 |
| LVOT meanGrad (mmHg) | 2.8±0.8 | 1.8±1 | <0.0001 |
| LVOT VTI (cm) | 23.4±3.1 | 21.6±5 | 0.114 |
| LVOT SV (ml) | 75.8±13.4 | 72.8±14.8 | 0.308 |
| AVO (ms) | 65.1±24.1 | 61.4±24.9 | 0.627 |
| AVC (mse) | 346.6±45.9 | 376.2±35.4 | <0.0001 |
| RV measurements | |||
| RVD basal (cm) | 3.3±0.3 | 3.1±0.4 | 0.263 |
| RVOT prox (cm) | 2.6±0.3 | 2.7±0.4 | 0.815 |
| TAPSE (mm) | 24.7±1.9 | 23.2±3.1 | 0.062 |
| Area RA (cm2) | 15.2±1.9 | 15±3.9 | 0.541 |
| TR max PG (mmHg) | 23.5±4 | 36.8±11 | <0.0001 |
| sPAP (mmHg) | 28.3±4 | 41.4±11.1 | <0.0001 |
| RVOT measurements | |||
| RVOT V mean (m/s) | 0.6±0.08 | 0.6±0.1 | 0.817 |
| RVOT meanGrad (mmHg) | 1.7±0.4 | 1.7±0.5 | 0.751 |
| RVOT VTI (cm) | 19.3±2.7 | 19.9±3.5 | 0.534 |
| RVOT SV (ml) | 77.4±13.9 | 81.1±18.1 | 0.518 |
| PV accel time (ms) | 132±28.9 | 88.7±20.1 | <0.0001 |
AS=Aortic stenosis, BSA=Body surface area, IVSd=Interventricular septum thickness at end-diastole, LV=Left ventricular, LVIDd=LV internal dimension at end-diastole, LVPWd=LV posterior wall thickness at end-diastole, IVSs=Interventricular septum thickness at end-systole, LVIDs=LV internal dimension ad end-systole, LVPWs=LV posterior wall thickness at end-systole, LVEDV=LV end diastolic volume, LVESV=LV end-systolic volume, LVEF=LV ejection fraction, LA=Left atrium, MV=Mitral valve, LVOT=LV outflow tract, AVO=Aortic valve opening, AVC=Aortic valve closure, RV=Right ventricular, RVD=RV dimension, RVOT=RV outflow tract, TAPSE=Tricuspid annular plane systolic excursion, TR=Tricuspid regurgitation, sPAP=Systolic pulmonary artery pressure, VTI=Velocity time integrals, SV=Stroke volume, PV=Pulmonary valve, RA=Right atrium, HR=Heart rate, PG=Peak gradient, 2D=Two dimensional
In AS patients, PV AccT (88.7 ± 20.1 vs. 132 ± 20.1; P < 0.0001) was significantly reduced in comparison with healthy controls, although TAPSE (23.2 ± 3.1 vs. 24.7 ± 1.9; P = 0.062) was not statistically different. Further, AS patients showed higher transtricuspid gradient (36.8 ± 11 vs. 23.5 ± 4; P < 0.0001) and consequently higher sPAP (41.4 ± 11.1 vs. 28.3 ± 4; P < 0.0001). All patients had the same mean RAP, estimated of 5 mmHg.
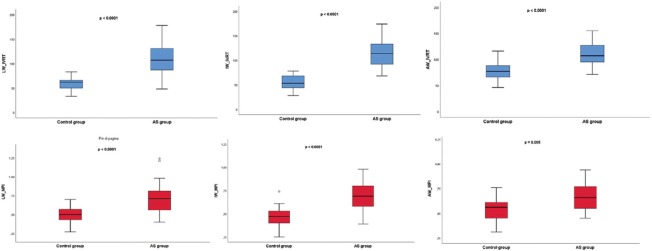
IVRTRV measured at anterior, inferior, and lateral tricuspid annulus was significantly longer and MPIRV was significantly higher in AS patients (P < 0.0001) [Figure 2]. Furthermore, E'RV was reduced if calculated in correspondence with inferior and anterior RV walls [Table 2]. In the control group, the anterior RV wall showed a significantly longer IVRTRV (P < 0.0001), a higher MPIRV (P = 0.039), and a higher A'RV wave (P < 0.0001) in comparison with the inferior and lateral RV wall. In AS patients, these differences could not have been observed, whereas only S'RV of the inferior RV wall was significantly lower (P = 0.028) in comparison with the lateral and anterior RV wall [Table 3].
