Abstract
Objective:
The aim of the present study was to evaluate the demographics, clinical characteristics, fatal dose, the efficacy of treatments, and prognosis in paraquat (PQ) poisoning in the Kerman Province of Iran.
Methods:
This analytical cross-sectional study was conducted on 126 PQ poisoned patients who were referred to Afzalipour Hospital during 2006–2015. Demographic variables such as age and gender, signs and symptoms of poisoning, the estimated ingested dosage of PQ, and clinical outcome were extracted from medical records. Patients were compared and categorized into two groups considering the outcome: survivors and nonsurvivors. Patients with nonoral exposures, combined drug exposures, PQ exposures more than 24 h before the presentation, and critical underlying diseases were not included in the study.
Findings:
Our results indicated that the mean dose of PQ used by all patients was 2358 mg, which was reported as 1846 and 2812 mg in females and males, respectively. Moreover, the results showed that the highest mortality rate was in patients with respiratory distress, followed by oral ulceration and excess salivation. In all PQ-poisoned patients, the dose of greater than approximately 2250 mg predicted death with 86.2% specificity and 75.7% sensitivity.
Conclusion:
Based on the results of the present study, the mortality rate in PQ-poisoned patients depended on the dose of poison, blood sugar level, and aspartate transaminase levels. Our results suggest that these parameters have excellent prognostic value for the prediction of mortality.
KEYWORDS: Mortality, paraquat, poisoning, prediction, prognosis, survival, treatment
INTRODUCTION
Paraquat (PQ) is a contact herbicide used to control a range of narrow or broadleaf plants and mostly used in weed growth control.[1] This chemical is highly toxic for humans and most animals[2,3] with poisoning mostly by accidental ingestion.[4] Poisoning, however, may also occur through other routes, including contact with skin or mucosae.[5] PQ is rapidly but incompletely absorbed and thereafter excreted in urine within 12–24 h. It has a severe effect on the lungs and in high doses may also damage other important organs such as the heart, kidneys, liver, adrenal glands, central nervous system, muscles, and spleen, causing multiple failures.[1,2] PQ selectively accumulates in the lungs resulting in severe oxidative injury and death of membrane epithelial cells due to the production of oxygen-free radicals; in other tissues, it may cause necrosis. PQ toxicity is more prevalent among those who use herbicides for suicide.[6]
To the best of our knowledge, there have been only a few studies on PQ poisoning in Iran. Sabzghabaee et al.[7] indicated 29 PQ poisonings in a 5-year period (2002–2006) in Isfahan with a 55.2% mortality rate. The exact number of deaths due to PQ poisoning in Iran is, however, unclear. Delirrad et al.[8] reported PQ poisoning during a period of 7 years (2007–2013) in Urmia with a 46.4% mortality rate which is less than that reported in some other studies.[6,9] Recently, Kavousi-Gharbi et al.[10] evaluated 104 cases of PQ poisoning, with a mortality rate of 43%. In many developing countries, in which the rules and regulations governing the sale and use of PQ are not strict, PQ intoxication is difficult to prevent.[11] PQ has also been banned in Germany since 2007. In Iran, PQ has been marketed as a 20% aqueous solution of dichloride salt (Gramoxone, Imperial Chemical Industries).[8] The people in the Kerman Province of Iran, however, usually dilute this product to a 2% solution. Three degrees of severity of PQ poisoning may occur: mild poisoning that initially leads to gastritis, inflammation of the mouth, and gastrointestinal upset but with eventual complete remission; moderate-to-severe poisoning, which usually results in acute renal failure and in severe cases leads to acute hepatitis, pneumonia, and pulmonary fibrosis, which often leads to death after 2–3 weeks; and acute severe poisoning with multiple organ failure and collapse of the cardiovascular system leading to death within a week.[8,12,13]
Some of the treatment approaches developed for PQ poisoning include administration of adsorbents, hypo-oxygenation, radiation therapy of the lungs, long-term detoxification, and lung transplantation.[5,9] The efficacy of these treatments, however, remains uncertain. Initial treatment for PQ poisoning includes prevention of further absorption of the poison and reduction of the amount of PQ in the blood by the use of hemodialysis.[14,15] Lee et al.[16] found that the route of PQ administration and dosage are the main prognostic factors. They found that with PQ doses above 50 mg/kg, patients died of circulatory failure within 72 h; doses between 35 and 50 mg/kg resulted in progressive pulmonary fibrosis. Therefore, our study aimed to assess the demographic and clinical characteristics, fatal dose, efficacy of treatments, and symptoms for predicting the medical outcomes in PQ poisoning in the Kerman Province of Iran from 2006 to 2015.
METHODS
Study design and setting
The population of this cross-sectional retrospective study included 126 PQ-poisoned patients from Jiroft and Kahnooj, the main agricultural regions in Kerman Province. These people were referred between 2006 and 2015 to Afzalipour Hospital, the main toxicology center in the southeast of Iran. The study was approved by the Kerman University of Medical Sciences Ethics Committee (IR.KMU.REC.1395.835). All methods were performed in accordance with the relevant guidelines and regulations. After explaining the study objectives, responsibilities of participants and researchers, data collecting, and confidentially of data, written and informed consent was obtained from participants or their family.
