Abstract
Background
We aimed to ascertain population-based practice patterns and survival outcomes of postoperative radiotherapy following breast conserving-surgery (BCS) in elderly women (aged ≥65 years) with early-stage pure mucinous breast carcinoma (PMBC).
Methods
Patients aged ≥65 years diagnosed with T1–2N0 and hormone receptor-positive PMBC between 1990 and 2010 were identified from the Surveillance, Epidemiology, and End Results database. Binomial logistic regression, Kaplan-Meier method, Multivariate Cox proportional hazards models, and propensity score matching (PSM) were used for statistical analysis.
Results
We enrolled 3416 patients, including 1225 (35.9%) and 2191 (64.1%) in the no-radiotherapy and radiotherapy cohorts, respectively. The percentage of patients receiving postoperative radiotherapy following BCS was significantly lower after 2004 (59.5% between 2004 and 2010), relative to that before 2004 (71.1% between 1990 and 2003; P < 0.001). Before PSM, the 10-year breast cancer-specific survival (BCSS) rates were 98.1 and 93.2% for patients with and without postoperative radiotherapy (log-rank test, P < 0.001), respectively. In the PSM cohort, receiving postoperative radiotherapy was associated with better BCSS rates, with 10-year BCSS rates of 97.6 and 94.5% in patients with and without postoperative radiotherapy, respectively (log-rank test, P = 0.001). Multivariate Cox proportional analysis indicated that receiving postoperative radiotherapy was an independent factor associated with better BCSS before (P < 0.001) and after PSM (P = 0.001), relative to those not receiving postoperative radiotherapy.
Conclusions
This study shows a decreasing utilization of postoperative radiotherapy following BCS of elderly PMBC patients over time. However, postoperative radiotherapy following BCS should be administered for elderly women with PMBC owing to independent association with better survival.
Keywords: Pure mucinous breast cancer, Radiotherapy, Elderly, Propensity score matching, Prognosis
Background
Pure mucinous breast carcinoma (PMBC) is a rare type of breast carcinoma involving abundant extracellular mucin production, which accounts for approximately 1–6% of all cases of breast cancer [1, 2]. PMBC has distinct clinicopathological and molecular features, including higher estrogen receptor (ER) and progesterone receptor (PR) expression, greater likelihood of human epidermal growth factor receptor-2 (HER2)-negative status, lower grade, and lower risk of nodal metastasis [3–5], which all contribute to better outcomes compared to invasive ductal carcinoma (IDC); indeed, the 10-year disease-free survival rate is > 90% [6–12]. In addition, the median age of PMBC patients was 70 years, which was significantly greater than the age of those with other histological subtypes [11]. In clinical practice, the recommendation for adjuvant treatment of PMBC differs from that for the usual breast cancer histology [13].
Prior studies have found that breast-conserving surgery (BCS) is an appropriate surgical procedure for most patients with early-stage PMBC [11, 14]. In patients with invasive breast carcinoma, several prospective clinical trials have indicated that the omission of postoperative radiotherapy (RT) following BCS is safe and associated with an acceptable low risk of local recurrence and without a detriment to overall survival (OS) among female patients who are elderly (aged ≥50, 65, or 70 years), tumor size ≤5 cm (T1–2), node-negative (N0) disease, and ER-positive tumors [15–18]. However, none of these trials specified whether PMBC patients were enrolled. In addition, in the trials evaluating the omission of postoperative RT, endocrine treatment was mandatory and in fact, there were statistically significant differences in local control rates, even though the recurrence rates were very low in general [15–18]. Until today, there is no clear recommendation on the best management of elderly PMBC patients with low risk for local recurrence. In light of this, we used data from the large and contemporary Surveillance, Epidemiology, and End Results (SEER) program to determine population-based practice patterns and survival outcomes in PMBC patients receiving postoperative RT, particularly among the elderly population.
Materials and methods
Patients
Patients diagnosed with PMBC between 1990 and 2010 were included from the SEER program. The SEER program is a population-based database maintained by the National Cancer Institute that includes the de-identified information of cancer incidence, demographics, first course of treatment, and vital status of approximately 28% of the United States (US) population [19]. Patients who met the following inclusion criteria were included in this study: 1) women with PMBC and aged ≥65 years; 2) treated with or without adjuvant external beam RT following BCS; 3) stage T1–2N0 disease; 4) ER- and PR-positive disease; and 5) available data on race/ethnicity, tumor grade, and chemotherapy. We defined aged ≥65 years as the elderly because this cut-off age regularly used in gerontology [20, 21]. Patients with metastatic disease, RT prior to surgery, receiving non- external beam RT, without positive histology, and unknown sequence of surgery and RT were excluded. This study was exempt from the approval process of the Institutional Review Board as it used de-identified data from the SEER program.
