Abstract
Objective
To compare a fixed-dose combination (FDC) of Rifampicin 450 mg, Isoniazid 300 mg, Pyrazinamide 1500 mg and Ethambutol 800 mg (usual care group) and regimen of Rifampicin 200 mg, Isoniazid 300 mg and piperine 10 mg along with Pyrazinamide 1500 mg and Ethambutol 800 mg (intervention care group).
Methods
A randomized, prospective, parallel group study was conducted on newly diagnosed tuberculosis patients. The drugs were given during intensive and continuous phase of treatment to newly diagnosed sputum positive pulmonary tuberculosis. All the patients were subjected to sputum examination, biochemical investigations followed by adverse drug event (ADE) monitoring.
Results
A total of 63 patients completed the study. No significant difference was observed in baseline characteristics of patients between the study groups. At the end of the continuous phase, both the groups showed zero bacteria detection. However, in the intervention group, the rate of sputum conversion was much faster than the usual care group. The rate of increase in SGOT and SGPT was much higher in the usual care group (p < 0.0001) than the interventional group (p < 0.05). Urea and creatinine has also increased from pre-treatment to end visit. The number of patients reported ADEs was less in the intervention care group (22.22%) when compared to the usual care group (36.84%).
Conclusion
Rifampicin 200 mg with piperine 10 mg FDC is compatible with the usual CAT-1 regimen.
Keywords: Tuberculosis, Intensive phase, Continuous phase, Piperine, Rifampicin
1. Introduction
Tuberculosis (TB) is a contagious lung disease affecting 40% Indian population and about 10% will develop active TB during their lifetime. In 2013, it is appraised 9 million people globally develops TB [1]. World Health Organization (WHO) recommends Directly Observed Treatment Short – Course (DOTS) therapy for control of TB. Standard DOTS Category I drugs comprise Isoniazid, Rifampicin, Ethambutol and Pyrazinamide [2]. Poor medication adherence to the treatment regimen is a major cause of treatment failure and of the emergence of drug-resistant TB. The major factors for non-adherence to TB treatment are long term duration of therapy and adverse effects of the drugs etc.
Recent data show that current pharmacotherapy for TB is inadequate to achieve therapeutic drug serum levels [3], [4]. Therefore, there is a necessity to identify a new molecule for the control of TB. The fixed drug combination (FDC) of Rifampicin, Piperine and Isoniazid is available for the treatment of tuberculosis. By adding Piperine, the dose of rifampicin is reduced to 200 mg which possesses to achieve the therapeutic serum level of Rifampicin as of 450 mg [5]. Since the dose of Rifampicin is reduced and the toxicity of Rifampicin is also reduced and thereby better patient compliance is expected. Thus, defaulter rate and development of drug resistance can be reduced. Many studies have considered this point and evaluated the relationship of Piperine and Rifampicin. However, limited data are available in clinical practice [6], [7], [8]. The present study was undertaken to find out the superiority of piperine included FDC in TB management.
2. Methods
2.1. Study design
The study was designed as a randomized, prospective, open-label, parallel group study. Human Institutional Ethics Committee approval was obtained prior to the commencement of the study (481/IEC/2013) and the study was registered in the Clinical Trial Registry – India (CTRI/2015/01/005475). The study was conducted in two centers (1) Department of Pulmonary Medicine, SRM Medical College Hospital and Research Center, Kattankulathur, Kanchipuram district, (2) Government Hospital – Kanchipuram, Thiruvallur district, Tamil Nadu, India.
2.2. Study criteria
Patients were aged between 18 and 60 years, either gender, without co-morbidities and newly diagnosed sputum positive pulmonary tuberculosis, according to RNTCP guidelines and willing to provide informed and written consent were enrolled for the study. A patient with a history of known cases of pulmonary tuberculosis, extra- pulmonary, severe alcoholic, cardiac disorders, HIV positive, pregnant women and lactating mothers, intolerance to FDC for the treatment of tuberculosis and voluntary withdrawal were excluded from the study.