Figure 2.
Comparison between AS (Group I) and controls (Group II) in terms of IVRTRV (above, in blue) and MPIRV (below, in red), calculated at lateral, inferior, and anterior tricuspid annulus, respectively. AS = Aortic stenosis; IVRT = Isovolumic relaxation time, MPI = Myocardial performance index
Table 2.
Tissue Doppler Imaging parameters measured at lateral, inferior, and anterior right ventricular wall
| Parameter | Control group (n=25) | AS group (n=25) | P |
|---|---|---|---|
| Lateral RV wall | |||
| IVCTRV (ms) | 68.2±13.1 | 72.4±19.3 | 0.662 |
| IVRTRV (ms) | 58.6±13.2 | 111.4±31.9 | <0.0001 |
| ETRV (ms) | 264.4±41.3 | 264.9±42.5 | 0.969 |
| MPIRV | 0.5±0.1 | 0.7±0.2 | <0.0001 |
| S’ wave RV (m/s) | 0.14±0.02 | 0.13±0.02 | 0.06 |
| E’ wave RV (m/s) | 0.13±0.03 | 0.11±0.03 | 0.08 |
| A’ wave RV (m/s) | 0.14±0.03 | 0.16±0.05 | 0.06 |
| Inferior RV wall | |||
| IVCTRV (ms) | 69.6±16.9 | 69.4±20.4 | 0.741 |
| IVRTRV (ms) | 54.7±14.7 | 114.6±29.3 | <0.0001 |
| ETRV (ms) | 271.9±33.1 | 272.7±40.6 | 0.869 |
| MPIRV | 0.4±0.1 | 0.6±0.1 | <0.0001 |
| S’ wave RV (m/s) | 0.13±0.02 | 0.11±0.02 | 0.02 |
| E’wave RV (m/s) | 0.13±0.03 | 0.11±0.07 | 0.03 |
| A’ wave RV (m/s) | 0.11±0.02 | 0.14±0.05 | 0.134 |
| Anterior RV wall | |||
| IVCTRV (ms) | 65.5±10.4 | 69.7±15.4 | 0.484 |
| IVRTRV (ms) | 78.4±17.5 | 109±23.1 | <0.0001 |
| ETRV (ms) | 268.2±35.1 | 271.1±43.7 | 0.614 |
| MPIRV | 0.5±0.1 | 0.6±0.1 | 0.005 |
| S’ wave RV (m/s) | 0.14±0.02 | 0.13±0.03 | 0.08 |
| E’ wave RV (m/s) | 0.15±0.03 | 0.12±0.04 | 0.034 |
| A’ wave RV (m/s) | 0.15±0.02 | 0.16±0.05 | 0.230 |
IVCT=Isovolumic contraction time, IVRT=Isovolumic relaxation time, ET=Ejection time, MPI=Myocardial performance index, RV=Right ventricular
Table 3.