Measures
The following variables were included in this study: age at diagnosis, race/ethnicity, grade, tumor stage, and whether chemotherapy and postoperative RT was administrated. The primary end-point of the present study was breast cancer-specific survival (BCSS), which was defined as the time from diagnosis to death from breast cancer.
Statistical analysis
Patient demographics, clinicopathological variables, and treatment variables were compared using the chi-squared test based on whether postoperative RT was administered. A 1:1 propensity score matching (PSM) method including 5 variables (age at diagnosis, grade, race/ethnicity, tumor stage, and chemotherapy) was used to create the matched cohorts to reduce any potential confounding in the retrospective studies [22, 23]. The predictors of postoperative RT administration were assessed using binomial logistic regression. The survival curves were plotted with the Kaplan-Meier method and compared using the log-rank test. Multivariate Cox proportional hazard models were used to investigate the independent prognostic indicators associated with BCSS. Statistical analyses were conducted using SPSS Statistical Software (version 22.0, IBM Corporation, Armonk, NY, USA), and a P value < 0.05 was considered to indicate statistical significance.
Results
Patient characteristics
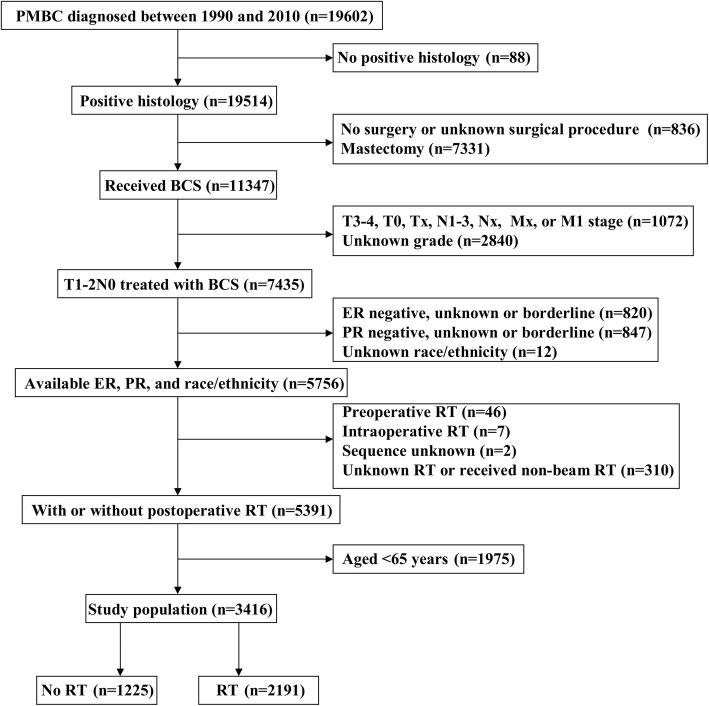
We enrolled 3416 patients with a median age of 75 years (range, 65–99 years). Figure 1 shows the patient selection flowchart for the present study. Most patients were Non-Hispanic White (n = 2829, 82.8%), well-to-moderately differentiated disease (n = 3326, 97.4%), T1 stage disease (n = 1672, 83.4%), and did not receive chemotherapy (n = 3343, 97.9%). A total of 1225 (35.9%) and 2191 (64.1%) patients were assigned to the no-RT and RT cohorts, respectively. Patients with older age (P < 0.001), Non-Hispanic White (P = 0.039), T2 stage disease (P = 0.041), and no receipt of chemotherapy (P < 0.001) were more likely to undergo postoperative RT. In addition, a total of 1010 pairs of patients were completely matched using PSM. Table 1 lists the patient characteristics before and after PSM.
Fig. 1.
Patient selection flowchart of the study
Table 1.