Participants
In the study period (2006–2015), patients with PQ poisoning were included based on the history of taking green liquid PQ (according to the patient's statement or closest relatives), clinical findings and physical examination (oral ulcers and vesicles and chemical damage of the gastrointestinal tract on endoscopy), and sodium dithionite urine test. The sodium dithionite test is based on the decline of PQ by sodium dithionite under alkaline circumstances. The implications are classified as grades 1–4: black (+4), deep blue (+3), light blue (+2), and hardly identifiable blue (+1): a “navy blue” or “dark blue” color demonstrating notable PQ poisoning.[17]
Patients who met any of the following criteria were not included in the study: combined drug exposures, discharge against medical advice, transferred to another hospital, PQ exposures >24 h previous to the presentation, and critical underlying diseases such as malignancy, heart, lung, renal, or liver diseases. The flowchart of patients' enrollment throughout the study is illustrated in Figure 1.
Figure 1.
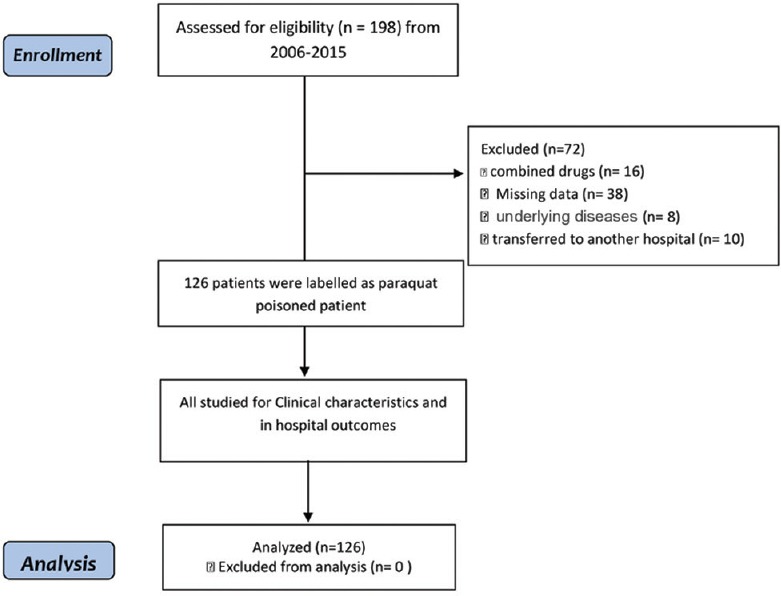
Flow diagram of the study patients
Most of the patients initially underwent gastric decontamination by lavage, adsorbent charcoal administration, intravenous fluid therapy for diuresis, and hemodialysis in the local hospital and after that were referred to our center. As PQ has no specific antidote, all patients subsequently received medication variably as follows: daily hemodialysis, antifibrosis and anti-inflammatory attempts with methylprednisolone succinate 15 mg/kg daily intravenous infusion after hemodialysis for three times, followed by intravenous dexamethasone 8 mg/ three times a day for 1 week, cyclophosphamide 15 mg/kg daily intravenous infusion after hemodialysis for three times, and mercaptoethanesulfonate 15 mg/kg daily continuous intravenous infusion after cyclophosphamide to prevent cyclophosphamide side effects such as hemorrhagic cystitis. In addition, antioxidant therapy with Vitamin C 1500 mg/intravenous infusion twice a day (q12 h) was given for 1 week, as well as Vitamin E 2 international units (IUs)/kg intramuscularly three times a day (q8 h) for 1 week, and N-acetylcysteine 150 mg/kg/daily continuous intravenous infusion for 1 week. Oxygen administration was avoided until the patient showed persistent hypoxemia. In 23 cases, phlebotomy was applied for hypo-oxygenation as well as the conventional therapy as mentioned above. After acquiring patients' approval, 500 ml of blood was removed daily and replaced by 500 ml of normal saline until hemoglobin concentration dropped below 9 mg/dl. All patients underwent endoscopic examination within 24 h of admission to the poisoning ward to determine the severity of gastrointestinal involvement and confirmation of PQ poisoning. These procedures were done without any complication or mortality (n = 0) even in cases of severe poisoning.
Data sources
Demographic variables such as age and gender, signs and symptoms of poisoning, the estimated ingested dosage of PQ, and clinical outcome were extracted from medical records. Patients were compared and categorized into two groups considering the outcome: survivors and nonsurvivors.
Statistical analysis
Statistical analysis was performed using SPSS software (version 22, IBM Corp, Chicago, USA) and R 3.3.1 with the pROC package (Insightful company, USA). Descriptive analysis was reported as frequency and percentage. The independent t-test was used to determine significance between the two groups (survival and nonsurvival) for continuous variables that were normally distributed, and the Chi-square test was used to compare the clinical characteristics of patients between the two groups. Sensitivity and specificity and the best cutoff on the dose of PQ for predicting mortality were calculated using the ROC curve. A level >5% was considered significant.
