Abstract
Setting
A prospective observational study conducted in Medical college hospital, in a high-TB- prevalence region of northern Telangana, India.
Objective
To know the diagnostic role of Xpert MTB/RIF assay in bronchoalveolar lavage fluid (BALF) in sputum-scarce, suspected pulmonary tuberculosis (PTB) patients.
Design
Study period was between October 2014 and March 2017. Suspected pulmonary tuberculosis patients aged 15 years or more, who were sputum-scarce and conforming to the inclusion criteria were submitted to bronchoscopy. BALF thus obtained was submitted to smear for acid fast bacilli (AFB) and Xpert MTB/RIF assay as index tests along with culture for Mycobacterium tuberculosis complex (MTBC). Culture for M. tuberculosis complex was considered as gold standard for the diagnosis of PTB. The sensitivity, specificity and predictive values were calculated for smear AFB and Xpert MTB/RIF assay.
Results
56 of the 81 patients were included and evaluated in the final analysis. In 10 of these 56 patients PTB was confirmed by culture positivity. The sensitivity and specificity of Xpert MTB/RIF assay was 90% (9/10,95%CI 59.6- 98.2) and 52.2% (24/46, 95%CI 38. 1-65.9) respectively and that of the smear AFB was 60% (6/10, 95%CI 31.2–83.1) and 67.4% (31/46, 95%CI 53.0–79.1). All the patients considered ‘probable’ PTB (pending culture results), were administered antituberculous treatment and showed complete clinicoradiological improvement on follow up. Three of the 31 Xpert MTB/RIF positive patients were detected as resistance to rifampicin (RR).
Conclusions
Xpert MTB/RIF assay of BALF in the study cohort provides rapid diagnosis of Mycobacterium tuberculosis, and detection of rifampicin resistance at the very outset, aiding in selection of appropriate ATT regimen. In this context, it can be recommended as the first line investigation. Xpert MTB/RIF assay aided by HRCT Chest and suggestive clinical presentation may be helpful in early institution of ATT especially in smear negative, culture negative cases.
Keywords: Xpert MTB/RIF assay, BAL fluid, Tuberculosis
1. Introduction
Global burden of tuberculosis continues to challenge its treatment and national programs into the 21st century. TB remained one of the top 10 causes of death worldwide in year 2016. An estimated 10.4 million people fell ill with TB in 2016: 90% were adults;65% male,10% were people living with HIV (74% in Africa) and 56% were in five countries: India, Indonesia, China, Philippines and Pakistan. [1].
The updated estimate of TB incidence in India is 211 cases per 100 000 population, 2.79 million cases for the year 2016 and the TB deaths being 32 per 100,000 population in 2016 [1]. Similar figures regarding the incidence of TB were noted regionally (northern Telangana) where the present study was conducted.
In 2016, there were 600 000 new cases with resistance to rifampicin (RRTB), of which 490 000 had multidrug-resistant TB (MDR-TB). Almost half (47%) of these cases were in India, China and the Russian Federation [1].
The above observations emphasize the need for not only early diagnosis of pulmonary tuberculosis but also its drug-sensitivity at the very outset. The infectious dose for transmission of tuberculosis is very low, usually 1–5 tubercle bacilli, thus reflecting the propensity of getting infection and need to aggressively diagnose and treat even sputum-scarce, suspect PTB patients, at the earliest [2]. A considerable proportion of the TB cases reported to WHO are still clinically diagnosed and in 2016, only 57% of the pulmonary cases reported to WHO were bacteriologically confirmed [1]. Up to one third of TB-HIV coinfected patients were found to be sputum-scarce in a study by Peter et al. [3]. In an Ethiopian study by Desta et al., it was noted that 82.6% of the cases were smear-negative and culture-negative, indicating the magnitude of the smear-negative PTB and the need for early and specific diagnosis [4]. In this context, a rapid test which enables early diagnosis of pulmonary tuberculosis and drug sensitivity, which aids in initiation of appropriate treatment regimen, is the need of the hour.
