Abstract
Introduction:
Rotavirus is the leading cause of hospitalizations and deaths from diarrhea. 33 African countries had introduced rotavirus vaccines by 2016. We estimate reductions in rotavirus hospitalizations and deaths for countries using rotavirus vaccination in national immunization programs and the potential of vaccine introduction across the continent.
Areas covered:
Regional rotavirus burden data were reviewed to calculate hospitalization rates, and applied to under-5 population to estimate baseline hospitalizations. Rotavirus mortality was based on 2013 WHO estimates. Regional pre-licensure vaccine efficacy and post-introduction vaccine effectiveness studies were used to estimate summary effectiveness, and vaccine coverage was applied to calculate prevented hospitalizations and deaths. Uncertainties around input parameters were propagated using boot-strapping simulations. In 29 African countries that introduced rotavirus vaccination prior to end 2014, 134,714 (IQR 112,321–154,654) hospitalizations and 20,986 (IQR 18,924–22,822) deaths were prevented in 2016. If all African countries had introduced rotavirus vaccines at benchmark immunization coverage, 273,619 (47%) (IQR 227,260–318,102) hospitalizations and 47,741 (39%) (IQR 42,822–52,462) deaths would have been prevented.
Expert commentary:
Rotavirus vaccination has substantially reduced hospitalizations and deaths in Africa; further reductions are anticipated as additional countries implement vaccination. These estimates bolster wider introduction and continued support of rotavirus vaccination programs.
Keywords: Rotavirus, rotavirus vaccine, Africa, diarrhea, healthcare burden
1. Introduction
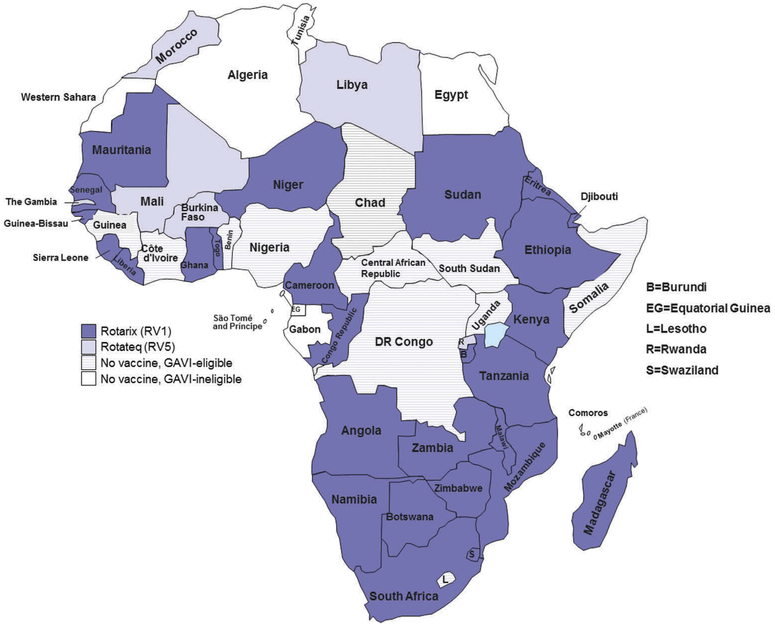
Rotavirus is the leading cause of severe diarrhea among children <5 years of age worldwide, and accounts for 39% of all childhood diarrhea deaths in African countries [1]. Over half of all global rotavirus deaths occur in the countries of sub-Saharan Africa; 10 of these countries had rotavirus mortality rates greater than 100 per 100,000 children in 2013 [1]. Two live attenuated, oral rotavirus vaccines (Rotarix™, GSK Biologics and RotaTeq®, Merck & Co) showed high efficacy (85–98%) against severe rotavirus gastroenteritis in high income countries during clinical trials[2,3], and in some high and middle income countries with established rotavirus vaccination programs, noroviruses have now replaced rotavirus as the leading cause of severe childhood diarrhea [4–7]. Subsequent trials have shown moderate efficacy (39–61%) in African and Asian countries known to have higher diarrheal disease burden and higher under-5 child mortality rates[8–10]. A range of hypotheses have been proposed to explain this phenomenon, including factors related to macro- and micro-nutrient deficiency, environmental enteric dysfunction, concomitant infections from bacterial, viral, parasitic and helminthic agents, and reduced immunogenicity of the vaccines related to interference with maternal antibodies and with coadministration of oral polio vaccine [11–18]. Despite moderate efficacy, the public health impact of rotavirus vaccination is expected to be substantial in African countries because of the tremendous burden of severe rotavirus diarrhea and rotavirus disease-associated death[19]. Therefore, in 2009, the World Health Organization (WHO) broadened its prior recommendation for rotavirus vaccines to include use in all countries and particularly in those countries with high levels of child mortality due to diarrhea [20]. By the end of 2016, thirty-three African countries had introduced rotavirus vaccines into their national immunization programs, many with the support of additional resources from Gavi: the Vaccine Alliance[21] (Figure 1). We estimate reductions in rotavirus hospitalizations and deaths for countries in Africa that had introduced rotavirus vaccination prior to 2015, and project benefits for all African countries if they had implemented rotavirus vaccination at the coverage level of benchmark vaccines.
Figure 1.
National immunization programs in Africa with rotavirus vaccination introduced, 2009–2016.