RESULTS
Among 198 patients, only 126 were included in the present study. Of these 126 people exposed to PQ, 63 (50%) were male, 64 (50.8%) were married, 2 (1.2%) had a history of previous attempted suicide, 3 (4.2%) had mental disorders, 61 (48.4%) had committed intentional self-poisoning, and 61 (48.4%) developed unintentional poisoning (consisting of accidental and occupational poisoning), mainly by ingestion. The mean age of all patients was 22.5 years (23 years in females and 22 years in males). The mean dose of PQ consumed by all patients was 2358 mg, with doses significantly higher in males (2812 mg) than in females (1846 mg) (P = 0.045). Of the 126 patients, 81 (63.6%) survived. In all PQ-poisoned patients, the dose of >2250 mg predicted death with 86.2% specificity and 75.7% sensitivity.
The majority of patients who received treatment survived. The frequencies of patients who survived and those who died were homogeneous in different treatments without any statistically significant difference. Respiratory distress, drooling, and abnormal lung sounds were significantly more frequent in fatal cases than in those who survived. The differences in other symptoms were not statistically significant between survivors and nonsurvivors [Table 1]. The frequency distribution of different treatments based on the outcomes illustrated that the most deaths occurred in people receiving methylprednisolone (n = 36), Vitamins E (n = 34), and Vitamin C (n = 34), with those who received phlebotomy comprising the lowest number of patients who survived (n = 14). The frequency distribution of different symptoms based on the observed outcomes illustrated that the highest number of deaths occurred in people presenting with respiratory distress (n = 41), followed by those with oral ulceration (n = 38) and those with excess salivation (n = 37). In addition, people with duodenal involvement had the lowest number of deaths (n = 0), followed by those with gastric involvement (n = 1). Thirty of the survivors (71.4%) received five of the different treatments, 20 (60.6%) received four treatments, and 12 (75%) received three treatments. Eight (61.5%), 3 (37.5%), and 8 (61.5%) of the survivors received six, two, and one treatment, respectively [Figure 2].
Table 1.
Clinical characteristics of survived and nonsurvived paraquat-poisoned patients
| Parameters | Total (n=126), n (%) | Survived (n=81), n (%) | Nonsurvived (n=45), n (%) | P≠ |
|---|---|---|---|---|
| Received treatment | ||||
| Gastric lavage | 97 (77.0) | 64 (66) | 33 (34) | 0.468 |
| Charcoal sorbitol | 79 (62.7) | 50 (63.3) | 29 (36.7) | 0.763 |
| Hemodialysis | 100 (79.4) | 67 (67) | 33 (33) | 0.303 |
| Methylprednisolone | 105 (83.3) | 69 (65.7) | 36 (34.3) | 0.454 |
| Cyclophosphamide | 91 (72.2) | 63 (69.2) | 28 (30.8) | 0.062 |
| Phlebotomy | 24 (19.0) | 14 (58.3) | 10 (41.7) | 0.499 |
| N-acetylcysteine | 90 (71.4) | 60 (66.7) | 30 (33.3) | 0.25 |
| Vitamin C | 102 (80.9) | 68 (66.7) | 34 (33.3) | 0.18 |
| Vitamin E | 103 (81.7) | 69 (67) | 34 (33) | 0.13 |
| Symptoms | ||||
| Oral ulcer | 109 (86.5) | 71 (65.1) | 38 (34.9) | 0.613 |
| Respiratory distress | 60 (47.6) | 19 (31.7) | 41 (68.3) | <0.001 |
| Excess salivation | 61 (48.4) | 24 (39.3) | 37 (60.7) | <0.001 |
| APA | 31 (24.6) | 7 (22.6) | 24 (77.4) | <0.001 |
| EEE | 15 (68.2) | 11 (73.3) | 4 (26.7) | 0.131 |
| GEE | 13 (61.9) | 12 (92.3) | 1 (7.7) | 0.271 |
| DI | 5 (25.0) | 5 (100) | 0 (0.0) | 0.278 |
≠Using Chi-square test. APA=Abnormal pulmonary auscultation, EEE=Esophageal erythema erosion, GEE=Gastric erythema erosion, DI=Duodenal involvement
Figure 2.