With the advent of Xpert MTB/RIF into the diagnostics of TB detection, it was noted that the test has much better accuracy than microscopy and culture methods [1]. The assay can give the rifampicin sensitivity results, simultaneously. In a recent study from India, which includes respiratory samples including sputum, Xpert MTB/RIF assay showed an overall sensitivity and specificity of 95.7% and 99.3% respectively and a sensitivity of 77.7% in smear negative-culture positive cases [5].
In the present study, we intended to find the diagnostic role of Xpert MTB/RIF assay in a specific group of cohorts of sputum-scarce, suspected PTB, wherein only the BALF could be obtained for the evaluation. In the absence of any specific National or regional guidelines regarding the diagnostic approach to be adopted in these subjects, we carried this study.
2. Subjects and methods
2.1. Study population
The present prospective observational study includes patients aged 15 years or more who either attended outpatient or admitted as inpatient in the department of Respiratory medicine, Prathima Institute of Medical Sciences (PIMS), Karimnagar, with suspect PTB, between 01/10/2014 and 31/3/2017. This is one of the private Institutes of Medical Sciences, providing tertiary medical services and a referral center to the northern Telangana region of India. Only those patients who are ‘sputum-scarce’ with suspected pulmonary TB were included in the study. All other patients with sputum production, either spontaneous or induced, and those with history of taking anti-TB treatment (ATT), and patients tested positive for HIV were excluded from the study. Similarly, patients who were moribund and could not withstand the bronchoscopic procedure for BALF, were excluded. The study was approved by the Institutional Ethics Committee. A fully informed written consent was obtained from all the subjects who are part of this study.
2.2. Procedure for BAL fluid
We used flexible bronchoscope (model: Olympus BF Type TE 2) for performing BAL procedure for obtaining the respiratory sample. We adopted the BAL procedure, in all patients to avoid any influence of the procedure on the diagnostic yield from the sample. The BAL fluid was obtained following the Official Clinical Practice Guideline of The American Thoracic Society (ATS) [6]. Of the samples thus obtained, half was submitted for AFB-smear and culture for Mycobacterium tuberculosis complex (MTBC) and the other half for Xpert MTB/RIF assay. All the samples were processed at SRL Diagnostics (College of American Pathologists accredited).
2.3. Microbiology and molecular biology
The decontamination of half the BAL fluid sent for smear and culture was done with the standard protocol of using sterile N-acetyl-l-cysteine/4% NAOH and centrifugation at a speed of 3000x g for 20 minutes. One smear was examined after Ziehl-Neelsen staining for AFB.
For culture, the above sample was inoculated into the BACTEC-MGIT 960 Instrument (culture system) for 6 weeks (Becton-Dickinson, Sparks, Maryland, USA).
Xpert MTB/RIF assay, an automated cartridge-based molecular technique, was performed on the other half of the BAL sample, according to the manufacturer's instructions. BAL fluid without prior decontamination was loaded into the Xpert-cartridge and test reported as ‘detected’ or ‘not detected’ (Cepheid GeneXpert System, Sunnyvale, US) [7].
2.4. Diagnosis of pulmonary tuberculosis
Diagnosis of active PTB was considered based on the positive culture of M. tuberculosis complex, which is the gold-standard.
Patients who were either positive for Xpert MTB/RIF assay of the BALF or those having suggestive clinicoradiological features of PTB and Xpert MTB/RIF negative were considered to have ‘probable PTB’. The latter group showed complete clinicoradiological recovery with ATT [8], [9]. Patients who tested negative for BALF Xpert MTB/RIF assay and smear AFB and without suggestive imaging features of PTB either on chest radiograph or HRCT chest were not considered for ATT and were evaluated for other causes and treated accordingly, pending culture AFB results. They were categorized as non-TB when their culture AFB was negative. HRCT Chest was taken where the chest radiograph was either indeterminate or for differential diagnosis and in considering ‘probable’ PTB.