Baseline characteristics of the two treatment cohorts before and after propensity score matching
| Variables | Before PSM | After PSM | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Non-RT (%) | RT (%) | P | n | Non-RT (%) | RT (%) | P | |
| Age (years) | ||||||||
| 65–69 | 681 | 123 (10.0) | 558 (25.5) | < 0.001 | 244 | 122 (12.1) | 122 (12.1) | 1 |
| 70–74 | 840 | 210 (17.1) | 630 (28.8) | 420 | 210 (20.8) | 210 (20.8) | ||
| 75–79 | 803 | 251 (20.5) | 552 (25.2) | 490 | 245 (24.3) | 245 (24.3) | ||
| > 79 | 1092 | 641 (52.3) | 451 (20.6) | 866 | 433 (42.9) | 433 (42.9) | ||
| Race/ethnicity | ||||||||
| Non-Hispanic White | 2829 | 1035 (84.5) | 1794 (81.9) | 0.039 | 1694 | 847 (83.9) | 847 (83.9) | 1 |
| Non-Hispanic Black | 214 | 76 (6.2) | 138 (6.3) | 116 | 58 (5.7) | 58 (5.7) | ||
| Hispanic (All Races) | 199 | 69 (5.6) | 130 (5.9) | 122 | 61 (6.0) | 61 (6.0) | ||
| Other | 174 | 45 (3.7) | 128 (5.9) | 88 | 44 (4.4) | 44 (4.4) | ||
| Grade | ||||||||
| Well-differentiated | 2279 | 837 (68.3) | 1442 (65.8) | 0.314 | 1358 | 679 (67.2) | 679 (67.2) | 1 |
| Moderately differentiated | 1047 | 356 (29.1) | 691 (31.5) | 622 | 311 (30.8) | 311 (30.8) | ||
| Poorly differentiated/undifferentiated | 90 | 32 (2.6) | 58 (2.6) | 40 | 20 (2.0) | 20 (2.0) | ||
| Tumor stage | ||||||||
| T1 | 2848 | 1000 (81.6) | 1848 (84.3) | 0.041 | 1672 | 836 (82.8) | 836 (82.8) | 1 |
| T2 | 568 | 225 (18.4) | 343 (15.7) | 348 | 174 (17.2) | 174 (17.2) | ||
| Chemotherapy | ||||||||
| No/unknown | 3343 | 1213 (99.0) | 2130 (97.2) | < 0.001 | 2000 | 1000 (99.0) | 1000 (99.0) | 1 |
| Yes | 73 | 12 (1.0) | 61 (2.8) | 20 | 10 (1.0) | 10 (1.0) | ||
PSM propensity score matching; RT radiotherapy
Trends of postoperative RT administration based on the period of diagnosis
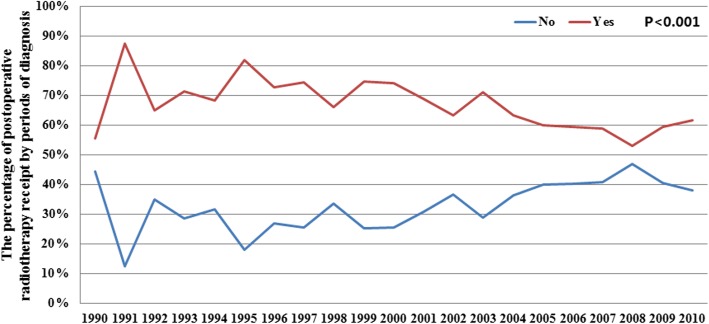
Figure 2 lists the temporal trends of postoperative RT administration from 1990 to 2010. We found that the percentage of patients receiving postoperative RT was significantly lower after 2004 (P < 0.001). In particular, the percentage of patients receiving postoperative RT was 71.1% from 1990 to 2003, and decreased to 59.5% from 2004 to 2010 (P < 0.001).
Fig. 2.
Change in the use of postoperative radiotherapy following breast-conserving surgery in patients with pure mucinous breast carcinoma, according to the period of diagnosis
Independent predictive factors associated with receiving postoperative RT
Binomial regression analysis confirmed that younger age, moderately differentiated disease, and receiving chemotherapy were independently associated with receiving postoperative RT (Table 2).
Table 2.
