Abstract
Tuberculosis involvement of the gastrointestinal tract, peritoneum, and associated viscera is an uncommon but well described entity. While peritoneal tuberculosis and tuberculous enteritis are more common, involvement of the esophagus, stomach, colon, rectum, anus, liver, bile ducts, gallbladder, and pancreas can occur. Diagnosis is challenging as cases often mimic neoplasm or inflammatory bowel disease. In this review we outline the pathogenesis, clinical presentation, diagnostic testing, and treatment strategies pertaining to such cases.
Keywords: Small bowel, Colorectal, Hepatobiliary, Pancreas, Gallbladder
Introduction
Tuberculosis (TB) is a global epidemic. In 2015, the WHO estimated there were 10.4 million new cases of TB and 1.4 million deaths worldwide. This included 1.2 million new cases and 0.4 million deaths in patients co-infected with human immunodeficiency virus (HIV) [1]. TB disproportionately affects patients afflicted by poverty, regardless of where they live in the world [2]. Although TB is much less common in the United States, it continues to be a public health concern. In 2014, the CDC reported 9421 new cases of TB in the United States (66% of which occurred among foreign born people) and in 2013 the CDC reported 555 deaths due to TB [3].
Given its prevalence and often non-specific presentation, cases of extrapulmonary tuberculosis (EPTB) are often difficult to diagnose and manage. Here, we present an overview of extra-pulmonary tuberculosis involving the peritoneum, gastrointestinal tract and associated viscera including the liver, bile ducts, pancreas, and gallbladder.
Overview
TB of the gastrointestinal tract, peritoneum, and associated viscera (collectively known as abdominal TB) is the sixth most frequent form of EPTB after lymphatic, genitourinary, bone, miliary, and CNS tuberculosis [4]. Peritoneal TB is the most common presentation of abdominal TB. Epidemiologic data suggests a predominance of peritoneal tuberculosis and tuberculous enteritis in younger patients less than 45 years of age [5], [6]. TB may manifest in any location throughout the luminal gastrointestinal tract from the oral cavity to the rectum, although certain locations, such as the ileocecum are more common. There are common features that pertain to abdominal TB regardless of the anatomical site involved.
Pathogenesis
Abdominal tuberculosis develops from invasion of pathogenic bacteria, triggering damaging granulomatous inflammation. Such invasion and inflammation can lead to ulceration, bleeding, and perforation. The spread of pathogenic bacteria to the gastrointestinal tract occurs via four main routes. These routes of acquisition include swallowing of contaminated respiratory tract secretions, hematogenous spread from active pulmonary infection, contiguous spread from adjacent infected viscera or lymph nodes, and uncommonly ingestion of contaminated unpasteurized dairy products [7], [8], [9]. When contaminated sputum or food is ingested pathogenic bacteria invade through the intestinal epithelium and into the submucosa. Areas within the gastrointestinal tract containing high concentrations of lymphoid tissue and M-cells, such as the terminal ileum, are particularly susceptible to invasion [5]. In addition to inflammatory damage of the gastrointestinal tract wall, pathologic involvement of the gastrointestinal vasculature occurs. This is evidenced by histopathologic studies of mesenteric vessels in patients with tuberculous enteritis. When examined microscopically these vessels show granulomatous inflammation within the arterial wall and thrombosis with the arterial lumen. Thus, ischemia may exacerbate the gastrointestinal damage initiated by this localized granulomatous inflammation [4].
Testing
Tuberculin skin testing (TST) and interferon gamma release assay (IGRA) are usually positive in cases of abdominal tuberculosis. However, a positive result cannot distinguish between latent and active infection, and negative TST or IGRA does not exclude active tuberculosis infection. This limits their utility in diagnosis of active abdominal TB [8]. Smear microscopy and mycobacterial culture should be performed in all cases of suspected TB infection. Histologic examination should be performed if tissue is obtained. The gold standard for diagnosis is positive culture growth. However, the clinical utility of culture is limited by its relative low yield and the prolonged period (often weeks) required for growth to be detected [10]. For example, in cases of peritoneal tuberculosis, culture of ascitic fluid and tissue sampling has a sensitivity of only 35%6. As such, adjunctive testing may be considered when possible. The Xpert MTB/RIF (Xpert) assay is a nucleic acid amplification test that identifies the presence of Mycobacterium tuberculosis DNA, while having the additive benefit of detecting gene mutations conferring rifampin resistance. The assay uses five molecular probes targeted to the 81 bp rpoB core region [11]. The pooled sensitivity of the Xpert assay in lymph node samples, gastric aspirates, and ascitic fluid samples is 96%, 78%, and 59% respectively [10], [12]. There is limited data regarding the sensitivity in fecal samples although one small study found sensitivity to be 100% and 50% in patients with sputum positive and sputum negative disease respectively [13]. Such data on testing extra pulmonary samples is not robust and limited to international studies, and the Xpert assay is currently only approved for use on respiratory samples in the United States. Due to such limitations, PCR testing of extra pulmonary samples is often done using ‘home grown’ laboratory developed molecular assays using unique proprietary primers. Such differences in testing make comparison of PCR testing used throughout the United States quite difficult.
Treatment
The current INDEX-TB guidelines for treatment of EPTB recommend standard treatment for all forms of abdominal TB. This consists of two months of four drug therapy (rifampin, isoniazid, pyrazinamide, ethambutol) followed by four months of two drug therapy (rifampin, isoniazid) [14]. These guidelines carefully note that this recommendation is largely extrapolated from study of pulmonary TB treatment, and that there is a paucity of data specific to treatment of abdominal TB. Providers should employ directly observed therapy (DOT) as outlined by the World Health Organization (WHO). Although DOT has not been extensively studied in abdominal TB, we advocate for the use of observed therapy based on its demonstrated benefit in patients with pulmonary tuberculosis. This recommendation aligns with the current Infectious Diseases Society of America (IDSA) and American Thoracic Society (ATS) guidelines [15].
A clinical dilemma that can occur is how to approach treating a patient with active pulmonary TB who also reports abdominal symptoms. While treatment of active pulmonary TB is adequate to treat most manifestations of coexisting abdominal disease, care should be taken to assess for abdominal complications that may not respond fully to drug therapy. Such complications may require endoscopic or surgical interventions, and are discussed in detail in the following sections.
Drug resistance
Single and multi-drug resistant TB (MDR-TB) infections are becoming more common. In 2017, the WHO estimated an incidence of 601,000 MDR-TB cases worldwide. Rapid molecular drug susceptibility testing is recommended for patients who have been previously treated for tuberculosis, have been in contact with patients with known MDR TB, those who were born in or spent more than one year in a country with a moderate TB incidence (≥20 cases per 100 000 people or a MDR TB prevalence greater than 2%), or HIV co-infected patients [16]. If culture growth is available causative organisms can be tested for drug susceptibility, allowing for tailored drug therapy. Recommendations regarding specific drug regimens for MDR-TB are beyond the scope of this review [17].
Tuberculosis of the gastrointestinal tract, peritoneum, and associated viscera
Peritoneal tuberculosis
Peritoneal tuberculosis is a manifestation of EPTB that requires a high degree of suspicion to diagnose. Diagnosis is often delayed weeks to months after the onset of symptoms [18,19]. One contributing factor to this delay is the presence of overlapping conditions (such as cirrhosis) that may provide an explanation for a multitude of patient symptoms. Peritoneal tuberculosis affects both sexes equally, and most commonly impacts those 35–45 years old [6]. Risk factors for developing peritoneal TB include states of immunosuppression (most prominently HIV/AIDS), kidney failure requiring dialysis, cirrhosis, and malnutrition [20,21].
Peritoneal infection most commonly results from hematogenous dissemination, although direct spread from involved areas of the gastrointestinal tract may occur. Concomitant pulmonary disease exists in 14% of patients 6. Once infected, the peritoneal membrane becomes thickened and hypervascular, and there is formation exudative proteinaceous ascites in most cases.
