Abstract
Rationale
Many high-burden countries are scaling-up Xpert MTB/RIF using a hub-and-spoke model. We evaluated the quality of care for patients undergoing TB evaluation at microscopy centers (spokes) linked to Xpert testing sites (hubs) in Uganda.
Objectives
To characterize the extent to which patients were receiving care in accordance with international and national guidelines.
Methods
We conducted a prospective cohort study of all adults with presumptive pulmonary TB at 24 health centers linked to Xpert testing sites. Health center staff photographed TB registers, and uploaded photos to a secure server bi-weekly. We assessed the proportion of patients (1) initiating testing; (2) completing testing; and (3) treated for confirmed TB within 14 days.
Measurements and Main Results
Between January to December 2017, 6744 patients underwent evaluation for pulmonary TB. Only 1316 patients had sputum referred for Xpert testing, including 1075/3229 (33.3%) people living with HIV and 241/3515 (6.9%) without HIV. Of 119 patients confirmed to have TB by Xpert testing, 44 (36%) did not initiate treatment. There were significant losses along the entire diagnostic cascade of care, with only 5330/6744 (79.0%) patients having samples referred for sputum-based testing, 2978/5330 (55.9%) patients completing recommended testing if referred, and 313/418 (74.9%) patients initiating treatment within 14 days if confirmed to have TB.
Conclusions
Although coverage of Xpert testing services across Uganda is high, the quality of care delivered to patients undergoing TB evaluation remains poor. Further research is needed to identify health system interventions to facilitate uptake of Xpert testing and high-quality care.
Keywords: Tuberculosis, Diagnosis, Quality of care
1. Introduction
According the World Health Organization (WHO), tuberculosis (TB) is now the leading cause of death from an infectious disease worldwide. An estimated 10.4 million people developed TB and 1.7 million died of the disease in 2016, which reflects an annual 2% decline in incidence and 3% decline in mortality [1]. Yet these estimates fall short of the progress needed to meet the ambitious targets of 95% reduction in TB mortality and 90% reduction in TB incidence by 2035 set forth by the End TB Strategy [2]. To accelerate progress, the End TB Strategy calls for enhanced implementation of current and novel tools emerging from the pipeline, including new diagnostics.
Xpert MTB/RIF® (Xpert), the first rapid molecular test for TB endorsed by the WHO, has been rapidly scaled-up in high burden countries since it was first introduced in 2010. Xpert is a semi-automated, cartridge-based molecular assay for diagnosis of pulmonary TB and detection of rifampicin resistance, and it is more sensitive than sputum smear microscopy [3]. The WHO now recommends Xpert as the initial diagnostic test for pulmonary TB in countries with high HIV prevalence and, where feasible, as a follow-on test for those with a negative smear microscopy result [4]. From 2010 to 2016, 29,865 Xpert modules and 23.1 million cartridges have been procured by low- and middle-income countries eligible for concessional pricing [5]. In addition, 28 of 48 high-burden TB, TB/HIV, or multidrug-resistant (MDR) TB countries have adopted national algorithms with Xpert as the initial diagnostic test for presumptive TB patients [6].
Although coverage of Xpert testing has increased dramatically, important questions related to Xpert implementation remain unanswered, including uptake in routine care and impact on quality of care for patients with presumptive TB. To address these questions, we collaborated with the Uganda National Tuberculosis and Leprosy Programme (NTLP) to evaluate the quality of care for patients undergoing TB evaluation at 24 health centers in Uganda that are linked to Xpert testing sites. Our objective was to characterize the extent to which patients were receiving care in accordance with national and international guidelines.
2. Methods
2.1. Study context
Uganda has been a leader in scaling up Xpert testing. As of December 2016, Uganda had procured 526 GeneXpert modules and 353,790 Xpert cartridges [5]. Since the majority of health centers in Uganda with TB diagnostic units lack adequate infrastructure to perform Xpert testing, the Uganda NTLP established a specimen-referral system in 2012, linking community-level TB microscopy centers (spokes) to regional- or district-level laboratories with GeneXpert devices (hubs) [7]. Sputum samples are transported from the spokes to the hubs via motorcycle (boda-boda) and results are returned the next time samples are collected. During the study period, Uganda NTLP guidelines, in accordance with the International Standards for TB Care (ISTC), recommended that Xpert be performed as a first-line test for high-risk groups including people living with HIV (PLHIV) [8], [9].