Independent predictive indicators of postoperative radiotherapy administration following breast-conserving surgery
| Variables | OR | 95% CI | P |
|---|---|---|---|
| Age (years) | |||
| 65–69 | 1 | ||
| 70–74 | 0.669 | 0.521–0.860 | 0.002 |
| 75–79 | 0.492 | 0.385–0.630 | < 0.001 |
| > 79 | 0.154 | 0.124–0.197 | < 0.001 |
| Race/ethnicity | |||
| Non-Hispanic White | 1 | ||
| Non-Hispanic Black | 0.917 | 0.673–1.249 | 0.582 |
| Hispanic (All Races) | 0.914 | 0.663–1.259 | 0.583 |
| Other | 1.432 | 0.990–2.069 | 0.056 |
| Grade | |||
| Well-differentiated | 1 | ||
| Moderately differentiated | 1.189 | 1.009–1.400 | 0.038 |
| Poorly differentiated/undifferentiated | 0.906 | 0.569–1.442 | 0.676 |
| Tumor stage | |||
| T1 | 1 | ||
| T2 | 0.931 | 0.762–1.136 | 0.480 |
| Chemotherapy | |||
| No/unknown | 1 | ||
| Yes | 2.219 | 1.158–4.251 | 0.016 |
CI confidence intervals; OR odds ratio
Independent prognostic factors associated with BCSS
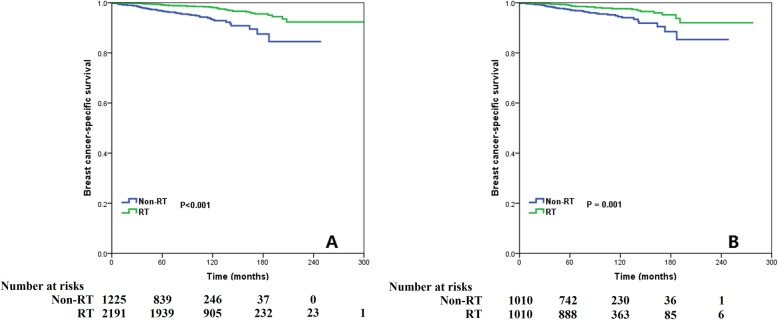
Over a median follow-up period of 95 months (range, 0–304 months) in the non-PSM cohort, we found that 1591 (46.6%) patients died, including 110 (3.2%) patients who died of breast cancer. The 5- and 10-year BCSS rates were 98.3 and 96.6%, respectively. The 10-year BCSS rates were 98.1 and 93.2% in patients with and without postoperative RT, respectively (log-rank test, P < 0.001; Fig. 3a). Over a median follow-up duration of 90 months in the PSM cohort, we found that receiving postoperative RT was associated with a better BCSS; in particular, the 10-year BCSS rates were 97.6 and 94.5% in patients with and without postoperative RT after PSM, respectively (log-rank test, P = 0.001; Fig. 3b).
Fig. 3.
Comparison of breast cancer-specific survival between the non-radiotherapy and adjuvant radiotherapy cohorts before (a) and after (b) propensity score matching
Multivariate Cox proportional analysis was used to determine the independent prognostic factors associated with BCSS (Table 3). After adjusting for age at diagnosis, tumor grade, race/ethnicity, tumor stage, and chemotherapy, we found that receiving postoperative RT was independently associated with better BCSS before (hazard ratio [HR]: 0.390, 95% confidence interval [CI]: 0.261–0.583, P < 0.001) and after PSM (HR: 0.445, 95%CI: 0.273–0.723, P = 0.001), compared to patients without postoperative RT.
Table 3.
