Abstract
Severe acute malnutrition (SAM) in children under five years is an important public health problem due to associated high mortality and long-term health consequences. Research on the dietary causes of SAM, especially the role and relative importance of dietary protein, in the aetiology of oedematous malnutrition, has led to considerable debates and controversies. The present article revisits some of the debates in this field, where the researchers at the National Institute of Nutrition (NIN), Hyderabad, India, with their pioneering work, have contributed to the global literature on the various facets of the disease. Highlighting the importance of energy as a bigger problem than protein malnutrition is a noteworthy contribution of NIN's research. It is, however, important to examine the protein quality of the diets in light of the new information on the lysine requirements. The article argues that the currently dominating hypothesis of free radical theory requires a critical review of the supporting evidence. Over the past few decades, the research has focused on low-cost diets using locally available foods. The article also argues that solutions based on local foods, being acceptable and sustainable, need to be strengthened for their effective delivery through the existing nutrition programmes. Recent evidence shows that the use of ready-to-use therapeutic foods (RUTF) with high micronutrient density may be linked with higher mortality possibly due to the high iron content, which could be counterproductive. There are several unaddressed concerns regarding the potential long-term impact of consumption of RUTF in children with SAM. More evidence and a cautious approach are, therefore, needed before implementing these solutions.
Keywords: Adaptation, energy malnutrition, India, Kwashiorkor, marasmus, oedematous malnutrition, protein, ready-to-use therapeutic foods, severe acute malnutrition
Introduction
Severe acute malnutrition (SAM), defined as severe wasting [weight-for-height Z score <−3 based on World Health Organization (WHO) reference standard] and/or the presence of nutritional oedema, is a life-threatening condition which needs urgent attention and appropriate management to reduce mortality and promote recovery. India has a high prevalence of SAM, representing a huge burden, and intriguingly, the recent National Family Health Survey-4 indicates a higher prevalence of severe wasting (7.5%) compared to the previous report (6.4%)1.
Over the last century, significant contributions have been made to the research on various aspects of severe life-threatening malnutrition in children that have been identified with different names such as protein-calorie malnutrition, protein-energy malnutrition (PEM), oedematous malnutrition, nutritional oedema, severe wasting or with names based on clinical manifestations such as marasmus, Kwashiorkor or Marasmic Kwashiorkor2. The legendary British physiologist, John Conrad Waterlow's early inventions of microbalances, his first impressions of fatty livers, his linking of the disease aetiology with deficiency of protein and calories and his foresight about protein requirements have placed our understanding of SAM on a sound footing3. Gopalan and Srikantia, his contemporaries from NIN, helped solve the major nutritional problems faced by the country during their time4,5,6. Gopalan and Waterlow also have a common lineage working with Professor B.S. Platt7 and, despite their differences on certain issues, their mutual collaboration has greatly benefitted the progress of knowledge regarding the pathogenesis, prevention and management of SAM. However, what was once considered protein deficiency leading to Kwashiorkor and calorie deficiency leading to marasmus is now being considered as deficiency of Type I nutrients leading to Kwashiorkor and Type II nutrients leading to marasmus or wasting8. Over the last century, research in this area has generated many theories, many controversies and, with it, many enigmas. It is important to review the evidence underlying these theories and reflect on the enigmas in the light of new information that has become available with advances in research in the past few decades. Srikantia9 discussed the adaptation theory proposed by Gopalan10, which has now been largely superseded by the free radical theory by Golden and Ramdath11. However, review of the information on the protein requirements in children12 and the controversies on some of the previous studies13,14, suggests that the free radical theory could in fact be a flipped version of protein deficiency theory with serious flaws.
This article aims to review important research carried out to improve our understanding of the aetiology and management of SAM and its relevance to the global literature.
Prevalence of protein-energy malnutrition (PEM)/severe acute malnutrition (SAM)
A large volume of research in the first few decades of the 20th century dealt with Kwashiorkor or oedematous malnutrition, which had very high mortality with median case fatality rates of 20-30 per cent15. Kwashiorkor, which means the 'disease of the deposed child' in the Ga language, was first reported by Williams in 193516. More than a decade later in 1947, Kwashiorkor was first reported from Assam in India as 'malignant malnutrition' by Hare17. In 1948, a case series was published reporting a differential diagnosis of infantile pellagra18. Kwashiorkor may have been reported under a different name much earlier in India, as early as 1931, as described by Lowe, who suggested pellagra-like condition of the skin among individuals suffering from leprosy. This was reported in 1942 by Wilson and Widdowson19, where a similar disease (Kwashiorkor) was seen in rice-growing districts of south India. Some of the earlier cases treated as malignant malnutrition at the NIN had case fatality rates of nearly 90 per cent (deaths/admissions: 9/10) with B-complex injections in 1948, but reduced to less than 10 per cent (deaths/admissions: 4/33) with skimmed milk in 1949 and were later reported as Kwashiorkor20.
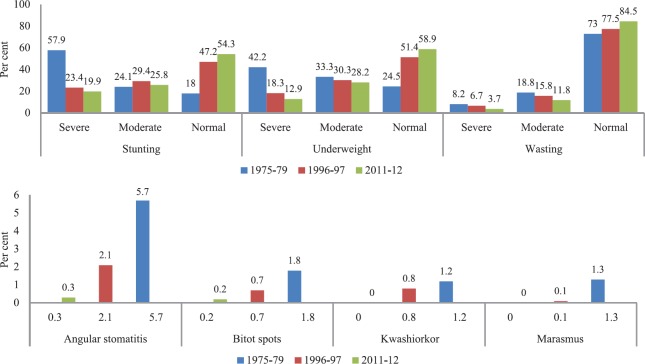
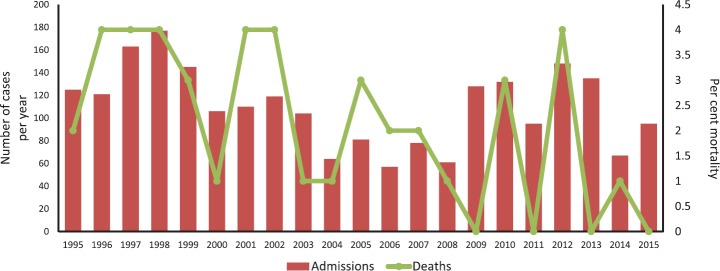
To assess the magnitude of the problem of PEM, the Nutrition Research Laboratories (NRL) in Coonoor (which was later shifted to Hyderabad and renamed as NIN in 1958) carried out its first large-scale survey in 1955 in 10 areas of four southern States and found that the prevalence of Kwashiorkor was about 0.8 per cent (43 cases out of 4536 children)21. The lower prevalence of Kwashiorkor was considered to be due to the afflicted children being treated at various hospitals. Further, in 12 per cent of the siblings of the affected children, a history of Kwashiorkor was reported. In another community study by Gopalan during the similar period, the prevalence of Kwashiorkor was 1.3 per cent (23 cases out of 1800 children) and 2-3 per cent of children showed severe degree of emaciation and were considered as marasmus cases10. Further, large-scale community-based surveys were done under the umbrella of National Nutrition Monitoring Bureau (NNMB), set up in 1975 at the NIN, which carried out periodic surveys in rural areas till 201222,23. Fig. 1 shows the time trends in various nutritional deficiencies in under-5 children as per the NNMB repeat surveys in rural areas of 10 States in India over four decades. The prevalence of Kwashiorkor (based on clinical signs) decreased from 1.2 per cent in 1975-1979 to 0.8 per cent in 1996-1997 and further to near zero by 2011-2012. Similarly, marasmus rates decreased from 1.3 per cent to near zero and severe wasting reduced from 8.2 to 3.7 per cent from the first to the last survey (Fig. 1). Underweight and stunting, especially the severe forms, have also decreased substantially during the above time period. The reduction in the moderate forms of undernutrition was relatively modest, possibly due to shift from severe-to-moderate and moderate-to-mild forms, with apparently low net effect on moderate undernutrition over this period. Figure 2 shows the number of cases since 1995 to 2015 at the Nutrition ward managed by NIN in a tertiary care hospital in Hyderabad. Of the total admissions during this period, 9 per cent of the cases were Kwashiorkor, 7.5 per cent were marasmic Kwashiorkor and 61 per cent were marasmus. During this time period, the overall mortality was 1.9 per cent. Of the total deaths, 16 per cent had Kwashiorkor, 11.3 per cent had marasmic Kwashiorkor and 54.5 per cent had marasmus.