2.2. Study setting and population
We identified potential study health centers by reviewing 2015 NTLP TB testing and treatment data for all community-level TB microscopy units (spokes) that were linked to Xpert testing sites (hubs) through the NTLP Xpert referral network. We included health centers that: (1) used sputum smear microscopy as the primary method of TB diagnosis; (2) participated in NTLP-sponsored external quality assurance for sputum smear microscopy; and (3) referred sputum samples to a district or regional health facility for Xpert testing. We excluded health centers that (1) performed sputum smear microscopy on less than 150 patients per year and (2) diagnosed less than 15 smear-positive TB cases per year. We worked with the Uganda NTLP to select 24 health centers from among those meeting eligibility criteria, excluding ones for which the District Health Officer did not consent to participate in the study. The 24 health centers referred sputum samples to a total of 17 Xpert testing hubs (see Supplementary Fig. 1).
We included data on all patients evaluated for pulmonary TB at the 24 health centers between January and December 2017. Patients were excluded if they (1) had sputum collected for monitoring of response to anti-TB therapy; (2) had sputum collected as part of active, community-based case finding (e.g., contact tracing, community outreach campaign); (3) had a documented prior history of TB treatment (e.g., reason for Xpert testing or TB treatment marked as treatment failure, relapse, or treatment after loss to follow-up); (4) were referred to a study health center for TB treatment after a diagnosis was established elsewhere; (5) were started on treatment for presumptive extra-pulmonary TB only; or (6) were less than 18 years old.
The study was approved by the University of California San Francisco Committee on Human Research, the Makerere University School of Medicine Research Ethics Committee, and by the Uganda National Council for Science and Technology. The requirement for informed consent was waived.
2.3. Study procedures
2.3.1. TB guideline training
A half-day refresher training on Uganda NTLP guidelines on TB diagnosis and treatment co-chaired by the District TB and Leprosy Supervisors and an NTLP/NTRL representative was held at each health center one to three months prior to the start of patient data collection. All health center staff directly involved in the care of patients with presumptive TB were invited to participate. Health center staff were also trained on how to complete TB registers, with emphasis on completion of frequently missing fields. In addition, Xpert testing hubs linked to study health centers were visited, and laboratory staff were provided with refresher training on GeneXpert device maintenance and operation.
2.3.2. Patient-level data collection
We collected patient-level demographic and clinical information from all health centers using existing data sources. During the health center visit to review guidelines, we trained two health center staff (one primary, one backup) identified by the health center director to take photos of data sources using a camera-equipped smartphone. They were instructed to upload the photos to a central secure server every two weeks for the duration of the study using Research Electronic Data Capture (REDCap) mobile software. Routine data sources included (1) NTLP Presumptive TB, Laboratory and Treatment registers and (2) Xpert laboratory requisition forms. Study staff reviewed each batch of photos from each health center for completeness and accuracy, and then extracted individual-level patient data using standardized REDCap forms (see Supplementary Materials 2 for details). Data extracted included demographics, HIV and ART status, TB test results and dates, and TB treatment date. If missing information or logical inconsistencies were identified, we first consulted the original data photographs, and then we phoned the health centers after every 2-week period of data extraction to resolve discrepancies and uncertainties. In addition to the primary data sources, we extracted Xpert testing data from the national GxAlert server hosted at NTLP to ensure complete capture of Xpert test results and reviewed pre-ART and ART registers to verify ART status for any patient living with HIV who was missing ART information. Finally, we checked other data sources (e.g., clinic registration logbooks) as needed to attempt to track down key information missing from primary sources during scheduled quarterly health center visits, including treatment status for all patients. If a patient was identified as RIF-resistant by Xpert, study staff called the referral MDR-TB treatment center to determine whether the patient was evaluated, if additional testing was performed, and/or if treatment was initiated.