Three patterns of peritoneal TB are classically described. This includes a pattern of thickened peritoneum with ascites and scattered tubercular nodules; thickened peritoneum with ascites but without tubercles; and markedly thickened peritoneum with extensive fibrous adhesions and a relative absence of ascites. This third type is also known as a fibroadhesive, dry, or plastic pattern and corresponds to the classically described ‘doughy abdomen’ on physical exmaination [22], [23]. This fibroadhesive pattern is least common, occurring in only 5–13% of peritoneal TB cases [22].
The clinical presentation of peritoneal TB is non-specific, often manifesting with an insidious development of systemic symptoms. The most common signs and symptoms include ascites (73%), abdominal pain (65%), weight loss (61%), fever (59%), diarrhea (21.4%), and constipation (11%). Lab testing is non-specific although normocytic anemia, thrombocytosis, monocytosis, and elevated erythrocyte sedimentation rate are characteristic [6].
Diagnostic paracentesis should be performed in all patients with ascites in whom peritoneal tuberculosis is a consideration. Ascitic fluid is typically straw colored but can be hemorrhagic in some cases. An ascites protein level ≥2.5 g/dL, serum albumin to ascitic fluid albumin ratio (SAAG) of less than 1.1 g/dL, elevated adenosine deaminase level >30 U/L, and a cell count of 500–1500 cells/mm3 with lymphocytic predominance is indicative of peritoneal TB. While ascitic fluid protein level ≥2.5 g/dL and SAAG < than 1.1 g/dL is present in essentially all cases of peritoneal TB, it is not necessarily unique to peritoneal TB. This can be due to the presence of a co-existing condition such as cirrhosis, heart and renal failure which can confound basic peritoneal fluid analysis. While positive culture is considered the gold standard, ascitic fluid and tissue culture have low yield (sensitivity 35%), and may take weeks to show growth. Only 3% of samples are smear positive (with Ziehl–Neelson stain) [6]. Molecular testing of ascitic fluid samples using a PCR assay can be considered as an adjunctive test [10,12].
Ultrasound and computerized tomography (CT imaging) are useful in identifying disease features, but perhaps the greatest utility of these modalities is identification of target sites for fluid and tissue sampling. Characteristic CT findings include ascites, mesenteric lymphadenopathy, a thickened hypervascular peritoneum, tubercular nodules, fibrous adhesions, and abnormal omental findings. Peritoneal carcinomatosis, Crohn's disease, and sarcoidosis may mimic the radiographic appearance of peritoneal TB, and these conditions should be considered when such radiographic findings are seen.
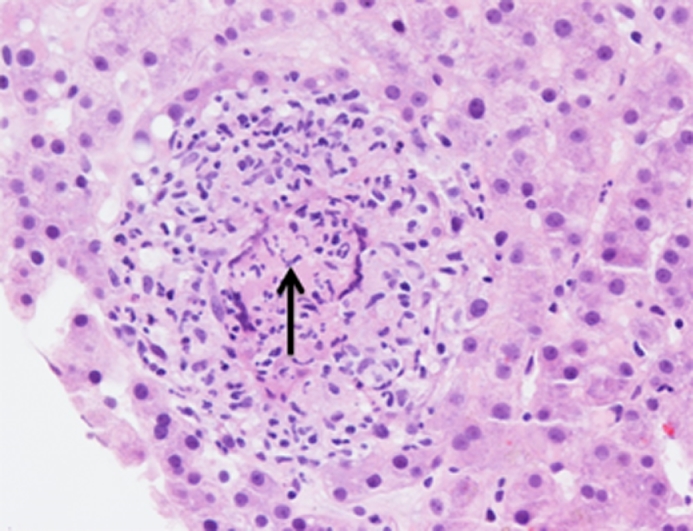
While clinical presentation, laboratory testing, peritoneal fluid testing, and imaging may confer a strong suspicion for peritoneal TB, diagnostic laparoscopy is often required to confirm the diagnosis. Forgoing laparoscopy until peritoneal fluid cultures result may portend increased mortality [24]. Laparoscopic visualization and tissue sampling for histologic examination have high diagnostic yield [22]. When visualization is coupled with histologic evaluation sensitivity is 98%6. Laparoscopy provides opportunity for direct observation of the aforementioned patterns that can include various combinations of elements including scattered tubercular nodules, thickened hypervascular peritoneum, fibrous adhesions, and omental abnormalities. Histologic examination demonstrates typical tubercular granulomas, and may show mycobacterial organisms with staining (Fig. 1).
Fig. 1.
H&E stain of TB granuloma with visible TB organism (arrow).
Despite low yield, mycobacterial cultures should still be obtained and if positive are particularly useful for drug susceptibility testing. In accordance with guidelines, standard therapy for peritoneal tuberculosis consists of two months of four drug therapy (rifampin, isoniazid, pyrazinamide, ethambutol) followed by four months of two drug therapy (rifampin, isoniazid) [14]. Complications of peritoneal TB including bowel perforation, intestinal obstruction secondary to fibrous adhesions, fistula formation, and hemorrhage can occur [6].
Small bowel tuberculosis (tuberculous enteritis)
Involvement of the small intestine by TB is commonly referred to as tuberculous enteritis. The ileocecal region is the most commonly involved area of the luminal gastrointestinal tract. The predilection for TB to involve the ileocecal region is dependent upon a multitude of factors that include physiologic stasis of bowel contents, intimate mucosal contact due to complete digestion, and the predilection of lymphoid tissue as discussed above [4].
Tuberculous enteritis progresses slowly, and patients may not seek medical care until complications occur. Symptoms are generally vague and consist of fever, abdominal pain (often chronic), night sweats, fatigue, weight loss, constipation, diarrhea, and bleeding. A palpable abdominal mass can sometimes be felt, most often in the right lower quadrant.
There are three main morphologic forms of tuberculous enteritis; ulcerative, hypertrophic, and ulcerohypertrophic [4]. Ulcerative morphology predominates in 60% of cases, followed by ulcerohypertrophic (30%), and hypertrophic (10%) [9]. Ulcerative tuberculous enteritis is characterized by single or multiple mucosal ulcerations which are typically oriented in the transverse direction, often circumferentially. This form typically affects the jejunum, ileum, and cecum. Complications include perforation, bleeding, fistula formation, and obstruction secondary to fibrotic stricture formation as ulcerations heal. Ulcerohypertrophic tuberculous enteritis is characterized by inflammatory psuedotumor formation accompanied by thickening and ulceration of the intestinal wall. Hypertrophic tuberculous enteritis manifests with scarring, fibrosis, and psuedotumor formation, most commonly involving the ileum and cecum. Complications of ulcerohypertrophic and hypertrophic forms are similar to the ulcerative form, although mass effect may cause mechanical obstruction regardless of stricture formation [25].
Laboratory abnormalities in tuberculous enteritis are also non-specific, although anemia and an elevated erythrocyte sedimentation rate are characteristic findings. Gastrointestinal or visceral tissue, ascitic fluid, and lymph node tissue can all be sent for smear microscopy, mycobacterial culture and PCR assay testing. Although not included in current guidelines, acid fast smear, mycobacterial cultures and PCR assay testing on stool samples can be considered as adjunctive testing. We recommend that patients with TB enteritis be tested with three spontaneous or induced sputum specimens to evaluate for concomitant pulmonary TB. If possible, molecular drug resistance testing should be performed to evaluate for rifampin and isoniazid resistance, as conventional culture based drug susceptibility typically takes several weeks. Rapid detection of resistance allows for earlier initiation of effective therapy for drug-resistant TB [26].
A variety of imaging modalities may be utilized in the diagnostic evaluation of suspected gastrointestinal TB. Barium studies are useful in demonstrating mucosal ulceration, stricture, a deformed cecum, or a dilated and incompetent ileocecal valve. Cross sectional imaging with computerized tomography (CT) is useful in identifying intra and extra-luminal pathology, as well as abdominal lymphadenopathy. Findings characteristic of TB associated abdominal lymphadenopathy include markedly enlarged lymph nodes with hypodense centers. These hypodense centers are representative of caseous liquefactive necrosis. CT may also show characteristic concentric intramural ileocecal thickening [27]. Endoscopy is perhaps the most useful modality and can identify ulcers, strictures, deformation of the cecum, ileocecal valve incompetence, and fistulas through direct visualization. Endoscopy provides the additional ability to perform diagnostic tissue sampling discussed above.
