Abstract
Metallic radionuclides have been instrumental in the field of nuclear imaging for over half a century. While recent years have played witness to a dramatic rise in the use of radiometals as labels for chelator-bearing biomolecules, imaging agents based solely on coordination compounds of radiometals have long played a critical role in the discipline as well. In this work, we seek to provide a brief overview of metal complex-based radiopharmaceuticals for positron emission tomography (PET) and single photon emission computed tomography (SPECT). More specifically, we have focused on imaging agents in which the metal complex itself rather than a pendant biomolecule or targeting moiety is responsible for the in vivo behavior of the tracer. This family of compounds contains metal complexes based on an array of different nuclides as well as probes that have been used for the imaging of a variety of pathologies, including infection, inflammation, cancer, and heart disease. Indeed, two of the defining traits of transition metal complexes — modularity and redox chemistry — have both been creatively leveraged in the development of imaging agents. In light of our audience, particular attention is paid to structure and mechanism, though clinical data is addressed as well. Ultimately, it is our hope that this review will not only educate readers about some of the seminal work performed in this space over the last 30 years but also spur renewed interest in the creation of radiopharmaceuticals based on small metal complexes.
Keywords: Nuclear imaging, positron emission tomography, PET, single photon emission computed tomography, SPECT, inorganic chemistry, metal complex, radiometals, 99mTc, 111In, 68Ga, 67Ga, 64Cu, 86Y, 89Zr
Graphical Abstract
In this review, we seek to provide a brief and accessible overview of metal complex-based radiopharmaceuticals for nuclear imaging.
INTRODUCTION
Over the past half-century, nuclear imaging has evolved from a largely experimental technique to a critical component of modern clinical practice. Single photon emission computed tomography (SPECT) and positron emission tomography (PET) have proven invaluable to oncology, cardiology, and neurology, providing functional imaging data that complements that produced by magnetic resonance imaging (MRI) and computed tomography (CT).
The centerpiece of any nuclear imaging technique is the radiopharmaceutical, the tracer designed to selectively and specifically localize in a tissue of interest. And the heart of any radiopharmaceutical is, of course, its gamma- or positron-emitting radionuclide. A wide array of radionuclides has been used in PET and SPECT tracers, including isotopes ranging from carbon-11 (11C; t1/2 ~ 20 min) to iodine-124 (124I; t1/2 ~ 4.2 d). The subset of metallic radionuclides — from here on called radiometals — has played an integral role in the field throughout its history (Table 1). Some of the earliest diagnostic imaging agents were complexes of technetium-99m (99mTc), while bioconjugates labeled with gallium-68 (68Ga), copper-64 (64Cu), and zirconium-89 (89Zr) represent some of the most promising tracers today.1 The vast majority of current research into radiometal-based imaging agents focuses on constructs in which a radiometal-chelate complex (e.g. [68Ga]Ga-DOTA or [89Zr]Zr-DFO) is appended to a delivery vector such as a small molecule, peptide, antibody, or nanoparticle.2 Yet this, of course, is not the only flavor of radiometal-based imaging agent. Another class of probes exists in which the metal complex itself is the functional component of the imaging agent. In these, no additional targeting vector is needed, as the radiometal complex alone is responsible for the in vivo localization of the tracer. Three facets of coordination chemistry make radiometals a unique platform for the creation of nuclear imaging agents. First, the ability of metals to act as an anchor for a three-dimensional scaffold of ligands enables control over the structural, physical, and biological properties of the probe. Second, the modularity of metal-ligand interactions facilitates the screening and optimization of potential imaging agents. And third, the flexible redox chemistry of some metal centers [e.g. Cu(I/II)] can provide an additional mechanism for the selective delivery of tracers to target tissues (though, to be fair, it has been a source of headaches as well).
Table 1.
Chemical and physical properties of selected metallic radionuclides.
| Modality | Nuclide | Half-Life | Source | Relevant Oxidation States | Common Coordination Number |
|---|---|---|---|---|---|
| PET | 68Ga | 68 min | generator | 3+ | 4, 5, 6 |
| 60Cu | 23.7 min | cyclotron | 1+, 2+ | 4, 5, 6 | |
| 61Cu | 3.3 h | cyclotron | 1+, 2+ | 4, 5, 6 | |
| 62Cu | 9.7 min | cyclotron | 1+, 2+ | 4, 5, 6 | |
| 64Cu | 12.7 h | cyclotron | 1+, 2+ | 4, 5, 6 | |
| 86Y | 14.7 h | cyclotron | 3+ | 8, 9 | |
| 89Zr | 78.4 h | cyclotron | 4+ | 8 | |
| SPECT | 99mTc | 6.0 h | generator | 1- to 7+ | 4, 5, 6 |
| 111In | 67.3 h | cyclotron | 3+ | 4, 5, 6 | |
| 67Ga | 78.2 h | cyclotron | 3+ | 5, 6, 7, 8 |
Coordination complexes of a variety of radiometals have been used as nuclear imaging agents, and a handful of metal complex-based imaging agents have been translated to the clinic with varying degrees of success (Table 2). 99mTc is far and away the most commonly represented radiometal in this group, a fact that likely stems from the nuclide’s popularity during the earliest days of nuclear medicine.3 Enthusiasm for PET and SPECT tracers based on metal complexes has waned somewhat in recent years, a trend which has coincided with the rise of bioconjugates in the field. However, we strongly believe that metal complexes still have much to offer nuclear imaging. Furthermore, we contend that an accessible — though far from exhaustive — review of the topic could help spur interest and innovation in the field. Indeed, while a plethora of excellent reviews on the role of radiometals in nuclear imaging have been published in recent years — especially those of Blower4, Donnelly5, Orvig6, 7, Bartholoma8, Holland9 and Boros10— the field has not seen an introductory, ‘one-stop-shop’ review specifically dedicated to the diversity of metal complex-based radiopharmaceuticals in quite a while.1, 2, 4–6, 8, 9, 11–17
Table 2.
Selected metal complex-based radiopharmaceuticals that have been translated to the clinic
| Radiopharmaceutical | Clinical Application |
|---|---|
| [99mTc][Tc(CO)3(NTA)]2- | Renal perfusion |
| [99mTc]TcN-DBODC5 | Myocardial perfusion |
| [99mTc]Tc-disofenin | Hepatobiliary imaging |
| [99mTc]Tc-DMSA | Renal clearance; glioblastoma |
| [99mTc]Tc-DTPA | Bone metastasis |
| [99mTc]Tc-ECD | Brain perfusion |
| [99mTc]Tc-ECDG | Lung cancer |
| [99mTc]Tc-HEDP | Bone metastasis |
| [99mTc]Tc-HMDP | Bone metastasis |
| [99mTc]Tc-HMPAO | Brain perfusion |
| [99mTc]Tc-lidofenin | Hepatobiliary imaging |
| [99mTc]Tc-MAEC | Renal imaging |
| [99mTc]Tc-MAG3 | Renal perfusion |
| [99mTc]Tc-MDP | Bone metastasis |
| [99mTc]Tc-mebrofenin | Hepatobiliary imaging |
| [99mTc]Tc-MIBI | Myocardial perfusion; glioblastoma |
| [99mTc]Tc-MPO | Myocardial perfusion |
| [99mTc]Tc-NOET | Myocardial perfusion |
| [99mTc]Tc-oxidronate | Altered osteogenesis; urothelial carcinoma |
| [99mTc]Tc-PnAO | Brain perfusion |
| [99mTc]Tc-Q12 | Myocardial perfusion |
| [99mTc]Tc-tetrafosmin | Myocardial perfusion |
| [64Cu]Cu-ATSM | Lung, cervical, and lung cancers |
| [64Cu]Cu-PTSM | Myocardial perfusion |
| [68Ga]Ga-Citrate | Wide variety of pathologies |
| [68Ga]Ga-EDTMP | Bone metastasis |
| [111In]In-(oxinate)3 | Platelet labeling |
| [111In]In-(tropolonate)3 | Leucocyte labeling |
In the pages that follow, we hope to offer an overview of the range of small metal complexes developed as PET and SPECT imaging agents, with a particular emphasis on work from the last twenty years. As we have discussed above, we have limited ourselves to agents in which the in vivo behavior of the tracer is predicated solely on the structure of the coordination complex itself rather than a pendant targeting functionality. This boundary condition excludes everything from radiometallated antibodies and nanoparticles to small metal complexes bearing hypoxia-targeting nitroimidazoles. While we have placed particular emphasis on structure and reactivity, clinical applications are discussed as well. Finally, in order to serve an audience of inorganic chemists, we have organized the work by radiometal rather than pathology, target, or mechanism. That said, further subdivisions were necessary within the section on 99mTc given the abundance of tracers based on the radiometal. Ultimately, it is our sincere hope that this work can play a part in fostering a revival of research into what we believe has become an underexplored class of nuclear imaging agents.
Technetium-99m
Introduction
The overwhelming majority of nuclear imaging agents based on small metal complexes are coordination compounds of 99mTc.18 99mTc is used in the vast majority of nuclear imaging scans and is presently the most widely used radionuclide in the clinic, largely due to its availability from the 99Mo/99mTc generator. Furthermore, the physical characteristics of the radionuclide (Eγ = 140.5 keV; t1/2 = 6.02 hours) are nearly ideal for SPECT imaging, and its short half-life and lack of particulate emissions result in minimal radiation burden to the patient. 99mTc decays to technetium-99 (99Tc), a very weak β- emitter with an extraordinarily long half-life (t1/2 ~ 211,000 y) that neither interferes with nor degrades image quality.19
The starting point for nearly all 99mTc-based radiopharmaceuticals is sodium pertechnetate: [99mTc]NaTcO4.20 Typically, pertechnetate is eluted from a generator and introduced directly into a “radiopharmaceutical kit”. These kits generally contain a ligand, a reductant — usually stannous ion — and a buffer, facilitating the creation of a solution that will yield the 99mTc-based imaging agent (it is important to note, however, that these kits often contain other components as well, including auxiliary ligands, catalysts, radical stabilizers, and compounds that aid in lyophilization). The advent of these radiopharmaceutical kits in the 1970s precipitated the rapid development of 99mTc-based SPECT agents for renal, myocardial, and hepatobiliary imaging. Unfortunately, however, the coordination chemistry of technetium was not well understood at the time, and, as a result, many early 99mTc-based radiopharmaceuticals were not fully characterized. Thankfully, studies of the coordination chemistry of technetium intensified in the late 1970s, leading to the discovery of an array of well-characterized 99mTc-based imaging agents with wide-ranging applications (Table 2).21
It is important to note that the concentration of [99mTc]TcO4− eluted from a generator is generally in the range of 10−7−10−10 M. These remarkably low, “no-carrier-added” concentrations and the short half-life of 99mTc combine to preclude the characterization of 99mTc-complexes using routine analytical techniques available to inorganic chemists. In many cases, 99mTc’s long-lived isotopologue 99Tc (β−: 243 KeV) has enabled the use of standard macroscopic methods to determine the structure and physiochemical properties of 99mTc radiopharmaceuticals by proxy. Most of the 99mTc-based radiopharmaceuticals on the market since the 1980s have been characterized by synthesizing the 99Tc isotopologue, studying the resulting complex using macroscopic analytical tools, and relating this data back to the 99mTc radiopharmaceutical via HPLC concordance experiments. Yet it is important to note that the discrete structures of some 99mTc-based radiopharmaceuticals remain unknown, particularly those based on 99mTc(III). In recent years, however, concerns over the storage and disposal of 99Tc have curtailed the use of this approach. Today, rhenium, the third row congener of Tc, is increasingly used for structural characterization. However, two key differences between Re and Tc can cloud these results: the inertness of Re compared to Tc and the former’s higher reduction potential.
Over the years, imaging agents have been developed which feature the 99mTc cation in a wide variety of oxidation states. Consequently, in the pages that follow, we have divided our discussion according to the oxidation state of the metal center, beginning with Tc(I) and ending with Tc(V).
Tc(I) Complexes
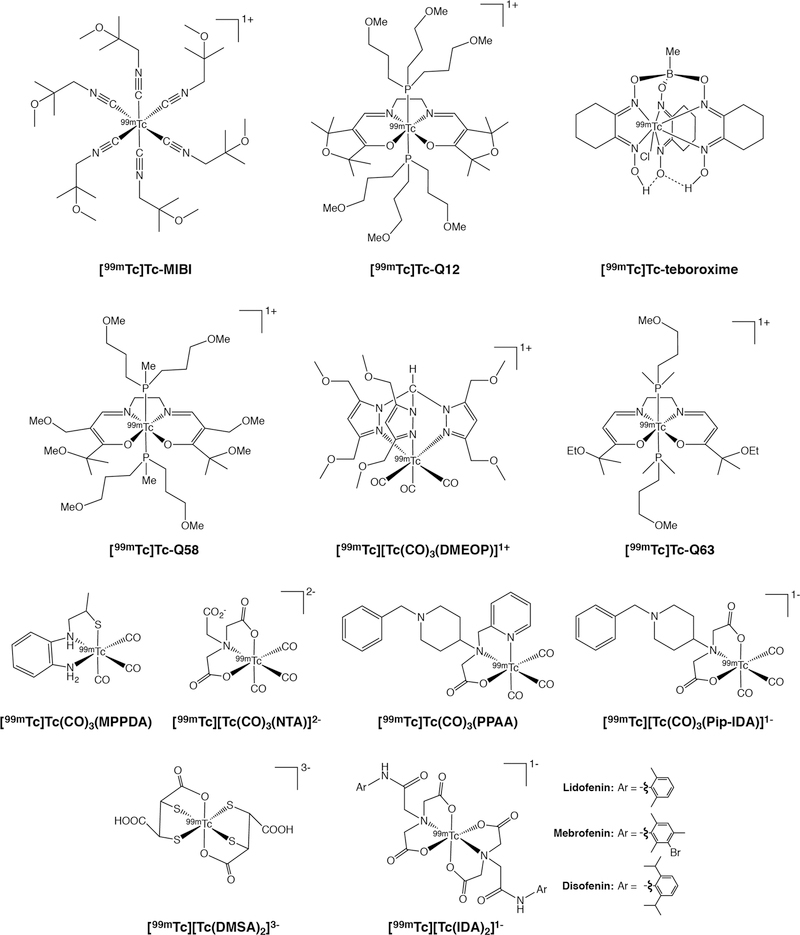
Complexes of Tc(I) are inert, stable, and tend to prefer octahedral coordination geometries. Without question, the most well-known 99mTc(I)-based tracer is 99mTc(I)-hexakis(2-methoxy-2-methylpropyl)nitrile, informally known as [99mTc]Tc-MIBI or [99mTc]Tc-sestamibi (Figure 1). [99mTc]Tc-MIBI features a coordinatively inert Tc(I) center with a low-spin d6 electron configuration surrounded by an octahedral array of six (2-methoxy-2-methylpropyl)nitrile ligands. The radiotracer is synthesized using a kit formulation in which the most important processes are metathesis and reduction reactions involving [99mTc]TcO4−, a preformed Cu(I)-tetrakis(2-methoxy-2-methylpropyl)nitrile complex, and a reducing agent (often SnCl2).22 The complex itself is positively charged (+1) and lipophilic and, as a result, accumulates in the mitochondria of cells.22, 23 [99mTc]Tc-MIBI stands as the most successful variant of a large series of related compounds that were developed and optimized over a period of years.
