Abstract
Background
The link between tuberculosis (TB) and malnutrition has long been recognized. Vitamin A and zinc deficiencies may reduce the host defenses and increase the risk for diseases.
Objective
The aim of the present study was to estimate the difference in vitamin A and zinc deficiencies together with dietary intakes among pulmonary TB patients and controls.
Materials and methods
A case-control study design was employed to undertake this study in North Shewa, Ethiopia. Sputum smear examination, high-performance liquid chromatography (HPLC), flame atomic absorption spectrometry (FAAS), and enzyme-linked immunosorbent assay (ELISA) were used to analyse acid fast bacilli (AFB), vitamin A, zinc, and C-reactive protein (CRP), respectively. Dietary intake was assessed using a 24-h recall questionnaire. Mann–Whitney U test, Kruskal–Wallis test, Chi-square, odds ratio (OR), Spearman correlation, and multinomial logistic regression model were computed for data analyses.
Results
In this study, 62 TB cases and 59 controls were included. The proportions of vitamin A deficiency among TB cases and controls were 56.4% and 39.0%, respectively. All TB cases and 92.5% controls were zinc deficient. The odds of TB cases with deficiencies of vitamin A and zinc was 2.3 (95% CI: 1.1 to 4.8)times more likely as compared to the controls. More than 80% of all participants had below average fulfilment of energy and vitamin A intakes.
Conclusion
Vitamin A and zinc deficiencies are severe problems among TB patients. Moreover, undernutrition determines the development of TB. Therefore, the management programs of TB need to address the problems of vitamin A and zinc deficiencies together with protein-energy malnutrition.
Keywords: Vitamin A, Zinc, Tuberculosis, Ethiopia
Abbreviations: AFB, Acid Fast Bacilli; BMI, Body Mass Index; CI, Confidence Interval; CRP, C-Reactive Protein; DDS, Dietary Diversity Score; ELISA, Enzyme-Linked Immunosorbent Assay; FAAS, Flame Atomic Absorption Spectrometry; HPLC, High Performance Liquid Chromatography; IQR, Inter Quartile Range; IZiNCG, International Zinc Nutrition Consultative Group; MUAC, Mid Upper Arm Circumference; SD, Standard Deviation; TB, Tuberculosis; VIF, Variance Inflation Factor
Introduction
Tuberculosis (TB) is an ancient disease caused by Mycobacterium tuberculosis. Despite the applied efforts for its control, TB is still one of the major public health challenges. According to the global TB report in 2017, 10.4 million TB cases and 1.67 million TB deaths occurred worldwide in 2016. Of whom, about 70% of the new cases and more than 85% of the deaths were reported from South East Asia and Africa [1]. The report also showed that Ethiopia is one of the top 30 TB burden countries globally with 182,000 new cases and 30,000 deaths in 2016 [1].
The link between TB and malnutrition has long been recognized; malnutrition may predispose people to the development of clinical disease, and TB can contribute to malnutrition [2]. Malnourished people are also susceptible to a new infection since their immune systems are debilitated [3]. As key contributors to immune function and cytokine kinetics, micronutrients such as vitamin A and zinc play a major role in combating TB [4].
Vitamin A is a fat-soluble vitamin which is needed in small quantities for several metabolic activities in the body [5]. Vitamin A and its active metabolites are important for growth and differentiation of a variety of cells, mainly in mucosa-associated epithelia [6], T and B lymphocytes, macrophages, and generation of antibodies [7]. Zinc is also an essential trace element with diverse physiologic and metabolic functions [8] such as maintaining immunological integrity, cellular immunity, and antioxidant activity [9]. Because of the absence of specialized zinc storage in the body, a daily intake is required to achieve its steady-state [10]. In the prechemotherapeutic era, cod liver oil rich in vitamin A was used regularly for the treatment of TB to strengthen the host defense system [11].
Vitamin A and zinc deficiencies are common features of pulmonary TB. Deficiencies of both micronutrients can reduce host defenses and immune responses [12]. A study done in Rwanda revealed that 29% of adult TB patients had serum vitamin A levels consistent with deficiency (less than 0.7 µmol/L) [13]. Deficiency in zinc (below 10.7 µmol/L) is thought to be one of the primary causes of morbidity in developing countries and yet little is known about the status of the world [14].
Until recent time, many of the epidemiological studies conducted on vitamin A and zinc deficiencies in Ethiopia were focusing on children and pregnant women [5], [15], [16]. However, there is a paucity of information on the magnitude of the deficiencies of these micronutrients in TB patients. The available studies were very few [17], [18] and conducted long years ago in some pocket areas of North West part of Ethiopia. Therefore, the present study was designed to estimate the difference in vitamin A and zinc deficiencies together with dietary intakes among pulmonary TB patients and non-TB controls.
Materials and methods
Study design, area and population
A facility-based case-control study was conducted in North Shewa Zone of Amhara Regional State, Central Ethiopia. In this study, one referral hospital and five health centers were involved. The study population included TB patients and non -TB controls who were living in the same geographic area. All TB cases who visited the health facilities between March and August 2015 were recruited.
Selection of TB cases and controls
The diagnosis of TB was performed microbiologically and radiologically as per the national standard diagnostic algorithm of Ethiopia [19]. As per the diagnostic algorithm, smear positive pulmonary TB is defined by at least two initial sputum smear examinations positive for acid fast bacilli (AFB) by direct microscopy, or a patient with one initial smear examination positive for AFB by direct microscopy and culture positive, or a patient with one initial smear examination positive for AFB by direct microscopy and radiographic abnormalities consistent with active TB as determined by a clinician.
Smear-negative pulmonary TB is also defined by a patient having symptoms suggestive of TB with at least three initial smear examinations negative for AFB by direct microscopy, and no response to a course of broad-spectrum antibiotics; radiological abnormalities consistent with pulmonary TB; decision by a clinician to treat with a full course of anti-TB; or a patient whose diagnosis is based on culture positive for M. tuberculosis [19], [20].