Histopathologic examination typically demonstrates large numerous caseating granulomas in submucosa and serosa with surrounding fibrosis, however noncaseating granulomas can also be found (Fig. 1). In patients with known active pulmonary TB and clinical symptoms, endoscopic, or radiographic findings suspicious for tuberculous enteritis, it may be acceptable to make a presumptive diagnosis without tissue culture or histopathology. However, careful consideration must be given not to overlook other potential etiologies that may mimic tuberculous enteritis. If a decision is made to treat empirically based on a presumptive diagnosis, follow up endoscopy may be considered to assess for resolution of abnormalities.
The differential diagnosis for patients suspected to have tuberculous enteritis includes Crohn's disease, typhlitis, infectious etiologies (amebiasis, yersiniosis, histoplasmosis, actinomycosis), and neoplasm (lymphoma, colon cancer). In particular, it can be very difficult to differentiate tuberculous enteritis from Crohn's disease. This is of great importance, as initiation of immunosuppression for presumptive inflammatory bowel disease can lead to exacerbation or dissemination of TB [28]. Contrasting clinical, radiographic, endoscopic, and histologic features is crucial to differentiate tuberculous enteritis from Crohn's disease (Table 1) [[29], [30], [31], [32], [33], [34], [35]]. When a definitive diagnosis remains in doubt after an extensive workup has been completed, a prudent strategy may be to consider empiric anti-tuberculous therapy before initiating immunosuppressant medications.
Table 1.
Tuberculous enteritis vs. Crohn's disease.
| Crohns | TB |
|---|---|
| No Ascites | Ascites |
| Linear ulcers, cobblestoning | Transverse or cirumferential ulcers |
| Normal mucosa adjacent to ulcer | Inflamed mucosa adjacent to ulcer |
| Mucosal granulomas predominate | Submucosal granulomas predominate |
| Granulomas small ≤ 200 micrometers) | Granulomas large (>200 μm) |
| Granulomas infrequent (<5 per biopsy) | Granulomas frequent (≥5 per biopsy) |
| Granulomas non-confluent, non-caseating | Granulomas confluent, caseating |
| Normal IC valves | Incompetent or patulous IC valve |
| No acid-fast bacilli | Acid-fast bacilli |
| No or low-grade fever | High grade fever |
| Small inflammtory lyphadenopathy | Large lymphadenopathy with necrotic centers |
In accordance with guidelines, standard treatment of tuberculous enteritis consists of two months of four drug therapy (rifampin, isoniazid, pyrazinamide, ethambutol) followed by four months of two drug therapy (rifampin, isoniazid) [14]. Concerns have been raised about drug absorption in the setting of active TB infection, in particular with regard to rifampin and isoniazid [36]. In theory this may be even more of a factor when there is extensive disease burden in the small bowel, and patients should be monitored closely for treatment failure. Clinical improvement can be expected in as soon as two weeks following initiation of therapy, while endoscopic improvement can be seen after three months [37]. If clinical symptoms persist beyond two weeks, an alternative diagnosis, disease complications, drug malabsorption, or drug resistant disease should be considered [38].
Adjunctive endoscopic and surgical interventions are employed to manage complications when necessary. Mucosal ulcerations, low grade obstructions and small fistulas typically respond to medical therapy, and surgery can be avoided. However, in some cases, mucosal healing may lead to scarring and late onset obstruction necessitating surgical resection. Surgery is indicated for complications such as perforation, massive bleeding, intestinal ischemia, or refractory obstruction. Short segment strictures can occasionally be managed with strictureplasty [39] preventing the need for bowel resection, or colonic balloon dilation [40]. Both options offer potential effective, minimally invasive therapeutic options.
Esophageal tuberculosis
Esophageal tuberculosis is uncommon, but multiple cases have been reported in the literature [41], [42]. Most cases of esophageal tuberculosis occur secondary to tuberculosis infection elsewhere in the body. In such cases the most common etiology of esophageal infection is spread of infection from the respiratory tract and mediastinum. Isolated primary esophageal tuberculosis infection is less common, but can occur [43]. Dysphagia is the most common presenting symptom [43], [44], although odynophagia can also occur [45]. Lesions most commonly occur in the mid and lower esophagus and are usually ulcerative. Infection may also present as an infiltrative growth that may be confused for an esophageal neoplasm [[46], [47], [48]]. Occasionally, esophageal symptoms may results for extrinsic compression by enlarged lymph nodes. Barium esophagram is useful to delineate ulcers and infiltrative growth, while CT is useful is characterizing existing thoracic lymphadenopathy [43]. Endoscopic ultrasound allows for evaluation of both the esophageal mucosa and mediastinal lymph node simultaneously. Additionally, it allows for tissue sampling and biopsy [49]. Severe complications such as esophageal abscess [50], perforation [51], massive hematemesis [52], and esophagotracheal, esophagobroncheal, and esophagomediastinal fistula formation [53], [54] have been reported. Definitive diagnosis is made through tissue sampling with standard smear microscopy, histologic examination, and culture. Standard anti-tuberculous therapy as outlined above is recommended in all cases [41].
Gastroduodenal tuberculosis
Gastroduodenal tuberculosis is an uncommon but well-known entity. Patients may present with gastric outlet obstruction, ulceration, upper gastrointestinal hemorrhage, or pseudotumor formation. Patients commonly report symptoms of dyspepsia for a prolonged period of time prior to diagnosis [55]. Instances of gastric outlet obstruction occur primarily because of TB invasion of the gut wall, although extrinsic compression by enlarged epigastric and periduodenal lymph nodes can occur. Fistula formation is possible, and choledocho-duodenal, pyeloduodenal, and aortoduodenal fistulas have been reported in the literature [56], [57], [58]. Barium studies may be useful in localizing areas of fistulization, luminal narrowing or ulceration [59]. Ultrasound and CT imaging are useful for the identification of mass lesions. Diagnosis of gastroduodenal TB is typically made using upper gastrointestinal endoscopy with tissue sampling with biopsy or mucosal resection for diagnostic testing. A surgical specimen is sometimes required for pathologic evaluation if endoscopic sampling is non-diagnostic. Treatment for gastric outlet obstruction may require surgical intervention if there is a poor response to anti-tuberculosis therapy. In the case of luminal narrowing, endoscopic balloon dilation can be useful. Surgical resection is considered if anti-tuberculosis therapy and endoscopic intervention fails to improve obstructive symptoms [60], [61], [62]. Standard anti-tuberculous therapy as outlined above is recommended in all cases.
Colorectal tuberculosis
Colonic tuberculosis is uncommon but is well described in the literature [63], [64]. Similar to tuberculous enteritis, the clinical presentation is non-specific and may include fever, weight loss, abdominal pain, gastrointestinal bleeding and diarrhea. A palpable abdominal mass is sometimes present. The cecum is most commonly involved, although any portion of the colon may be affected. Endoscopic findings include ulceration, bleeding, nodules, strictures, and fibrous bands. Polypoid lesions mimicking colonic neoplasia may also be present [[65], [66]]. The most common complication is colonic perforation [67], [68] requiring urgent surgical intervention. Definitive diagnosis is made utilizing the testing described above. Standard anti-tuberculous therapy as outlined above is recommended in all cases.
In addition to colonic involvement, tuberculous involvement of the rectum and anus has been reported. This is quite rare, with involvement of the anus representing only 1% of abdominal TB [69]. Anorectal tuberculosis typically occurs in the setting of colonic tuberculosis, although isolated disease has been reported [70]. Presentation includes anal fissure, perirectal fistula, perirectal abscess, and non-healing or recurrent peri-anal lesions [25], [71]. Complications of massive rectal bleeding [72] and rectal stricture [73] have been reported. Initial diagnosis can be difficult due to disease rarity. It should be considered in at risk populations, particularly when rectal or anal lesions continue to recur and fail to respond to conservative treatment. As discussed above, such a presentation may mimic Crohn's disease, and great care must be given to make a correct diagnosis. Severe disease may necessitate surgical intervention, and standard anti-tuberculous therapy as outlined above is recommended in all cases.