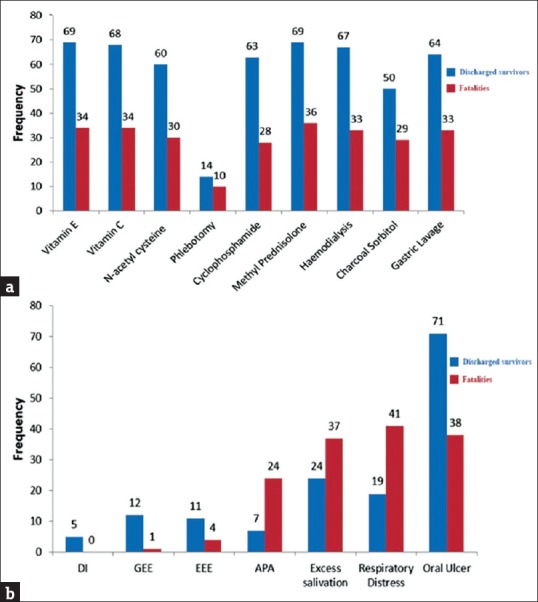
Distribution of treatment methods with outcome categories (a) and distribution of symptoms relative to outcome categories (b); APA: Abnormal Pulmonary Auscultation, EEE: Esophageal Erythema Erosion, GEE: Gastric Erythema Erosion, DI: Duodenal Involvement; Frequency = number of patients, with total n=126
There were significant differences between the surviving and nonsurviving groups according to the dose of poison, white blood cell count, blood sugar (BS) level, serum aspartate transaminase (AST), serum alanine transaminase (ALT), and creatinine (Cr) levels [Table 2]. The median doses of poison for survival and nonsurvival were 1500 (300–1500) and 3000 (2000–5000) mg, respectively (P < 0.001). As Table 2 shows, patients who died had significantly higher levels versus survivors of BS (124.03 ± 48.07 mg/dl vs. 100.17 ± 26.0 mg/dl, P = 0.004), AST (88.0 IU/L [47.0–75.0] vs. 32.0 IU/L [25.0–38.0], P < 0.001), and ALT (54.5 IU/L [25.0–190.0] vs. 22.0 IU/L [17.0–30.0], P < 0.001). Using the multiple logistic regression analysis model, it was determined that three factors were significant for predicting mortality, including: dose of poison (odds ratio [OR] [95% confidence interval (CI)]: 1.006 [1.001–1.01], P = 0.04), BS (OR [95% CI]: 1.02 [1.01–1.03], P = 0.04), and AST (OR [95% CI]: 1.04 [1.01–1.06], P = 0.002). Moreover, the death rate rose 1.04 times per unit of AST increase [Table 3]. The Hosmer–Lemeshow goodness-of-fit was derived as insignificant (χ2 = 8.27, df = 8, P = 0.41). Therefore, the null hypothesis of the model's fitting the data well is not rejected. Cox and Snell R-squared was derived as 61.3%, indicating that independent variables were effective in predicting mortality.
Table 2.
Comparison of different variables in the survived and nonsurvived groups according to outcome
| Variable | Survived (n=81) | Nonsurvived (n=45) | t/Z/χ2 | P |
|---|---|---|---|---|
| Age (years) | 21.59±5.66** | 24.34±11.68 | t=1.48 | 0.14 |
| Gender, n (%) | ||||
| Male | 40 (31.7) | 23 (18.3) | χ2=0.03 | 0.85 |
| Female | 41 (32.5) | 22 (17.5) | ||
| Dose of poison (mg of 2% PQ) | 1500 (300-1500)*** | 3000 (2000-5000) | Z=5.22 | <0.001 |
| WBC (103 μL) | 10.3 (8.15-12.5) | 15.5 (10.8-21.9) | Z=4.45 | <0.001 |
| Hb (g/dL) | 13.16±3.55 | 13.20±2.41 | t=0.37 | 0.70 |
| HCT (%) | 39.95 (35.45-44.92) | 38.7 (33.85-45.7) | Z=0.45 | 0.65 |
| BS (mg/dL) | 100.17±26.00 | 124.23±48.07 | t=3.0 | 0.004 |
| AST (IU/L) | 32.0 (25.0-38.0) | 88.0 (47.0-175.0) | Z=6.54 | <0.001 |
| ALT (IU/L) | 22.0 (17.0-30.0) | 54.5 (28.0-190.0) | Z=5.73 | <0.001 |
| Cr (mg/dL) | 1.2 (0.8-1.6) | 2.6 (1.5-3.5) | Z=5.06 | <0.001 |
| Na (mEq/l) | 141.61±4.99 | 141.85±7.14 | t=0.22 | 0.83 |
| K (mEq/l) | 3.91±0.67 | 4.11±0.87 | t=1.34 | 0.18 |
| pH | 7.41±0.11 | 7.37±0.16 | t=1.19 | 0.24 |
| HCO3 (mEq/l) | 22.85±4.60 | 20.64±6.22 | t=1.56 | 0.12 |
| PaCO2 (mmHg) | 38.41±8.74 | 35.78±12.70 | t=0.88 | 0.37 |
t=Test statistics independent t-test, Z=Test statistics Mann-Whitney, χ2=Test statistics Chi-square, **Mean±SD, ***Median (IQR). SD=Standard deviation, IQR=Interquartile range, ALT=Alanine aminotransferase, AST=Aspartate aminotransferase, BS=Blood sugar, Cr=Creatinine, Hb=Hemoglobin, HCO3=Serum bicarbonate, HCT=Hematocrit, K=Potassium, Na=Sodium, PaCO2=Arterial partial pressure of carbon dioxide, WBC=White blood cell, PQ=Paraquat
Table 3.