In this study, both smear positive and negative pulmonary TB cases who were willing to participate in the study were included. All selected TB cases were used as a point of contact to recruit controls. Individuals who were apparently healthy, non-TB, living in the same geographic areas with TB cases and interested in participating in the study were selected as controls. As vitamin A and zinc concentrations in the serum are affected by many physiological and pathological states and drugs, TB cases and controls with pregnancy, lactation, chronic or degenerative diseases, and who were taking vitamin A and zinc supplements, corticosteroid drugs and oral contraceptives were excluded from the study.
Duration of treatment
The treatment of TB lasts for a total of six months for newly diagnosed TB patients or for those who had taken previously the anti-TB drugs for less than one month as per the standard treatment guidelines for the general hospital in Ethiopia. The treatment regimen consists of 2 months treatment with Rifampicin (R), Isoniazid (H), Pyrazinamide (Z) and Ethambutol (E) during the intensive phase, followed by four months with Rifampicin and Isoniazid in the continuation phase (2RHZE/4RH) [21].
Data collection
A structured questionnaire, comprised of socio-demographic characteristics, health status and diet factors, was prepared, translated into Amharic (the local language) and administered to each of the study participant. Medical records were also used to collect clinical characteristics.
Anthropometric measurements
Standardized procedures were employed to measure body weight, height, and mid upper arm circumference (MUAC). All study participants were weighed while wearing light clothes using an electronic platform weighing scale to the nearest 0.1 kg. Height was measured to the nearest 0.1 cm by means of a seca stadiometer.
Body mass index (BMI) was calculated as body weight (kg) divided by height (m) squared (kg/m2). BMI values of 18.5, 17.0, and 16.0 kg/m2 were used as the cut-off values below which patients were classified as having mild, moderate, or severe malnutrition [21]. MUAC was measured half way between the olecranon and acromion processes of the left arm using a flexible non-stretch measuring tape to the nearest 0.1 cm while the arm is hanging relaxed, without compressing the tissues. MUAC less than 23 cm for male and 22 cm for female is used to define undernutrition as per FANTA III [22].
Dietary intake assessment
The dietary intake was assessed by means of a 24-h recall to estimate the intake of energy, protein, vitamin A and zinc. Each 24-h recall was performed using a standardized four-stage protocol [23], [24]. First, a complete list of all food and beverages consumed over the 24 h was obtained. Second, detailed descriptions of the food and beverages consumed, including the cooking methods and brand names were recorded, together with the time and place of consumption. Third, estimates of the portion sizes of all consumed food items were determined by referring to household measuring and serving utensils (e.g., spoons, plates or cups), and food packages. Finally, the food recall was reviewed to ensure that all items had been recorded correctly. The amount of nutrient intake from dietary recalls was calculated using nutrisurvey software (Dr.Juergen Erhardt SEAMEO-TROPMED RCCN-University of Indonesia copy right© 2007).
After the dietary intake assessment, the individual dietary diversity score (DDS) was calculated as the number of food groups consumed over the 24-h recall. These food groups were based on the guidelines for measuring the household and individual dietary diversity. The score for individual diet diversity goes from 0 to 9 [25], [26]. Considering four food groups as the minimum acceptable dietary diversity, the individual DDS less than four was classified as having a poor dietary diversity [26], [27].
Blood collection and serum separation
After overnight fasting, 10 mL of venous blood was withdrawn from antecubital fossa vein into a non-heparinized vacutainer tube between 8:00 and 10:00am at the selected health facilities. Blood samples were allowed to clot for about 1 h in the dark and subjected to centrifugation at 4000 rpm for 10 min at room temperature. Samples with visible haemolysis were discarded.
The sera were separated immediately into aliquots of sterile Eppendorf tubes by means of sterile Pasteur pipettes and stored at −20 °C in the laboratory of the health facility. The sera were later transported in the ice box to Armauer Hansen Research Institute (AHRI) where they were stored at −80 °C until analysis. Aliquots of the sera were transferred to Ethiopian Public Health Institute (EPHI) for the analysis of serum vitamin A concentration and to Natural Sciences College of Addis Ababa University for the determination of the serum zinc concentration.
Measurements of vitamin A, zinc and C-reactive protein (CRP)
Vitamin A
Serum vitamin A concentration was determined using high performance liquid chromatography (HPLC) as explained in the method of Arroyave et al. [28] with slight modifications. In brief, 100 µL of ethanol and an equal volume of retinyl acetate (reconstituted in ethanol) as internal standard were added into a 2 mL Eppendorf tube containing 100 µL of serum sample. The solution was then mixed for 1 min using vortex mixer. In the first round, 750 µL of n-hexane was added into the solution, vortex mixed for 1 min and centrifuged at 3000 rpm for 10 min. The supernatant was transferred into a new Eppendorf tube. In second round, 750 µL of n-hexane was added and the whole steps were repeated. The pooled supernatant was then evaporated into dryness under a steam of nitrogen, reconstituted in 200 µL of methanol, and transferred into the injection vial.
A Shimadzu HPLC system (Shimadzu, Tokoyo) which composed of a reverse phase Supelco LC-18 column (250 × 4.6 mm, 5 µm particle size) and UV–vis detector was used to separate and detect the retinol at 325 nm with a column temperature set at 40 °C. Series of standards (having a concentration of 60, 40, 20, 10 and 5 µg/dL) and samples were loaded into the autosampler tray of the HPLC. Methanol was used as a mobile phase with injection volume of 40 µL, flow rate of 1 mL/min and retention time of 15 min.