Hepatobiliary
Tuberculous of the hepatobiliary tract is uncommon, and accounts for about 1% of all tuberculous infections [74]. TB involvement of the hepatobiliary tract may be isolated, associated with additional enteric involvement, or occur in the setting of miliary tuberculosis. It may manifest in a variety of ways including tuberculous pseudotumor [75], tuberculous cholangitis [76], tuberculous liver abscess [77], and tuberculous hepatitis [74]. Fulminant hepatic failure has been reported but is exceedingly rare [78]. Biliary involvement may result from extrinsic compression of the biliary tree by enlarged tuberculous lymph nodes, hepatic granulomas, or from direct involvement of biliary epithelium. Cases of obstructive jaundice secondary to TB are often mistaken for pancreatic adenocarcinoma or cholangiocarcinoma [79].
Symptoms of hepatobiliary tuberculosis may be non-specific. The most common presenting symptoms include upper abdominal pain (45–66%), fever (63–90%), and weight loss (55–75%). Clinical findings include hepatomegaly (80–96%), splenomegaly (25–55%), and jaundice (20–35%) [[74], [80], [81], [82], [83]]. On examination the liver may be hard and nodular (55%) and tender (36%) [81]. Liver function tests including aspartate aminotransferase, alanine transaminase, alkaline phosphatase, and bilirubin are often abnormal, however not diagnostic of hepatobiliary tuberculosis. Derangements in markers of synthetic liver function such as prolonged prothrombin time, decreased albumin, and decrease platelet count are less common. Overall, laboratory testing is nonspecific [84].
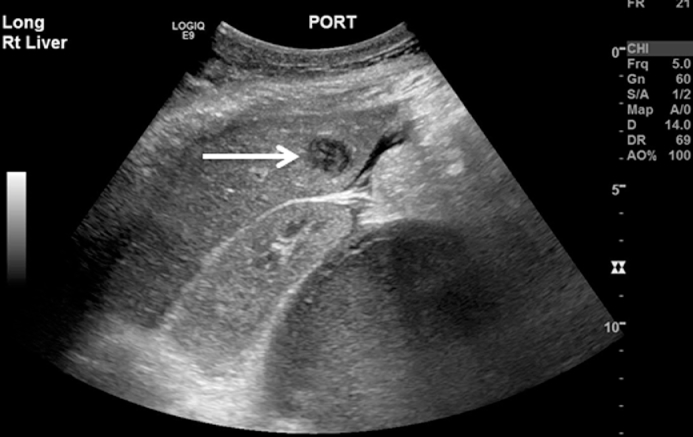
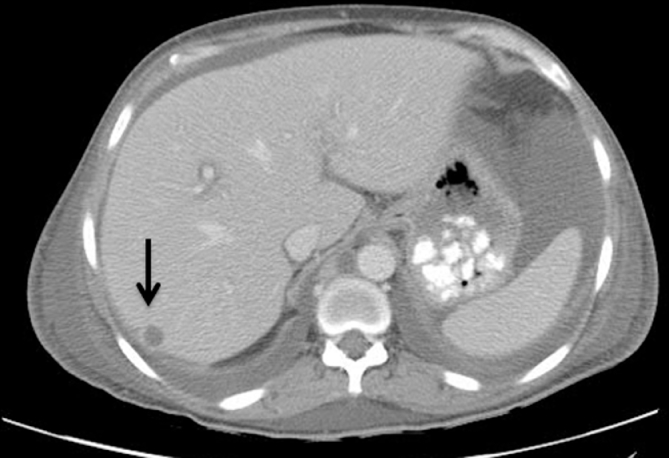
Various imaging modalities can be useful in the evaluation of hepatobiliary TB. Hepatomegaly is commonly seen is all forms of imaging. Abdominal X-ray may show areas of hepatic calcification indicative of large calcified tuberculomas. Abnormal chest x-ray demonstrating pulmonary tuberculosis has been reported in 75% of patients with confirmed hepatobiliary TB [83]. In isolated haptic tuberculosis diagnostic yield is higher with ultrasound, CT or laparoscopic assisted liver biopsy. Ultrasound may show bile duct dilation when obstruction is present, and may show hypoechoic or complex masses representing an abscess or pseudotumor (Fig. 2). Such ultrasound findings may be confused with hepatic neoplasm [85].CT imaging may show caseating tuberculous granulomas which are characterized as non-enhancing, low density lesions with peripheral rim enhancement. Such lesions may be indistinguishable from primary hepatocellular tumors or metastatic disease (Fig. 3) [[86], [87]]. Laparoscopy can be utilized for targeted liver biopsy and macroscopic visualization of the liver with tuberculomas appearing as white irregular nodules. Endoscopic retrograde cholangiopancreatography (ERCP) has diagnostic and therapeutic role and is indicated in patients presenting with obstructive jaundice. Alvarez et al described ERCP findings in 26 patients and reported hilar stricture in 61.5%, beaded appearance of the common bile duct with segmental areas of dilation and constriction in 19%, dilation of intrahepatic bile duct in 27%, and beaded appearance of the intrahepatic bile ducts in 23% patients [80]. The utility of ERCP may be augmented with the concomitant use of endoscopic ultrasound [88].
Fig. 2.
Ultrasound image showing hepatic abscess (arrow).
Fig. 3.
CT Image showing hepatic abscess (arrow) and abdominal ascites.
Definitive diagnosis is made through tissue sampling with standard smear microscopy, histologic examination, and culture. Caseating granuloma formation is characteristic, which may coalesce to form larger, calcified tuberculomas. These large calcified tuberculomas are more common in the liver than in other areas of the gastrointestinal tract afflicted with TB [84]. AFB stains have been reported to be positive in 7–59% patients, with positive staining being more common in those with tuberculous abscess and liquified caseous material [80].
Treatment with standard anti-tuberculous therapy is indicated in all cases of hepatobiliary involvement [[14], [89]]. Careful monitoring is required to assess for drug induced hepatotoxicity, given that patients will already have some degree of liver injury from the underlying TB [90]. This should be done with frequent checks of liver function tests (monthly at a minimum). In cases in which there is significant liver injury at treatment onset, or in which drug induced hepatoxicity occurs during the treatment course, stopping isoniazid in favor of alternative drug therapy should be strongly considered [84].
In patients with obstructive jaundice, antituberculous therapy is recommended in combination with decompression of the biliary tract. The can be achieved by endoscopic retrograde cholangiopancreatography (ERCP) and stent placement, percutaneous trans-hepatic biliary drainage (when expertise in therapeutic biliary stenting is unavailable), or surgical intervention [91], [92]. Tuberculous liver abscess is managed with antituberculous therapy and percutaneous drainage [86], [93].
Gallbladder
Rarely, EPTB may manifest within the gallbladder. Tuberculous involvement of the gallbladder may be isolated, associated with additional enteric involvement, or occur in the setting of disseminated tuberculosis. Most frequently, TB involvement of the gallbladder will present with symptoms of biliary colic or cholecystitis. The diagnosis is often unexpected and only discovered after cholecystectomy and surgical pathology is reviewed [94], [95]. Ultrasound is the imaging modality of choice to identify features of cholecystitis, but findings are not unique to tuberculosis infection [96]. Rare complications have been reported including gallbladder perforation [97] and post-cholecystectomy biliary fistula formation [98]. Completion of a treatment course with standard anti-tuberculous therapy is indicated regardless of whether or not definitive cholecystectomy is performed [95].
Pancreas
Abdominal tuberculosis may manifest in the pancreas, although uncommonly. It may be isolated, associated with additional enteric involvement, or occur in the setting of disseminated tuberculosis [99]. Most commonly, TB involvement of the pancreas presents as a pancreatic mass most often misdiagnosed as pancreatic adenocarcinoma. TB involvement of the pancreas may also masquerade as intraductal pancreatic mucinous tumor [100] or focal pancreatitis [101]. TB of the pancreas is sometimes only identified after surgical resection of the affected pancreas [102]. Complications including gastrointestinal bleeding [103] and pancreatic abscess [104] have been reported. Cross sectional imaging with CT or MRI may show focal mass or diffuse pancreatic enlargement [105]. Endoscopic ultrasound, with diagnostic FNA has been shown to be effective as a means of obtaining a diagnosis [106], [107]. Treatment with standard anti-tuberculous therapy is indicated, with invasive interventions reserved for select complications.