Comparison among tissue Doppler Imaging parameters evaluated at the three right ventricular walls in aortic stenosis patients and in controls
| Parameters | Lateral wall RV | Inferior wall RV | Anterior wall RV | P |
|---|---|---|---|---|
| Control group | ||||
| IVCTRV (ms) | 68.2±13.1 | 69.6±16.9 | 65.5±10.4 | 0.654 |
| IVRTRV (ms) | 58.6±13.2 | 54.7±14.7 | 78.4±17.5 | <0.0001 |
| ETRV (ms) | 264.4±41.3 | 271.9±33.1 | 268.2±35.1 | 0.818 |
| MPIRV | 0.4±0.1 | 0.4±0.1 | 0.5±0.1 | 0.039 |
| S’ wave RV (m/s) | 0.14±0.02 | 0.13±0.02 | 0.14±0.02 | 0.036 |
| E’wave RV (m/s) | 0.13±0.03 | 0.13±0.03 | 0.14±0.03 | 0.272 |
| A’ wave RV (m/s) | 0.14±0.03 | 0.11±0.02 | 0.15±0.02 | <0.0001 |
| AS group | ||||
| IVCTRV (ms) | 72.4±19.3 | 69.4±20.4 | 69.7±15.4 | 0.769 |
| IVRTRV (ms) | 111.4±31.9 | 114.6±29.3 | 109±23.1 | 0.874 |
| ETRV (ms) | 264.9±42.5 | 272.7±40.6 | 271.1±43.7 | 0.805 |
| MPIRV | 0.7±0.2 | 0.6±0.1 | 0.6±0.1 | 0.846 |
| S’ wave RV (m/s) | 0.13±0.02 | 0.11±0.02 | 0.13±0.03 | 0.028 |
| E’ wave RV (m/s) | 0.11±0.03 | 0.11±0.07 | 0.12±0.04 | 0.150 |
| A’ wave RV (m/s) | 0.16±0.05 | 0.14±0.05 | 0.16±0.05 | 0.140 |
IVCT=Isovolumic contraction time, IVRT=Isovolumic relaxation time, ET=Ejection time, MPI=Myocardial performance index, RV=Right ventricular
In AS patients, a significant correlation between regional IVRTRV and the following parameters of RV function was found: sPAP, TRmax PG, and PV AccT [Table 4]. A linear correlation between IVRTRV-free wall/inferior and LV mass/BSA and between IVRTRV-free wall/inferior and LA volumes was found. In addition, the correlation between diastolic dysfunction, documented by the increase of ELV/e'LV ratio, and IVRTRV was observed in AS patients. A strong correlation was found between IVRTRV-freewall/inferior and AS severity, evaluated by AV velocities, AV gradient, and AVA-I [Table 4].
Table 4.
Correlations of isovolumic relaxation time and myocardial performance index, measured at lateral, inferior, and anterior right ventricular wall, with aortic valvular parameters in aortic stenosis patients
| AS parameters | LW IVRTRV (ms) | IW IVRTRV (ms) | AW IVRTRV (ms) | LW MPIRV | IW MPIRV | AW MPIRV |
|---|---|---|---|---|---|---|
| V max (r, P) | 0.521**, 0.003 | 0.483**, 0.006 | 0.318, 0.081 | 0.394*, 0.028 | 0.488**, 0.005 | 0.216, 0.242 |
| AV meanGr (r, P) | 0.570**, 0.001 | 0.456**, 0.010 | 0.331, 0.069 | 0.437*, 0.014 | 0.443*, 0.013 | 0.174, 0.349 |
| VTI (r, P) | 0.595**, <0.0001 | 0.558**, 0.001 | 0.338, 0.063 | 0.316, 0.083 | 0.396*, 0,027 | 0.017, 0.928 |
| AVA ind (r, P) | −0.377**, 0.036 | −0.456**, 0.01 | −0.191, 0.304 | −0.299, 0.103 | −0.428*, 0.016 | −0.250, 0.175 |
*Significant (−0.05), **Highly significant (−0.01). AS=Aortic stenosis, IVRT=Isovolumic relaxation time, MPI=Myocardial performance index, AV=Aortic valve, VTI=Velocity time integrals, AVA=Aortic valve area, RV=Right ventricular, LW=Lateral wall, IW=Inferior wall, AW=Anterior wall
Reproducibility analysis
Intra- and interobserver variability expressed as the mean percentage error (absolute difference/mean) and the intraclass correlation coefficients (ICC) were very good for all studied parameters: IVRTRV-freewall (7% ±6% and 8% ±6%; ICC: 0.99 and 0.97); IVRTRV-anterior (7% ±7% and 6% ±7%; ICC: 0.99 and 0.98); IVRTRV-inferior (6% ±6% and 6% ±5%; ICC: 0.99 and 0.98); MPIRV-freewall (8% ±6% and 7% ±7%; ICC: 0.99 and 0.97); MPIRV-anterior (9% ±10% and 10% ±8%; ICC: 0.99 and 0.98); and MPIRV-inferior (6% ±5% and 7% ±7%; ICC: 0.99 and 0.97).
