Abstract
Background
Several radiological features have been reported in association with latent tuberculosis infection (LTBI) but it has not been studied which are specific. The aim of this study was to evaluate allegedly characteristic abnormalities on chest radiography (CXR) in patients with LTBI compared to uninfected controls.
Methods
From 236 patients tested with QuantiFERON-TB Gold In-Tube (QFT), the CXR was re-evaluated in a blinded fashion for fibrotic scarring, (non-)calcified nodules and pleural thickening. LTBI was defined as presence of a positive QFT result and/or positive tuberculin skin test result stratified by Bacille Calmette-Guérin-vaccination status.
Results
Any predefined abnormality of LTBI was observed in 116/236 (49.2%) patients, the frequency not being different between groups. However, the specificity for LTBI of a fibrotic scar ≥ 2 cm2 was 100% [95% CI: 92.0%–100%] and of a calcified nodule ≥1.5 mm was 95.7% [95% CI: 85.2%–99.5%]. The frequency of non-calcified nodules and pleural thickening did not differ between groups.
Conclusion
Only a fibrotic scar ≥ 2 cm2 and/or a calcified nodule ≥1.5 mm were significantly associated with LTBI. This finding is clinically relevant mainly in patients who are at significant risk of TB reactivation and in whom indirect diagnostic tests may be unreliable.
Keywords: Latent tuberculosis, Thoracic radiography, Diagnostic imaging, Sensitivity, Specificity, Pulmonary nodule
1. Introduction
Adequate screening for latent tuberculosis (TB) infection (LTBI) has become increasingly important in low TB-endemic countries such as The Netherlands, particularly due to a growing number of immigrants coming from high TB-endemic countries [1] and increasing use of immunosuppressive therapies [2]. For the diagnosis of LTBI the World Health Organisation (WHO) recommends using either the tuberculin skin test (TST) or interferon-gamma release assays (IGRA). In case of a positive TST or IGRA (either QuantiFERON Gold® or T-SPOT.TB® test) result, chest radiography (CXR) is indicated to evaluate for possible signs of active (subclinical) TB, followed by initiation of treatment for LTBI [3]. According to the Dutch national guideline regarding screening for LTBI before initiating immunosuppressive therapy, a CXR is always indicated regardless of immunological results [4]. From the literature it is known that certain allegedly characteristic abnormalities on CXR, such as (calcified) nodule(s), pleural thickening and/or fibrotic lesions, may be present in case of a prior Mycobacterium tuberculosis (Mtb) infection [5], [6]. Thus, the CXR could have additional value for LTBI screening. However, there are no validated criteria for interpretation of such abnormal findings on a CXR.
Both TST and IGRA, being dependent on the patient's immune system, can be false negative or indeterminate in patients with chronic diseases and/or immunosuppressed individuals [7], [8]. An advantage of the CXR is that abnormalities due to past infection are independent of the immune status. In previous studies, the prevalence of any of the mentioned radiological signs in individuals with LTBI ranged from 6.1% in a low TB-endemic area to 63.0% in a high TB-endemic area [9], [10], suggesting that repeated infections and/or higher infection burden may be associated with more detectable radiological lesions. Specific radiological signs such as non-calcified nodules and fibrotic lesions have been associated with a high risk of progression to active TB [5], [11], [12]. Most studies that assessed radiological signs of LTBI, however, lacked a control group of uninfected individuals. Three studies that did include a control group only referred to ‘radiological abnormalities’ but did not describe which lesions were assessed, nor were the radiologists blinded to the infection status of the study participants [11], [13], [14]. Thus, all past studies had a high risk of bias.
The aims of the present study were to assess the sensitivity and specificity of abnormalities previously reported as being suggestive for LTBI on CXR in individuals with or without LTBI. LTBI diagnosis was based on a positive QuantiFERON TB Gold In-tube® (QFT) and/or TST. The radiological assessment was performed in a blinded fashion, in the sense that the reader was blinded to the QFT and TST results and the diagnosis.
2. Methods
2.1. Setting
All CXRs were performed at either Leiden University Medical Center (LUMC), which is a tertiary care teaching hospital, the occupational health service (OHS) or at the regional municipal health service (MHS). The protocol of this retrospective observational study using anonymised data was evaluated by the Medical Ethics Committee of LUMC and waived from the requirement of informed consent (protocol G16.105).
2.2. Study design
Data were retrieved between October 2016 and June 2018. Collected data included demographics, immigration status, Bacille Calmette-Guérin (BCG)-vaccination status, and, if available, relevant comorbidities, risk factors, reported TB contact, past TB, QFT results, TST results if available and microbiological results.
2.3. Study participants
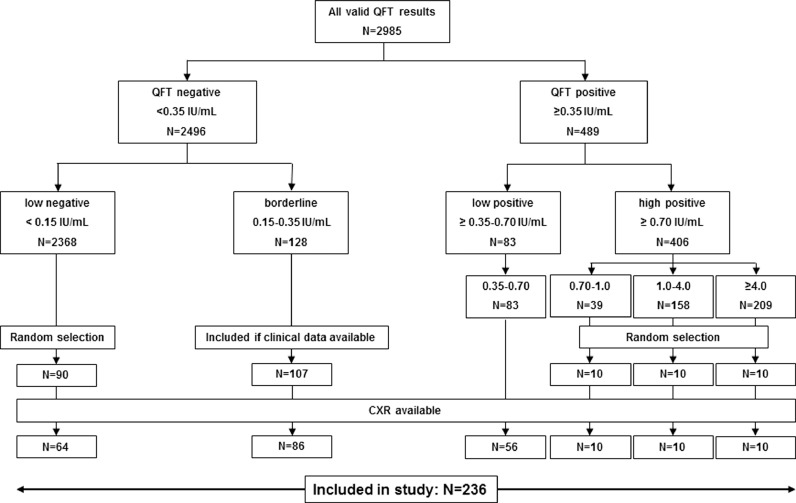
The study population has been previously described in a study which was focused on evaluation of QFT results near the cut-off [15]. The study population consists of 90 randomly selected individuals with a low-negative QFT result (<0.15 IU/ml), 107 subjects with a borderline (0.15–0.35 IU/ml), 83 with a low positive (0.35–0.70 IU/ml and a random selection of 30 patients with a high-positive (≥0.70 IU/ml) QFT result. Excluded were individuals <18 years old and those in whom no CXR had been performed. A flow diagram of the study population is shown in Fig. 1.