Conclusion
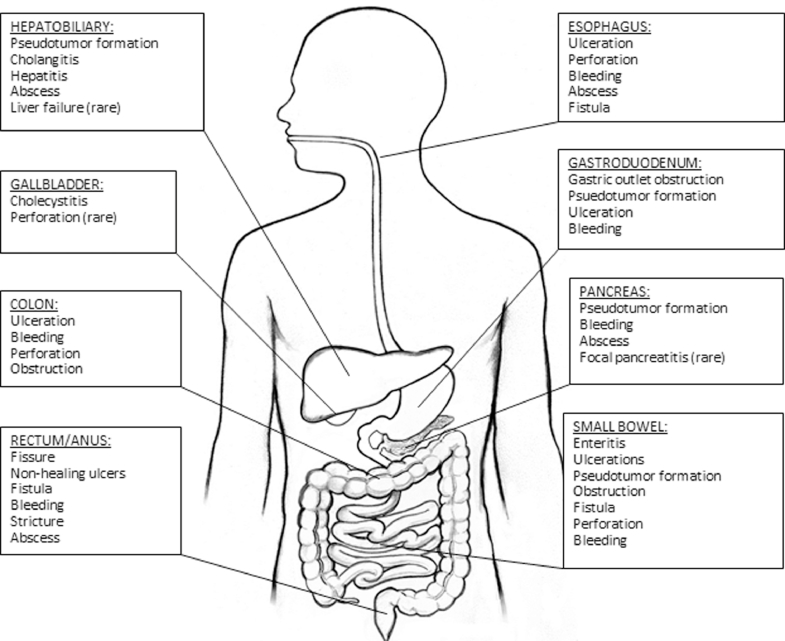
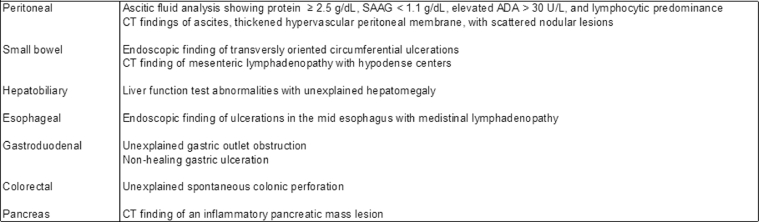
In conclusion, tuberculous involvement of the peritoneum, gastrointestinal tract and associated viscera is an uncommon but well described entity. While peritoneal tuberculosis and tuberculous enteritis are most common, involvement of the esophagus, stomach, colon, rectum, anus, liver, bile ducts, gallbladder, and pancreas can occur (Fig. 4). Diagnosis is challenging as cases often mimic neoplasm or inflammatory bowel disease. Clinicians should incorporate abdominal TB in their differential diagnosis, with key disease features in mind (Fig. 5). Definitive diagnosis is made via fluid and tissue analysis. All cases regardless of anatomic involvement warrant standard anti-tuberculous therapy. Invasive and specialized interventions are reserved for select complications.
Fig. 4.
Reported complications of tuberculous involving the gastrointestinal tract and associated viscera.
Fig. 5.
Findings that should increase suspicion of abdominal tuberculosis infection.
Contributor Information
Thomas Malikowski, Email: Malikowski.thomas@mayo.edu.
Thomas Smyrk, Email: Smyrk.Thomas@mayo.edu.
Laura Raffals, Email: Raffals.Laura@mayo.edu.
Vandana Nehra, Email: Nehra.Vandana@mayo.edu.
References
- 1.Global tuberculosis report. 2016. Geneva: World Health Organization.
- 2.Zumla A., Raviglione M., Hafner R., Fordham von Reyn C. Tuberculosis. N Engl J Med. 2013;368:745–755. doi: 10.1056/NEJMra1200894. [DOI] [PubMed] [Google Scholar]
- 3.Tuberculosis. Centers for Disease Control and Prevention.
- 4.Dasgupta A., Singh N., Bhatia A. Abdominal tuberculosis: a histopathological study with special reference to intestinal perforation and mesenteric vasculopathy. J Lab Phys. 2009;1:56–61. doi: 10.4103/0974-2727.59700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donoghue H.D., Holton J. Intestinal tuberculosis. Curr Opin Infect Dis. 2009;22:490–496. doi: 10.1097/QCO.0b013e3283306712. [DOI] [PubMed] [Google Scholar]
- 6.Sanai F.M., Bzeizi K.I. Systematic review: tuberculous peritonitis–presenting features, diagnostic strategies and treatment. Aliment Pharmacol Therap. 2005;22:685–700. doi: 10.1111/j.1365-2036.2005.02645.x. [DOI] [PubMed] [Google Scholar]
- 7.Horvath K.D., Whelan R.L. Intestinal tuberculosis: return of an old disease. Am J Gastroenterol. 1998;93:692–696. doi: 10.1111/j.1572-0241.1998.207_a.x. [DOI] [PubMed] [Google Scholar]
- 8.Kapoor V.K. Abdominal tuberculosis. Postgrad Med J. 1998;74:459–467. doi: 10.1136/pgmj.74.874.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall J.B. Tuberculosis of the gastrointestinal tract and peritoneum. Am J Gastroenterol. 1993;88:989–999. [PubMed] [Google Scholar]
- 10.Maynard-Smith L., Larke N., Peters J.A., Lawn S.D. Diagnostic accuracy of the Xpert MTB/RIF assay for extrapulmonary and pulmonary tuberculosis when testing non-respiratory samples: a systematic review. BMC Infect Dis. 2014;14:709. doi: 10.1186/s12879-014-0709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lawn S.D., Nicol M.P. Xpert(R) MTB/RIF assay: development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future Microbiol. 2011;6:1067–1082. doi: 10.2217/fmb.11.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott L.E., Beylis N., Nicol M. Diagnostic accuracy of Xpert MTB/RIF for extrapulmonary tuberculosis specimens: establishing a laboratory testing algorithm for South Africa. J Clin Microbiol. 2014;52:1818–1823. doi: 10.1128/JCM.03553-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kokuto H., Sasaki Y., Yoshimatsu S., Mizuno K., Yi L., Mitarai S. Detection of mycobacterium tuberculosis (MTB) in fecal specimens from adults diagnosed with pulmonary tuberculosis using the Xpert MTB/rifampicin test. Open Forum Infect Dis. 2015;2:ofv074. doi: 10.1093/ofid/ofv074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma S.K., Ryan H., Khaparde S. Index-TB guidelines: guidelines on extrapulmonary tuberculosis for India. Indian J Med Res. 2017;145:448–463. doi: 10.4103/ijmr.IJMR_1950_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nahid P., Dorman S.E., Alipanah N. Executive summary: official american thoracic society/centers for disease control and prevention/infectious diseases society of america clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2016;63:853–867. doi: 10.1093/cid/ciw566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewinsohn D.M., Leonard M.K., LoBue P.A. Official American thoracic society/infectious diseases society of America/centers for disease control and prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2017;64:111–115. doi: 10.1093/cid/ciw778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horsburgh C.R.J., Barry C.E.I., Lange C. Treatment of tuberculosis. N Engl J Med. 2015;373:2149–2160. doi: 10.1056/NEJMra1413919. [DOI] [PubMed] [Google Scholar]
- 18.Gitt S., Haddad F., Levenson S. Tuberculous peritonitis: an overlooked diagnosis. Hosp Pract (Off Ed) 1992;27:224–228. doi: 10.1080/21548331.1992.11705351. [DOI] [PubMed] [Google Scholar]
- 19.Lisehora G.B., Peters C.C., Lee Y.T., Barcia P.J. Tuberculous peritonitis–do not miss it. Dis Colon Rectum. 1996;39:394–399. doi: 10.1007/BF02054053. [DOI] [PubMed] [Google Scholar]
- 20.Aguado J.M., Pons F., Casafont F., San Miguel G., Valle R. Tuberculous peritonitis: a study comparing cirrhotic and noncirrhotic patients. J Clin Gastroenterol. 1990;12:550–554. [PubMed] [Google Scholar]
- 21.Shakil A.O., Korula J., Kanel G.C., Murray N.G., Reynolds T.B. Diagnostic features of tuberculous peritonitis in the absence and presence of chronic liver disease: a case control study. Am J Med. 1996;100:179–185. doi: 10.1016/s0002-9343(97)89456-9. [DOI] [PubMed] [Google Scholar]
- 22.Bhargava D.K., Shriniwas ChopraP., Nijhawan S., Dasarathy S., Kushwaha A.K. Peritoneal tuberculosis: laparoscopic patterns and its diagnostic accuracy. Am J Gastroenterol. 1992;87:109–112. [PubMed] [Google Scholar]
- 23.Manohar A., Simjee A.E., Haffejee A.A., Pettengell K.E. Symptoms and investigative findings in 145 patients with tuberculous peritonitis diagnosed by peritoneoscopy and biopsy over a five year period. Gut. 1990;31:1130–1132. doi: 10.1136/gut.31.10.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chow K.M., Chow V.C., Hung L.C., Wong S.M., Szeto C.C. Tuberculous peritonitis-associated mortality is high among patients waiting for the results of mycobacterial cultures of ascitic fluid samples. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2002;35:409–413. doi: 10.1086/341898. [DOI] [PubMed] [Google Scholar]
- 25.Ha H.K., Ko G.Y., Yu E.S. Intestinal tuberculosis with abdominal complications: radiologic and pathologic features. Abdom Imaging. 1999;24:32–38. doi: 10.1007/s002619900436. [DOI] [PubMed] [Google Scholar]
- 26.Tuberculosis (TB) Guidelines. Centers for Disease Control and Prevention.