2.5. Statistical analysis
Demographic and clinical characteristics were presented in number and percentages. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for the diagnosis of sputum-scarce, suspect PTB patients were calculated for BALF AFB smear and Xpert assay, and their 95% confidence intervals (CIs) were estimated using Wilson's score method by OpenEpi Diagnostic calculator, Version 3. McNemar's test was performed for comparison of sensitivities.
3. Results
3.1. Characteristics of study population
Of the 81 patients considered for the study, 56 patients were included for final analysis after excluding the rest not conforming to the inclusion criteria. Demographic and clinical features of the study subjects were shown in Table 1.
Table 1.
Demographic and clinical characteristics of 56 patients with suspected PTB.
| Characteristics | n (%) |
|---|---|
| Age, years (Mean ± SD) | 47 ± 20 |
| Male | 35 (63) |
| Urban Residents | 25 (45) |
| Chest symptoms (present) | |
| Cough | 52 (93) |
| Fever | 46 (82) |
| Chest pain | 10 (18) |
| Dyspnea | 15 (27) |
| Hemoptysis | 07 (13) |
| Diabetes Mellitus | 08 (14) |
| Mycobacterium Tuberculosis | |
| Culture positive | 10 (18) |
| Xpert MTB/RIFpositive | 31 (55) |
| AFB smear positive | 21 (38) |
| Clinico-radiologically suggested | 10* (18) |
| Non-TB | 15 (27) |
| NTM† | 01 |
| Lung Cancer | 01 |
| Acute Exacerbation Of | 03 |
| Bronchiectasis | |
| Non-Mycobacterial (Pneumonia) | 10 |
one of the ten clinicoradiologically diagnosed patient was culture positive
NTM-Nontuberculous mycobacteria
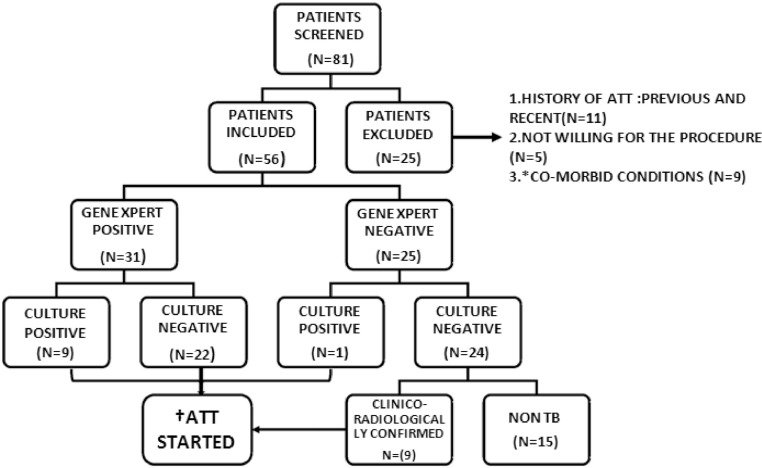
Of the 56 patients thus included, 41 (73.2%) were considered ‘probable’ PTB and treated with ATT. Thus, pending culture AFB results, 31 patients based on positive Xpert and 10 based on clinicoradiographic features received treatment with ATT (Figure). All the patients who received ATT showed complete clinicoradiological recovery. Remaining 15 patients were considered non- tuberculous and the details of which were briefed in Table 1.
Figure.
Flow chart depicting analysis of subjects included in the study.
* - Coronary artery disease, Chronic Kidney disease, Exacerbation of airway disease.
† - Anti tuberculosis treatment.
3.2. Microbiology
BALF of 10 of the 56 patients was culture positive for M. tuberculosis complex, which was taken as gold-standard in the final analysis of the results. The BALF of remainder 46 subjects was culture negative; 22 (55.4%) of them tested positive for Xpert MTB/RIF and 24 (44.6%) were negative. 9 of the ten culture positive cases were also Xpert positive. BALF smear for AFB was positive in 21 patients and Xpert assay detected 10 of the smear negatives in the diagnostic evaluation. The latter observation enabled 10 more patients to receive specific treatment with ATT without further delay in the diagnosis.