2. Methodology to estimate rotavirus hospitalizations and deaths prevented with rotavirus vaccination
2.1. Baseline estimates of hospitalizations and deaths due to rotavirus in Africa
Estimates of rotavirus hospitalizations were derived from published studies of active, population-based surveillance from districts in Kenya [22–24], Libya [25], Malawi [26], Mozambique [27], and South Africa [28] conducted before rotavirus vaccine introduction (Table 1). These studies calculated hospitalization rates for children under 5 years of age for a minimum of one rotavirus season prior to rotavirus vaccine introduction in their respective national immunization programs, typically as part of a Health and Demographic Surveillance System (HDSS) [29]. Given the higher burden of illness in younger children [30–32], hospitalization rates for rotavirus were stratified by the following age groups: 0–11 months, 12–23 months, and 24–59 months old. The median hospitalization rates per 100,000 were 887 (inter-quartile range (IQR) 305–1514) for the 0–11 month, 484 for the 12–23 month, and 41 for the 24–59 month age groups. To determine the number of rotavirus hospitalization in absence of vaccination, the age group-specific rotavirus hospitalization rates were applied to the World Population Prospects (WPP) 2015 population estimates for each country [33].
Table 1.
Population-based rates for children hospitalized with rotavirus diarrhea from community surveillance systems in Africa prior to rotavirus vaccination.
| Country | Surveillance years | Rotavirus prevalence | Rotavirus hospitalization rate per 100,000 (95% CI) | Reference |
|---|---|---|---|---|
| 0–11 months | ||||
| Kenya | 2002–2004 | 38% | 1431 (1275–1600) | Nokes [22] |
| South Africa | 2003–2005 | 25% | 1597 (1451–1743) | Seheri [28] |
| Mozambique | 2007–2011 | 42% | 342 (282–403) | Nhampossa [27] |
| Malawi | 2012 | 49% | 269 | Bar Zeev [26] |
| 12–23 months | ||||
| South Africa | 2003–2005 | 18% | 484 (392–576) | Seheri [28] |
| 24–59 months | ||||
| South Africa | 2003–2005 | 10% | 41 (25–57) | Seheri [28] |
| 0–59 months | ||||
| Kenya | 2002–2004 | 30% | 478 (437–521) | Nokes [22] |
| Kenya | 2003–2005 | 19% | 107 | Tate [23] |
| South Africa | 2003–2005 | 21% | 466 (428–504) | Seheri [28] |
| Kenya | 2010–2011 | 27% | 501 (443–558) | Khagayi [24] |
| Libya | 2012–2013 | 58% | 418 (405–431) | Alkoshi [25] |
The age group (0–11, 12–23 and 24–59 months) distribution for rotavirus hospitalizations was applied to previously published rotavirus mortality estimates for 2013[1] to determine the number of rotavirus deaths in each age strata. The 2013 mortality estimates applied country-specific estimates of rotavirus prevalence in moderate-to-severe diarrhea cases to WHO estimates of the number of diarrhea deaths in children[34].
2.2. Rotavirus vaccine effectiveness
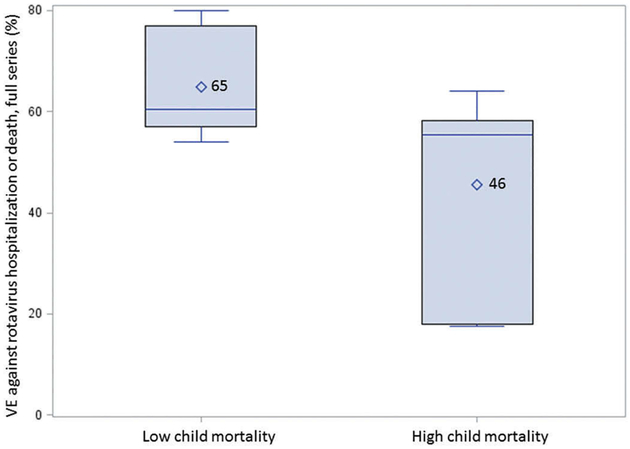
Data from published clinical efficacy trials and post-introduction vaccine effectiveness evaluations from African countries were reviewed[8,9,26,35–41]. In the post-introduction evaluations, investigators estimated vaccine effectiveness using a case–control design, with children enrolled from surveillance sites with stool samples that tested positive by enzyme-linked immunosorbent assay (ELISA) for rotavirus as cases, and with samples that tested negative by ELISA for rotavirus as controls [42,43]. Where multiple scenarios for vaccine effectiveness were available, the estimate against hospitalization or moderate-to-severe diarrhea was used. While the African region has higher child mortality than other regions, individual countries report a range in under-5 mortality from 13 to 157 per 1000 live births in 2015 [44]. As vaccine effectiveness studies show a lower performance in higher-mortality countries, even when limited to those conducted in African countries, we used the median under-5 mortality rate for the continent (67 per 1000 live births) to classify countries to a higher or lower child mortality rate group (Table 2). The mean vaccine effectiveness in the higher-mortality group was 46% (95% CI: 28–64%), compared to 65% (95% 53–77%) for the lower-mortality group (Figure 2).
Table 2.
Clinical trials of vaccine efficacy and post-introduction evaluations of vaccine effectiveness for prevention of moderate-to-severe rotavirus diarrhea in African countries.
| Country | Study type | Vaccine | Vaccine effectiveness mean (95% CI) | Reference |
|---|---|---|---|---|
| Lower child mortality rate group (<67 deaths per 1000 live births) | ||||
| Botswana | Effectiveness, post-introduction | Rotarix | 54% (23–73) | Gastañaduy [35] |
| Kenya | Efficacy, clinical trial | RotaTeq | 64% (−6–90) | Armah [9] |
| Rwanda | Effectiveness, post-introduction | RotaTeq | 80% (28–94) | Ngabo [36] |
| South Africa | Effectiveness, post-introduction | Rotarix | 57% (40–68) | Groome [37] |
| South Africa | Efficacy, clinical trial | Rotarix | 77% (56–88) | Madhi [8] |
| Tanzania | Effectiveness, post-introduction | Rotarix | 57% (14–78) | Abeid [38] |
| Higher child mortality rate group (≥67 deaths per 1000 live births) | ||||
| Ghana | Effectiveness, post-introduction | Rotarix | 18% (−81–63) | Armah [39] |
| Ghana | Efficacy, clinical trial | RotaTeq | 56% (28–73) | Armah [9] |
| Malawi | Effectiveness, post-introduction | Rotarix | 64% (24–83) | Bar Zeev [40] |
| Malawi | Effectiveness, post-introduction | Rotarix | 58% (20–78) | Bar Zeev [26] |
| Malawi | Efficacy, clinical trial | Rotarix | 49% (19–68) | Madhi [8] |
| Mali | Efficacy, clinical trial | RotaTeq | 18% (−23–45) | Armah [9] |
| Zambia | Effectiveness, post-introduction | Rotarix | 56% (−34–86) | Beres [41] |
Figure 2.