Fig. 1.
Overview of all included patients
NOTE. The selection of patients is described in detail in a previous publication [15].
Abbreviations: QFT: QuantiFERON-TB Gold In-Tube; CXR: chest radiography.
2.4. Classification criteria for LTBI
Patients were considered Mtb infected in case of a positive QFT result (≥0.35 IU/ml) and/or positive TST, defined as induration ≥15 mm irrespective of BCG-vaccination status or ≥10 mm in BCG unvaccinated individuals or those vaccinated up to one year of age. Mtb infection was considered possible in case of a borderline QFT result (0.15–0.35 IU/ml) in association with a negative TST result, if performed, or with no TST performed. In a separate analysis, the criteria for LTBI were broadened to include immunological proof and/or all available evidence for an infection with Mtb including history and microbiological results.
2.5. Radiography
Because the routine evaluation of the CXR investigations had often not primarily been focused on identification of characteristics of possible LTBI, all CXR were re-assessed by an expert thoracic radiologist (LK, who has >20 years of experience in CXR evaluation). The radiologist was blinded to all data except for age and clinical history of lung diseases, and thus unaware of QFT and TST results and final clinical diagnosis. All CXRs, both posteroanterior and lateral, were evaluated for signs compatible with prior TB, being fibrous scarring (including size and presence of volume loss), pleural thickening, calcified nodules (including calcified lymph nodes) with size and number, and non-calcified nodules. STROBE guidelines for reporting observational studies were followed [16].
2.6. Statistical analysis
Differences between categorical data were evaluated with chi square tests or Fisher's Exact Probability Test if appropriate. Sensitivity and specificity of abnormalities considered suggestive for LTBI on CXR were calculated. Continuous data were compared between groups using one-way ANOVA or nonparametrically with Mann-Whitney U or Kruskall-Wallis test as appropriate. Using two-sided testing, differences were considered significant at p < 0.05. Statistical analysis was performed using IBM SPSS Statistics version 23 and GraphPad Prism 7.
3. Results
3.1. Study population
Of 293 selected patients, no CXR was available for 57 individuals. In the remaining 236 the CXR was reassessed. The characteristics of all 236 included individuals are shown in Table 1. Roughly 75% originated from the hospital setting, the remaining 25% from the MHS/OHS. Patients from the hospital setting were significantly older, more often immunocompromised and had a higher frequency of TB-related abnormalities on CXR. Patients from the MHS/OHS were more frequently BCG vaccinated, TST positive and more often reported TB contact. The proportion of patients with LTBI was significantly higher in those originating from the MHS/OHS.
Table 1.
Characteristics of the study population.
| Leiden University Medical Center | Municipal or occupational health services | All | ||
|---|---|---|---|---|
| Characteristics | N = 179 | N = 57 | N = 236 | p value |
| Age (y) | 51.7 ± 19.4 | 39.7 ± 14.0 | 48.8 ± 18.9 | <0.001 |
| Sex (male) | 87/179 (48.6%) | 24/57 (42.1%) | 111/236 (47.0%) | 0.39 |
| Immigrant | 79/179 (44.1%) | 33/57 (57.9%) | 112/236 (47.5%) | 0.07 |
| Sub-Saharan Africa | 11/79 (13.9%) | 6/33 (18.2%) | 17/112 (15.2%) | |
| North Africa | 14/79 (17.7%) | 4/33 (12.1%) | 18/112 (16.1%) | |
| Asia/Indonesia | 35/79 (44.3%) | 8/33 (24.2%) | 43/112 (38.4%) | |
| Middle and S. America | 10/79 (12.7%) | 1/33 (3.0%) | 11/112 (9.8%) | |
| Eastern-Europe/Russia | 1/79 (1.3%) | 0/33 (0%) | 1/112 (0.9%) | |
| Other | 8/79 (10.1%) | 14/33 (42.4%) | 22/112 (19.6%) | |
| Originating from a high TB-endemic countrya | 43/179 (24.0%) | 11/57 (19.3%) | 54/236 (22.9%) | 0.46 |
| Travel to TB-endemic country | 65/159 (40.9%) | 25/50 (50.0%) | 90/209 (43.1%) | 0.26 |
| Reported past active TB | 5/179 (2.8%) | 0/57 (0%) | 5/236 (2.1%) | 0.34 |
| treated | 4/5 (80.0%) | 0/0 (0%) | 4/5 (80.0%) | |
| Reported contact with active TB | 26/179 (14.5%) | 38/57 (66.7%) | 64/236 (27.1%) | <0.001 |
| Professional risk | 32/179 (17.9%) | 13/57 (22.8%) | 45/236 (19.1%) | 0.41 |
| Immunocompromised | 54/179 (30.2%) | 1/57 (1.8%) | 55/236 (23.3%) | <0.001 |
| BCG-vaccinated | 73/179 (40.8%) | 37/57 (64.9%) | 110/236 (46.6%) | 0.001 |
| TST performed | 87/179 (61.7%) | 54/57 (94.7%) | 141/236 (59.7%) | <0.001 |
| TST ≥10 mm | 53/87 (60.9%) | 47/54 (87.0%) | 100/141 (70.9%) | <0.001 |
| QFT-GIT | 0.41 | |||
| <0.15 IU/ml | 52/179 (29.1%) | 12/57 (21.1%) | 64/236 (27.1%) | |
| 0.15–0.35 IU/ml | 63/179 (35.2%) | 20/57% (35.1%) | 83/236 (35.2%) | |
| ≥0.35 IU/ml | 64/179 (35.8%) | 25/57 (43.9%) | 89/236 (37.7%) | |
| LTBI (based on TST & IGRAb) | 0.006 | |||
| No | 42/179 (23.5%) | 4/57 (7.0%) | 46/236 (19.5%) | |
| Possible | 52/179 (29.1%) | 3/57 (5.3%) | 55/236 (23.3%) | |
| Yes | 85/179 (47.5%) | 50/57 (87.7%) | 135/236 (57.2%) | |
| Any prespecified abnormality on CXR | 96/179 (53.6%) | 20/57 (35.1%) | 116/236 (49.2%) | 0.015 |
Continuous variables are displayed as mean ± SD, categorical values are displayed as numerator over denominator (%).