The overall sensitivity of the BALF GeneXpert MTB assay was 90% (9/10, 95%CI 59.6- 98.2), and overall specificity was 52.2% (24/46, 95%CI38.1- 65.9). The positive predictive value
(PPV) was 29.03% (95%CI16.1–46.6), while the negative predictive value (NPV) was 96% (95%CI 80.5- 99.3). Sensitivity of smear AFB of BALF was 60% (6/10, 95%CI 31.2–83.1) and the specificity was 67.4% (31/46, 95%CI 53.0–79.1) (Table 2).
Table 2.
Diagnostic accuracy of Xpert MTB/RIF assay and AFB smear using BALF with AFB culture as gold standard.
| Variables | Sensitivity* % (95 CI) | Specificity % (95 CI) | Positive Predictive Value % (95 CI) | Negative Predictive Value % (95 CI) |
|---|---|---|---|---|
| Xpert MTB/RIF assay | 90.0 (59.6–98.2) | 52.2 (38.1–65.9) | 29.03 (16.1–46.6) | 96.0 (80.5–99.3) |
| AFB smear | 60.0 (31.2–83.1) | 67.4 (53.0–79.1) | 28.6 (13.8–50.0) | 88.6 (74.1–95.5) |
(McNemar's Test: *p = 0.25). BALF: Bronchoalveolar lavage fluid.
Overall, BALF of 35 patients (62.5%) was AFB smear-negative. The sensitivity and specificity of Xpert MTB/RIF assay in AFB smear-negative BALF samples were 75% (3/4, 95% CI 30.06- 95.44) and 77.42% (24/31, 95%CI 60.19- 88.61) respectively. All twenty-one (37.5%) patients with AFB smear-positive BALF samples had tested positive for Xpert MTB/RIF.
3.3. Detection of rifampicin resistance
Three of the 31 patients with Xpert MTB/RIF assay positive detected rifampicin resistance and these patients were referred to Revised National Tuberculosis Control Program (RNTCP) center for further management.
3.4. Radiographic profile
Most of the radiographic features noted, both in GeneXpert positive and negative patients, were nodular and consolidative lesions, whether diffuse or localized. Amongst the 31 GeneXpert- positive cases 22 had nodular lesions and six were consolidations and only three had cavitating lesions. Of the ten GeneXpert- negative cases five were nodular and three were consolidations and rest of the two were mixed lesions.
4. Discussion
Smear AFB-negative or sputum-scarce PTB masquerades as various non-tuberculous disorders, thus contributing to delay in diagnosis causing transmission and drug resistance. In these patients the fibreoptic bronchoscopy is the only tool to obtain the respiratory samples like bronchial washings or BALF for rapid and specific diagnosis.
To make an early diagnosis and prompt treatment, minimizing empiricism, we intended to study the diagnostic role of Xpert MTB/RIF assay of BALF in the study cohort.
The studies of Theron et al. and Lee et al. prompted and guided us to undertake this study, which are also from a high-TB incidence area [10], [11]. The present study revealed 90% sensitivity of Xpert assay and 60% for the AFB smear in BALF, though these results were not statistically significant. Overall specificity of Xpert MTB/RIF is 52.2% and the latter was enhanced to 77.4% when only smear negative cases were taken into consideration. Xpert assay helped in the diagnosis of additional 10 cases where smear was negative.
Despite the culture of MTBC being the gold standard in the diagnosis of active PTB, the inherent problem of delay in obtaining the results cannot aid in the rapid and early diagnosis of TB and detection of drug resistance and initiating prompt treatment.
In our study,10 of the 41 ‘probable’ - PTB patients were culture positive for M. tuberculosis complex.
