Abstract
Background
Cambodia has one of the highest tuberculosis (TB) prevalence rates in the world. People aged 55 years and over account for an estimated 50% of the country's TB burden, yet this group has a low notification rate owing to specific barriers in accessing health services. One-off active case finding (ACF) days with mobile GeneXpert and X-ray systems were organized at 75 government health facilities in four operational districts. Symptomatic community members with an abnormal chest X-ray were tested using the Xpert MTB/RIF assay. People with TB were then treated at health facilities after screening services moved onto the next site.
Methods
De-identified project data were analysed to produce descriptive statistics about the people tested on Xpert and those diagnosed with TB. A linear regression was fit through the 12 quarters of National TB Program (NTP) TB case notification data immediately prior to ACF. The regression was used to calculate trend-expected notifications during and after the ACF quarters. Notifications from the ACF quarters were then compared to actual notifications from the previous year and to the trend-expected notifications during the ACF quarter by age group and type of TB. Finally, NTP TB treatment outcomes for the patients started on treatment during the ACF quarter were compared to those from a year prior.
Results
2068 individuals submitted sputum for Xpert MTB/RIF testing, resulting in the identification of 319 (15.4%) bacteriologically-positive TB patients and an additional 574 people who were clinically diagnosed with TB. In the ACF quarters, new bacteriologically-positive notifications increased +119.2% for all ages and +262.7% for people aged 55 and over compared with trend-expected notifications. Treatment initiation figures remained above trend-expected notifications for three full quarters after ACF. The treatment success rate across all operational districts was significantly higher for patients detected in the ACF quarters (88.8% vs 94.5%, p = 0.012).
Conclusion
A series of roving, one-off ACF days at government health facilities were able to increase TB diagnosis, treatment initiation and treatment outcomes in a key population with high TB prevalence. Targeted ACF interventions such as this could be used to reduce a backlog of untreated, prevalent TB.
Keywords: Tuberculosis, Active case finding, Elderly, Chest X-ray, Xpert, GeneXpert
List of Abbreviations: ACF, Active case finding; Bac+, Bacteriologically-positive; CR, Computer radiography; NNS, Number needed to screen; TB, Tuberculosis; VHSG, Village health support group
1. Background
Cambodia has seen a significant reduction in its estimated tuberculosis (TB) burden over the past decade. Full DOTS coverage at government health centres was achieved in 2004 and since then, community DOTS and public-private mix initiatives have expanded access to TB care services across the country [1]. As a result, the number of TB case notifications each year more than doubled from 18,891 in 2000 to an average of 37,930 between 2012–2016 [2]. In addition to rapid economic growth, this expansion of care was responsible for an almost 60% decline in TB prevalence, from an estimated 1619 people with TB per 100,000 population in 2000 to 668 per 100,000 in 2014 [2].
Despite these impressive achievements, Cambodia still has one of the highest TB prevalence rates in the world and growth in TB case notifications each year has stagnated since 2008 [3]. A comparison of the country's estimated prevalence rate in 2014 (668 per 100,000) against the case notification rate (279 per 100,000) shows that up to 58% of people with TB were still being missed [2]. And many of those who do seek care for TB, only do so after a delay, allowing transmission to continue in homes and communities [4].
As in many other Asian settings [5], the prevalence of pulmonary TB among people aged 55 and over is extremely high in Cambodia, exceeding 2400 per 100,000 population [1]. Two thirds of this burden is smear-negative TB and this estimate does not include extrapulmonary disease, a form of TB which is more common among older people [6], [7], [8]. Individuals who present with atypical and smear-negative TB are more difficult and expensive to diagnose accurately, as they require additional screening and diagnostic tests (e.g. chest X-ray, Xpert MTB/RIF assay and/or culture), multiple visits to health facilities, antibiotic trails and expert clinical care [9]. This more involved clinical work up presents many barriers for diagnosis and cure which can result in older individuals either never entering or dropping out of the TB care pathway [10]. Other factors, including social marginalization, impaired mobility and financial dependency, can also contribute to the low case detection and high mortality rates among older people [11].
New ways of implementing TB care are needed to overcome the stagnation of progress and to reach key populations. The new Global Plan to End TB 2016–2020 calls for such a paradigm shift and sets high targets for reaching and treating 90% of people with TB and curing 90% of those started on treatment [12]. Achievement of these targets will not be possible without wide-scale implementation of active case finding (ACF) – reaching out to individuals who are less likely to self-present at health services and/or who have poor access to care. However, the most impactful and cost-effective ways to integrate ACF to existing TB care programs are not well understood [13]. Many ACF initiatives simply measure yield, or number of people identified with TB as a direct result of case finding activities [14], [15], [16], [17]. However, some active case finding approaches may not be identifying people with TB who would go undiagnosed, untreated and/or unreported in the absence of enhanced screening services. Thus it is also important to measure population-level impact of ACF interventions [18], [19]. In addition, several ACF initiatives have suffered from high loss to follow-up and others have not measured treatment outcomes [20].