- 27.Suri S., Gupta S., Suri R. Computed tomography in abdominal tuberculosis. Br J Radiol. 1999;72:92–98. doi: 10.1259/bjr.72.853.10341698. [DOI] [PubMed] [Google Scholar]
- 28.Wagner T.E., Huseby E.S., Huseby J.S. Exacerbation of Mycobacterium tuberculosis enteritis masquerading as Crohn's disease after treatment with a tumor necrosis factor-alpha inhibitor. Am J Med. 2002;112:67–69. doi: 10.1016/s0002-9343(01)01035-x. [DOI] [PubMed] [Google Scholar]
- 29.Almadi M.A., Ghosh S., Aljebreen A.M. Differentiating intestinal tuberculosis from Crohn's disease: a diagnostic challenge. Am J Gastroenterol. 2009;104:1003–1012. doi: 10.1038/ajg.2008.162. [DOI] [PubMed] [Google Scholar]
- 30.Dilauro S., Crum-Cianflone N.F. Ileitis: when it is not Crohn's disease. Curr Gastroenterol Rep. 2010;12:249–258. doi: 10.1007/s11894-010-0112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X., Liu X., Zou Y. Predictors of clinical and endoscopic findings in differentiating Crohn's disease from intestinal tuberculosis. Dig Dis Sci. 2011;56:188–196. doi: 10.1007/s10620-010-1231-4. [DOI] [PubMed] [Google Scholar]
- 32.Makharia G.K., Srivastava S., Das P. Clinical, endoscopic, and histological differentiations between Crohn's disease and intestinal tuberculosis. Am J Gastroenterol. 2010;105:642–651. doi: 10.1038/ajg.2009.585. [DOI] [PubMed] [Google Scholar]
- 33.Pulimood A.B., Amarapurkar D.N., Ghoshal U. Differentiation of Crohn's disease from intestinal tuberculosis in India in 2010. World J Gastroenterol. 2011;17:433–443. doi: 10.3748/wjg.v17.i4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pulimood A.B., Peter S., Ramakrishna B. Segmental colonoscopic biopsies in the differentiation of ileocolic tuberculosis from Crohn's disease. J Gastroenterol Hepatol. 2005;20:688–696. doi: 10.1111/j.1440-1746.2005.03814.x. [DOI] [PubMed] [Google Scholar]
- 35.Pulimood A.B., Ramakrishna B.S., Kurian G. Endoscopic mucosal biopsies are useful in distinguishing granulomatous colitis due to Crohn's disease from tuberculosis. Gut. 1999;45:537–541. doi: 10.1136/gut.45.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinheiro V.G., Ramos L.M., Monteiro H.S. Intestinal permeability and malabsorption of rifampin and isoniazid in active pulmonary tuberculosis. Braz J Infect Dis: Off Publ Braz Soc Infect Dis. 2006;10:374–379. doi: 10.1590/s1413-86702006000600003. [DOI] [PubMed] [Google Scholar]
- 37.Park Y.S., Jun D.W., Kim S.H. Colonoscopy evaluation after short-term anti-tuberculosis treatment in nonspecific ulcers on the ileocecal area. World J Gastroenterol. 2008;14:5051–5058. doi: 10.3748/wjg.14.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tony J., Sunilkumar K., Thomas V. Randomized controlled trial of DOTS versus conventional regime for treatment of ileocecal and colonic tuberculosis. Indian Journal Of Gastroenterology: Official Journal of the Indian Society of Gastroenterology 2008;27:19–21. [PubMed]
- 39.Katariya R.N., Sood S., Rao P.G., Rao P.L. Stricture-plasty for tubercular strictures of the gastro-intestinal tract. Br J Surg. 1977;64:496–498. doi: 10.1002/bjs.1800640713. [DOI] [PubMed] [Google Scholar]
- 40.Bhasin D.K., Sharma B.C., Dhavan S., Sethi A., Sinha S.K., Singh K. Endoscopic balloon dilation of ileal stricture due to tuberculosis. Endoscopy. 1998;30:S44. doi: 10.1055/s-2007-1001274. [DOI] [PubMed] [Google Scholar]
- 41.Rosario M.T., Raso C.L., Comer G.M. Esophageal tuberculosis. Digest Dis Sci. 1989;34:1281–1284. doi: 10.1007/BF01537279. [DOI] [PubMed] [Google Scholar]
- 42.Mokoena T., Shama D.M., Ngakane H., Bryer J.V. Oesophageal tuberculosis: a review of eleven cases. Postgrad Med J. 1992;68:110–115. doi: 10.1136/pgmj.68.796.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jain S.K., Jain S., Jain M., Yaduvanshi A. Esophageal tuberculosis: is it so rare? Report of 12 cases and review of the literature. Am J Gastroenterol. 2002;97:287–291. doi: 10.1111/j.1572-0241.2002.05456.x. [DOI] [PubMed] [Google Scholar]
- 44.Welzel T.M., Kawan T., Bohle W., Richter G.M., Bosse A., Zoller W.G. An unusual cause of dysphagia: esophageal tuberculosis. J Gastrointest Liver Dis: JGLD. 2010;19:321–324. [PubMed] [Google Scholar]
- 45.Changal K.H., Raina A.H., Parra R., Khan M.A. Esophageal tuberculosis; a rare cause of odynophagia: a case report. Egypt J Chest Dis Tuberc. 2013;62:349–351. [Google Scholar]
- 46.Musoglu A., Ozutemiz O., Tekin F., Aydin A., Savas R., Ilter T. Esophageal tuberculosis mimicking esophageal carcinoma. Turk J Gastroenterol: Off J Turk Soc Gastroenterol. 2005;16:105–107. [PubMed] [Google Scholar]
- 47.Leung V.K., Chan W.H., Chow T.L., Luk I.S., Chau T.N., Loke T.K. Oesophageal tuberculosis mimicking oesophageal carcinoma. Hong Kong Med J = Xianggang yi xue za zhi. 2006;12:473–476. [PubMed] [Google Scholar]
- 48.Huang Y.K., Wu Y.C., Liu Y.H., Liu H.P. Esophageal tuberculosis mimicking submucosal tumor. Interact Cardiovasc Thorac Surg. 2004;3:274–276. doi: 10.1016/j.icvts.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 49.Puri R., Khaliq A., Kumar M., Sud R., Vasdev N. Esophageal tuberculosis: role of endoscopic ultrasound in diagnosis. Dis esophagus: Off J Int Soc Dis Esophagus. 2012;25:102–106. doi: 10.1111/j.1442-2050.2011.01223.x. [DOI] [PubMed] [Google Scholar]
- 50.Eroglu A., Kurkcuoglu C., Karaoglanoglu N., Yilmaz O., Gursan N. Esophageal tuberculosis abscess: an unusual cause of dysphagia. Dis Esophagus: Off J Int Soc Dis Esophagus. 2002;15:93–95. doi: 10.1046/j.1442-2050.2002.00235.x. [DOI] [PubMed] [Google Scholar]
- 51.Grubbs B.C., Baldwin D.R., Trenkner S.W., McCabe R.P.,Jr., Maddaus MA. Distal esophageal perforation caused by tuberculosis. J Thorac Cardiovasc Surg. 2001;121:1003–1004. doi: 10.1067/mtc.2001.111196. [DOI] [PubMed] [Google Scholar]