Abbreviations: TB: tuberculosis; BCG: Bacille Calmette-Guérin; TST: tuberculin skin test; QFT-GIT: QuantiFERON-TB Gold In-Tube; LTBI: latent tuberculosis infection; IGRA: interferon-gamma release assays; CXR: chest radiography.
Defined as country with TB incidence ≥50 cases of active tuberculosis/100,000 inhabitants.
As explained in the methods.
3.2. Comparison between patients with LTBI and uninfected individuals
Patients classified as having LTBI were compared to those with possible LTBI (as based on only a borderline QFT result) and to those considered uninfected (Table 2). Any prespecified abnormality on CXR was observed in 116/236 (49.2%) of all included persons, in similar frequencies among patients with LTBI, possible LTBI or no LTBI. Pleural thickening was the most prevalent abnormality (24.2%), but not different between groups. Fibrotic scarring, both with and without volume loss was even significantly more often observed in individuals without LTBI. However, a fibrotic scar ≥ 2 cm2 was observed in 27 patients, all of whom were diagnosed with LTBI or possible LTBI (p = 0.003). At least one calcified nodule ≥ 1.5 mm was observed in 25 patients, of whom 23 (92.0% [95% CI: 74%−99%]) were diagnosed with LTBI or possible LTBI (p = 0.002). Examples of a fibrotic scar ≥ 2 cm2 without and with a calcified nodule ≥ 1.5 mm are shown in Figs. 2 and 3, respectively. Both features were very specific but not sensitive for LTBI (Table 3). Thus, positive findings were informative but absence of such lesions does not exclude LTBI. Four individuals had both a fibrotic scar ≥ 2 cm2 and a calcified nodule ≥ 1.5 mm, all of whom were diagnosed with either LTBI or possible LTBI. The frequency of non-calcified nodules did not differ between groups. If the criteria for LTBI were broadened including microbiological results and a positive history of latent or active TB, the results were not different.
Table 2.
Radiological characteristics stratified by Mtb infection status.
| No LTBI | Possible LTBI (borderline QFT only) | LTBI | All | ||
|---|---|---|---|---|---|
| Characteristics | N = 46 | N = 55 | N = 135 | N = 236 | p value |
| Age (y) | 49.0 ± 19.7 | 51.0 ± 21.2 | 47.8 ± 17.7 | 48.8 ± 18.9 | 0.55 |
| Sex (male) | 25/46 (54.3%) | 31/55 (56.4%) | 55/135 (40.7%) | 111/236 (47.0%) | 0.08 |
| Immigrant | 13/46 (28.3%) | 24/55 (43.6%) | 75/135 (55.6%) | 112/236 (47.5%) | 0.005 |
| BCG | 13/46 (28.3%) | 22/55 (40.0%) | 75/135 (55.6%) | 110/236 (46.6%) | 0.003 |
| Immunocompromised | 11/46 (23.9%) | 15/55 (27.3%) | 29/135 (21.5%) | 55/236 (23.3%) | 0.69 |
| Reported past active TB | 1/46 (2.2%) | 2/55 (3.6%) | 2/135 (1.5%) | 5/236 (2.1%) | 0.70 |
