Abstract
Background
Emergence and spread of β-lactamase resistant Klebsiella pneumoniae have posed a serious threat, especially in paediatric patients globally. The present study focuses on explore drug resistance profile and molecular characterization of carbapenemase and extended-spectrum β-lactamase producing K. pneumoniae isolated from paediatric patients in Shenzhen, China.
Methods
Present study, a total of 31 isolates of multi-drug resistant K. pneumoniae were collected from Shenzhen Children’s Hospital, China during Jan 2014 to December 2015. ESBLs production was confirmed by using the combination disc diffusion method followed by antimicrobial susceptibility. In addition, β-lactamase encoding genes were determined by PCR assay and sequencing. The genotypic diversity and phylogenetic relationship were determined by multi-locus sequence typing (MLST) method and pulsed-field gel electrophoresis (PFGE).
Results
We examined 31, unique K. pneumoniae isolates collected from 2014 and 2015 in Shenzhen Children’s Hospital, China. All the 31 isolates 100% were resistant to ceftazidime, ertapenem, ampicillin, cefazolin and ampicillin-sulbactam followed by ceftriaxone 94% (n = 29), aztreonam 89% (n = 26), cefepime 84% (n = 26), nitrofurantoin 75% (n = 24), piperacillin 52% (n = 16), and levofloxacin 49% (n = 15). Of the 31 β-lactamase gene coding isolates, blaCTX-M was mainly detected in about 100% (n = 31), followed by blaKPC 71% (n = 22), blaSHV 61% (n = 19), blaNDM 25% (n = 8), blaCYM 13% (n = 4), blaOXA-48 9% (n = 3), blaGES 9% (n = 3) and blaTEM 6% (n = 2). Seventeen distinct sequences type were observed with ST20 being mostly identified 16% (n = 5). Pulsed-field gel electrophoresis (PFGE) typing showed that identical profile for the isolates recovered from the Department of Intensive Care Unit and Department of Neurology of our hospital. Plasmid replicon typing result indicates the presence of IncFIS type as highest in all isolates as 61% (n = 19), followed by IncFIB 23% (n = 7), IncFIA and IncFIC 16% (n = 5) each.
Conclusion
Our study reports the occurrence and spread of extended β-lactamase K. pneumoniae ST20 and ST2407 for the first time, in Shenzhen, particularly in paediatric patients. To prevent and control the infection by limiting the spread of infection-causing organisms it is very crucial to detect the presence of resistant genes at an early stage.
Keywords: Klebsiella pneumoniae, ESBLs, Antimicrobial susceptibility, Molecular characterization
Introduction
Klebsiella pneumoniae (K. pneumoniae) has most common pathogen intricate in healthcare-associated infections particularly in children that cause a wide variety of infections consisting pneumonia, urinary tract infections, bloodstream infections, intra-abdominal infection and bacteraemia and such conditions treat by antibiotics [1–3]. Indiscriminate use of antibiotics has led to the global spread of extended-spectrum β-lactamases (ESBLs) and K. pneumoniae carbapenemase (KPC) in K. pneumoniae being reported globally [4]. ESBLs have the ability to hydrolyse broad spectrum β-lactams antibiotics including cephalosporin. Since 1983, ESBLs-producing K. pneumoniae infections have been proved to be difficult to treat. ESBLs encoding genes such as blaSHV, blaCTX-M, blaTEM, blaOXA, blaPER, and blaVEB were generally reported from such infections. Eventually, several genes encoding for carbapenemase have also been reported in K. pneumoniae which includes class-A β-lactamase K. pneumoniae carbapenemase (KPC), class-B β-lactamases, IMP, VIM, New