Figure 1.
Selected [99mTc]Tc(I) and [99mTc]Tc(III) complexes
Cardiac imaging is the principal clinical application of [99mTc]Tc-MIBI, and the uptake of the radiotracer in the myocardium is proportional to blood flow.24 Consequently, clinicians can compare two [99mTc]Tc-MIBI scans taken under different conditions — one at rest, one during stress — in order to differentiate between induced and persistent abnormalities in myocardial perfusion. In the years since its emergence in nuclear cardiology, [99mTc]Tc-MIBI has found applications in other fields as well. For example, the uptake and retention of [99mTc]Tc-MIBI in breast cancer tissue has been explored as a predictive marker for response to chemotherapy.25–27 Remarkably, [99mTc]Tc-MIBI — as well as a handful of other 99mTc(I) hexa-isonitrile compounds — has also been explored for both the imaging of parathyroid abnormalities as well as the delineation of the expression of p-glycoprotein (Pgp; multidrug resistance protein 1) (Figure 2).28–31
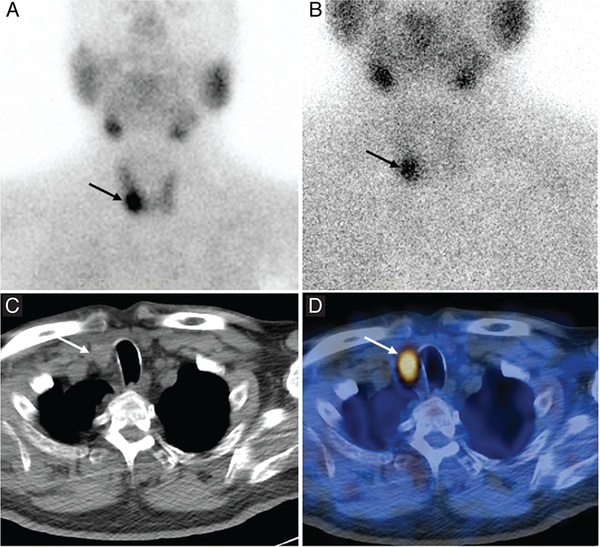
Figure 2.
Planar [99mTc]Tc-MIBI scintigraphy (A and B), CT (C), and SPECT/CT (D) of a patient with primary hyperparathyroidism, showing focal tracer uptake in the lower part of the right lobe of the thyroid gland. Reprinted from Keidar, et al. Preoperative [99mTc]Tc-MIBI SPECT/CT interpretation criteria for localization of parathyroid adenomas — correlation with surgical findings. Molecular Imaging and Biology 2017, 19, 265–270. Copyright by the World Molecular Imaging Society.31
Of course, [99mTc]Tc-MIBI is not the only metal complex-based SPECT agent with a Tc(I) center. A handful of others have been developed, though none has advanced beyond preclinical investigations. For example, three different Tc(I)-tricarbonyl complexes have been reported as potential brain imaging agents: [99mTc]Tc-tricarbonyl 4-(N-benzylpiperdine)-pyridine-2-ylmthyl-amino acetic acid [99mTc][Tc(CO)3PPAA], 99mTc tricarbonyl 4-iminodiacetato-N-benzylpiperdine [99mTc][Tc(CO)3PipIDA]−, and 99mTc tricarbonyl N-(2-mercapto-propyl)-1,2-phenylenediamine [99mTc][Tc(CO)3MPPDA] (Figure 1).32, 33 Unfortunately, however, initial preclinical investigations have revealed that each compound suffers from suboptimal uptake and retention in brain tissue. Perhaps more hopefully, another Tc(I)-tricarbonyl complex — [99mTc][Tc(CO)3(NTA)]2- — was recently developed as an imaging agent to measure renal plasma flow (Figure 1).34 The dianionic nature of the complex is thought to aid with plasma clearance, tubular extraction, and renal extraction. In preclinical studies, [99mTc][Tc(CO)3(NTA)]2- has displayed higher uptake and retention in failed kidneys than the clinical gold standard: 131I-o-iodohippurate. Finally, a family of 99mTc(I)-tricarbonyl complexes bearing tripodal tris(pyrazolyl)methane ligands — e.g. [99mTc][Tc(CO)3(DMEOP)]+ — has also been explored for imaging Pgp expression in preclinical models of breast and lung cancer (Figure 1).35–37
Tc(III) Complexes
A range of 99mTc(III) complexes has also been developed for SPECT imaging. 99mTc(III) complexes have a d4 electron configuration and have been known to form 6- and 7-coordinate species with a wide variety of ligands. The best known of these tracers are the myocardial perfusion imaging agents [99mTc]Tc-teboroxime and [99mTc]Tc-Q12 (Figure 1).38, 39 [99mTc]Tc-Teboroxime is the most successful agent to emerge from the boronic acid adducts of technetium dioximes (BATO) family of compounds.38 The complex is heptacoordinate, featuring six imine donors and a (labile) chloride arranged around a 99mTc(III) center in a trigonal prismatic geometry.40 Practically speaking, the neutral, lipophilic complex boasts rapid blood clearance and high uptake in myocardial tissue at early time points. In recent years, the Liu laboratory at Purdue University has sought to improve upon [99mTc]Tc-teboroxime through the creation of ‘second-generation’ variants with sulfonyl-containing boronate caps. One of these tracers — [99mTc]Tc-3SPboroxime — has displayed improved myocardial imaging properties compared to [99mTc]Tc-teboroxime, while another — [99mTc]Tc-4ASboroxime — has been shown to label red blood cells efficiently and displayed promise as a blood pool imaging agent in preclinical experiments.41, 42 [99mTc]Tc-Q12 — also known as [99mTc]Tc-furifosmin — is a member of the “Q-series” of six-coordinate compounds bearing two trans monodentate trialkylphosphine ligands as well as a tetradentate N2O2-based Schiff base ligand.39 Like [99mTc]Tc-MIBI, [99mTc]Tc-Q12 is cationic and lipophilic and produces high uptake in myocardial tissue.39, 43 Furthermore, like [99mTc]Tc-MIBI, [99mTc]Tc-Q12 — and the structurally related [99mTc]Tc-Q58 and [99mTc]Tc-Q63 complexes (Figure 1) — has also been used as a tracer for the delineation of Pgp expression.44–46
Moving on, [99mTc]Tc-DMSA is a hexacoordinate complex in which the 99mTc(III) center is bound by two tridentate dimercaptosuccinic acid ligands (Figure 1). The anionic, hydrophilic complex — which can be synthesized in a single step using a kit formulation — was developed as a renal imaging agent, as it accumulates in the cortical tubules and is eliminated via the urinary tract.47 A 99mTc(V) version of [99mTc]Tc-DMSA is also accessible from the kit via an additional oxidation step (Figure 3).48 Finally, a large family of iminodiacetic acid (IDA)-based complexes of 99mTc(III) — including the FDA-approved [99mTc]Tc-lidofenin, [99mTc]Tc-mebrofenin, and [99mTc]Tc-disofenin — have been developed for the SPECT imaging of the biliary tract (Figure 1).49, 50 These tracers can be synthesized via a kit formulation and feature two tridentate diaryl iminodiacetic acid ligands arrayed around a 99mTc(III) center. The ability of this family of agents to image liver function arises from their uptake in hepatocytes and subsequent secretion into the bile.51
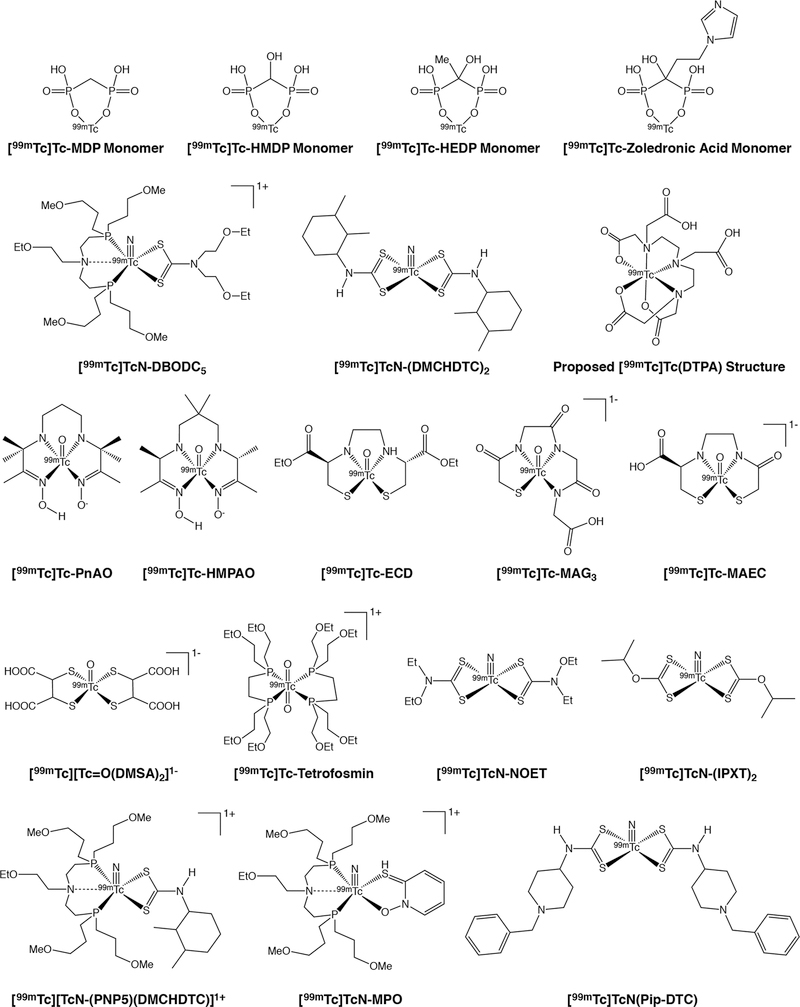
Figure 3.
Selected [99mTc]Tc(IV) and [99mTc]Tc(V) complexes
Tc(IV) Complexes
The members of the [99mTc]Tc-diphosphonate family of bone imaging agents are the best known tracers based on 99mTc(IV). [99mTc]Tc-MDP is the simplest member of this group, though its structure — most likely some sort of polymer in which methylenediphosphonate acts as a bridging ligand — remains elusive (Figure 3).52 [99mTc]Tc-MDP, like all members of this family, relies upon the affinity of its phosphonate ligands for Ca2+ as the source of its affinity for bone.53 A wide range of other 99mTc-labeled diphosphonates have been developed, including variants based on hydroxyethylidene diphosphonate (HEDP), hydroxymethylidene diphosphonate (HMDP), and pyrophosphate (Figure 3).54 The quality of images obtained with these tracers can be ordered as follows: [99mTc]Tc-HMDP >> [99mTc]Tc-MDP > [99mTc]Tc-HEDP >> [99mTc]Tc-pyrophosphate (Figure 3). Recently, a new class of 99mTc-labeled diphosphonate tracers based on zoledronic acid has emerged, though the marginal improvement provided by these agents remains to be seen (Figure 3).55, 56
The reduction of [99mTc]TcO4− by reducing agents in the presence of weak ligands often forms 99mTc colloids that are presumed to be composed of 99mTc(IV). Thin layer chromatography methods have been developed to separate 99mTc-labeled complexes from both 99mTc-based colloids and [99mTc]TcO4−.57 Though few 99mTc(IV) complexes have been identified for radiopharmaceutical purposes, a series of monocationic 99mTc(IV) complexes of N-substituted-3-hydroxy-2-methyl-4-pyridinones have been explored for renal imaging. Biodistribution studies in rabbits and mice show high uptake and retention in the kidneys, suggesting that these compounds may prove useful for kidney imaging.58, 59 Finally, the 99mTc complex of the ubiquitous chelator diethylenetriaminepentaacetic acid ([99mTc]Tc-DTPA; Figure 3) has also been used clinically as a SPECT tracer for both kidney and brain imaging.60, 61 Somewhat surprisingly, however, both the structure and the oxidation state of [99mTc]Tc-DTPA have yet to be reliably determined, with the latter variously proposed to be 99mTc(III), 99mTc(IV), or 99mTc(V).62
Tc(V) Complexes
99mTc(V) complexes form the largest family of 99mTc-based SPECT tracers, and — not coincidentally — their history stretches back to the earliest days of 99mTc-SPECT. 99mTc(V) complexes have a d2 electron configuration and, generally speaking, assume either 5-coordinate square pyramidal or 6-coordinate octahedral geometries in aqueous environments. Furthermore, most 99mTc(V) compounds can be divided into two categories: those containing [99mTc][Tc(V)=O]3+ bonds and those containing [99mTc][Tc(V)≡N]2+ bonds.