2.3. Treatments
Patients satisfying above study criteria were enrolled in the study. Clinical information relevant for the study was collected from the patients, health care professionals, necessary records and as well as from patient representatives in few cases. Patients were randomized into two groups, namely Usual Care (UC) and Intervention Care (IC) groups. UC group patients received CAT – I regimen (Rifampicin 450 mg, Isoniazid 300 mg, Pyrazinamide 1500 mg and Ethambutol 800 mg) in intensive phase. Isoniazid 300 mg and Rifampicin 450 mg in the continuous phase. IC group patients received Rifampicin 200 mg, Isoniazid 300 mg and piperine 10 mg along with Pyrazinamide 1500 mg and Ethambutol 800 mg in intensive phase. Isoniazid 300 mg and Rifampicin 200 mg and piperine 10 mg in continuation phase.
2.4. Clinical investigation
Both the group patients were subjected to sputum examination and biochemical investigations. The primary efficacy evaluation was based on assessing sputum conversion. The standard bacteriological assessment was performed by a sputum acid fast bacillus (AFB) test. Ziehl-Neelsen's staining of sputum smear was performed. Grading of slides in AFB microscopy was done according to Revised National Treatment Control Program (RNTCP).
Assessment of liver, kidney functions and adverse drug event monitoring were considered to be the secondary tolerability outcome measures. The tolerability of the liver is very important during the treatment of tuberculosis. Rifampicin is a potent drug and the risk of liver damage is high. In order to monitor the tolerability and functionality of the liver, Serum Glutamic Oxaloacetic Transaminase (SGOT) and Serum Glutamic Pyruvic Transaminase (SGPT) parameters were taken into consideration. In the current study, along with liver tolerability, kidney tolerability was also considered. The chances of renal impairment are seen in many studies during the chemotherapy of tuberculosis. To monitor the tolerability and normal functionality of kidney, serum urea and creatinine levels was measured. All the assessments were performed at baseline, End of Intensive Phase (END IP) i.e. on Day 60, Middle of Continuous Phase (MID CP) i.e. on Day 120 and at the End of Continuous Phase (END CP) i.e. on Day 180. On every follow-up, patient's medication adherence and adverse drug events (if any) were monitored. During the study period, patients were assessed for adverse events (nature, severity and casual relationship), which were further classified according to their type, severity and possible associations with the treatments.
2.5. Statistical analysis
Quantitative variables were expressed as mean (SD). The probability value less than 0.05 was considered for statistical significance. Paired t test was used for the comparisons within the groups. Student t test was used to compare the scores between the groups using GraphPad Prism Version 4.03. Per protocol analysis has been performed.
3. Results
A total of 72 patients attended the screening phase for sputum positive newly diagnosed pulmonary tuberculosis, out of which 66 patients met the study criteria. The patients who got enrolled after giving written informed consent were randomized into two groups. In the UC group, out of 34 patients, 32 patients completed the study and in the IC group, out of 32 patients, 31 patients completed the study. Finally, 63 patients completed all the follow-up visits (Fig. 1).
Fig. 1.
CONSORT diagram for flow of patients.
Baseline characteristics like age, body mass index (BMI), gender, sputum conversion rate, liver and renal function tests are shown in Table 1. No significant difference was observed with respect to baseline characteristics of patients between the study groups (p > 0.05).
Table 1.