Delhi Metallo-β lactamase (NDM) and class-D β-lactamase-oxacillinase type 48 (OXA-48) [5]. Co-production of KPC and NDM in K. pneumoniae was reported to have notably increased in Canada, USA, and China has reported a notable increase in the co-occurrence of KPC and NDM in K. pneumoniae while Africa reported having a major spread of OXA-48 like producing strains [6]. Among paediatric cases from China, only NDM-producing K. pneumoniae is primarily reported in maximum cases despite the presence of wide-spread KPC-producing strains [7]. According to the CHINET antimicrobial surveillance program conducted for 2005–2014, reports of carbapenem-resistant K. pneumoniae have increased from 5.3 to 15.9% in paediatric patients in China [8]. The co-harbouring of two or more clusters of resistance genes in K. pneumoniae have added to poses a challenge for prescribing efficient antibiotic medication. Unfortunately, the lack of effective treatment therapy and extensive application of invasive treatment methods have resulted in the rise of the infections caused by multi-drug resistant K. pneumoniae, ultimately causing mortality in some cases [9, 10]. Co-existence of ESBLs and KPC in K. pneumoniae has world-wide dissemination causing life-threatening clinical outcomes in pediatric patients however, very no more data is available on the susceptibility and molecular characteristics of this pathogen in China. Identification of drug resistance genes and pattern of resistance aids in the targeted drug use which acts as a barrier for further spread of the infection. Hence, we propose to study phenotype and genotype of multi-drug resistant K. pneumoniae isolates obtained from paediatric clinical cases which are first of its kind study in the Shenzhen, China.
Materials and methods
Bacterial isolation and identification
Thirty-one non-duplicate (one isolate from one patient) clinical isolates of K. pneumoniae were collected during January 2014 to December 2015 from Shenzhen Children’s Hospital. The hospital comprises of 1220 beds, offering state of the art health facilities to the surrounding population of the southern part of China. Among the 31 ESBLs producing K. pneumoniae isolates 17 (55%) were from male and 14 (45%) were from female, patients, with age ranging from 1 month to 12 years. The clinical isolation site for specimens were as follows, sputum n = 11, blood n = 6, urine n = 5, catheter-associated and stool n = 3 each, cerebral-spinal fluid n = 2 and throat swab n = 1 (Fig. 2). All isolates were primarily identified by VITEK@2-(Biomerieux, Ref. No. 27530/275660) automated system and confirmed by using 16s RNA gene sequencing.
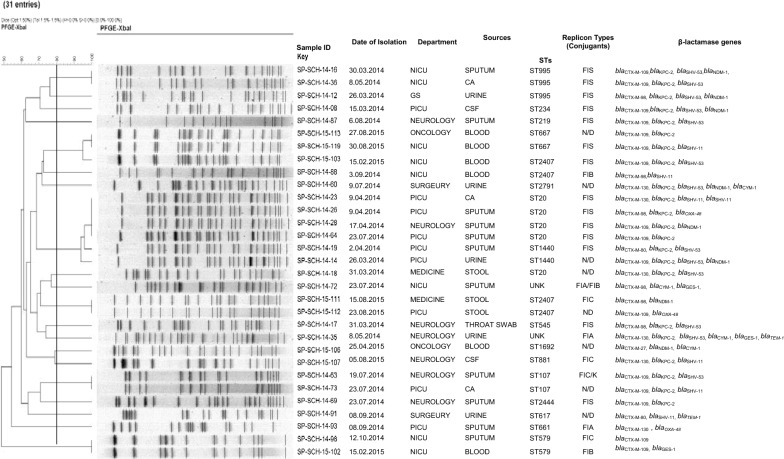
Fig. 2.
Dendrogram of the 31-PFGE-Xbal identified extended spectrum β-lactamase producing Klebsiella pneumoniae isolates recovered from paediatric patients showing their clustering by date of isolation, department of isolation, sources of specimen, sequences types, producing beta-lactamases and clonal relatedness
Phenotypic detection of ESBLs production
The combination disc test was done for phenotypic detection of ESBLs production. The test was performed by using a disc of both cefotaxime and ceftazidime, separately and in combination with clavulanic acid. Control strain, which was selected from the characterized strain collection of our laboratory while ATCC25922 used as a negative control strain. The ESBLs production result was analysed as per the Clinical and Laboratory Standards Institute (CLSI) guideline (CSLI, 2010).