Multivariate analysis of the prognostic factors associated with breast cancer-specific survival before and after propensity score matching
| Variables | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (years) | ||||||
| 65–69 | 1 | 1 | ||||
| 70–74 | 0.878 | 0.479–1.612 | 0.675 | 1.261 | 0.534–2.977 | 0.597 |
| 75–79 | 0.885 | 0.469–1.671 | 0.707 | 0.997 | 0.409–2.429 | 0.995 |
| > 79 | 1.985 | 1.135–3.473 | 0.016 | 2.004 | 0.936–4.433 | 0.086 |
| Race/ethnicity | ||||||
| Non-Hispanic White | 1 | 1 | ||||
| Non-Hispanic Black | 1.382 | 0.695–2.747 | 0.356 | 0.822 | 0.256–2.636 | 0.742 |
| Hispanic (All Races) | 0.676 | 0.248–1.842 | 0.444 | 0.713 | 0.223–2.288 | 0.570 |
| Other | 0.426 | 0.105–1.732 | 0.233 | 0.717 | 0.175–2.937 | 0.644 |
| Grade | ||||||
| Well-differentiated | 1 | 1 | ||||
| Moderately differentiated | 1.195 | 0.797–1.791 | 0.388 | 1.211 | 0.736–1.922 | 0.452 |
| Poorly differentiated/undifferentiated | 2.429 | 1.111–5.311 | 0.026 | 2.256 | 0.693–7.350 | 0.177 |
| Tumor stage | ||||||
| T1 | 1 | 1 | ||||
| T2 | 1.626 | 1.051–2.517 | 0.029 | 1.871 | 1.106–3.167 | 0.020 |
| Chemotherapy | ||||||
| No/unknown | 1 | 1 | ||||
| Yes | 1.572 | 0.492–5.019 | 0.445 | 3.143 | 0.745–13.256 | 0.119 |
| Radiotherapy | ||||||
| No/unknown | 1 | 1 | ||||
| Yes | 0.390 | 0.261–0.583 | < 0.001 | 0.445 | 0.273–0.723 | 0.001 |
CI confidence intervals; HR, hazard ratio; PSM propensity score matching
Discussion
In the present study, we aimed to assess the practice patterns and survival outcomes of postoperative RT following BCS in elderly PMBC patients, and found that the use of postoperative RT has decreased in recent years, even though it was associated with better BCSS before and after PSM. To our knowledge, this is the first study to assess the effect and administration of postoperative RT after BCS among elderly PMBC patients.
Although the present study only included patients aged ≥65 years, the patient characteristics in the selection flowchart showed that most patients had small tumor size, lower tumor grade, and ER- and PR-positive disease, consistent with that noted in previous studies [3–5]. In addition, most patients also had HER2-negative disease [3, 5]. Due to the less aggressive behavior of PMBC, the prognosis was excellent, with a 10-year BCSS rate of up to 96.6% in the present study. A retrospective analysis from a single institute or population-based study also showed an excellent BCSS of > 90% [3, 24].
Furthermore, the period of diagnosis impacted the decision of surgical procedure. A study by Sas-Korczyńska et al. included patients from 1952 to 2002 (n = 94) found that most patients were treated with mastectomy (95.7%) and only 4.3% received BCS [12]. Anan et al. also found that only 14.5% of patients received BCS between 1976 and 1998 (n = 76) [25]. However, 79.3, 51.3, and 64.1% of patients diagnosed in 1990–2010, 2014–2016, and 1983–2014, respectively, at 3 Chinese institutes still received mastectomy [10, 26, 27]. Several recent studies from the US showed that > 60% of patients were treated with BCS, and that this rate remained relatively constant between 1998 and 2018 [6, 11, 28]. The selection flowchart of our study also confirmed that approximately 60% of patients were treated with BCS, and that the prognosis in this patient subset was excellent. Our results also confirmed that BCS is an appropriate treatment for low-risk early-stage PMBC.
Previous clinical trials have sought to compare the outcomes between adjuvant endocrine treatment alone and adjuvant RT plus endocrine treatment following BCS in elderly patients with invasive breast carcinoma [15–18]; however, the histological subtypes of the enrolled patients were not recorded in those studies. As there are significant differences in the clinicopathological and molecular features between IDC and PMBC, it is unclear whether RT can be safely omitted in PMBC patients. However, in the present study, 64.1% of elderly patients were treated with RT, and the percentage of receiving postoperative RT significantly decreased from 2004 to 2010 (59.5%), relative to that from 1990 to 2003 (71.1%). The results of 2 prospective clinical trials were published in 2004, which showed that adjuvant RT plus tamoxifen was associated with better locoregional control but no significant difference in the rates of distant recurrence and OS compared to tamoxifen alone [15, 16], which could impact the treatment decision of postoperative RT after BCS in PMBC. Given the decrease in the use of postoperative RT in recent years, these findings could impact patient counseling, which is currently supported by findings from existing randomized studies conducted in IDC patients and may not be suitable due to the inherent lower aggressiveness of PMBC.