We will address complexes containing [99mTc][Tc(V)=O]3+ bonds first, as these compounds have been more prevalent through the years. [99mTc]Tc(V)-propylene amine oxime ([99mTc]Tc-PnAO) was the first commercially available 99mTc-based cerebral blood flow agent approved for use in humans.63 The neutral, distorted square pyramidal complex consists of a [99mTc][Tc(V)=O]3+ motif and a diaminedioxime ligand (Figure 3).63, 64 In order to compensate for the suboptimal pharmacokinetics of [99mTc]Tc-PnAO, a second-generation complex with a slightly altered ligand structure was created: [99mTc]Tc-hexamethylpropyleneamine oxime ([99mTc]Tc-HMPAO; Figure 3).65, 66 [99mTc]Tc-HMPAO exists as two diastereomers: a meso compound and a pair of d,l enantiomers.67 While the meso form diffuses quickly through the blood brain barrier, the d,l enantiomers decompose into hydrophilic products and are trapped in the brain, facilitating the imaging of cerebral perfusion.67, 68 Interestingly, even after other agents have surpassed [99mTc]Tc-HMPAO’s performance for brain imaging, its ability to label leukocytes has given the complex a second life as a whole-cell labeling reagent for intra-abdominal infections and inflammatory bowel disease.69
During the continued search for improved brain imaging agents, [99mTc]Tc-L,L-ethylenedicysteine diethylester ([99mTc]Tc-ECD) was identified as a lead candidate and subsequently approved for clinical use (Figure 3).70 [99mTc]Tc-ECD is a neutral, lipophilic [99mTc][Tc(V)=O]3+-containing diamide dimercaptide (N2S2) complex that was found to pass through the blood brain barrier and was subsequently approved for clinical use.70 After the complex passes through the blood brain barrier, its two ester moieties are hydrolyzed by intracellular esterases, thereby creating a complex with a net negative charge.71, 72 Due to differences in hydrolysis rates, the l,l isomer is retained in the brain, but the d,d isomer is not.71, 72 Both isomers are hydrolyzed in the blood, however, ensuring rapid blood and renal clearance.68, 73–75
Continuing our discussion of NxSy complexes, in 1986, a series of 99mTc complexes with N2S2 ligands were explored as potential renal imaging agents.76 Due to a variety of limitations, however, none of the complexes proved suitable. In response to this setback, the same team developed a 99mTc(V) complex bearing a triamide mercaptide (N3S) ligand — mercaptoacetylglycylglycylglycine (MAG3) — as an alternative (Figure 3).76 [99mTc]Tc-MAG3 is a negatively charged, square pyramidal complex boasting a [99mTc][Tc(V)=O]3+ bond and a square pyramidal geometry.76–78 Although 90% of [99mTc]Tc-MAG3 is rapidly bound to plasma protein after injection, 50% of the blood activity is excreted from the kidneys on each pass, making the radiotracer useful for measuring renal plasma flow.79
Despite the approval of [99mTc]Tc-MAG3 by the U.S. FDA for renal perfusion imaging, its relatively high plasma protein binding meant that there was room for improvement.80 During a study of [99mTc]Tc-ECD metabolites, it was found that the most polar of these metabolites — [99mTc]Tc-L,L-ethylenedicysteine ([99mTc]Tc-EC) — is rapidly excreted in the urine.81 A subsequent study revealed that [99mTc]Tc-EC has a significantly lower blood plasma protein binding than [99mTc]Tc-MAG3.82 One drawback, however, was that it was found that at physiological pH, [99mTc]Tc-EC exists in both dianionic and monoanionic states, each of which exhibits a distinct clearance rate and protein binding affinity.83 This complication was later overcome with the development of [99mTc]Tc-D-mercaptoacetamide-ethylene-cysteine ([99mTc]Tc-MAEC), which is an N3S complex with a [99mTc][Tc(V)=O]3+ motif that combines the most advantageous features of [99mTc]Tc-MAG3 and [99mTc]Tc-EC (Figure 3).84
To wind down our discussion of agents containing [99mTc][Tc(V)=O]3+ bonds, [99mTc][Tc(1,2-bis[bis(2-ethoxyethyl)phosphino]ethane)2O2]+ — [99mTc]Tc-tetrofosmin — was developed in an effort to address concerns surrounding the non-target tissue uptake of 99mTc-based myocardial perfusion agents (Figure 3). [99mTc]Tc-tetrofosmin is a lipophilic, cationic, 99mTc(V) trans-dioxo bisdiphosphine complex bearing two terminal ethoxyethyl groups on each of its four phosphines.85 The lipophilicity and dipole moment of the complex allows it to permeate the cytosolic membrane of heart cells.86 After promising preclinical investigations, [99mTc]Tc-tetrofosmin was approved for use as a myocardial perfusion agent in the U.S. in 1996. Perhaps not surprisingly, [99mTc]Tc-tetrofosmin, like many myocardial perfusion imaging agents, has also been examined for the imaging of Pgp expression.87, 88
Shifting gears, in the mid-1990s, Pasqualini, et al. identified [99mTc]TcN[Et(EtO)NCS2]2 ([99mTc]Tc-NOET) as a potential myocardial perfusion imaging agent from a series of [99mTc][Tc(V)≡N]2+-containing bisthiocarbamate complexes (Figure 3).89 Although [99mTc]Tc-NOET was found to be reasonably effective for cardiac imaging, its clinical utility was ultimately limited by high non-target uptake in the lungs and liver as well as slow blood clearance.90, 91 More recently, a wide range of [99mTc][Tc(V)≡N]2+-based complexes has been explored as imaging agents. For example, the neutral, lipophilic complex containing a [99mTc][Tc(V)≡N]2+ motif and two isopropyl xanthate (IPXT) ligands — [99mTc]TcN(IPXT)2 — was found to have high brain uptake and retention (Figure 3).92 In addition, the neutral lipophilic [99mTc][Tc(V)≡N]2+-based complex formed from two 2,3-dimethylcyclohexl dithiocarbamato (DMCHDTC) ligands — [99mTc]TcN(DMCHDTC)2 — and the cationic, lipophilic [99mTc][Tc(V)≡N]2+-based complex containing one DMCHDTC ligand and one bis(dimethoxypropylphosphinoethyl)ethoxyethylamine (PNP5) ligand — [99mTc][TcN(PNP5)(DMCHDTC)]+ — have both shown localization and retention in the heart, suggesting potential as myocardial imaging agents (Figure 3).93, 94 In still another study, a [99mTc][Tc(V)≡N]2+-based complex bearing two 4-dithiocarbamato-N-benylpiperidine (Pip-DTC) ligands was synthesized to create [99mTc][TcN]Pip-DTC, a tracer that displays affinity for sigma receptors (Figure 3).32
Another member of the [99mTc][Tc(V)≡N]2+ family — [99mTc]Tc-[bis(dimethoxypropylphosphinoethyl)-ethoxyethylamine-[bis(N-ethoxyethyl)-dithiocarbamato] nitride ([99mTc]TcN-DBODC5) — has also been used as a myocardial perfusion imaging agent (Figures 3 and 4).95–97 [99mTc]TcN-DBODC5 is a monocationic 99mTc-nitrido complex containing a bidentate dithiocarbamate ligand and a tridentate PNP bisphosphine ligand arranged in an octahedral geometry. In preclinical experiments, [99mTc]TcN-DBODC5 produced high uptake in the myocardium as well as lower liver-to-heart activity concentration ratios and faster blood clearance rates than both [99mTc]Tc-tetrosfosmin and [99mTc]Tc-MIBI.98 Interestingly, [99mTc]TcN-DBODC5’s selective mitochondrial accumulation and activity as a substrate for Pgp suggest that it may have a place in tumor imaging protocols as well.99, 100 Like its compatriots, [99mTc]TcN-DBODC5 is synthesized via kit formulation, though the need for post-kit purification has limited its clinical application. Another 99mTc(V)-based dithiocarbamate myocardial perfusion tracer — [99mTc]TcN-(DMCHDTC)2 — was reported in 2009 by Zhang, et al (Figure 3).94 Like [99mTc]TcN-DBODC5, [99mTc]TcN-(DMCHDTC)2 contains a [99mTc][Tc(V)≡N]2+ motif, though the latter eschews phosphines for two tridentate dimethylcyclohexyl dithiocarbamate ligands. While preclinical investigations suggest that the in vivo performance of [99mTc]TcN-(DMCHDTC)2 may compare favorably to other members of the [99mTc]Tc-dithiocarbamate family, no clinical studies have appeared to date. Finally, a related compound — [99mTc][TcN(N-ethoxyethyl-N,N-bis[2-(bis(3-methoxypropyl)phosphino)ethyl]amine)(2-mercaptopyridine oxide)]+ ([99mTc]TcN-MPO) — has also recently been found to produce favorable myocardial uptake and improved liver-to-heart activity concentration ratios in both animals and humans (Figure 3).101–103
Figure 4.
Planar, whole-body [99mTc]TcN-DBODC5 scintigraphy (left to right: collected 5, 30, 60, and 240 min post-injection) of a healthy under (A) rest and (B) stress conditions. Adapted and reprinted from Cittanti et al. Whole-body biodistribution and radiation dosimetry of the new cardiac tracer 99mTcN-DBODC. Journal of Nuclear Medicine 2008, 49, 1299–1304. Copyright by the Society of Nuclear Medicine and Molecular Imaging.98
The Radioisotopes of Copper
Four radionuclides of copper have been used for PET imaging: 60Cu (t1/2 = 0.4 h; β+ yield = 93%; Eβ+ = 3.9 and 3.0 MeV), 61Cu (t1/2 = 3.32 h; β+ yield = 62%; Eβ+ = 1.2 and 1.15 MeV), 62Cu (t1/2 = 0.16 h; β+ yield = 98%; Eβ+ = 2.19 MeV), and 64Cu (t1/2 = 12.7 h; β+ yield = 19%; Eβ+ = 0.656 MeV).104, 105 64Cu is easily the most often employed because it has a half-life that allows for facile handling and shipment and can be produced rather easily using a cyclotron via the bombardment of an enriched 64Ni target. It is typically coordinated by chelators such as NOTA, SarAr, or CB-TE2A as part of a biomolecular conjugate. Regardless of the isotope, Cu(II) is the most biologically relevant oxidation state, though as we will see, reduction to Cu(I) is possible — and in some cases, desirable — in vivo. In light of its a d9 electron configuration, Cu(II) can accommodate a variety of coordination numbers and geometries, including square planar, square pyramidal, trigonal bipyramidal, and octahedral.104
Thiosemicarbazones
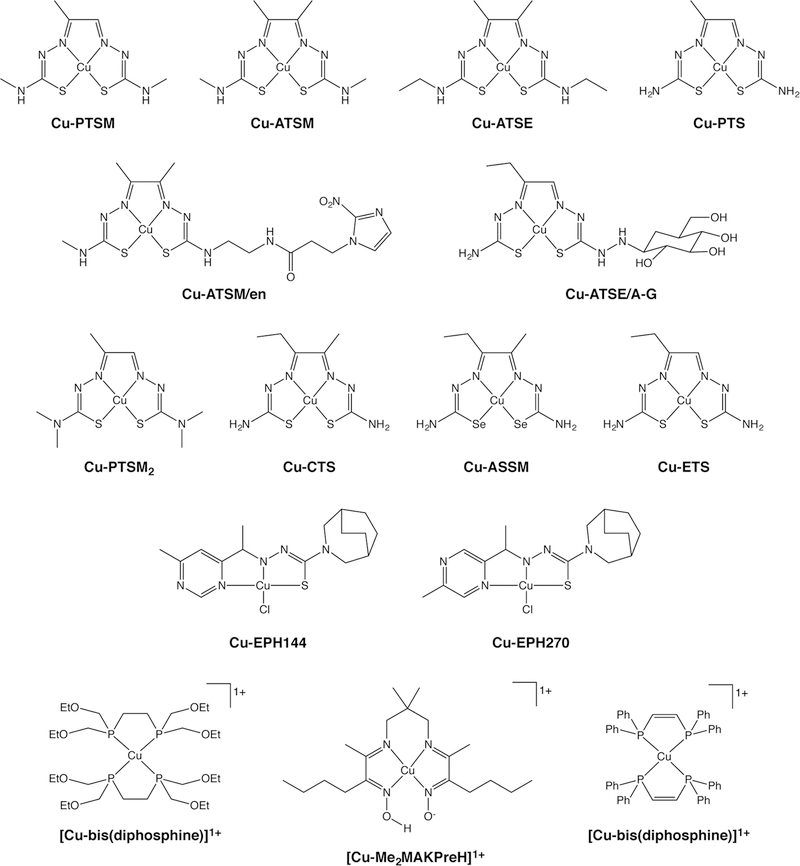
The two most well-known Cu-based PET imaging agents are both small metal complexes of thiosemicarbazone ligands: Cu(II)-diacetyl-bis(N4-thiosemicarbazone) [Cu-PTSM] and Cu(II)-diacetyl-bis(N4-methylthiosemicarbazone) [Cu-ATSM] (Figure 5). In both cases, a Cu(II) center is coordinated in a square planar geometry by the two nitrogen atoms and two sulfur atoms of the thiosemicarbazone. Remarkably, while the structures of Cu-PTSM and Cu-ATSM only differ by a single methyl group, they have completely different applications: the former is a perfusion tracer, while the latter is used for hypoxia imaging. Both complexes are prepared for the clinic using kit formulations and have been labeled and used with the entire gamut of radioisotopes of Cu.
Figure 5.