Effectiveness of rotavirus vaccination in children younger than 5 years by child mortality rate strata.
2.3. Estimate of 2016 reductions in diarrheal hospitalizations and deaths for countries introducing rotavirus vaccine into national immunization programs by end of 2014
Estimates of current reductions in rotavirus burden were limited to the 29 countries who introduced rotavirus vaccination (23 introduced RV1 and six RV5) by end of 2014 to allow for at least one full year after vaccine introduction to realize the impact of the vaccination program. For these 29 countries, the vaccine coverage for rotavirus last dose reported to WHO in 2015[45] was applied to the WPP population[33] for the age groups described earlier (0–11, 12–23, and 24–59 months) to estimate the number of vaccinated individuals in each country. The time since vaccine introduction until end of 2016 was used to determine the number of full and partial birth cohorts that would have been eligible for rotavirus vaccination in each country. The number of hospitalizations prevented was calculated as the product of the number vaccinated and the hospitalization rate for each age group, multiplied by the vaccine effectiveness stratified by child mortality rate. The number of deaths prevented was calculated by multiplying the proportion of vaccinated individuals by the baseline number of deaths in each age group and the mortality-stratified vaccine effectiveness. A boot-strapping simulation of 1000 cases using the IQR for hospitalization rate and 95% confidence interval of the mean vaccine effectiveness rate was performed to propagate mean and interquartile estimates for each country. Country-level estimates of baseline and prevented rotavirus hospitalization and deaths for 2016 are presented in Supplemental Table 1. For all African countries that introduced rotavirus vaccines by the end of 2014, we estimate that 134,714 (IQR 112,321–154,654) rotavirus hospitalizations and 20,986 (IQR 18,924–22,822) rotavirus deaths were prevented in 2016 (Table 3).
Table 3.
Estimates of rotavirus hospitalizations and deaths in African countries in 2016 with hypothetical full introduction of rotavirus vaccination into national immunization programs.
| Rotavirus hospitalizations | Rotavirus deaths | |||||
|---|---|---|---|---|---|---|
| Expected No. | Prevented No. | Prevented % | Expected No. | Prevented No. | Prevented % | |
| All Countries (n = 54) | 586,568 (524,970–645,674) | 273,619 (227,260–318,102) | 47 (43–49) | 122,889 (120,370–125,305) | 47,741 (42,822–52,462) | 39 (36–42) |
| Countries introducing rotavirus vaccination by 2014 end (n = 29) | 294,044 (262,947–321,736) | 134,714 (112,321–154,654) | 46 (43–48) | 55,168 (53,938–56,263) | 20,986 (18,924–22,822) | 38 (35–41) |
| Additional reductions in introducing countries with benchmark vaccination coverage (n = 29) | 20,082 (17,234 – 23,037) | 6.9 (6.6–7.1) | 4473 (4126–4832) | 8.1 (7.7–8.6) | ||
| Countries without rotavirus vaccination by 2014 end (n = 25) | 293,801 (265,942–320,279) | 119,594 (100,350–137,883) | 41 (37–43) | 67,771 (66,594–68,890) | 22,351 (20,065–24,523) | 33 (30–36) |
| Child mortality rate strata | ||||||
| High (n = 27) | 314,681 (282,126–342,696) | 110,958 (89,207–129,676) | 35 (32–38) | 95,366 (93,422–97,040) | 31,642 (27,837–34,916) | 33 (30–36) |
| Low (n = 27) | 271,218 (242,972–296,954) | 162,046 (138,063–183,897) | 60 (57–62) | 27,520 (26,973–28,018) | 16,095 (15,033–17,063) | 58 (56–61) |
| Subregion | ||||||
| Eastern (n = 18) | 202,004 (181,579–221,192) | 110,467 (93,870–126,059) | 55 (52–57) | 32,490 (31,902–33,042) | 16,181 (14,989–17,300) | 50 (47–52) |
| Western (n = 16) | 186,832 (169,496–206,337) | 66,591 (55,217–79,389) | 36 (33–38) | 52,223 (51,383–53,167) | 17,009 (15,282–18,953) | 33 (30–36) |
| Northern (n = 6) | 87,901 (79,029–97,421) | 51,713 (43,904–60,093) | 59 (56–62) | 4757 (4611–4914) | 2395 (2151–2656) | 50 (47–54) |
| Central (n = 9) | 86,376 (77,141–95,172) | 32,468 (26,004–38,624) | 38 (34–41) | 31,927 (31,177–32,642) | 11,137 (9740–12,468) | 35 (31–38) |
| Southern (n = 5) | 18,892 (17,142–20,695) | 9183 (7942–10,463) | 49 (46–51) | 1301 (1259–1345) | 632 (581–684) | 49 (46–51) |
| World Bank Income Group | ||||||
| Low (n = 26) | 283,313 (254,174–310,547) | 132,893 (110,735–153,602) | 47 (44–49) | 61,426 (60,248–62,527) | 25,008 (22,559–27,297) | 41 (37–44) |
| Lower-middle (n = 17) | 247,727 (222,802–274,461) | 112,563 (94,040–132,429) | 45 (42–48) | 49,962 (49,048–50,942) | 18,523 (16,696–20,482) | 37 (34–40) |
| Upper-middle (n = 9) | 52,579 (47,171–57,938) | 26,238 (22,127–30,311) | 50 (47–52) | 11,328 (10,982–11,669) | 4019 (3536–4497) | 35 (32–39) |
| High (n = 2) | 435 (390–474) | 59 (49–69) | 14 (13–15) | 77 (75–78) | 8 (7–9) | 10 (9–11) |
| GAVI eligibility | ||||||
| Yes (n = 40) | 492,756 (445,651–542,888) | 216,980 (182,355–253,831) | 44 (41–47) | 119,622 (117,436–121,949) | 45,644 (41,324–50,241) | 38 (35–41) |
| No (n = 14) | 89,671 (80,233–98,262) | 53,584 (45,562–60,886) | 60 (57–62) | 3078 (2982–3165) | 1725 (1592–1847) | 56 (53–58) |
Estimates presented as median (interquartile range).