Baseline characteristics of the patients.
| Demographic variables | Usual care | Intervention care |
|---|---|---|
| Age (years) | 43.89 (13.05) | 40.0 1 (11.44) |
| BMI (kg/m2) | 25.62 (1.86) | 26.15 (1.33) |
| Gender, n (%) | ||
| Male | 18 (56.25) | 16 (51.61) |
| Female | 14 (43.75) | 15 (48.39) |
| Sputum conversion rate | 1.947 (0.84) | 1.912 (0.87) |
| LFT (U/L) | ||
| SGOT | 19.00 (4.02) | 21.11 (3.53) |
| SGPT | 20.05 (3.17) | 22.58 (4.41) |
| RFT (mg/dl) | ||
| Urea | 18.21 (2.32) | 20.02 (5.35) |
| Creatinine | 0.64 (0.16) | 0.54 (0.18) |
BMI: Body Mass Index; LFT: Liver Function Test; SGOT: Serum Glutamic Oxaloacetic Transaminase; SGPT: Serum Glutamic Pyruvic Transaminase; RFT: Renal Function Test
Data expressed as Mean (SD), No significant differences were found in the baseline characteristics between the groups.
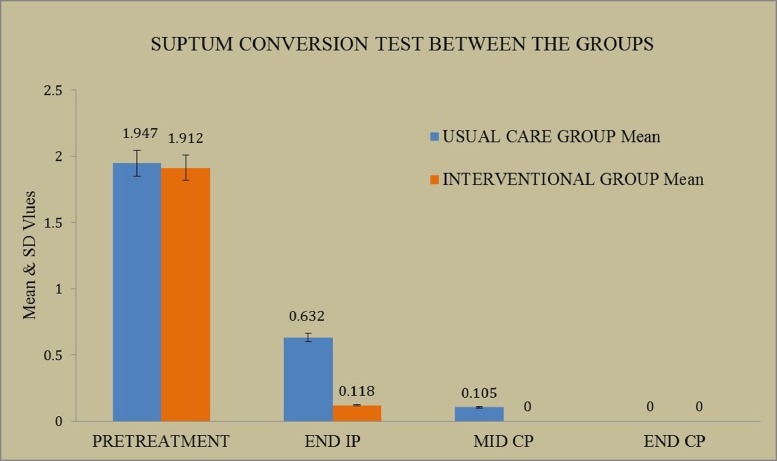
Measurement of sputum conversion rate was the primary efficacy outcome. The mean (SD) values of UC and IC group patients during baseline were found to be 1.947 (0.84) and 1.912 (0.87) respectively. The same on END IP was found to be 0.632 (0.91) and 0.118 (0.48). During MID CP, the value of UC group was 0.105 (0.31) whereas in the intervention group it was zero. During END CP period, both the groups showed zero bacteria detection (Fig. 2).
Fig. 2.
Comparisons of sputum conversion test between usual and intervention care groups.
In IC group, only 1 patient has not completed their intensive phase by day 60, since their sputum conversion test was positive, due to patient non-compliance, whereas in UC group, 8 patients could not complete intensive phase by day 60 in spite of their regular medication. By the mid of the continuous phase, sputum AFB test is negative in all the patients of IC group where as in UC group 2 patients were left with positive results. This may reflect that in terms of bacteriological success, IC group patients were found to be clinically as well as statistically higher in sputum conversion rate when compared to UC group patients.
Assessment of Liver Function Tests (LFT) and Renal Function Tests (RFT) values were the secondary measures to assess the tolerability of liver and kidney. From Table 2, it is shown that, the base value (pre-treatment) and final review value (END CP) for SGOT and SGPT have significantly increased to higher values, but when we observe both values, the rate of increase is much higher in the UC group (p < 0.0001) than the Interventional group (p < 0.05) indicating that Rifampin 200 mg with Piperine 10 mg (FDC) is safer to liver than the Rifampin 450 mg (control group regimen).
Table 2.