Selected Cu(II) complexes
Cu-PTSM is a radiotracer for the imaging of blood perfusion in the brain and myocardium.105 Due to its small size, planarity, and lipophilicity, Cu-PTSM is able to move into cells via passive diffusion. While the exact mechanism of cellular uptake and retention remain subject to some debate, it is generally agreed upon that once inside the cell, the Cu(II) center is reduced to Cu(I) and subsequently dissociates from the PTSM ligand.106, 107 Subsequently, this free Cu(I) binds to intracellular proteins, trapping Cu(I) within the cell. Preclinical studies in mice, non-human primates, and dogs all support the in vivo efficacy of Cu-PTSM for cerebral and myocardial perfusion imaging. In the clinic, Cu-PTSM has proven effective for the imaging of tumoral blood flow as well as both myocardial and cerebral perfusion. However, it has not been proven to provide any substantive advantages over other, more commonly-used perfusion imaging agents.108–112
As we have noted, Cu-PTSM’s close cousin Cu-ATSM has an entirely different application: the imaging of hypoxia.113 Briefly, the term ‘hypoxic’ is used to describe tissues that are deprived of normal, physiological levels of oxygen. In cancer, hypoxia is often associated with increased tumor aggressiveness, increased metastatic potential, higher rates of recurrence, and resistance to radiation therapy and chemotherapy. The selective uptake and retention of Cu-ATSM in hypoxic cells was first discovered in the late 1990s. Since then, a wealth of in vitro and in vivo data has supported the selectivity of the radiotracer, though variation has been observed between cell lines and tissue types.114–120
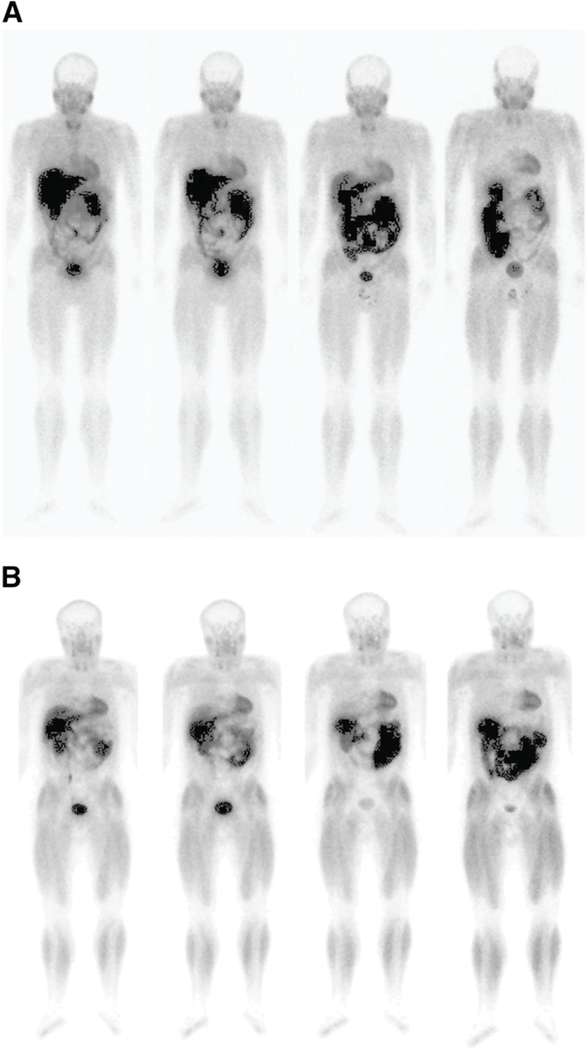
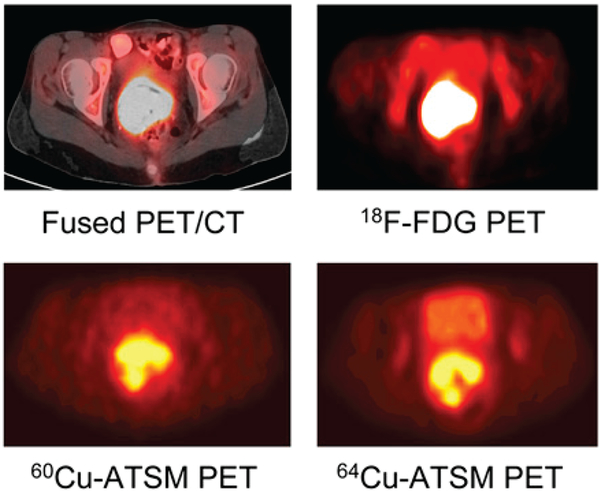
The precise mechanism of Cu-ATSM’s uptake and retention in hypoxic cells has been subject to significant debate, though most agree that the reduction of the initial Cu(II) complex is a critical step.120–122 Initially, it was believed that Cu(II)-ATSM could only be reduced and subsequently trapped in hypoxic tissues. However, Dearling et al. proposed a more nuanced mechanism. In this model, Cu(II)-ATSM is reduced in both hypoxic and normal tissues, producing a labile [Cu(I)-ATSM]− complex. In normoxic cells, this [Cu(I)-ATSM]− complex can be oxidized back to Cu(II)-ATSM, allowing the intact complex to diffuse out of the cell.9 In hypoxic tissues, however, [Cu(I)-ATSM]− is not reoxidized rapidly enough to prevent its acid-catalyzed dissociation through [Cu(I)-ATSMH] or [Cu(I)-ATSMH2]+ intermediates, ultimately leading to the release and trapping of the radioactive Cu(I) by intracellular proteins.123, 124 In 2005, Burgman et al. offered a hypothesis to explain why the uptake of Cu-ATSM varies between cell lines. The authors posited that because chaperones, transporters, and other proteins are required for the efflux and trapping of the radiotracer, variations in the expression levels of these proteins across cell lines will naturally lead to variations in hypoxia-selective uptake and retention.125 Given the likely role of protonation in the acid-catalyzed dissociation reactions, intracellular pH differences between cell lines may also play a role in this variation. In light of this mechanistic work, it becomes clear that the disparity in hypoxia-selectivity between Cu-ATSM and Cu-PTSM is not solely predicated on the differing redox potentials of the two complexes (−0.59 and −0.51 V vs. Ag/AgCl, respectively) but also upon differences in their stabilities, protonation rates, and LUMO energies.121 Mechanistic details aside, 60Cu-, 62Cu-, and 64Cu-ATSM have all shown some promise in the clinic. In 2008, for example, Lewis et al. published a trial comparing [60Cu]Cu-ATSM and [64Cu]Cu-ATSM PET in 10 patients with cervical carcinoma (Figure 6).126 Increased uptake of both [60Cu]Cu-ATSM and [64Cu]Cu-ATSM was observed in the tumors of all 10 patients, though the authors reported that [64Cu]Cu-ATSM produced higher quality images with superior tumor-to-background activity concentration ratios. However, neither [60Cu]Cu-, [62Cu]Cu-, nor [64Cu]Cu-ATSM have been widely adopted for clinical hypoxia imaging to date.
Figure 6.
Transaxial [18F]FDG-PET/CT, [18F]FDG PET, [60Cu]Cu-ATSM PET, and [64Cu]Cu-ATSM images of a patient with cancer of the uterine cervix. 18F-FDG = [18F]fluorodeoxyglucose = 2-deoxy-2-[18F]fluoroglucose. Adapted and reprinted from Lewis et al. An imaging comparison of [64Cu]Cu-ATSM and [60Cu]Cu-ATSM in cancer of the uterine cervix. Journal of Nuclear Medicine 2008, 49 (7), 1177–1182. Copyright by the Society of Nuclear Medicine and Molecular Imaging.131
In light of the limitations of Cu-ATSM and Cu-PTSM, a number of groups have worked on developing improved versions of these two tracers (Figure 5).114, 116, 127–130 For example, Cu-ATSE, Cu-CTS, Cu-ASSM, Cu-ATSE/A-G, and Cu-ATSM/en were created as second-generation hypoxia imaging agents. Cu-ETS, Cu-PTSM2, and Cu-PTS, on the other hand, were created as second-generation perfusion tracers. These novel tracers proved unable to appreciably improve upon the performance of their noteworthy precursors.
Interestingly, an additional family of 64Cu-thiosemicarbazone complexes — Cu azabicyclo[3.2.2]nonane thiosemicarbazones — has been explored for yet another application: the in vivo delineation of topoisomerase II (Topo-II) expression (Figure 5).131 Topoisomerase II is a DNA repair enzyme linked to increased tumor aggressiveness and metastatic potential in an array of cancers, and a number of Cu-thiosemicarbazone complexes have been shown to effectively inhibit Topo-II in vitro. In vivo experiments in mice bearing L1210 (Topo-II over-expressing) and PC-3 (Topo-II under-expressing) xenografts revealed that two of these 64Cu-labeled complexes — [64Cu]Cu-EPH144 and [64Cu]Cu-EPH270 — produce specific tumoral uptake in the Topo-II over-expressing tumors. The translational potential of these two agents is limited by their very high accumulation in non-target tissues, but they nonetheless could serve as starting points for a second generation of optimized probes.132
Beyond Thiosemicarbazones
Moving away from thiosemicarbazone complexes, copper(I)-bis(diphosphine) complexes are well-characterized, lipophilic compounds with established tumoricidal properties (Figure 5). In 2000, Lewis et al. evaluated a series of 64Cu-labeled Cu(I)-bis(diphosphine) complexes for the imaging of the multidrug resistance phenotype (MDR) in rats implanted with fibrocarcinoma.133, 134 One of the complexes in question, [64Cu][CuL52]+, was determined to accumulate in tumors with rapid clearance from the blood, renal excretion, and low uptake in non-target tissues. Unfortunately, however, no follow-up studies on this class of compounds have been published. Finally, a family of 64Cu-based complexes bearing diiminedioxime ligands was also investigated as agents for both myocardial perfusion and MDR imaging, but few subsequent studies have emerged.135, 136
The Radioisotopes of Gallium
As with other radiometals, much of the recent work on 67Ga- and 68Ga-based imaging agents has made use of bifunctional chelators, placing it beyond the scope of this review. However, it is worth mentioning that one such agent — [68Ga]Ga-DOTA-TATE — was recently approved by the FDA for the PET imaging of neuroendocrine tumors.137, 138 Yet even though bioconjugate-based systems remain the primary focus of the field, the increased availability and ease of use of 68Ge/68Ga-generators has sparked a surge of interest in 68Ga-PET.139 This trend, in turn, has led to the development of a handful of promising imaging agents based on small metal complexes.
Prior to the advent of [68Ga]Ga-DOTA-TATE and its many cousins, the first prominent imaging agent based on gallium was [67/68Ga]Ga-citrate.140 [67Ga]Ga-citrate was a common imaging agent for inflammation, infection, and certain types of tumors, including lung cancer, lymphoma, and leukemia, while [68Ga]Ga-citrate has been used to visualize vascular permeability in lung disease as well as infections in the bone.141 [67/68Ga]Ga-citrate, however, is not stable in vivo; rather, the metal itself is quickly transchelated by transferrin to create [67/68Ga]Ga-transferrin.142 Consequently, the Ga-citrate complex itself is not essential to the in vivo localization of the radiopharmaceutical, thus excluding it from discussion here. Similarly, we will likewise eschew other unstable gallium complexes that rely upon transferrin for targeting.
Ligands with Oxygen, Nitrogen, and Sulfur Donors
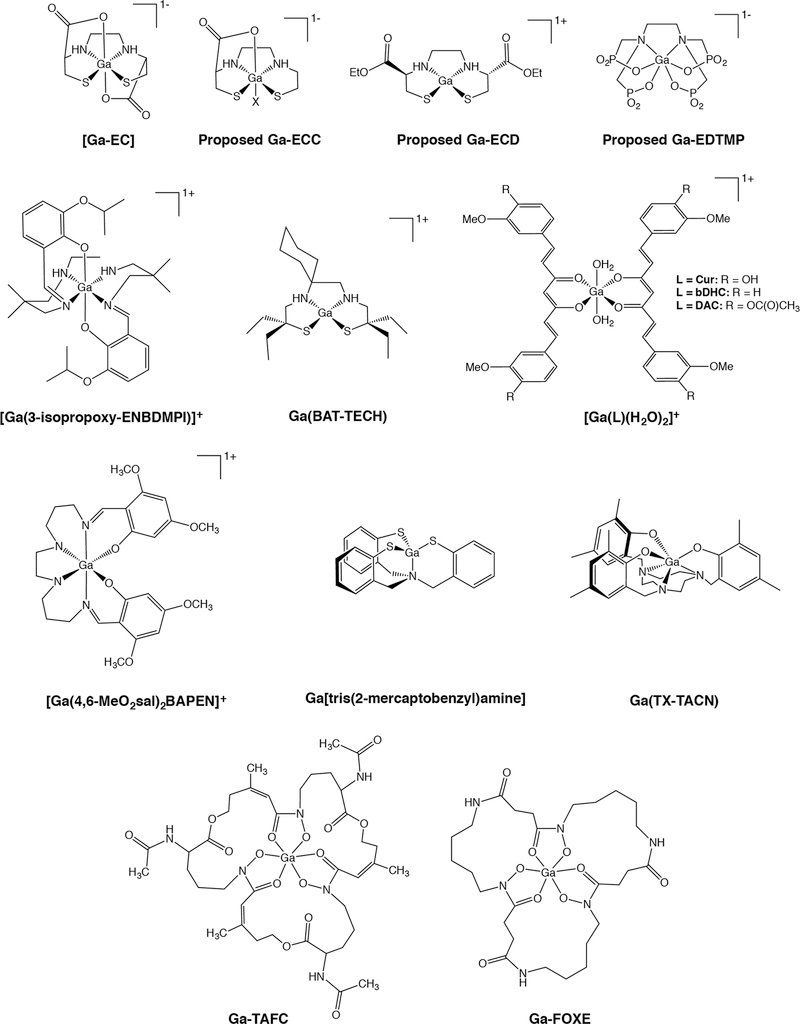
Shifting gears to imaging agents that do fit our parameters, Li et al. first characterized gallium(III) N,N′-ethylene-di-L-cysteine (Ga-EC) in hopes of developing a new family of 67Ga SPECT agents (Figure 7).143 Ga-EC was found to have an N2S2O2 distorted octahedral coordination in which nitrogen and sulfur atoms occupy the equatorial positions and oxygen atoms occupy the axial positions.143 Although Ga-EC was shown to be stable under physiological conditions, [67Ga]Ga-EC exhibited high liver uptake in vivo and was consequently abandoned as a prospective radiotracer. Years later, a mono-carboxylate derivative of EC — ethylenecysteamine cysteine (ECC) — was complexed with [68Ga]Ga3+ to test its potential as a renal imaging agent homologous to [99mTc]Tc-ECC (Figure 7).144 Although the structure of [67Ga]Ga-ECC was not investigated, the authors proposed that the complex maintained the distorted octahedral geometry of Ga-EC, with an unknown ligand replacing the second carboxylate oxygen. Unlike [67Ga]Ga-EC, [67Ga]Ga-ECC primarily clears through the kidneys, a trait that led the investigators to test [68Ga]Ga-ECC as a PET tracer for renal imaging.145
Figure 7.
