Abstract
Clinical uncertainty exists regarding which assay should be designated as the standard monitoring coagulation test for intravenous unfractionated heparin (UFH). Several studies have compared the use of activated partial thromboplastin time (aPTT) and antifactor-Xa (anti-Xa) and have come out with varying results. The correlation between these 2 tests varied, markedly from strong to weak. Some have demonstrated that monitoring with anti-Xa heparin assay leads to fewer dose adjustments, resulting in fewer laboratory tests, while others have not. In the current study, we evaluated the correlation between aPTT and anti-Xa values to guide clinical management of UFH, with the intention to develop a new correlation nomogram.
Keywords: bleeding, anticoagulants, heparins
Background
Intravenous unfractionated heparin (UFH) has been a cornerstone for anticoagulant therapy for more than 60 years.1,2 Various properties including unpredictable pharmacokinetics, interpatient variability, and a narrow therapeutic index necessitate close monitoring of its anticoagulant effect.3–6 Two different strategies are commonly used to monitor the therapeutic effects of UFH: the antifactor-Xa (anti-Xa) heparin assay and the activated partial thromboplastin time (aPTT).3,7 There is debate regarding which assay should be designated as the standard monitoring strategy or whether a combination of strategies should be utilized based on specific populations.
Both aPTT and anti-Xa assays differ. Activated partial thromboplastin time is a global assessment of coagulation that reflects both the intrinsic and common pathways of the clotting cascade. Conversely, the anti-Xa is a chromogenic assay that measures the inhibition of clotting factor Xa, which reflects the plasma heparin concentration. However, both assays have limitations when used for monitoring UFH therapy. Activated partial thromboplastin time lacks standardization, and sensitivity of reagents to heparin and coagulation factors varies among manufacturers. Activated partial thromboplastin time, unlike the prothrombin time, lacks the equivalent of the international normalized ratio, which corrects for reagent variation. It is also influenced by fluctuations in other clotting factors that impact heparin activity.8,9 Although the anti-Xa has demonstrated less variability, it is more likely to be affected by recent use of low-molecular-weight heparins, fondaparinux, direct factor Xa inhibitors, as well as by hyperbilirubinemia and hypertriglyceridemia.8
This study was conducted to evaluate the correlation between aPTT and anti-Xa laboratory values at Brigham and Women’s Hospital in patients receiving UFH. Our intention was to develop a new correlation nomogram to use the anti-Xa to titrate UFH similar to our existing aPTT dose adjustment nomogram. Additionally, we evaluated the frequency of major bleeding and thrombotic events as they relate to aPTT and anti-Xa values to determine whether discordance in laboratory values was associated with adverse clinical outcomes.
Methods and Materials
This was a single-center, quality improvement study performed between January 1, 2017, and March 31, 2017, in which data were collected prospectively. Patients being treated with UFH therapy for at least 24 hours were identified using a daily report generated from the electronic health record. Consecutive patients were included in the analysis if they were receiving UFH and had documented aPTT and anti-Xa laboratory values drawn simultaneously as per study protocol. The heparin nomogram used is shown in Figure 1. Blood for aPTT testing was collected every 6 hours per standard hospital guidelines. Patients were excluded if they were pregnant, younger than 18 years, had a baseline aPTT greater than 40 seconds, had an aPTT less than 40 seconds despite UFH for greater than 6 hours without a change in dose, currently receiving treatment with fondaparinux or a low-molecular-weight heparin, or currently using a factor Xa inhibitor. Patients were also excluded if they were receiving support via extracorporeal membrane oxygenation (ECMO) or a ventricular assist device (VAD). The primary end point of this study was to evaluate the correlation between aPTT and anti-Xa laboratory values in patients receiving UFH, and secondarily to evaluate the frequency of major bleeding and thrombotic events as they related to aPTT and anti-Xa values.
Figure 1.
Unfractionated heparin (UFH) titration nomogram for goal PTT 60-80. PTT indicates partial thromboplastin time.
Secondary end points, including major bleeding and thrombotic events, were evaluated for the duration of hospitalization. We defined major bleeding according to the International Society on Thrombosis and Haemostasis.10 Thrombotic events were defined as deep vein thrombosis confirmed by venography or Doppler ultrasound, pulmonary embolism confirmed by perfusion lung scan or pulmonary angiography, and stroke defined as an acute episode of focal or global neurological dysfunction caused by brain, spinal cord, or retinal vascular injury because of hemorrhage, infarction, or embolism. For all bleeding and thrombotic events, we also evaluated aPTT and anti-Xa values during the treatment period with UFH.