| treated | 0/1 (0%) | 2/2 (100%) | 2/2 (100%) | 4/5 (80%) | |
| Reported contact with active TB | 6/46 (13.0%) | 11/55 (20.0%) | 47/135 (34.8%) | 64/236 (27.1%) | 0.007 |
| Past pulmonary diseases nonTB | 13/43 (30.2%) | 6/26 (18.8%) | 11/56 (19.6%) | 30/131 (22.9%) | 0.38 |
| COPD/emfysema | 0/13 (0%) | 0/6 (0%) | 4/11 (36.4%) | 4/30 (13.3%) | |
| pneumonia nonTB | 6/13 (46.2%) | 3/6 (50.0%) | 5/11 (45.5%) | 14/30 (46.7%) | |
| other | 7/13 (53.8%) | 3/6 (50.0%) | 2/11 (18.2%) | 12/30 (40.0%) | |
| Prespecified signs of latent TB on CXR | 28/46 (60.9%) | 23/55 (41.8%) | 65/135 (48.1%) | 116/236 (49.2%) | 0.15 |
| Fibrotic scar any size | 13/46 (28.3%) | 15/55 (27.3%) | 19/135 (14.1%) | 47/236 (19.9%) | 0.034 |
| Fibrotic scar ≥ 2 cm2 | 0/44 (0%) | 12/54 (22.2%) | 15/135 (11.1%) | 27/233 (11.6%)a | 0.003 |
| Fibrotic scar any size with volume loss | 11/46 (23.9%) | 9/55 (16.4%) | 13/135 (9.6%) | 33/236 (14.0%) | 0.046 |
| Calcified nodules | 8/46 (17.4%) | 9/55 (16.4%) | 18/135 (13.3%) | 35/236 (14.8%) | 0.75 |
| Size of largest nodule | 0.053 | ||||
| <1.5 mm | 6/8 (75.0%) | 0/9 (0%) | 4/18 (22.2%) | 10/35 (28.6%) | |
| ≥1.5 mm | 2/8 (25.0%) | 9/9 (100%) | 14/18 (77.8%) | 25/35 (71.4%) | 0.002 |
| 1.5–3 mm | 1/8 (12.5%) | 4/9 (44.4%) | 6/18 (33.3%) | 11/35 (31.4%) | |
| 3–10 mm | 1/8 (12.5%) | 4/9 (44.4%) | 6/18 (33.3%) | 11/35 (31.4%) | |
| ≥10 mm | 0/8 (0%) | 1/9 (11.1%) | 2/18 (11.1%) | 3/35 (8.6%) | |
| Number | 0.72 | ||||
| 1 | 5/8 (62.5%) | 5/9 (55.6%) | 11/18 (61.1%) | 21/35 (60.0%) | |
| 2–5 | 2/8 (25.0%) | 4/9 (44.4%) | 4/18 (22.2%) | 10/35 (28.6%) | |
| >5 | 1/8 (12.5%) | 0/9 (0%) | 3/18 (16.7%) | 4/35 (11.4%) | |
| Non-calcified nodule | 3/46 (6.5%) | 2/55 (3.6%) | 10/135 (7.4%) | 15/236 (6.4%) | 0.71 |
| Pleural thickening | 12/46 (26.1%) | 8/55 (14.5%) | 37/135 (27.4%) | 57/236 (24.2%) | 0.16 |
Continuous variables are displayed as mean ± SD, categorical values are displayed as numerator over denominator (%).
Abbreviations: LTBI: latent tuberculosis infection; QFT: QuantiFERON-TB Gold In-Tube; BCG: Bacille Calmette-Guérin; TB: tuberculosis; COPD: chronic obstructive pulmonary disease.
The size of the fibrotic scar could not be assessed reliably in three cases due to other acute pathology (N = 2) or pre-existent familial pulmonary fibrosis (N = 1).
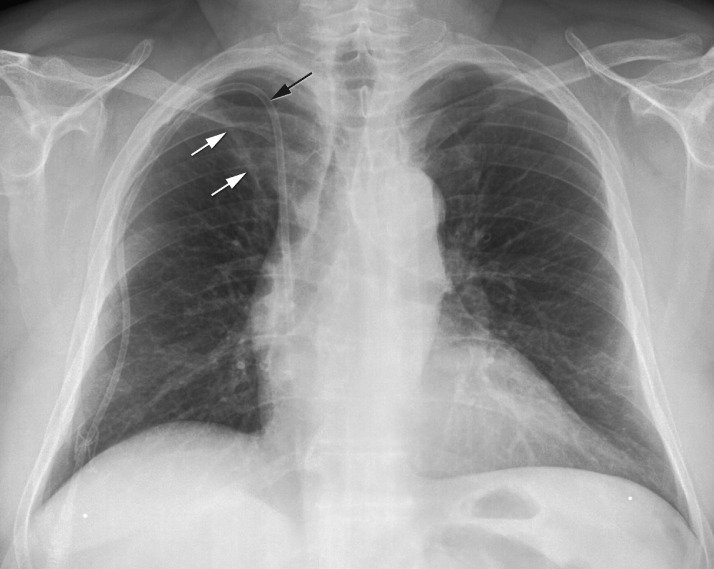
Fig. 2.
Fibrotic scar ≥ 2 cm2
Posteroanterior chest X-ray of a 52-year old Turkish man with diabetes mellitus and on hemodialysis. Central line in dialysis patient (black arrow). Fibrotic scar larger than 2 cm2 in the right upper lobe (white arrows).
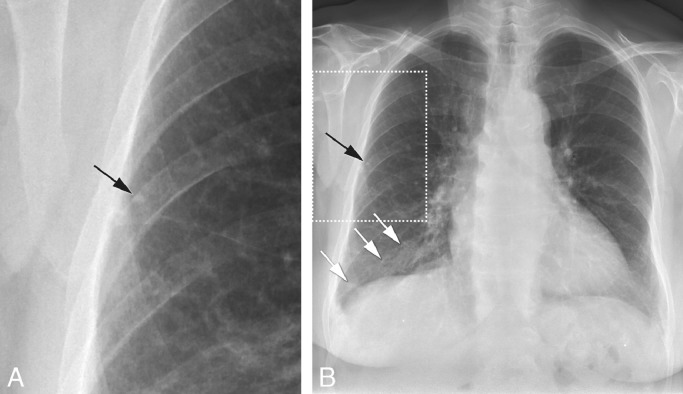
Fig. 3.