Selected Ga(III) complexes
Ligands with Nitrogen and Sulfur Donors
More recently, the same laboratory that developed [67Ga]Ga- and [68Ga]Ga-ECC published a study focused on the use of a 68Ga-labeled ethylcysteinate dimer ([68Ga]Ga-ECD; Figure 7) as a cerebral blood flow PET tracer.146 Generator-produced [68Ga]GaCl3 was reacted with a commercially available ECD kit to form the proposed — though not confirmed — cationic N2S2 complex. Although [68Ga]Ga-ECD did not have sufficient uptake in the brain to allow for cerebral blood flow imaging, its relatively high cardiac uptake and fast urinary excretion indicate potential for myocardial perfusion imaging. 67/68Ga also forms stable complexes with the N2S2 ligand BAT-TECH, producing complexes that have exhibited high myocardial uptake in mice and non-human primates (Figure 7).147, 148 X-ray crystallography of non-radioactive Ga-BAT-TECH revealed a 5-coordinate distorted square pyramidal geometry in which Ga3+ is bound to the tetradentate ligand as well as an axial chloride. While this structure was determined in the solid-state, it is likely that the chloride anion dissociates under physiological conditions, though this has not been investigated. Finally, Cutler, et al. explored the use of 68Ga complexes bearing tripodal NS3 ligands for both brain and heart imaging (Figure 7)149, while Moore and coworkers created an N3S3 framework — TX-TACNH3 — that proved suitable for the stable coordination of both 111In and 67/68Ga (Figure 7).150, 151
Ligands with Oxygen and Nitrogen Donors
Moving on to nitrogen- and oxygen-based ligands, gallium(III)-(bis(3-isopropoxy2-phenolate-benzylidene)-N,N’-bis(2,2-dimethyl-3-amino-propyl) ethylenediamine) — Ga[3-isopropoxy-ENBDMPI]+ — is a hydrophobic, monocationic Ga(III)-complex with an N4O2 coordination sphere and a pseudo-octahedral geometry that has also been explored as a myocardial perfusion imaging agent (Figure 7).152 Both [67Ga]Ga- and [68Ga]Ga-[3-isopropoxy-ENBDMPI]+ have displayed high retention in the heart and rapid clearance from the liver, suggesting that these complexes could be deployed for cardiac SPECT and PET, respectively. In addition, Ga-[3-isopropoxy-ENBDMPI]+ and several other structurally-related N4O2-based 67/68Ga complexes have also been investigated as functional imaging agents for multidrug resistance (MDR) and Pgp expression.153–158
Along similar lines, more than two decades ago, Green, et al. developed a series of 67/68Ga-labeled tracers using bis- and tris(salicylaldimine) platforms, most notably [67/68Ga][Ga(4,6-MeO2sal)2BAPEN]+ (Figure 7).159–162 Several of these highly lipophilic, pseudo-octahedral N3O3-and N4O2-based complexes exhibited high myocardial uptake and rapid blood clearance in rats and dogs, though the in vivo results for a handful of these radiotracers in pigs revealed that the myocardial uptake was not proportional to perfusion, thereby precluding clinical translation.162
Interestingly, a series 68Ga complexes with ligands based on curcumin ((1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione) has recently attracted attention for imaging β-amyloid plaques and fibrils in Alzheimer’s disease. The three most promising of these ligands — curcumin (CUR), diacetyl-curcumin (DAC), and bis(dehydroxy)curcumin (bDHC) — were each found to form octahedral complexes with gallium(III) (Figure 7).163–165 Although [68Ga][Ga(CUR)2(H2O)2]+, [68Ga][Ga(DAC)2(H2O)2]+, and [68Ga][Ga(bDHC)2(H2O)2]+ all demonstrated a high affinity for β-amyloid plaques and fibrils in vitro, they unfortunately proved unable to produce useful images of amyloid aggregates in vivo. Yet in light of the potential benefits of PET imaging during early-stage Alzheimer’s disease, it is likely that second-generation 68Ga-curcuminoid complexes will be explored in search of a probe that combines high affinity for β-amyloid plaques and fibrils with favorable pharmacokinetic behavior.
In a very recent entry to this compendium, Greiser, et al. created a new class of 68Ga-labeled radiotracers based on 1,4-diazepan-6-amine (DAZA) ligands.166 These N3O3-based complexes are inert and stable, and while the preclinical data is sparing, in ovo imaging suggests that these compounds could serve as liver imaging agents. Finally, following the FDA approval of 153Sm- and 177Lu-ethylenediamine tetra(methylene phosphonic acid) (EDTMP) as bone-targeted radiotherapeutics, a 68Ga-labeled variant of the compound ([68Ga]Ga-EDTMP) has been tested as a PET imaging agent for bone scans (Figure 7).167 [68Ga]Ga-EDTMP has a putative octahedral geometry and an N2O4 coordination sphere and has shown rapid kidney clearance and high bone uptake in rat models.
Siderophores
Siderophore-derived ligands have also been used in conjunction with 67/68Ga to create bacteria-specific imaging agents.168 Siderophores are molecules secreted into the extracellular space by some bacterial and fungal cells to scavenge essential ferric ions. Once these molecules find and bind the sought-after cations, they are actively transported back into the cell where the iron can be utilized for various cellular processes. In light of the metal ion-binding ability of many siderophores, inorganic radiochemists have jumped at the chance to harness these molecules for applications with radiometals. Some siderophores — most notably desferrioxamine — have emerged as the basis of bifunctional chelators. Others, however, have been leveraged to create infection-targeted imaging agents.
One laboratory in particular has made impressive preclinical advances in the radiolabeling of the fungal siderophore triacetylfusarinine C (TAFC) and the bacteria-derived siderophore ferrioxamine E (FOXE) for the imaging of invasive pulmonary aspergillosis (IPA).168, 169 Both siderophores exhibit excellent binding to 68Ga3+, which boasts coordination chemistry similar to their natural target Fe3+ (Figure 7). In fact, it was found that minimal degradation of [68Ga]Ga-TAFC and [68Ga]Ga-FOXE occurred even during competitive binding assays with Fe(III). In vivo PET imaging with these tracers in a rat model of IPA consistently showed uptake in the primary sites of infection (lungs) that was proportional to the severity of infection.169 Just as importantly, both radiometal chelates showed visible accumulation in the lungs and clearance from the blood as soon as 20 minutes after their administration. However, assays testing the uptake of these 68Ga-labeled siderophores by other species of bacteria and fungi found that not all had the same affinity for the imaging agents.170 This suggests that different 68Ga-siderophore complexes may be required to image different types of infections.
Indium-111 and Zirconium-89
Far and away the most common metal complex-based radiopharmaceuticals featuring 111In (t1/2 ~ 2.8 d) are reagents for cell labeling (Figure 8). The use of white blood cells for targeted imaging is predicated on the natural inclination of leukocytes and macrophages to migrate to sites of infection and inflammation within the body (Figure 9). Labeling these cells with 111In-based metal complexes allows them to be tracked in vivo via SPECT. Clinically, this approach has been used to non-invasively visualize a range of pathologies, including fevers of unknown origin, post-operative infections, diabetic infections, and inflammation associated with cancer.171–174 More recently, cell-labeling techniques have been harnessed as theranostic imaging strategies in conjunction with cell-based cancer therapies.175 The incorporation of radionuclides into live, patient-derived neutrophils has been achieved by coordinating radiometals with lipophilic ligands, producing neutral, hydrophobic complexes that are passively taken up by cells. Upon diffusing through the cell membrane, the subsequent dissociation of the ligands frees the metal cation and allows it to bind to intracellular macromolecules, trapping it within the cytosol. These labeled autologous cells can then be administered to the patient, where they will resume their role as critical components of the immune system.
Figure 8.
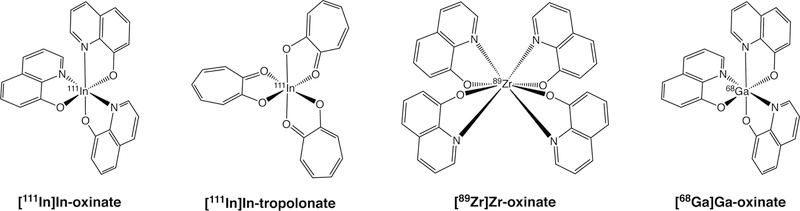
Selected [111In]In(III), [89Zr]Zr(IV), and [68Ga]Ga(III) complexes
Figure 9.
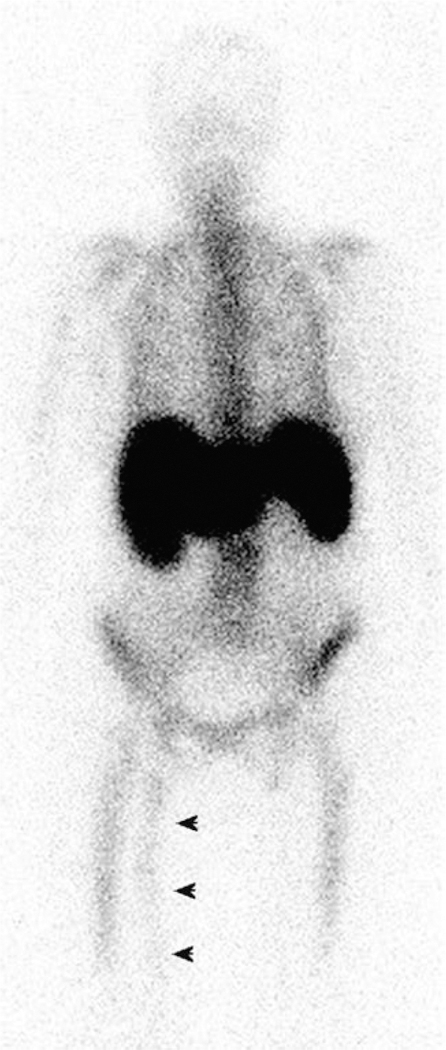
Planar scintigraphy using [111In]In-oxine-labeled leukocytes revealing an area of increased uptake in an infected prosthetic vascular graft in the right thigh (black arrows). Adapted and reprinted from Love et al. Radionuclide imaging of infection. Journal of Nuclear Medicine Technology 2004, 32, 47–57. Copyright by the Society of Nuclear Medicine and Molecular Imaging.174
Two complexes have proven particularly effective for the labeling of a wide range of cell types: [111In]In(oxinate)3 and [111In]In(tropolonate)3 (Figure 8).176–178 Though there has been some debate regarding the relative merits of the two compounds, [111In]In(oxinate)3 — commonly known as 111In-oxime — has been the more widely deployed in the clinic. The tropolonate complex labels cells somewhat more efficiently, which necessitates less preparation of the isolated neutrophils prior to labeling.179 However, this difference has not proven significant enough to prompt a shift in clinical preference. It is important to note that while radioactive cell labeling has proven effective in the clinic, the use of 111In has inherent limitations, most notably the nuclide’s compatibility with SPECT rather than PET. To circumvent this issue, oxinate complexes of the positron-emitting radiometals 89Zr and 68Ga have recently been developed and studied in preclinical mouse models (Figure 8).180–183
CONCLUSION
In the preceding pages, we have sought to illuminate the remarkable breadth of metal complexes that have been used for PET and SPECT imaging. While we have tried to include many important and compelling examples, we are nonetheless sure that we have left out some excellent work. To the creators of these inadvertently neglected compounds, we humbly apologize and appeal to their understanding of the exigencies of writing introductory reviews.
As we mentioned in the introduction, the last two decades have been marked by a gradual decline in the development of radiopharmaceuticals based on metal complexes. Not surprisingly, this trend has run counter-parallel to the advent of biomolecular imaging agents labeled with radiometals. While we are firm believers in the power of peptides, proteins, and antibodies as targeting vectors, we nonetheless wonder if the pendulum has swung too far, leaving metal complex-based radiotracers with a regrettable lack of attention.