Retinol was determined by comparing the retention times with the external standard and quantified based on externally drawn calibration curve using the series concentrations of standards. All extraction procedures were performed under dim light to avoid oxidation of the compound. Vitamin A concentration below 0.7 µmol/L was considered as deficient.
Zinc
Serum zinc concentration was determined by means of flame atomic absorption spectrometry (FAAS) with a micro-sampling technique in AA-7000 Atomic Absorption Spectrophotometer (Shimadzu, Japan) using the method described in Sepehri et al. [29]. In brief, 10 mL of concentrated nitric acid was added to 1 mL of serum in a beaker and heated for 3 h, below boiling point, on a hot plate. When the volumes of the samples reduced to about one-third, 5 mL of 30% hydrogen peroxide solution was added. The samples were further heated almost to dryness at the same temperature. Finally, the residues were dissolved in 50 mL of 1% nitric acid and filtered. The prepared samples were transferred into 50 mL polyethylene tubes for zinc analysis. The concentration of 10.7 µmol/L was used as a cut-off value below which was considered as zinc deficient.
C-reactive protein (CRP)
CRP was measured at AHRI, Ethiopia using enzyme-linked immunosorbent assay (ELISA) (Human CRP ELISA Kit, HK 358, Hycult biotech) method according to the manufacturer's instruction as indicated in the manual of the kit. For each TB patient, CRP was measured in duplicate. The mean value ≥10 mg/L was considered as CRP positive.
Ethical consideration
This study is a part of our project entitled ‘’effect of micronutrients on the treatment outcome of TB’’, which has been ethically approved by the ethical approval committee of AHRI- ALERT (All Africa Leprosy Rehabilitation and Training) Centre. Supportive letters were also obtained from the zonal and districts’ health bureau of Amhara Regional State. This study was carried out in accordance with the principle of Helsinki declaration. After explaining the purpose and objective of the study, written informed consent was obtained from each of the study participant.
Statistical analysis
Statistical analysis was conducted using IBM SPSS version 23 statistical program. All continuous data were checked for a normal distribution using a Shapiro-Wilk test. Descriptive statistics, including frequency, proportion, and median (IQR-Inter Quartile Range) were employed to summarize the study variables. Mean and standard deviation (SD) were used to describe concentrations of vitamin A and zinc, BMI, and MUAC. Odds ratio (OR) together with 95% confidence interval (CI) was computed to assess the strength of association.
The significance of the difference in continuous and categorical variables between groups was compared using Mann–Whitney U test and Chi-square test, respectively. Kruskal–Wallis test was used to assess the difference in the serum vitamin A and zinc concentrations of TB patients at different duration of treatment. The association between MUAC, BMI, and DDS was examined using Spearman correlation test.
Bivariate analysis was done to explore the crude association between different predictor variables and TB. To control for possible confounding factors and identify factors that are independently associated with TB, multinomial logistic regression analysis was performed for those variables with p-value of less than 0.2 in the bivariate analysis. The major assumptions of logistic regression model were multicollinearity and interaction among independent variables. The absence of multicollinearity was checked using variance inflation factor (VIF)/tolerance and the fitness of logistic regression model was checked using Hosmer-Lemeshow statistical test. Unless specified, p-value < 0.05 was considered as statistically significant.
Results
Socio-demographic characteristics
The present study was conducted on 62 TB patients and 59 controls. The median (IQR) age was 26 (14) years with the minimum and maximum age of 14 and 77 years. Most of the participants were Orthodox Tewahido Christians in religion (89.3%) and farmers in occupation (31.1%) (Table 1).
Table 1.
Socio-demographic characteristics of TB patients and Controls.
| Type | Variables | TB-cases (n) | Control (n) | P-value |
|---|---|---|---|---|
| Sex | Female | 26 | 36 | 0.036 |
| Male | 36 | 23 | ||
| Residence | Urban | 24 | 22 | 0.391 |
| Rural | 29 | 37 | ||
| Age (years) | 14–18 | 9 | 9 | 0.805 |
| 19–30 | 37 | 32 | ||
| 31–50 | 12 | 15 | ||
| 51–70 | 3 | 3 | ||
| More than 70 | 1 | 0 | ||
| Marital status | Single | 31 | 26 | 0.807 |
| Married | 30 | 32 | ||
| Widowed | 1 | 1 | ||
| Educational level | Illiterate | 8 | 8 | 0.644 |
| Primary | 20 | 16 | ||
| Secondary | 23 | 21 | ||
| Tertiary | 11 | 12 | ||
| Religious Teaching | 0 | 2 | ||
| Religion | Orthodox Tewahido Christian | 56 | 52 | 0.176 |
| Muslim | 1 | 5 | ||
| Protestant | 3 | 2 | ||
| Catholic | 2 | 0 | ||
| Occupation | Student | 9 | 15 | 0.002 |
| Private | 12 | 19 | ||
| Government employees | 8 | 7 | ||
| Non-Government employees | 0 | 1 | ||
| Farmer | 29 | 8 | ||
| Housewife | 2 | 8 | ||
| Retired | 1 | 0 |
There was statistically significant difference in sex between TB patients and the controls (P < 0.05). The odds of males with TB was 2.2 (95% CI: 1.05 to 4.48) times more likely as compared to the odds of females. The most (85%) TB afflicted age group was found between 19 and 50 years.
Clinical signs and symptoms of TB
TB patients showed various clinical signs and symptoms. The identified clinical signs and symptoms at the time of diagnosis were fatigue (100%), cough (83.3%), malaise (83.3%), night sweating (83.3%), fever (75%), anorexia (75%), productive cough (75%), chest pain (70.8%), dyspnoea (54.2%), haemoptysis (29.2%) and tachycardia (25%).