Fibrotic scar ≥ 2 cm2 with a calcified nodule ≥ 1.5 mm
78-year old female with diabetes mellitus and auto-immune disease on immunosuppression. Zoomed-in part (a) of posterioanterior chest X-ray (b) showing small 3 mm sized calcified nodule (black arrow in a, b). Fibrotic scar larger than 2 cm2 in the right lower lobe (white arrows, b).
Table 3.
Specific findings associated with latent tuberculosis infection.
| LTBI or possible LTBI N = 189 | No LTBI N = 44 | ||
|---|---|---|---|
| Fibrotic scar ≥ 2 cm2 | 27 | 0 | Positive predictive value: 100% |
| No fibrotic scar ≥ 2 cm2 | 162 | 44 | Negative predictive value: 21.4% (95% CI: 20.4%−22.4%) |
| Sensitivity: 14.3% (95% CI: 9.6%−20.1%) | Specificity: 100% (95% CI: 92.0%−100%) |
| LTBI or possible LTBI N = 190 | No LTBI N = 46 | ||
|---|---|---|---|
| Calcified nodule ≥ 1.5 mm | 23 | 2a | Positive predictive value: 92.0% (95% CI: 73.8%−97.9%) |
| No calcified nodule ≥ 1.5 mm | 167 | 44 | Negative predictive value: 20.9% (95% CI: 19.6%−22.2%) |
| sensitivity: 12.1% (95% CI: 7.8%−17.6%) | specificity: 95.7% (95% CI: 85.2%−99.5%) |
Abbreviations: LTBI: latent tuberculosis infection; CI: confidence interval.
In both cases no tuberculin skin test had been performed.
3.3. Assessment by QFT or TST result
Individuals were also compared by QFT and TST result (Tables S1 and S2). Patients with a positive, borderline or negative QFT result were similar with regard to the presence of most abnormalities on CXR, except for a fibrotic scar ≥ 2 cm2 which was more often observed in patients with a borderline or positive QFT result (p = 0.01). If one or more calcified nodules were present (N = 35), then presence of a calcified nodule ≥1.5 mm was strongly associated with a borderline or positive QFT result (p = 0.008). No significant differences in radiological abnormalities were observed between individuals with a positive or negative TST result.
4. Discussion
In this study, 236 CXRs of patients in whom a QFT had been performed for various reasons were re-evaluated for prespecified lesions by a radiologist who was unaware of corresponding IGRA and TST result and final clinical diagnosis. Presence of a fibrotic scar ≥2 cm2 and/or a calcified nodule ≥1.5 mm were specific for LTBI or possible LTBI. In our study, all other abnormalities on CXR that have previously been reported to be characteristic of LTBI occurred in similar frequencies in patients with or without LTBI.
It is plausible that a fibrotic scar ≥2 cm2 and/or a calcified nodule ≥1.5 mm on CXR were specific for LTBI because these lesions reflect the result of the Mtb-host interaction during infection in the lung. After inhalation, Mtb is phagocytosed by alveolar macrophages. At the site of infection, a granuloma is formed through interaction between infected macrophages, neutrophils, B cells and T cells [17]. In case of a non-progressor, the immune response will contain bacillary growth, which eventually can result in the development of fibrotic tissue in or around the granuloma [18], [19]. This fibrotic tissue is sometimes visible on CXR and can be (partly) calcified. The size of granulomas varies depending on the extent of granulomatous inflammatory response before the containment is achieved. A calcified granuloma at the initial place of infection in the lung is named a Ghon focus, while this lesion in combination with an ipsilateral calcified hilar node is named a Ranke complex [17], [19]. Nevertheless, both a fibrotic scar and a calcified nodule have an extensive differential diagnosis. and may be present in e.g. granulomatous diseases such as sarcoidosis, bacterial or other pulmonary infections or interstitial lung diseases [12], and/or occupational lung diseases such as silicosis or pneumoconiosis, or may represent metastasis [19].
Although not pathognomonic, our data suggest that a fibrotic scar and/or a calcified nodule in combination with an increased a priori risk, e.g. due to Mtb-exposure or history of TB or LTBI, is highly suggestive of TB infection. This finding can be of added value in patients in whom it is important not to miss LTBI because of a very high risk of TB reactivation and in whom the TST and IGRA may be unreliable as a result of an impaired immune status. Only two previous studies evaluated the difference between a fibrotic scar larger or smaller than 2 cm2, but both lacked uninfected controls. In the first study among TST positive healthcare workers, a fibrotic scar ≥ 2 cm2 was associated with a positive QFT [10]. In the second study among TST positive untreated individuals, it was associated with a higher risk of development of active TB [20]. To our knowledge, our study is the first to identify calcified nodules or calcified nodules ≥ 1.5 mm as being specific for LTBI.
Roughly 50% of all included patients in our study had any prespecified abnormality on CXR considered previously associated with LTBI. Previous studies specifically aimed at assessing radiological abnormalities in individuals with or without LTBI reported a prevalence of any suggestive lesion between 4.1% and 67.9% [9], [10], [21], [22], [23], [24]. This large range was probably related to the setting and the type of lesions that were assessed in these studies. In our cohort, the prevalence of certain radiological abnormalities was higher than reported in the literature, with the exception of calcified nodules. Five previous studies assessed the frequency of predefined abnormalities on CXR [9], [10], [22], [24], [25]. In these studies, the prevalence of fibrotic scarring, calcified nodules, non-calcified nodules, and pleural thickening varied from 0% to 3.0%, 1.8% to 59.8%, 0% to 3.0%, and 0.3% to 2.7%, respectively. However, none of these studies included uninfected controls, precluding conclusions with regard to the specificity of these lesions. The differences in the prevalence rates for each abnormality between our study and earlier literature could be caused by several characteristics of our study population and the method of assessment. Firstly, nearly 20% of all patients in our study had a history of pulmonary disease, even more in the group without LTBI, which could account for various non-TB related lesions on CXR that look similar to those due to past Mtb infection. Secondly, except for one study [25], earlier literature described a population that consisted of asymptomatic patients screened for occupational purposes, whereas patients in our study were screened for different reasons such as possible active TB, or detecting LTBI in case of eligibility for immunosuppressive therapy. Finally, the mean age of our population was at least 10 years higher than that of patients in previous studies, resulting in a higher chance of having other unreported pulmonary conditions reflected in various lesions on CXR.