Fig. 1.
Time trends in under-5 undernutrition and nutritional deficiencies from the baseline survey till the final repeat survey carried out by the National Nutrition Monitoring Bureau23.
Fig. 2.
Time trends in admissions of various nutritional cases and mortality rates from 1995 to 2015 at nutrition ward (the nutrition ward is maintained by the National Institute of Nutrition in collaboration with the State Government of Telangana) in a tertiary care hospital in Hyderabad.
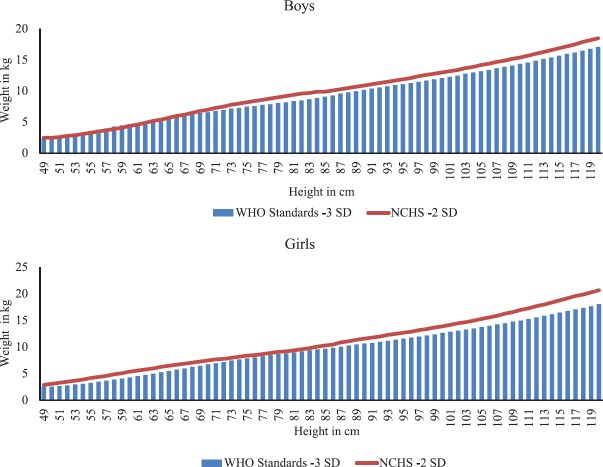
While assessing the time trends over years, it is important to note that the standards for identification of SAM have changed over the years, and what has been earlier considered moderate wasting using National Center for Health Statistics (NCHS) standards may in most cases be classified as severe wasting using the WHO growth reference standards24,25 (Fig. 3), thus contributing to the seemingly increased prevalence estimates of SAM.
Fig. 3.
Cut-off values for moderate wasting (as represented by red lines) at various heights as per the older National Center for Health Statistics (NCHS) standards used by 199924 and 2013 World Health Organization guidelines25 as well as the cut-off values of severe wasting (as represented by the bars).
Aetiology of protein-energy malnutrition
Research on the dietary causes of Kwashiorkor and marasmus, especially the role and relative importance of dietary protein in the aetiology of oedematous malnutrition, has led to considerable debates and controversies. It was triggered by Gopalan's work which demonstrated that the antecedent dietary intakes of children presenting with Kwashiorkor and marasmus were not significantly different from each other10. In general, the dietary intakes of the study children were inadequate in calories but not in protein, and the rates of marasmus were twice as high as that of Kwashiorkor. These findings were confirmed in follow up studies on 300 children, among which seven children developed frank Kwashiorkor10. Gopalan hypothesized that the primary reason for the development of Kwashiorkor or marasmus in a particular child was adaptation, whereby the integrity of liver was maintained in children with marasmus but not in those with Kwashiorkor. This work led to a substantial shift in the prevalent understanding of the relative magnitude of calorie and protein malnutrition by highlighting that calorie malnutrition was a much bigger problem than protein malnutrition. This also marked the beginning of the fall in the support for protein malnutrition, which McLaren later described as 'the great protein fiasco', quoting many of the studies carried out at the NIN26.
There are, however, three important issues with the above approach of dietary assessment. First, the average dietary intakes may not be truly representative of the vulnerable segments of the population and the intakes have a wide variability within the same individual27. Second, the estimates of adequacy of proteins and calories were based on recommended dietary allowances (RDAs) which have been constantly updated28 and cannot be considered definitive for true adequacy. Moreover, the absence of quantitative or qualitative differences between the diets of children who developed Kwashiorkor or marasmus can be explained in terms of known individual variability in nutrient requirements. On intakes that are marginal in both energy and protein, those children with a relatively high energy requirement may become marasmic, whereas those with a high protein requirement may present with Kwashiorkor. The above issues were pointed out by Waterlow, who showed that the best marker for protein deficiency was the protein-energy (PE) ratio and reduction in PE ratio was a better marker for understanding protein inadequacy of a calorie-sufficient diet29. He further showed that in case of children in Uganda, where Kwashiorkor was more common than in Gambian children, the PE ratio of diets of children below the 10th centile was lower than the critical level of 5, which was much lower than the PE ratio of breast milk (7.2). This was not so in case of Gambian children. However, an important question is whether it is true for the cereal-based diets in India, where marasmic Kwashiorkor is more common than Kwashiorkor (discussed below). The third important issue pertains to the contribution of breastmilk to the dietary intakes of children less than two years which was not measured. There is considerable uncertainty regarding the estimated calorie and protein intakes of breastfed children, and the interpretation of dietary intakes has to be viewed with caution.
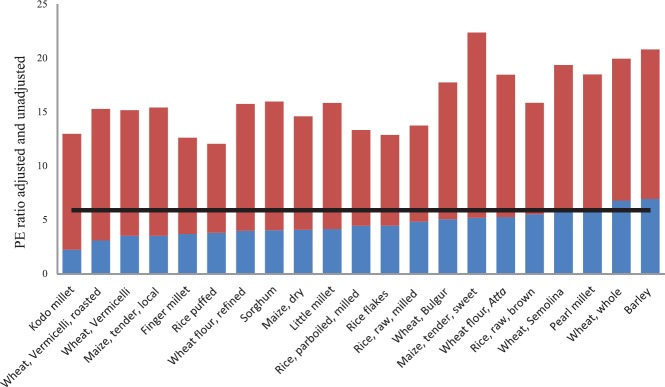
The newer RDA for protein set by the Indian Council of Medical Research (ICMR) in 201030 is considerably higher than before due to changes in the estimated lysine requirements (a rate-limiting amino acid for most cereals) from 12 to 30 mg/kg/day28. The current recommended energy intake for infants is 80 kcal/kg/day30. The PE ratios of Indian diets, therefore, have to be adjusted for the lysine score as well as for the protein digestibility [PE ratio adjusted for (Protein Digestibility-Corrected Amino Acid Score) PDCAAS]. Fig. 4 shows the PE ratios of commonly consumed cereals published in Indian Food Composition Tables31, with and without adjusting for PDCAAS score for children aged 6-24 months. PE ratios were calculated based on average lysine requirements of 54.5 mg/g of protein for six (57 mg/g) to 24 month (52 mg/g) children, based on RDA guidelines by the ICMR30. Protein digestibility was taken at 80 per cent for all cereals and millets except for wheat, which was taken at 90 per cent32. While the unadjusted PE ratios were higher than the recommended level of 5.930, the adjusted PE ratios for many cereals, except wheat and barley, were lower than 5.9. This PE ratio may further decrease by the addition of vegetables, fats and sugars in case of diets that lack high-quality protein from animal source foods such as milk, eggs and meat. Thus, there is theoretical possibility of diets being deficient in protein and yet be adequate in calories in children from low socio-economic groups when the quality of protein is taken into consideration. It is also important to note that the protein requirements can increase by almost 20 per cent in children with infections, due to rise in acute-phase proteins33. Metabolic adaptation, on the other hand, may decrease the requirements, although the extent of this adaptation is unclear34.
Fig. 4.
Unadjusted (entire bar) and adjusted (blue bar) protein-energy (PE) ratios for commonly consumed cereals and millets in India (in ascending order of adjusted PE ratios from left to right)31.