Antimicrobial susceptibility test
Antimicrobial susceptibility was performed by using VITEK@2 compact system (Biomerieux, Ref. No. 27530/275660) for 18 antimicrobial agents namely, ampicillin/sulbactam, piperacillin, ertapenem, amikacin, levofloxacin, nitrofurantoin, ampicillin, cefazolin, ceftazidime, ceftriaxone, cefepime, imipenem, cefotetan, tobramycin, gentamicin, and ciprofloxacin. The results were construed according to the Clinical and Laboratory Standards Institute (CLSI) guideline (CSLI, 2010).
Detection of β-lactamase genes
The standard PCR was performed to detect the presence of ESBLs producing genes: blaTEM, blaSHV, blaCTX-M (variant), blaGES, blaCYM and blaVEB using specific primers previously described [11]. Carbapenemase genes including blaKPC, blaNDM, and blaOXA were detected by PCR as previously described [12]. The purified PCR products were sequenced commercially (Sangon Biotech-Shanghai, China). DNA Sequences were analysed by (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and (https://bigsdb.pasteur.fr/klebsiella/klebsiella.html).
PFGE and multi-locus sequence typing (MLST)
We performed PFGE to check whether there is the presence of any clonal transmission within the hospital and MLST was applied to assess the genetic relatedness of the identified isolates. Post extraction, DNA was digested with 45U Xbal (Takara Biotech) for 2 h at 37 °C. We used CHEFDRIII apparatus (Bio-Rad Laboratories, Hercules, CA, USA) to perform PFGE for K. pneumoniae isolates as per earlier described [13]. Amplified fragments of seven housekeeping genes (gapA, infB, mdh, pgi, phoE, rpoB, and tonB) were sequenced to MLST analysis and MLST website MLST website-http://www.pasteur.fr/recherche/genopole/PF8/mlst/Kpneumoniae was referred to assign the STs.
Plasmid transferability
Streptomycin-resistant E. coli C600 used as the recipient strain in conjugation experiments to analyse the horizontal gene transfer of blaCTX-M and blaKPC for ESBLs and carbapenemase producing respectively in E. coli isolates. We used a liquid mating assay as described earlier [14]. Transconjugants were selected Luria–Bertani agar containing streptomycin 2000 (µg/ml) and cefotaxime (32 µg/ml). Transconjugants were further tested for ESBLs and KPC enzyme production after performing a phenotypic test.
PCR-based replicon typing
PCR-based replicon typing was performed for both plasmids from parental and transconjugant isolates. The Inc (incompatibility) groups were determined by using specific primer introduced by Carattoli et al. [15].
Results
Antimicrobial resistance profile of K. pneumoniae
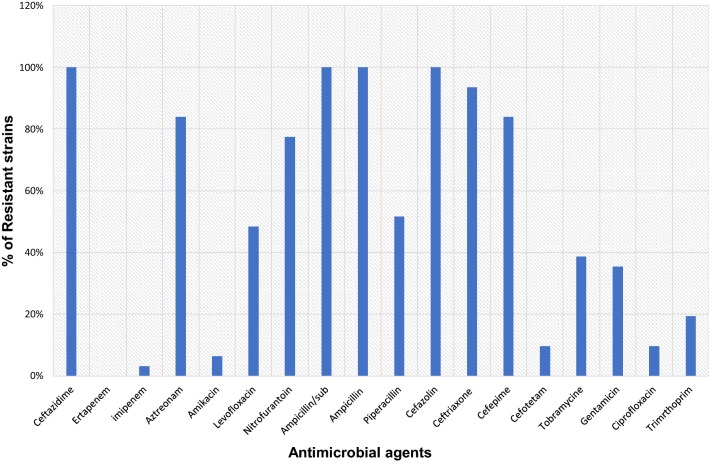
All the 31 isolates were confirmed as K. pneumoniae by VITEK@2-(Biomerieux, Ref. No. 27530/275660) automated system and by using 16s RNA gene sequencing. Primarily all the isolates were confirmed for ESBLs production by combination disc test followed by susceptibility. Antimicrobial susceptibility tests reflected that all of the 31 ESBLs producing K. Pneumoniae isolates 100% were resistant to ceftazidime, ertapenem, ampicillin, cefazolin and ampicillin-sulbactam followed by ceftriaxone 94% (n = 29), aztreonam 89% (n = 26), cefepime 84% (n = 26), nitrofurantoin 75% (n = 24), piperacillin 52% (n = 16), levofloxacin 49% (n = 15), tobramycin 39% (n = 12), gentamycin 35% (n = 11), trimethoprim 19% (n = 6), cefotetan and ciprofloxacin 10% (n = 3) each and amikacin 6% (n = 2). However, all of the isolates were susceptible to imipenem (Fig. 1). All the 30 ESBLs encoding gene K. pneumoniae isolates exhibited a multi-drug resistant phenotype (resistant to at least one agent in three or more antimicrobial group), hence proves the term ‘superbugs’.