Vitamin A and zinc deficiencies
The proportions of vitamin A, zinc and the combination of the two micronutrient deficiencies were 47.9%, 96.5% and 47.8%, respectively. The mean serum retinol and zinc concentrations in females (0.80 ± 0.47 µmol/L and 2.10 ± 1.20 µmol/L, respectively) were not significantly different from that of males (0.77 ± 0.60 µmol/L and 2.50 ± 2.50 µmol/L, respectively) (p > 0.05). The proportions of vitamin A deficiencies among TB patients and the controls were 56.4% and 39.0%, respectively.
All TB patients and 92.5% of the controls were identified as zinc deficient. There were statistically significant differences in all proportions of the deficiencies between TB patients and the controls at p < 0.1. The odds of TB patients who were vitamin A deficient was 2.03 (90% CI: 1.1 to 3.7) times greater than the odds of the controls. In the same line, TB patients had vitamin A and zinc deficiencies 2.3 (95% CI: 1.1 to 4.8) times more likely as compared to the controls.
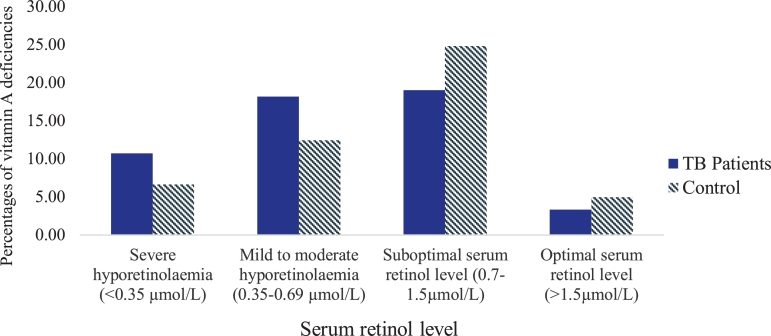
Fig. 1 shows a wide-ranging of serum retinol levels. The proportions of severe and, mild to moderate hyporetinolaemia were higher in TB patients than the controls. About 19% of TB patients and 25% of the controls had suboptimal retinol levels, whereas the optimal level in both groups was below 5%.
Fig. 1.
Percentages of vitamin A deficiencies in TB patients and controls. The categories of vitamin A deficiencies were indicated in WHO global report [30], Olang et al. [31] and Kassaye et al. [32].
CRP
About 62% of TB patients were CRP positive. The mean ± SD of retinol and zinc concentration in CRP positive TB patients were 0.62 ± 0.50 µmol/L and 2.05 ± 0.74 µmol/L, respectively. Whereas, these values in CRP negative TB patients were 0.75 ± 0.69 µmol/L and 2.48 ± 1.20 µmol/L, respectively. Excluding CRP positives, the prevalence of vitamin A deficiencies in CRP negative TB patients became 66.7%. Both CRP negative and positive TB patients did not have statistically significant differences in serum retinol (p = 0.25) and zinc (p = 0.09) concentrations.
Durations of TB treatment
Fig. 2 shows the mean serum retinol and zinc concentrations in different durations of TB treatment. During the first month of TB treatment, the retinol level was below the cut-off value of 0.70 µmol/L but increased slightly afterward. Similarly, the zinc level increased slightly up to 2months of treatment but its concentration throughout the entire courses of the treatment was below the cut-off value of 10.70 µmol/L. Per the result of the analysis of variance, the retinol and zinc concentrations were not significantly different in both within and between the durations of the treatment.
Fig. 2.
Mean concentrations of serum retinol (μmol/L) and zinc (μmol/L) in different duration of anti-TB treatment.
Dietary intakes
The mean DDS was 3.56. Most (84%) of TB patients and the controls (86%) had below average DDS (4.5 out of 9 food groups). DDS had statistically significant correlation with BMI (γ= −0.22; p = 0.019). There was also a significant difference in DDS between males and females (p = 0.031). While analysing the nutrients intake, about 87% of TB patients and 80% of the controls had below 50% fulfilment of energy intake. Almost all participants had below 50% fulfilment of vitamin A intake. But, less than 30% of participants had protein and zinc intakes below 50% fulfilments. In other words, many participants had above the average fulfilments of protein and zinc intakes, albeit much cereal-based diet.
Nutritional status
More than 29% of the participants had BMI below 18.50 kg/m2. The mean BMI of female TB patients (18.80 ± 3.80 kg/m2) was significantly lower than females in the controls (20.40 ± 2.92 kg/m2) (p < 0.05). There was a statistically significant difference in the proportions of undernutrition (BMI < 18.50 kg/m2) between TB patients (40.3%) and the controls (19.3%) (P < 0.05). Undernourished individuals had 2.8 times the odds of TB disease compared to the controls. As defined by MUAC, undernutrition for both male and female was 44.2%. The mean MUAC of females in TB patients (20.60 ± 2.20 cm) was significantly different from those females in the controls (21.80 ± 2.20 cm) at p < 0.10. MUAC was significantly correlated with BMI (γ= 0.56, P < 0.001).
Determinants of TB
The multinomial logistic regression model in Table 2 indicated that sex and BMI had statistically significant association with TB patients (p < 0.05). Adjusting for BMI, serum retinol level, religion, and occupation, females had 0.2 times less the odds of TB compared to males. Similarly, undernourished individuals had 3.3 times the odds of TB compared to well-nourished individuals holding sex, serum retinol level, religion and occupation constant.
Table 2.