DISCUSSION
The present study focuses on the impact of moderate and severe AS on global and regional RV function. The following results can be summarized. (1) A correlation between the severity of AS and the increase of IVRTRV and MPIRV was reported. (2) Regional RV function detected by a multisegmental approach using TDI was different in normal probands. IVRTRV of the anterior RV wall, MPIRV, and A'RV were significantly increased in comparison with other RV segments. (3) These differences were not observed in AS patients, but only S'RV of the inferior RV was reduced in AS patients in comparison with the normal probands. (4) A significant correlation between regional IVRTRV and sPAP, TRmax PG, PV AccT, and ELV/e'LV ratio and a strong correlation between IVRTRV and AS severity were found.
Data of the analysis of RV remodeling and RV function are very limited in patients with AS in the literature.[12] Recent studies have demonstrated that RV has a key role in cardiac mechanics highlighting the importance of an accurate evaluation RV function and regional RV wall motion.[13] RV performance has been demonstrated to be a predictor of mortality and morbidity not only in patients with RV diseases but also in those with LV dysfunction, myocardial infarction, dilated cardiomyopathy, and valvular heart diseases.[14] The visualization of the RV is still challenging by echocardiography. Thus, qualitative assessment of the RV shape, size wall motion abnormalities, and RV volumes is generally limited and actually improved by 3D echocardiography.[15] However, the acquisition of the complete RV is often not possible in TTE in the clinical scenario. A more accurate quantitative assessment of the RV mechanics requires a more standardized echocardiographic examination. Surkova et al. have suggested six standardized 2D echocardiographic views to obtain a more comprehensive assessment of the different RV segments.[16] In the present study, an additional view was used to evaluate the inferior RV wall. Certainly, the analysis of RV function by a multisegmental approach, when it is possible and feasible, is clinically important because some pathological conditions may be characterized by specific patterns of RV segmental contraction.[17] This is the first study which specifically addressed the evaluation of regional RV function detected by TDI velocities of the regional basal RV myocardium in a very selected AS population. In our AS patients, the anterior, inferior, and free RV wall showed a similar TDI pattern in terms of IVRTRV and MPIRV. We do not know the pattern of RV segmental contraction in diseases that are characterized by RV involvement. Probably, regional TDI analysis may have an added value for a more comprehensive echocardiographic evaluation and further monitoring. Moreover, the additional analysis, performed to test if the variability in IVRTRV and MPIRV values was meaningful, confirmed the good reproducibility of findings. MPIRV is already known as an indicator of RV myocardial performance, which is unaffected by RV geometry.[18] It has been reported that RVD is associated with an increase of MPIRV, which correlates well with RV EF, RV FAC, and mean pulmonary artery pressure (mPAP).[19,20] Moreover, some studies have used IVRTRV measured by TDI at the lateral region to distinguish patients with increased sPAP.[21] Zimbarra Cabrita et al. have shown that IVRTRV was significantly increased in patients with pulmonary hypertension.[22] Lindqvist et al.[23] and Dambrauskaite et al.[24] have proposed the correlation between sPAP and IVRTRV as an additional noninvasive tool for the assessment of sPAP. In the present study, IVRTRV and MPIRV at all three RV wall regions were increased in AS patients, suggesting that all segments of the anterior, inferior, and lateral RV wall achieve a complete relaxation in patients with AS within a distinct time delay in comparison with normal controls. In addition, RV performance might be modified in AS patients due to impaired early and late diastolic velocities (E'RV and A'RV). These findings highlight that AS has an impact on RV function. Thus, especially MPIRV and IVRTRV might be useful as a useful indicators of RVD that may identify high-risk patients with AS in clinical routine. In normal controls, the anterior RV wall showed a prolonged IVRTRV and an increased MPIRV, in comparison with the inferior and lateral RV wall. Moreover, the strongest correlation observed between RV IVRT and AS severity was found in correspondence with RV free wall and inferior wall. Because, probably, the mechanics of the RV segments are different, this finding is interesting and highlights the importance of an accurate and multisegmental evaluation of regional RV TDI pattern.