Several ACF initiatives in Cambodia have had success in increasing the number of people receiving TB treatment among key populations. In the urban slums of Phnom Penh, door-to-door symptom screening, sputum transportation, introduction of new diagnostic tests and mHealth data collection tools resulted in the detection and treatment of over 780 previously undiagnosed TB patients over a 14 month period [3]. In rural areas, chest X-rays were used to identify people for further testing among household and neighbourhood contacts of index TB patients. This approach identified TB patients earlier in their disease course [21], resulted in a 46% increase in TB treatment in a population of 2.9 million [22], and may be cost-effective way to reduce TB mortality [23].
Because of the extremely high TB burden and suboptimal case detection rate among the older population in Cambodia, we rigorously evaluated a programmatic ACF intervention targeting people aged 55 and over.
2. Methods
2.1. Study setting
This ACF intervention was implemented in four rural districts of Cambodia with a combined population of just over one million people: Ang Roka, Battambang, Kong Pisey, and Sampovmeas. These districts were selected because health services in these rural areas are relatively weak, owing to the large catchment areas and limited infrastructure of the health facilities. In addition, the notification rate for all forms TB in the districts was 299 patients per 100,000 population in 2012, compared to national incidence and prevalence estimates (411 and 764 per 100,000 respectively), indicating there was potential for significant improvements in TB notification [2]. Between 1 July 2013 and 31 March 2014, a series of roving, one-day ACF events were organized at 75 of the 78 government health facilities (96%) in these four districts.
2.2. Active case finding approach
An initial team of village health support groups (VHSGs) sensitized the communities living in the catchment area of participating health facilities about TB for one to two weeks prior to each of the ACF days. Individuals with TB-related symptoms (cough, chest pain, weight loss, fatigue, fever or night sweats) were referred to participating health facilities for further testing and were followed up to ensure they arrived at the health facility. Small transport enablers were provided to those in need to improve participation. VHSG outreach efforts were focused on people aged 55 years and over, but anyone with a cough of any duration was encouraged to avail screening and testing services. During each ACF day, a mobile X-ray, computer radiography (CR) reader and GeneXpert system were installed at the participating health facility. Individuals arriving at the health facility were systematically re-screened using a verbal symptoms questionnaire and then by chest X-ray. Symptomatic individuals with an abnormal chest X-ray were then asked to submit a spot sputum specimen for testing with the Xpert MTB/RIF assay. Test results were returned within a day and bacteriologically-positive (Bac+) patients were started on treatment at the health facility under the supervision of National Center for Tuberculosis and Leprosy Control (CENAT) clinicians and staff. A project physician reviewed the chest X-ray results of bacteriologically-negative individuals and initiated treatment based on a clinical evaluation. The initiative provided USD 2 (KHR 8000) per patient started on treatment to each participating health facility to support initiation of treatment and follow-up of patients. Beyond this minimal support, TB treatment and care was provided by the CENAT and its partners under routine conditions.
2.3. Data collection and analysis
Patient demographic, symptom, Xpert MTB/RIF test and chest X-ray
result data were collected using paper forms in the field, which were later transported to Phnom Penh and digitized. De-identified data were abstracted from the project's database to determine the characteristics of people presenting for TB screening and resultant test yields. P-values were calculated using the Fisher's exact test to measure the significance of different yields by key demographic variables. In addition, case notification data from CENAT were also collected and analysed using a monitoring and evaluation framework developed by the Stop TB Partnership's TB REACH initiative [19]. Evaluation was based on the concept of additional notifications – meaning the capacity of an intervention to increase TB diagnosis, treatment and reporting above what was recorded in a defined baseline period and after adjusting for secular trends. A 12-quarter (3 year) pre-intervention period was established before the quarter in which ACF days were hosted for each district. A linear regression analysis was used to control for trends in pre-intervention notifications, which was then projected forward to calculate the expected notifications for the intervention quarters and beyond. Additional notifications and departure from expected notifications were calculated by comparing actual notifications against trend-expected notifications during the intervention quarter. A summary graph was generated, standardizing the intervention quarters at one point on the x-axis regardless of the quarter in which the ACF days were hosted to show the overall effect of the intervention. Finally, treatment outcome data were obtained from CENAT for the cohorts of patients started on treatment during the intervention quarters and those started on treatment in the same quarter one year prior for each district. P-values were calculated using the Fisher's exact test to measure the significance of changes in treatment success rates using Stata 13 (StataCorp, College Station, TX, USA).