The anecdotal evidence, as originally reported by Williams16 that Kwashiorkor was seen in elder sibling when a younger one was born was indicative of drastic lowering of PE ratio of diets with tapering of breastfeeding and reliance on starchy foods as observed by the Ghanian nurses16. Similar studies reported from India and other countries have suggested the role of displaced breast milk due to working mothers, early weaning and starchy diets in the aetiology of Kwashiorkor35,36. In some of the earlier studies carried out by the NIN in tea plantation areas, where the mothers of the children were at work, the prevalence of Kwashiorkor was high, and the authors reported a cereal-based diet with only a few breast milk feeds given to the younger child37. Further epidemiological evidence suggested that Kwashiorkor was less common in the poor communities with occupations related to the production of animal source foods such as milk38. The case series of Kwashiorkor reported from North America included children consuming diets that were low in quality protein such as rice milk39. Cases of Kwashiorkor induced by dietary protein restriction due to perceived milk intolerance or food faddism have been reported in the US also40.
Golden has argued that experimental animal models of induced Kwashiorkor do not exist and the protein-deficient diets given to the animals also lacked other nutrients such as zinc, thus questioning the role of protein deficiency as the sole aetiology of Kwashiorkor11,41. Further, it has been said that the protein content of the diets given to the animals was too low (contributing to <3% of energy) to be consumed by children from poor households in developing countries41. However, Kwashiorkor-like syndrome has been successfully reproduced in pigs42, rats43 and monkeys44,45 with protein-deficient diets over a relatively short period of time. Some of the animal studies also used diets which were typically consumed by poor children in Africa46 and India47, suggesting the causal role of diets consumed by low-income children in Kwashiorkor.
Alternate hypotheses regarding the pathogenesis of Kwashiorkor and marasmus
Many alternate hypotheses about the pathogenesis of Kwashiorkor and marasmus have been postulated10,11,48,49,50. Of these, two hypotheses have gained special significance10,11. The theory of adaptation states that children consuming diets deficient in proteins and calories may either develop marasmus or Kwashiorkor based on their adaptability. A child with a better adaptability preserves the structural and functional integrity of liver at the expense of less important organs such as muscle and skin, whereas a child lacking this adaptability develops Kwashiorkor10. However, in some parts of the world, the mortality rates in children with marasmus were found to be higher51 or only slightly lower15 than those in children with Kwashiorkor, thus raising questions about the better adaptability in marasmus cases as per this theory. Further, the integrity of liver was found to vary with the severity of protein and calorie deficiency apart from the percentage of energy intakes contributed by fat. Animal studies have shown that on a low-protein, normal calorie diet typically consumed in low-income households, there was increased accumulation of fat in the periportal areas of liver44,45. The deposited fat content was found to be as high as 50 per cent of the total weight of the liver and held a long-lasting impression with Waterlow29. The reasons for increased fat accumulation in liver are unlikely to be due to the reduced synthesis of lipoprotein as has been suggested52 and more likely to be due to increase in the autophagy of peroxisomes and mitochondrial dysfunction due to protein deficiency53. Further, the accumulated fat was found to disappear after rehabilitation with protein-rich diets44,45. In contrast, when given a low-protein, low-calorie diet, as is believed to be the aetiology of marasmus, the structural and functional integrity of liver was preserved without any fat accumulation29. Thus, the development of marasmus or Kwashiorkor appears to be linked with environmental factors, in this case diet, rather than an individual's ability of adaptation. Apart from the hepatic changes, skin and hair changes seen in Kwashiorkor are generally not seen in marasmus. The high turnover rates of skin and hair necessitate higher protein requirements. In Kwashiorkor, both qualitative and quantitative changes are seen in the skin, with less nitrogen as well as less collagen and hydroxyproline content54. The crazy pavement dermatosis, typically seen in Kwashiorkor due to the inelasticity of skin, was found to reverse on a high-protein diet55. Similarly, the hair changes linked with deficiency of important amino acids such as cysteine also reversed during nutrition rehabilitation, suggesting that majority of the changes in Kwashiorkor could be explained with the classical theory of protein deficiency.
The other alternate hypothesis regarding the aetiology of Kwashiorkor was the free radical theory proposed by Golden and Ramdath11. The theory has started with a premise that it is difficult to experimentally produce Kwashiorkor in animal models. The theory further states that the free radicals produced due to various noxae, such as infections and toxins, cause hepatic damage resulting in a fatty liver and the free radical production is linked to high iron stores and raised ferritin levels seen in Kwashiorkor. However, as Waterlow explained, the fatty liver produced by protein-deficient diet was entirely different from that produced from free radical damage due to peroxidation56. Moreover, the high iron stores and raised ferritin levels seen in Kwashiorkor are also seen in marasmus57 and in many other conditions that do not produce Kwashiorkor58. The high ferritin levels seen in Kwashiorkor may in fact be an effect of liver damage rather than its cause58.
As per the free radical theory, absence of protective pathways leads to ineffective defence of the free radicals due to deficiencies of various micronutrients such as vitamins A and E and various biochemical pathways involving free radical scavenging. The theory states that the oedema seen in Kwashiorkor is due to vitamin E deficiency leading to the peroxidation of membranes in capillaries and renal cell membrane11. However, deficiencies of the micronutrients and trace elements in Kwashiorkor are linked with low levels of albumin and other proteins that are transporters of many of the fat-soluble vitamins such as vitamins A and E and trace elements59. Studies have also shown higher vitamin E levels in Kwashiorkor, and administration of vitamin E to children with marasmus and Kwashiorkor did not show any benefit in terms of duration of oedema or rate of weight gain60. In a randomized controlled trial antioxidant therapy did not reduce the incidence of Kwashiorkor in children from Malawi61. Glutathione levels, a central feature of the protective pathway, were found to be markedly reduced in Kwashiorkor compared to marasmus, but glutathione levels are reduced in protein deficiency62 and can be reversed by the supplementation of amino acid cysteine63, thus suggesting a cause rather than effect. Further, protein-deficient diets in rats have been shown to increase oxidative stress64. Thus, oxidative stress seen in Kwashiorkor may be the effect of protein deficiency rather than its cause. The free radical theory thus fails to explain many of the characteristic features seen in Kwashiorkor and could possibly be an effect of protein deficiency.
Protein supplementation in the treatment of Kwashiorkor
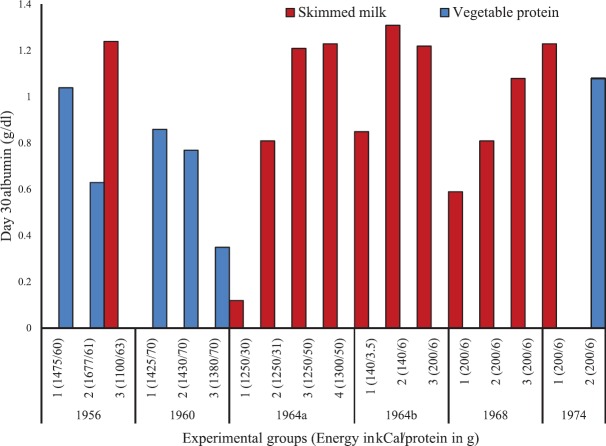
Studies from various centres across the world showed that milk-based diets, rich in high-quality protein, resulted in the cure of Kwashiorkor65,66. Some earlier experiments have demonstrated initial recovery with balanced amino acid mixture and glucose67. Studies at the NIN have also confirmed the cure of Kwashiorkor by the provision of milk-based and mixed protein-based diets68. Studies were also carried out to ascertain the role of vegetable protein in the treatment of Kwashiorkor to make these diets affordable to the poor after discharge from the hospital as well as for community-level prevention of Kwashiorkor69,70,71,72,73. Fig. 5 shows various studies conducted at the NIN facility to assess the impact of various milk and vegetable protein-based diets on reducing oedema and increasing serum albumin levels in children with oedematous malnutrition. In general, vegetable protein-based diets were inferior to the milk-based diets in terms of clearance of oedema and rise in serum albumin. However, mixed diets including vegetable and milk proteins were equally effective in terms of cure rates compared to milk protein alone70.
Fig. 5.
Rise in serum albumin on day 30 in various studies from 1956 to 1974 at the nutrition ward. Blue colour represents diet based on vegetable protein and the red colour represents diet based on skimmed milk protein. X-axis represents the experimental group and the calorie and protein given in parenthesis. Due to large number of experimental groups (n=7) in 196470 study, it has been split in two parts (1964a and 1964b)70 for easy interpretation of the results. For the years 195674, 196069 and 1964a, calories and proteins are given as total per day, while in 1964b, 196872 and 197473, intakes are given per kilogram body weight per day. The methodologies on various papers have been mentioned elsewhere68.