2.4. Study outcomes
We defined a presumptive TB patient to be any eligible adult listed in any NTLP TB register, Xpert laboratory requisition form, or GxAlert database. Three quality indicators were assessed, each representing an ISTC guideline-recommended step along the diagnostic cascade of care for patients with presumptive TB(9): (1) proportion of presumptive TB patients referred for sputum-based TB testing (i.e., had sputum collected for smear or Xpert testing); (2) proportion of patients referred who completed testing per Uganda NTLP guidelines (i.e., one valid Xpert result or, if HIV-negative, one positive or two negative smear results); and (3) proportion of microbiologically-confirmed TB patients who were initiated on TB treatment within 14 days of presenting to the health center. In addition, we evaluated a summary metric of the entire TB diagnostic process: the proportion of presumptive TB patients who were evaluated in accordance with ISTC recommendations (i.e., had samples referred for testing, completed testing, and treated within 14 days if test-positive).
2.5. Statistical analysis
All analyses were carried out using STATA 14 (StataCorp LP, College Station, TX). Xpert utilization and quality indicators were assessed using binomial regression with robust standard errors accounting for clustering by health center to give overall proportions and 95% confidence intervals (CI) and proportions by month. Binomial regression was also used to assess differences in indicators proportions by test type. Time-to-treatment were reported overall and by test type using median and interquartile range (IQR), and we plotted a Kaplan–Meier failure function to visually assess cumulative proportion of patients initiating treatment by test.
3. Results
3.1. Patient characteristics
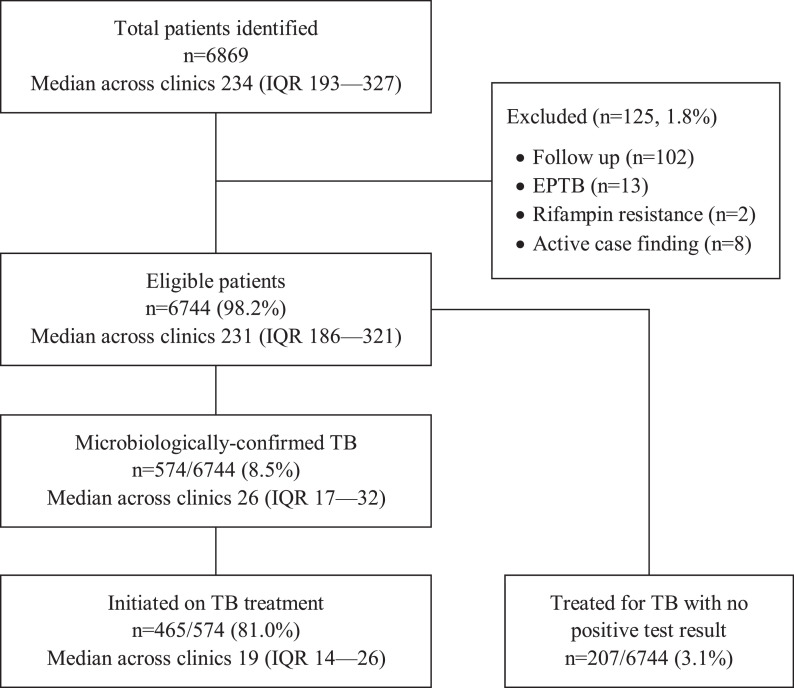
Of 6869 adults identified across the TB-related data sources, 125 (1.8%) were excluded from the analysis: 102 (1.5%) follow-up patients, 13 (0.2%) patients with extra-pulmonary TB only, 2 (0.03%) patients identified as having rifampin resistance on Xpert testing, and 8 (0.1%) patients identified through community-based active case finding campaigns. Thus, 6744 (98.2%) adults with presumptive pulmonary TB at the 24 health centers were included (median across the 24 clinics of 231, interquartile range [IQR] 186–321) (Fig. 1). The median age was 38 years (IQR 28–50), 3381 (50.1%) were female, and 3229 (47.9%) were documented as being HIV seropositive. TB was confirmed in 574 (8.5%) patients, of whom 465 (81.0%) were initiated on TB treatment. In addition, 207 of 6744 (3.1%) patients without microbiologic confirmation were treated for TB (Fig. 1).
Fig. 1.