Furthermore, present study noted that Xpert MTB/RIF could detect 9 of the 10 culture positive cases. Before the advent of Xpert MTB/RIF assay the BALF smear AFB showed better sensitivity over the sputum smears, though the latter has the disadvantage of not being specific for M. tuberculosis. Of the 25 patients with a negative sputum smear, BALF led to rapid diagnosis in 14 (56%) patients in a study by Tueller et al. (eight BAL MTC-PCR and BALF smear positive) [12]. Present study constitutes specific subgroup of suspect PTB patients with no sputum and involves the outcomes pertaining to BALF only. We did not obtain respiratory samples from transbronchial lung biopsy (TBLB) in view of the attendant morbidity, higher cost implications and with no added advantage shown from the previous studies [13]. The poor yield of culture in the present study might be from the mycobactericidal effect of the lidocaine used during the bronchoscopy procedure for obtaining the BALF. During the procedure, we tried our best to restrain from excessive indulgence of its use except for very uncooperative patients. The study by Conte and Laforet showed that the lidocaine (1 ml of 2%), which was half the amount required for bronchoscopy, carried a significant inhibitory effect on Mycobacterium tuberculosis. Limiting the quantity of lidocaine for this purpose would also compromise with the quality and quantity of the sample and the patient cooperation during the procedure [14], [15]. In our study, Xpert assay detected 22 culture negative patients who were successfully treated with ATT. Lee et al. in their retrospective study involving similar cohort noted that four of the 12 culture negative patients tested positive for Xpert assay. This could suggest that the Xpert assay may be more sensitive than the culture AFB in this clinical scenario [11].
Theron et al., in their study observed 16 of the 19 Xpert MTB/RIF-positive, culture- negative patients were deemed likely to be true positives and designated them as “highly likely TB”. Their data suggests, as they noted, that the sensitivity in negative TB was limited by bacterial load and Xpert MTB/RIF Cycle threshold values correlating smear with bacterial load would be important. They noted the limit of detection in their sputum samples below 100 cfu/ml [16]. Similar observations were noted regarding the Xpert MTB/RIF positive, culture negative results by Barnard et al. [17].
Reflection of these observations may be implicated in most of the radiographic patterns being nodular and/or consolidative lesions in the present study, which in turn might reflect a low-bacillary burden of the lesions. Where chest X-ray findings were indeterminate, HRCT Chest scanning enabled in early diagnosis and treatment of our smear negative and Xpert negative patients. As noted by the studies of Lee et al. and Hatipoglu et al., it can be accurate in specific diagnosis and management of active pulmonary tuberculosis in these smear- negative, Xpert-negative patients [18], [19].
10 patients of the study who were diagnosed as ‘probable’ PTB based on clinicoradiographic features were Xpert- negative and smear-negative. Subsequently one of them proved culture positive. All these patients showed complete clinicoradiological improvement on ATT. Thus, in our smear negative, Xpert negative and culture negative patients we still had to depend on the clinicoradiographic features and treat these patients empirically.
In a study by Shin et al., the combination of bronchoscopically obtained respiratory specimens with HRCT, in sputum smear negative PTB, increased the sensitivity to 96.3% and NPV to 96.2% [20].
Though our study did not intend to compare diagnostic role of radiographic features of PTB with Xpert MTB/RIF assay, we did not find any correlation nor any statistical significance between the assay and pattern and extent of radiographic lesions.
Three patients were detected as Xpert MTB/RIF- Rifampicin resistance, emphasizing the added advantage of the assay.
Smaller sample size of the study cohort might be the plausible cause for the sensitivity of Xpert assay and that of the smear not being significant statistically. Other weakness of our study might be that the various demographic variables, like age and gender patterns, association of diabetes and alcoholism, radiological patterns and their correlation with duration of the symptoms and bacillary load in the form of threshold values of the Xpert assay, were not accounted for.
Most of these patients who are sputum-scarce and culture negative, probably represents an early disease state with few symptoms and paucity of radiographic abnormalities. In a high-TB burden region this invites empirical treatment with ATT and consequent adverse drug effects and drug resistance, which reemphasizes the need for a test like Xpert MTB/RIF assay which aid in rapid diagnosis of the study cohort [21]. Future larger studies, in high-prevalence setting of TB, may further clarify the diagnostic role of Xpert MTB/RIF assay in similar cohorts.