Prognostic factors in the prediction of mortality using multiple logistic regression analysis
| Variable | B (SE) | OR (95% CI) | P |
|---|---|---|---|
| Dose of poison (mg) | 0.006 (0.003) | 1.006 (1.001-1.01) | 0.04 |
| WBC (103 μL) | 0.11 (0.07) | 1.11 (0.97-1.26) | 0.11 |
| BS (mg/dL) | 0.02 (0.009) | 1.02 (1.01-1.03) | 0.04 |
| AST (IU/L) | 0.04 (0.01) | 1.04 (1.01-1.06) | 0.002 |
| Cr (mg/dL) | 0.23 (0.18) | 1.26 (0.88-1.80) | 0.20 |
B (SE)=Coefficient (standard error), OR=Odds ratio, CI=Confidence ınterval, WBC=White blood cell, BS=Blood sugar, AST=Aspartate transaminase, Cr=Creatinine
DISCUSSION
Acute PQ poisoning is considered as an important health issue in many developing countries due to the lack of specific antidote and effective treatment and the high rate of associated mortality.[18] In the present study, the mortality rate was 36.7% (in 126 patients). Zhou et al.[19] reported that 51.98% of the people they studied died within 1 month. The rates of mortality in Wu et al.,[20] Banday et al.,[21] Goudarzi et al.,[22] and Waikhom et al.[23] studies were 52%, 70%, 73.6%, and 91.7%, respectively. Conversely, Kim et al.[18] reported a lower PQ mortality rate of 9.7% in 2009–2010 and 6.5% in 2012–2013. The severity of PQ intoxication, different approaches to treatment, the difference in capacity levels of hospitals, and the amount of PQ ingested would be considered contributing factors to differing results of poison-related deaths. It has also been seen that many PQ-poisoned patients died even at low PQ concentrations, whereas others with similar levels survived.[24,25] The high rate of mortality due to PQ poisoning is related to its intrinsic toxicity. The ingested dose and quick access to health care, however, are two factors that affect the prognosis of PQ poisoning.[22] Half of the patients in the present study were male, and in other studies, males ranged between 55% and 70%.[7,8,10,26] In Delirrad et al. study, the majority of the patients were male, with a male: female ratio of 1.28.[8]
The mean age of the PQ-poisoned patients in our study was 22.5 years. Similarly, other studies reported the incidence of this poisoning in the youth who comprise the community's most active and reproductive population.[8,10] The consequences of these people's deaths can be adverse for their families and the community, necessitating investigation of possible predisposing causes. Of the participants of the current study, 48.4% committed intentional self-poisoning. Delirrad et al.[8] reported that 89.7% of patients with poisoning were suicide related; the corresponding suicide figures in Amiri et al.[26] and Sabzghabaee et al.[7] studies were 76.9% and 100%, respectively. Overall, suicidal poisoning is much more severe than accidental poisoning due to the consumption of higher doses of poison.[1]
The prevalence of PQ poisoning is most likely due to its easy access in Kerman. Conversely, some countries (e.g., France) have forbidden the sale and use of PQ due to its toxic properties. The economic implication of not using these pesticides in Iran's largest province will result in lower crop yields, thus influencing their widespread use by farmers. Over the past four decades, this province has depended on agricultural revenues as its primary source for economic growth. In addition to being the world's largest producer of pistachios, this region is most prominently known in Iran for its citrus, date palm, vegetables, walnuts, and varieties of grain crops.[27] Subsequently, numerous types of pesticides are utilized due to this wide assortment, as well as the fact that Kerman simply is a vast region of agricultural production. Our study showed that the highest rate of mortality occurred in people presenting with respiratory distress. Consistently, Goudarzi et al.[22] reported that respiratory distress was one of the most potent predictors of death. In addition, Agrawal et al.,[28] Lee et al.,[16] and Sandhu et al.[29] reported respiratory distress to be a main cause of death. Banday et al.[21] reported hypoxemia secondary to pulmonary fibrosis to be the main cause of death in patients with PQ poisoning. Zhou et al.[19] demonstrated that the partial pressure of CO2 in arterial blood (PaCO2) was significantly lower in fatal cases. Irrespective of its route of absorption, lesions due to PQ are seen mainly in the lungs. Lung is the only organ in which the poison is further accumulated in a time-dependent manner, leading to damage to alveolar epithelial cells, hemorrhage, and edema.[1,22,30,31] Lung injury in early stages presents as respiratory distress and later undergoes fibrosis.[22]
The dose of consumed PQ was significantly higher in the nonsurvivors than in the survivors. The primary outcomes of PQ poisoning depend on the consumed dose of this poison. Delirrad et al.,[8] Sabzghabaee et al.,[7] Zhou et al.,[19] and Goudarzi et al.[22] also considered the amount of consumed PQ to be a predictor of mortality. Kavousi-Gharbi et al.[10] study showed, based on poison consumption rate and ROC curve, that the best cutoff point was calculated at 22.5 ml or higher for the 20% solution. Amiri et al.[26] reported the cutoff point of the undiluted poison for death to be 22 ml. Delirrad's study[8] showed, however, that doses of <25 ml were associated with acceptable prognosis. It is noted that PQ used in Iran is in a solution of 20%; however, in the Kerman Province of Iran, from where the participants of the current study originated, it is usually diluted by the people to 2%. Hence, the increased volumes were used. Surprisingly, in our study, the mean cutoff point of the poison for death was a dose of 2250 mg. This may have been due to various early treatments and treatment combinations that were used in the management of our poisoned patients.