LV pressure overload due to AS will lead to LV hypertrophy, small LV cavities, an increase of LV end-diastolic pressure (LVEDP), and diastolic dysfunction in a compensated stage. These LV alterations reduce the LV wall stress according to Laplace's law and induce an increase of LV mass, LA volume, and LVEDP as confirmed in the present study.[25]
Noteworthy, in our study, we found statistically significant differences between the two groups in terms of diastolic and systolic IVS and LVPW and LV mass/BSA, suggesting an adaptive compensatory mechanism of LV remodeling. According to the new classification of LV remodeling patterns in AS patients proposed by Gaasch and Zile,[26] in which beside LV mass index and relative wall thickness (RWT), LVEDV index was included, eight possible remodeling patterns may be described in severe AS. In our AS patients, using this classification, more prevalent remodeling pattern was concentric hypertrophy reported in 63% of cases. Furthermore, in the study population of 286 patients analyzed by Di Nora et al.,[27] concentric hypertrophy was more frequently (in 57.3%) described, and in addition, increased values of IVS, LVPW, and RWT were more often found in symptomatic patients compared to asymptomatic ones.
Secondary changes on pulmonary circulation due to AS have been observed by an increase of TRmax PG and sPAP and by a reduction of PV AccT, leading to impaired RV wall motion, although TAPSE was still in normal range. Thus, it was documented that increased LV afterload due to chronic diseases will lead to RV alterations with possible subsequent RVD.[28] Although TAPSE is the most widely used RV parameter in clinical routine,[29] TAPSE might not be an appropriate parameter to detect early changes of RVD due to AS. Interestingly, in our study population, no difference in terms of TAPSE between the two groups was reported. Thus, in addition to TAPSE, RV TDI parameters should be established in clinical practice and be routinely introduced for analysis of RV function to identify subclinical RVD. Furthermore, it is crucial to evaluate the possible different etiologies of RVD and RV remodeling pattern, analyzing accurately regional RV wall motion abnormalities, RV volumes and function, and the presence or not of late gadolinium enhancement or fat at cardiac magnetic resonance (CMR). A great challenge is to distinguish between RV adaptive remodeling and early pathological changes seen in inherited or acquired cardiomyopathies. In this, an integrated multi-imaging approach could be very helpful and needed in differential diagnosis, especially with the support of tissue characterization provided by CMR imaging.[30]
The correlation between IVRTRV and LV mass, LA volume, and ELV/e'LV ratio suggests that the RV/LV interdependence might have a key role in the pathophysiology of RVD in AS patients.
By our analysis, we can state that increased mLAP/SPAP secondary to severe AS effects RV function through increased IVRT-RV and MPI.[31] The increase of IVRTRV in relation to AS severity suggests also that a progression of RVD may occur with increasing AS severity. It can be assumed that early treatment of AS can induce restoration of load-dependent RVD.
Galli et al.[32] have shown that LV and RVD were associated with increased cardiovascular mortality in AS.[33] At present, the decision-making for the treatment of AS patients is based on the assessment of AS severity, LVEF, and symptom onset. As shown by the present data, comprehensive RV evaluation seems to be necessary in AS patients in addition to the conventional evaluation of the LV and the AV.[34] Regional RV TDI parameters can be proposed to be systematically assessed in AS patients to characterize RVD. In addition to advanced echocardiographic techniques, such as the quantification of the RV myocardial longitudinal strain,[35] it could provide new insights into the RV remodeling in the near future. However, further studies are needed to clarify whether or not improvement of RV function can be documented by restoration of TDI parameters to normal values after treatment of AS. It can be hypothesized that RV reverse remodeling[36] will occur after successful treatment of AS implying a better prognosis for clinical outcomes in these patients.