Patient Flow Diagram. Of 6869 adults identified at 24 health centers through review of the TB-related data sources, 6744 (98.2%) with presumptive pulmonary TB were included in the analysis. TB treatment was initiated 465 of 574 (81.0%) patients with a confirmed TB diagnosis and 207 of 6744 (3.1%) without a positive sputum smear or Xpert result. Of the 207 patients treated empirically, 20 (9.7%) were Xpert-negative, 91 (44.0%) were smear-negative, and 96 (46.4%) were not tested.
3.2. Xpert utilization and outcomes
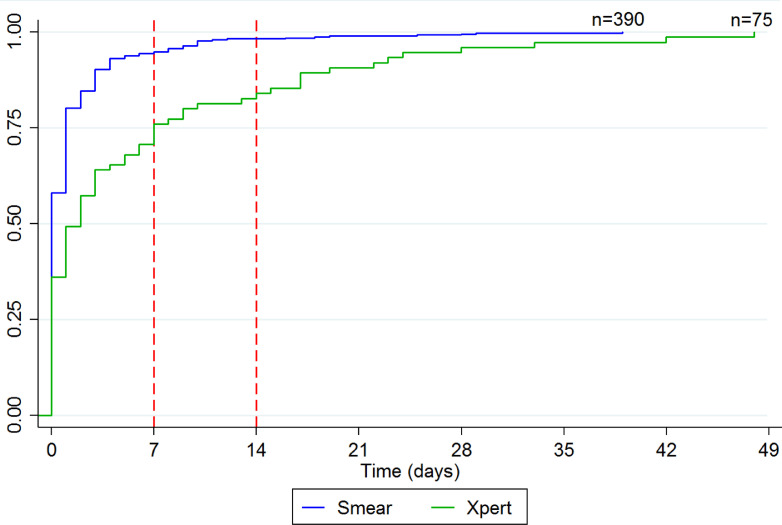
Of the 6744 adults with presumptive TB, 1316 (19.5%, 95% CI 18.6–20.5%) had samples referred for Xpert testing, including 1075 of 3229 (33.3%, 95% CI 31.5–34.8) with and 241 of 3515 (6.9%, 95% CI 6.0–7.7) without documented HIV infection. Uptake of Xpert testing was low throughout the study period, with as few as 10.5% and as much as 29.4% having samples referred for Xpert testing during the study period. There was no increasing trend over time (p < 0.05, chi-squared test for trend). Xpert was requested as the first-line test for only 81 of 6744 (1.2%) patients overall and 60 of 3229 (1.9%) patients with HIV infection. Of the 119 patients confirmed to have TB by Xpert testing, 75 (63.0%) were initiated on treatment overall and 63 (52.9%) within 14 days. Median time-to-treatment was longer for patients confirmed to have TB by Xpert testing (2 days, IQR 0–7) compared to smear microscopy (0 days, IQR 0—1). The cumulative proportion started on treatment was lower at both 7 days (75.0% vs. 94.1%, p < 0.001) and 14 days (84.4% vs. 97.1%, p < 0.001) for patients confirmed to have TB by Xpert testing (Fig. 2).
Fig. 2.
Cumulative proportion initiating TB treatment, by test. Among the 465 patients who initiated TB treatment, median time-to-treatment was 0 days (IQR 0–1) for the 390 patients diagnosed via smear microscopy and 2 days (IQR 0–7) for the 75 patients diagnosed via Xpert. The cumulative proportion starting treatment by 7 days (94.1% vs. 75.0%, p < 0.001) and 14 days (97.1% vs. 84.4%, p < 0.001) was higher for patients diagnosed via smear microscopy.