To evaluate concordance between aPTT and anti-Xa values, all therapeutic aPTT values were identified and subsequently compared to their corresponding anti-Xa values. A therapeutic aPTT for atrial fibrillation (AF) and venous thromboembolism (VTE) was defined as 60 to 80 seconds, while the reference range aPTT at our institution is 23.8 to 36.6 seconds. This range was based on historical data. A therapeutic anti-Xa value was defined as 0.3 to 0.7 U/mL (for AF and VTE indications).9 Corresponding laboratory values that had an aPTT value between 60 and 80 seconds while the anti-Xa value fell between the range of 0.3 to 0.7 U/mL were considered concordant. Any paired values in which the aPTT ranged between 60 and 80 seconds and the corresponding anti-Xa value fell outside the range 0.3 to 0.7 U/mL were considered discordant.
During the study period, a single lot number of Diagnostica Stago—STA PTT-A reagent and a mechanical clot detection coagulometer were used (Parsippany, NJ). When the aPTT exceeded 150 seconds, the results were reported as greater than 150 seconds. To measure anti-Xa, a colorimetric anti-Xa assay, the Diagnostica Stago—STA-liquid anti-Xa (which does not utilize antithrombin as a reagent), and the STA-R Evolution analyzer were used. Anti-Xa values less than 0.10 U/mL were reported as less than 0.10 U/mL.
Continuous variables were presented as mean (standard deviation); ordinal variables presented as median (interquartile range); and categorical variables were presented as proportions. Pearson correlation coefficient (r) was used to measure the linear relationship between aPTT and anti-Xa values. A P value of less than .05 was considered significant.
Results
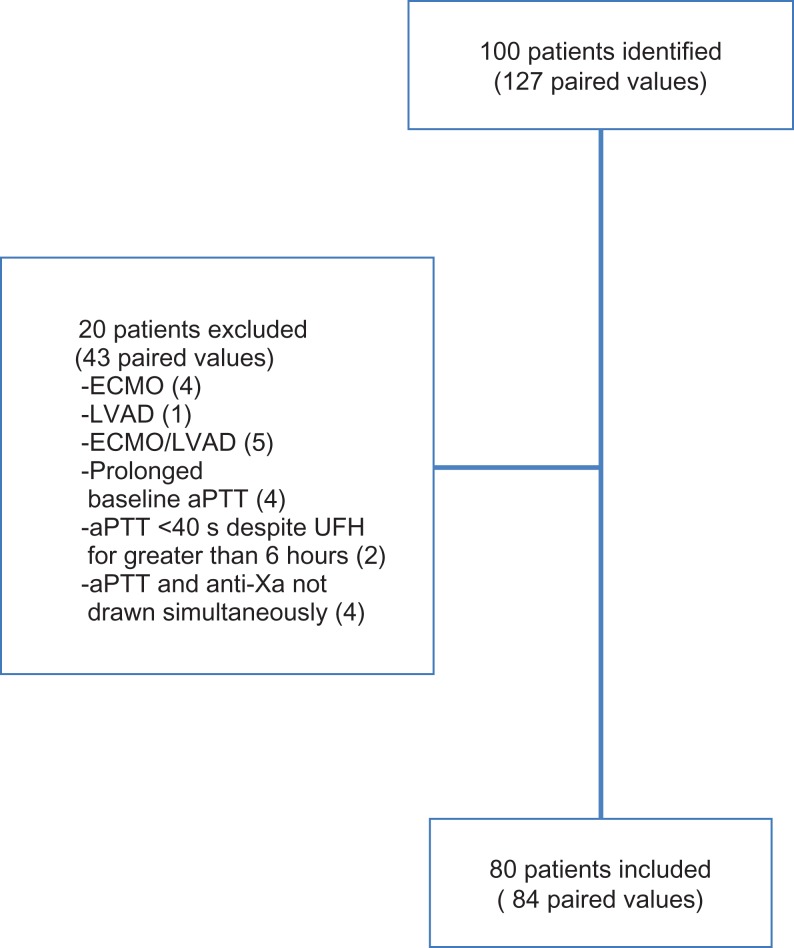
From January 1, 2017, until March 31, 2017, 127 paired aPTT and anti-Xa values from 100 patients were identified. Twenty patients were excluded from the final analysis, with 4 patients excluded for prolonged baseline aPTT, 2 patients with aPTT less than 40 seconds despite receiving a heparin infusion for greater than 6 hours, 4 patients with anti-Xa and aPTT labs that were not drawn simultaneously. Ten patients receiving ECMO and/or VAD support were also excluded. Eighty-four paired aPTT and anti-Xa values from 80 patients who met predefined inclusion criteria were included in the final analysis (Figure 2).