5. Conclusion
Since Xpert MTB/RIF assay of BALF helps in rapid diagnosis of sputum scarce, suspect PTB patients, and in detection of rifampicin resistance, it can be the first line diagnostic modality. In Xpert assay negative patients with pending culture results, other diagnostic modalities like HRCT chest along with suggestive clinical presentation may be reinforced for an early diagnosis and treatment with ATT. These observations may help in future guidelines for the clinicians handling this subgroup of cohorts and in formulating the National guidelines.
Conflict of interest
None.
Acknowledgments
Acknowledgements
No acknowledgements.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Global tuberculosis report 2017. Geneva: world health organization.
- 2.Balasubramanian V., Wiegeshaus E.H., Taylor B.T., Smith D.W. Pathogenesis of tuberculosis: pathway to apical localization. Tubercle Lung Dis. 1994;75(3):168–178. doi: 10.1016/0962-8479(94)90002-7. Jun 1. [DOI] [PubMed] [Google Scholar]
- 3.Peter J.G., Theron G., Muchinga T.E., Govender U., Dheda K. The diagnostic accuracy of urine-based Xpert MTB/RIF in HIV-infected hospitalized patients who are smear-negative or sputum scarce. PloS One. 2012;7(7):e39966. doi: 10.1371/journal.pone.0039966. Jul 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desta K., Asrat D., Lemma E., Gebeyehu M., Feleke B. Prevalence of smear negative pulmonary tuberculosis among patients visiting St. Peter's Tuberculosis Specialized Hospital, Addis Ababa, Ethiopia. Ethiop Med J. 2009;47(1):17–24. Jan. [PubMed] [Google Scholar]
- 5.Sharma S.K., Kohli M., Yadav R.N., Chaubey J., Bhasin D., Sreenivas V., Sharma R., Singh B.K. Evaluating the diagnostic accuracy of Xpert MTB/RIF assay in pulmonary tuberculosis. PloS One. 2015;10(10) doi: 10.1371/journal.pone.0141011. Oct 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer K.C., Raghu G., Baughman R.P., Brown K.K., Costabel U., Du Bois R.M., Drent M., Haslam P.L., Kim D.S., Nagai S., Rottoli P. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2012;185(9):1004–1014. doi: 10.1164/rccm.201202-0320ST. May 1. [DOI] [PubMed] [Google Scholar]
- 7.Xpert MTB/RIF, Package insert, Cepheid, CA, USA (2009).
- 8.Kanaya A.M., Glidden D.V., Chambers H.F. Identifying pulmonary tuberculos in patients with negative sputum smear results. Chest J. 2001;120(2):349–355. doi: 10.1378/chest.120.2.349. Aug 1. [DOI] [PubMed] [Google Scholar]
- 9.Tozkoparan E., Deniz O., Ciftci F., Bozkanat E., Bicak M., Mutlu H., Ors F., Bilgic H., Demirci N. The roles of HRCT and clinical parameters in assessing activity of suspected smear negative pulmonary tuberculosis. Arch Med Res. 2005;36(2):166–170. doi: 10.1016/j.arcmed.2004.12.010. Mar 4. [DOI] [PubMed] [Google Scholar]
- 10.Theron G., Peter J., Meldau R., Khalfey H., Gina P., Matinyena B., Lenders L., Calligaro G., Allwood B., Symons G., Govender U., Setshedi M., Dheda K. Accuracy and impact of Xpert MTB/RIF for the diagnosis of smear-negative or sputum-scarce tuberculosis using bronchoalveolar lavage fluid. Thorax. 2013;68(11):1043–1051. doi: 10.1136/thoraxjnl-2013-203485. Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee H.Y., Seong M.W., Park S.S, Hwang S.S., Lee J., Park Y.S., Lee C.H., Lee S.M., Yoo C.G., Kim Y.W., Han S.K., Yim J.J. Diagnostic accuracy of Xpert® MTB/RIF on bronchoscopy specimens in patients with suspected pulmonary tuberculosis. Int J Tuberc Lung Dis. 2013;17(7):917–921. doi: 10.5588/ijtld.12.0885. Jul 1. [DOI] [PubMed] [Google Scholar]