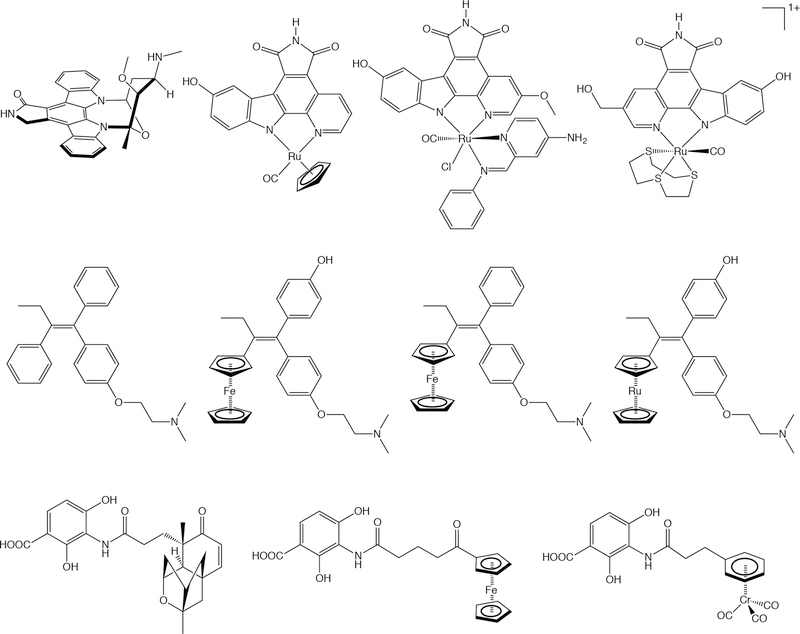
Meanwhile, the field of medicinal inorganic chemistry has recently undergone a fascinating surge.184, 185 Researchers have increasingly harnessed the unique properties of transition metals to produce a wide range of complexes — including compounds centered on Ru, Au, Os, Ga, Co, and Fe — with potential as therapeutics for an array of diseases, most notably cancer.186, 187, 188 For example, Meggers, et al. have developed a series of organometallic Ru(II) complexes that are potent kinase inhibitors (Figure 10, top).189–191 In addition, the Jaouen laboratory has developed a family of organometallic Fe-based estrogen receptor modulators that has shown therapeutic potential in preclinical models of breast cancer (Figure 10, middle).192–194 And finally, Patra, et al. have recently reported a novel class of organometallic iron- and chromium-based antibiotics based on the structure of platensimycin (Figure 10, bottom).195–197 We contend that these compounds — and many others like them — represent fertile ground for the development of new diagnostic and theranostic radiopharmaceuticals. Some of these complexes are centered upon a metal (e.g. Cu) with at least one imaging-relevant isotopologue. Others, unfortunately, are not. In these cases, however, it would be possible to create a radiolabeled variant of the metal complex by incorporating a radionuclide such as 18F or 11C into a ligand either before or, preferably, after complexation. The radiolabeling of a ligand in the presence of a metal center may prove challenging, but we are confident that any difficulties could be circumvented, especially in light of the exciting recent advances in both 11C and 18F radiosynthesis.
Figure 10.
Selected medicinal inorganic and organometallic compounds. Top: Ru-based kinase inhibitors inspired by the structure of staurosporine (far left). Middle: Fe- and Ru-based selective estrogen receptor modulators inspired by the structure of tamoxifen (far left). Bottom: Fe- and Cr-based antibiotics based on the structure of platensimycin (far left).
In the end, it is our sincere hope that the coming years will play witness to a creative renaissance in the study of metal complexes for nuclear imaging and that work at the intersection of radiochemistry, molecular imaging, and medicinal inorganic chemistry will produce new generations of innovative and useful imaging agents.
ACKNOWLEDGEMENTS
The authors are grateful for the generous financial support of the National Institutes of Health (BMZ: R01CA204167, U01CA221046, and R01CA240963) and the National Science Foundation (DSM: HRD-1547830). We would also like to thank Professor John Babich (Weill Cornell Medical College) for helpful discussions and sage advice.
REFERENCES
- 1.Wadas TJ, Wong EH, Weisman GR and Anderson CJ, Chem. Rev, 2010, 110, 2858–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeglis BM and Lewis JS, Dalton Trans, 2011, 40, 6168–6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu S and Chakraborty S, Dalton Trans, 2011, 40, 6077–6086. [DOI] [PubMed] [Google Scholar]
- 4.Blower PJ, Dalton Trans, 2015, 44, 4819–4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paterson BM and Donnelly PS, Chem. Soc. Rev, 2011, 40, 3005–3018. [DOI] [PubMed] [Google Scholar]
- 6.Price EW and Orvig C, Chem. Soc. Rev, 2014, 43, 260–290. [DOI] [PubMed] [Google Scholar]
- 7.Kostelnik TI and Orvig C, Chem. Rev, 2019, 119, 902–956. [DOI] [PubMed] [Google Scholar]
- 8.Bartholomä MD, Louie AS, Valliant JF and Zubieta J, Chem. Rev, 2010, 110, 2903–2920. [DOI] [PubMed] [Google Scholar]
- 9.Boros E and Holland JP, J Labelled Comp Radiopharm, 2018, 61, 652–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boros E and Packard AB, Chem. Rev, 2019, 119, 870–901. [DOI] [PubMed] [Google Scholar]
- 11.Morais GR, Paulo A and Santos I, Organometallics, 2012, 31, 5693–5714. [Google Scholar]
- 12.Kniess T, Laube M, Wüst F and Pietzsch J, Dalton Trans, 2017, 46, 14435–14451. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharyya S and Dixit M, Dalton Trans, 2011, 40, 6112–6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeglis BM, Houghton JL, Evans MJ, Viola-Villegas N and Lewis JS, Inorg. Chem, 2014, 53, 1880–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velikyan I, Med. Chem, 2011, 7, 345–379. [DOI] [PubMed] [Google Scholar]
- 16.Brandt M, Cardinale J, Aulsebrook ML, Gasser G and Mindt TL, J. Nucl. Med, 2018, 59, 1500–1506. [DOI] [PubMed] [Google Scholar]
- 17.Jurisson S, Cutler C and Smith SV, Q. J. Nucl. Med. Mol. Imaging, 2008, 52, 222–234. [PubMed] [Google Scholar]
- 18.Mahmood A and Jones AG, Handbook of Radiopharmaceuticals, 2003, 323–362. [Google Scholar]
- 19.Holland JP, Williamson MJ and Lewis JS, Mol. Imaging, 2010, 9, 1–20. [PMC free article] [PubMed] [Google Scholar]
- 20.Welch MJand Redvanly CS, eds., Handbook of Radiopharmaceuticals: Radiochemistry and Applications, Wiley, New York, 2003. [Google Scholar]
- 21.Banerjee S, Pillai MR and Ramamoorthy N, Semin. Nucl. Med, 2001, 31, 260–277. [DOI] [PubMed] [Google Scholar]
- 22.Piwnica-Worms D, Kronauge JF and Chiu ML, Circulation, 1990, 82, 1826–1838. [DOI] [PubMed] [Google Scholar]
- 23.Chiu ML, Kronauge JF and Piwnica-Worms D, J. Nucl. Med, 1990, 31, 1646–1653. [PubMed] [Google Scholar]
- 24.Jain D, Semin. Nucl. Med, 1999, 29, 221–236. [DOI] [PubMed] [Google Scholar]
- 25.Ciarmiello A, Del Vecchio S, Silvestro P, Potena MI, Carriero MV, Thomas R, Botti G, D’Aiuto G and Salvatore M, J. Clin. Oncol, 1998, 16, 1677–1683. [DOI] [PubMed] [Google Scholar]
- 26.Taillefer R, Semin. Nucl. Med, 1999, 29, 16–40. [DOI] [PubMed] [Google Scholar]
- 27.Waxman AD, Semin. Nucl. Med, 1997, 27, 40–54. [DOI] [PubMed] [Google Scholar]
- 28.Hendrikse NH, Franssen EJF, van der Graaf WTA, Meijer C, Piers DA, Vaalburg W and de Vries EGE, Br. J. Cancer, 1998, 77, 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McBiles M, Lambert AT, Cote MG and Kim SY, Semin. Nucl. Med, 1995, 25, 221–234. [DOI] [PubMed] [Google Scholar]
- 30.Mendes F, Paulo A and Santos I, Dalton Trans, 2011, 40, 5377–5393. [DOI] [PubMed] [Google Scholar]
- 31.Keidar Z, Solomonov E, Karry R, Frenkel A, Israel O and Mekel M, Mol. Imag. Biol, 2017, 19, 265–270. [DOI] [PubMed] [Google Scholar]
- 32.Satpati D, Bapat K, Dev Sarma H, Venkatesh M and Banerjee S, J. Labelled Compd. Radiopharmaceut, 2010, 53, 198–204. [Google Scholar]
- 33.Rattat D, Cleyhens B, Bormans G, Terwinghe C and Verbruggen A, Bioorg. Med. Chem. Lett, 2005, 15, 4192–4195. [DOI] [PubMed] [Google Scholar]
- 34.Lipowska M, Marzilli LG and Taylor AT, J. Nucl. Med, 2009, 50, 454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandes C, Maria L, Gano L, Santos IC, Santos I and Paulo A, J. Organomet. Chem, 2014, 760, 138–148. [Google Scholar]
- 36.Maria L, Fernandes C, Garcia R, Gano L, Paulo A, Santos IC and Santos I, Dalton Trans, 2009, DOI: 10.1039/b817451b, 603–606. [DOI] [PubMed] [Google Scholar]
- 37.Maria L, Cunha S, Videira M, Gano L, Paulo A, Santos IC and Santos I, Dalton Trans, 2007, DOI: 10.1039/b705226, 3010–3019. [DOI] [PubMed] [Google Scholar]
- 38.Zheng Y, Ji S, Tomaselli E, Ernest C, Freiji T and Liu S, Nucl. Med. Biol, 2014, 41, 813–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossetti C, Vanoli G, Paganelli G, Kwiatkowski M, Zito F, Colombo F, Bonino C, Carpinelli A, Casati R, Deutsch K and et al. , J. Nucl. Med, 1994, 35, 1571–1580. [PubMed] [Google Scholar]
- 40.Treher EN, Francesconi LC, Gougoutas JZ, Malley MF and Nunn AD, Inorg. Chem, 1989, 28, 3411–3416. [Google Scholar]
- 41.Zhao Z-Q, Liu M, Fang W and Liu S, J. Med. Chem, 2018, 61, 319–328. [DOI] [PubMed] [Google Scholar]
- 42.Liu M, Zhao Z-Q, Fang W and Liu S, Bioconj. Chem, 2017, 28, 2998–3006. [DOI] [PubMed] [Google Scholar]
- 43.Bernard BF, Krenning EP, Breeman WAP, Ensing G, Benjamins H, Bakker WH, Visser TJ and de Jong M, Nucl. Med. Biol, 1998, 25, 233–240. [DOI] [PubMed] [Google Scholar]
- 44.Ballinger JR, Muzzammil T and Moore MJ, J. Nucl. Med, 1997, 38, 1915–1919. [PubMed] [Google Scholar]
- 45.Crankshaw CL, Marmion M, Luker GD, Rao V, Dahlheimer J, Burleigh BD, Webb E, Deutsch KF and Piwnica-Worms D, J. Nucl. Med, 1998, 39, 77–86. [PubMed] [Google Scholar]
- 46.Luker GD, Crankshaw CL and Piwnica-Worms D, J. Nucl. Med, 1997, 38, 321–321. [PubMed] [Google Scholar]
- 47.Enlander D, Weber PM and dos Remedios LV, J. Nucl. Med, 1974, 15, 743–749. [PubMed] [Google Scholar]
- 48.Ugur O, Kostakoglu L, Guler N, Caner B, Uysal U, Elahi N, Haliloglu M, Yuksel D, Aras T, Bayhan H and Bekdik C, Eur. J. Nucl. Med, 1996, 23, 1367–1371. [DOI] [PubMed] [Google Scholar]
- 49.Loberg MD, Cooper M, Harvey E, Callery P and Faith W, J. Nucl. Med, 1976, 17, 633–638. [PubMed] [Google Scholar]
- 50.Chervu LR, Nunn AD and Loberg MD, Semin. Nucl. Med, 1982, 12, 5–17. [DOI] [PubMed] [Google Scholar]
- 51.Lamdie H, Cook AM, Scarsbrook A. f., Lodge JPA, Robinson PJ and Chowdhury FU, Clin. Radiol, 2011, 66, 1094–1105. [DOI] [PubMed] [Google Scholar]
- 52.Libson K, Deutsch E and Barnett BL, J. Am. Chem. Soc, 1980, 102, 2476–2478. [Google Scholar]
- 53.Pauwels EKJ and Stokkel MPM, Q. J. Nucl. Med, 2001, 45, 18–26. [PubMed] [Google Scholar]
- 54.Wong KK and Piert M, J. Nucl. Med, 2013, 54, 590–599. [DOI] [PubMed] [Google Scholar]
- 55.Qiu L, Cheng W, Lin J, Luo S, Xue L and Pan J, Molecules, 2011, 16, 6165–6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiu L, Lin J, Cheng W, Wang Y and Luo S, Med. Chem. Res, 2013, 22, 6154–6162. [Google Scholar]
- 57.Larson SK, Solomon HF, Caldwell WW and Abrams MJ, Bioconj. Chem, 1995, 6, 635–638. [DOI] [PubMed] [Google Scholar]
- 58.Edwards DS, Liu S, Lyster DM, Poirier MJ, Vo C, Webb GA, Zhang Z and Orvig C, Nucl. Med. Biol, 1993, 20, 857–863. [DOI] [PubMed] [Google Scholar]
- 59.Edwards DS, Liu S, Poirier MJ, Zhang Z, Webb GA and Orvig C, Inorg. Chem, 1994, 33, 5607–5609. [Google Scholar]
- 60.Lorberboym M, Lampl Y and Sadeh M, J. Nucl. Med, 2003, 44, 1898–1904. [PubMed] [Google Scholar]