Within group analysis of LFT and RFT parameters.
| Parameters | DAY 0 |
END IP |
MID CP |
END CP |
||||
|---|---|---|---|---|---|---|---|---|
| UC | IC | UC | IC | UC | IC | UC | IC | |
| SGOT | 19.00 (4.02) | 21.11 (3.53) | 26.36 (2.77)c | 24.82 (3.57) | 30.10 (3.41)c | 24.76 (4.29) | 32.63 (4.24)c | 25.58 (4.25)a |
| SGPT | 20.05 (3.17) | 22.58 (4.41) | 26.68 (2.53)c | 26.17 (4.00) | 29.78 (2.71)c | 27.64 (2.76)b | 31.94 (2.95)c | 26.05 (5.01) |
| Urea | 18.21 (2.32) | 20.02 (5.35) | 21.15 (2.58) | 23.23 (5.11) | 22.00 (2.82) | 22.58 (4.55) | 23.0 (2.72)b | 23.29 (5.09) |
| Creatinine | 0.64 (0.16) | 0.54 (0.18) | 0.65 (0.12) | 0.69 (0.27) | 0.73 (0.12) | 0.66 (0.16) | 0.77 (0.12) | 0.66 (0.15) |
SGOT: Serum Glutamic Oxaloacetic Transaminase; SGPT: Serum Glutamic Pyruvic Transaminase; UC: Usual Care; IC: Internvention Care; END IP: End of Intensive Phase; MID CP: Middle of Continuous Phase; END CP: End of Continuous Phase.
p < 0.05.
p < 0.01.
p < 0.0001, Data expressed as Mean (SD).
Urea and creatinine has also increased from pre-treatment to END CP (Table 2). However, the significance is shown only in urea level in the UC group (p < 0.01). No significant increase was observed in creatinine level in both groups. It is evident from Table 3 that IC group is safer to liver and kidney. A significant change is observed in SGOT and SGPT parameters in between group analysis (Table 3) and no significant difference with respect to urea and creatinine.
Table 3.
Between group analysis of LFT and RFT parameters.
| Parameters | Mean difference | 95% CI |
|
|---|---|---|---|
| Lower | Upper | ||
| SGOT | 7.050 | 2.842 | 11.260 |
| SGPT | 5.890 | 1.682 | 10.100 |
| Urea | 0.290 | −4.498 | 3.918 |
| Creatinine | 0.110 | −4.098 | 4.318 |
CI: Confidence Interval; SGOT: Serum Glutamic Oxaloacetic Transaminase; SGPT: Serum Glutamic Pyruvic Transaminase.
Tolerability assessment is also performed by monitoring adverse drug events (ADEs). ADEs gauged during six month treatment period are summarized in Table 4. A total of 5 different ADEs was reported by the study patients. All the ADEs were of minor clinical importance in both the groups. The number of patients reported ADEs was less in the IC group (22.22%) when compared to UC group (36.84%). All the suspected adverse events were assessed for causality using Naranjo's algorithm and it was confirmed by the panel of experts that most of the ADEs were possibly related to the study medications. No patients withdrew their consent from the study due to adverse events.
Table 4.
Adverse Drug Events in study patients.
| Organ system involved | Type of ADEs | Usual care | Intervention care | Total ADEs |
|---|---|---|---|---|
| Gastrointestinal | Nausea | 1 | 1 | 2 |
| Vomiting | 2 | 1 | 3 | |
| Diarrhea | 1 | – | 1 | |
| Abdominal pain | 2 | 1 | 3 | |
| Cutaneous | Itching | 1 | 1 | 2 |
| Total | 7 | 4 | 11 |
ADEs: Adverse Drug Events.
4. Discussion
Tuberculosis (TB), a complex socioeconomic disease, remains a global health threat in India and accounts nearly one third of prevalent cases worldwide. TB remains as a major cause of morbidity and mortality. TB is a curable disease if diagnosed and treated properly with anti-tuberculosis therapy (ATT). In addition to benefits, poor and variable bioavailability of ATT drugs produces a challenge to successful anti-tubercular program [9].