3.3. Quality of TB diagnosis
3.3.1. Referral for sputum-based TB testing
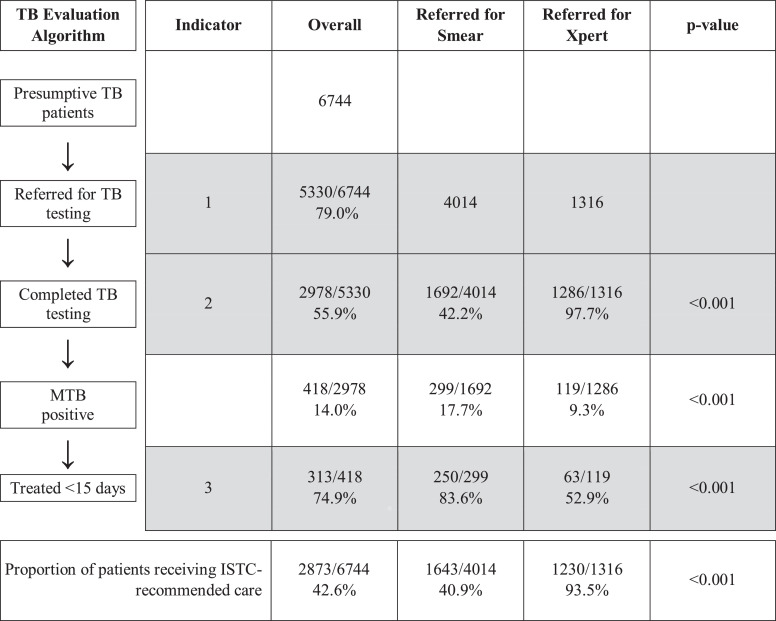
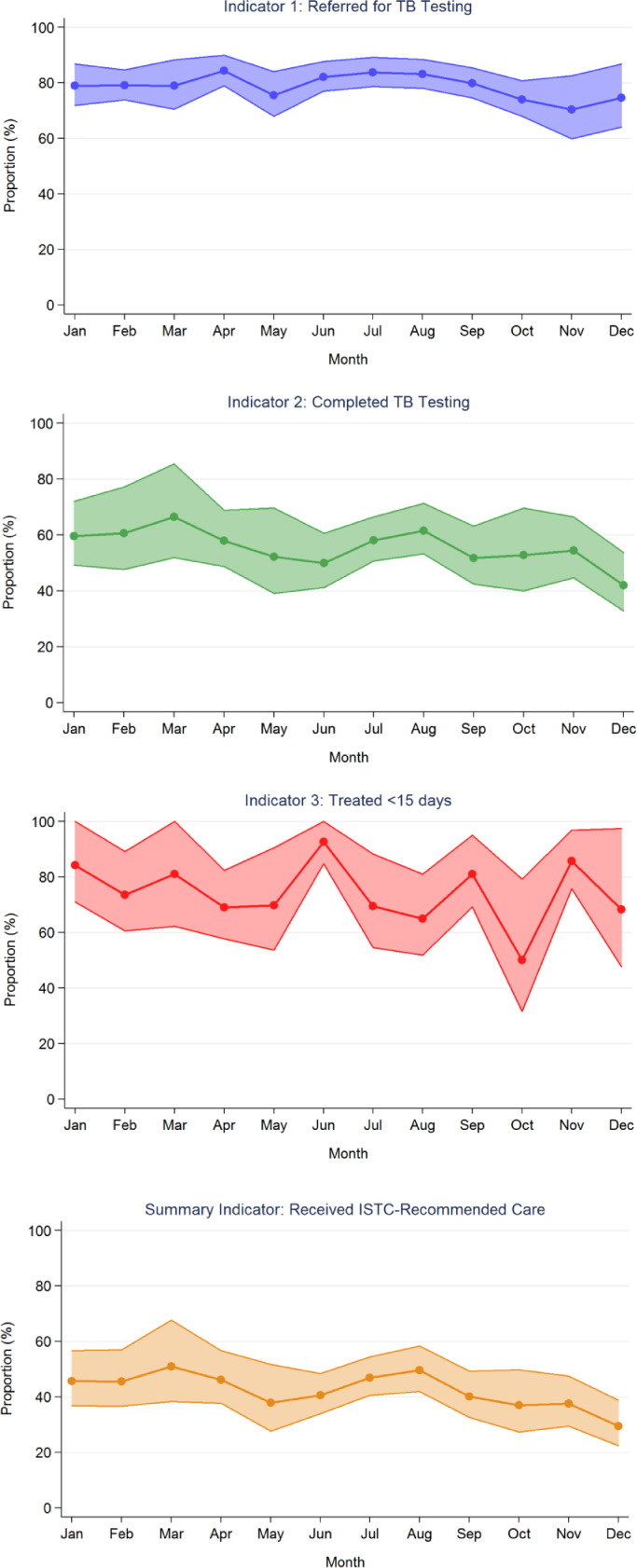
Of the 6744 patients with presumptive TB, 5330 had samples referred for sputum-based testing (i.e., had sputum collected for onsite sputum smear microscopy and/or hub-based Xpert testing). The proportion of patients with samples referred for testing was 79.0% (95% CI: 75.2–83.0%) overall (Fig. 3) and ranged across health centers from 58.7% to 92.0% (Fig. 4). The proportion of patients with samples referred for testing ranged across months from 70.3% to 84.3% (Fig. 5).