Fig. 1.
Antimicrobial susceptibility of 31 extended spectrum β-lactamase producing Klebsiella pneumoniae isolates recovered from paediatric patients to commonly used antibiotics
Molecular analysis of drug resistance genes
All of the 31 ESBLs-genes encoding K. pneumoniae isolates were carrying blaCTX-M genes, with the most common being blaCTX-M-109 (52%, n = 16), followed by blaCTX-M-130 (19%, n = 6) and blaCTX-M-98 (19%, n = 6), blaCTX-M-80 (7%, n = 2) and blaCTX-M-27, 109 (3%, n = 1). Additionally, co-existence of other β-lactamase genes were detected, blaKPC-2 (71%, n = 22), blaSHV-53 (42%, n = 13), blaSHV-11 (19%, n = 6), blaNDM-1 (25%, n = 8), blaCYM-1 (13%, n = 4), blaOXA-48 (9%, n = 3), blaGES-1 (9%, n = 3) and blaTEM (6%, n = 2) (Fig. 2). The blaVAB gene was not find in this study. There was no noteworthy difference in the occurrence of blaCTX-M genes among the ESBLs-producing K. pneumoniae isolated from the different isolation sites or even samples.
Multi-locus sequences typing and PFGE
The extensive diversity of MLST was recorded from ESBLs producing K. pneumoniae isolates, with a total of 17 different STs of which, ST20 (16%) was highly prevalent in Shenzhen, China. All the ST20 isolates were recovered from the Department of Neurology and Department of Intensive Care Unit, the results indicated that K. pneumoniae ST20 is dominant in these two wards and a key transporter for the blaCTX-M-109 gene. Our particular concern is that the blaCTX-M-109 gene was reported in nine different STs in Shenzhen Children’s Hospital. This observation suggests that ESBLs-producing K. pneumoniae isolates carrying blaCTM-M-109 gene might have spread in the Shenzhen region and may be widespread in Southern China. The 31 ESBLs-producing K. pneumoniae isolates were allocated to 20 distinct PFGE clusters sharing ≥ 80% band similarity. The PFGE results showed that the clonal transmission was often observed in the same department and within different departments in the hospital (Fig. 2).
Plasmid profiling
The successful transconjugants were selected from Luria–Bertani agar containing streptomycin 2000 (µg/ml) and cefotaxime (32 µg/ml). PCR based replicon type assay results showed that Inc (Incompatibility) plasmids groups IncFIS (n = 14), IncFIC (n = 4), IncFIB and IncFIA (n = 3) each, were carrying the blaCTX-M group gene (Fig. 2). Conjugants were not obtained from seven parental strains.
