INTRODUCTION
Genetic manipulation methods allow fluorescent labeling of virtually any cell type or protein of interest in developing embryos, providing powerful insights into morphogenetic events at cellular and subcellular resolutions. The development of ex vivo embryo culture methods combined with high-resolution imaging provides a strong platform for observing morphogenetic events as they occur within the developing embryo. This protocol details the dissection and immobilization of postimplantation mouse embryos in preparation for live imaging.
RELATED INFORMATION
For additional information, see Live Imaging of Mouse Embryos (Garcia et al. 2011a). Protocols for Preparation of Rat Serum for Culturing Mouse Embryos (Garcia et al. 2011b) and Time-Lapse Imaging of Postimplantation Mouse Embryos (Garcia et al. 2011c) are also available.
MATERIALS
RECIPES: Please see the end of this article for recipes indicated by <R>.
It is essential that you consult the appropriate Material Safety Data Sheets and your institution’s Environmental Health and Safety Office for proper handling of equipment and hazardous materials used in this protocol.
Reagents
-
Mammalian embryo culture medium
The appropriate culture medium depends on the stage of the embryo to be cultured.
For younger embryos (5.5–7.5 days post coitum [dpc]), culture in a 1:1 medium of Dulbecco’s modified Eagle’s medium (DMEM) and heat-inactivated rat serum (prepared as in Preparation of Rat Serum for Culturing Mouse Embryos [Garcia et al. 2011b]).
For embryos 7.5 dpc and beyond, use DMEM/F-12 instead of DMEM, and supplement the culture medium with 10 mM penicillin-streptomycin solution.
Prepare the appropriate medium in a sterile tissue culture hood and filter it through a 0.45-μm filter to sterilize. Always heat and equilibrate the pH of the culture medium by placing it in a tissue culture incubator (5% CO2, 37° C) for at least 1 h before use. Use 2 mL of culture media with the Lab-Tek culture chamber, and use 3-4 mL with the MatTek glass bottom dish.
<R>Mammalian embryo dissection medium
Mice, capable of mating
Equipment
-
Culture chamber
Use either a Lab-Tek II chambered coverglass (Nunc 155379) or a MatTek 35-mm glass bottom culture dish (MatTek P35G-1-10-C).
Dissection instruments
Embryo immobilizer (see Step 8)
Forceps (watchmaker’s Dumont #5)
Incubator, tissue culture
Pasteur pipettes
METHOD
Dissection
Perform timed matings and dissections as described by Nagy et al. (2003), except make every effort to keep the embryos at 37° C during the dissection. We use a heated dissecting stage and preheated media. (For a step-by-step description of embryo dissections, refer to the chapter “Isolation, Culture and Manipulation of Postimplantation Embryos” in Nagy et al. [2003].)
-
Perform timed matings.
The presence of a vaginal plug is taken as 0.5 dpc.
On the day of interest, kill the pregnant mouse according to local animal ethics guidelines. Make a V-shaped incision into the abdominal cavity through the skin and body wall.
Remove the uterine horns and place in the warmed mammalian embryo dissection medium (Fig. 1). To make dissection of embryos easier, separate the uterus into individual embryo segments using small scissors (Fig. 1B,C). Gently peel away the muscle of the uterus (Fig. 1D) and remove the individual embryos, which are surrounded by decidua (Fig. 1E).
Carefully remove the deciduum using Dumont #5 watchmaker’s forceps to expose the embryo (Fig. 1F).
-
Using standard embryo dissection technique (as described by Nagy et al. 2003), remove Reichert’s membrane and the trophoblast layer (Fig. 1G,H). Leave all other extraembryonic tissues intact up until 9.5 dpc. (Fig. 1I).
The ectoplacental cone is usually left on the embryo. The yolk sac can be removed from 8.5 dpc embryos and must be removed from 9.5 dpc embryos. However, if studying vascular morphology of the yolk sac during 8.5–9.5 dpc, the yolk sac must remain intact with the ectoplacental cone still attached.
-
After dissection, use a Pasteur pipette to transfer the embryos to the culture chambers containing prewarmed mammalian embryo culture medium.
Do not culture embryos individually or in overcrowded conditions. They will not develop well. Each embryo requires 0.5–1 mL of heated medium. Limit older embryos (>7.5 dpc) to a maximum of three embryos per chamber.
-
Place the culture chambers in a tissue culture incubator for at least 30 min to allow the embryos to recover from dissection.