- 61.Arnold RW, Subramanian G, McAfee JG, Blair RJ and Thomas FD, J. Nucl. Med, 1975, 16, 357–367. [PubMed] [Google Scholar]
- 62.Nosco D and Beaty-Nosco J, Coord. Chem. Rev, 1999, 184, 91–123. [Google Scholar]
- 63.Volkert WA, Hoffman TJ, Seger RM, Troutner DE and Holmes RA, Eur. J. Nucl. Med, 1984, 9, 511–516. [DOI] [PubMed] [Google Scholar]
- 64.Fair CK, Troutner DE, Schlemper EO, Murmann RK and Hoppe ML, Acta Crystallographica Section C, 1984, 40, 1544–1546. [Google Scholar]
- 65.Leonard Holman B., Jones AG, Lister-James J, Davison A, Abrams MJ, Kirshenbaum JM, Tumeh SS and English RJ, J. Nucl. Med, 1984, 25, 1350–1355. [PubMed] [Google Scholar]
- 66.Leonard J-P, Nowotnik DP and Neirinckx RD, J. Nucl. Med, 1986, 27, 1819–1823. [PubMed] [Google Scholar]
- 67.Nowotnik DP, Canning LR, Cumming SA, Harrison RC, Higley B, Nechvatal G, Pickett RD, Piper IM, Bayne VJ, Forster AM, Weisner PS, Neirinckx RD, Volkert WA, Troutner DE and Holmes RA, Nucl. Med. Commun, 1985, 6, 499–506. [DOI] [PubMed] [Google Scholar]
- 68.Sharp PF, Smith FW, Gemmell HG, Lyall D, Evans NTS, Gvozdanovic D, Davidson J, Tyrrell DA, Pickett RD and Neirinckx RD, J. Nucl. Med, 1986, 27, 171–177. [PubMed] [Google Scholar]
- 69.Roddie ME, Peters AM, Danpure HJ, Osman S, Henderson BL, Lavender JP, Carroll MJ, Neirinckx RD and Kelly JD, Radiology, 1988, 166, 767–772. [DOI] [PubMed] [Google Scholar]
- 70.Vallabhajosula S, Zimmerman RE, Picard M, Stritzke P, Mena I, Hellman RS, Tikofsky RS, Stabin MG, Morgan RA and Goldsmith SJ, J. Nucl. Med, 1989, 30, 599–604. [PubMed] [Google Scholar]
- 71.Walovitch RC, Cheesman EH, Maheu LJ and Hall KM, J. Cereb. Blood Flow Metab, 1994, 14. [PubMed] [Google Scholar]
- 72.Walovitch RC, Hill TC, Garrity ST, Cheesman EH, Burgess BA, O’Leary DH, Watson AD, Ganey MV, Morgan RA and Williams SJ, J. Nucl. Med, 1989, 30, 1892–1901. [PubMed] [Google Scholar]
- 73.Holman BL, Hellman RS, Goldsmith SJ, Mena IG, Leveille J, Gherardi PG, Moretti J-L, Bischof-Delaloye A, Hill TC, Rigo PM, Van heertum RL, Ell PJ, Buell U, De Roo MC and Morgan RA, J. Nucl. Med, 1989, 30, 1018–1024. [PubMed] [Google Scholar]
- 74.Leveille J, Demonceau G, De Roo M, Rigo P, Taillefer R, Morgan RA, Kupranick D and Walovitch RC, J. Nucl. Med, 1989, 30, 1902–1910. [PubMed] [Google Scholar]
- 75.Leveille J, Demonceau G and Walovitch RC, J. Nucl. Med, 1992, 33, 480–484. [PubMed] [Google Scholar]
- 76.Eshima D, Taylor A, Fritzberg AR, Kasina S, Hansen L and Sorenson JF, J. Nucl. Med, 1987, 28, 1180–1186. [PubMed] [Google Scholar]
- 77.Kasina S, Fritzberg AR, Johnson DL and Eshima D, J. Med. Chem, 1986, 29. [DOI] [PubMed] [Google Scholar]
- 78.Rao TN, Adhikesavalu D, Camerman A and Fritzberg AR, J. Am. Chem. Soc, 1990, 112, 5798–5804. [Google Scholar]
- 79.Jafri RA, Britton KE, Nimmon CC, Solanki K, Al-Nahhas A, Bomanji J, Fettich J and Hawkins LA, J. Nucl. Med, 1988, 29, 147–158. [PubMed] [Google Scholar]
- 80.Bubeck B, Brandau W, Weber E, Kalble T, Parekh N and Georgi P, J. Nucl. Med, 1990, 31, 1285–1293. [PubMed] [Google Scholar]
- 81.Verbruggen AM, Nosco DL, Van Nerom CG, Bormans GM, Adriaens PJ and De Roo MJ, J. Nucl. Med, 1992, 33, 551–557. [PubMed] [Google Scholar]
- 82.Van Nerom CG, Bormans GM, De Roo MJ and Verbruggen AM, Eur. J. Nucl. Med, 1993, 20, 738–746. [DOI] [PubMed] [Google Scholar]
- 83.Taylor A, Hansen L, Eshima D, Malveaux E, Folks R, Shattuck L, Lipowska M and Marzilli LG, J. Nucl. Med, 1997, 36, 821–826. [PubMed] [Google Scholar]
- 84.Taylor AT, Lipowska M, Hansen L, Malveaux E and Marzilli LG, J. Nucl. Med, 2004, 45, 885–891. [PubMed] [Google Scholar]
- 85.Kelly JD, Forster AM, Higley B, Archer CM, Booker FS, Canning LR, Chiu KW, Edwards B, Gill HK, McPartlin M, Nagle KR, Latham IA, Pickett RD, Storey AE and Webbon PM, J. Nucl. Med, 1993, 34, 222–227. [PubMed] [Google Scholar]
- 86.Platts EA, North TL, Pickett RD and Kelly JD, J. Nucl. Cardiol, 1995, 2, 317–326. [DOI] [PubMed] [Google Scholar]
- 87.Muzzammil T, Moore MJ and Ballinger JR, Cancer Biother. Radiopharm, 2000, 15, 339–346. [DOI] [PubMed] [Google Scholar]
- 88.Ballinger JR, J. Clin. Pharmacol, 2001,39S–47S. [PubMed] [Google Scholar]
- 89.Pasqualini R, Duatti A, Bellande E, Comazzi V, Brucato V, Hoffschir D, Fagret D and Comet M, J. Nucl. Med, 1994, 35, 334–341. [PubMed] [Google Scholar]
- 90.Uccelli L, Giganti M, Duatti A, Bolzati C, Pasqualini R, Cittanti C, Colamussi P and Piffanelli A, J. Nucl. Med, 1995, 36, 2075–2079. [PubMed] [Google Scholar]
- 91.Johnson G III, Nguyen KN, Liu Z, Gao P, Pasqualini R and Okada RD, J. Nucl. Cardiol, 1997, 4, 217–225. [DOI] [PubMed] [Google Scholar]
- 92.Zhang J, Lin Y, Sheng X and Wang X, Appl. Radiat. Isot, 2009, 67. [DOI] [PubMed] [Google Scholar]
- 93.Zhang J, Song Z, Jinfeng C and Wang X, Appl. Radiat. Isot, 2009, 67, 1661–1663. [DOI] [PubMed] [Google Scholar]
- 94.Zhang J, Song Z and Wang X, Appl. Radiat. Isot, 2009, 67, 577–580. [DOI] [PubMed] [Google Scholar]
- 95.Hatada K, Riou LM, Ruiz M, Yamamichi Y, Duatti A, Lima RL, Goode AR, Watson DD, Beller GA and Glover DK, J. Nucl. Med, 2004, 45, 2095–2101. [PubMed] [Google Scholar]
- 96.Hatada K, Ruiz M, Riou LM, Lima RL, Goode AR, Watson DD, Beller GA and Glover DK, J. Nucl. Cardiol, 2006, 13, 779–790. [DOI] [PubMed] [Google Scholar]
- 97.Zhang W-C, Fang W, Li B, Wang X-B and He Z-X, Cardiology, 2009, 112, 89–97. [DOI] [PubMed] [Google Scholar]
- 98.Cittanti C, Uccelli L, Pasquali M, Boschi A, Flammia C, Bagatin E, Casali M, Stabin MG, Feggi L, Giganti M and Duatti A, J. Nucl. Med, 2008, 49, 1299–1304. [DOI] [PubMed] [Google Scholar]
- 99.Bolzati C, Carta D, Gandin V, Marzano C, Morellato N, Salvarese N, Cantore M and Colabufo NA, J. Biol. Inorg. Chem, 2013, 18, 523–538. [DOI] [PubMed] [Google Scholar]
- 100.Bolzati C, Cavazza-Ceccato M, Agostini S, Tokunaga S, Casara D and Bandoli G, J. Nucl. Med, 2008, 49, 1336–1344. [DOI] [PubMed] [Google Scholar]
- 101.Kim Y-S, Shi J, Zhai S, Hou G and Liu S, J. Nucl. Cardiol, 2009, 16, 571–579. [DOI] [PubMed] [Google Scholar]
- 102.Kim Y-S, Wang J, Broisat A, Glover DK and Liu S, J. Nucl. Cardiol, 2008, 15, 535–546. [DOI] [PubMed] [Google Scholar]
- 103.Gao S, Zhao G, Wen Q, Bai L, Chen B, Ji T, Ji B and Ma Q, Clin. Nucl. Med, 2014, 39, 14–19. [DOI] [PubMed] [Google Scholar]
- 104.Wadas TJ, Wong EH, Weisman GR and Anderson CJ, Curr. Pharm. Des, 2007, 13, 3–16. [DOI] [PubMed] [Google Scholar]
- 105.Blower PJ, Lewis JS and Zweit J, Nucl. Med. Biol, 1996, 23, 957–980. [DOI] [PubMed] [Google Scholar]
- 106.Fujibayashi Y, Taniuchi H, Wada K, Yonekura Y, Konishi J and Yokoyama A, Ann. Nucl. Med, 1995, 9, 1–5. [DOI] [PubMed] [Google Scholar]
- 107.Green MA, Mathias CJ, Welch MJ, McGuire AH, Perry D, Fernandez-Rubio F, Perlmutter JS, Raichle ME and Bergmann SR, J. Nucl. Med, 1990, 31, 1989–1996. [PubMed] [Google Scholar]
- 108.Herrero P, Hartman JJ, Green MA, Anderson CJ, Welch MJ, Markham J and Bergmann SR, J. Nucl. Med, 1996, 37, 1294–1300. [PubMed] [Google Scholar]
- 109.Herrero P, Markham J, Weinheimer CJ, Anderson CJ, Welch MJ, Green MA and Bergmann SR, Circulation, 1993, 87, 173–183. [DOI] [PubMed] [Google Scholar]
- 110.Holschneider D, Yang J, Sadler T, Galifianakis N, Bozorgzadeh M, Bading J, Conti P and Maarek J-M, Brain Res, 2008, 1234, 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Flower MA, Zweit J, Hall AD, Burke D, Davies MM, Dworkin MJ, Young HE, Mundy J, Ott RJ, McCready VR, Carnochan P and Allen-Mersh TG, Eur. J. Nucl. Med, 2001, 28, 99–103. [DOI] [PubMed] [Google Scholar]
- 112.Liu J, Hajibeigi A, Ren G, Lin M, Siyambalapitiyage W, Liu ZS, Simpson E, Parkey RW, Sun XK and Oz OK, J. Nucl. Med, 2009, 50, 1332–1339. [DOI] [PubMed] [Google Scholar]
- 113.Vavere AL and Lewis JS, Dalton Trans, 2007, 4893–4902. [DOI] [PubMed] [Google Scholar]
- 114.Basken NE and Green MA, Nucl. Med. Biol, 2009, 36, 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Basken NE, Mathias CJ and Green MA, J. Pharm. Sci, 2009, 98, 2170–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Blower PJ, Castle TC, Cowley AR, Dilworth JR, Donnelly PS, Labisbal E, Sowrey FE, Teat SJ and Went MJ, Dalton Trans, 2003, DOI: 10.1039/B307499D, 4416–4425. [DOI] [Google Scholar]
- 117.Bourgeois M, Rajerison H, Guerard F, Mougin-Degraef M, Barbet J, Michel N, Cherel M and Faivre-Chauvet A, Nuc. Med. Rev. Cent. East. Eur, 2011, 14, 90–95. [DOI] [PubMed] [Google Scholar]
- 118.Carlin S, Zhang H, Reese M, Ramos NN, Chen Q and Ricketts S-A, J. Nucl. Med, 2014, 55, 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fujibayashi Y, Cutler C, Anderson C, McCarthy D, Jones L, Sharp T, Yonekura Y and Welch M, Nucl. Med. Biol, 1999, 26, 117–121. [DOI] [PubMed] [Google Scholar]
- 120.Fujibayashi Y, Taniuchi H, Yonekura Y and Ohtani H, J. Nucl. Med, 1997, 38, 1155. [PubMed] [Google Scholar]
- 121.Holland JP, Barnard PJ, Collison D, Dilworth JR, Edge R, Green JC and McInnes EJ, Chem. Eur. J, 2008, 14, 5890–5907. [DOI] [PubMed] [Google Scholar]
- 122.Vāvere AL and Lewis JS, Nucl. Med. Biol, 2008, 35, 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dearling JLJ, Lewis JS, Mullen GED, Rae MT, Zweit J and Blower PJ, Eur. J. Nucl. Med, 1998, 25, 788–792. [DOI] [PubMed] [Google Scholar]
- 124.Dearling JLJ, Lewis JS, Mullen GED, Welch MJ and Blower PJ, J. Biol. Inorg. Chem, 2002, 7, 249–259. [DOI] [PubMed] [Google Scholar]
- 125.Burgman P, O’Donoghue JA, Lewis JS, Welch MJ, Humm JL and Ling CC, Nucl. Med. Biol, 2005, 32, 623–630. [DOI] [PubMed] [Google Scholar]
- 126.Lewis JS, Laforest R, Dehdashti F, Grigsby PW, Welch MJ and Siegel BA, J. Nucl. Med, 2008, 49, 1177–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bayly SR, King RC, Honess DJ, Barnard PJ, Betts HM, Holland JP, Hueting R, Bonnitcha PD, Dilworth JR, Aigbirhio FI and Christlieb M, J. Nucl. Med, 2008, 49, 1862–1868. [DOI] [PubMed] [Google Scholar]
- 128.Green MA, Mathias CJ, Willis LR, Handa RK, Lacy JL, Miller MA and Hutchins GD, Nucl. Med. Biol, 2007, 34, 247–255. [DOI] [PubMed] [Google Scholar]