Figure 2.
Inclusion/exclusion.
Patient baseline characteristics are noted in Table 1. The patient population was 65% male, with a median age of 65.9 years and a mean body mass index of 28.8 kg/m2. Most (83.8%) patients were admitted to non–intensive care units and receiving UFH for the management of VTE (22.5%), AF (22.5%), and acute coronary syndrome (28.8%). The remaining patients were being treated for other indications, including lower limb ischemia, peripheral artery disease, and arterial thromboembolism, as noted in Table 1. Most patients used an aPTT goal of 60 to 80 seconds, while others were modified to physician preference.
Table 1.
Baseline Characteristics.a
| n = 80 | |
|---|---|
| Age, years | 65.9 (54.8-76) |
| Male | 52 (65.0%) |
| Weight, kg | 82.1 ± 22.7 |
| BMI, kg/m2 | 28.8 ± 7.6 |
| ICU admission | 13 (16.3%) |
| Indication for anticoagulation | |
| Venous thromboembolism | 18 (22.5) |
| Atrial fibrillation | 18 (22.5) |
| Acute coronary syndrome | 23 (28.8) |
| Stroke | 0 (0) |
| Arterial thromboembolism | 3 (3.8) |
| Peripheral artery disease | 11 (13.8) |
| Other | 7 (8.8) |
Abbreviations: aPTT, activated partial thromboplastin time; BMI, body mass index; ICU, intensive care unit; IQR, interquartile range; SD, standard deviation.
a Data presented as median (IQR), mean ± SD, or n (%).
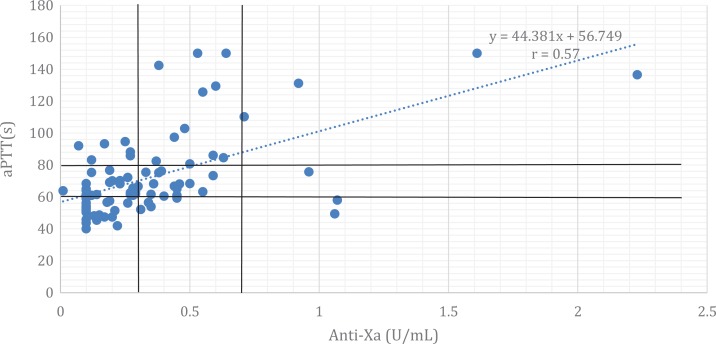
A scatter plot of aPTT and anti-Xa values was generated (Figure 3). The correlation nomogram corresponds to a line of best fit equal to y = 44.381x + 56.749, as shown in Figure 3. The aPTT range corresponding to anti-Xa values of 0.3 to 0.7 U/mL, as calculated from the linear regression equation, was 70.6 to 87.8 seconds. The Pearson correlation coefficient (r) was 0.57 (P value <.05).
Figure 3.
Anti-Xa: aPTT correlation nomogram. Anti-Xa indicates antifactor-Xa; aPTT, activated partial thromboplastin time.
For the purposes of evaluating concordance between aPTT and anti-Xa, 35 therapeutic aPTTs, defined as 60 to 80 seconds, were identified. Paired anti-Xa and aPTT values were simultaneously therapeutic 45.7% of the time (Table 2). When paired values were examined, the most common reason for discordance among the values was a therapeutic aPTT (range 60-80 seconds) with a corresponding subtherapeutic anti-Xa (<0.3 U/mL). In one instance, a therapeutic aPTT was recorded while the anti-Xa was supertherapeutic at 0.96 U/mL.
Table 2.