Embryos at early stages of development (8.5 dpc and younger) should recover quickly. Embryos obtained at 9.5 dpc are less likely to remain viable for longer than 12 h. In this case, look for a normal heartbeat and blood flow to ensure an optimal culture.
FIGURE 1.
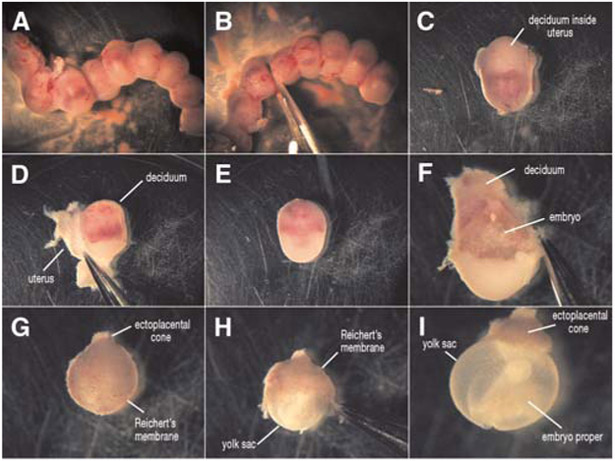
Dissection of an E9.5 mouse embryo. The uterine horn is removed from the mother and separated into individual embryos surrounded by the uterus and deciduum (A–C). The uterus is peeled away (D), leaving the embryo surrounded by a thick decidual layer (E). The deciduum is then removed (F) and the Reichert’s membrane/trophoblast layer (G,H) is carefully separated from the embryo, exposing the intact embryo with the yolk sac available for live imaging (I).
Embryo Immobilization
-
8.
Immobilize the embryos using one of the following techniques to prevent them from drifting out of focus during culturing.
Embryo drift is especially significant at higher magnifications. At stage 8.5 dpc, the yolk sac has expanded and is quite buoyant. This makes the embryos susceptible to small currents in the medium, and the ectoplacental cone, which is heavier than the yolk sac, sinks toward the bottom of the dish, reorienting the embryo. At stage 9.5 dpc, the yolk sac can be removed, allowing the embryo to sit nicely on the bottom of the Lab-Tek chamber.- Orient embryos at all stages, as required, using a suction-holding pipette attached to a micromanipulator.
- Tie a piece of human hair or fine platinum wire in a knot around the ectoplacental cone and immobilize the embryo by “propping” it up.
- Make a wire hook and place it around the ectoplacental cone. Before medium is added, anchor the hook into wax or agarose that has been fixed to the bottom of the chamber.
-
Allow a small piece of decidua to remain attached to the ectoplacental cone when performing dissections.This piece of tissue will allow the embryo to be weighed down to the bottom of the dish, limiting its movement as the embryo develops.
-
If using a MatTek culture dish, allow the ectoplacental cone of the embryo to stick to the slightly raised outer ring where the glass bottom coverslip attaches to the plastic dish.This will immobilize the ectoplacental cone and limit movement of the embryo.If the yolk sac has expanded, immobilize the embryos in a way that avoids any pressure on the yolk sac or the growing embryo. Even slight pressure from a thin layer of agar or Nitex grating overlying the embryo will prevent circulation in the yolk sac.
RECIPES
NOTE: Recipes for reagents marked with the <R> symbol not listed below can be found online at http://www.cshprotocols.org/recipes.
Mammalian embryo dissection medium
DMEM/F-12 (90% v/v)
Heat-inactivated fetal bovine serum (FBS) (10% v/v)
Penicillin-streptomycin antibiotic solution (10 mM)
Prepare fresh medium (50 mL) before dissection and allow it to warm to 37°C in a water bath.
REFERENCES
- Garcia MD, Udan RS, Hadjantonakis A-K, Dickinson ME. 2011a. Live imaging of mouse embryos. Cold Spring Harb Protoc doi: 10.1101/pdb.top104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MD, Udan RS, Hadjantonakis A-K, Dickinson ME. 2011b. Preparation of rat serum for culturing mouse embryos. Cold Spring Harb Protoc doi: 10.1101/pdb.prot5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MD, Udan RS, Hadjantonakis A-K, Dickinson ME. 2011c. Time-lapse imaging of postimplantation mouse embryos. Cold Spring Harb Protoc doi: 10.1101/pdb.prot5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Gertsenstein M, Vintersten K, Behringer R. 2003. Manipulating the mouse embryo: A laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]