Multinomial logistic regression model for different variables in TB patients.
| Variables | β | Wald | DF | P-value | Odds Ratio | 95% CI |
||
|---|---|---|---|---|---|---|---|---|
| Lower Limit | Upper Limit | |||||||
| Sex | Females | −1.64 | 10 | 1 | 0.002* | 0.19 | 0.07 | 0.53 |
| Males | ||||||||
| BMI | < 18.50 kg/m2 | 1.2 | 5.06 | 1 | 0.024* | 3.33 | 1.17 | 9.50 |
| ≥ 18.50 kg/m2 | ||||||||
| Retinol | < 0.70 µmol/L | 0.62 | 1.77 | 1 | 0.184 | 1.86 | 0.74 | 4.64 |
| ≥ 0.70 µmol/L | ||||||||
| Religion | Orthodox Tewahido | 0.7 | 0.22 | 1 | 0.64 | 2.01 | 0.10 | 38.56 |
| Muslim | −0.48 | 0.07 | 1 | 0.796 | 0.62 | 0.02 | 23.34 | |
| Protestant | ||||||||
| Occupations | Students | −0.92 | 2.01 | 1 | 0.16 | 0.40 | 0.11 | 1.42 |
| Private workers | −0.84 | 1.77 | 1 | 0.183 | 0.43 | 0.13 | 1.48 | |
| Government employees | −0.97 | 1.59 | 1 | 0.207 | 0.38 | 0.09 | 1.71 | |
| Housewives | 1.73 | 2.91 | 1 | 0.088 | 1.26 | 0.35 | 4.51 | |
| Farmers | ||||||||
NB: The reference category is the controls.
*Statistically significant at p < 0.05.
Discussion
In most countries, TB notification is twice as high in men as in women [33]. The study done in central part of Ethiopia also showed a high rate of TB notification in male (60.9%) and people with the age group of 14 to 54 years (87.3%) [20]. In line with this, the odds of males with TB in the present study was 2.2 times more likely as compared to the odds of females. The most (85%) TB afflicted age group was also found between 19 and 50 years. The reason why males and the productive age groups are highly afflicted by TB could be associated with their sociocultural, behavioral and biological components [33].
Micronutrient deficiencies are rampant in Ethiopia. More than 47% of TB patients and controls were affected by the deficiencies of either vitamin A, zinc or their combination. This was corroborated by the report of Keflie et al. [26] who identified the presence of a high risk of micronutrient deficiency in central part of Ethiopia (>60%). According to WHO and International Zinc Nutrition Consultative Group (IZiNCG), the risks of vitamin A and zinc deficiencies are of public health concern when the prevalence of low serum retinol and zinc concentrations are greater than 20% [34]. This implied that the results of the present study were more than twice of the indicator of the public health concern.
Comparing with the control, the odds of TB patients who were vitamin A deficient was 2 times higher. The proportion of vitamin A deficiency in TB patients was 56.4% which was closer to the report of nearly 60% among TB patients in Gondar, Northwest Ethiopia [18]. Severe and mild to moderate types of vitamin A deficiency was more common in TB patients than the controls. The low concentration of vitamin A in the serum of TB patients could be resulted from TB induced anorexia that lead to low consumption of vitamin A rich food items; reduced absorption of dietary vitamin A owing to parasites co-infection; decreased mobilization of hepatic reserves of retinol; rapid utilization of vitamin A by target tissues; and increased urinary losses of vitamin A associated with fever [18], [35]. This justification was strengthened by the occurrence of fever and anorexia together with fatigue, cough, malaise, and night sweating in more than 74% of TB patients in the present study.
Likewise, there was a significant difference in the proportion of zinc deficiency among TB patients and controls. All TB patients were identified as zinc deficient. This result was much higher than the report of the previous study done in Northwest Ethiopia (32.1%) [17]. Such a proportional difference could be owing to variations in the dietary patterns and seasons together with the availability and accessibility of food items rich in zinc. In line with the present study, the low serum zinc concentration in TB patients was also reported in Indonesia [24], Ecuador [36], Malaysia [37], and Korea [38].
The lowered concentration of zinc among TB patients could probably be associated with reduction in hepatic production of a zinc carrier protein (α2-macroglobulin); redistribution of zinc from blood circulation to other tissues; and/or a rise in the production of metallothionein, a protein that transports zinc to the liver [17], [37], [39]. The concomitant deficiencies of vitamin A and zinc were very high in TB patients as compared to the control. This could be due to the high magnitude of zinc deficiency in TB patients. Vitamin A and zinc deficiencies are usually occurred together, as zinc deficiency impairs the synthesis of retinal binding proteins and reduces plasma retinal concentration [40].
Several studies indicated the essence of measuring CRP in TB patients to get a clear picture on the serum retinol and zinc concentrations [18], [36], [41]. CRP is a tissue protein which is produced at the time of acute phase response and its concentration changes rapidly as a result of infection or inflammation. Visser et al. [41] showed the relationship between the low retinol concentration in active TB and the acute phase response. Likewise, Cuevas and Koyanagi [9] described the low concentration of zinc in TB patients with and without a raised CRP.
In the present study, we did not observe any difference in the concentrations of vitamin A and zinc in the serum of TB patients with and without CRP. In line with our finding, Karyadi, et al. [24] reported that retinol concentration did not correlate significantly with markers of acute phase response. This suggested that CRP is not the best option to control the change in the acute phase response in the concentrations of vitamin A and zinc in the serum of TB patients.
We observed a fluctuation of vitamin A and zinc concentrations in TB patients at different durations of anti-TB treatment. During the first month, the retinol concentration was below the cut-off value but later increased slightly. The gradual increment of zinc concentration was also observed up to the second month of the treatment, but such a concentration was below the cut-off value throughout the whole course of anti-TB treatment. The improvement of vitamin A was in accordance with the reports of Mugusi et al. [7] in Tanzania and Kassu et al. [18] in Ethiopia. It is believed generally as patients improve and fever subsides, the loss of vitamin A declines, and its serum concentration resume to the reference ranges [18].