Discussion
ESBLs producing K. pneumoniae is a serious threat to pediatric patients, particularly in new-borns, owing to restricted therapeutic options [16]. This pathogen is now considered a reservoir for virulence and resistance genes due to the acquisition of β-lactamase and recently reported colistin resistance mcr-1 gene and so make this a serious threat to human and animal [17, 18]. Our study revealed that CTX-M109 was the most prevalent β-lactamase in Shenzhen, China. So far, no data available on ESBLs producing K. pneumoniae in paediatric clinical cases particularly those caused by CTX-M109 producing K. pneumoniae. In our study, we first determined the phenotype and genotype of multi-drug resistant K. pneumoniae isolates obtained from the paediatric clinical cases in Shenzhen, China. The present study showed that K. pneumoniae were highly resistant to commonly used antibiotics, except for imipenem, amikacin and cefotetan. We did not find any significant antimicrobial resistance profile among the ESBLs producing K. pneumoniae which is different from studies in Shanghai [19]. We found blaCTX-M-109 as the predominant genotype of ESBLs-producing K. pneumoniae in Shenzhen followed by blaCTX-M-9, blaCTX-M-130, blaCTX-M-80, and blaCTX-M-27 revealing the diversity of CTX-M genotype of ESBLs producing E. coli in Shenzhen, China. Similar results were reported from across China [20]. We first report the high presence of co-existing carbapenem-resistant genes blaKPC-2 (71%), blaNDM-1 (25%) with ESBLs encoding gene in Shenzhen, China as well co-existence of carbapenem resistance genes in ESBLs-producing K. pneumoniae has flagged concern about the spread of such superbugs in the Shenzhen area. Several reports have shown the co-existence of carbapenem resistance genes and ESBLs encoding genes in K. pneumoniae on a global scale [21]. Our MLST results demonstrated that ST20 and ST2407 were highly prominent in Shenzhen area which encodes ESBLs genes. Several countries such as New Zealand [22], Greece [23], Canada [5], Korea [24] have reported infection caused by K. pneumoniae ST20 with ESBLs production. Jin et al. first reported an outbreak of ESBLs producing K. pneumoniae ST20 during August 2012 to September 2013, among neonates in Shandong province, China [25]. We find the clonal transmission within the hospital, which is comparable with previous studies by Dongxing Tian et al., in 2018 at Shanghai, China [19]. The transmission may occur due to direct contacts with the patients or hospital staff (hands, saliva, other body fluids etc.) or environmental sources such as water, food, other body fluids. We strictly follow the standard operating procedure for patient handling in our hospital to restrict such superbugs transmission. The limitation of our study is that we did not study isolates from other hospitals to observe whether there is clonal transmission between the hospitals. Plasmid replicon typing and conjugation experiment results exposed that IncFIS, IncFIB and IncFIA replicons were existing in the transconjugants and blaCTX-M genes co-transfer with carbapenemase coding genes such as blaKPC-2, and blaNDM-1. No apparent relationship between replicon and sequence type was observed among the current isolates. We did not the dissemination of any particular clones in Shenzhen, China. Our finding stresses the significance of continuous monitoring to detect multi-drug resistant isolates so as to promote therapeutic strategies for treating infections in paediatric patients. We propose a new study to assess the presence of other resistance-related determinants, such as the outer-membrane permeability and also to augment sample size for the molecular study.
Conclusion
Earlier studies in China have reported the occurrence of ESBL-producing K pneumoniae however, information regarding the susceptibility pattern and description of molecular structure in paediatric patients is very limited. Best of our knowledge, For the first time, we report the emergence of ESBLs from a major Children’s Hospital in Shenzhen, China. Our results highlight the occurrence of ESBLs in K. pneumoniae belonging to the CTX-M-109 type.
Acknowledgements
Not available.
Abbreviations
- MLST
multi-locus sequence typing (MLST) method and (PFGE)
- PFGE
pulsed-field gel electrophoresis
- ESBLs
extended-spectrum beta-lactamases
- Inc
incompatibility
- STs
sequences types
Authors’ contributions
SP and FW contributed to the conception and designed the work; SP carries out all the experiments, XC collection of all isolates; SP, XC, and FW contributed to the final analysis of data. All authors have contributed to the drafting and revision of the manuscript. All authors read and approved the final manuscript.