That the frequency of most predefined lesions on the CXR in individuals with and without LTBI was similar is in line with the results of several previous studies [13], [26], [27], [28], [29], [30], most of which specified the lesions considered suggestive for LTBI [13], [26], [27], [29], [30], although only one was specifically aimed at finding abnormalities on CXR [13], and the radiologist was blinded in only two studies [27], [30]. In other studies, however, predefined lesions considered suggestive for LTBI were observed significantly more frequently in patients with LTBI than in uninfected controls [11], [23], [27], [29], [31]. The assessment by the radiologist was blinded in four of these studies [11], [23], [27], [31]. However, only one study was specifically aimed to find LTBI-related abnormalities on CXR [23]. Studies in which the radiologist is aware of immunological results prior to evaluating a CXR are subject to potential observer bias, as a radiologist can be more inclined to register minor abnormalities in patients with a positive TST or IGRA result. The strength of our study was that the radiologist was blinded to all immunological results and final clinical diagnosis. The lack of a second observer was a limitation, but a previous study demonstrated high inter-observer agreement between radiologists [10].
Another limitation of our study was that the study population included a disproportionally large number of individuals with a QFT result around the cut-off (0.15–0.70 IU/ml) and only a limited number of patients with a high positive QFT, as participants had been selected with the focus on borderline QFT results [15]. Therefore, it would be useful to repeat our study in a population reflecting the natural distribution of QFT results. Inclusion of more patients with a high positive QFT is expected to result in more pronounced differences between groups and may reveal additional lesions specific for LTBI. Interestingly, the presence of a fibrotic scar ≥2 cm2 and/or a calcified nodule ≥1.5 mm on CXR was found as often in individuals with possible LTBI (borderline QFT result with negative or no TST) as in those with LTBI. This corroborates the findings from previous studies in which it was demonstrated that most borderline QFT results reflect infection with Mtb as opposed to random assay variability [15], [32], [33].
In general, finding abnormalities of LTBI on a CXR can occur in three different settings. First, if a CXR is made to assess for possible active TB after a positive TST or IGRA result during screening for LTBI. Here, findings suggestive for LTBI have no added value since preventive therapy is guided by the positive immune test. A second setting is when lesions on CXR suspect for possible LTBI can be an incidental finding in patient in whom CXR is made for other purposes. If the patient is or will be at risk of TB reactivation, e.g. as a result of immunosuppressive therapy, treatment of LTBI may be indicated. The third situation, in which CXR can be of particular value, is when patients are screened for LTBI because of (planned) immunosuppression with drugs such as TNF antagonists or those used for prevention of rejection after organ transplantation, and in whom a suppressed or waned immune response can result in false negative TST or IGRA results [8], [34], [35]. Importantly, negative IGRA results include borderline QFT results and it has previously been demonstrated that the majority of individuals with a borderline QFT are actually infected with Mtb, which is highly relevant if immunosuppression is planned [15], [32], [33]. A case of disseminated TB during treatment with infliximab, and who in retrospect had a borderline QFT result, illustrates that the sensitivity of screening methods in this setting can be improved [36]. Nevertheless, the CXR has suboptimal sensitivity for LTBI, even for calcified nodules [37]. In that regard, computed tomography (CT) had a higher sensitivity and a high concordance rate with IGRA results [6], [38]. Because CT findings carry the risk of more false positive results it is of utmost importance to better define which lesions on CT are specific for TB infection. Computer-aided detection of active pulmonary TB on CXR has been studied [39], [40]. Thus far this has not yet been done for detection of LTBI, but this could be considered if the highly specific lesions observed in our study can be confirmed.
5. Conclusion
In conclusion, a fibrotic scar ≥ 2 cm2 and/or a calcified nodule ≥1.5 mm on CXR were specifically associated with prior TB infection. Finding such lesions is clinically relevant in patients who are at high risk of TB reactivation and in whom results of TST and IGRA may be false negative.
Acknowledgments
Conflict of interest
None.
Acknowledgements
This work was supported by supported by EC HORIZON2020 TBVAC2020 (contract no. 643381). The study sponsor was not involved in the study design; collection, analysis and interpretation of data; the writing of the manuscript nor the decision to submit the manuscript for publication.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jctube.2019.01.004.