2.4. Estimate of potential reductions in diarrheal hospitalizations and deaths
To determine the potential benefit of rotavirus vaccination in Africa, we estimated the reductions in rotavirus hospitalizations and deaths assuming that all countries had already implemented rotavirus vaccination at coverage equivalent to more established vaccinations in the Expanded Program on Immunization (EPI) [46]. This coverage goal has been shown feasible in Latin American countries, where even Gavi-eligible countries have achieved high coverage rates for rotavirus vaccination similar to routine EPI vaccinations [47,48]. We chose the reported coverage for diphtheria-tetanus-pertussis (DTP) containing vaccines, which are recommended to be administered on the same schedule as rotavirus vaccines, and are the benchmark for the 2011–2020 Global Vaccine Action Plan [49]. For countries that have introduced the three-dose RotaTeq® formulation, the reported coverage[45] for the third dose of DTP vaccine was used. As coverage for the second dose of DTP vaccine is not routinely reported, we estimated DTP2 coverage by calculating the mean of the first and third dose of DTP, and applied this estimate for countries that have introduced the two-dose Rotarix™ formulation. The two-dose estimate was also used for countries that have not yet introduced rotavirus vaccination into their national immunization program, based on the global preference for Rotarix™ that is currently available.
In total, we estimate that 273,619 (IQR 227,260–318,102) hospitalizations and 47,741 (42,822–52,462) deaths from rotavirus would have been prevented in 2016 if all countries in Africa had implemented rotavirus vaccination at current routine immunization coverage (Table 3, country-specific estimates available in Supplemental Table 2). These estimates represent a 47% reduction in hospitalizations (IQR 43–49%) and 39% reduction in deaths (IQR 36–42%) from the burden in 2013.
The gap between the reductions estimated for full implementation across the continent and the estimates for countries that introduced by 2014 highlights the potential benefits of rotavirus vaccination in Africa. For countries that have already introduced rotavirus vaccination, an additional 20,082 (IQR 17,234–23,037) hospitalizations and 4473 (4126–4832) deaths would have been prevented if rotavirus vaccination coverage matched DTP coverage. For the 25 countries that had not yet introduced rotavirus vaccination, full coverage would have resulted in an additional 119,594 (IQR 100,350–137,883) hospitalizations and 22,351 (IQR 20,065–24,523) deaths prevented.
The overall reductions are further stratified by child mortality rate[44], African region[50], World Bank Income Group level[51], and 2016 eligibility for Gavi assistance[21]. These stratified estimates highlight some important findings. Despite lower vaccine effectiveness, almost twice as many rotavirus deaths would have been prevented in higher-mortality countries compared to lower-mortality countries, due to the higher burden of baseline deaths. Regional estimates of the percent hospitalizations and deaths prevented are tempered by large countries with lower routine immunization coverage: Nigeria (Western region, 70% DTP1 and 56% DTP3 coverage), Democratic Republic of Congo (Middle region, 82% DTP1 and 81% DTP3 coverage), and South Africa (southern region, 72% DTP1 and 69% DTP3 coverage)[45]. Strengthening of these countries’ routine immunization programs would substantially enhance the impact of rotavirus vaccination and likely equalize regional differences. Over half of prevented rotavirus deaths would have occurred in low-income countries, and another 40% in low-middle income countries. There are only two high-income countries, where there is low existing burden but also low composite percent reduction due to low immunization coverage in Equatorial Guinea (28% DTP1 and 16% DTP3). Finally, almost 80% of prevented hospitalizations and over 95% of prevented deaths would have occurred in Gavi-eligible countries.
3. Conclusion
Using current data on regional rotavirus disease burden, vaccine effectiveness, and vaccine coverage, we estimate that over 130,000 hospitalizations and almost 21,000 deaths due to rotavirus in children <5 years were prevented in 2016 in 29 African countries that introduced rotavirus vaccines into their national immunization programs before 2015. Furthermore, if all African countries had implemented rotavirus vaccines at current routine immunization coverage, an additional 139,000 rotavirus hospitalizations and 27,000 rotavirus deaths would have been prevented, resulting in overall reductions of over 270,000 rotavirus hospitalizations (47%) and almost 48,000 rotavirus deaths (39%). Absolute reductions in rotavirus mortality would have occurred predominately in countries with high childhood mortality, low and low-middle income, and countries eligible for Gavi assistance.
Our estimates are subject to several caveats. Available data for African region-specific rotavirus hospitalizations are sparse, with global estimates[52] often cited as surrogates. Our estimates used age-based hospitalization rates based on data from five African countries of varying sub-region, childhood mortality rate, population size and density, and income level. Ideally, further active surveillance from more population settings would allow for more robust, localized hospitalization rate estimates. Even with further data, local practice and hospital admission patterns for diarrhea change over time, likely to affect the hospitalization rates independent of trends in actual rotavirus illnesses[53,54].