Fig. 3.
Quality of TB diagnostic evaluation (January–December 2017). The table displays the proportion of adults with presumptive TB at 24 health centers who completed each step of the diagnostic process, generated through binomial regression models adjusted for clustering by health center. Overall, 79.0% of presumptive TB patients had samples referred for sputum-based testing, 55.9% completed testing if referred, and 74.9% were treated within 14 days if TB was microbiologically-confirmed. The proportion completing all steps in accordance with the International Standards for TB Care was 42.6%. Compared to patients with samples referred for smear microscopy, patients with samples referred for Xpert testing were more likely to complete testing (97.7% vs 42.3%) but less likely to initiate treatment within 14 days if test results were positive (52.9% vs. 83.6%).
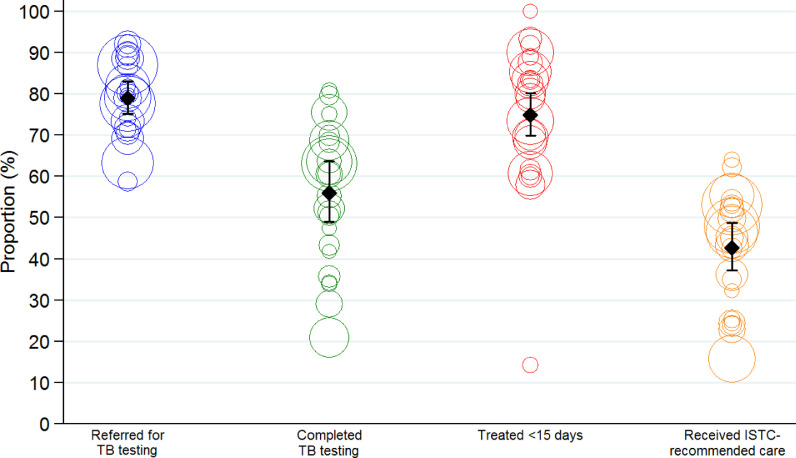
Fig. 4.
Health center-level variation in quality of TB diagnostic evaluation (January–December 2017). The graph plots each quality indicator for the 24 health centers, along with the summary estimate and 95% confidence interval adjusted for health center. Each circle represents the relative size of each health center by indicator. Across the health centers, the proportions with samples referred for TB testing, completing test if referred, initiating treatment if TB was confirmed and receiving ISTC-recommended care varied from 58.7–92.0%, 20.9–80.8%, 14.3–100%, and 15.7–64.0%, respectively.
Fig. 5.
Quality indicators over time (January–December 2017). The graphs plot the proportion and 95% confidence intervals, adjusted for health center, for each indicator over the 12-month study period. The proportions with samples referred for TB testing, completing test if referred, initiating treatment if TB was confirmed and receiving ISTC-recommended care varied from 70.3–84.3%, 41.9–66.5%, 50.0–92.7%, and 29.5–50.9%, respectively.
3.3.2. Completion of TB testing
Of the 5330 patients with samples referred for testing, 2978 (55.9; 95% CI: 49.0–63.7%) completed testing per Uganda NTLP guidelines, ranging across health centers from 20.9% to 80.8% (Figs. 3 and 4). The proportion of patients completing testing ranged across months from 41.9% to 66.5% (Fig. 5).