Corroborative evidence for oedema reduction before the recovery of serum albumin was provided by animal studies carried out by Srikantia. An experiment on six monkeys (Macaca radiata) demonstrated that serum albumin dropped from 4.1 to 3.5 g/dl within two weeks of feeding low-protein diet (case in 2.5%) and thereafter declined slowly to 2.3 g/dl till tenth week with plateau thereafter44. Oedema developed in all the monkeys between 13th and 16th wk. When the monkeys were re-fed with standard 16 per cent protein diet, visible oedema disappeared in 8-12 days, but serum albumin rose to 2.8 g/dl even after four weeks of feeding. In the same study, ferritin appeared at 12th wk coinciding with the appearance of oedema and disappeared with the disappearance of oedema. The poor correlation of serum albumin with disappearance of oedema and extracellular fluid volume in the above studies led Srikantia and other researchers with similar findings, to question the role of albumin in the aetiology of oedema in Kwashiorkor75.
Further, Golden in his two studies76,77, showed that it was possible to correct oedema in children on low-protein (2.5% of energy from protein) diets and showed poor correlation between dietary protein and disappearance of oedema76, thereby questioning the role of protein deficiency in the development of Kwashiorkor. However, this has been questioned by Coulthard13 based on careful re-examination of the evidence on the poor correlation of serum albumin with oedema. Moreover, as explained by Coward in great detail78, to expect a good correlation between two variables, in this case albumin and colloid pressure, the relationship has to be linear for all range of values, which is not the case in Kwashiorkor. In children with Kwashiorkor, Coward78 found the relationship between serum albumin and colloid pressure to be cubic, and therefore, the poor correlation between albumin and colloid pressure observed in the above studies can be explained by their non-linear relationship. Further, the normal negative interstitial pressure, which results in a small amount of fluid filtered from the capillaries, increases to zero due to decreased protein in the interstitial space, which allows the normal pressure gradients to be maintained. This is best explained in case of congenital analbuminaemia, where the serum albumin levels are <1 g/dl, but only mild ankle oedema is seen79.
The reasons for disappearance of oedema before the correction of serum albumin are, however, complex. A closer look at the studies carried out at the NIN to test the impact of various diets (Fig. 6) revealed that the mean duration of oedema was lower in those children who had achieved the maximum increase in serum albumin. An inverse association between mean duration of oedema and serum albumin (r=−0.55, P<0.05) was also observed, suggesting that the rise in albumin may contribute to the clearance of oedema13. Further, changes in total plasma colloid pressure are reflective of total albumin and total plasma volume. Serum albumin is a poor indicator of total albumin, as the rise in total circulating albumin has been shown to be twice than that in serum albumin due to simultaneous increase in plasma volume80. The rise in serum albumin in a study conducted in Jamaica by Golden et al76 providing a diet with low protein (0.6 g/kg/day) and maintenance energy (≈96 KCal/kg/day) was 0.3 g/dl lower than that of average rise in albumin found in studies at the NIN (0.5 g/dl)68. This small study (n=12), although reported correlation of serum albumin with clearance of oedema, has not provided information on the correlation of clearance of oedema with the rate of change of albumin, which would have been helpful in understanding the role of increase in serum albumin in the reduction of oedema76. In another study, Golden reported lack of correlation between protein intakes and rate of change of oedema77. However, based on the reanalysis of the data and review of literature, Coulthard demonstrated that this was a mistaken conclusion13.
Fig. 6.
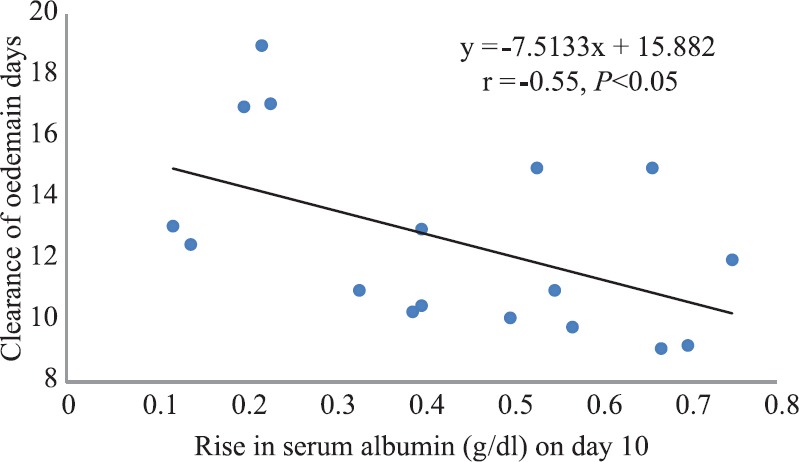
Scatter plot graph and linear regression with clearance of oedema in days as dependent variable and rise in albumin (g/dl) on day 10 as independent variable. All the data points of the Fig. 5 have been used for the analysis except for Group 3 in 1960 where there was a decrease in albumin. The methodologies on various papers have been mentioned elsewhere68.
Type II nutrients: Potassium, magnesium, zinc
Type II nutrients are those nutrients whose deficiencies cannot be measured and symptoms are not obvious81. One such example is growth failure. The idea is that an individual can have normal concentrations of a nutrient such as zinc and yet be deficient in that nutrient as identified by growth failure; therefore, their requirements can only be understood through the therapeutic approach on optimal growth. However, this leads to an uncharted territory of the unknown, and such an approach may be dangerous as studies on supplementation of zinc, a Type II nutrient, started with an objective of improving the catch-up growth, particularly linear growth, in SAM children resulted in higher mortality rates with high-dose zinc82. McCance et al83 in the review of seven balance studies (including his study) on retention during recovery phase have shown a median retention of protein at 1.46 mg/kg (range 0.23-3.0 as calculated from nitrogen with a correction factor of 6.25), potassium retention at 1.2 mEq/kg (range 0.15-2.3) and magnesium retention at 0.6 mg/kg (range 0.22-2.47). This study also included other nutrients, presented as mean ± standard deviation such as retention of zinc at 0.8±0.4 mg/kg, copper at 0.02±0.04 mg, manganese at 0.04±0.03 mg and chromium at 9.0±60 mg. The retention rates of all the nutrients were much lower than the intakes in the studies and also the WHO recommendations for these nutrients. This approach provided a better picture of the tissue accretion rates than a blind therapeutic approach and associated problems.
Potassium plays a key role in the resolution of oedema, though its effects are less clear84. Potassium levels are low in Kwashiorkor and marasmus, but when corrected for acidosis, potassium levels are much lower in Kwashiorkor and are also one of the reasons of apathy in these children85. The low potassium levels are due to decrease in the number of cells, cellular organelle and also loss of functional capacity to pump sodium out and potassium in by energy-dependent sodium potassium ATPase mechanism86. As a result, there is increased sodium and water retention in the cells and also a compensatory rise in sodium pumps87. The cause of potassium deficiency is surprisingly less clear in these children. It is assumed that the deficiency is less likely to be due to dietary inadequacy, but may be due to gastrointestinal losses as malnourished children are relatively inefficient in retaining the potassium85. The normal level of potassium content in the body is 45 mEq/kg body weight and level <35 mEq/kg has been considered to be potassium deficiency. When given at doses 2-4 mEq/kg, about 3-4 wk are taken for complete recovery. Alleyne has suggested three phases of recovery86. In the first phase (5 days), the functional capacity improves, thereby correcting the true potassium deficiency. This is followed by the lag phase and the phase of rapid growth, where potassium is retained in the amount appropriate for deposition of new tissue. The diets based on locally produced foods are usually low in potassium, magnesium, zinc and other micronutrients, known to affect recovery in SAM and has been described elsewhere68. The WHO recommends potassium supplementation with 2-4 mEq/kg/day for the first two weeks of rehabilitation25.
