We read with great interest the recent systematic review and meta-analysis of high-flow nasal cannula (HFNC) oxygen therapy versus conventional oxygen therapy (COT) in patients after planned extubation [1]. We greatly appreciate Zhu Y and colleagues’ efforts, but some important issues may better be discussed.
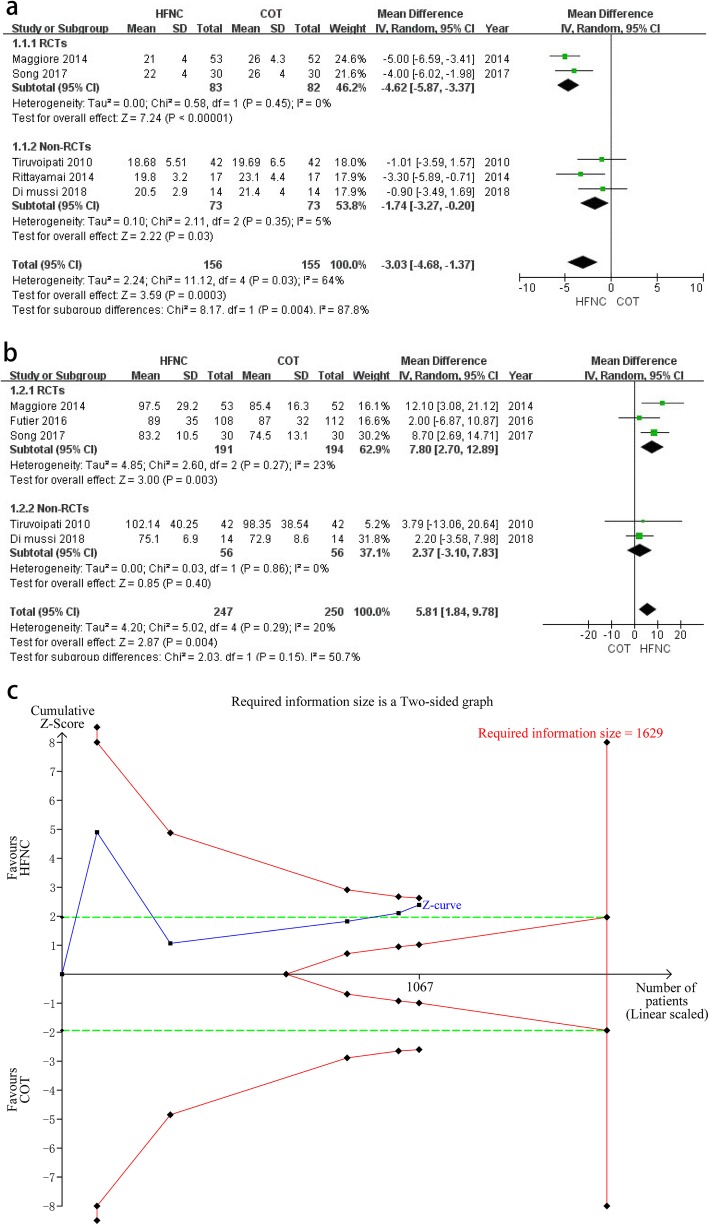
First, randomized controlled trials (RCTs) and non-RCTs may be inappropriately combined together in the meta-analysis, which goes against the principle of pooling studies with the similar design [2, 3]. Thus, results from RCTs and non-RCTs may better be separately pooled (Fig. 1a, b). Our Fig. 1 a and b show that the pooled results of RCTs and non-RCTs were not entirely consistent and subgroup analyses significantly decreased the heterogeneity, which suggested that the heterogeneity may originate from pooling studies with the different design. While we found that the pooled results of RCTs were even more biased in favor of HFNC than non-RCTs.
Fig. 1.
a Comparison of respiratory rates between the HFNC group and COT group. b Comparison of PaO2 between the HFNC group and COT group. c Trial sequential analysis for comparison of postextubation respiratory failure between the two groups
Second, using the standardized mean difference as the summary statistic for the meta-analyses of PaO2 and respiratory rates may be improper. The standardized mean difference is utilized as the summary statistic in the meta-analysis when the trials all assess the same outcome, but measure it in various ways [3]. Moreover, the standardized mean difference is unitless, which only shows the difference in a relatively measurement scale rather than a real difference in variability [3]. Therefore, the mean difference may better be used as the summary statistic to pool data (Fig. 1a, b).
Third, trial sequential analysis for comparison of postextubation respiratory failure between two groups may better be drawn based on the accurate relative risk reduction (RRR) of 37.17% (). Then, Fig. 1c is drawn to show that the line of cumulative Z-curve neither crossed the line of the trial sequential monitoring boundary for benefit nor the required information size boundary, which established inconclusive evidence [4]. But in the authors’ Figure S7, the line of cumulative Z-curve obviously crossed the line of the trial sequential monitoring boundary for benefit, which may mislead the interpretation because of the inaccurate trial sequential analysis. Therefore, the figure of trial sequential analysis may better be not drawn based on a rough estimated RRR.
Acknowledgements
We are grateful to Dr. Hui-Zi Li for his statistical support in this study.
Abbreviations
- HFNC
High-flow nasal cannula
- COT
Conventional oxygen therapy
- RCT
Randomized clinical trial
- RRR
The relative risk reduction
Authors’ contributions
M-SL was responsible for conception of the letter and wrote the manuscript. G-JH and LW conceived and revised this manuscript. All authors had read and approved this final manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
This comment refers to the article available at https://doi.org/10.1186/s13054-019-2465-y.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Guan-Jiang Huang, Email: hgj719471594@zju.edu.cn.
Lun Wu, Email: lunwuzhongshan@outlook.com.
References
- 1.Zhu Y, Yin H, Zhang R, et al. High-flow nasal cannula oxygen therapy versus conventional oxygen therapy in patients after planned extubation: a systematic review and meta-analysis. Crit Care. 2019;23:180. doi: 10.1186/s13054-019-2465-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. In: The Cochrane Collaboration, 2011. https://handbook-5-1.cochrane.org.
- 4.Schoenfeld P. Evidence based medicine (EBM) in practice: applying relative risk and relative risk reduction. Am J Gastroenterol. 2004;99:2084–2085. doi: 10.1111/j.1572-0241.2004.40949.x. [DOI] [PubMed] [Google Scholar]
- 5.Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). In: Copenhagen Trial Unit, 2017. http://www.ctu.dk/tsa/downloads.aspx.
- 6.Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in a random-effects meta-analysis. BMC Med Res Methodol. 2009;9:86. [DOI] [PMC free article] [PubMed]