Despite the low improvement, our observation on the changes of zinc concentration was corroborated by the findings of Khanna et al. [42] and Kassu et al. [17]. On the contrary, Edem and others [39] revealed that trace elements and vitamins were lowest at 2 and 4 months of anti-TB drug therapy. This may be due to drug-micronutrient interactions or drug induced nutrient depletion. Further, the effects of anti-TB drugs on the absorption of zinc was presented by Karyadi et al. [11] in their study done in Indonesia. Ethambutol was shown in rats to increase not only zinc absorption but also urinary zinc losses, resulting in reduced circulating zinc concentrations [43].
In the comparison made on nutritional status as measured by BMI and MUAC, the results were significantly lower in TB patients than the controls. Undernourishment in TB patients was almost three times higher than that of the controls. In agreement with our results, several studies demonstrated undernutrition as a well-recognized clinical sign of active TB [24], [44], [45]. Pakasi et al. [46] described undernutrition as a reflection of two processes. One would be protein-energy malnutrition (which severely affects host defense) and the other was wasting due to the catabolism induced by the acute phase response. The causes of undernutrition in patients with active TB could be anorexia, impaired absorption of nutrients, or increased catabolism associated with the inflammatory and immune response [24], [44]. Our study identified undernutrition and sex as significant determinants of active TB.
The dietary intake assessment revealed that the mean DDS was 3.56, and above 80% of TB patients and the controls had below the average DDS. These results were in accordance with the previous study done in the same area [26]. A diet of at least 4 DDS was valid as nutritionally adequate, but below 4 DDS represented a poor dietary diversity [26], [47]. This suggested that both TB patients and controls had poor dietary diversity. The intakes of energy and vitamin A rich food items were also poor. More than 85% of TB patients could not fulfil even half of their daily requirements of energy and vitamin A. Poor dietary intake of vitamin A rich food is an important predictor of vitamin A deficiency [16].
Although the intakes of protein and zinc looked better, the main dietary sources were cereal based. The previous study in the same area also indicated a high reliance on the consumption of cereals, vegetables and legumes with less animal source foods [26]. As our body does not store zinc, it requires a constant dietary intake. Most plant-based diets are not good sources of zinc owing to the presence of phytate, a component of plants that chelates zinc and prevents its absorption [48]. The low content of zinc in Ethiopian soil could be another factor for the inadequate intake of zinc [15]. Hence, plant- based diets with low animal source proteins were accounted for the high deficiency of zinc in both TB patients and controls.
Limitation
Although this study had the strength of dealing with the serum vitamin A and zinc concentrations in TB patients together with CRP, nutritional status and dietary intakes, it was a facility- based study and the participants could not represent the general population. In addition, the 24-h recall method, which was used to assess the dietary intake, could probably miss some of the feeding behaviours particularly in the case of altered dietary patterns.
Conclusion
In conclusion, vitamin A and zinc deficiencies are severe problems among TB patients and non-TB controls. The cause-effect relationship between undernutrition and TB is just like the puzzle of the hen or the egg, of the two which comes first. Our study underlines that undernutrition determines the development of TB. Most TB patients have DDS below average and, very poor dietary intakes of vitamin A, zinc, protein, and energy. During the duration of anti-TB treatment, the concentrations of vitamin A and zinc improve but not to the extent of curbing their deficiencies. Therefore, to claim success, TB management program needs to give emphasis on addressing the problems of vitamin A and zinc deficiencies together with protein-energy malnutrition. In the future, we recommend further community- based studies to be conducted at different parts of Ethiopia to substantiate our findings.
Acknowledgments
Acknowledgment
We are grateful to all study participants, data collectors, health facilities, and the health bureau of North Shewa Zone of Amhara Region. We are also indebted to Mr. Alexander Koza, Mr. Hulumtaye Tefera, Mr. Meseret Woldeyohannes and Mr. Debebe Hailu for their technical assistance during the processes of the laboratory analyses. This study was financially supported by the Dr. Hermann Eiselen Ph.D. grant from the foundation fiat panis. The first author obtained a scholarship from Food Security Centre (FSC) of University of Hohenheim, which is supported by the German Academic Exchange Service (DAAD) with funds of the Federal Ministry of Economic Cooperation and Development (BMZ) of Germany, and thus, highly acknowledged.
Conflict of interest
The authors declared that they have no competing interest.
Author's contribution
TSK designed the study, collected the data, analyzed, interpreted and drafted the manuscript. AS, AZ, AM, MA and HKB critically reviewed the manuscript. All the authors read and approved the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jctube.2018.05.002.
Appendix. Supplementary materials
References
- 1.WHO. Global TB report 2017. Annex 4: TB burden estimates, notifications and treatment ; outcomes for individual countries and territories, WHO regions and the world. 2017. Accessed on February 26, 2018. http://www.who.int/tb/publications/global_report/gtbr2017_annex4.pdf?ua=1.