Appendix. Supplementary materials
References
- 1.Lonnroth K., Mor Z., Erkens C., Bruchfeld J., Nathavitharana R.R., van der Werf M.J. Tuberculosis in migrants in low-incidence countries: epidemiology and intervention entry points. Int J Tuberc Lung Dis. 2017;21(6):624–637. doi: 10.5588/ijtld.16.0845. [DOI] [PubMed] [Google Scholar]
- 2.Burmester G.R., Bijlsma J.W.J., Cutolo M., McInnes I.B. Managing rheumatic and musculoskeletal diseases - past, present and future. Nat Rev Rheumatol. 2017;13(7):443–448. doi: 10.1038/nrrheum.2017.95. [DOI] [PubMed] [Google Scholar]
- 3.Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. Geneva: World Health Organization; 2018. https://www.who.int/tb/publications/2018/latent-tuberculosis-infection/en/. Last accessed on January 28th, 2019. [PubMed]
- 4.Nederlandse Vereniging van Artsen voor Longziekten en Tuberculose. Tuberculose en TNF-α blokkerende therapie. 2014 https://www.nvalt.nl/kwaliteit/richtlijnen/overige-relevante-documenten//Overige%20relevante%20documenten/NVALT-Statement-Tuberculose-en-TNF-a-blokkerende-therapie%20April%202014.pdf Available at: 2014. [Google Scholar]
- 5.Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000;161(4 Pt 2):S221–SS47. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 6.Lyu J., Lee S.G., Hwang S., Lee S.O., Cho O.H., Chae E.J. Chest computed tomography is more likely to show latent tuberculosis foci than simple chest radiography in liver transplant candidates. Liver Transpl. 2011;17(8):963–968. doi: 10.1002/lt.22319. [DOI] [PubMed] [Google Scholar]
- 7.Cattamanchi A., Smith R., Steingart K.R., Metcalfe J.Z., Date A., Coleman C. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals: a systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2011;56(3):230–238. doi: 10.1097/QAI.0b013e31820b07ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sester M., van Leth F., Bruchfeld J., Bumbacea D., Cirillo D.M., Dilektasli A.G. Risk assessment of tuberculosis in immunocompromised patients. A TBNET study. Am J Respir Crit Care Med. 2014;190(10):1168–1176. doi: 10.1164/rccm.201405-0967OC. [DOI] [PubMed] [Google Scholar]
- 9.Eisenberg R.L., Pollock N.R. Low yield of chest radiography in a large tuberculosis screening program. Radiology. 2010;256(3):998–1004. doi: 10.1148/radiol.10100485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joshi R., Patil S., Kalantri S., Schwartzman K., Menzies D., Pai M. Prevalence of abnormal radiological findings in health care workers with latent tuberculosis infection and correlations with T cell immune response. PLoS One. 2007;2(8):e805. doi: 10.1371/journal.pone.0000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christopoulos A.I., Diamantopoulos A.A., Dimopoulos P.A., Goumenos D.S., Barbalias G.A. Risk factors for tuberculosis in dialysis patients: a prospective multi-center clinical trial. BMC Nephrol. 2009;10:36. doi: 10.1186/1471-2369-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solsona Peiro J., de Souza Galvao M.L., Altet Gomez M.N. Inactive fibrotic lesions versus pulmonary tuberculosis with negative bacteriology. Arch Bronconeumol. 2014;50(11):484–489. doi: 10.1016/j.arbres.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Foster R., Ferguson T.W., Rigatto C., Lerner B., Tangri N., Komenda P. A retrospective review of the two-step tuberculin skin test in dialysis patients. Can J Kidney Health Dis. 2016;3:28. doi: 10.1186/s40697-016-0119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunimoto D., Der E., Beckon A., Thomas L., Egedahl M., Beatch A. Use of the QuantiFERON-TB Gold test to confirm latent tuberculosis infection in a Canadian tuberculosis clinic. Int J Tuberc Lung Dis. 2009;13(6):726–730. [PubMed] [Google Scholar]
- 15.Uzorka J.W., Kroft L.J.M., Bakker J.A., van Zwet E.W., Huisman E., Knetsch-Prins C. Proof of concept that most borderline Quantiferon results are true antigen-specific responses. Eur Respir J. 2017;50(5) doi: 10.1183/13993003.01630-2017. [DOI] [PubMed] [Google Scholar]
- 16.von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Bull World Health Organ. 2007;85(11):867–872. doi: 10.2471/BLT.07.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeong Y.J., Lee K.S. Pulmonary tuberculosis: up-to-date imaging and management. AJR Am J Roentgenol. 2008;191(3):834–844. doi: 10.2214/AJR.07.3896. [DOI] [PubMed] [Google Scholar]
- 18.Dannenberg A.M., Jr. Pathogenesis of pulmonary tuberculosis. Am Rev Respir Dis. 1982;125(3 Pt 2):25–29. doi: 10.1164/arrd.1982.125.3P2.25. [DOI] [PubMed] [Google Scholar]
- 19.Khan A.N., Al-Jahdali H.H., Allen C.M., Irion K.L., Al Ghanem S., Koteyar S.S. The calcified lung nodule: what does it mean? Ann Thorac Med. 2010;5(2):67–79. doi: 10.4103/1817-1737.62469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease C Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. International Union Against Tuberculosis Committee on Prophylaxis. Bull World Health Organ. 1982;60(4):555–564. [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer M., Clarke P., O'Regan A.W. Utility of the lateral chest radiograph in the evaluation of patients with a positive tuberculin skin test result. Chest. 2003;124(5):1824–1827. doi: 10.1378/chest.124.5.1824. [DOI] [PubMed] [Google Scholar]