Rifampicin is the first line drug of choice in the chemotherapy of TB since 1960s and it is a potent inducer of CYP-450 enzymes. This leads to a number of adverse drug interactions [10]. In addition, this results in over expression of P-glycoprotein-drug efflux pumps [11]. Both these effects lead to sub-therapeutic levels, which are seen usually after continuous use of rifampicin for 10–14 days [12]. Apart from this, conventional rifampicin is poorly tolerable. Nausea, vomiting, upper abdominal discomfort, hepatotoxicity and gastrointestinal are most common adverse effects of rifampicin [13].
In order to overcome the above discussed limitations of conventional rifampicin, a new formulation of rifampicin was developed. A boosted rifampicin containing a fixed dose combination (FDC) product (Rifampicin 200 mg + Piperine 10 mg) that has been approved in India for use as an anti-tubercular drug. Piperine is an alkaloid obtained from black pepper (Piper nigrum Linn) and long pepper (Piper longum Linn). It inhibits auto-induction processes in the liver and thereby maintains the same level of rifampicin throughout therapy [14].
Preclinical studies carried out in M. tuberculosis infected mice model reported that the Th-1 up-regulatory effect of piperine can augment the therapeutic efficacy outcome of Rifampicin treatment. In addition, in-vitro studies reported that piperine enhances the anti-mycobacterial activity of Rifampicin and significantly prolong its post antibiotic effect [15].
Covic et al. [16] examined the relationship between serum concentration levels of piperine and Rifampicin in pulmonary tuberculosis patients. They found a significant increase in Cmax (10.2 µg/ml from 8.15 µg/ml) and AUC (81 µg.h/ml from 47.49 µg.h/ml) of Rifampicin. They concluded that administration of piperine along with Rifampicin out-turn in increased bioavailability of Rifampicin.
A clinical trial conducted in newly diagnosed smear positive pulmonary tuberculosis patients, reported that the plasma levels of rifampicin declines after two weeks of administration of standard Rifampicin (450 mg). Nevertheless, the plasma levels of piperine with rifampicin (200 mg) maintains after two weeks of administration [17], [18].
In the present study, though the values of SGOT, SGPT between the groups seem meager in magnitude, a statistically significant difference was observed between the groups. The study concludes that intervention care group was found to be compatible with the usual care group by not only considering the SGOT, SGPT statistical significance, but also the faster sputum conversion rate, the protective effect of liver and reduced number of ADRs in intervention care group patients.
The outcome of the present study can be of immense clinical utility while managing patients of tuberculosis with liver and kidney abnormalities. Limited sample size, short duration of the period and the open label study design are limitation of the study. Since we get promising result, we continue this study for longer duration with large population to authenticate the observations. We also planned to examine the level of rifampicin in the blood and cavitary lung lesion of patients.
Conflict of interest
None.
Ethical statement
Human Institutional Ethics Committee approval was obtained prior to the commencement of the study and the study was registered in the Clinical Trial Registry – India (CTRI/2015/01/005475).
Acknowledgments
Dr Nageswari AD would like to thank Cadila Pharmaceuticals for providing the drug samples. Authors are thankful to the management of SRM Institute of Science and Technology, Deemed University for proving necessary facilities including PFT to carry out the study.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jctube.2018.07.002.
Contributor Information
A.D. Nageswari, Email: dradnageswari@yahoo.com.
M.G. Rajanandh, Email: mgrpharm@gmail.com.
Appendix. Supplementary materials
References
- 1.World Health Organization. Global tuberculosis report. WHO/HTM/TB/2014.420. WHO, 2014.