- 12.Tueller C., Chhajed P.N., Buitrago-Tellez C., Frei R., Frey M., Tamm M. Value of smear and PCR in bronchoalveolar lavage fluid in culture positive pulmonary tuberculosis. Eur Respir J. 2005;26(5):767–772. doi: 10.1183/09031936.05.00046105. Nov 1. [DOI] [PubMed] [Google Scholar]
- 13.Mok Y., Tan T.Y., Tay T.R., Wong H.S., Tiew P.Y., Kam J.W., Siao C. Do we need transbronchial lung biopsy if we have bronchoalveolar lavage Xpert® MTB/RIF? Int J Tuberc Lung Dis. 2016;20(5):619–624. doi: 10.5588/ijtld.15.0463. May 1. [DOI] [PubMed] [Google Scholar]
- 14.Conte B.A., Laforet E.G. The role of the topical anesthetic agent in modifying bacteriologic data obtained by bronchoscopy. New Engl J Med. 1962;267(19):957–960. doi: 10.1056/NEJM196211082671903. Nov 8. [DOI] [PubMed] [Google Scholar]
- 15.Iyer V.N., Joshi A.Y., Boyce T.G., Brutinel M.W., Scalcini M.C., Wilson J.W., McCoy K., Aksamit T.R. Bronchoscopy in suspected pulmonary TB with negative induced-sputum smear and MTD® Gen-probe testing. Respir Med. 2011;105(7):1084–1090. doi: 10.1016/j.rmed.2011.03.003. Jul 31. [DOI] [PubMed] [Google Scholar]
- 16.Theron G., Peter J., van Zyl-Smit R., Mishra H., Streicher E., Murray S., Dawson R., Whitelaw A., Hoelscher M., Sharma S., Pai M. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med. 2011;184(1):132–140. doi: 10.1164/rccm.201101-0056OC. Jul 1. [DOI] [PubMed] [Google Scholar]
- 17.Barnard D.A., Irusen E.M., Bruwer J.W., Plekker D., Whitelaw A.C., Deetlefs J.D., Koegelenberg C.F. The utility of Xpert MTB/RIF performed on bronchial washings obtained in patients with suspected pulmonary tuberculosis in a high prevalence setting. BMC Pulm Med. 2015;15(1):103. doi: 10.1186/s12890-015-0086-z. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee K.S., Hwang J.W., Chung M.P., Kim H., Kwon O.J. Utility of CT in the evaluation of pulmonary tuberculosis in patients without AIDS. Chest. 1996;110(4):977–984. doi: 10.1378/chest.110.4.977. Oct 31. [DOI] [PubMed] [Google Scholar]
- 19.Hatipoğlu O.N., Osma E., Manisali M., Ucan E.S., Balci P., Akkoclu A., Akpinar O., Karlikaya C., Yüksel C. High resolution computed tomographic findings in pulmonary tuberculosis. Thorax. 1996;51(4):397–402. doi: 10.1136/thx.51.4.397. Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin J.A., Chang Y.S., Kim T.H., Kim H.J., Ahn C.M., Byun M.K. Fiberoptic bronchoscopy for the rapid diagnosis of smear-negative pulmonary tuberculosis. BMC Infect Dis. 2012;12(1):141. doi: 10.1186/1471-2334-12-141. Jun 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen M.-V.H., Jenny-Avital E.R., Burger S., Leibert E.M., Achkar J.M. Clinical and radiographic manifestations of sputum culture negative pulmonarytuberculosis. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0140003. [DOI] [PMC free article] [PubMed] [Google Scholar]