According to the current study, increasing the number of different treatment approaches has a positive effect on the prognosis. The primary approaches to the treatment of PQ poisoning are based on the rapid early removal of the poison from the gastrointestinal tract (prevention of absorption), increased release and excretion of toxin from the blood (diuresis), and prevention of lung injury using anti-inflammatory and antioxidant agents.[1] Li et al.[32] reviewed three clinical trials including 164 participants. All three studies compared routine treatments (induction of vomiting, dosing of activated charcoal, and blood purification) with standard treatments alongside glucocorticoids and cyclophosphamide for patients with PQ poisoning. The findings demonstrated that mortality rate decreased by 28% in patients under combination treatment compared to those undergoing routine treatments alone. Consistently, He et al.[33] meta-analysis on efficacy and safety of pulse immunosuppressive therapy with glucocorticoid and cyclophosphamide reported promising findings. In Wu et al.[20] study on 1811 PQ-poisoned people under treatment with hemoperfusion, the three-drug (cyclophosphamide, methylprednisolone, and dexamethasone) treatment was added to the treatment of 42.2% of the patients. That study showed that the combination treatment was associated with the highest survival rate (48%, P = 0.001). Lin et al.[34,35] studies also reported that this combination treatment led to enhanced survival rate.[34,35] These combination treatments involve several mechanisms to treat the patients, including anti-inflammatory, antioxidant, and decrease in lipid peroxidation.[5,30] It is noteworthy mentioning that none of the therapeutic agents can be used for the patients in case of gastrointestinal bleeding.[36]
Multiple logistic regression analysis showed that the mortality rates in PQ-poisoned patients depended on dose of poison, BS, and AST, so that these parameters have significantly increased the prediction of mortality rates of poisoned peoples (P < 0.05), which is consistent with the findings of Liu et al.[37] Moreover, our results clearly showed that nonsurvivors had significantly higher activities of AST and BS counts. Our results suggest that BS and AST have excellent prognostic value for the prediction of mortality and may play a significant role in evaluating the severity of acute PQ poisoning. The findings by Huang et al.[38] showed that by multiple logistic regression analysis, the APACHE II score and peak data of BS at 24 h after admission were capable of predicting inhospital mortality in acute PQ poisoning. Liu et al.[39] also illustrated that the dose of poison and arterial blood lactate was more sensitive in predicting the mortality risk of PQ poisoning. However, in the same study, Zhou et al.[19] stated that neutrophil–lymphocyte ratio (NLR), leukocyte, and neutrophil counts might be useful and simple parameters in predicting the prognosis of PQ poisoning.
In our study, the data were collected from a single tertiary center. Thus, our findings may not be accurately generalized to all settings. Moreover, no data were available concerning the outcomes after hospital discharge, and specifically, death occurring after discharge may have been missed. We retrospectively reviewed medical records for patients with acute PQ poisoning, and incomplete documentation in the medical records may cause unmeasured systematic biases, so individuals with incomplete medical files were excluded. However, the value of this finding was limited, as the volume of poison consumed could only be obtained from the history given by the patients or their relatives, and serum PQ concentration was not available in our clinical setting. To improve the predictive accuracy, larger data sets, novel biomarkers, more sophisticated modeling methods, and enhanced data collection methods are suggested in the future.
According to the findings of the current study, the effects of PQ poisoning primarily depended on the consumed dose, early medical interventions, and subsequent combinations of treatment approaches. The fatal dose (mg) in PQ-poisoned patients was >2250 mg of a 2% dilution of the marketed 20% solution. In addition, the highest number of deaths occurred in people presenting with respiratory distress, despite various treatments. The findings of this study may provide more reliable information for physicians to allow them a more informed perspective on the severity of PQ poisoning and to predict the patients' prognosis more appropriately. In addition, therapeutic guidelines are essential. Finally, ongoing reviews should be consulted, and meta-analyses should be conducted to gain further information on this poisoning, and further clinical trials should be conducted on different available and new or novel treatments.
AUTHORS' CONTRIBUTION
Omid Mehrpour was the overall coordinator. Zohreh Oghabian, June Williams, Mohammad Mohajeri, Samaneh Nakhaee, Saeedeh Shojaeepour, Alireza Amirabadizadeh, Samira Elhamirad, Morteza Hajihosseini, and Borhan Mansouri were responsible for the design and preparation of the manuscript. Zohreh Oghabian, Mohammad Mohajeri, and Saeedeh Shojaeepour conducted the data collection. Alireza Amirabadizadeh and Morteza Hajihosseini designed the study's analytic strategy and helped with the interpretation of the data. Samaneh Nakhaee, Omid Mehrpour, Alireza Amirabadizadeh, and Borhan Mansouri made substantial contributions in drafting the manuscript and revising it critically for important intellectual content. All authors have read and approved the final version of the manuscript.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This research was funded by the Kerman University of Medical Sciences. Hereby, the authors express their deep gratefulness to this organization for granting the funding for the study. Furthermore, our sincere thanks go to all the participants who took part in this project.