Similarly, reductions in rotavirus hospitalizations and deaths are dependent on existing data of vaccine effectiveness and vaccine coverage. Given the small number of available vaccine effectiveness evaluations, summary statistics may be influenced by outlier results, leading to conservative estimates in hospitalization and death reductions. This is evident in the wide confidence interval we estimate for vaccine effectiveness in lower-mortality countries. We also only estimated reductions based on full-series coverage. Additional modest reductions in deaths and hospitalization may also occur in partially immunized children or as the result of indirect protection resulting from reduced circulation of rotavirus in the community. Thus, we may have underestimated the full impact of the vaccination program. We also assumed that coverage of rotavirus vaccine would achieve coverage levels similar to that of other routine childhood vaccines. However, in some countries, rotavirus vaccine coverage has lagged behind coverage of other vaccines, and therefore may take longer for the full impact of rotavirus vaccination to be observed. Furthermore, strategies to improve the performance of oral rotavirus vaccines in high-mortality countries are currently being explored, including the use of zinc supplementation[55], timing of breastfeeding[37,56,57], improved nutrition[35], and alternate dosing schedules[58,59]. Country-level estimates of vaccine coverage, as used in this analysis, can mask within-country disparities in coverage, with infants that are most at risk to die from rotavirus also less likely to receive vaccination[60,61]. Improvements in vaccine effectiveness and equitable strengthening of national immunization programs systems are likely to lead to greater reductions in rotavirus burden than estimated here. Expansion of rotavirus vaccination in Africa is being coupled with ongoing surveillance for vaccine safety, following existing protocols for monitoring of adverse effects following immunization and specifically for intussusception [62–64]. A review of naturally occurring intussusception in African countries found that intussusception reports were infrequent at the age when rotavirus vaccine is first administered, peaked around 5–8 months of age, and that delayed presentation was common and associated with worse outcomes [65].
Despite these caveats, our estimates show the remarkable impact of rotavirus vaccination in Africa, the continent with the highest rotavirus disease burden. The gap in potential reduction in disease burden can be closed with vaccine introduction in all African countries and with vaccine coverage at routine EPI immunization levels.
4. Expert commentary
Rotavirus is the leading cause of severe diarrhea in children globally, with a particularly high burden of disease in sub-Saharan Africa[1]. We estimate that rotavirus currently causes almost 600,000 hospitalizations and over 120,000 deaths in African countries. Vaccines against rotavirus have been shown to reduce rotavirus disease in diverse populations[66], especially severe disease that leads to hospitalizations and deaths. Currently licensed and globally commercially available rotavirus vaccines have high acceptability at the country level, have demonstrated robust effectiveness against severe rotavirus disease and have a track record of safety[67]. Since 2009, thirty-three African countries have introduced rotavirus vaccination into their national immunization programs. In the 29 countries that introduced prior to 2015, over 130,000 rotavirus hospitalizations and almost 21,000 rotavirus deaths were prevented in 2016. In one decade, rotavirus vaccination has gone from national licensure in the country of manufacture, to broad introduction and measurable impact in low and lower-middle income countries, a remarkable leap forward in equity from the historic pace of vaccine introductions[49].
Despite these successes, potential further reductions in hospitalizations and deaths caused by rotavirus highlight opportunities for action. Several large countries with a high burden of illness have yet to introduce rotavirus vaccination, but are expected to do so in 2018, including Nigeria and the Democratic Republic of Congo. Optimized use of existing oral rotavirus vaccinations, coupled with vaccines in discovery, is likely to lead to higher vaccine effectiveness than is currently measured. Improved treatment of diarrhea and strengthening of national immunization programs, especially in large countries with current low coverage for routine immunizations, will also lead to benefits greater than we currently estimate. Licensure of new live-attenuated, oral rotavirus vaccines from additional manufacturers in India, will help to stabilize global vaccine supply and potentially lower the cost of vaccine, which will help improved stability of national rotavirus vaccinations programs. Importantly, as many countries transition from Gavi-funding support over the next 5 years, having a broader market of rotavirus vaccines with lower price point will be critical for the sustainability of rotavirus immunization.
Finally, the availability of external resource support, through organizations such as WHO, UNICEF and Gavi, make the broad adoption of newer vaccines in low and lower-middle income countries an achievable goal.
5. Five-year view
Continued introduction of rotavirus vaccination into national immunization programs in additional African countries is anticipated over the next five years. Nigeria and the Democratic Republic of Congo, the two highest-burden countries in Africa that together account for 140,000 hospitalizations and 43,000 deaths annually from rotavirus, are expected to introduce rotavirus vaccines in 2018 with Gavi support. In addition to the existing WHO-approved rotavirus vaccines, several new vaccines are available in local national markets[68,69] and are likely to seek prequalification status[70] from WHO for broader use. Other, novel vaccines are currently undergoing preclinical and clinical trial testing[67], including vaccines using novel reassortant rotavirus strains, and vaccines using alternative delivery methods such as a heat stable oral formulation[69] and intramuscular or intradermal formulations[71]. Strategies to improve the performance of oral rotavirus vaccines in high-mortality countries are currently being explored, including the use of zinc supplementation[55], use of the antisecretory agent racecadotril[72,73], timing of breastfeeding[37,56,57], improved nutrition[35], and alternate dosing schedules[74–76]. These developments in the rotavirus vaccine marketplace and improved effectiveness of oral vaccines are likely to encourage broader and more effective introduction of rotavirus vaccines in African countries, thus providing greater reductions in the burden of rotavirus infection than are estimated in this report. Continued monitoring of rotavirus trends, circulating rotavirus genotypes, and safety through existing surveillance networks[63,77], as well as rigorous studies to measure mortality reduction, are important to fully understanding the impact of rotavirus vaccination.
Supplementary Material
Key issues.