- 2.Macallan DC. Malnutrition in tuberculosis. Diagn Microbiol Infect Dis. 1999;34(2):153–157. doi: 10.1016/s0732-8893(99)00007-3. [DOI] [PubMed] [Google Scholar]
- 3.Sinclair D, Abba K, Grobler L, Sudarsanam TD. Nutritional supplements for people being treated for active tuberculosis. Cochrane Database Syst Rev. 2011:11. doi: 10.1002/14651858.CD006086.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Bhaskaram P. Micronutrient malnutrition, infection, and immunity: An overview. Nutr Rev. 2002;60:S40–S45. doi: 10.1301/00296640260130722. [DOI] [PubMed] [Google Scholar]
- 5.Demissie T, Ali A, Mekonen Y, Haider J, Umeta M. Magnitude and distribution of vitamin A deficiency in Ethiopia. Food Nutr Bull. 2010;31(2) doi: 10.1177/156482651003100206. [DOI] [PubMed] [Google Scholar]
- 6.Biesalski HK, Donatus N. New aspects in vitamin A metabolism: the role of retinyl esters as systemic and local sources for retinol in mucous epithelia. J Nutr. 2004;134(Suppl 12) doi: 10.1093/jn/134.12.3453S. 3453S–3457S. [DOI] [PubMed] [Google Scholar]
- 7.Mugusi FM, Rusizoka O, Habib N, Fawzi W. Vitamin A status of patients presenting with pulmonary tuberculosis and asymptomatic HIV-infected individuals, Dar es Salaam, Tanzania. Int J Tuberc Lung Dis. 2003;7(8):804–807. [PubMed] [Google Scholar]
- 8.Hambidge M. Zinc and health: current status and future directions. J Nutr. 2000;130(Suppl):1344–1349. [Google Scholar]
- 9.Cuevas L, Koyanagi A. Zinc and infection: a review. Ann Trop Paediatr. 2005;25:149–160. doi: 10.1179/146532805X58076. [DOI] [PubMed] [Google Scholar]
- 10.Bonaventura P, Benedetti G, Albarède F, Miossec P. Zinc and its role in immunity and inflammation. Autoimmun Rev. 2015;14:277–285. doi: 10.1016/j.autrev.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Karyadi E, West CE, Schultink W, Nelwan RH, Gross R, Amin Z, Dolmans WMV, Schlebusch H, van der Meer JWM. A double blind, placebo-controlled study of vitamin A and zinc supplementation in persons with tuberculosis in Indonesia: effects on clinical response and nutritional status. Am J Clin Nutr. 2002;75:720–727. doi: 10.1093/ajcn/75.4.720. [DOI] [PubMed] [Google Scholar]
- 12.Armijos RX, Weigel MM, Chacon R, Flores L, Campos A. Adjunctive micronutrient supplementation for pulmonary tuberculosis. Salud Publica Mex. 2010;52:185–189. doi: 10.1590/s0036-36342010000300001. [DOI] [PubMed] [Google Scholar]
- 13.Rwangabwoba JM, Fischman H, Semba RD. Serum Vitamin A levels during tuberculosis and human immunodeficiency virus infection. Int J Tuberc Lung Dis. 1998;2:771–773. [PubMed] [Google Scholar]
- 14.de Benoist B Darnton-HillI, Davidsson L, Davidsson L, Fontaine O, Hotz C. Conclusions of the Joint WHO/UNICEF/IAEA/IZiNCG interagency meeting on zinc status indicators. Food Nutr Bull. 2007;28(3 suppl):S480–S484. doi: 10.1177/15648265070283S306. [DOI] [PubMed] [Google Scholar]
- 15.Kumera G, Awoke T, Melese T, Eshetie S, Mekuria G, Mekonnen F, Ewunetu T, Gedle D. Prevalence of zinc deficiency and its association with dietary, serum albumin and intestinal parasitic infection among pregnant women attending antenatal care at the University of Gondar Hospital, Gondar, Northwest Ethiopia. BMC Nutr. 2015;1:31. [Google Scholar]
- 16.Tariku A, Fekadu A, Ferede AT, Abebe SM, Adane AA. Vitamin A deficiency and its determinants among preschool children: a community-based cross-sectional study in Ethiopia. BMC Res Notes. 2016;9:323. doi: 10.1186/s13104-016-2134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kassu A, Yabutani T, Mahmud ZH, Mohammad A, Nguyen N, Huong BT, Hailemariam G, Diro E, Ayele B, Wondmikun Y, Motonaka J, Ota F. Alterations in serum levels of trace elements in tuberculosis and HIV infections. Eur J Clin Nutr. 2006;60:580–586. doi: 10.1038/sj.ejcn.1602352. [DOI] [PubMed] [Google Scholar]
- 18.Kassu A, Van Nhien N NakamoriM, Diro E, Ayele B, Mengistu G, WondmikunY NishikawaT, Yamamoto S, Ota F. Deficient serum retinol levels in HIV-infected and uninfected patients with tuberculosis in Gondar, Ethiopia. Nutr Res. 2007;27(2):86–91. [Google Scholar]
- 19.FMOH. Federal democratic republic of ethiopia ministry of health guidelines for clinical and programmatic management of TB, TB/HIV and Leprosy. 2013. Addis Ababa, 5th ed.
- 20.Keflie TS, Ameni G. Microscopic examination and smear negative pulmonary tuberculosis in Ethiopia. Pan African Med J. 2014;19:162. doi: 10.11604/pamj.2014.19.162.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.EFMACA. Food, Medicine and healthcare administration and control authority of Ethiopia. Standard treatment guidelines for general hospital. 2014. 3rd ed. Addis Abeba, Ethiopia.