Concordant and Discordant Values.a,b
| Values | n = 35 | |
|---|---|---|
| Concordant values | Therapeutic aPTT and anti-Xa | 16 (45.71%) |
| Discordant values | Therapeutic aPTT: Subtherapeutic anti-Xa | 18 (51.43%) |
| Therapeutic aPTT: Supertherapeutic anti-Xa | 1 (2.86%) |
Abbreviations: aPTT, activated partial thromboplastin time; anti-Xa, antifactor-Xa.
a Therapeutic aPTT defined as 60 to 80 seconds.
b Therapeutic anti-Xa defined as 0.3 to 0.7 U/mL.
There were no new thrombotic events observed in any patients during the study period; however, there was one major bleed. One patient, while following aPTT, experienced a retroperitoneal bleed, which occurred while the aPTT was supertherapeutic (125.7 seconds) and the anti-Xa was therapeutic (0.55 U/mL). The patient reported abdominal pain and experienced a 2.9 g/dL drop in hemoglobin within 24 hours of becoming symptomatic in the setting of a persistently supertherapeutic aPTT (94.6 seconds). There were 13 other patients with anti-Xa ≥0.55 U/mL who did not have bleeding events.
Discussion
We present our correlation nomogram, with the mathematical formula displayed in Figure 3. In our analysis, 84 paired values from 80 patients, we observed a moderate, positive correlation (r = 0.57; P value <.05). These findings are in concert with previously published literature.9,11–13 The residual discordance could be expected, given that the aPTT and anti-Xa assays measure different outcomes of heparin effects, both laboratory tests can be affected by different patient-specific factors, and the existence of both intralaboratory and interlaboratory factors including different sensitivities of the varying assays. Therefore, anti-Xa and aPTT laboratory tests should be correlated with heparin levels (0.2-0.4 U/mL) at each institution.
Our concordance rate of 45.7% is consistent with prior studies, demonstrating concordance rates from 35% to 60%.14–16 Despite exhibiting moderate correlation and levels of concordance that are similar to several previously published reports, our institution continues to use the aPTT to monitor UFH. Prior to performing our correlation study, several colleagues published data regarding our use of clinical monitoring programs and the ability to improve time to a therapeutic aPTT. First, Alsuliaman et al investigated the effects of the implementation of a pharmacy clinical surveillance system (PCSS) with an alert specific to subtherapeutic aPTT values in patients receiving UFH. This investigation demonstrated that the implementation and use of a PCSS resulted in a decrease in the average time to obtain a therapeutic aPTT, from 21.8 to 15.4 hours (P value = .002).17 Shortly thereafter, Schurr et al published data on the implementation of a nurse-driven, weight-based heparin nomogram in which the mean time to a therapeutic aPTT was 11.7 hours, without an increase in supertherapeutic aPTT values.18 This time to achieve a therapeutic aPTT using the aPTT nurse-driven dosing protocol is similar to a recently published study that demonstrated a time to achieve therapeutic anticoagulation using the anti-Xa of 16 hours. This is a marked decrease from the results a previous study which reported mean time to therapeutic anticoagulation of 28 hours using anti-Xa monitoring.8,19
Use of a therapeutic anti-Xa range of 0.3 to 0.7 U/mL remains controversial, although cited in the American College of Chest Physicians (CHEST) and College of American Pathologists (CAP) guidelines, it is based on results from studies conducted 20 years ago.5,20,21 In one study, patients with acute VTE were randomized to have UFH titrated with a goal anti-Xa level of 0.35 to 0.67 U/mL versus a goal aPTT of 60 to 85 seconds (both values were calibrated to a heparin level of 0.2-0.4 U/mL). The group managed with anti-Xa monitoring had fewer recurrent thrombotic events and fewer bleeding episodes.20 Similarly, another study showed that bleeding was absent at the lower range of therapeutic anti-Xa values (0.24-0.36 U/mL) and subsequently observed at higher anti-Xa values.21
One bleed was observed in our study. It was associated with an aPTT of 125 seconds and a paired anti-Xa value of 0.55 U/mL. Although this anti-Xa value is considered therapeutic per the CHEST and CAP guidelines, equivalency between heparin levels as measured by a neutralization assay and a functional assay remains questionable.22 Several studies have reported anti-Xa values of 0.29 to 0.47 U/mL and 0.25 to 0.45 U/mL which were equivalent to protamine titration levels of 0.2 to 0.4 U/mL.23,24 The possibility exists that an anti-Xa value of 0.55 U/mL may represent a supertherapeutic heparin level based on accepted protamine titration levels, although 13 other patients had an anti-Xa ≥0.55 U/mL and did not experience a bleeding event. Of note, despite 51.4% of paired values having a subtherapeutic anti-Xa, there were no new thrombotic events in our study population. Based on the correlation nomogram devised in this analysis, 3 scenarios may arise where the clinical interpretation of the anti-Xa and aPTT is not consistent.