To our knowledge, no study has assessed the rates and patterns of local recurrence stratified based on whether postoperative RT was administered. Barkley et al. examined 111 PMBC patients (median age, 56 years), including 67% (n = 64) treated with BCS and 95.5% treated with RT following BCS. Over a median follow-up duration of 63 months, they found that 2 patients with stage T1 N0 and HR-positive disease developed local recurrence after adjuvant RT [26]. In addition, Vo et al. examined 61 patients (median age, 60 years) treated with BCS, including 90% treated with RT, and 3 patients were found to have developed local recurrence [14]. Gwark et al. assessed 471 patients from Korea (mean age, 46 years), including 65.6% who were treated with RT, and found a total of 34 relapses, including 10 patients of local recurrence [4]. Although we could not compare the effect of RT on local recurrence, based on the above results, the local recurrence rate of PMBC should be very low after postoperative RT. Hence, the administration of postoperative RT could potentially improve the outcomes of PMBC patients.
Limited studies were available to compare the outcomes of PMBC based on the administration of postoperative RT. A prior SEER study created a clinical nomogram to predict the outcomes of early PMBC [24]; however, the role of postoperative RT was not included in the nomogram. Gwark et al. found that RT was related to survival outcomes in univariate analysis, whereas no significant association with outcomes was observed in the multivariate analysis [4]; however, the surgical procedure was not specified in that study. Hence, we were unable to determine the role of RT on the outcomes on different surgical procedures. Another study from the SEER database that included 11,400 PMBC patients indicated that the addition of postoperative RT did not significantly improve OS; however, in that study as well, the surgical procedure was not analyzed [9]. To our knowledge, this is the first study to indicate the benefit of postoperative RT on BCSS in elderly PMBC patients, compared to those without postoperative RT. A large retrospective cohort study found that approximately 30% of PMBC patients did not receive postoperative RT following BCS [11], consistent with our findings in the elderly population. Thus, our findings show that postoperative RT should not be omitted following BCS in elderly PMBC patients.
Our study has certain limitations. First, inherent bias is possible in any retrospective study. Second, a centralized pathology review of the SEER database was lacking. Nevertheless, the survival rate of patients was similar to that of previous studies [6–12]. Thus, our study might still be representative of the relevant population. In addition, the details regarding endocrine therapy, chemotherapy regimen, radiotherapy dose, completeness of chemotherapy and radiotherapy, and the pattern of locoregional and distant recurrence were also not recorded in the SEER database. Finally, the percentage of patients receiving RT was under-reported in the SEER database [29]. Despite these limitations, our study offers new insights on the impact of postoperative RT on prognosis after BCS in PMBC patients. The SEER program provides us with a large sample size that can be used to analyze rare breast cancer histologies using robust statistical methods with sufficient power to draw conclusions. Thus, we believe that our findings will contribute to the current knowledge on the effect of postoperative RT following BCS in low-risk elderly PMBC patients.
Conclusion
In conclusion, although the use of postoperative RT following BCS has been decreasing over time in elderly PMBC patients, we believe that postoperative RT may be beneficial in this patient subset; however, additional prospective studies are needed to confirm these results.
Acknowledgments
The authors acknowledge the efforts of the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER database.
Abbreviations
- BCS
Breast-conserving surgery
- BCSS
Breast cancer-specific survival
- CI
Confidence interval
- CSS
Cause-specific survival
- ER
Estrogen receptor
- HER2
Human epidermal growth factor receptor-2
- HR
Hazard ratio
- IDC
Invasive ductal carcinoma
- OS
Overall survival
- PMBC
Pure mucinous breast carcinoma
- PR
Progesterone receptor
- PSM
Propensity score matching
- RT
Radiotherapy
- SEER
Surveillance, Epidemiology, and End Results
- US
United States
Authors’ contributions
SGW and FYL drafted the manuscript. SGW performed the statistical analyses. JW and CLL participated in the design of the study. JZ and ZYH conceived of the study. SGW acquired the datasets. All authors read and approved the final manuscript.
Funding
This work was partly supported by the National Natural Science Foundation of China (81872459, 81803050), the Commission Young and Middle-aged Talents Training Project of Fujian Health Commission (No. 2019-ZQNB-25), and the Science and Technology Planning Projects of Xiamen Science & Technology Bureau (No. 3502Z20174070).
Availability of data and materials
Any request of data and material may be sent to the corresponding author.