- 129.Bonnitcha PD, Bayly SR, Theobald MBM, Betts HM, Lewis JS and Dilworth JR, J. Inorg. Biochem, 2010, 104, 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bonnitcha PD, Va̅vere AL, Lewis JSand Dilworth JR, J. Med. Chem, 2008, 51, 2985–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wei L, Easmon J, Nagi RK, Muegge BD, Meyer LA and Lewis JS, J. Nucl. Med, 2006, 47, 2034–2041. [PubMed] [Google Scholar]
- 132.Zeglis BM, Divilov V and Lewis JS, J. Med. Chem, 2011, 54, 2391–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lewis JS, Dearling JL, Sosabowski JK, Zweit J, Carnochan P, Kelland LR, Coley HM and Blower PJ, Eur. J. Nucl. Med, 2000, 27, 638–646. [DOI] [PubMed] [Google Scholar]
- 134.Lewis JS, Zweit J, Dearling JLJ, Rooney BC and Blower PJ, Chem. Commun, 1996, DOI: 10.1039/CC9960001093, 1093–1094. [DOI] [Google Scholar]
- 135.Packard AB, Barbarics E, Kronauge JF, Treves ST and Jones A, Abstracts of Papers of the American Chemical Society, 1998, 215, U957–U958. [Google Scholar]
- 136.Packard AB, Kronauge JF, Day PJ and Treves ST, Nucl. Med. Biol, 1998, 25, 531–537. [DOI] [PubMed] [Google Scholar]
- 137.Otte A, Jermann E, Behe M, Goetze M, Bucher HC, Roser HW, Heppeler A, Mueller-Brand J and Maecke HR, Eur. J. Nucl. Med, 1997, 24, 792–795. [DOI] [PubMed] [Google Scholar]
- 138.Kayani I, Bomanji JB, Groves A, Conway G, Gacinovic S, Win T, Dickson J, Caplin M and Ell PJ, Cancer, 2008, 112, 2447–2455. [DOI] [PubMed] [Google Scholar]
- 139.Zhernosekov KP, Filosofov DV, Baum RP, Aschoff P, Bihl H, Razbash AA, Jahn M, Jennewein M and Rosch F, J. Nucl. Med, 2007, 48, 1741–1748. [DOI] [PubMed] [Google Scholar]
- 140.Banerjee SRand Pomper MG, Appl. Radiat. Isot, 2013, 76, 2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nanni C, Errani C, Boriani L, Fantini L, Ambrosini V, Boschi S, Rubello D, Pettinato C, Mercuri M, Gasbarrini A and Fanti S, J. Nucl. Med, 2010, 51, 1932–1936. [DOI] [PubMed] [Google Scholar]
- 142.Gunasekera SW, King LJ and Lavender PJ, Clin. Chim. Acta, 1972, 39, 401–406. [DOI] [PubMed] [Google Scholar]
- 143.Li Y, Martell AE, Hancock RD, Reibenspies JH, Anderson CJ and Welch MJ, Inorg. Chem, 1996, 35, 404–414. [DOI] [PubMed] [Google Scholar]
- 144.Jalilian AR, Yousefnia H, Zolghadri S, Khoshdel MR, Bolourinovin F and Rahiminejad A, J. Radioanal. Nucl. Chem, 2010, 284, 49–54. [Google Scholar]
- 145.Mirzaei A, Jalilian AR, Aghanejad A, Mazidi M, Yousefnia H, Shabani G, Ardaneh K, Geramifar P and Beiki D, Nucl. Med. Mol. Imaging (2010), 2015, 49, 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mirzaei A, Jalilian AR, Shabani G, Fakhari A, Akhlaghi M and Beiki D, J. Radioanal. Nucl. Chem, 2016, 307, 725–732. [Google Scholar]
- 147.Francesconi LC, Liu BL, Billings JJ, Carroll PJ, Graczyk G and Kung HF, J. Chem. Soc., Chem. Commun, 1991, 2, 94–95. [Google Scholar]
- 148.Kung HF, Liu BL, Mankoff D, Kung MP, Billings JJ, Francesconi L and Alavi A, J. Nucl. Med, 1990, 31, 1635–1640. [PubMed] [Google Scholar]
- 149.Cutler CS, Giron MC, Reichert DE, Snyder AZ, Herrero P, Anderson CJ, Quarless DA, Koch SA and Welch MJ, Nucl. Med. Biol, 1999, 26, 305–316. [DOI] [PubMed] [Google Scholar]
- 150.Moore DA, Fanwick PE and Welch MJ, Inorg. Chem, 1990, 29, 672–676. [Google Scholar]
- 151.Moore DA, Fanwick PE and Welch MJ, Inorg. Chem, 1989, 28, 1504–1506. [Google Scholar]
- 152.Sharma V, Sivapackiam J, Harpstrite SE, Prior JL, Gu H, Rath NP and Piwnica-Worms D, PLoS One, 2014, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fellner M, Dillenburg W, Buchholz HG, Bausbacher N, Schreckenberger M, Renz F, Rosch F and Thews O, Mol. Imag. Biol, 2011, 13, 985–994. [DOI] [PubMed] [Google Scholar]
- 154.Piwnica-Worms D and Sharma V, Curr. Top. Med. Chem, 2010, 10, 1834–1845. [DOI] [PubMed] [Google Scholar]
- 155.Sharma V, Beatty A, Wey SP, Dahlheimer J, Pica CM, Crankshaw CL, Bass L, Green MA, Welch MJ and Piwnica-Worms D, Chem. Biol, 2000, 7, 335–343. [DOI] [PubMed] [Google Scholar]
- 156.Sharma V, Prior JL, Belinsky MG, Kruh GD and Piwnica-Worms D, J. Nucl. Med, 2005, 46, 354–364. [PubMed] [Google Scholar]
- 157.Thews O, Dillenburg W, Fellner M, Buchholz HG, Bausbacher N, Schreckenberger M and Rosch F, Eur. J. Nucl. Med. Mol. Imag, 2010, 37, 1935–1942. [DOI] [PubMed] [Google Scholar]
- 158.Sharma V, Beatty A, Wey SP, Dahlheimer J, Pica CM, Crankshaw CL, Bass L, Green MA, Welch MJ and Piwnica-Worms D, Chem Biol, 2000, 7, 335–343. [DOI] [PubMed] [Google Scholar]
- 159.Tsang BW, Mathias CJ, Fanwick PE and Green MA, J. Med. Chem, 1994, 37, 4400–4406. [DOI] [PubMed] [Google Scholar]
- 160.Green MA, Welch MJ, Mathias CJ, Fox KAA, Knabb RM and Huffman JC, J. Nucl. Med, 1985, 26, 170–180. [PubMed] [Google Scholar]
- 161.Tsang BW, Mathias CJ and Green MA, J. Nucl. Med, 1993, 34, 1127–1131. [PubMed] [Google Scholar]
- 162.Tarkia M, Saraste A, Saanijoki T, Oikonen V, Vähäsilta T, Strandberg M, Stark C, Tolvanen T, Teräs M, Savunen T, Green MA, Knuuti J and Roivainen A, Nucl. Med. Biol, 2012, 39, 715–723. [DOI] [PubMed] [Google Scholar]
- 163.Asti M, Ferrari E, Croci S, Atti G, Rubagotti S, Iori M, Capponi PC, Zerbini A, Saladini M and Versari A, Inorg. Chem, 2014, 53, 4922–4933. [DOI] [PubMed] [Google Scholar]
- 164.Rubagotti S, Croci S, Ferrari E, Iori M, Capponi PC, Lorenzini L, Calza L, Versari A and Asti M, Int. J. Mol. Sci, 2016, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rubagotti S, Croci S, Ferrari E, Orteca G, Iori M, Capponi PC, Versari A and Asti M, J. Inorg. Biochem, 2017, 173, 113–119. [DOI] [PubMed] [Google Scholar]
- 166.Greiser J, Kühnel C, Görls H, Weigand W and Freesmeyer M, Dalton Trans, 2018, 47, 9000–9007. [DOI] [PubMed] [Google Scholar]
- 167.Mirzaei A, Jalilian AR, Badbarin A, Mazidi M, Mirshojaei F, Geramifar P and Beiki D, Ann. Nucl. Med, 2015, 29, 506–511. [DOI] [PubMed] [Google Scholar]
- 168.Petrik M, Haas H, Schrettl M, Helbok A, Blatzer M and Decristoforo C, Nucl. Med. Biol, 2012, 39, 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Petrik M, Haas H, Dobrozemsky G, Lass-Florl C, Helbok A, Blatzer M, Dietrich H and Decristoforo C, J. Nucl. Med, 2010, 51, 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Petrik M, Haas H, Laverman P, Schrettl M, Franssen GM, Blatzer M and Decristoforo C, Mol. Imag. Biol, 2014, 16, 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schmidt KG, Rasmussen JW, Sorensen PG and Wedebye IM, Scand. J. Infect. Dis, 1987, 19, 339–345. [DOI] [PubMed] [Google Scholar]
- 172.Ascher NL, Ahrenholz DH, Simmons RL, Weiblen B, Gomez L, Forstrom LA, Frick MP, Henke C and McCullough J, Ann. Surg, 1979, 114, 386–392. [DOI] [PubMed] [Google Scholar]
- 173.Johnson JE, Kennedy EJ, Shereff MJ, Patel NC and Collier BD, Foot Ankle Int, 1996, 17, 10–16. [DOI] [PubMed] [Google Scholar]
- 174.Love C and Palestro CJ, J. Nucl. Med. Technol, 2004, 32, 47–57. [PubMed] [Google Scholar]
- 175.Stanton SE, Eary JF, Marzbani EA, Mankoff D, Salazar LG, Higgins D, Childs J, Reichow J, Dang YS and Disis ML, J. Immunother. Cancer, 2016, 4, 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Segal AW, Arnot RN, Thakur ML and Lavender JP, Lancet, 1976, 2, 1056–1058. [DOI] [PubMed] [Google Scholar]
- 177.Peters AM, Saverymuttu SH, Reavy HJ, Danpure HJ, Osman S and Lavender JP, J. Nucl. Med, 1983, 24, 39–44. [PubMed] [Google Scholar]
- 178.Kotze HF, Heyns AD, Lotter MG, Pieters H, Roodt JP, Sweetlove MA and Badenhorst PN, J. Nucl. Med, 1991, 32, 62–66. [PubMed] [Google Scholar]
- 179.Intenzo CM, Desai AG, Thakur ML and Park CH, J. Nucl. Med, 1987, 28, 438–441. [PubMed] [Google Scholar]
- 180.Charoenphun P, Meszaros LK, Chuamsaamarkkee K, Sharif-Paghaleh E, Ballinger JR, Ferris TJ, Went MJ, Mullen GED and Blower PJ, Eur. J. Nucl. Med. Mol. Imag, 2015, 42, 278–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ferris TJ, Charoenphun P, Meszaros LK, Mullen GED, Blower PJ and Went MJ, Dalton Trans, 2014, 43, 14851–14857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Enyedy EA, Doemoetoer O, Varga E, Kiss L, Trondl R, Hartinger CG and Keppler BK, J. Inorg. Biochem, 2012, 117, 189–197. [DOI] [PubMed] [Google Scholar]
- 183.Chitambar CR, Future Med. Chem, 2012, 4, 1257–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Louie AY and Meade TJ, Chem. Rev, 1999, 99, 2711–2734. [DOI] [PubMed] [Google Scholar]
- 185.Mjos KD and Orvig C, Chem. Rev, 2014, 114, 4540–4563. [DOI] [PubMed] [Google Scholar]
- 186.Gianferrara T, Bratsos I and Alessio E, Dalton Trans, 2009, 7588–7598. [DOI] [PubMed] [Google Scholar]
- 187.Gasser G, Ott I and Metzler-Nolte N, J. Med. Chem, 2011, 54, 3–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bruijnincx PCA and Sadler PJ, Curr. Opin. Chem. Biol, 2008, 12, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Debreczeni JE, Bullock AN, Atilla GE, Williams DS, Bregman H, Knapp S and Meggers E, Angew. Chem. Int. Ed, 2006, 45, 1580–1585. [DOI] [PubMed] [Google Scholar]
- 190.Feng L, Geisselbrecht Y, Blanck S, Wilbuer A, Atilla-Gokcumen GE, Filippakopoulos P, Kraeling K, Celik MA, Harms K, Maksimoska J, Marmorstein R, Frenking G, Knapp S, Essen L-O and Meggers E, J. Am. Chem. Soc, 2011, 133, 5976–5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Smalley KSM, Contractor R, Haass NK, Kulp AN, Atilla-Gokcumen GE, Williams DS, Bregman H, Flaherty KT, Soengas MS, Meggers E and Herlyn M, Cancer Res, 2007, 67, 209–217. [DOI] [PubMed] [Google Scholar]
- 192.Jaouen G, Top S, Vessieres A, Leclercq G and McGlinchey MJ, Curr. Med. Chem, 2004, 11, 2505–2517. [DOI] [PubMed] [Google Scholar]
- 193.Top S, Vessieres A, Cabestaing C, Laios I, Leclercq G, Provot C and Jaouen G, J. Organomet. Chem, 2001, 637, 500–506. [Google Scholar]
- 194.Vessieres A, Top S, Beck W, Hillard E and Jaouen G, Dalton Trans, 2006, DOI: 10.1039/b509948f, 529–541. [DOI] [PubMed] [Google Scholar]
- 195.Patra M, Gasser G and Metzler-Nolte N, Dalton Trans, 2012, 41, 6350–6358. [DOI] [PubMed] [Google Scholar]
- 196.Patra M, Gasser G, Pinto A, Merz K, Ott I, Bandow JE and Metzler-Nolte N, ChemMedChem, 2009, 4, 1930–1938. [DOI] [PubMed] [Google Scholar]
- 197.Patra M, Gasser G, Wenzel M, Merz K, Bandow JE and Metzler-Nolte N, Organometallics, 2010, 29, 4312–4319. [Google Scholar]