- 52.Fang H.Y., Lin T.S., Cheng C.Y., Talbot A.R. Esophageal tuberculosis: a rare presentation with massive hematemesis. Ann Thorac Surg. 1999;68:2344–2346. doi: 10.1016/s0003-4975(99)01160-1. [DOI] [PubMed] [Google Scholar]
- 53.Devarbhavi H.C., Alvares J.F., Radhikadevi M. Esophageal tuberculosis associated with esophagotracheal or esophagomediastinal fistula: report of 10 cases. Gastrointest Endosc. 2003;57:588–592. doi: 10.1067/mge.2003.140. [DOI] [PubMed] [Google Scholar]
- 54.Griga T., Duchna H.W., Orth M. Tuberculous involvement of the oesophagus with oesophagobroncheal fistula. Digest Liver Dis: Off J Ital Soc Gastroenterol Ital Assoc Stud Liver. 2002;34:528–531. doi: 10.1016/s1590-8658(02)80113-x. [DOI] [PubMed] [Google Scholar]
- 55.Rao Y.G., Pande G.K., Sahni P., Chattopadhyay T.K. Gastroduodenal tuberculosis management guidelines, based on a large experience and a review of the literature. Can J Surg. 2004;47:364–368. [PMC free article] [PubMed] [Google Scholar]
- 56.Chaudhary A., Bhan A., Malik N., Dilawari J.B., Khanna S.K. Choledocho-duodenal fistula due to tuberculosis. Indian J Gastroenterol: Off J Indian Soc Gastroenterol. 1989;8:293–294. [PubMed] [Google Scholar]
- 57.Kitagawa T., Sato K., Maetani I. Pyeloduodenal fistula diagnosed by esophagogastroduodenoscopy. Ann Gastroenterol: Q Publ Hellenic Soc Gastroenterol. 2015;28:287. [PMC free article] [PubMed] [Google Scholar]
- 58.Kodaira Y., Shibuya T., Matsumoto K. Primary aortoduodenal fistula caused by duodenal tuberculosis without an abdominal aortic aneurysm: report of a case. Surg Today. 1997;27:745–748. doi: 10.1007/BF02384989. [DOI] [PubMed] [Google Scholar]
- 59.Chavhan G.E., Ramakantan R. Duodenal tuberculosis: radiological features on barium studies and their clinical correlation in 28 cases. J Postgrad Med. 2003;49:214–217. [PubMed] [Google Scholar]
- 60.Puri A.S., Sachdeva S., Mittal V.V. Endoscopic diagnosis, management and outcome of gastroduodenal tuberculosis. Indian J Gastroenterol: Off J Indian Soc Gastroenterol. 2012;31:125–129. doi: 10.1007/s12664-012-0203-3. [DOI] [PubMed] [Google Scholar]
- 61.Negi S.S., Sachdev A.K., Chaudhary A., Kumar N., Gondal R. Surgical management of obstructive gastroduodenal tuberculosis. Trop Gastroenterol: Off J Digest Dis Found. 2003;24:39–41. [PubMed] [Google Scholar]
- 62.Padussis J., Loffredo B., McAneny D. Minimally invasive management of obstructive gastroduodenal tuberculosis. Am Surg. 2005;71:698–700. [PubMed] [Google Scholar]
- 63.Alvares J.F., Devarbhavi H., Makhija P., Rao S., Kottoor R. Clinical, colonoscopic, and histological profile of colonic tuberculosis in a tertiary hospital. Endoscopy. 2005;37:351–356. doi: 10.1055/s-2005-861116. [DOI] [PubMed] [Google Scholar]
- 64.Misra S.P., Misra V., Dwivedi M., Gupta S.C. Colonic tuberculosis: clinical features, endoscopic appearance and management. J Gastroenterol Hepatol. 1999;14:723–729. doi: 10.1046/j.1440-1746.1999.01940.x. [DOI] [PubMed] [Google Scholar]
- 65.Kalvaria I., Kottler R.E., Marks I.N. The role of colonoscopy in the diagnosis of tuberculosis. J Clin Gastroenterol. 1988;10:516–523. doi: 10.1097/00004836-198810000-00008. [DOI] [PubMed] [Google Scholar]
- 66.Kim K.M., Lee A., Choi K.Y., Lee K.Y., Kwak J.J. Intestinal tuberculosis: clinicopathologic analysis and diagnosis by endoscopic biopsy. Am J Gastroenterol. 1998;93:606–609. doi: 10.1111/j.1572-0241.1998.173_b.x. [DOI] [PubMed] [Google Scholar]
- 67.Garcia-Diaz R.A., Ruiz-Gomez J.L., Rodriguez-Sanjuan J.C., Garcia-Palomo D., Gomez-Fleitas M. Perforation of the colon caused by intestinal tuberculosis. Dis Colon Rectum. 2006;49:927. doi: 10.1007/s10350-006-0542-1. author reply. [DOI] [PubMed] [Google Scholar]
- 68.Masood I., Majid Z., Rafiq A., Rind W., Zia A., Raza S. Multiple, pan-enteric perforation secondary to intestinal tuberculosis. Case Rep Surg. 2015;2015 doi: 10.1155/2015/318678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sultan S., Azria F., Bauer P., Abdelnour M., Atienza P. Anoperineal tuberculosis: diagnostic and management considerations in seven cases. Dis Colon Rectum. 2002;45:407–410. doi: 10.1007/s10350-004-6191-3. [DOI] [PubMed] [Google Scholar]
- 70.Puri A.S., Vij J.C., Chaudhary A. Diagnosis and outcome of isolated rectal tuberculosis. Dis Colon Rectum. 1996;39:1126–1129. doi: 10.1007/BF02081413. [DOI] [PubMed] [Google Scholar]
- 71.Tago S., Hirai Y., Ainoda Y., Fujita T., Takamori M., Kikuchi K. Perianal tuberculosis: a case report and review of the literature. World J Clin Cases. 2015;3:848–852. doi: 10.12998/wjcc.v3.i9.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Monkemuller K.E., Lewis J.B.Jr. Massive rectal bleeding from colonic tuberculosis. Am J Gastroenterol. 1996;91:1439–1441. [PubMed] [Google Scholar]
- 73.Das P.C., Radhakrishna K., Rao P.L. Rectal stricture: a complication of tuberculosis. J Pediatr Surg. 1996;31:983–984. doi: 10.1016/s0022-3468(96)90429-7. [DOI] [PubMed] [Google Scholar]
- 74.Essop A.R., Posen J.A., Hodkinson J.H., Segal I. Tuberculosis hepatitis: a clinical review of 96 cases. Q J Med. 1984;53:465–477. [PubMed] [Google Scholar]
- 75.Gallinger S., Strasberg S.M., Marcus H.I., Brunton J. Local hepatic tuberculosis, the cause of a painful hepatic mass: case report and review of the literature. Can J Surg. 1986;29:451–452. [PubMed] [Google Scholar]
- 76.Ozin Y., Parlak E., Kilic Z.M., Temucin T., Sasmaz N. Sclerosing cholangitis-like changes in hepatobiliary tuberculosis. Turk J Gastroenterol: Off J Turk Soc Gastroenterol. 2010;21:50–53. doi: 10.4318/tjg.2010.0049. [DOI] [PubMed] [Google Scholar]
- 77.Goh K.L., Pathmanathan R., Chang K.W., Wong N.W. Tuberculous liver abscess. J Trop Med Hyg. 1987;90:255–257. [PubMed] [Google Scholar]