Catch-up growth and composition of weight gain
After the initial stabilization phase when the metabolic machinery gets back to normalcy and oedema subsides, high-energy dense foods are recommended for rapid catch-up growth and the management is similar for oedematous and non-oedematous malnutrition25. The most important limiting factor for promoting weight gain with the older milk-based diets was energy. Studies by Ashworth in Jamaica showed increased weight gain after increasing the energy content without increasing the protein content88. Based on the seminal studies carried out in Jamaica and elsewhere, calorie intakes of 160-220 kcal/kg/day and protein intakes of 2-4 g/kg/day have been recommended during nutrition rehabilitation phase by the WHO25. Studies carried out at the NIN have also shown that the mixed protein diets providing about 200 kcal/kg/day energy and 4-6 g/kg/day of protein were associated with the highest rate of weight gain70. Analysis of the data based on the catch-up growth of 309 children admitted at the NIN during 2001-2005 showed that the average rate of weight gain was about 6 g/kg/day89. Relatively lower rates of weight gain observed in this study could be due to multiple factors such as frequent morbidities in hospitalized children, reducing the weight gain by almost about 40 per cent; admission of moderately wasted children (due to being at high risk of SAM) and lower intake of potassium, magnesium, etc, and have been discussed elsewhere68,89. Studies at various Nutritional Rehabilitation Centres (NRCs) have shown rates of weight gain in the range of about 5-10 g/kg/day90,91. Due to concerns about disproportionately higher amounts of body fat deposition during rapid weight gain of children recovering from SAM, a study at our ward examined the composition of weight gain during nutrition rehabilitation in 80 children (aged 6-60 months)92. Overall, the average rate of weight gain was 6.1 g/kg/day (total weight gain of about 1.09 kg in one month) and fat mass contributed to about 40 per cent of the weight gain. The children with most severe wasting had significantly higher weight gain and higher fat-free mass gain, probably in an attempt to recover the lost tissue. On the other hand, the fat mass gain did not differ in relation to the rate of weight gain or baseline severity of wasting, demonstrating that it is possible to achieve rapid weight gain with recovery of the lost tissue in severely malnourished children with diets based on local foods92.
Community-based management of severe acute malnutrition
An important problem with facility-based nutrition rehabilitation is the high default rates, mainly because families find it difficult to stay away from their homes for longer periods due to economic and other constraints. In community- or home-based management of children with uncomplicated SAM, an estimated 85 per cent of the total cases can lead to recovery rates similar to that of facility-based management and are better accepted by the community at large93.
Most of the studies on community-based nutrition rehabilitation, mainly conducted in Africa, have used centrally or locally produced ready-to-use therapeutic foods (RUTFs) and other locally produced nutrient-dense foods. A Cochrane review in 2013 has concluded that RUTF may improve recovery slightly (risk ratio 1.32; 95% CI 1.16-1.50; based on low-quality evidence), but it is unclear whether RUTF improves relapse, mortality or weight gain94. In a small study conducted at our centre, when rates of weight gain of children receiving RUTF and those receiving local foods were compared, it was observed that the weight gains were higher in the RUTF group than that of the other group only for the first two weeks, but in later weeks, the rates of weight gain were similar in the two groups68. It is likely that the nutrients which were replete by the use of RUTF in the first two weeks may not have been limiting during the latter part of the catch up68.
Of particular interest in this regard is an Indian study by Bhandari et al95 which compared (n=906) the efficacy of centrally produced RUTF (RUTF-C), locally prepared RUTF (RUTF-L) and micronutrient-enriched (augmented) energy-dense home-prepared foods (A-HPF) for home-based management of uncomplicated SAM. The intervention lasted for 16 wk, followed by 16 wk of sustenance by linking with the existing programmes. At the end of 16 wk period, the recovery rates with RUTF-L, RUTF-C and A-HPF group were 56.9, 47.5 and 42.8 per cent, respectively. Group receiving RUTF-L had significantly higher rate of weight gain than A-HPF, and time to recovery was shorter in both the RUTF groups, suggesting the higher efficacy of RUTF compared to micronutrient-enriched home-based diets. However, as early as 16 wk after the end of the treatment phase, there was a relapse in many of the children and the recovery rates in the RUTF-L, RUTF-C and A-HPF groups were 17.3, 12.1 and 14.6 per cent, respectively, and were not significantly different from each other. This is an important finding of the study and raises questions about the sustainability of community-based nutrition rehabilitation programmes using RUTFs and other nutrient-dense foods and highlights that the projected benefits of community-based management using these solutions are most likely overestimated96. The case fatality rates in the three groups also show intriguing trends. During the total period of 32 wk of intervention and sustenance, five children from the two RUTF groups died, but no mortality was reported in the group receiving home-based foods95. The likely reasons for higher mortality rates in the groups that received RUTFs need further exploration. Moreover, about 10 per cent of children in all the groups were hospitalized for various reasons, >40 per cent of children had an episode of diarrhoea and about 70 per cent had an episode of fever during the study95. In another randomized controlled trial, where the iron content of RUTFs was increased compared to the standard RUTF, there was a rise in haemoglobin, but the mortality rates were almost twice as compared to the standard RUTF97. It is likely that the high iron content in RUTF may have resulted in increased infections and mortality. Micronutrient supplements with iron are known to increase the risk of inflammation and infections as iron is also an indispensable nutrient for many pathogenic bacteria in the gut98.
Several other concerns have been raised regarding the potential long-term impact of consumption of RUTF in children with SAM including alteration of epigenome and associated metabolic functions, gut microbiome and body composition that are not adequately examined and are currently not well understood99. Studies assessing the cost-benefit ratios of RUTFs versus local foods should also assess costs related to the displacement of resources from other long-term strategies for improving nutrition such as nutrition-sensitive agriculture and social and behavioural changes99. The above concerns also need to be examined in the background of an observational study conducted in Meerut, Uttar Pradesh96, which evaluated survival and recovery of children with SAM without the existing community-based management programme (n=409) and showed that, after median duration of follow up of 7.4 months, the case fatality was 1.2 per cent and spontaneous recovery occurred in about 31 per cent of children96. The authors speculated that the benefits of investing in community-based management of severe wasting in India are considerably overestimated.
Future research and way forward
The WHO guidelines for the management of SAM children are based on extensive studies carried out by various centres across the world that helped advancing the knowledge regarding the disease pathogenesis and its management. However, only one-third of the recommendations in these guidelines were supported by the results of directly relevant research (intervention or observational studies), whereas almost half of the recommendations were based on expert opinion, unsupported by published evidence100. Although the recommendations based on expert opinion are not necessarily incorrect, need for further epidemiological and clinical research cannot be overemphasized to improve treatment outcomes in a large number of children with SAM that continue to get admitted in tertiary care hospitals. Tackling the problem of high defaulter rates and strengthening linkages with the existing health and nutrition programmes are especially important.
The newer ICMR30 and WHO12 guidelines on the requirements of protein re-emphasize the importance of protein quality in line with the studies carried out by the NIN in the early years of its existence23. Improvement in protein quality of supplements provided in the nutrition programmes, by taking due care of the PE ratio adjusted for PDCAAS, is therefore needed.
Uncomplicated SAM is an extension of moderate wasting and may be treated as a continuum of the same disease. Compared to RUTF, locally available nutrient-dense foods offer advantages of lower cost, wider acceptability and availability; the existing programmes in the country such as Integrated Child Development Services, therefore, need to be strengthened for effective delivery of nutrient-dense local foods to the children with uncomplicated SAM. Further research is needed to better understand the long-term impact of RUTF consumption on metabolic functions and body composition of children with SAM. Moreover, careful studies estimating the cost-benefit ratios of RUTFs versus local foods are needed to inform policy.
Footnotes
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.International Institute for Population Sciences. National Family Health Survey (NFHS-4) Mumbai: International Institute for Population Sciences; 2016. [Google Scholar]
- 2.Semba RD. The rise and fall of protein malnutrition in global health. Ann Nutr Metab. 2016;69:79–88. doi: 10.1159/000449175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Guardian. John Waterlow obituary. 2010. [accessed on November 10, 2018]. Available from: https://www.theguardian.com/science/2010/nov/15/john-wat erlow-obituary .