Rotavirus is the leading cause of severe diarrhea in children, and is the cause of an estimated 587,000 hospitalizations and 123,000 deaths yearly in African countries.
Since 2009, rotavirus vaccines have been recommended by the World Health Organization for use in all countries globally to prevent severe diarrhea. By the end of 2016, 33 African countries had introduced rotavirus vaccines into their national immunization programs.
In 2016, an estimated 135,000 rotavirus hospitalizations and 21,000 rotavirus deaths were prevented in the 29 countries that introduced rotavirus vaccine before 2015.
If all African countries implemented rotavirus vaccination in routine immunization, almost 274,000 rotavirus hospitalizations (47%) and 48,000 rotavirus deaths (39%) would have been prevented in 2016. Improved vaccine effectiveness and strengthened immunization programs could lead to further gains. Most of the mortality reductions occur in higher-mortality, lower-income countries.
Funding
The manuscript was not funded.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Tate JE, Burton AH, Boschi-Pinto C, et al. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013. Clin Infect Dis. 2016;62:S96–S105. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Most widely-cited current estimates of rotavirus mortality.
- 2.Vesikari T, Do M, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. [DOI] [PubMed] [Google Scholar]
- 3.Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. [DOI] [PubMed] [Google Scholar]
- 4.Payne DC, Vinje J, Szilagyi PG, et al. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med. 2013;368:1121–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemming M, Räsänen S, Huhti L, et al. Major reduction of rotavirus, but not norovirus, gastroenteritis in children seen in hospital after the introduction of RotaTeq vaccine into the National Immunization Programme in Finland. Eur J Pediatr. 2013;172:739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bucardo F, Reyes Y, Svensson L, et al. Predominance of norovirus and sapovirus in Nicaragua after implementation of universal rota-virus vaccination. PLoS One. 2014;9:e98201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koo HL, Neill FH, Estes MK, et al. Noroviruses: the most common pediatric viral enteric pathogen at a Large University Hospital after introduction of rotavirus vaccination. J Pediatric Infect Dis Soc. 2013;2:57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madhi SA, Cunliffe NA, Steele D, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Engl J Med. 2010;362:289–298. [DOI] [PubMed] [Google Scholar]; •• Randomized controlled trial of Rotarix (RV1) vaccine in African countries.
- 9.Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2010;376:606–614. [DOI] [PubMed] [Google Scholar]; •• Randomized controlled trial of RotaTeq (RV5) vaccine in African countries.
- 10.Zaman K, Dang DA, Victor JC, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2010;376:615–623. [DOI] [PubMed] [Google Scholar]
- 11.Pasetti MF, Simon JK, Sztein MB, et al. Immunology of gut mucosal vaccines. Immunol Rev. 2011;239:125–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosek M, Guerrant RL, Kang G, et al. Assessment of environmental enteropathy in the MAL-ED cohort study: theoretical and analytic framework. Clin Infect Diseases Off Publ Infect Dis Soc America. 2014;59:S239–S47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang V, Jiang B, Tate J, et al. Performance of rotavirus vaccines in developed and developing countries. Hum Vaccin. 2010;6:532–542. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Review of hypotheses for moderate vaccine efficacy of oral vaccines in developing countries.
- 14.Stelekati E, Wherry EJ. Chronic bystander infections and immunity to unrelated antigens. Cell Host Microbe. 2012;12:458–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naylor C, Lu M, Haque R, et al. Environmental enteropathy, oral vaccine failure and growth faltering in infants in Bangladesh. EBioMedicine 2015;2:1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moon SS, Tate JE, Ray P, et al. Differential profiles and inhibitory effect on rotavirus vaccines of nonantibody components in breast milk from mothers in developing and developed countries. Pediatr Infect Dis J. 2013;32:863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emperador DM, Velasquez DE, Estivariz CF, et al. Interference of monovalent, bivalent, and trivalent oral poliovirus vaccines on monovalent rotavirus vaccine immunogenicity in rural Bangladesh. Clin Infect Dis. 2016;62:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramani S, Mamani N, Villena R, et al. Rotavirus serum iga immune response in children receiving rotarix coadministered with bOPV or IPV. Pediatr Infect Dis J. 2016;35:1137–1139. [DOI] [PubMed] [Google Scholar]
- 19.Gessner BD, Feikin DR. Vaccine preventable disease incidence as a complement to vaccine efficacy for setting vaccine policy. Vaccine. 2014;32:3133–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization. Rotavirus vaccines: an update. Weekly Epidemiological Record. 2009;84:533–540.20034143 [Google Scholar]
- 21.Gavi: The Vaccine Alliance. Countries eligible for support; cited 2017 Mar 3 Available from: http://www.gavi.org/support/sustainability/countries-eligible-for-support/
- 22.Nokes DJ, Abwao J, Pamba A, et al. Incidence and clinical characteristics of group A rotavirus infections among children admitted to hospital in Kilifi, Kenya. PLoS Med. 2008;5:e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tate JE, Rheingans RD, O’Reilly CE, et al. Rotavirus disease burden and impact and cost-effectiveness of a rotavirus vaccination program in Kenya. J Infect Dis. 2009;200:S76–S84. [DOI] [PubMed] [Google Scholar]
- 24.Khagayi S, Burton DC, Onkoba R, et al. High burden of rotavirus gastroenteritis in young children in rural western Kenya, 2010–2011. Pediatr Infect Dis J. 2014;33(Suppl 1):S34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alkoshi S, Leshem E, Parashar UD, et al. Anticipating rotavirus vaccines–a pre-vaccine assessment of incidence and economic burden of rotavirus hospitalizations among children < 5 year of age in Libya, 2012–13. BMC Public Health. 2015;15:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bar-Zeev N, Jere KC, Bennett A, et al. Population impact and effectiveness of monovalent rotavirus vaccination in urban Malawian children 3 years after vaccine introduction: ecological and case-control analyses. Clin Infect Diseases: Off Publ Infect Dis Soc America. 2016;62:S213–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nhampossa T, Mandomando I, Acacio S, et al. Diarrheal disease in rural Mozambique: burden, risk factors and etiology of diarrheal disease among children aged 0–59 months seeking care at health facilities. Plos One. 2015;10:e0119824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seheri ML, Dewar JB, van der Merwe L, et al. Prospective hospital-based surveillance to estimate rotavirus disease burden in the Gauteng and North West Province of South Africa during 2003–2005. J Infect Dis. 2010;202:S131–S8. [DOI] [PubMed] [Google Scholar]
- 29.Odhiambo FO, Laserson KF, Sewe M, et al. Profile: the KEMRI/CDC health and demographic surveillance system–Western Kenya. Int J Epidemiol. 2012;41:977–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Global Burden of Disease Pediatrics Collaboration. Global and national burden of diseases and injuries among children and adolescents between 1990 and 2013: findings from the global burden of disease 2013 study. JAMA Pediatr. 2016;170(3):267–287. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Global Burden of Disease estimates for children and adolescents.