REFERENCES
- 1.Dinis-Oliveira RJ, Duarte JA, Sánchez-Navarro A, Remião F, Bastos ML, Carvalho F. Paraquat poisonings: Mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol. 2008;38:13–71. doi: 10.1080/10408440701669959. [DOI] [PubMed] [Google Scholar]
- 2.Chen HW, Tseng TK, Ding LW. Intravenous paraquat poisoning. J Chin Med Assoc. 2009;72:547–50. doi: 10.1016/S1726-4901(09)70426-5. [DOI] [PubMed] [Google Scholar]
- 3.Fathi N, Hossinipanah M, Hajihossini M, Ranjbar A, editors. Effects of Paraquat on Testicular Histomorphometry of Male Rats. Biological Forum– An International Journal. 2015;7:573–5. [Google Scholar]
- 4.Pavan M. Acute kidney injury following paraquat poisoning in İndia. Iran J Kidney Dis. 2013;7:64–6. [PubMed] [Google Scholar]
- 5.Gawarammana IB, Buckley NA. Medical management of paraquat ingestion. Br J Clin Pharmacol. 2011;72:745–57. doi: 10.1111/j.1365-2125.2011.04026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolilekas L, Ghizopoulou E, Retsou S, Kourelea S, Hadjistavrou C. Severe paraquat poisoning. A long-term survivor. Respir Med Extr. 2006;2:67–70. [Google Scholar]
- 7.Sabzghabaee AM, Eizadi-Mood N, Montazeri K, Yaraghi A, Golabi M. Fatality in paraquat poisoning. Singapore Med J. 2010;51:496–500. [PubMed] [Google Scholar]
- 8.Delirrad M, Majidi M, Boushehri B. Clinical features and prognosis of paraquat poisoning: A review of 41 cases. Int J Clin Exp Med. 2015;8:8122–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Dorooshi G, Zolfaghari S, Eizadi-Mood N, Gheshlaghi F. A new treatment approach for acute paraquat poisoning. J Res Pharm Pract. 2018;7:115–6. doi: 10.4103/jrpp.JRPP_18_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kavousi-Gharbi S, Jalli R, Rasekhi-Kazerouni A, Habibagahi Z, Marashi SM. Discernment scheme for paraquat poisoning: A five-year experience in Shiraz, İran. World J Exp Med. 2017;7:31–9. doi: 10.5493/wjem.v7.i1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elenga N, Merlin C, Le Guern R, Kom-Tchameni R, Ducrot YM, Pradier M, et al. Clinical features and prognosis of paraquat poisoning in French Guiana: A review of 62 cases. Medicine (Baltimore) 2018;97:e9621. doi: 10.1097/MD.0000000000009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts DM, Wilks MF, Roberts MS, Swaminathan R, Mohamed F, Dawson AH, et al. Changes in the concentrations of creatinine, cystatin C and NGAL in patients with acute paraquat self-poisoning. Toxicol Lett. 2011;202:69–74. doi: 10.1016/j.toxlet.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahmani AH, Forouzandeh H, Tadayon Khatibi M. Medical management and outcome of paraquat poisoning in Ahvaz, Iran: A hospital-based study. Asia Pac J Med Toxicol. 2015;4:74–8. [Google Scholar]
- 14.Liang LP, Kavanagh TJ, Patel M. Glutathione deficiency in Gclm null mice results in complex I inhibition and dopamine depletion following paraquat administration. Toxicol Sci. 2013;134:366–73. doi: 10.1093/toxsci/kft112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moudgil K, Sreeja R, Rose A, Ponnusankar S. Paraquat induced acute renal failure: A case report. Int Res J Pharm. 2018;9:125–7. [Google Scholar]
- 16.Lee EY, Hwang KY, Yang JO, Hong SY. Predictors of survival after acute paraquat poisoning. Toxicol Ind Health. 2002;18:201–6. doi: 10.1191/0748233702th141oa. [DOI] [PubMed] [Google Scholar]
- 17.Seok S, Kim YH, Gil HW, Song HY, Hong SY. The time between paraquat ingestion and a negative dithionite urine test in an independent risk factor for death and organ failure in acute paraquat intoxication. J Korean Med Sci. 2012;27:993–8. doi: 10.3346/jkms.2012.27.9.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J, Shin SD, Jeong S, Suh GJ, Kwak YH. Effect of prohibiting the use of paraquat on pesticide-associated mortality. BMC Public Health. 2017;17:858. doi: 10.1186/s12889-017-4832-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou DC, Zhang H, Luo ZM, Zhu QX, Zhou CF. Prognostic value of hematological parameters in patients with paraquat poisoning. Sci Rep. 2016;6:36235. doi: 10.1038/srep36235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu WP, Lai MN, Lin CH, Li YF, Lin CY, Wu MJ. Addition of immunosuppressive treatment to hemoperfusion is associated with improved survival after paraquat poisoning: A nationwide study. PLoS One. 2014;9:e87568. doi: 10.1371/journal.pone.0087568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banday T, Bhat Sadaf B, Bhat Sabreen B. Manifestation, complications and clinical outcome in paraquat poison: A hospital based study in a rural area of Karnataka. J Environ Occup Sci. 2014;3:21–4. [Google Scholar]