- 4.Gopalan C. The contribution of nutrition research to the control of undernutrition: The Indian experience. Annu Rev Nutr. 1992;12:1–7. doi: 10.1146/annurev.nu.12.070192.000245. [DOI] [PubMed] [Google Scholar]
- 5.Krishnaswamy K, Bamji MS. Coluthur Gopalan: a legend in nutrition science. Current Sci. 2014;107:1184–9. [Google Scholar]
- 6.Gavaravarapu SM, Hemalatha R. National Institute of Nutrition: 100 years of empowering the nation through nutrition. Indian J Med Res. 2018;148:477–87. doi: 10.4103/ijmr.IJMR_2061_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waterlow JS. Reflections on stunting. In: Gopalan C, editor. Recent trends in nutrition. Delhi: Oxford University Press; 1993. [Google Scholar]
- 8.Golden MH. Evolution of nutritional management of acute malnutrition. Indian Pediatr. 2010;47:667–78. doi: 10.1007/s13312-010-0103-5. [DOI] [PubMed] [Google Scholar]
- 9.Srikantia S. Protein calorie malnutrition in Indian children. Indian J Med Res. 1969;57:36–53. [Google Scholar]
- 10.Gopalan C. Kwashiorkor and marasmus: Evolution and distinguishing features 1968. Natl Med J India. 1992;5:145–51. [PubMed] [Google Scholar]
- 11.Golden MH, Ramdath D. Free radicals in the pathogenesis of Kwashiorkor. Proc Nutr Soc. 1987;46:53–68. doi: 10.1079/pns19870008. [DOI] [PubMed] [Google Scholar]
- 12.Joint FAO/WHO/UNU Expert Consultation on Protein and Amino Acid Requirements in Human Nutrition. Protein and amino acid requirements in human nutrition: Report of a joint FAO/WHO/UNU expert consultation. Geneva, Switzerland: Food and Agriculture Organization of the United Nations, World Health Organization & United Nations University; 2007. [Google Scholar]
- 13.Coulthard MG. Oedema in Kwashiorkor is caused by hypoalbuminaemia. Paediatr Int Child Health. 2015;35:83–9. doi: 10.1179/2046905514Y.0000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golden MH. Nutritional and other types of oedema, albumin, complex carbohydrates and the interstitium – A response to Malcolm Coulthard's hypothesis: Oedema in Kwashiorkor is caused by hypo-albuminaemia. Paediatr Int Child Health. 2015;35:90–109. doi: 10.1179/2046905515Y.0000000010. [DOI] [PubMed] [Google Scholar]
- 15.Schofield C, Ashworth A. Why have mortality rates for severe malnutrition remained so high? Bull World Health Organ. 1996;74:223–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Williams CD. Kwashiorkor: A nutritional disease of children associated with a maize diet. Lancet. 1935;2:1151–2. [PMC free article] [PubMed] [Google Scholar]
- 17.Hare KP. Kwashiorkor (malignant malnutrition) arising in Assam. J Trop Med Hyg. 1947;50:63–8. [PubMed] [Google Scholar]
- 18.Ramalingaswami V, Menon PS, Venkatachalam PS. Infantile pellagra; report on five cases. Indian Physician. 1948;7:229–37. [PubMed] [Google Scholar]
- 19.Wilson DC, Widdowson EM. A comparative nutritional survey of various Indian communities. Indian Med Res Mem. 1942;34:6. [Google Scholar]
- 20.Report for the Year 1949-50. Coonoor (South India): The Nutrition Research Laboratories. Indian Research Fund Association; 1950. pp. 16–8. [PubMed] [Google Scholar]
- 21.Rao KS, Swaminathan MC, Swarup S, Patwardhan VN. Protein malnutrition in South India. Bull World Health Organ. 1959;20:603–39. [PMC free article] [PubMed] [Google Scholar]
- 22.National Nutrition Monitoring Bureau. Report of year 1979 and pooled report for 1974-79. National Institute of Nutrition. Hyderabad: Indian Council of Medical Research; 1980. [Google Scholar]
- 23.National Nutrition Monitoring Bureau. National Institute of Nutrition. Hyderabad: ICMR; 2012. Diet and nutritional status of rural population, prevalence of hypertension & diabetes among adults and infants & young child feeding practices – Report of third repeat survey; Technical report No.26. [Google Scholar]
- 24.World Health Organization. Management of severe malnutrition: A manual for physicians and other senior health workers. Geneva: WHO; 1999. [Google Scholar]
- 25.World Health Organization. Guideline: Updates on the management of severe acute malnutrition in infants and children. Geneva: WHO; 2013. [PubMed] [Google Scholar]
- 26.McLaren DS. The great protein fiasco. Lancet. 1974;2:93–6. doi: 10.1016/s0140-6736(74)91649-3. [DOI] [PubMed] [Google Scholar]
- 27.Palaniappan U, Cue RI, Payette H, Gray-Donald K. Implications of day-to-day variability on measurements of usual food and nutrient intakes. J Nutr. 2003;133:232–5. doi: 10.1093/jn/133.1.232. [DOI] [PubMed] [Google Scholar]
- 28.Kurpad A. Overview of changing protein and amino acid requirements and application to pregnancy requirements. Food Nutr Bull. 2013;34:234–6. doi: 10.1177/156482651303400214. [DOI] [PubMed] [Google Scholar]
- 29.Waterlow JC. Childhood malnutrition in developing nations: Looking back and looking forward. Annu Rev Nutr. 1994;14:1–9. doi: 10.1146/annurev.nu.14.070194.000245. [DOI] [PubMed] [Google Scholar]
- 30.ICMR Expert Committee. A report of the expert group of the Indian Council of Medical Research. 2nd ed. Vol. 2010. New Delhi: Indian Council of Medical Research; 2010. Nutrient requirements and recommended dietary allowances for Indians. [Google Scholar]
- 31.Longvah T, Ananthan I, Bhaskarachary K, Venkaiah K. Indian food composition tables. Hyderabad: National Institute of Nutrition. Indian Council of Medical Research; 2017. [Google Scholar]
- 32.Millward DJ, Jackson AA. Protein/energy ratios of current diets in developed and developing countries compared with a safe protein/energy ratio: implications for recommended protein and amino acid intakes. Public Health Nutr. 2004;7:387–405. doi: 10.1079/PHN2003545. [DOI] [PubMed] [Google Scholar]
- 33.Kurpad AV. The requirements of protein & amino acid during acute & chronic infections. Indian J Med Res. 2006;124:129–48. [PubMed] [Google Scholar]
- 34.Waterlow JC. The nature and significance of nutritional adaptation. Eur J Clin Nutr. 1999;53(Suppl 1):S2–5. doi: 10.1038/sj.ejcn.1600739. [DOI] [PubMed] [Google Scholar]
- 35.Gopalan C, Ramalingaswamy V. Kwashiorkor in India 1955. Indian J Med Res. 2012;136:108. [PMC free article] [PubMed] [Google Scholar]
- 36.Scrimshaw NS, Behar M, Arroyave G, Tejada C, Viteri F. Kwashiorkor in children and its response to protein therapy. J Am Med Assoc. 1957;164:555–61. doi: 10.1001/jama.1957.62980050003014. [DOI] [PubMed] [Google Scholar]
- 37.Report for the Year 1952-53. Coonoor (South India): The Nutrition Research Laboratories. Indian Research Fund Association; 1953. [PubMed] [Google Scholar]
- 38.Brock JF, Autret M. Kwashiorkor in Africa. Bull World Health Organ. 1952;5:1–71. [PMC free article] [PubMed] [Google Scholar]
- 39.Rossouw JE. Kwashiorkor in North America. Am J Clin Nutr. 1989;49:588–92. doi: 10.1093/ajcn/49.4.588. [DOI] [PubMed] [Google Scholar]