Funding
Sciences and Technology Project from the science technology and innovation committee of Shenzhen Municipality (Grant No. JCYJ20170817170110940) and Sanming Project of Medicine in Shenzhen (Grant No. SZSM201512033)
Availability of data and materials
Not available.
Ethics approval and consent to participate
Present study approved form Shenzhen Children’s Hospital (Research) ethical committee 2018(013).
Consent for publication
The clinical isolate samples used in this research were part of the routine hospital laboratory procedure. Oral consent was taken from the patients. We do not use patients name or personal information so no need to take writing consent.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Paterson D, Ko W, Von A, Mohapatra S, Casellas J, Goossens H, et al. International prospective study of Klebsiella pneumoniae bacteraemia: implications of extended-spectrum beta-lactamase reduction in Nosoc- omial infections. Ann Intern Med. 2004;140:26–32. doi: 10.7326/0003-4819-140-1-200401060-00008. [DOI] [PubMed] [Google Scholar]
- 2.Raymond J, Aujard Y. Nosocomial infections in pediatric patients: a European multicentre prospective study. European Study Group. Infect Control Hosp Epidemiol. 2000;21:260–263. doi: 10.1086/501755. [DOI] [PubMed] [Google Scholar]
- 3.Kang C, Kim S, Kim D, Park W, Lee K, Kim H, et al. Risk factors for and clinical outcomes of bloodstream infections caused by extended-spectrum β-lactamase-producing Klebsiella pneumoniae. Infect Control Hosp Epidemiol. 2004;25:860–867. doi: 10.1086/502310. [DOI] [PubMed] [Google Scholar]
- 4.Mosqueda J, Montano-Loza A, Rolon A, Cervantes C, Bobadilla J, Silva J, et al. Molecular epidemiology and risk factors of bloodstream infections caused by extended-spectrum β-lactamase-producing Klebsiella pneumoniae: a case–control study. Int J Infect Dis. 2008;12:653–659. doi: 10.1016/j.ijid.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Peirano G, Sang J, Pitondo-Silva A, Laupland K, Pitout J. Molecular epidemiology of extended-spectrum-β-lactamase-producing Klebsiella pneumoniae over a 10-year period in Calgary, Canada. J Antimicrob Chemother. 2012;67:1114–1120. doi: 10.1093/jac/dks026. [DOI] [PubMed] [Google Scholar]
- 6.David VD, Yohei D. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence. 2017;8:460–469. doi: 10.1080/21505594.2016.1222343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X, Li X, Wang M, Yue H, Li P, Liu Y, et al. Outbreak of NDM-1-producing Klebsiella pneumoniae causing neonatal infection in a teaching hospital in mainland China. Antimicrob Agents Chemother. 2015;59:4349–4435. doi: 10.1128/AAC.03868-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu F, Guo Y, Zhu D, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005–2014. Clin Microbiol Infect. 2016;22:S9–S14. doi: 10.1016/j.cmi.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Ulu-Kilic A, Alp E, Percin D, et al. Risk factors for carbapenem-resistant Klebsiella pneumoniae rectal colonization in pediatric units. J Infect Dev Countri. 2014;8:1361–1364. doi: 10.3855/jidc.4593. [DOI] [PubMed] [Google Scholar]
- 10.Kontopidou F, Giamarellou H. Group for the Study of KPC-producing Klebsiella pneumoniae infections in intensive care units. Infections caused by carbapenem-resistant Klebsiella pneumoniae among patients in intensive care units in Greece: a multi-centre study on clinical outcome and therapeutic options. Clin Microbiol Infect. 2014;20:117–123. doi: 10.1111/1469-0691.12341. [DOI] [PubMed] [Google Scholar]
- 11.Tian B, Huang M, Fang L, Qing Y, Zhang F, Huang X. CTX-M-137, a hybrid of CTX-M-14-like and CTX-M-15-like beta-lactamases identified in an Escherichia coli clinical isolate. J Antimicrob Chemother. 2014;69:2081–2085. doi: 10.1093/jac/dku126. [DOI] [PubMed] [Google Scholar]