3. Results
Table 1 shows the demographics of people tested with the Xpert MTB/RIF assay and the resultant yields. A total of 2068 people provided sputum specimens for testing, which resulted in the detection of 319 (15.4%) Bac+patients. Nine of the Bac+results (2.8%) were resistant to rifampicin. Although 25% more females were tested compared with males, test yields were significantly higher in males (18.3% vs 13.1%, p = 0.001). More than 75% of the people tested with the Xpert MTB/RIF assay were aged 55 or over, but this group had the lowest test yields of all the age groups at 14.5%. Most of the people tested had no history of TB treatment (85.9%) and yields were significantly higher among those without a history of TB treatment (17.4% vs 3.4%, p < 0.001). All but ten of the individuals tested with the Xpert MTB/RIF assay were positive on the multi-symptom screening questionnaire, and 93.0% of these symptomatic individuals had a cough lasting two weeks or longer and/or haemoptysis. Although the diagnostic algorithm called for chest X-ray screening in parallel with Xpert MTB/RIF testing, only 1777 chest X-rays were performed and the majority of chest X-rays had abnormalities consistent with a pulmonary infection (83.1%). No Bac+TB patients were detected among those who had normal chest X-ray findings.
Table 1.
Demographic characteristics of people tested and Bac + TB patients detected.
| Xpert MTB/RIF tests performed | Bac + TB yield | |
|---|---|---|
| All individuals tested | 2068 (100%) | 319 (15.4%) |
| Health facilities (ACF Days) | ||
| Ang Roka (n = 11) | 313 (15.1%) | 35 (11.2%) |
| Battambong (n = 23) | 604 (29.2%) | 68 (11.3%) |
| Kong Pisey (n = 19) | 674 (32.6%) | 110 (16.3%) |
| Sampovmeas (n = 22) | 477 (23.1%) | 106 (22.2%) |
| Gender | ||
| Male | 918 (44.4%) | 168 (18.3%) |
| Female | 1150 (55.6%) | 151 (13.1%) |
| Age | ||
| 0–14 Years | 2 (0.1%) | 0 (0.0%) |
| 15–34 Years | 93 (4.5%) | 24 (25.8%) |
| 35–54 Years | 380 (18.4%) | 64 (16.8%) |
| ≥55 Years | 1593 (77.0%) | 231 (14.5%) |
| History of TB treatment | ||
| No | 1777 (85.9%) | 309 (17.4%) |
| Yes | 291 (14.1%) | 10 (3.4%) |
| Self-reported symptoms | ||
| Cough ≥ 2 Weeks and/or haemoptysis | 1913 (92.5%) | 298 (15.6%) |
| Any TB symptom | 2058 (99.5%) | 317 (15.4%) |
| Asymptomatic | 10 (0.5%) | 2 (20.0%) |
| Chest X-ray result | ||
| Not performed | 291 (14.1%) | 42 (14.4%) |
| Normal | 59 (2.9%) | 0 (0.0%) |
| Any abnormality | 1718 (83.1%) | 277 (16.1%) |
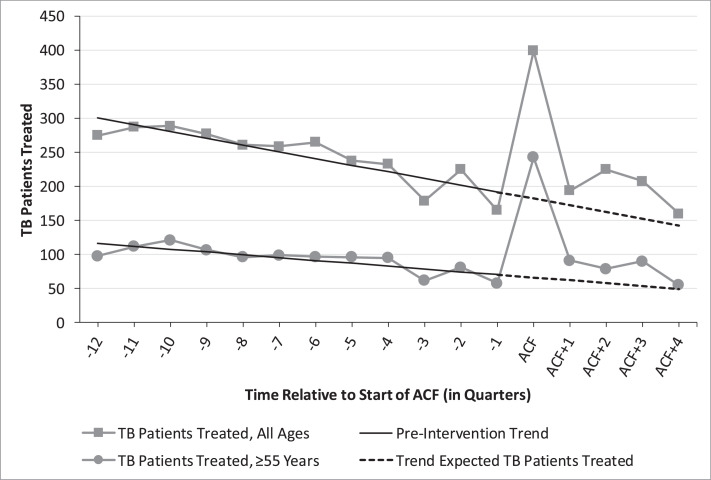
Fig. 1 and Table 2 describe the impact that ACF activities had on the number of people starting treatment for TB and being reported to the CENAT. During the intervention quarter, there were 217 (+119.2%) additional notifications of new Bac + TB among all ages compared with trend-expected notifications, and 176 (+262.7%) among those aged 55 and over. There were 579 (+88.8%) additional All Forms notifications across all ages compared with trend-expected notifications. The additional notification to yield ratio was 68.0% for Bac + TB among all ages, 76.2% for Bac + TB among those aged 55 and over, and 73.1% for All Forms of TB among all ages. In the four quarters post ACF activities, new Bac + TB notifications were 25.1% higher than trend-expected notifications for all ages and 40.8% higher for those aged 55 and over.