In scenario 1, an anti-Xa of 0.1 to 0.2 U/mL (subtherapeutic) on our correlation nomogram would correspond to an aPTT of 61.8 to 65.6 seconds (therapeutic), resulting in conflicting interpretations of the patient’s degree of anticoagulation. The clinician should evaluate the individual patient’s risk of thrombosis and bleeding to determine whether UFH should be uptitrated. If the patient has multiple risk factors for bleeding, the intensity of anticoagulation may be deemed adequate. However, if the patient is at high risk of thrombosis, such as patients with a VAD or acute VTE, then the UFH should be uptitrated.
In scenario 2, an anti-Xa of 0.3 to 0.5 U/mL would result in a simultaneously therapeutic aPTT between 70.1 and 78.9 seconds based on the correlation nomogram. The clinician should continue to monitor for clinical signs and symptoms of appropriate anticoagulation intensity, such as signs and symptoms of bleeding as well as increase in thrombosis burden, clot propagation, or development of new thromboses.
Lastly, in scenario 3, an anti-Xa of 0.7 U/mL (therapeutic) on our correlation nomogram would correspond to an aPTT of 88 seconds (supertherapeutic), again resulting in conflicting interpretations. In this case, the patient may be within the target intensity of anticoagulation or be supertherapeutic, depending on which value is interpreted. Patient comorbidities that may affect monitoring UFH should be evaluated. For example, a patient with an active infection may have a large burden of inflammatory mediators that elevate the aPTT. In this case, the anti-Xa may be considered more reliable and reflective of the degree of anticoagulation. Thus, adjusting UFH, particularly in critically ill patients, requires an assessment of the clinical scenario and may warrant the use of multiple coagulation tests, with the understanding that each has advantages and limitations.
Our study has several strengths, including its prospective nature, simultaneously obtained paired lab samples analyzed in a hospital laboratory, and narrowly defined therapeutic ranges. We were limited by the small sample size.
Conclusion
The correlation nomogram presented here demonstrates a moderate correlation and reflects the unique differences between the anti-Xa and aPTT assays. There is uncertainty about the stated therapeutic values for each of these assays. We continue to utilize aPTT to adjust UFH at our institution for the majority of patients, but will use both laboratory assays to guide UFH therapy in specific patient cases. Further studies should correlate aPTT and anti-Xa levels directly with the UFH concentration to create a nomogram that will be accurate when adjusting UFH dosing for therapeutic administration.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Kevin McLaughlin  https://orcid.org/0000-0002-3259-3547
https://orcid.org/0000-0002-3259-3547
References
- 1. Kernohan RJ, Todd C. Heparin therapy in thromboembolic disease. Lancet. 1966;1(7438):621–623. [DOI] [PubMed] [Google Scholar]
- 2. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315–352. doi:10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 3. Francis JL, Groce JB. Challenges in variation and responsiveness of unfractionated heparin. Pharmacotherapy. 2004;24:108S–119S. doi:10.1592/phco.24.12.108 S.36114. [DOI] [PubMed] [Google Scholar]
- 4. Bussey HI. Problems with monitoring heparin anticoagulation. Pharmacotherapy. 1999;19(1):2–5. doi:10.1592/phco.19.1.2.30519. [DOI] [PubMed] [Google Scholar]
- 5. Olson JD, Arkin CF, Brandt JT, et al. College of American Pathologists Conference XXXI on laboratory monitoring of anticoagulant therapy: laboratory monitoring of unfractionated heparin therapy. Arch Pathol Lab Med. 1998;122(9):782–798. [PubMed] [Google Scholar]
- 6. Rosenberg AF, Zumberg MS, Taylor LM, LeClaire AC, Harris NS. The use of anti-Xa assay to monitor intravenous unfractionated heparin therapy. J Pharm Pract. 2010;23(3):210–216. doi:10.1177/0897190010362172. [DOI] [PubMed] [Google Scholar]