- 12.Poirel L, Walsh T, Cuvillier V, Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbio Infect Dis. 2011;70:119–123. doi: 10.1016/j.diagmicrobio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Han H, Zhou H, Li H, Gao Y, Lu Z, Hu K, Xu B. Optimization of pulse-field gel electrophoresis for subtyping of Klebsiella pneumoniae. Int J Environ Res Public Health. 2013;10:2720–2731. doi: 10.3390/ijerph10072720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian B, Yi-Qi J, Ying-Min H, et al. Characterization of CTX-M-140, a variant of CTX-M-14 extended-spectrum β-lactamase with decreased cephalosporin hydrolytic activity, from cephalosporin-resistant proteus mirabilia’s. Antimicrob Agents Chemother. 2016;60:6121–6126. doi: 10.1128/AAC.00822-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall E. Identification of plasmids by PCR-based replicon typing. J Microbio Meth. 2005;63:219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Logan L. Carbapenem-resistant Enterobacteriaceae: an emerging prob- lem in children. Clin Infect Dis. 2012;55:852–859. doi: 10.1093/cid/cis543. [DOI] [PubMed] [Google Scholar]
- 17.Holt K, Wertheim H, Zadoks R, et al. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgentthreat to public health. Proc Natl Acad Sci USA. 2015;112:3574–3581. doi: 10.1073/pnas.1501049112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ridolfo L, Rimoldi G, Pagani C, et al. Diffusion and transmission of carbapenem-resistant Klebsiella pneumoniae in the medical and surgical wards of a university hospital in Milan Italy. J Infect Public Health. 2016;9:24–33. doi: 10.1016/j.jiph.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Tian D, Pan F, Wang C, Sun Y, Zhang H. Resistance phenotype and clinical molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae among pediatric patients in Shanghai. Infect Drug Resist. 2018;11:1935–1943. doi: 10.2147/IDR.S175584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Zhou K, Zheng B, Zhao L, et al. High prevalence of esbl-producing Klebsiella pneumoniae causing community-onset infections in China. Front Microbiol. 2016;7:1830. doi: 10.3389/fmicb.2016.01830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arabaghian H, Salloum T, Alousi S, Panossian B, Araj GF, Tokajian S. Molecular characterization of carbapenem-resistant Klebsiella pneumoniae and Klebsiella quasipneumoniae isolated from Lebanon. Sci Rep. 2019;9:531. doi: 10.1038/s41598-018-36554-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freeman J, Rubin J, McAuliffe G, Peirano G, Roberts S, Drinković D, Pitout J. Differences in risk-factor profiles between patients with ESBL-producing Escherichia coli and Klebsiella pneumoniae: a multicentre case-case comparison study. Antimicrob Resist Infect Control. 2014;3:27. doi: 10.1186/2047-2994-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mavroidi A, Liakopoulos A, Gounaris A, Goudesidou M, Gaitana K, Miriagou V, Petinaki E. Successful control of a neonatal outbreak caused mainly by ST20 multidrug-resistant SHV-5-producing Klebsiella pneumoniae, Greece. BMC Pediatr. 2014;14:105. doi: 10.1186/1471-2431-14-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeom J, Lee M, Peck K, Song J. Clonal dissemination of extended-spectrum beta-lactamase (ESBL)-producing Klebsiella pneumoniae isolates in a Korean hospital. J Korean Med Sci. 2008;23:53–60. doi: 10.3346/jkms.2008.23.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin Y, Shao C, Li J, Fan H, Bai Y, Wang Y. The outbreak of Multidrug-Resistant NDM-1-producing Klebsiella pneumoniae from a neonatal unit in Shandong Province, China. PLoS ONE. 2015;10:e0119571. doi: 10.1371/journal.pone.0119571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not available.