- 31.Walker CL, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet (London, England). 2013;381:1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lanata CF, Fischer-Walker CL, Olascoaga AC, et al. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One. 2013;8(9):e72788. doi: 10.1371/journalpone.0072788.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.United Nations Department of Economic and Social Affairs, Population Division. World Population Prospects: the 2015 Revision, DVD Edition. 2015; [cited 2017 Mar].
- 34.World Health Organization. Child cause of death estimates 2000–2013; [cited 2017 Mar]. Available from: http://www.who.int/healthinfo/global_burden_disease/estimates_child_cod_2013/en/
- 35.Gastanaduy PA, Steenhoff AP, Mokomane M, et al. Effectiveness of monovalent rotavirus vaccine after programmatic implementation in Botswana: a multisite prospective case-control study. Clin Infect Dis. 2016;62(Suppl 2):S161–7. [DOI] [PubMed] [Google Scholar]
- 36.Ngabo F, Tate JE, Gatera M, et al. Effect of pentavalent rotavirus vaccine introduction on hospital admissions for diarrhoea and rotavirus in children in Rwanda: a time-series analysis. Lancet Glob Health. 2016;4:e129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Groome MJ, Moon SS, Velasquez D, et al. Effect of breastfeeding on immunogenicity of oral live-attenuated human rotavirus vaccine: a randomized trial in HIV-uninfected infants in Soweto, South Africa. Bull World Health Organ. 2014;92:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abeid KA, Jani B, Cortese MM, et al. Monovalent rotavirus vaccine effectiveness and impact on rotavirus hospitalizations in Zanzibar. Tanzania: Data from the First 3 Years Post-Introduction. The Journal of infectious diseases; 2016. [DOI] [PubMed] [Google Scholar]
- 39.Armah G, Pringle K, Enweronu-Laryea CC, et al. Impact and effectiveness of monovalent rotavirus vaccine against severe rotavirus diarrhea in Ghana. Clin Infect Dis. 2016;62(Suppl 2):S200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bar-Zeev N, Kapanda L, Tate JE, et al. Effectiveness of a monovalent rotavirus vaccine in infants in Malawi after programmatic roll-out: an observational and case-control study. Lancet Infect Dis. 2015;15:422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beres LK, Tate JE, Njobvu L, et al. A preliminary assessment of rotavirus vaccine effectiveness in Zambia. Clin Infect Dis. 2016;62 (Suppl 2):S175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tate JE, Patel MM, Cortese MM, et al. Use of patients with diarrhea who test negative for rotavirus as controls to estimate rotavirus vaccine effectiveness through case-control studies. Clin Infect Dis. 2016;62(Suppl 2):S106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz LM, Halloran ME, Rowhani-Rahbar A, et al. Rotavirus vaccine effectiveness in low-income settings: an evaluation of the test-negative design. Vaccine. 2017;35:184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.World Health Organization. WHO mortality database. Geneva, Switzerland; [cited 2017 Mar]. Available from: http://www.who.int/healthinfo/mortality_data/en/ [Google Scholar]
- 45.World Health Organization. WHO vaccine-preventable diseases: monitoring system. Geneva, Switzerland; [cited 2017 Mar]. Available from: http://www.who.int/immunization/monitoring_surveillance/data/en/ [Google Scholar]
- 46.World Health Organization. Recommendations for routine immunization - summary tables. Geneva, Switzerland; [cited 2017 Mar]. Available from: http://www.who.int/immunization/policy/immunization_tables/en/ [Google Scholar]
- 47.De Oliveira LH, Danovaro-Holliday MC, Sanwogou NJ, et al. Progress in the introduction of the rotavirus vaccine in Latin America and the Caribbean: four years of accumulated experience. Pediatr Infect Dis J. 2011;30:S61–6. [DOI] [PubMed] [Google Scholar]
- 48.Progress in the introduction of rotavirus vaccine–Latin America and the Caribbean, 2006–2010. MMWR Morbidity and mortality weekly report. 2011;60:1611–1614. [PubMed] [Google Scholar]
- 49.World Health Organization. Global vaccine action plan 2011–2020. Geneva, Switzerland; [cited 2017 Mar]. Available from: http://www.who.int/immunization/global_vaccine_action_plan/GVAP_doc_2011_2020/en/ [Google Scholar]
- 50.United Nations Department of Economic and Social Affairs, Statistics Division. Composition of macro geographical (continental) regions, geographical sub-regions, and selected economic and other groupings; [cited 2017 Mar]. Available from: https://millenniumindicators.un.org/unsd/methods/m49/m49regin.htm#africa
- 51.The World Bank. World Bank Country and Lending Groups; [cited 2017 Mar]. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- 52.Parashar UD, Hummelman EG, Bresee JS, et al. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Breiman RF, Zaman K, Armah G, et al. Analyses of health outcomes from the 5 sites participating in the Africa and Asia clinical efficacy trials of the oral pentavalent rotavirus vaccine. Vaccine. 2012;30 (Suppl 1):A24–9. [DOI] [PubMed] [Google Scholar]
- 54.Feikin DR, Laserson KF, Ojwando J, et al. Efficacy of pentavalent rotavirus vaccine in a high HIV prevalence population in Kenya. Vaccine. 2012;30(Suppl 1):A52–60. [DOI] [PubMed] [Google Scholar]