- 7. Tahir R. A review of unfractionated heparin and its monitoring. US pharmacist. 2007;32(7):26–36. http://www.uspharmacist.com/index.asp?show=article&page=8_2073.htm. Accessed May 10, 2017. [Google Scholar]
- 8. Guervil DJ, Rosenberg AF, Winterstein AG, Harris NS, Johns TE, Zumberg MS. Activated partial thromboplastin time versus antifactor Xa heparin assay in monitoring unfractionated heparin by continuous intravenous infusion. Ann Pharmacother. 2011;45(7-8):861–868. [DOI] [PubMed] [Google Scholar]
- 9. Vandiver JW, Vondracek TG. Antifactor Xa levels versus activated partial thromboplastin time for monitoring unfractionated heparin. Pharmacotherapy. 2012;32(6):546–558. [DOI] [PubMed] [Google Scholar]
- 10. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692–694. [DOI] [PubMed] [Google Scholar]
- 11. Koerber JM, Smythe MA, Begle RL, Mattson JC, Kershaw BP, Westley SJ. Correlation of activated clotting time and activated partial thromboplastin time to plasma heparin concentration. Pharmacotherapy. 1999;19(8):922–931. [DOI] [PubMed] [Google Scholar]
- 12. Price EA, Jin J, Nguyen HM, Krishnan G, Bowen R, Zehnder JL. Discordant aPTT and anti-Xa values and outcomes in hospitalized patients treated with intravenous unfractionated heparin. Ann Pharmacother. 2013;47(2):151–158. [DOI] [PubMed] [Google Scholar]
- 13. Baker BA, Adelman MD, Smith PA, Osborn JC. Inability of the activated partial thromboplastin time to predict heparin levels. Time to reassess guidelines for heparin assays. Arch Intern Med. 1997;157(21):2475–2479. [PubMed] [Google Scholar]
- 14. Samuel S, Allison TA, Sharaf S, et al. Antifactor Xa levels vs. activated partial thromboplastin time for monitoring unfractionated heparin. A pilot study. J Clin Pharm Ther. 2016;41(5):499–502. [DOI] [PubMed] [Google Scholar]
- 15. Takemoto CM, Streiff MB, Shermock KM, et al. Activated partial thromboplastin time and anti-Xa measurements in heparin monitoring: biochemical basis for discordance. Am J Clin Pathol. 2013;139(4):450–456. [DOI] [PubMed] [Google Scholar]
- 16. Byun JH, Jang IS, Kim JW, Koh EH. Establishing the heparin therapeutic range using aPTT and anti-Xa measurements for monitoring unfractionated heparin therapy. Blood Res. 2016;51(3):171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alsulaiman D, Sylvester K, Stevens C, Carter D. Comparison of time to therapeutic aPTT in patients who received continuous unfractionated heparin after implementation of pharmacy-wide intervention alerts. Hosp Pharm. 2016;51(8):656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schurr JW, Stevens CA, Bane A, et al. Description and evaluation of the implementation of a weight-based, nurse-driven heparin nomogram in a tertiary academic medical center. Clin Appl Thromb Hemost. 2018;24(2):248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Whitman-Purves E, Coons JC, Miller T, et al. Performance of anti-factor Xa versus activated partial thromboplastin time for heparin monitoring using multiple nomograms. Clin Appl Thromb Hemost. 2018;24(2):310–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levine MN, Hirsh J, Gent M, et al. A randomized trial comparing activated thromboplastin time with heparin assay in patients with acute venous thromboembolism requiring large daily doses of heparin. Arch Intern Med. 1994;154(1):49–56. [PubMed] [Google Scholar]
- 21. Holm HA, Abildgaard U, Kalvenes S. Heparin assays and bleeding complications in treatment of deep venous thrombosis with particular reference to retroperitoneal bleeding. Thromb Haemost. 1985;53(2):278–281. [PubMed] [Google Scholar]
- 22. Smythe MA, Koerber JL, Mattson JC. The heparin anti-Xa therapeutic range: are we there yet? Chest. 2002;121:303–304. [DOI] [PubMed] [Google Scholar]
- 23. Kitchen S, Preston FE. The therapeutic range for heparin therapy: relationship between six activated partial thromboplastin time reagents and two heparin assays. Thromb Haemost. 1996;75(5):734–739. [PubMed] [Google Scholar]
- 24. Taylor CT, Petros WP, Ortel TL. Two instruments to determine activated partial thromboplastin time: implications for heparin monitoring. Pharmacotherapy. 1999;19(4):383–387. [DOI] [PubMed] [Google Scholar]