Limitations
We recognize as limitation the fact that our population has been prospectively screened only in one institution; thus, our study suffers the problems related to a single-center analysis in terms of potential generalizability of its observations. Furthermore, we enrolled a high selected study population. This was due to the strict selection criteria and to the inclusion of only patients with excellent echocardiographic windows and good quality of images; further studies collecting a wider sample of patients should provide confirmation of these results.
We recognize that AS is common in the elderly people, whereas its prevalence is very low among adults aged <60 years. In younger AS patients, rheumatic disease and bicuspid AV were the most common etiologies. For this reason, in our study population, we found a statistically significant difference in terms of age between two groups. Anyway, elderly patients with completely normal structural and functional findings on echocardiography are poorly represented in clinical scenario. We also excluded by analysis patients with diastolic dysfunction secondary to hypertensive heart disease (HHD) because it has been documented that RV systolic dysfunction, evaluated using several echocardiographic parameters, is also common in patients with HHD and normal EF.[37] Thus, we have avoided potential bias in results. We have not explored the correlation of regional TDI RV parameters and the presence of symptoms in AS patients, how these measures of RV performance may be correlated or associated with outcome, the potential changes after specific treatment, and their role in follow-up. Certainly, further studies are needed to clarify these points.
CONCLUSIONS
The present study highlights the role of the analysis of RV function in AS patients. A correlation between the severity of AS and the increase of IVRTRV and MPIRV was reported, although TAPSE was still in normal range. It can be concluded that distinct analysis of RV performance is important for echocardiographic evaluation of patients with AS.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
SUPPLEMENTARY MATERIAL
Study design. ECG = Electrocardiogram, AF = Atrial fibrillation, RBBB = Right bundle branch block, LBBB = Left bundle branch block, LV = Left ventricular, RV = Right ventricle, COPD = Chronic obstructive pulmonary disease, MI = Myocardial infarction, AS = Aortic stenosis
REFERENCES
- 1.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Hatle L, Sutherland GR. Regional myocardial function – A new approach. Eur Heart J. 2000;21:1337–57. doi: 10.1053/euhj.2000.2251. [DOI] [PubMed] [Google Scholar]
- 3.Cavalcante JL, Rijal S, Althouse AD, Delgado-Montero A, Katz WE, Schindler JT, et al. Right ventricular function and prognosis in patients with low-flow, low-gradient severe aortic stenosis. J Am Soc Echocardiogr. 2016;29:325–33. doi: 10.1016/j.echo.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–91. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 5.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Pavlicek M, Wahl A, Rutz T, de Marchi SF, Hille R, Wustmann K, et al. Right ventricular systolic function assessment: Rank of echocardiographic methods vs. cardiac magnetic resonance imaging. Eur J Echocardiogr. 2011;12:871–80. doi: 10.1093/ejechocard/jer138. [DOI] [PubMed] [Google Scholar]
- 7.Yildirim N, Saricam E, Ozbakir C, Bozboga S, Ocal A. Assessment of the relationship between functional capacity and right ventricular ultrasound tissue characterization by integrated backscatter in patients with isolated mitral stenosis. Int Heart J. 2007;48:87–96. doi: 10.1536/ihj.48.87. [DOI] [PubMed] [Google Scholar]
- 8.Baumgartner H Chair, Hung J Co-Chair, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: A focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2017;18:254–75. doi: 10.1093/ehjci/jew335. [DOI] [PubMed] [Google Scholar]