Ethics approval and consent to participate
This study was based on the publicly available SEER database and we have got the permission to access them on purpose of research only (Reference number: 11025-Nov2016). As the SEER database consists of de-identified information, the study was exempt from the approval process of Institutional Review Boards of the First Affiliated Hospital of Xiamen University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
San-Gang Wu and Feng-Yan Li contributed equally to this work.
Contributor Information
San-Gang Wu, Email: unowu12345@hotmail.com.
Feng-Yan Li, Email: lify@sysucc.org.cn.
Jun Wang, Email: 13882713780@163.com.
Chen-Lu Lian, Email: 846589561@qq.com.
Juan Zhou, Email: juanzhou12345@163.com.
Zhen-Yu He, Email: hezhy@sysucc.org.cn.
References
- 1.Komaki K, Sakamoto G, Sugano H, Morimoto T, Monden Y. Mucinous carcinoma of the breast in Japan. A prognostic analysis based on morphologic features. Cancer. 1988;61(5):989–996. doi: 10.1002/1097-0142(19880301)61:5<989::AID-CNCR2820610522>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 2.André S, Cunha F, Bernardo M, Menesese Sousa J, Cortez F, Soares J. Mucinous carcinoma of the breast: a pathologic study of 82 cases. J Surg Oncol. 1995;58(3):162–167. doi: 10.1002/jso.2930580305. [DOI] [PubMed] [Google Scholar]
- 3.Roux P, Knight S, Cohen M, Classe JM, Mazouni C, Chauvet MP, et al. Tubular and mucinous breast cancer: results of a cohort of 917 patients. Tumori. 2019;105(1):55–62. doi: 10.1177/0300891618811282. [DOI] [PubMed] [Google Scholar]
- 4.Gwark SC, Lee HS, Lee Y, Lee SB, Sohn G, Kim J, et al. Clinical implication of HER2 status in hormone receptor-positive mucinous breast Cancer. Ann Surg Oncol. 2019;26(7):2166–2174. doi: 10.1245/s10434-019-07332-9. [DOI] [PubMed] [Google Scholar]
- 5.Pareja Fresia, Lee Ju Youn, Brown David N, Piscuoglio Salvatore, Gularte-Mérida Rodrigo, Selenica Pier, Da Cruz Paula Arnaud, Arunachalam Sasi, Kumar Rahul, Geyer Felipe C, Silveira Catarina, da Silva Edaise M, Li Anqi, Marchiò Caterina, Ng Charlotte K Y, Mariani Odette, Fuhrmann Laetitia, Wen Hannah Y, Norton Larry, Vincent-Salomon Anne, Brogi Edi, Reis-Filho Jorge S, Weigelt Britta. The Genomic Landscape of Mucinous Breast Cancer. JNCI: Journal of the National Cancer Institute. 2019;111(7):737–741. doi: 10.1093/jnci/djy216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li CI. Risk of mortality by histologic type of breast cancer in the United States. Horm Cancer. 2010;1(3):156–165. doi: 10.1007/s12672-010-0016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park S, Koo J, Kim JH, Yang WI, Park BW, Lee KS. Clinicopathological characteristics of mucinous carcinoma of the breast in Korea: comparison with invasive ductal carcinoma-not otherwise specified. J Korean Med Sci. 2010;25(3):361–368. doi: 10.3346/jkms.2010.25.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson WF, Chu KC, Chang S, Sherman ME. Comparison of age-specific incidence rate patterns for different histopathologic types of breast carcinoma. Cancer Epidemiol Biomark Prev. 2004;13(7):1128–1135. [PubMed] [Google Scholar]
- 9.Di Saverio S, Gutierrez J, Avisar E. A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma. Breast Cancer Res Treat. 2008;111(3):541–547. doi: 10.1007/s10549-007-9809-z. [DOI] [PubMed] [Google Scholar]
- 10.Cao AY, He M, Liu ZB, Di GH, Wu J, Lu JS, et al. Outcome of pure mucinous breast carcinoma compared to infiltrating ductal carcinoma: a population-based study from China. Ann Surg Oncol. 2012;19(9):3019–3027. doi: 10.1245/s10434-012-2322-6. [DOI] [PubMed] [Google Scholar]
- 11.Wasif N, McCullough AE, Gray RJ, Pockaj BA. Influence of uncommon histology on breast conservation therapy for breast cancer-biology dictates technique? J Surg Oncol. 2012;105(6):586–590. doi: 10.1002/jso.22132. [DOI] [PubMed] [Google Scholar]
- 12.Sas-Korczyńska B, Mituś J, Stelmach A, Ryś J, Majczyk A. Mucinous breast cancer - clinical characteristics and treatment results in patients treated at the oncology Centre in Kraków between 1952 and 2002. Contemp Oncol (Pozn) 2014;18(2):120–123. doi: 10.5114/wo.2014.42727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NCCN clinical Practice guidelines in oncology V.2.2019. Breast Cancer. (2019) Available online at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf [Access Jul 12, 2018].