Fig. 1.
New Bac + TB patients started on treatment by age group, three years before and one year after ACF started.
Table 2.
Comparison between people detected with TB and actual and expected notifications.
| People detected with TB (project yield) | Actual intervention quarter notifications | Trend expected intervention quarter notifications | Additional notifications | Additional notifications to yield ratio | |
|---|---|---|---|---|---|
| New Bac+, All Ages | 319 | 399 | 182 | 217 (+119.2%) | 68.0% |
| Ang Roka | 35 | 58 | 24 | 34 (+141.7%) | 97.1% |
| Battambong | 68 | 115 | 56 | 59 (+105.4%) | 86.8% |
| Kong Pisey | 110 | 105 | 45 | 60 (+133.3%) | 54.5% |
| Sampovmeas | 106 | 121 | 57 | 64 (+112.3%) | 60.4% |
| New Bac+, ≥55 Years | 231 | 243 | 67 | 176 (+262.7%) | 76.2% |
| Ang Roka | 29 | 34 | 8 | 26 (+325.0%) | 89.7% |
| Battambong | 62 | 61 | 13 | 48 (+369.2%) | 66.7% |
| Kong Pisey | 68 | 71 | 24 | 47 (+195.8%) | 69.1% |
| Sampovmeas | 62 | 77 | 22 | 55 (+250.0%) | 88.7% |
| All forms of TB, all ages | 893 | 1,232 | 653 | 579 (+88.7%) | 73.1% |
| Ang roka | 98 | 152 | 68 | 84 (+122.4%) | 69.4% |
| Battambong | 230 | 445 | 196 | 249 (+126.6%) | 85.2% |
| Kong pisey | 318 | 344 | 245 | 99 (+40.3%) | 77.0% |
| Sampovmeas | 247 | 291 | 143 | 148 (+103.6%) | 57.9% |
Table 3 shows the treatment outcomes for new Bac + TB patients initiated on treatment during the intervention quarters and the same quarters in each district one year prior to ACF activities. Across all districts, the treatment success rate (outcomes of cure or treatment completed) significantly increased from 88.8% among patients started on treated in the pre-intervention quarters to 94.5% among patients started on treatment during the intervention quarters (p = 0.012). District level analysis showed that the improvement was primarily due to improvements in a single district. In Battambang district, treatment success increased from 74.4% among patients started on treatment in the pre-intervention quarter to 87.0% for patients started on treatment in the intervention quarter (p = 0.039). In the other three districts, treatment success rates were above 95% in the pre-intervention quarter and no significant changes in the treatment success rate was observed in the intervention quarter.
Table 3.
TB treatment outcomes for new Bac + TB patients in the pre-intervention and intervention quarters.
| Pre-intervention quarters | Intervention quarters | ||||||
|---|---|---|---|---|---|---|---|
| Quarter | New Bac+notifications | Treatment success | Quarter | New Bac+ notifications | Treatment success | P value | |
| All districts | N/A | 233 | 207 (88.8%) | N/A | 399 | 377 (94.5%) | 0.012 |
| Ang Roka | 2012 Q3 | 39 | 38 (97.4%) | 2013 Q3 | 58 | 56 (96.6%) | 1.000 |
| Battambang | 2013 Q1 | 82 | 61 (74.4%) | 2014 Q1 | 115 | 100 (87.0%) | 0.039 |
| Kong Pisey | 2012 Q3 | 53 | 51 (96.2%) | 2013 Q3 | 105 | 102 (97.1%) | 1.000 |
| Sampovmeas | 2012 Q4 | 59 | 57 (96.6%) | 2013 Q4 | 121 | 119 (98.3%) | 0.598 |
4. Discussion
We have shown that there are still many people, especially among the elderly, with undiagnosed TB in rural communities in Cambodia despite the fact the country has a robust and well-functioning TB program. This ACF intervention focused on expanding access to quality diagnostics and strengthened treatment services for people aged 55 and over was able to identify many people with previously undiagnosed TB. With minimal additional support, CENAT and its partners were able to treat an increased patient load, while also improving TB treatment outcomes.