- 9.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90:1284–9. doi: 10.1016/s0002-9149(02)02864-3. [DOI] [PubMed] [Google Scholar]
- 11.Hoit BD. Left atrial size and function: Role in prognosis. J Am Coll Cardiol. 2014;63:493–505. doi: 10.1016/j.jacc.2013.10.055. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz LA, Rozenbaum Z, Ghantous E, Kramarz J, Biner S, Ghermezi M, et al. Impact of right ventricular dysfunction and tricuspid regurgitation on outcomes in patients undergoing transcatheter aortic valve replacement. J Am Soc Echocardiogr. 2017;30:36–46. doi: 10.1016/j.echo.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 13.Asami M, Stortecky S, Praz F, Lanz J, Räber L, Franzone A, et al. Prognostic value of right ventricular dysfunction on clinical outcomes after transcatheter aortic valve replacement. JACC Cardiovasc Imaging. 2019;12:577–87. doi: 10.1016/j.jcmg.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part II: Pathophysiology, clinical importance, and management of right ventricular failure. Circulation. 2008;117:1717–31. doi: 10.1161/CIRCULATIONAHA.107.653584. [DOI] [PubMed] [Google Scholar]
- 15.Surkova E, Muraru D, Iliceto S, Badano LP. The use of multimodality cardiovascular imaging to assess right ventricular size and function. Int J Cardiol. 2016;214:54–69. doi: 10.1016/j.ijcard.2016.03.074. [DOI] [PubMed] [Google Scholar]
- 16.Surkova E, Peluso D, Kasprzak JD, Badano LP. Use of novel echocardiographic techniques to assess right ventricular geometry and function. Kardiol Pol. 2016;74:507–22. doi: 10.5603/KP.a2016.0041. [DOI] [PubMed] [Google Scholar]
- 17.La Gerche A, Jurcut R, Voigt JU. Right ventricular function by strain echocardiography. Curr Opin Cardiol. 2010;25:430–6. doi: 10.1097/HCO.0b013e32833b5f94. [DOI] [PubMed] [Google Scholar]
- 18.Tei C, Dujardin KS, Hodge DO, Bailey KR, McGoon MD, Tajik AJ, et al. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr. 1996;9:838–47. doi: 10.1016/s0894-7317(96)90476-9. [DOI] [PubMed] [Google Scholar]
- 19.Blanchard DG, Malouf PJ, Gurudevan SV, Auger WR, Madani MM, Thistlethwaite P, et al. Utility of right ventricular tei index in the noninvasive evaluation of chronic thromboembolic pulmonary hypertension before and after pulmonary thromboendarterectomy. JACC Cardiovasc Imaging. 2009;2:143–9. doi: 10.1016/j.jcmg.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Eidem BW, O'Leary PW, Tei C, Seward JB. Usefulness of the myocardial performance index for assessing right ventricular function in congenital heart disease. Am J Cardiol. 2000;86:654–8. doi: 10.1016/s0002-9149(00)01047-x. [DOI] [PubMed] [Google Scholar]
- 21.Bréchot N, Gambotti L, Lafitte S, Roudaut R. Usefulness of right ventricular isovolumic relaxation time in predicting systolic pulmonary artery pressure. Eur J Echocardiogr. 2008;9:547–54. doi: 10.1093/ejechocard/jen121. [DOI] [PubMed] [Google Scholar]
- 22.Zimbarra Cabrita I, Ruisanchez C, Dawson D, Grapsa J, North B, Howard LS, et al. Right ventricular function in patients with pulmonary hypertension; the value of myocardial performance index measured by tissue Doppler imaging. Eur J Echocardiogr. 2010;11:719–24. doi: 10.1093/ejechocard/jeq051. [DOI] [PubMed] [Google Scholar]
- 23.Lindqvist P, Waldenström A, Wikström G, Kazzam E. Right ventricular myocardial isovolumic relaxation time and pulmonary pressure. Clin Physiol Funct Imaging. 2006;26:1–8. doi: 10.1111/j.1475-097X.2005.00639.x. [DOI] [PubMed] [Google Scholar]
- 24.Dambrauskaite V, Delcroix M, Claus P, Herbots L, Palecek T, D'hooge J, et al. The evaluation of pulmonary hypertension using right ventricular myocardial isovolumic relaxation time. J Am Soc Echocardiogr. 2005;18:1113–20. doi: 10.1016/j.echo.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 25.Jurcut R, Giusca S, La Gerche A, Vasile S, Ginghina C, Voigt JU. The echocardiographic assessment of the right ventricle: What to do in 2010? Eur J Echocardiogr. 2010;11:81–96. doi: 10.1093/ejechocard/jep234. [DOI] [PubMed] [Google Scholar]