- 14.Vo T, Xing Y, Meric-Bernstam F, Mirza N, Vlastos G, Symmans WF, et al. Long-term outcomes in patients with mucinous, medullary, tubular, and invasive ductal carcinomas after lumpectomy. Am J Surg. 2007;194(4):527–531. doi: 10.1016/j.amjsurg.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Fyles AW, McCready DR, Manchul LA, Trudeau ME, Merante P, Pintilie M, et al. Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N Engl J Med. 2004;351(10):963–970. doi: 10.1056/NEJMoa040595. [DOI] [PubMed] [Google Scholar]
- 16.Hughes KS, Schnaper LA, Berry D, Cirrincione C, McCormick B, Shank B, et al. Lumpectomy plus tamoxifen with or without irradiation in women 70 years of age or older with early breast cancer. N Engl J Med. 2004;351(10):971–977. doi: 10.1056/NEJMoa040587. [DOI] [PubMed] [Google Scholar]
- 17.Kunkler IH, Williams LJ, Jack WJ, Cameron DA. Dixon JM; PRIME II investigators. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;16(3):266–273. doi: 10.1016/S1470-2045(14)71221-5. [DOI] [PubMed] [Google Scholar]
- 18.Hughes KS, Schnaper LA, Bellon JR, Cirrincione CT, Berry DA, McCormick B, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31(19):2382–2387. doi: 10.1200/JCO.2012.45.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Custom Data (with chemotherapy recode), Nov 2015 Sub (2000–2013) <Katrina/Rita Population Adjustment> − Linked To County Attributes - Total U.S., 1969–2014 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released July 2016, based on the November 2015 submission.
- 20.Shenoy P, Harugeri A. Elderly patients' participation in clinical trials. Perspect Clin Res. 2015;6(4):184–189. doi: 10.4103/2229-3485.167099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zizza CA, Ellison KJ, Wernette CM. Total water intakes of community-living middle-old and oldest-old adults. J Gerontol A Biol Sci Med Sci. 2009;64(4):481–486. doi: 10.1093/gerona/gln045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33–38. [Google Scholar]
- 23.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu J, Wu L, Jiang M, Li D, Jiang T, Hong Z, et al. Clinical Nomogram for predicting survival outcomes in early mucinous breast Cancer. PLoS One. 2016;11(10):e0164921. doi: 10.1371/journal.pone.0164921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anan K, Mitsuyama S, Tamae K, Nishihara K, Iwashita T, Abe Y, et al. Pathological features of mucinous carcinoma of the breast are favourable for breast-conserving therapy. Eur J Surg Oncol. 2001;27(5):459–463. doi: 10.1053/ejso.2001.1142. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Chen X, Lin L, Fei X, Garfield DH, Hong J, et al. Distribution and clinical utility of the 21-gene recurrence score in pure mucinous breast Cancer patients: a case-control study. J Cancer. 2018;9(18):3216–3224. doi: 10.7150/jca.27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan B, Yao R, Shi J, Xu QQ, Zhou YD, Mao F, et al. Prognosis of subtypes of the mucinous breast carcinoma in Chinese women: a population-based study of 32-year experience (1983-2014) Oncotarget. 2016;7(25):38864–38875. doi: 10.18632/oncotarget.8778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barkley CR, Ligibel JA, Wong JS, Lipsitz S, Smith BL, Golshan M. Mucinous breast carcinoma: a large contemporary series. Am J Surg. 2008;196(4):549–551. doi: 10.1016/j.amjsurg.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Noone AM, Lund JL, Mariotto A, Cronin K, McNeel T, Deapen D, et al. Comparison of SEER treatment data with Medicare claims. Med Care. 2016;54:e55–e64. doi: 10.1097/MLR.0000000000000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Any request of data and material may be sent to the corresponding author.