- 40.Tierney EP, Sage RJ, Shwayder T. Kwashiorkor from a severe dietary restriction in an 8-month infant in suburban Detroit, Michigan: Case report and review of the literature. Int J Dermatol. 2010;49:500–6. doi: 10.1111/j.1365-4632.2010.04253.x. [DOI] [PubMed] [Google Scholar]
- 41.Golden MH. Transport proteins as indices of protein status. Am J Clin Nutr. 1982;35(5 Suppl):1159–65. doi: 10.1093/ajcn/35.5.1159. [DOI] [PubMed] [Google Scholar]
- 42.Lowrey RS, Pond WG, Barnes RH, Krook L, Loosli JK. Influence of caloric level and protein quality on the manifestations of protein deficiency in the young pig. J Nutr. 1962;78:245–53. doi: 10.1093/jn/78.2.245. [DOI] [PubMed] [Google Scholar]
- 43.Lago ES, Teodósio NR, Araújo CR, Azevedo MC, Pessoa DC, Campos FA, et al. Rat models of protein and protein-energy malnutrition. Int J Vitam Nutr Res. 1993;63:52–6. [PubMed] [Google Scholar]
- 44.Srikantia SG, Gopalan C. Role of ferritin in nutritional edema. J Appl Physiol. 1959;14:829–33. doi: 10.1152/jappl.1959.14.5.829. [DOI] [PubMed] [Google Scholar]
- 45.Deo MG, Ramalingaswami V. Production of periportal fatty infiltration of the liver in the rhesus monkey by a protein-deficient diet. Lab Invest. 1960;9:319–29. [PubMed] [Google Scholar]
- 46.May T, Klatt KC, Smith J, Castro E, Manary M, Caudill MA, et al. Choline supplementation prevents a hallmark disturbance of Kwashiorkor in weanling mice fed a maize vegetable diet: Hepatic steatosis of undernutrition. Nutrients. 2018;10 doi: 10.3390/nu10050653. pii: E653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Report for the Year 1948-49. Coonoor (South India): The Nutrition Research Laboratories, Indian Research Fund Association Report No. 5. 1953 [PubMed] [Google Scholar]
- 48.Jackson AA. Severe undernutrition in Jamaica. Kwashiorkor and marasmus: The disease of the weanling. Acta Paediatr Scand Suppl. 1986;323:43–51. doi: 10.1111/j.1651-2227.1986.tb10349.x. [DOI] [PubMed] [Google Scholar]
- 49.Hendrickse RG. The influence of aflatoxins on child health in the tropics with particular reference to Kwashiorkor. Trans R Soc Trop Med Hyg. 1984;78:427–35. doi: 10.1016/0035-9203(84)90052-x. [DOI] [PubMed] [Google Scholar]
- 50.Forrester TE, Badaloo AV, Boyne MS, Osmond C, Thompson D, Green C, et al. Prenatal factors contribute to the emergence of Kwashiorkor or marasmus in severe undernutrition: Evidence for the predictive adaptation model. PLoS One. 2012;7:e35907. doi: 10.1371/journal.pone.0035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gernaat HB, Dechering WH, Voorhoeve HW. Mortality in severe protein-energy malnutrition at Nchelenge, Zambia. J Trop Pediatr. 1998;44:211–7. doi: 10.1093/tropej/44.4.211. [DOI] [PubMed] [Google Scholar]
- 52.Badaloo A, Reid M, Soares D, Forrester T, Jahoor F. Relation between liver fat content and the rate of VLDL apolipoprotein B-100 synthesis in children with protein-energy malnutrition. Am J Clin Nutr. 2005;81:1126–32. doi: 10.1093/ajcn/81.5.1126. [DOI] [PubMed] [Google Scholar]
- 53.van Zutphen T, Ciapaite J, Bloks VW, Ackereley C, Gerding A, Jurdzinski A, et al. Malnutrition-associated liver steatosis and ATP depletion is caused by peroxisomal and mitochondrial dysfunction. J Hepatol. 2016;65:1198–208. doi: 10.1016/j.jhep.2016.05.046. [DOI] [PubMed] [Google Scholar]
- 54.Vasantha L. Labile collagen content in the skin in Kwashiorkor. Clin Chim Acta. 1969;26:277–80. doi: 10.1016/0009-8981(69)90378-7. [DOI] [PubMed] [Google Scholar]
- 55.Vasantha L, Srikantia SG, Gopalan C. Biochemical changes in the skin in Kwashiorkor. Am J Clin Nutr. 1970;23:78–82. doi: 10.1093/ajcn/23.1.78. [DOI] [PubMed] [Google Scholar]
- 56.Waterlow JC. Protein-energy malnutrition: The nature and extent of the problem. Clin Nutr. 1997;16(Suppl 1):3–9. doi: 10.1016/s0261-5614(97)80043-x. [DOI] [PubMed] [Google Scholar]
- 57.Velásquez Rodríguez CM, Parra Sosa B, Morales Mira G, Agudelo Ochoa G, Cardona Henao O, Bernal Parra C, et al. “Free” iron, transferrin and ferritin levels in serum and their relation with severe malnutrition. An Pediatr (Barc) 2007;66:17–23. doi: 10.1157/13097353. [DOI] [PubMed] [Google Scholar]
- 58.Koperdanova M, Cullis JO. Interpreting raised serum ferritin levels. BMJ. 2015;351:h3692. doi: 10.1136/bmj.h3692. [DOI] [PubMed] [Google Scholar]
- 59.Ingenbleek Y, Van Den Schrieck HG, De Nayer P, De Visscher M. The role of retinol-binding protein in protein-calorie malnutrition. Metabolism. 1975;24:633–41. doi: 10.1016/0026-0495(75)90143-2. [DOI] [PubMed] [Google Scholar]
- 60.Beau JP, Sy A. Vitamin E supplementation in Senegalese children with Kwashiorkor. Sante. 1996;6:209–12. [PubMed] [Google Scholar]
- 61.Ciliberto H, Ciliberto M, Briend A, Ashorn P, Bier D, Manary M, et al. Antioxidant supplementation for the prevention of Kwashiorkor in Malawian children: Randomised, double blind, placebo controlled trial. BMJ. 2005;330:1109. doi: 10.1136/bmj.38427.404259.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–92. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 63.Badaloo A, Reid M, Forrester T, Heird WC, Jahoor F. Cysteine supplementation improves the erythrocyte glutathione synthesis rate in children with severe edematous malnutrition. Am J Clin Nutr. 2002;76:646–52. doi: 10.1093/ajcn/76.3.646. [DOI] [PubMed] [Google Scholar]
- 64.Huang CJ, Fwu ML. Degree of protein deficiency affects the extent of the depression of the antioxidative enzyme activities and the enhancement of tissue lipid peroxidation in rats. J Nutr. 1993;123:803–10. doi: 10.1093/jn/123.5.803. [DOI] [PubMed] [Google Scholar]
- 65.Behar M, Viteri F, Scrimshaw NS. Treatment of severe protein deficiency in children (Kwashiorkor) Am J Clin Nutr. 1957;5:506–15. doi: 10.1093/ajcn/5.5.506. [DOI] [PubMed] [Google Scholar]
- 66.Pretorius PJ, Hansen JD, Davel JG, Brock JF. Skimmed milk and Kwashiorkor. S Afr Med J. 1956;30:447–50. [PubMed] [Google Scholar]
- 67.Scrimshaw NS, Viteri FE. INCAP studies of Kwashiorkor and marasmus. Food Nutr Bull. 2010;31:34–41. doi: 10.1177/156482651003100105. [DOI] [PubMed] [Google Scholar]
- 68.Mamidi RS, Kulkarni B, Radhakrishna KV. Hospital-based nutrition rehabilitation of children with severe acute malnutrition-experiences from a nutrition centre in India. Nutr Ther Metab. 2011;29:107–18. [Google Scholar]
- 69.Srikantia S, Gopalan C. Clinical trials with vegetable protein foods in Kwashiorkor. Indian J Med Res. 1960;48:637–44. [PubMed] [Google Scholar]
- 70.Srikantia SG, Venkatachalam PS, Reddy V, Gopalan C. Protein-calorie needs in Kwashiorkor. Indian J Med Res. 1964;52:1104–10. [PubMed] [Google Scholar]
- 71.Srikantia SG, Gopalan C. Fish protein concentrates in the treatment of Kwashiorkor. Am J Clin Nutr. 1966;18:34–7. doi: 10.1093/ajcn/18.1.34. [DOI] [PubMed] [Google Scholar]