- 26.Gaasch WH, Zile MR. Left ventricular structural remodeling in health and disease: With special emphasis on volume, mass, and geometry. J Am Coll Cardiol. 2011;58:1733–40. doi: 10.1016/j.jacc.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 27.Di Nora C, Cervesato E, Cosei I, Ravasel A, Popescu BA, Zito C, et al. New classification of geometric ventricular patterns in severe aortic stenosis: Could it be clinically useful? Echocardiography. 2018;35:1077–84. doi: 10.1111/echo.13892. [DOI] [PubMed] [Google Scholar]
- 28.Todiere G, Neglia D, Ghione S, Fommei E, Capozza P, Guarini G, et al. Right ventricular remodelling in systemic hypertension: A cardiac MRI study. Heart. 2011;97:1257–61. doi: 10.1136/hrt.2010.221259. [DOI] [PubMed] [Google Scholar]
- 29.Ghio S, Recusani F, Klersy C, Sebastiani R, Laudisa ML, Campana C, et al. Prognostic usefulness of the tricuspid annular plane systolic excursion in patients with congestive heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am J Cardiol. 2000;85:837–42. doi: 10.1016/s0002-9149(99)00877-2. [DOI] [PubMed] [Google Scholar]
- 30.Antonini-Canterin F, Di Nora C. Arrhythmogenic right ventricular cardiomyopathy or athlete's heart? Challenges in assessment of right heart morphology and function. Monaldi Arch Chest Dis. 2019;89:1. doi: 10.4081/monaldi.2019.1047. [DOI] [PubMed] [Google Scholar]
- 31.Biering-Sørensen T, Mogelvang R, Schnohr P, Jensen JS. Cardiac time intervals measured by tissue doppler imaging M-mode: Association with hypertension, left ventricular geometry, and future ischemic cardiovascular diseases. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002687. pii: e002687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galli E, Guirette Y, Feneon D, Daudin M, Fournet M, Leguerrier A, et al. Prevalence and prognostic value of right ventricular dysfunction in severe aortic stenosis. Eur Heart J Cardiovasc Imaging. 2015;16:531–8. doi: 10.1093/ehjci/jeu290. [DOI] [PubMed] [Google Scholar]
- 33.Melby SJ, Moon MR, Lindman BR, Bailey MS, Hill LL, Damiano RJ, Jr, et al. Impact of pulmonary hypertension on outcomes after aortic valve replacement for aortic valve stenosis. J Thorac Cardiovasc Surg. 2011;141:1424–30. doi: 10.1016/j.jtcvs.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pibarot P, Dumesnil JG. Aortic stenosis: Look globally, think globally. JACC Cardiovasc Imaging. 2009;2:400–3. doi: 10.1016/j.jcmg.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Muraru D, Onciul S, Peluso D, Soriani N, Cucchini U, Aruta P, et al. Sex- and method-specific reference values for right ventricular strain by 2-dimensional speckle-tracking echocardiography. Circ Cardiovasc Imaging. 2016;9:e003866. doi: 10.1161/CIRCIMAGING.115.003866. [DOI] [PubMed] [Google Scholar]
- 36.Calcutteea A, Holmgren A, Lindqvist P, Henein MY. Organised right ventricular remodelling in aortic stenosis even after valve replacement. Int J Cardiol. 2013;168:1549–50. doi: 10.1016/j.ijcard.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 37.Oketona OA, Balogun MO, Akintomide AO, Ajayi OE, Adebayo RA, Mene-Afejuku TO, et al. Right ventricular systolic function in hypertensive heart failure. Vasc Health Risk Manag. 2017;13:353–60. doi: 10.2147/VHRM.S142429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study design. ECG = Electrocardiogram, AF = Atrial fibrillation, RBBB = Right bundle branch block, LBBB = Left bundle branch block, LV = Left ventricular, RV = Right ventricle, COPD = Chronic obstructive pulmonary disease, MI = Myocardial infarction, AS = Aortic stenosis