Several initiatives have focused on scaling up and optimizing the use of the Xpert MTB/RIF assay, yet they have failed to increase All Forms TB case notifications [24], [25], [26]. In settings where empiric treatment is commonplace, testing individuals already presenting for care with a more sensitive diagnostic test can result in a shift of patients from one notification category to another; people who formerly would have been clinically diagnosed after a negative result on the less sensitive test (e.g. smear microscopy), now receive bacteriological confirmation of their disease on the more sensitive test and are notified as Xpert/Bac + TB. This intervention did not simply implement a new diagnostic tool; its community engagement activities focused on increasing the number of people evaluated for TB in a key population above and beyond ‘business as usual’ service delivery. By targeting groups with poor access to care, we were able to increase new Bac + notifications across all ages, particularly among those aged 55 and over. The increase in people placed on treatment was not the result of a shift in notifications, as there was also a large increase in all forms of TB notifications driven by the gains in new Bac + detection and also clinical diagnoses made possible by the use of chest X-ray. These findings further demonstrate the need to increase the number of people evaluated for TB with new diagnostic tests, rather than replacing existing tests in a passive system with new ones, to find people with TB who are ‘missed’ by public health services [18], [21], [27]. The introduction of new screening and diagnostic tools into rural areas which have traditionally relied on smear microscopy for diagnosis and have had very limited access to chest X-ray, the efforts of VHSG's community sensitization work, the active follow up of referrals and the distribution of small transportation enablers ensured that large numbers of our target population were willing to attend and be screened at these ACF days.
Historically, many ACF interventions have simply measured yield and did not make any effort to evaluate changes in the total number of people treated for TB at the population level [14], [28], [29]. Monitoring the ratio of additional notifications to yield can be highly insightful, as it shows the proportion of individuals identified by an initiative who would likely have been diagnosed, treated and reported in the absence of additional efforts. Of the new Bac + TB among people aged 55 and over by this intervention, more than two thirds translated to additional notifications, indicating that there was large and true expansion of treatment. The monitoring of additional notifications to yield will become more important as National TB Programs reorient activities towards the targets in Global Plan to End TB 2016–2020 and End TB Strategy [12], [30].
Though this ACF intervention provided one-time screening and testing services at health facilities, it appears to have had an effect on new Bac + TB case notifications in post-intervention quarters as well. The number of people treated in the four quarters after ACF was +25.1% than trend-expectations for all ages and 40.8% higher for those aged 55 and over. By the fourth quarter post-intervention, the number of people treated for TB returned to trend-expected levels for both all ages and those aged 55 and over. The lingering effect of ACF activities was unexpected and was possibly due to the raised awareness about TB in the community resulting from VHSG outreach and a resultant increase in health seeking behaviour. It is also possible that since significantly more people in the community were receiving TB treatment, more contacts with TB symptoms accompanied their family and friends to clinics and were evaluated for TB. In addition, health facility staff received direct monetary support from the project for follow up care, which included household contact tracing efforts, and they were sensitized to the need for improved reporting.
Additional people with TB may have been diagnosed by this ACF if a more sensitive screening algorithm was employed. Chest X-rays were not used as a screening tool to identify people who do not report TB-related symptoms, but are still in need of diagnostic testing. Modern TB prevalence surveys in Asia have shown that asymptomatic individuals comprise 40–79% of people with Bac + TB [5], and the 2011 Cambodia prevalence survey found that 56% of Bac + TB patients were asymptomatic [1]. This means that a substantial proportion of people with TB did not benefit from this ACF intervention since TB symptoms were the entry point for testing. Further, 93% of those tested on the Xpert MTB/RIF assay had a cough for two or more weeks and/or haemoptysis. Few individuals with a cough of short duration and/or other TB-related symptoms alone (e.g. unexplained weight loss without cough) were tested, despite 67% of all Bac + TB patients detected in the 2011 Cambodia prevalence survey having such profiles [1]. This initiative was not able to use chest X-ray as a screening tool because of throughput issues with the X-ray machine and finite testing capacity on the GeneXpert system, but other Cambodian studies have shown this to be feasible [21], [31]. The next generation of molecular tests, including the Xpert MTB/RIF Ultra assay [32], [33], could also further improve Bac + yield. Future ACF initiatives should prioritize their use, particularly when dealing with asymptomatic and weakly symptomatic individuals who likely have paucibacilliary disease.