3.3.3. Treatment initiation within 14 days
Of the 418 patients who completed testing and had microbiologically-confirmed TB, 313 (74.9%; 95% CI: 69.9–80.2%) were initiated on TB treatment within 14 days of presenting to the health center (Fig. 3). This ranged across health centers from 14.3% to 100% (Fig. 4), and across months from 50.0% to 92.7% (Fig. 5). Of the remaining patients, 88 (21.1%) never started treatment and 17 (4.1%) started treatment between 15 and 48 days of presenting to the health center.
3.3.4. ISTC-recommended care
Of the 6744 patients with presumptive TB, 2873 received ISTC-adherent care. The proportion who received ISTC-recommended care was 42.6% (95% CI: 37.2–48.7%) overall (Fig. 3) and ranged across health centers from 15.7% to 64.0% (Fig. 4). The range across months was 29.5%–50.9% (Fig. 5).
4. Discussion
Considerable resources have been devoted to scaling-up Xpert devices and cartridges in high TB burden countries, with many countries achieving high coverage. However, little attention has been paid to the impact of scale-up on the utilization of Xpert and quality of care for patients with presumptive TB, particularly at lower-level facilities without onsite Xpert testing capacity. At health centers linked to Xpert testing hubs across Uganda, we found that there was limited uptake of Xpert testing. Less than one-quarter of all patients and only 33.3% of patients with known HIV infection had samples referred for Xpert testing, and only 52.9% of patients confirmed to have TB by Xpert testing were initiated on treatment rapidly (within 14 days). Adherence to ISTC guidelines for TB diagnostic evaluation was poor, with less than half of patients receiving guideline-recommended care. Additional attention and resources are needed to ensure that high coverage of Xpert testing capacity translates into improvements in patient care.
Our findings are consistent with previous studies suggesting low uptake of Xpert testing. Hanrahan et al. also found Xpert utilization to be poor in Uganda, with only 21% of smear-negative patients receiving Xpert testing at 18 health centers, of which eight had onsite Xpert testing capacity [10]. Clouse et al. studied Xpert utilization among HIV/TB co-infected adults in 18 countries and found that <15% received Xpert testing even when onsite testing was available [11]. To our knowledge, however, our study is the first to assess the entire cascade of care for TB diagnostic evaluation, from referral for TB testing to TB treatment initiation for patients who test positive, in the context of Xpert scale-up. If generated in real-time, such data could be used to drive facility-level quality improvement.
Our study demonstrates that quality of care for patients undergoing TB evaluation remains poor despite high coverage of Xpert devices and cartridges. The vast majority of patients had samples referred for sputum smear examination as the first-line test, regardless of HIV status. When sputum was collected and sent for Xpert testing, a valid result was obtained for 97.7% of patients. However, there were significant delays in testing and notification of results, leading to only 52.9% of patients confirmed to have TB by Xpert testing being treated within 14 days and 37.0% not initiating treatment. In contrast, the vast majority (83.6%) of smear-positive TB patients initiated treatment at their initial health center visit.
This study has several potential limitations. First, we focus on impact of Xpert scale-up on diagnosis and treatment of drug susceptible TB. Although rapid identification of patients with likely MDR TB is a major advantage of Xpert testing, only two patients were found to have RIF-resistance in our study. Both patients were referred to an MDR TB treatment center and initiated MDR TB treatment within twelve days. Second, it is possible that our pragmatic data collection methods resulted in underestimation of Xpert utilization and its impact on quality of care. We believe this is unlikely as any Xpert testing missed through review of TB registers at the health centers was captured through review of the national GxAlert database. Finally, while our study highlights key gaps in the quality of care, further mixed methods research is needed to identify health system-, provider- and patient-level factors contributing to the gaps [12].
In summary, despite massive scale-up of Xpert testing capacity in Uganda, quality of care remains poor at peripheral TB diagnostic units. Funding agencies and National TB Programs in low- and middle- income countries should focus resources not just on procuring GeneXpert devices and Xpert cartridges, but also on ensuring that testing and communication of results to providers and patients occurs rapidly. Implementation and operational research is needed to identify barriers to rapid testing and treatment initiation. The development and testing of interventions to overcome these barriers will allow the scale-up of novel diagnostics to realize its full potential.
Acknowledgments
Conflict of interest
None.