- 2.Qureshi D, Kausar H. Adverse effects of first line antituberculosis drugs in patients on DOTS CAT-1 under revised national tuberculosis control programme (RNTCP) IJBPAS. 2013;2:2267–2280. [Google Scholar]
- 3.Mehta JB, Harsha S, Ryland P. Utility of rifampin blood levels in the treatment and follow-up of active pulmonary tuberculosis in patients who were slow to respond to routine directly observed therapy. Chest. 2001;120:1520–1524. doi: 10.1378/chest.120.5.1520. [DOI] [PubMed] [Google Scholar]
- 4.Navin A, Bedi KL. Bioenhancers: revolutionary concept to market. J Ayurveda Integr Med. 2010;1:2. doi: 10.4103/0975-9476.65073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurpreet KR, Jagdev SK, Rajkumar Bioenhancers from mother nature and their applicability in modern medicine. Int J Appl Basic Med Res. 2011;1:5–10. doi: 10.4103/2229-516X.81972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jindani A, Nunn AJ, Enarson DA. Two 8-month regimens of chemotherapy for treatment of newly diagnosed pulmonary tuberculosis: international multicenter randomized trial. Lancet. 2004;364:1244–1251. doi: 10.1016/S0140-6736(04)17141-9. [DOI] [PubMed] [Google Scholar]
- 7.Sandeep S, Manoj K. Piperine as an inhibitor of Rv1258c, a putative multidrug efflux pump of Mycobacterium tuberculosis. J Antimicro Chemother. 2010;65:1694–1701. doi: 10.1093/jac/dkq186. [DOI] [PubMed] [Google Scholar]
- 8.Helen M, Peter W, Andre B. Determinants of Rifampin, Isoniazid, Pyrazinamide, and Ethambutol Pharmacokinetics in a Cohort of Tuberculosis Patients. Antimicrob Agents Chemother. 2006;50:1170–1177. doi: 10.1128/AAC.50.4.1170-1177.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chia HC, Yen FC. Acute kidney injury due to anti-tuberculosis drugs: a five-year experience in an aging population. BMC Infect Dis. 2014;14:23. doi: 10.1186/1471-2334-14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horita Y, Doi N. Comparative study of the effects of antituberculosis drugs and antiretroviral drugs on cytochrome P450 3A4 and P-glycoprotein. Antimicrob Agents Chemother. 2014;58:3168–3176. doi: 10.1128/AAC.02278-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jawahar MS. Current trends in chemotherapy of tuberculosis. Indian J Med Res. 2004;120:398–417. [PubMed] [Google Scholar]
- 12.Babalik A, Babalik A, Mannix S. Therapeutic drug monitoring in the treatment of active tuberculosis. Can Respir J. 2011;18:225–229. doi: 10.1155/2011/307150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhingra VK, Rajpal S, Aggarwal N. Adverse drug reactions observed during DOTS. J Commun Dis. 2004;36 251-229. [PubMed] [Google Scholar]
- 14.Rajanandh MG, Nageswari AD, Ramasamy C. Side effects of antitubercular drugs on directly observed treatment strategy under revised national tuberculosis control programme in a teaching hospital. Global J Pharmacol. 2012;6:29–32. [Google Scholar]
- 15.Vriese AS, Robbrecht DL, Vanholder RC, Vogelaers DP, Lameire NH. Rifampicin-associated acute renal failure: pathophysiologic, immunologic, and clinical features. Am J Kidney Dis. 1998;31:108–115. doi: 10.1053/ajkd.1998.v31.pm9428460. [DOI] [PubMed] [Google Scholar]
- 16.Covic A, Goldsmith DJ, Segall L. Rifampicin-induced acute renal failure: a series of 60 patients. Nephrol Dial Transpl: Off Publ European Dial Transpl Assoc European Renal Assoc. 1998;13:924–929. doi: 10.1093/ndt/13.4.924. [DOI] [PubMed] [Google Scholar]
- 17.Muthukumar T, Jayakumar M, Fernando EM, Muthusethupathi MA. Acute renal failure due to rifampicin: a study of 25 patients. Am J kidney Dis: Off J Nat Kidney Found. 2002;40:690–696. doi: 10.1053/ajkd.2002.35675. [DOI] [PubMed] [Google Scholar]
- 18.Schubert C, Bates WD, Moosa MR. Acute tubule interstitial nephritis related to anti-tuberculous drug therapy. Clin Nephrol. 2010;73:413–419. doi: 10.5414/cnp73413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.