There is also no concrete evidence that ACF alone will improve treatment outcomes due to finding people earlier in their disease development [20]. However, ACF offers the opportunity to reach people in need of care, to offer them a comprehensive package of services and ultimately, to improve treatment outcomes [34]. An ACF initiative in rural Ethiopia, was able to more than double the number of people treated for TB while also improving treatment outcomes by engaging a large, all-female cadre of community health workers for follow up care [35]. This initiative strengthened follow up care services by providing USD 2 per person started on treatment to health facilities which enabled them to hire extra staff (or pay overtime for existing staff) and covered some of the increased costs of follow up care aimed at limiting loss to follow up, such as home visits. However, the reasons for the significant improvement in treatment outcomes in the Battambang district are not fully understood. It was not possible to disaggregate CENAT treatment outcome data to analyse for differences in success rates between individuals found via the intervention and those found passively during the intervention quarters.
The analysis of official CENAT data to evaluate this intervention has several limitations. We were unable to select an appropriate control area against which changes in the intervention area could be measured. Numerous other active case finding projects affected CENAT notification data to varying degrees in either the pre-intervention or intervention periods. However, since the ACF activities in each district were staggered across different quarters, the absence of notification peaks during non-intervention quarters and the high additional notifications to yield ratios indicate that outside forces were very unlikely to have caused the increase in notifications. Additionally, we feel the comparison of actual against expected notifications is valid since the linear regression trends have fairly high R-squared values (0.78 for New Bac + TB in all ages and 0.66 among people aged 55 and over). Official CENAT case notification data are not disaggregated by age for All Forms of TB, and we believe the project's impact is diluted when analysing notifications for all ages. Since over three quarters of the people tested by the Xpert MTB/RIF assay were aged 55 and over, we have presented the age disaggregated new Bac + TB case notifications, in addition to All Forms of TB. In addition, it was not possible to quantify the accuracy of clinical diagnoses. Although we have demonstrated increases in diagnosis and treatment of TB, the initiative did not systematically collect information about the number of people screened by VHSG workers, and thus it is impossible to comment on the coverage of the intervention in the catchment area of each health facility or to calculate numbers needed to screen (NNS).
5. Conclusions
Roving, one-off upgrades of health facility screening and diagnostic infrastructure can have a significant impact on the number of people diagnosed and treated for TB, even in the context of a well-functioning TB program with declining notifications. With minimal support for treatment follow up activities, treatment success rates remained stable or increased despite the increased patient load. The approaches of this ACF initiative in rural Cambodia can serve as a model for reaching key populations with limited access to health services to improve detection and treatment for everyone with TB.
Declarations
Availability of data and materials
The datasets during and/or analysed during the current study available from the corresponding author on reasonable request.
Funding
This work was supported by a grant from the Stop TB Partnership's TB REACH initiative using funds from Global Affairs Canada and UNITAID.
Authors’ contributions
CM, MK and MTE conceived of the study design. CM and MK supervised the implementation of project activities and programmatic data collection. LG and AJC were responsible for additional data collection, verification and analysis. AJC and JC drafted the manuscript. All authors reviewed the manuscript and read and approved the final manuscript.
Competing interests
Andrew Codlin and Jacob Creswell are members of TB REACH at Stop TB Partnership, but do not make funding decisions.
Ethics approval and consent to participate
Approval to conduct this programmatic intervention was obtained from the National Center for Tuberculosis and Leprosy Control (CENAT). De-identified intervention data and publically-available, aggregate data from CENAT were used for these analyses. Only verbal consent was obtained; individuals were able to refuse participation at any point during the screening, diagnosis or treatment process without compromising their access to the routine care provided by CENAT.
Acknowledgments
We would also like to acknowledge the support of CENAT in providing drugs and infrastructure to execute this intervention.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jctube.2018.11.001.
Contributor Information
Andrew James Codlin, Email: andrewc@stoptb.org.
Chry Monyrath, Email: rath@thecata.org.kh.
Mom Ky, Email: momky@thecata.org.kh.
Lisanne Gerstel, Email: l.gerstel@kit.nl.
Jacob Creswell, Email: jacobc@stoptb.org.
Mao Tan Eang, Email: mao@online.com.kh.