- 22.Eisenberg R.L., Romero J., Litmanovich D., Boiselle P.M., Bankier A.A. Tuberculosis: value of lateral chest radiography in pre-employment screening of patients with positive purified protein derivative skin test results. Radiology. 2009;252(3):882–887. doi: 10.1148/radiol.2523082019. [DOI] [PubMed] [Google Scholar]
- 23.Jeong Y.J., Yoon S., Koo H.K., Lim H.J., Lee J.S., Lee S.M. Positive tuberculin skin test or interferon-gamma release assay in patients with radiographic lesions suggesting old healed tuberculosis. J Korean Med Sci. 2012;27(7):761–766. doi: 10.3346/jkms.2012.27.7.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gottridge J.A., Meyer B.R., Schwartz N.S., Lesser R.S. The nonutility of chest roentgenographic examination in asymptomatic patients with positive tuberculin test results. Arch Intern Med. 1989;149(7):1660–1662. [PubMed] [Google Scholar]
- 25.Eisenberg R.L., Heidinger B.H. Low yield of chest radiography in general inpatients and outpatients with "positive PPD" results in a country with low prevalence of TB. Acad Radiol. 2017;24(7):846–850. doi: 10.1016/j.acra.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Bonfiglioli K.R., Ribeiro A.C., Moraes J.C., Saad C.G., Souza F.H., Calich A.L. LTBI screening in rheumatoid arthritis patients prior to anti-TNF treatment in an endemic area. Int J Tuberc Lung Dis. 2014;18(8):905–911. doi: 10.5588/ijtld.13.0755. [DOI] [PubMed] [Google Scholar]
- 27.Seyhan E.C., Sokucu S., Altin S., Gunluoglu G., Trablus S., Yilmaz D. Comparison of the QuantiFERON-TB Gold In-Tube test with the tuberculin skin test for detecting latent tuberculosis infection in hemodialysis patients. Transpl Infect Dis. 2010;12(2):98–105. doi: 10.1111/j.1399-3062.2009.00469.x. [DOI] [PubMed] [Google Scholar]
- 28.Triverio P.A., Bridevaux P.O., Roux-Lombard P., Niksic L., Rochat T., Martin P.Y. Interferon-gamma release assays versus tuberculin skin testing for detection of latent tuberculosis in chronic haemodialysis patients. Nephrol Dial Transplant. 2009;24(6):1952–1956. doi: 10.1093/ndt/gfn748. [DOI] [PubMed] [Google Scholar]
- 29.Vassilopoulos D., Tsikrika S., Hatzara C., Podia V., Kandili A., Stamoulis N. Comparison of two gamma interferon release assays and tuberculin skin testing for tuberculosis screening in a cohort of patients with rheumatic diseases starting anti-tumor necrosis factor therapy. Clin Vaccine Immunol. 2011;18(12):2102–2108. doi: 10.1128/CVI.05299-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wauters A., Peetermans W.E., Van den Brande P., De Moor B., Evenepoel P., Keuleers H. The value of tuberculin skin testing in haemodialysis patients. Nephrol Dial Transplant. 2004;19(2):433–438. doi: 10.1093/ndt/gfg569. [DOI] [PubMed] [Google Scholar]
- 31.Roelsgaard E., Nyboe J. A tuberculosis survey in Kenya. Bull World Health Organ. 1961;25:851–870. [PMC free article] [PubMed] [Google Scholar]
- 32.Nemes E., Rozot V., Geldenhuys H., Bilek N., Mabwe S., Abrahams D. Optimization and interpretation of serial QuantiFERON testing to measure acquisition of Mycobacterium tuberculosis Infection. Am J Respir Crit Care Med. 2017;196(5):638–648. doi: 10.1164/rccm.201704-0817OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uzorka J.W., Bossink A.W.J., Franken W.P.J., Thijsen S.F.T., Leyten E.M.S., van Haeften A.C. Borderline QuantiFERON results and the distinction between specific responses and test variability. Tuberculosis (Edinb) 2018;111:102–108. doi: 10.1016/j.tube.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Leyten E.M., Arend S.M., Prins C., Cobelens F.G., Ottenhoff T.H., van Dissel J.T. Discrepancy between Mycobacterium tuberculosis-specific gamma interferon release assays using short and prolonged in vitro incubation. Clin Vaccine Immunol. 2007;14(7):880–885. doi: 10.1128/CVI.00132-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belard E., Semb S., Ruhwald M., Werlinrud A.M., Soborg B., Jensen F.K. Prednisolone treatment affects the performance of the QuantiFERON gold in-tube test and the tuberculin skin test in patients with autoimmune disorders screened for latent tuberculosis infection. Inflamm Bowel Dis. 2011;17(11):2340–2349. doi: 10.1002/ibd.21605. [DOI] [PubMed] [Google Scholar]
- 36.Uzorka J.W., Delfos N.M., Witte A.M.C., Scheper H., van Soolingen D., Arend S.M. Tuberculosis after a borderline QuantiFERON result during screening before infliximab. Eur Respir J. 2018;52(2) doi: 10.1183/13993003.00913-2018. [DOI] [PubMed] [Google Scholar]
- 37.Ketai L., Malby M., Jordan K., Meholic A., Locken J. Small nodules detected on chest radiography: does size predict calcification? Chest. 2000;118(3):610–614. doi: 10.1378/chest.118.3.610. [DOI] [PubMed] [Google Scholar]
- 38.Song D.J., Tong J.L., Peng J.C., Cai C.W., Xu X.T., Zhu M.M. Tuberculosis screening using IGRA and chest computed tomography in patients with inflammatory bowel disease: a retrospective study. J Digest Dis. 2017;18(1):23–30. doi: 10.1111/1751-2980.12437. [DOI] [PubMed] [Google Scholar]
- 39.Santosh K.C., Vajda S., Antani S., Thoma G.R. Edge map analysis in chest X-rays for automatic pulmonary abnormality screening. Int J CARS. 2016;11(9):1637–1646. doi: 10.1007/s11548-016-1359-6. [DOI] [PubMed] [Google Scholar]
- 40.Lakhani P., Sundaram B. Deep learning at chest radiography: automated classification of pulmonary tuberculosis by using convolutional neural networks. Radiology. 2017;284(2):574–582. doi: 10.1148/radiol.2017162326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.