Acknowledgments
The authors thank staff at the Uganda Ministry of Health facilities who participated in data collection. The study was supported by a grant from the U.S. National Institutes of Health (R01 HL130192).
Author contributions
The contributions of the study authors included study design (K.F., T.N., P.S., A.K., A.C.); data collection (K.F., T.N., C.O., M.N., S.N., D.O., A.H.); data analysis (K.F., K.F., A.C.); and manuscript preparation (K.F., T.N., K.F., A.C.). All authors reviewed and approved the final manuscript. K.F. had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding
Supported by the National Institutes of Health (A.C., R01 HL130192). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jctube.2019.100099.
Contributor Information
Katherine Farr, Email: katherine.farr@ucsf.edu.
Talemwa Nalugwa, Email: talemwan@yahoo.co.uk.
Priya B. Shete, Email: priya.shete@ucsf.edu.
Alvina H. Han, Email: ahh2142@columbia.edu.
Katherine Fielding, Email: katherine.fielding@lshtm.ac.uk.
David W. Dowdy, Email: ddowdy1@jhmi.edu.
DAJ Moore, Email: david.moore@lshtm.ac.uk.
J. Lucian Davis, Email: lucian.davis@yale.edu.
Achilles Katamba, Email: axk95@case.edu.
Adithya Cattamanchi, Email: adithya.cattamanchi@ucsf.edu.
Appendix. Supplementary materials
References
- 1.World Health Organization . World Health Organization; Geneva: 2015. Global tuberculosis report 2015. [Google Scholar]
- 2.WHO . World Health Organization; Geneva, Switzerland: 2013. Global strategy and targets for tuberculosis prevention, care and control after 2015. No. EB134/12. [Google Scholar]
- 3.Steingart K.R., Schiller I., Horne D.J., Pai M., Boehme C.C., Dendukuri N. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;1 doi: 10.1002/14651858.CD009593.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Global TB Programme . World Health Organization, Global TB Program; Geneva, Switzerland: 2013. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children; p. 1. online resource (1 PDF file (xv, 79 pages)) [PubMed] [Google Scholar]
- 5.World Health Organization. Annual number of xpert MTB/RIF cartridges procured under concessional pricing. 2016. Available from: http://www.who.int/tb/areas-of-work/laboratory/mtb-rif-rollout/en/.
- 6.World Health Organization. Global tuberculosis report 2017. 2017. Available from: http://www.who.int/tb/publications/global_report/en/.
- 7.Ministry of Health. National TB and leprosy program guidelines. 2012. http://library.health.go.ug/publications/tuberculosis/uganda-national-guidelines-tuberculosis-infection-control-health-care.
- 8.Ministry of Health. Manual for management and control of TB and leprosy 2017. 2017. Available from: http://library.health.go.ug/publications/service-delivery-diseases-control-prevention-communicable-diseases/tuberculosis/manual.
- 9.TB CARE I. International standards for tuberculosis care. 2014. Available from: http://www.who.int/tb/publications/ISTC_3rdEd.pdf.
- 10.Hanrahan C.F., Haguma P., Ochom E., Kinera I., Cobelens F., Cattamanchi A., Davis L., Katamba A., Dowdy D. Implementation of xpert MTB/RIF in Uganda: missed opportunities to improve diagnosis of tuberculosis. Open Forum Infect Dis. 2016;3:1–6. doi: 10.1093/ofid/ofw068. ofw068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clouse K., Blevins M., Lindegren M.L., Yotebieng M., Nguyen D.T., Omondi A., Michael D., Zannou D.M., Carriquiry G., Pettit A. International epidemiologic databases to evaluate ac. low implementation of xpert MTB/RIF among HIV/TB co-infected adults in the international epidemiologic databases to evaluate AIDS (IeDEA) program. PloS One. 2017;12 doi: 10.1371/journal.pone.0171384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cattamanchi A., Miller C.R., Tapley A., Haguma P., Ochom E., Ackerman S., Davis J.L., Katamba A., Handley M.A. Health worker perspectives on barriers to delivery of routine tuberculosis diagnostic evaluation services in Uganda: a qualitative study to guide clinic-based interventions. BMC Health Serv Res. 2015;15:10. doi: 10.1186/s12913-014-0668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.