- 22.Goudarzi F, Armandeh J, Jamali K, Rahmati H, Meisami A, Abbasi H. Mortality analysis of patients with paraquat poisoning treated at two university hospitals in Shiraz, Iran. Asia Pac J Med Toxicol. 2014;3:141–5. [Google Scholar]
- 23.Waikhom R, Vedanti P, Iboton Y, Buddhachandra N, Yohenba K. The role of early haemoperfusion/haemodialysis with steroids, cyclophosphamide, N acetyl cysteine and everolimus in acute paraquat poisoning: İs there still hope? J Evol Med Dent Sci. 2016;5:272–5. [Google Scholar]
- 24.Gil HW, Kang MS, Yang JO, Lee EY, Hong SY. Association between plasma paraquat level and outcome of paraquat poisoning in 375 paraquat poisoning patients. Clin Toxicol (Phila) 2008;46:515–8. doi: 10.1080/15563650701549403. [DOI] [PubMed] [Google Scholar]
- 25.Hong JR, Seok SJ, Jeong DS, Lee SG, Gil HW, Yang JO, et al. Association of the superoxide dismutase (V16A) and catalase (C262T) genetic polymorphisms with the clinical outcome of patients with acute paraquat intoxication. Korean J Intern Med. 2010;25:422–8. doi: 10.3904/kjim.2010.25.4.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amiri AH, Delfan B, Jaferian S. Paraquat poisoning cases treated at Shohada Ashayer hospital of Khorramabad in 2001-2006. Res J Biol Sci. 2008;3:525–9. [Google Scholar]
- 27.Amizadeh M, Safari-Kamalabadi M, Askari-Saryazdi G, Amizadeh M, Reihani-Kermani H. Pesticide exposure and head and neck cancers: A case-control study in an agricultural region. Iran J Otorhinolaryngol. 2017;29:275–85. [PMC free article] [PubMed] [Google Scholar]
- 28.Agarwal R, Srinivas R, Aggarwal AN, Gupta D. Experience with paraquat poisoning in a respiratory intensive care unit in North İndia. Singapore Med J. 2006;47:1033–7. [PubMed] [Google Scholar]
- 29.Sandhu J, Dhiman A, Mahajan R, Sandhu P. Outcome of paraquat poisoning-a five year study. Indian J Nephrol. 2003;13:64–8. [Google Scholar]
- 30.Hedaiaty M, Sabzghabaee AM, Gheshlagh F, Eizadi-Mood N. Paraquat poisoning management in Iran (Isfahan): Devising a protocol. Journal of Advances in Medicine and Medical Research. 2016;16:1–10. [Google Scholar]
- 31.Wang L, Huang J. Paraquat induces acute respiratory distress syndrome by promoting the lung epithelial-mesenchymal transition. Int J Clin Exp Med. 2017;10:657–65. [Google Scholar]
- 32.Li LR, Sydenham E, Chaudhary B, You C. Glucocorticoid with cyclophosphamide for paraquat-induced lung fibrosis. Cochrane Database Syst Rev. 2010;6:CD008084. doi: 10.1002/14651858.CD008084.pub2. [DOI] [PubMed] [Google Scholar]
- 33.He F, Xu P, Zhang J, Zhang Q, Gu S, Liu Y, et al. Efficacy and safety of pulse immunosuppressive therapy with glucocorticoid and cyclophosphamide in patients with paraquat poisoning: A meta-analysis. Int Immunopharmacol. 2015;27:1–7. doi: 10.1016/j.intimp.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 34.Lin JL, Lin-Tan DT, Chen KH, Huang WH. Repeated pulse of methylprednisolone and cyclophosphamide with continuous dexamethasone therapy for patients with severe paraquat poisoning. Crit Care Med. 2006;34:368–73. doi: 10.1097/01.ccm.0000195013.47004.a8. [DOI] [PubMed] [Google Scholar]
- 35.Lin JL, Lin-Tan DT, Chen KH, Huang WH, Hsu CW, Hsu HH, et al. Improved survival in severe paraquat poisoning with repeated pulse therapy of cyclophosphamide and steroids. Intensive Care Med. 2011;37:1006–13. doi: 10.1007/s00134-010-2127-7. [DOI] [PubMed] [Google Scholar]
- 36.Reihani H, Zarmehri B. Multi-organ failure due to paraquat poisoning: Case report and review of literature. Asia Pac J Med Toxicol. 2016;5:98–100. [Google Scholar]
- 37.Liu JY, Liu QM, Guo YJ, Lin DJ. Risk factors for pulmonary fibrosis in patients with paraquat poisoning. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2016;34:520–2. doi: 10.3760/cma.j.issn.1001-9391.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Huang NC, Hung YM, Lin SL, Wann SR, Hsu CW, Ger LP, et al. Further evidence of the usefulness of acute physiology and chronic health evaluation II scoring system in acute paraquat poisoning. Clin Toxicol (Phila) 2006;44:99–102. doi: 10.1080/15563650500514251. [DOI] [PubMed] [Google Scholar]
- 39.Liu XW, Ma T, Li LL, Qu B, Liu Z. Predictive values of urine paraquat concentration, dose of poison, arterial blood lactate and APACHE II score in the prognosis of patients with acute paraquat poisoning. Exp Ther Med. 2017;14:79–86. doi: 10.3892/etm.2017.4463. [DOI] [PMC free article] [PubMed] [Google Scholar]