- 72.Srikantia SG, Sahgal S. Use of cottonseed protein in protein-calorie malnutrition. Am J Clin Nutr. 1968;21:212–6. doi: 10.1093/ajcn/21.3.212. [DOI] [PubMed] [Google Scholar]
- 73.Reddy V, Gupta CP. Treatment of Kwashiorkor with opaque-2 maize. Am J Clin Nutr. 1974;27:122–4. doi: 10.1093/ajcn/27.2.122. [DOI] [PubMed] [Google Scholar]
- 74.Gopalan C, Mehta G, Srikantia SG, Venkatachalam PS. Treatment of nutritional oedema syndrome (Kwashiorkor) with vegetable protein diets. Indian J Med Res. 1956;44:539–45. [PubMed] [Google Scholar]
- 75.Montgomery RD. The relation of oedema to serum protein and pseudocholinesterase levels in the malnourished infant. Arch Dis Child. 1963;38:343–8. doi: 10.1136/adc.38.200.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Golden MH, Golden BE, Jackson AA. Albumin and nutritional oedema. Lancet. 1980;1:114–6. doi: 10.1016/s0140-6736(80)90603-0. [DOI] [PubMed] [Google Scholar]
- 77.Golden MH. Protein deficiency, energy deficiency, and the oedema of malnutrition. Lancet. 1982;1:1261–5. doi: 10.1016/s0140-6736(82)92839-2. [DOI] [PubMed] [Google Scholar]
- 78.Coward WA. Serum colloidal osmotic pressure in the development of Kwashiorkor and in recovery: Its relationship to albumin and globulin concentrations and oedema. Br J Nutr. 1975;34:459–67. doi: 10.1017/s0007114575000517. [DOI] [PubMed] [Google Scholar]
- 79.Del Ben M, Angelico F, Loffredo L, Violi F. Treatment of a patient with congenital analbuminemia with atorvastatin and albumin infusion. World J Clin Cases. 2013;1:44–8. doi: 10.12998/wjcc.v1.i1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Viart P. Hemodynamic findings in severe protein-calorie malnutrition. Am J Clin Nutr. 1977;30:334–48. doi: 10.1093/ajcn/30.3.334. [DOI] [PubMed] [Google Scholar]
- 81.Golden MH. Specific deficiencies versus growth failure: Type I and type II nutrients. SCN News. 1995:10–4. [PubMed] [Google Scholar]
- 82.Doherty CP, Sarkar MA, Shakur MS, Ling SC, Elton RA, Cutting WA, et al. Zinc and rehabilitation from severe protein-energy malnutrition: Higher-dose regimens are associated with increased mortality. Am J Clin Nutr. 1998;68:742–8. doi: 10.1093/ajcn/68.3.742. [DOI] [PubMed] [Google Scholar]
- 83.McCance RA, Rutishauser IH, Boozer CN. Effect of Kwashiorkor on absorption and excretion of N, fat, and minerals. Arch Dis Child. 1970;45:410–6. doi: 10.1136/adc.45.241.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Waterlow JC. Kwashiorkor revisited: The pathogenesis of oedema in Kwashiorkor and its significance. Trans R Soc Trop Med Hyg. 1984;78:436–41. doi: 10.1016/0035-9203(84)90053-1. [DOI] [PubMed] [Google Scholar]
- 85.Garrow JS. Total body-potassium in Kwashiorkor and marasmus. Lancet. 1965;2:455–8. doi: 10.1016/s0140-6736(65)91420-0. [DOI] [PubMed] [Google Scholar]
- 86.Alleyne GA. Studies on total body potassium in malnourished infants. Factors affecting potassium repletion. Br J Nutr. 1970;24:205–12. doi: 10.1079/bjn19700021. [DOI] [PubMed] [Google Scholar]
- 87.Kaplay SS. Na+ 'pump' and cell energetics in protein-energy malnutrition. Nutr Res. 1984;4:935–48. [Google Scholar]
- 88.Ashworth A. Growth rates in children recovering from protein-calorie malnutrition. Br J Nutr. 1969;23:835–45. doi: 10.1079/bjn19690094. [DOI] [PubMed] [Google Scholar]
- 89.Mamidi RS, Kulkarni B, Radhakrishna KV, Shatrugna V. Hospital based nutrition rehabilitation of severely undernourished children using energy dense local foods. Indian Pediatr. 2010;47:687–93. doi: 10.1007/s13312-010-0101-7. [DOI] [PubMed] [Google Scholar]
- 90.Burza S, Mahajan R, Marino E, Sunyoto T, Shandilya C, Tabrez M, et al. Community-based management of severe acute malnutrition in India: New evidence from Bihar. Am J Clin Nutr. 2015;101:847–59. doi: 10.3945/ajcn.114.093294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chaturvedi A, Patwari AK, Soni D, Pandey S, Prost A, Gope RK, et al. Progress of children with severe acute malnutrition in the malnutrition treatment centre rehabilitation program: Evidence from a prospective study in Jharkhand, India. Nutr J. 2018;17:69. doi: 10.1186/s12937-018-0378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Radhakrishna KV, Kulkarni B, Balakrishna N, Rajkumar H, Omkar C, Shatrugna V, et al. Composition of weight gain during nutrition rehabilitation of severely under nourished children in a hospital based study from India. Asia Pac J Clin Nutr. 2010;19:8–13. [PubMed] [Google Scholar]
- 93.Ashworth A, Khanum S. Cost-effective treatment for severely malnourished children: What is the best approach? Health Policy Plan. 1997;12:115–21. doi: 10.1093/heapol/12.2.115. [DOI] [PubMed] [Google Scholar]
- 94.Schoonees A, Lombard M, Musekiwa A, Nel E, Volmink J. Ready-to-use therapeutic food for home-based treatment of severe acute malnutrition in children from six months to five years of age. Cochrane Database Syst Rev. 2013:CD009000. doi: 10.1002/14651858.CD009000.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bhandari N, Mohan SB, Bose A, Iyengar SD, Taneja S, Mazumder S, et al. Efficacy of three feeding regimens for home-based management of children with uncomplicated severe acute malnutrition: A randomised trial in India. BMJ Glob Health. 2016;1:e000144. doi: 10.1136/bmjgh-2016-000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sachdev HS, Sinha S, Sareen N, Pandey RM, Kapil U. Survival and recovery in severely wasted under-five children without community management of acute malnutrition programme. Indian Pediatr. 2017;54:817–24. doi: 10.1007/s13312-017-1142-y. [DOI] [PubMed] [Google Scholar]
- 97.Bahwere P, Akomo P, Mwale M, Murakami H, Banda C, Kathumba S, et al. Soya, maize, and sorghum-based ready-to-use therapeutic food with amino acid is as efficacious as the standard milk and peanut paste-based formulation for the treatment of severe acute malnutrition in children: A noninferiority individually randomized controlled efficacy clinical trial in Malawi. Am J Clin Nutr. 2017;106:1100–12. doi: 10.3945/ajcn.117.156653. [DOI] [PubMed] [Google Scholar]
- 98.Soofi S, Cousens S, Iqbal SP, Akhund T, Khan J, Ahmed I, et al. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: A cluster-randomised trial. Lancet. 2013;382:29–40. doi: 10.1016/S0140-6736(13)60437-7. [DOI] [PubMed] [Google Scholar]
- 99.Bazzano AN, Potts KS, Bazzano LA, Mason JB. The life course implications of ready to use therapeutic food for children in low-income countries. Int J Environ Res Public Health. 2017;14 doi: 10.3390/ijerph14040403. pii: E403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tickell KD, Denno DM. Inpatient management of children with severe acute malnutrition: A review of WHO guidelines. Bull World Health Organ. 2016;94:642–51. doi: 10.2471/BLT.15.162867. [DOI] [PMC free article] [PubMed] [Google Scholar]