Appendix. Supplementary materials
References
- 1.Report: second national tuberculosis prevalence survey Cambodia, 2011 . National Center for Tuberculosis and Leprosy Control, Ministry of Health; Phnom Penh, Cambodia: 2012. [Google Scholar]
- 2.Global tuberculosis report 2017 . World Health Organization; Geneva: 2017. [Google Scholar]
- 3.Lorent N., Choun K., Thai S., Kim T., Huy S., Pe R. Community-based active tuberculosis case finding in poor urban settlements of phnom penh, cambodia: a feasible and effective strategy. PLoS ONE. 2014 Mar 27;9(3) doi: 10.1371/journal.pone.0092754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Storla D.G., Yimer S., Bjune G.A. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008;8(January):15. doi: 10.1186/1471-2458-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onozaki I., Law I., Sismanidis C., Zignol M., Glaziou P., Floyd K. National tuberculosis prevalence surveys in Asia, 1990-2012: an overview of results and lessons learned. Trop Med Int Health. 2015;20(September (9)):1128–1145. doi: 10.1111/tmi.12534. [DOI] [PubMed] [Google Scholar]
- 6.Pratt R.H., Winston C.A., Kammerer J.S., Armstrong L.R. Tuberculosis in older adults in the United States, 1993-2008. J Am Geriatr Soc. 2011;59(May (5)):851–857. doi: 10.1111/j.1532-5415.2011.03369.x. [DOI] [PubMed] [Google Scholar]
- 7.Yoshikawa T.T. Tuberculosis in aging adults. J Am Geriatr Soc. 1992;40(February (2)):178–187. doi: 10.1111/j.1532-5415.1992.tb01941.x. [DOI] [PubMed] [Google Scholar]
- 8.Pérez-Guzmán C., Vargas M.H., Torres-Cruz A., Villarreal-Velarde H. Does aging modify pulmonary tuberculosis?: A meta-analytical review. Chest. 1999;116(October (4)):961–967. doi: 10.1378/chest.116.4.961. [DOI] [PubMed] [Google Scholar]
- 9.Negin J., Abimbola S., Marais B.J. Tuberculosis among older adults—time to take notice. Int J Infect Dis. 2015;32(March):135–137. doi: 10.1016/j.ijid.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 10.MacPherson P., Houben R.M.G.J., Glynn J.R., Corbett E.L., Kranzer K. Pre-treatment loss to follow-up in tuberculosis patients in low- and lower-middle-income countries and high-burden countries: a systematic review and meta-analysis. Bull World Health Organ. 2014;92(February (2)):126–138. doi: 10.2471/BLT.13.124800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhushan B., Kajal N.C., Maske A., Singh S.P. Manifestations of tuberculosis in elderly versus young hospitalised patients in Amritsar, India. Int J Tuberc Lung Dis. 2012;16(September (9)):1210–1213. doi: 10.5588/ijtld.11.0778. [DOI] [PubMed] [Google Scholar]
- 12.2015. http://www.stoptb.org/assets/documents/global/plan/GlobalPlanToEndTB_TheParadigmShift_2016-2020_StopTBPartnership.pdf The Global Plan to End TB 2016-2020 [Internet]. Geneva, Switzerland: Stop TB Partnership.
- 13.Golub J.E., Mohan C.I., Comstock G.W., Chaisson R.E. Active case finding of tuberculosis: historical perspective and future prospects. Int J Tuberc Lung Dis. 2005;9(November (11)):1183–1203. [PMC free article] [PubMed] [Google Scholar]
- 14.Fox G.J., Barry S.E., Britton W.J., Marks G.B. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2013;41(January (1)):140–156. doi: 10.1183/09031936.00070812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison J., Pai M., Hopewell P.C. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8(June (6)):359–368. doi: 10.1016/S1473-3099(08)70071-9. [DOI] [PubMed] [Google Scholar]
- 16.Baussano I., Williams B.G., Nunn P., Beggiato M., Fedeli U., Scano F. Tuberculosis incidence in prisons: a systematic review. PLoS Med. 2010;7(December (12)) doi: 10.1371/journal.pmed.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kranzer K., Houben R.M., Glynn J.R., Bekker L.-G., Wood R., Lawn S.D. Yield of HIV-associated tuberculosis during intensified case finding in resource-limited settings: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(February (2)):93–102. doi: 10.1016/S1473-3099(09)70326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Creswell J., Sahu S., Blok L., Bakker M.I., Stevens R., Ditiu L. A multi-site evaluation of innovative approaches to increase tuberculosis case notification: summary results. PLoS ONE. 2014;9(4) doi: 10.1371/journal.pone.0094465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blok L., Creswell J., Stevens R., Brouwer M., Ramis O., Weil O. A pragmatic approach to measuring, monitoring and evaluating interventions for improved tuberculosis case detection. Int Health. 2014;6(September (3)):181–188. doi: 10.1093/inthealth/ihu055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kranzer K., Afnan-Holmes H., Tomlin K., Golub J.E., Shapiro A.E., Schaap A. The benefits to communities and individuals of screening for active tuberculosis disease: a systematic review. Int J Tuberc Lung Dis. 2013;17(April (4)):432–446. doi: 10.5588/ijtld.12.0743. [DOI] [PubMed] [Google Scholar]