- 55.Colgate ER, Haque R, Dickson DM, et al. Delayed dosing of oral rotavirus vaccine demonstrates decreased risk of rotavirus gastroenteritis associated with serum zinc: a randomized controlled trial. Clin Infect Dis. 2016;63:634–641. [DOI] [PubMed] [Google Scholar]
- 56.Ali A, Kazi AM, Cortese MM, et al. Impact of withholding breast-feeding at the time of vaccination on the immunogenicity of oral rotavirus vaccine–a randomized trial. PLoS One. 2015;10:e0127622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rongsen-Chandola T, Strand TA, Goyal N, et al. Effect of withholding breastfeeding on the immune response to a live oral rotavirus vaccine in North Indian infants. Vaccine. 2014;32(Suppl 1):A134–9. [DOI] [PubMed] [Google Scholar]
- 58.Tissera MS, Cowley D, Bogdanovic-Sakran N, et al. Options for improving effectiveness of rotavirus vaccines in developing countries. Hum Vaccin Immunother. 2017;13(4):921–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burnett E, Yen C, Tate JE, et al. Rotavirus vaccines: current global impact and future perspectives. Future Virol. 2016;11:699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Restrepo-Mendez MC, Barros AJ, Wong KL, et al. Inequalities in full immunization coverage: trends in low- and middle-income countries. Bull World Health Organ. 2016;94:794–805b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rheingans R, Atherly D, Anderson J. Distributional impact of rotavirus vaccination in 25 GAVI countries: estimating disparities in benefits and cost-effectiveness. Vaccine. 2012;30(Suppl 1):A15–23. [DOI] [PubMed] [Google Scholar]
- 62.Bines JE, Patel M, Parashar U. Assessment of postlicensure safety of rotavirus vaccines, with emphasis on intussusception. J Infect Dis. 2009;200(Suppl 1):S282–90. [DOI] [PubMed] [Google Scholar]
- 63.Mandomando I, Weldegebriel G, de Deus N, et al. Feasibility of using regional sentinel surveillance to monitor the rotavirus vaccine impact, effectiveness and intussusception incidence in the African Region. Vaccine. 2017;35:1663–1667 [DOI] [PubMed] [Google Scholar]
- 64.Mwenda JM, Tate JE, Steele AD, et al. Preparing for the scale-up of rotavirus vaccine introduction in Africa: establishing surveillance platforms to monitor disease burden and vaccine impact. Pediatr Infect Dis J. 2014;33(Suppl 1):S1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mpabalwani EM, Mwenda JM, Tate JE, et al. Review of naturally occurring intussusception in young children in the WHO African region prior to the era of rotavirus vaccine utilization in the expanded programme of immunization. J Trop Pediatr. 2017;63:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tate JE, Parashar UD. Rotavirus vaccines in routine use. Clin Infect Dis. 2014;59:1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yen C, Tate JE, Hyde TB, et al. Rotavirus vaccines: current status and future considerations. Hum Vaccin Immunother. 2014;10:1436–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhandari N, Rongsen-Chandola T, Bavdekar A, et al. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian infants: a randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2014;383:2136–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Isanaka S, Guindo O, Langendorf C, et al. Efficacy of a low-cost, heat-stable oral rotavirus vaccine in Niger. New England J Med. 2017;376:1121–1130. [DOI] [PubMed] [Google Scholar]
- 70.World Health Organization. Immunization standards: vaccine quality. Geneva, Switzerland; [cited 2017 Mar]. Available from: http://www.who.int/immunization_standards/vaccine_quality/en/ [Google Scholar]
- 71.Fix AD, Harro C, McNeal M, et al. Safety and immunogenicity of a parenterally administered rotavirus VP8 subunit vaccine in healthy adults. Vaccine. 2015;33:3766–3772. [DOI] [PubMed] [Google Scholar]
- 72.Kang G, Thuppal SV, Srinivasan R, et al. Racecadotril in the management of rotavirus and non-rotavirus diarrhea in under-five children: two randomized, double-blind, placebo-controlled trials. Indian Pediatr. 2016;53:595–600. [DOI] [PubMed] [Google Scholar]
- 73.Gharial J, Laving A, Were F. Racecadotril for the treatment of severe acute watery diarrhoea in children admitted to a tertiary hospital in Kenya. BMJ. 2017;4:e000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Armah G, Lewis KD, Cortese MM, et al. A randomized, controlled trial of the impact of alternative dosing schedules on the immune response to human rotavirus vaccine in rural Ghanaian infants. J Infect Dis. 2016;213:1678–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ali SA, Kazi AM, Cortese MM, et al. Impact of different dosing schedules on the immunogenicity of the human rotavirus vaccine in infants in Pakistan: a randomized trial. J Infect Dis. 2014;210:1772–1779. [DOI] [PubMed] [Google Scholar]
- 76.Bines JE, Danchin M, Jackson P, et al. Safety and immunogenicity of RV3-BB human neonatal rotavirus vaccine administered at birth or in infancy: a randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2015;15:1389–1397. [DOI] [PubMed] [Google Scholar]
- 77.Mwenda JM, Tate JE, Parashar UD, et al. African rotavirus surveillance network: a brief overview. Pediatr Infect Dis J. 2014;33(Suppl1):S6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.