- 78.Hussain W., Mutimer D., Harrison R., Hubscher S., Neuberger J. Fulminant hepatic failure caused by tuberculosis. Gut. 1995;36:792–794. doi: 10.1136/gut.36.5.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kok K.Y., Yapp S.K. Tuberculosis of the bile duct: a rare cause of obstructive jaundice. J Clin Gastroenterol. 1999;29:161–164. doi: 10.1097/00004836-199909000-00012. [DOI] [PubMed] [Google Scholar]
- 80.Alvarez S.Z. Hepatobiliary tuberculosis. J Gastroenterol Hepatol. 1998;13:833–839. doi: 10.1111/j.1440-1746.1998.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 81.Alvarez S.Z., Carpio R. Hepatobiliary tuberculosis. Dig Dis Sci. 1983;28:193–200. doi: 10.1007/BF01295113. [DOI] [PubMed] [Google Scholar]
- 82.Hersch C. Tuberculosis of the liver. a study of 200 cases. S Afr Med J = Suid-Afrikaanse tydskrif vir geneeskunde. 1964;38:857–863. [PubMed] [Google Scholar]
- 83.Maharaj B., Leary W.P., Pudifin D.J. A prospective study of hepatic tuberculosis in 41 black patients. Q J Med. 1987;63:517–522. [PubMed] [Google Scholar]
- 84.Chaudhary P. Hepatobiliary tuberculosis. Ann Gastroenterol: Q Publ Hellenic Soc Gastroenterol. 2014;27:207–211. [PMC free article] [PubMed] [Google Scholar]
- 85.Blangy S., Cornud F., Sibert A., Vissuzaine C., Saraux J.L., Benacerraf R. Hepatitis tuberculosis presenting as tumoral disease on ultrasonography. Gastrointest Radiol. 1988;13:52–54. doi: 10.1007/BF01889024. [DOI] [PubMed] [Google Scholar]
- 86.Reed D.H., Nash A.F., Valabhji P. Radiological diagnosis and management of a solitary tuberculous hepatic abscess. Br J Radiol. 1990;63:902–904. doi: 10.1259/0007-1285-63-755-902. [DOI] [PubMed] [Google Scholar]
- 87.Chan H.S., Pang J. Isolated giant tuberculomata of the liver detected by computed tomography. Gastrointest Radiol. 1989;14:305–307. doi: 10.1007/BF01889223. [DOI] [PubMed] [Google Scholar]
- 88.Alvarez S.Z., Sollano J.D.,Jr. ERCP in hepatobiliary tuberculosis. Gastrointest Endosc. 1998;47:100–104. [PubMed] [Google Scholar]
- 89.Chong VH. Hepatobiliary tuberculosis: a review of presentations and outcomes. South Med J. 2008;101:356–361. doi: 10.1097/SMJ.0b013e318164ddbb. [DOI] [PubMed] [Google Scholar]
- 90.Saukkonen J.J., Cohn D.L., Jasmer R.M. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174:935–952. doi: 10.1164/rccm.200510-1666ST. [DOI] [PubMed] [Google Scholar]
- 91.Bearer E.A., Savides T.J., McCutchan J.A. Endoscopic diagnosis and management of hepatobiliary tuberculosis. Am J Gastroenterol. 1996;91:2602–2604. [PubMed] [Google Scholar]
- 92.Poon R.T., Lo C.M., Fan S.T. Diagnosis and management of biliary obstruction due to periportal tuberculous adenitis. Hepatogastroenterology. 2001;48:1585–1587. [PubMed] [Google Scholar]
- 93.Mustard R.A., Mackenzie R.L., Gray R.G. Percutaneous drainage of a tuberculous liver abscess. Can J Surg. 1986;29:449–450. [PubMed] [Google Scholar]
- 94.Gowrinath K., Ashok S., Thanasekaran V., Rao K.R. Tuberculous cholecystitis. Int J Tuberc Lung Dis: Off J Int Union Tuberc Lung Dis. 1997;1:484–485. [PubMed] [Google Scholar]
- 95.Kumar K., Ayub M., Kumar M., Keswani N.K., Shukla H.S. Tuberculosis of the gallbladder. HPB Surg: World J Hepat Pancr Biliary Surg. 2000;11:401–404. doi: 10.1155/2000/21392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jain R., Sawhney S., Bhargava D., Berry M. Gallbladder tuberculosis: sonographic appearance. J Clin Ultrasound: JCU. 1995;23:327–329. doi: 10.1002/jcu.1870230511. [DOI] [PubMed] [Google Scholar]
- 97.Hahn S.T., Park S.H., Shin W.S., Kim C.Y., Shinn K.S. Gallbladder tuberculosis with perforation and intrahepatic biloma. J Clin Gastroenterol. 1995;20:84–86. doi: 10.1097/00004836-199501000-00022. [DOI] [PubMed] [Google Scholar]
- 98.Gupta N.M., Khaitan A., Singh V., Radotra B. Isolated gallbladder tuberculosis with postoperative biliary fistula. Endoscopy. 1998;30:S73–S74. doi: 10.1055/s-2007-1001357. [DOI] [PubMed] [Google Scholar]
- 99.Baraboutis I., Skoutelis A. Isolated tuberculosis of the pancreas. JOP: J Pancreas. 2004;5:155–158. [PubMed] [Google Scholar]
- 100.Bhatia V., Garg P.K., Arora V.K., Sharma R. Isolated pancreatic tuberculosis mimicking intraductal pancreatic mucinous tumor. Gastrointest Endosc. 2008;68:610–611. doi: 10.1016/j.gie.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 101.Rana S.S., Bhasin D.K., Rao C., Singh K. Isolated pancreatic tuberculosis mimicking focal pancreatitis and causing segmental portal hypertension. JOP: J Pancreas. 2010;11:393–395. [PubMed] [Google Scholar]
- 102.Mansoor J., Umair B. Primary pancreatic tuberculosis: a rare and elusive diagnosis. J Coll Phys Surg–Pak: JCPSP. 2013;23:226–228. [PubMed] [Google Scholar]
- 103.Fan S.T., Yan K.W., Lau W.Y., Wong K.K. Tuberculosis of the pancreas: a rare cause of massive gastrointestinal bleeding. Br J Surg. 1986;73:373. doi: 10.1002/bjs.1800730517. [DOI] [PubMed] [Google Scholar]
- 104.Jenney A.W., Pickles R.W., Hellard M.E., Spelman D.W., Fuller A.J., Spicer W.J. Tuberculous pancreatic abscess in an HIV antibody-negative patient: case report and review. Scand J Infect Dis. 1998;30:99–104. doi: 10.1080/003655498750003438. [DOI] [PubMed] [Google Scholar]
- 105.De Backer A.I. MorteleK.J., Bomans P., De Keulenaer B.L., Vanschoubroeck I.J., Kockx M.M. Tuberculosis of the pancreas: MRI features. AJR Am J Roentgenol. 2005;184:50–54. doi: 10.2214/ajr.184.1.01840050. [DOI] [PubMed] [Google Scholar]
- 106.Ahlawat S.K., Charabaty-Pishvaian A., Lewis J.H., Haddad N.G. Pancreatic tuberculosis diagnosed with endoscopic ultrasound guided fine needle aspiration. JOP: J Pancreas. 2005;6:598–602. [PubMed] [Google Scholar]
- 107.Gupta P., Guleria S., Agarwal S. Role of endoscopic ultrasound guided FNAC in diagnosis of pancreatic TB presenting as mass lesion: a case report and review of literature. Indian J Tuberc. 2011;58:120–124. [PubMed] [Google Scholar]