- 21.Eang M.T., Satha P., Yadav R.P., Morishita F., Nishikiori N., van-Maaren P. Early detection of tuberculosis through community-based active case finding in Cambodia. BMC Public Health. 2012;12(June):469. doi: 10.1186/1471-2458-12-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morishita F., Eang M.T., Nishikiori N., Yadav R.-P. Increased case notification through active case finding of tuberculosis among household and neighbourhood contacts in Cambodia. PLoS ONE. 2016;11(3) doi: 10.1371/journal.pone.0150405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yadav R.P., Nishikiori N., Satha P., Eang M.T., Lubell Y. Cost-effectiveness of a tuberculosis active case finding program targeting household and neighborhood contacts in Cambodia. Am J Trop Med Hyg. 2014;90(May (5)):866–872. doi: 10.4269/ajtmh.13-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theron G., Zijenah L., Chanda D., Clowes P., Rachow A., Lesosky M. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet. 2014;383(February (9915)):424–435. doi: 10.1016/S0140-6736(13)62073-5. [DOI] [PubMed] [Google Scholar]
- 25.Durovni B., Saraceni V., van den Hof S., Trajman A., Cordeiro-Santos M., Cavalcante S. Impact of replacing smear microscopy with Xpert MTB/RIF for diagnosing tuberculosis in brazil: a stepped-wedge cluster-randomized trial. PLoS Med. 2014;11(December (12)) doi: 10.1371/journal.pmed.1001766. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4260794/ [Internet][cited 2018 Apr 2]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Creswell J., Rai B., Wali R., Sudrungrot S., Adhikari L.M., Pant R. Introducing new tuberculosis diagnostics: the impact of Xpert(®) MTB/RIF testing on case notifications in Nepal. Int J Tuberc Lung Dis. 2015;19(May (5)):545–551. doi: 10.5588/ijtld.14.0775. [DOI] [PubMed] [Google Scholar]
- 27.John S., Gidado M., Dahiru T., Fanning A., Codlin A.J., Creswell J. Tuberculosis among nomads in Adamawa, Nigeria: outcomes from two years of active case finding. Int J Tuberc Lung Dis. 2015;19(April (4)):463–468. doi: 10.5588/ijtld.14.0679. [DOI] [PubMed] [Google Scholar]
- 28.Sekandi J.N., Neuhauser D., Smyth K., Whalen C.C. Active case finding of undetected tuberculosis among chronic coughers in a slum setting in Kampala, Uganda. Int J Tuberc Lung Dis. 2009;13(April (4)):508–513. [PMC free article] [PubMed] [Google Scholar]
- 29.Kranzer K., Lawn S.D., Meyer-Rath G., Vassall A., Raditlhalo E., Govindasamy D. Feasibility, yield, and cost of active tuberculosis case finding linked to a mobile HIV service in Cape Town, South Africa: a cross-sectional study. PLoS Med. 2012;9(8) doi: 10.1371/journal.pmed.1001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geneva, Switzerland: World Health Organization; 2015.
- 31.Creswell J., Codlin A.J., Andre E., Micek M.A., Bedru A., Carter E.J. Results from early programmatic implementation of Xpert MTB/RIF testing in nine countries. BMC Infect Dis. 2014;14(January):2. doi: 10.1186/1471-2334-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chakravorty S., Simmons A.M., Rowneki M., Parmar H., Cao Y., Ryan J. The New Xpert MTB/RIF Ultra: improving detection of Mycobacterium tuberculosisand resistance to rifampin in an assay suitable for point-of-care testing. MBio. 2017;8(August (4)) doi: 10.1128/mBio.00812-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dorman S.E., Schumacher S.G., Alland D., Nabeta P., Armstrong D.T., King B. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis. 2018;18(January (1)):76–84. doi: 10.1016/S1473-3099(17)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Datiko D.G., Lindtjørn B. Health extension workers improve tuberculosis case detection and treatment success in southern Ethiopia: a community randomized trial. PLoS ONE. 2009;4(5) doi: 10.1371/journal.pone.0005443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yassin M.A., Datiko D.G., Tulloch O., Markos P., Aschalew M., Shargie E.B. Innovative community-based approaches doubled tuberculosis case notification and improve treatment outcome in Southern Ethiopia. PLoS ONE. 2013;8(May (5)) doi: 10.1371/journal.pone.0063174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets during and/or analysed during the current study available from the corresponding author on reasonable request.