- 22.FANTA III . Adolescents and adults: a systematic review. 2013. Food and nutrition technical assistance. use of cut-offs for mid-upper arm circumference (MUAC) as an indicator or predictor of nutritional and health-related outcomes. FHI 360 1825 Connecticut Avenue, NW. [Google Scholar]
- 23.Gibson RS. Oxford University Press; New York, NY: 1993. Nutritional assessment. [Google Scholar]
- 24.Karyadi E, Schultink JW, Newan RH, Gross R, Amin Z, Dolmans WM, van der Meer JW, Hautvast JG, West CE. Poor micronutrient status of active pulmonary tuberculosis patients in Indonesia. J Nutr. 2000;130:2953–2958. doi: 10.1093/jn/130.12.2953. [DOI] [PubMed] [Google Scholar]
- 25.FAO. Food and Agriculture Organization . 2007. Guidelines for measuring household and individual dietary diversity. Version 2. Rome, Italy. [Google Scholar]
- 26.Keflie TS, Samuel A, Christine L, Nohr D, Biesalski HK. Dietary Patterns and Risk of Micronutrient Deficiencies: their Implication for Nutritional Intervention in Ethiopia. J Nutrit Health Food Sci. 2018;6(1):1–16. [Google Scholar]
- 27.WHO. World Health Organization . Food and nutrition technical assistance; 2007. Indicators for assessing infant and young child feeding practices Part 1 Definitions. [Google Scholar]
- 28.Arroyave G, Chichester CO, Flores H, Glover J, Mejia L, Oslen JA, Simpson KL, Underwood BA. Nutrition Foundation; Washington DC: 1982. Biochemical methodology for the assessment of vitamin a status: a report of the international vitamin a consultative group. [Google Scholar]
- 29.Sepehri Z, Mirzaei N, Sargazi A, Sargazi A, Mishkar AP, Kiani Z, Oskoee HO, Arefi D, Ghavami S. Essential and toxic metals in serum of individuals with active pulmonary tuberculosis in an endemic region. J Clin Tuberculosis Other Mycobact Dis. 2017;6:8–13. doi: 10.1016/j.jctube.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO. World Health Organization . World Health Organization; Geneva, Switzerland: 2009. Global prevalence of vitamin a deficiency in populations at risk 1995–2005: WHO global database on vitamin a deficiency. [Google Scholar]
- 31.Olang B, Abdollahi Z, Neshati R, Ali MA, Naghavi M, Agneta Yngve. Vitamin A status in pregnant women in Iran in 2001 and its relationship with province and gestational age. Food Nutr Res. 2014;58:25707. doi: 10.3402/fnr.v58.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kassaye T, Receveur O, Johns T, Becklake MR. Prevalence of vitamin A deficiency in children aged 6–9 years in Wukro, northern Ethiopia. Bull World Health Organ. 2001;79:415–422. [PMC free article] [PubMed] [Google Scholar]
- 33.Neyrolles O, Quintana-Murci L. Sexual inequality in tuberculosis. PLoS Med. 2009;6(12) doi: 10.1371/journal.pmed.1000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO. World Health Organization . 1996. Indicators for assessing vitamin A deficiency and their application in monitoring and evaluation of intervention programs. Geneva. [Google Scholar]
- 35.Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 36.Koyanagi A, Kuffo D, Gresely L, Shenkin A, Cuevas LE. Relationships between serum concentrations of C-reactive protein and micronutrients, in patients with tuberculosis. Ann Trop Med Parasitol. 2004;98:391–399. doi: 10.1179/000349804225003424. [DOI] [PubMed] [Google Scholar]
- 37.Rohini K, Srikumar PS, Saxena J, Kumar MA. Alteration in the levels of serum micronutrients in tuberculosis patients. Int J Biol Med Res. 2013;4(1):2958–2961. [Google Scholar]
- 38.Choi R, Kim HT, Lim Y, Kim MJ, Kwon OJ, Jeon K, Park HY, Jeong BH, Koh WJ, Lee SY. Serum concentrations of trace elements in patients with tuberculosis and its association with treatment outcome. Nutrients. 2015;7:5969–5981. doi: 10.3390/nu7075263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edem VF, Ige O, Arinola OG. Plasma vitamins and essential trace elements in newly diagnosed pulmonary tuberculosis patients and at different durations of anti-tuberculosis chemotherapy. Egypt J Chest Dis Tuberculosis. 2015;64:675–679. [Google Scholar]
- 40.Taylor CG, Bray TM. Effect of hyperoxia on oxygen free radical defense enzymes in the lung of zinc-deficient rats. J Nutr. 1991;121:460–466. doi: 10.1093/jn/121.4.460. [DOI] [PubMed] [Google Scholar]
- 41.Visser ME, Grewal HMS, Swart EC, Dhansay MA, Walzl G, Swanevelder S, Lombard C, Maartens G. The effect of vitamin A and zinc supplementation on treatment outcomes in pulmonary tuberculosis: a randomized controlled trial. Am J Clin Nutr. 2011;93:93–100. doi: 10.3945/ajcn.110.001784. [DOI] [PubMed] [Google Scholar]
- 42.Khanna BK, Kumar RL, Mukherjee PK, Chaudhary AR, Kamboj VP. Plasma copper and zinc levels in pulmonary tuberculosis, Indian. J. Tuberculosis. 1982;29:179–181. [Google Scholar]
- 43.King AB, Schwartz R. Effects of the antituberculous drug ethambutol on zinc absorption, turnover and distribution in rats fed diet marginal and adequate in zinc. J Nutr. 1987;117:704–708. doi: 10.1093/jn/117.4.704. [DOI] [PubMed] [Google Scholar]
- 44.Paton NI, Chua YK, Earnest A, Chee CB. Randomized controlled trial of nutritional supplementation in patients with newly diagnosed tuberculosis and wasting. Am J Clin Nutr. 2004;80(2):460–465. doi: 10.1093/ajcn/80.2.460. [DOI] [PubMed] [Google Scholar]
- 45.Dargie B, Tesfaye G, Worku A. Prevalence and associated factors of undernutrition among adult tuberculosis patients in some selected public health facilities of Addis Ababa, Ethiopia: a cross-sectional study. BMC Nutrition. 2016;2:7. [Google Scholar]
- 46.Pakasi TA, Karyadi E, Suratih NMD, Salean M, Darmawidjaja N, Bor H, van der Velden K, Dolmans WMV, van der Meer JWM. Zinc and vitamin A supplementation fails to reduce sputum conversion time in severely malnourished pulmonary tuberculosis patients in Indonesia. Nutr J. 2010;9:41. doi: 10.1186/1475-2891-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Habte TY, Krawinkel M. Dietary diversity score: a measure of nutritional adequacy or an indicator of healthy diet. JNH. 2016;3(3) [Google Scholar]
- 48.Arsenault JE, Brown KH. Zinc intake of preschool children exceeds new dietary reference intakes. Am J Clin Nutr. 2003;78:1011–1017. doi: 10.1093/ajcn/78.5.1011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.